Abstract
Objective:
To characterize the clinical research landscape of pediatric drug resistant epilepsy (DRE) with a focus on neurotechnology.
Method:
We searched the ClinicalTrials.gov registry using the terms ‘epilepsy’ and ‘drug resistant’ for studies including participants age 0–17 years. Returns were grouped by intervention (e.g., neurotechnological, drug). Key trial features such as age range, trial status and outcomes were compared across interventions.
Results:
We identified 101 registered trials with pediatric DRE patients. Thirty-two (32%) investigate neurotechnological interventions, devices, or diagnostic procedures; 13 (41%) are currently active. Among neurotechnology trials, 15 (46%) investigate vagus nerve stimulation, transcranial direct current stimulation, or deep brain stimulation; few are specific to children. Of the remaining 69 trials, 37 investigate a drug, 17 investigate a dietary therapy, and 15 investigate another intervention. Seizure frequency is the most frequent primary outcome measured in the trials identified.
Significance:
The landscape of registered trials pertaining to pediatric DRE reflects a lag between clinical research and clinical practice, and highlights the need for timely evidence before novel neurotechnological interventions are widely adopted into clinical practice.
Keywords: Clinical Trials, drug resistant epilepsy, neurotechnological interventions
Introduction
More than 500,000 children in the USA and Canada suffer from epilepsy today.1 Unmanaged, epilepsy can result in cognitive decline, social isolation and poor quality of life, and has substantial economic impact on families and society.2 Up to 30% of children with epilepsy continue to have seizures while on anti-seizure medication, a condition known as drug resistant epilepsy (DRE). Novel neurotechnologies, including minimally invasive and neuromodulatory options for seizure control, represent a new frontier for improving the subjective and objective quality of life for pediatric DRE patients3, but best practices for treatment do not currently exist. Properly selected, up to 70% of DRE patients can become seizure-free after surgery.4
Neurosurgical interventions to treat pediatric DRE have evolved significantly over recent decades. The rapid emergence of different technologies with varied attributes compounds the complexity of decision-making and counseling, especially for patients whose brains are still developing.5 There is evidence that many of these novel neurotechnologies are already being used in clinical practice,6,7 amplified by industry pressure on providers and familes to make use of them.8,9 Unlike novel pharmaceutical interventions, however, innovations involving neurosurgical treatment of DRE are less likely to undergo rigorous testing through randomized controlled trials, leaving health care providers and parents with gaps and uncertainty in evidence and knowledge regarding the efficacy, safety and long-term side effects. The objective of this work was to examine and characterize the clinical research landscape of pediatric DRE, with a special focus on neurotechnological interventions. We hypothezised that within the clinical area of drug-resistant epilepsy, there are comparatively few trials evaluating novel neurotechnology relative to pharmaceuticals, and that even fewer focus exclusively on pediatric patients.
Methods
Data Source
We searched ClinicalTrials.gov, an NIH-hosted public listing of publically and privately supported clinical studies on a wide range of diseases and conditions,10 specifically for records of studies using the search words ‘epilepsy’ and ‘drug-resistant’ (last update: July 2019). We retained any trial that included participants aged to 0–17, regardless of epilepsy/seizure type, study design, location or trial status for analysis.
Two reviewers (KJK and MA) reviewed the retrieved data independently, assessed their eligibility for inclusion, and grouped them into categories according to the intervention studied.
Variables
Trials were categorized into one of four categories (neurotechnology, drug, dietary, or other) according to the intervention under evaluation. Trial status was categorized as active, not active, completed, and unknown status. The ‘active’ category comprised trials listed as ‘available’ (i.e., for expanded access), ‘recruiting’, ‘active, not yet recruiting’, ‘approved for marketing’, and ‘enrolling by invitation’. The ‘not active’ category comprised trials listed as ‘terminated’, ‘not yet recruiting’, ‘no longer available’ and ‘withdrawn’.
The age inclusion criteria for each trial was grouped according to specific age range included in the trial: child only (0–17 years of age), child and adult (0–65 years of age), and child, adult and older adult (0–65 years and older).
Intervention endpoints were classified according to their primary and secondary outcome measures. Primary outcomes were grouped into seizure-related outcome (e.g., frequency, reduction in number, percentage of seizure free patients), adverse events, quality of life, cognition, tolerability, other and missing. Each trial was also assessed and grouped into one of 13 different categories according to the combination of primary and secondary outcome endpoints included in the trial.
The start year of each trial was determined by the date listed in the individual trial record. If no start date was available, a start date was imputed from the first posted date. The trial location was grouped by continent/region of the trial, and a separate classification was included for multinational and international trials. The location of each trial was based on the country listed in the individual trial record. If no location was listed, the location listed for the sponsor of the trial was used.
Analysis
We conducted a comparative analysis of the trials based on specific intervention grouping, trial status, age ranges, and intervention endpoints. Analyses were conducted using RStudio and Excel.
Results
We identified 101 registered trials/studies in which pediatric patients with DRE were eligible to participate. There were 37 drug trials, 32 neurotechnology trials, 17 dietary trials and 15 trials evaluating miscellaneous interventions, such as a behavioral intervention, a genetic screening program and an education/training program. There were no coding disagreements between the reviewers.
Trials classified as neurotechnology consist of a range of interventions, including MRI-guided laser interstitial thermal therapy (LITT), robot assisted stereo EEG (rSEEG), stereotactic radiosurgery (SRS), vagus nerve stimulation (VNS), deep brain stimulation (DBS), and transcranial direct current stimulation (TDCS). The most commonly investigated neurotechnological intervention was VNS (n=8, 25%), followed by TDCS (n=4, 13%), and DBS (n=3, 9%). Cannabidiol (n=17, 46%) was the most common drug intervention. Trials coded as dietary interventions include the Low Glycemic Index Therapy (n=12, 71%), Modified Atkins Diet (n=4, 23%) and a dietary supplement (n=1, 6%). A full list of trials categorized as ‘other’ is acessible through the online supplement.
The highest number of trials were registered in Europe (n= 41), with almost 50% (n=20) of those located in France. Over two thirds of those trials (n=28, 68%) were drug trials. North America (n=37) has the second highest number of registered trials, of which 31 (84%) are being conducted in the United States. In contrast with Europe, trials conducted in North America are primarily neurotechnological (n=20, 54%).
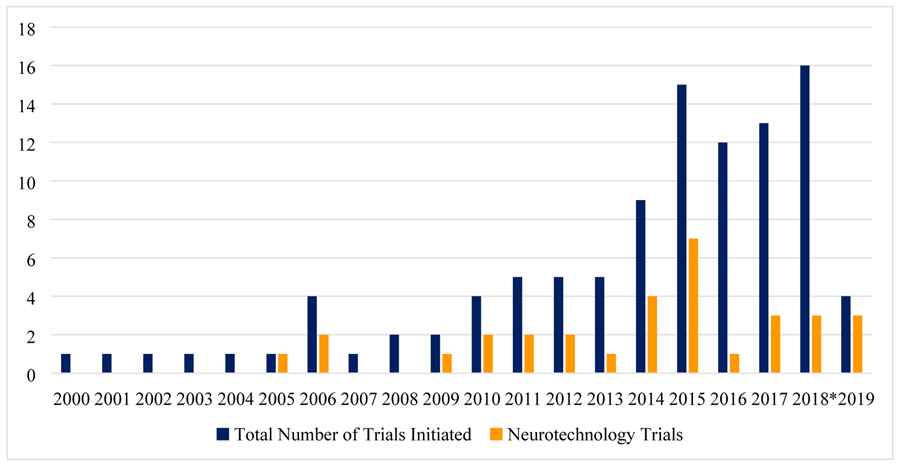
Overall, the number of registered DRE-related trials increased steadily over the last decade (Figure 1). The absolute number of neurotechnology trials increased, although at a slower rate than trials investigating a drug or dietary therapy. Proportionally, neurotechnology trials account for 18–23% of all DRE trials in 2017 and 2018 compared with 40–50% of all trials in most years between 2009 and 2015. Of trials investigating neurotechnology (i.e., neurotechnological intervention, device, or diagnostic procedure) 13 (41%) are listed as active, five (n=16%) as inactive, and eight (25%) are listed as complete, of which three have posted results.
Figure 1:
Number of initiated trials over time (last update - July 2019)
The eligible age range of trials varies depending on the type of intervention. Trials investigating a drug, dietary or another intervention tend to be more age-specific and restrictive in their age eligibility criteria; between 26% and 53% of the trials we identified in these areas recruited only pediatric populations. In contrast, trials involving neurotechnology seem to have extended age ranges that include adults and older adults. Only 13% are conducted with children and youth exclusively.
Seizure activity (e.g., percentage of seizure free participants, reduction in number of seizures) is the most common primary outcome of neurotechnology (n=18, 56%) and dietary trials (n=14, 82%). In the DRE drug trials we evaluated, there was an almost even split between seizure activity (n=13, 35%) and adverse events (n=14, 37%) as primary endpoint. None of the trials we classified as neurotechnology evaluated adverse events as their primary endpoint. Ten trials classified as neurotechnology evaluated a combination of primary and secondary outcome endpoints that included seizures, adverse events and quality of life; three trials classified as neurotechnological assessed cognitive change such as IQ and memory improvement as a secondary outcome endpoint. Trial outcome endpoints classified as ‘other’ included a qualtitative genetic analysis, a lateralization index and assessment of allelic frequency of genes involved in epileptogenesis (see online supplement for full list of trial outcome endpoints categorized as ‘other’).
Discussion
The number of DRE-related clinical trials has significantly expanded over the last decade with many new trials initiated. The majority of trials were/are evaluating drug interventions. The absolute number of DRE-related neurotechnology trials was steady, after a temporary increase in 2014 and 2015, but declined as a proportion of all identified trials in DRE. Neurotechnology DRE trials are less likely to be exclusively pediatric, which may suggest that the quality and specificity of evidence in this area will lag behind other interventions. The main outcome endpoint evaluated was seizure frequency, but the variety of primary and secondary outcomes endpoints reflects the range of impacts of DRE, the potential benefits of treatment, and the complexity of factors that need to be taken into consideration by parents and health care providers in decision-making. While not the objective of this work, little is known about what is most important to patients, caregivers and physicians in making treatment decisions. Better understanding of the priorities for outcomes of these groups is needed to guide the design of future research involving neurotechnology and better support informed treatment decision-making.
Overall, the landscape of registered neurotechnology trials reflects a lag between clinical research and clinical practice, and highlights the need for timely evidence before novel interventions are widely adopted into clinical practice.
Limitations
The use of the ClinicalTrials.gov registry for research purposes has several limitations and our results have to be interpreted within the respective context of these. First, registered studies are an incomplete sample of the clinical research enterprise as incentives and norms for registering, updating information, and posting results for trials/studies vary by intervention type, funding source, date of study initiation, geographic location and regulatory jurisdiction.10 Second, change in recruitment status after registering a trial is technically challenging and may bias the classification of the ‘trial status’ variable.
Conclusions
To our knowledge, this is the first study to assess and describe the landscape of clinical research involving neurotechnological interventions in the treatment of pediatric DRE using data retrieved from the ClinicalTrials.gov registry. Our findings suggest that high quality information from clinical research to support decision-making about the use of neurotechnological interventions likely lags behind their introduction and use in clinical practice. We also found that seizure frequency is the primary focus of trials involving a neurotechnological intervention, device or diagnostic procedure. These findings highlight a need for timely, comprehensive evidence before novel interventions are widely adopted into clinical practice.
Supplementary Material
Table 1:
Trial Characteristics by Intervention Group (last update - July 2019)
| Intervention Groups | ||||
|---|---|---|---|---|
| Neurotechnology | Drug | Diet | Other | |
| N= 32 (32%) | N= 37 (37%) | N= 17 (17%) | N= 15 (15%) | |
| Trial Status | ||||
| Active | 13 | 20 | 2 | 9 |
| Not active | 5 | 9 | 1 | 2 |
| Completed (*Results available) | 8 (*3) | 7 (*5) | 10 | 2 |
| Unknown Status | 6 | 1 | 4 | 2 |
| Age Group | ||||
| Child (0 – 17 years) | 4 | 10 | 9 | 4 |
| Child, Adult (0 – 60 years) | 8 | 19 | 5 | 3 |
| Child, Adult, Older Adult (0 – 65+ years) | 20 | 8 | 3 | 8 |
| Primary Outcome | ||||
| Seizure | 18 | 13 | 14 | 4 |
| Adverse events | 0 | 14 | 1 | 1 |
| Quality of life | 1 | 0 | 0 | 2 |
| Cognition | 0 | 1 | 0 | 1 |
| Tolerability | 1 | 1 | 1 | 0 |
| Other | 12 | 5 | 1 | 7 |
| Missing | 0 | 3 | 0 | 0 |
| All Outcomes | ||||
| Seizure only | 6 | 8 | 3 | 1 |
| Seizure and adverse events | 1 | 13 | 6 | 1 |
| Seizure and quality of life | 5 | 1 | 1 | 0 |
| Seizure and cognition | 1 | 0 | 1 | 1 |
| Seizure, adverse events and quality of life | 4 | 2 | 1 | 2 |
| Seizure, adverse events and cognition | 1 | 1 | 1 | 0 |
| Seizure, adverse events, quality of life and cognition | 1 | 1 | 2 | 0 |
| Quality of life only | 0 | 0 | 0 | 1 |
| Adverse events only | 0 | 4 | 0 | 1 |
| Cognition only | 0 | 0 | 0 | 1 |
| Tolerability | 1 | 1 | 1 | 0 |
| Other | 12 | 3 | 1 | 7 |
| Missing | 0 | 3 | 0 | 0 |
| Location | ||||
| Europe | 20 | 5 | 6 | 10 |
| North America | 6 | 28 | 0 | 3 |
| Asia | 4 | 2 | 7 | 0 |
| Middle East | 0 | 1 | 3 | 0 |
| Multi-National/International | 2 | 1 | 1 | 2 |
ACKNOWLEGMENTS
JI is Canada Research Chair in Neuroethics. PJM is the Alcan Chair in Neurosciences at UBC. This work was supported by the National Institutes of Health (NIH/NIMH) 1RF1MH117805 Informing Choice for Neurotechnological Innovation in Pediatric Epilepsy Surgery, J. Illes, PI and P. McDonald, Co-PI.
Footnotes
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
References
- 1.Prasad AN, Sang X, Corbett BA, Burneo JG. Prevalence of Childhood Epilepsy in Canada. Can J Neurol Sci J Can Sci Neurol 2011. September;38(5):719–22. [DOI] [PubMed] [Google Scholar]
- 2.Prasad AN, Burneo JG, Corbett B. Epilepsy, comorbid conditions in Canadian children: Analysis of cross-sectional data from Cycle 3 of the National Longitudinal Study of Children and Youth. Seizure 2014. November;23(10):869–73. [DOI] [PubMed] [Google Scholar]
- 3.Joshi SM, Singh RK, Shellhaas RA. Advanced Treatments for Childhood Epilepsy: Beyond Antiseizure Medications. JAMA Pediatr 2013. January 1;167(1):76. [DOI] [PubMed] [Google Scholar]
- 4.Muh CR. Current and Emerging Surgical Therapies for Severe Pediatric Epilepsies. Semin Pediatr Neurol 2016. May;23(2):143–50. [DOI] [PubMed] [Google Scholar]
- 5.Berg AT, Baca CB, Loddenkemper T, Vickrey BG, Dlugos D. Priorities in pediatric epilepsy research: Improving children’s futures today. Neurology. 2013. September 24;81(13):1166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McHugh JC, Singh HW, Phillips J, Murphy K, Doherty CP, Delanty N. Outcome Measurement after Vagal Nerve Stimulation Therapy: Proposal of a New Classification. Epilepsia. 2007. February;48(2):375–8. [DOI] [PubMed] [Google Scholar]
- 7.Hader WJ, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L, Wiebe S, Kwon C-S, et al. Complications of epilepsy surgery-A systematic review of focal surgical resections and invasive EEG monitoring. Epilepsia. 2013. May;54(5):840–7. [DOI] [PubMed] [Google Scholar]
- 8.Grundy Q, Hutchison K, Johnson J, Blakely B, Clay-Wlliams R, Richards B, et al. Device representatives in hospitals: are commercial imperatives driving clinical decision-making? J Med Ethics. 2018. September;44(9):589–92. [DOI] [PubMed] [Google Scholar]
- 9.Hines JZ, Lurie P, Yu E, Wolfe S. Left to Their Own Devices: Breakdowns in United States Medical Device Premarket Review. PLoS Med 2010. July 13;7(7):e1000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tse T, Fain KM, Zarin DA. How to avoid common problems when using ClinicalTrials.gov in research: 10 issues to consider. BMJ 2018. May 25;k1452. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.