Abstract
In this study, we sought to determine the burden and characteristics of orgasmic dysfunction (OD) and concomitant erectile dysfunction (ED) in men with type 1 diabetes (T1D) enrolled in the Epidemiology of Diabetes Interventions and Complications (EDIC) study. In 2010, we assessed orgasmic and erectile function using the International Index of Erectile Function (IIEF). Sociodemographic, clinical and diabetes characteristics were compared by OD status (OD only, OD and ED, no ED or OD). Age-adjusted associations between risk factors and OD status were examined. OD and ED information was available from 563 men. Eighty-three men (14.7%) reported OD of whom 21 reported OD only and 62 reported OD and ED. Age-adjusted odds ratios demonstrated that men who reported OD only had higher odds of depression, low sexual desire and decreased alcohol use compared to men reporting no dysfunction. Men with OD concomitant with ED had greater odds of elevated hemoglobin A1C, peripheral and autonomic neuropathy, and nephropathy. Men reporting both dysfunctions were also more likely to report smoking, lower urinary tract symptoms and had greater odds of androgen deficiency than men with no sexual dysfunction. Men with longstanding T1D suffer from an increased burden of OD. Psychogenic factors predominate in men reporting OD only while men who present with concomitant ED report increased burden of diabetes severity, characteristics previously observed with incident ED. ED may be the central impediment to sexual function in men with OD and ED. Longitudinal studies to characterize OD and ED experience over time are warranted.
Keywords: Diabetes, Orgasmic Dysfunction, Erectile Dysfunction, Type 1 diabetes
INTRODUCTION
Increased rates of diabetes and aging of the diabetic population are likely to lead to an increase in diabetes-related complications, including those related to sexual function.1 While the negative effect of diabetes on erectile dysfunction (ED) has been studied extensively,2 studies examining the burden of other aspects of sexual dysfunction, specifically orgasmic dysfunction (OD) in men with diabetes and its association with diabetes-related factors are far more limited.3,4 ED is defined as the inability to achieve or sustain an erection suitable for sexual intercourse while OD is defined as the inability to ejaculate and/or have feelings of orgasm or climax during sexual arousal and stimulation. (NIH consensus,Jenkins) To our knowledge, no prior studies have highlighted the burden of and risk factors for OD independent of and concomitant with ED in men with type 1 diabetes (T1D).
The Diabetes Control and Complications Trial (DCCT), the landmark multicenter, randomized controlled trial designed to determine whether intensive treatment regimen of tight glucose control could impact development of diabetic complications, demonstrated that an average of 6.5 years of intensive glycemic control reduced the risk of proliferative retinopathy, nephropathy, and cardiovascular disease by 35-76%.5 All men enrolled in the ongoing follow-up study, the Epidemiology of Diabetes Interventions and Complicatons (EDIC) were invited to participate in the UroEDIC Study, an ancillary study to examine the presence of urologic complications, including erectile dysfunction during EDIC year 10 (2003). Intensive glycemic control reduced the onset of ED in this cohort of participants5. To better understand the impact of T1D on orgasmic function, we evaluated the association between diabetes- related factors and OD independently and concomitantly with ED, in men with T1D.
MATERIALS AND METHODS
Study Population
The DCCT and EDIC studies have been previously described in detail.6,7 Briefly, 1,441 subjects with T1D for 1-15 years with no (primary prevention cohort) or minimal diabetic retinopathy (secondary intervention cohort) were enrolled in DCCT. Subjects were randomly assigned to either intensive or conventional treatment and were followed for 3-9 years (mean 6.5 years). At the end of DCCT, intensive therapy was recommended for all subjects, subjects in the conventional treatment group were trained in intensive therapy, and all subjects returned to their own health care providers for ongoing diabetes care. Annual EDIC examinations began in 1994, one year after completion of the DCCT, and 1,375 (96%) of former DCCT subjects consented to participate in EDIC, including 720 men. A detailed description of EDIC study procedures and baseline characteristics has been published.7 At EDIC year 17 in 2010, 644 of 669 men in EDIC (96% response rate) agreed to participate in UroEDIC II, the followup to UroEDIC, an ancillary study designed to examine the urologic complications of diabetes in 2003. Among the men who agreed to participate in UroEDIC II, 563 provided information on erectile and orgasmic dysfunction. Burden and correlates of ED in this cohort have been examined and previously published,8 therefore the current analyses excluded the 177 men who reported ED only and the remaining 386 comprise the study cohort for this report (Figure 1). Distribution of OD and ED in the 386 male participants in presented in Figure 2. Institutional review boards of all participating centers approved all DCCT/EDIC procedures and all participants provided written informed consent.
Figure 1.
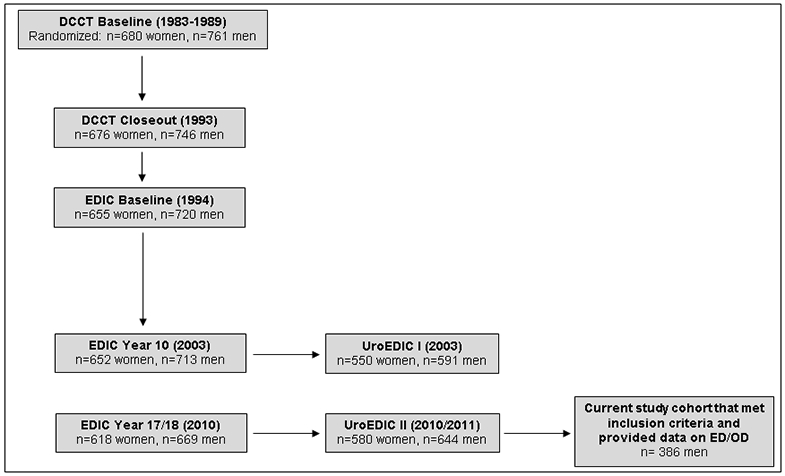
DCCT, EDIC, UroEDIC and Current Study Population
Figure 2.
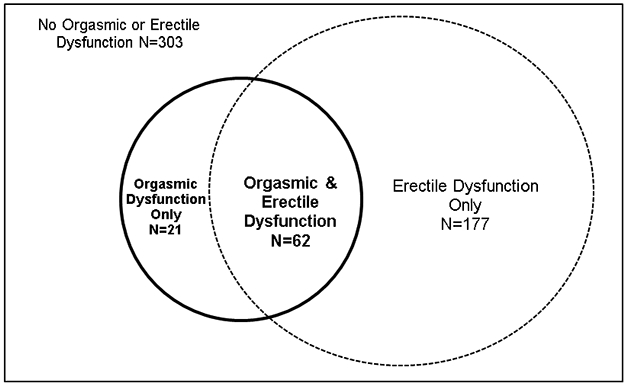
Distribution of Orgasmic and Erectile Dysfunction in Male UroEDIC Participants at EDIC Year 17
Data Sources and Variables of Interest
Information on sexual function was collected using the International Index of Erectile Function (IIEF), a validated questionnaire that assesses erectile function, sexual desire, and orgasmic function as well as quality of life as related to overall sexual function.9 ED was ascertained based on a single question from the IIEF: Over the past 4 weeks, how would you rate your confidence to get and keep an erection? This single question, which assesses the confidence of a man in his ability to get and keep an erection, has been shown in prior studies to strongly correlate with the IIEF erectile function domain composite scores.10 In addition, this item correlates well with bother due to erectile problems and global sexual bother, and thus serves as a proxy for global sexual function and bother.10 Additionally, this item has been found to be suitable for use in clinical and epidemiologic studies of male sexual dysfunction and it’s utilization avoids respondent burden and missing data. Reponses range from “very low” (1 points) to “very high” (5 points), with ED defined as a score of ≤2. OD was defined using the validated orgasmic function domain of the IIEF questionnaire that consists of 2 questions examining orgasmic function over the past 4 weeks: Over the past 4 weeks, when you had sexual stimulation or intercourse, how often did you ejaculate? and Over the past 4 weeks, when you had sexual stimulation or intercourse, how often did you have the feeling of orgasm or climax?” Responses range from “no sexual stimulation/intercourse” (0 points) to “almost always/always” (5 points). Responses of 0 (no sexual stimulation/intercourse) to either question were removed with OD defined in the remaining sample as a total score of ≤6 (moderate/severe) on the two items.
Hemoglobin A1c (HbA1c) was measured at baseline and quarterly during DCCT and annually in EDIC as previously described.7 For purposes of this analysis, we used time-weighted HbA1c levels, representing total glycemic exposure during DCCT/EDIC with weights of 0.25 and 1 for quarterly DCCT and annual EDIC values, respectively. Retinopathy was assessed using fundus photographs that were centrally graded using the Early Treatment Diabetic Retinopathy Study (ETDRS) scale and defined as proliferative diabetic retinopathy (PDR) or worse through EDIC year 14. Nephropathy was defined as microalbuminuria (AER 30-300 mg/24hr) or albuminuria (AER >300 mg/24hr) or end stage renal disease at EDIC year 15/16. Peripheral neuropathy was determined at EDIC year 17 using the Michigan Neuropathy Screening Instrument (MNSI)11 and defined as greater than 6 positive responses on the MNSI questionnaire or a score of greater than 2 on the MNSI examination. Abnormal cardiovascular autonomic neuropathy (CAN) function was defined as: either R-R variation<15 or R-R variation between 15-19.9 plus either a Valsalva ratio≤1.5 or a supine-to-standing drop in diastolic blood pressure of ≥10 mm Hg.12 Hypertension was defined as sitting SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg or the use of antihypertensive medication.13
Androgen deficiency was defined as total testosterone concentration <300mg/dL(Bhasin) based on serum samples obtained in the morning for a majority of the participants. Lower urinary tract symptom (LUTS) severity was determined using the American Urological Association Symptom Index (AUASI).14 Moderate or severe LUTS was defined as an AUASI score ≥8. Depression was defined by a composite depression variable based on study coordinator ratings of clinical depression using DSM-IV criteria and patient self-report of use of antidepressant medications and/or psychological counseling for depressive symptoms. General health status was measured using two subscales on physical and social function from the 36-Item Short Form Health Survey (SF-36).15
Statistical Analyses
Descriptive analyses examined the distribution of sociodemographic and clinical characteristics, markers of diabetes control, treatment, and complications by OD status (OD only, ED and OD, no ED or OD). The Kruskal-Wallis test assessed differences in quantitative variables and the contingency chi-square test assessed categorical variables. Separate logistic regression models estimated the associations between each risk factor and OD status at EDIC year 17 after adjustment for age. All analyses were performed using SAS® version 9.4.
RESULTS
Table 1 presents sociodemographic, clinical, and sexual characteristics of the study population by OD status. Overall, men reporting OD only (n=21) or both OD & ED (n=62) were significantly older, less often married, reported greater use of tobacco and lower use of alcohol and had lower SF-36 physical and social function scores compared to those reporting no OD or ED (n=303). In addition, we observed increased HbA1c levels and higher rates of nephropathy, peripheral and autonomic neuropathy and other comorbidities including hypertension and LUTS in men with OD only or both OD & ED compared to the men with no OD or ED. Further, pairwise tests suggest men with OD and concomitant ED are significantly older and have higher HbA1c levels compared to men with OD only. (data not shown)
Table 1.
Characteristics of the study population by prevalent cases of orgasmic (OD) and erectile (ED) dysfunction at EDIC year 17
| No OD or ED (n=303) |
OD only (n=21) |
OD & ED (n=62) |
P-value | |
|---|---|---|---|---|
| Sociodemographic/Clinical | ||||
| Age (years) | 49.5±6.4 | 50.1±5.5 | 54.6±6.0 | <0.0001 |
| Married | 235 (78) | 14 (67) | 47 (77) | 0.4632 |
| College education | 199 (66) | 14 (67) | 34 (56) | 0.2823 |
| Current smoker | 28 (9) | 2 (10) | 13 (21) | 0.0254 |
| Current drinker | 159 (53) | 6 (29) | 28 (46) | 0.0705 |
| Body Mass Index (BMI) (kg/m2) | 28.3±4.3 | 28.3±3.6 | 29.4±5.7 | 0.5075 |
| Body Mass Index category | ||||
| Normal (BMI<25) | 67 (23) | 4 (20) | 13 (22) | 0.5872 |
| Overweight (25≤BMI<30) | 138 (46) | 9 (45) | 21 (36) | |
| Obese (BMI≥30) | 92 (31) | 7 (35) | 24 (41) | |
| Hypertension* | 188 (63) | 15 (71) | 49 (80) | 0.0255 |
| Total testosterone (ng/dL) | 560.0±192.8 | 539.7±190.9 | 524.7±234.0 | 0.3151 |
| Androgen deficient (Testosterone<300) | 16 (5) | 3 (14) | 8 (14) | 0.0327 |
| AUA Symptom Index (points) | 4.7±4.2 | 6.5±3.8 | 8.4±6.3 | <0.0001 |
| Moderate/Severe LUTS (AUASI≥8) | 53 (17) | 6 (29) | 30 (48) | <0.0001 |
| BPH medication use | 2 (1) | 0 (0) | 4 (6) | ----- |
| Depression | 46 (15) | 7 (33) | 19 (31) | 0.0036 |
| Antidepressant medication use | 71 (23) | 10 (48) | 27 (44) | 0.0007 |
| SF-36 sub-scales† | ||||
| Physical function | 92.5±13.2 | 82.2±19.2 | 75.3±22.2 | <0.0001 |
| Social function | 82.7±13.4 | 71.7±17.5 | 73.4±18.6 | <0.0001 |
| Diabetes Control and Treatment | ||||
| DCCT cohort (primary prevention) | 165 (54) | 11 (52) | 29 (47) | 0.5422 |
| DCCT treatment group (intensive) | 154 (51) | 8 (38) | 32 (52) | 0.5150 |
| Duration of T1D (years) | 29.3±4.9 | 28.7±4.5 | 29.8±4.6 | 0.6535 |
| Time-weighted DCCT/EDIC HbA1c (%) | 7.7±0.9 | 7.9±1.0 | 8.3±1.0 | 0.0002 |
| Insulin dose (U/kg/day) | 0.7±0.3 | 0.8±0.3 | 0.8±0.3 | 0.3191 |
| Diabetes Complications | ||||
| Retinopathy‡ | 50 (17) | 4 (19) | 16 (26) | 0.2216 |
| Nephropathy§ | 16 (5) | 2 (10) | 9 (16) | <0.0001 |
| Neuropathy | ||||
| Abnormal MNSI¶ | 100 (34) | 10 (50) | 37 (62) | 0.0002 |
| Composite CAN∥ | 77 (26) | 8 (38) | 35 (49) | <0.0001 |
| Sexual Characteristics | ||||
| Engaged in sexual activity | 288 (95) | 21 (100) | 50 (81) | 0.0001 |
| ED medication use | 0 (0) | 0 (0) | 11 (18) | ----- |
| Other ED treatment | 0 (0) | 0 (0) | 10 (16) | ----- |
| Low sexual desire | 90 (30) | 13 (62) | 29 (55) | <0.0001 |
Note: Data are Mean±Std or N (%). P-values based the Kruskal-Wallis test for quantitative variables or the Contingency chi-square for qualitative variables. Sample sizes may vary due to missing data.
Hypertension defined as sitting systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP)≥90 mmHg or the use of antihypertensive medication.
Microvascular complications:
SF-36 scores range from 0 to 100, where 100 indicates a more favorable quality of life.
Retinopathy defined as PDR or worse up through EDIC year 14 using the Early Treatment Diabetic Retinopathy Study on a scale of 0-23 (≥12 proliferative diabetic retinopathy).
Nephropathy defined as any AER≥300 mg/24hr or ESRD at EDIC year 15/16.
Neuropathy defined as an abnormal MNSI at EDIC year 17 by the Michigan Neuropathy Screening Instrument >6 responses on the 4questionnaire or a score of >2 on the exam.
Composite CAN function defined as either R-R variation<15 or R-R variation between 15-19.9 plus either a Valsalva ratio ≤1.5 or a supine-to-standing drop of 10 mm Hg in diastolic blood pressure at EDIC year 16/17.
Table 2 presents age-adjusted odds ratios estimating associations between sociodemographic, clinical and diabetes characteristics among men with OD only or with OD and ED, each compared to men without OD or ED. Compared to men without OD or ED, men reporting OD only had a significantly higher odds of depression (OR=2.82, 95%CI=1.08,7.37), an increased odds of low sexual desire (OR=3.74, 95%CI=1.50,9.34), and a decreased report of alcohol consumption (OR=0.35, 95%CI=0.13,0.92). While there were statistically significant associations between report of OD only and physical and social role function as measured by the short form health survey (SF36), the effects were small. Beyond associations observed for men reporting OD only, men with concomitant OD and ED had significantly higher odds of androgen deficiency (OR=3.53, 95%CI=1.29,9.71), LUTS (OR=3.52, 95%CI=1.92,6.47), and smoking (OR=2.37, 95%CI=1.10,5.09). Concomitant OD and ED was also associated with several diabetes factors including time-weighted DCCT/EDIC HbA1C% (OR=2.47, 95%CI=1.74, 3.52), nephropathy (OR=4.04, 95%CI=1.53, 10.64), peripheral neuropathy (OR=2.28, 95%CI=1.25, 4.17) and cardiovascular autonomic neuropathy (OR=2.89, 95%CI=1.57, 5.32).
Table 2.
Age-adjusted odds ratios (95% CI) estimating associations between sociodemographic, clinical and diabetes characteristics and OD and/or ED at EDIC year 17
| Characteristic | OD only (N=21) vs. No OD or ED (N=303) |
OD & ED (N=62) vs. No ED or OD (N=303) |
|---|---|---|
| Sociodemographic/Clinical | ||
| Age (years) | 1.01 (0.95,1.09) | 1.14 (1.08,1.20) |
| Married (yes vs. no) | 0.53 (0.20,1.39) | 0.60 (0.30,1.24) |
| College education (yes vs. no) | 1.02 (0.40,2.62) | 0.64 (0.36,1.15) |
| Current smoker (yes vs. no) | 1.00 (0.22,4.56) | 2.37 (1.10,5.09) |
| Current drinker (yes vs. no) | 0.35 (0.13,0.92) | 0.65 (0.36,1.16) |
| Body Mass Index category | ||
| Overweight (25≤BMI<30) vs. Normal (BMI<25) | 1.09 (0.32,3.68) | 0.82 (0.37,1.80) |
| Obese (BMI≥30) vs. Normal (BMI<25) | 1.28 (0.36,4.53) | 1.41 (0.64,3.09) |
| Hypertension* (yes vs. no) | 1.47 (0.55,3.92) | 1.96 (0.97,3.94) |
| Androgen deficient (Testosterone<300) (yes vs. no) | 3.00 (0.80,11.27) | 3.53 (1.29,9.71) |
| Moderate/Severe LUTS (AUASI≥8) (yes vs. no) | 1.86 (0.68,5.05) | 3.52 (1.92,6.47) |
| Depression (yes vs. no) | 2.82 (1.08,7.37) | 2.69 (1.38,5.24) |
| Antidepressant medication use (yes vs. no) | 3.00 (1.22,7.33) | 2.54 (1.40,4.64) |
| SF-36 sub-scales† | ||
| Physical function | 0.97 (0.95,0.99) | 0.95 (0.93,0.96) |
| Social function | 0.96 (0.94,0.98) | 0.96 (0.94,0.97) |
| Diabetes Control and Treatment | ||
| DCCT cohort (secondary intervention vs. primary prevention) | 1.08 (0.45,2.62) | 1.26 (0.71,2.23) |
| DCCT treatment group (conventional vs. intensive) | 1.71 (0.69,4.27) | 1.07 (0.60,1.91) |
| Duration of T1D (years) | 0.97 (0.88,1.07) | 1.00 (0.95,1.06) |
| Time-weighted DCCT/EDIC Hemoglobin A1c (%) | 1.21 (0.74,1.98) | 2.47 (1.74,3.52) |
| Insulin dose (1U/kg/day) | 1.76 (0.51,6.05) | 1.54 (0.65,3.65) |
| Diabetes Complications | ||
| Retinopathy‡ (yes vs. no) | 1.21 (0.39,3.76) | 1.89 (0.96,3.75) |
| Nephropathy§ (yes vs. no) | 1.89 (0.40,8.89) | 4.04 (1.53,10.64) |
| Neuropathy | ||
| Abnormal MNSI¶ (yes vs. no) | 1.94 (0.77,4.91) | 2.28 (1.25,4.17) |
| Composite CAN∥ (yes vs. no) | 1.74 (0.67,4.50) | 2.89 (1.57,5.32) |
| Sexual Characteristics | ||
| Engaged in sexual activity (yes vs. no) | - | 0.19 (0.07,0.46) |
| Low sexual desire (yes vs. no) | 3.74 (1.50,9.34) | 2.64 (1.41,4.94) |
Note: Data are odds ratios and 95% confidence intervals from age-adjusted logistic regression models.
Hypertension defined as sitting systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP)≥90 mmHg or the use of antihypertensive medication.
Microvascular complications:
SF-36 scores range from 0 to 100, where 100 indicates a more favorable quality of life.
Retinopathy defined as PDR or worse up through EDIC year 14 using the Early Treatment Diabetic Retinopathy Study on a scale of 0-23 (≥12 proliferative diabetic retinopathy).
Nephropathy defined as any AER≥300 mg/24hr or ESRD at EDIC year 15/16.
Neuropathy defined as an abnormal MNSI at EDIC year 17 by the Michigan Neuropathy Screening Instrument >6 responses on the questionnaire or a score of >2 on the exam.
Composite CAN function defined as either R-R variation<15 or R-R variation between 15-19.9 plus either a Valsalva ratio≤1.5 or a supine-to-standing drop of 10 mm Hg in diastolic blood pressure at EDIC year 16/17.
DISCUSSION
This is the first study to our knowledge that highlights the burden of and risk factors for OD independent of and with concomitant ED in men with T1D. Our results suggest that men reporting OD only present a predominantly psychogenic phenotype as demonstrated by high associations with depression, decreased alcohol intake and low sexual desire while men with OD with concomitant ED demonstrate a mixed phenotype with a physiological predominance, characterized by increased diabetes severity and metabolic dysfunction.
Our report of overall OD prevalence in men with T1D (14.7%) is indeed greater than estimates reported in community-based samples of men, suggesting an impact of diabetes on orgasmic function.16,17 Data from the National Health and Social Life Survey (NHSLS) report that 9% of men 40-59 years of age indicate an inability to achieve orgasm with estimates as low as 7% in men younger than 39 years of age.18 Our estimate of the burden of OD, however, is lower than that reported among men with diabetes in other cohorts. Specifically, in the National Social Life, Health and Aging Project (NSHAP), 26.1% of men with diagnosed diabetes with age 57-84 years, report an inability to climax compared to 15.9% of men without diabetes (p<0.05).3 Similarly, in a population-based sample of men aged 40-79 years from the Olmsted County Study (OCS), Burke et al. demonstrated that 31% of the cohort with diabetes reported ejaculatory dysfunction compared to 7% of the cohort without diabetes.4 Our lower estimate may be explained by the older ages of the NSHAP and OCS cohorts. Further, it is likely that the majority of the NSHAP and OCS participants had type 2 diabetes. While hyperglycemia is a common feature of both type 1 and type 2 diabetes, a review of studies examining ED in diabetic animal models suggest that there are distinct mechanisms underlying the ED phenotype by type of diabetes.19 However, literature on the effects of diabetes type on orgasmic function in humans is lacking.
Among men who report OD only, we observed a near 3-fold increased odds of depression based on a composite definition of diagnosis and medication use compared to men reporting no sexual dysfunction. These observations are consistent with others who also demonstrated significant correlations of OD with depression and associated antidepressant medication use.20,21 While it might be expected that the impact of depression on OD is psychogenic, the physiological contribution of selective serotonin reuptake inhibitors (SSRIs) specifically on ejaculatory function is consistent with inhibitory effects of descending serotonergic neurons from the brainstem on ejaculatory responses. In fact 48% of men reporting OD only indicated antidepressent medication use.22 In addition, we observed that men who endorsed current alcohol intake had lower odds of OD only, which can be attributed to a delay in orgasm. A prior study demonstrated that increased alcohol consumption was associated with an increased latency of ejaculation which may in some men alleviate anxiety and prolong sexual intercourse.23 Finally, consistent with the literature,24 we observed a near 4-fold increased odds of low sexual desire in men with OD only relative to men reporting no sexual dysfunction.
While factors associated with report of OD only in this cohort may be considered primarily psychogenic, men reporting OD with concomitant ED portray a more severe diabetes phenotype (i.e. poorer glycemic control, presence of micro/macrovascular complications). This is supported by the significant associations observed between time-weighted HbA1c levels, presence of nephropathy, peripheral and autonomic neuropathy, and self-report of concomitant OD and ED. We also observed that men with OD with concomitant ED had increased odds of androgen deficiency. Low testosterone has been associated with weaker orgasms in prior reports and may also explain the significant increased reports of low sexual desire in this group of men as reduced libido is considered to be the most prominent symptom associated with low testosterone.25,26 Finally, we observed a 3.5-fold greater odds of moderate/severe LUTS in men with concomitant OD and ED. It is possible that increased noradrenergic nerve activity associated with bladder outlet obstruction, an important component of LUTS, may also interfere with the normal process of erection and ejaculation.27 In addition, LUTS-associated discomfort itself may impede psychological processes necessary for orgasm.
Interestingly, all of the factors observed to be important in men with concomitant OD and ED in the current report were previously demonstrated to be significantly associated with onset of ED in a prior analysis of this cohort which examined risk factors for the development of ED over time.8 Specifically, in our previous report, men who developed ED had significantly higher HbA1c levels, and greater prevalence of peripheral neuropathy and self-report of LUTS compared to men who did not develop ED. The similar findings of associated risk factors in the prior report and the significant overlap of OD and ED in the current report (75% of men with OD reported concomitant ED) suggest that ED may be a central impediment to healthy sexual function and risk factors observed to be elevated in men with concomitant OD and ED may in fact just represent the ED experience. Erections are often antecedent to orgasms and difficulty with erections may translate to orgasmic dysfunction. Additionally, men and healthcare providers are often unaware that orgasm can be achieved without erections. The lack of awareness and subsequent lack of sexual stimulation in presence of ED may contribute to an increase patient-reported concomitant OD and ED. Longitudinal studies examining the natural history and temporal sequence of co-occurring OD and ED are warranted.
While the orgasmic function domain of the IIEF was validated to be scored as the sum of the responses to questions on ejaculation frequency and climax/orgasm, the two questions do in fact describe different functions. While we observed significant concordance between the responses to the ejaculation and orgasm questions (Kappa=0.73, chi-square p-value<0.0001), up to 15% of men with no or decreased sensation of orgasm reported normal ejaculation frequency. This underscores the importance of the differentiation between orgasm and ejaculation, which in clinical practice are often considered interchangeable phenomena. While the sample size to comprehensively examine these functions independently among the 21 men who reported OD only was too small, descriptive analyses suggests factors identified among these men overall to be associated with OD only were the same among men reporting ejaculation function and orgasm/climax function separately (data not shown). Additional studies with larger samples examining these individual functions are warranted.
While strengths of this report include its large and well-characterized population of men with type 1 diabetes, several limitations should be noted. First, the DCCT/EDIC participants are a highly motivated group of individuals who have been followed for many years, therefore, these results may not be generalizable to a broader population of men with T1D. Second, this study lacks a control group of men without diabetes and thus does not allow for a true comparative analysis. Third, ED was ascertained based on a single question from the IIEF which assesses the confidence of a man in his ability to get and keep an erection regardless of partnership. Importantly, this item has been shown in prior studies to strongly correlate with the IIEF erectile function domain composite scores and correlates well with bother due to erectile problems and global sexual bother.10 Fourth, the limited use of the Likert scale for items of the orgasm domain (removal of responses of 0) may have an impact on face validity of the domain score. This approach was taken as we were unable to distinguish whether an answer of 0 indicated dysfunction or lack of interest or partner. Further, given the relatively young age of our cohort and narrow age range, we were limited in our ability to better understand the effect of declining sexual function with age. Finally, our overall sample size of participants reporting OD is small, particularly in the OD only group, which affected our ability to build multivariable models adjusting for potential confounders and potentially detect other statistically significant associations. However, our estimates of effect provide insight into the potential impact of diabetes related factors on sexual function to be vetted in further larger studies.
CONCLUSIONS
Men with longstanding type 1 diabetes suffer from an increased burden of OD both independently and with concomitant ED. Psychogenic factors predominate in men reporting OD only while men who report concomitant OD and ED have worse glycemic control and more diabetic-related complications. These characteristics have previously been associated with increased ED severity suggesting that ED may be the central impediment to sexual function in this group of men. Increasing patient and physician awareness of factors associated with sexual dysfunction in this popuation may lead to increased self-efficacy and improved diabetes management. Future longitudinal studies to characterize OD and ED as well as other domains of sexuality such as sexual desire and satisfaction in type 1 diabetes are warranted.
Supplementary Material
ACKNOWLEDGMENTS
A complete list of participants in the DCCT/EDIC Research Group is presented in the Supplementary Material published online for the article in N Engl J Med 2017;376:1507-16.
Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Insulet Corporation (Bedford, MA) , Lifescan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Ft. Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN) , and Sanofi-Aventis (Bridgewater, NJ).
Funding/Support: The DCCT/EDIC has been supported by cooperative agreement grants (1982-1993, 2012-2017, 2017-2022), and contracts (1982-2012) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Disease (current grant numbers U01 DK094176 and U01 DK094157), and through support by the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the General Clinical Research Centers Program (1993-2007), and Clinical Translational Science Center Program (2006-present), Bethesda, Maryland, USA.
The authors acknowledge Brandon Haynes contributions to preliminary analyses.
Trial Registration: clinicaltrials.gov NCT00360815 and NCT00360893
Additional statement for collaborators: Additional support for this DCCT/EDIC collaborative study (UroEDIC) was provided by an R01 grant (2009-2013) with the National Institute of Diabetes and Digestive and Kidney Disease (5R01DK083927-03).
Footnotes
Conflict of interest: Authors have no conflicts of interest to disclose.
REFERENCES
- 1.Brown JS, Wessells H, Chancellor MB, et al. : Urologic Complications of Diabetes. Diabetes Care 2005; 28: 177–185. [DOI] [PubMed] [Google Scholar]
- 2.Wessells H: Insights and interventions in diabetes associated erectile dysfunction. J. Urol 2013; 190: 15–16. [DOI] [PubMed] [Google Scholar]
- 3.Lindau ST, Tang H, Gomero A, et al. : Sexuality among middle-aged and older adults with diagnosed and undiagnosed diabetes: A national, population-based study. Diabetes Care 2010; 33: 2202–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke JP, Jacobson DJ, McGree ME, et al. : Diabetes and sexual dysfunction: results from the Olmsted County study of urinary symptoms and health status among men. J Urol 2007; 177: 1438–1442. [DOI] [PubMed] [Google Scholar]
- 5.Nathan DM: The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 Years: Overview. Diabetes Care 2014; 37: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Diabetes Control and Complications Trial Research Group: Diabetes Control and Complications Trial (DCCT): Results of Feasibility Study. The DCCT Research Group. Diabetes Care 1987; 10: 1–19. [DOI] [PubMed] [Google Scholar]
- 7.Shamoon H, Cleary P, Barnie A, et al. : Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999; 22: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wessells H, Penson DF, Cleary P, et al. : Effect of Intensive Glycemic Therapy on Erectile Function in Men With Type 1 Diabetes. J. Urol 2011; 185: 1828–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen RC, Riley A, Wagner G, et al. : The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997; 49: 822–830. [DOI] [PubMed] [Google Scholar]
- 10.Penson DF, Wessells H, Cleary P, et al. : Sexual dysfunction and symptom impact in men with long-standing type 1 diabetes in the DCCT/EDIC cohort. J Sex Med 2009; 6: 1969–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herman WH, Pop-Busui R, Braffett BH, et al. : Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet. Med 2012; 29: 937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin CL, Albers JW, Pop-Busui R, et al. : Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014; 37: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Boer IH, Kestenbaum B, Rue TC, et al. : Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Arch. Intern. Med. 2008; 168: 1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barry MJ, Fowler FJ Jr., O’Leary MP, et al. : The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J. Urol 1992; 148: 1549–57; discussion 1564. [DOI] [PubMed] [Google Scholar]
- 15.Ware JEJ: SF-36 Health Survey: Manual and Interpretation Guide. Boston, MA: The Health Institute; 1993. [Google Scholar]
- 16.Spector IP and Carey MP: Incidence and prevalence of the sexual dysfunctions: A critical review of the empirical literature. Arch. Sex. Behav 1990. [DOI] [PubMed]
- 17.Simons JS and Carey MP: Prevalence of sexual dysfunctions: Results from a decade of research. Arch. Sex. Behav 1990; 19: 389–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laumann EO, Paik A and Rosen RC: Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999; 281: 537–544. [DOI] [PubMed] [Google Scholar]
- 19.Chitaley K: Type 1 and Type 2 diabetic-erectile dysfunction: same diagnosis (ICD-9), different disease? J Sex Med 2009; 6 Suppl 3: 262–268. [DOI] [PubMed] [Google Scholar]
- 20.Sadovsky R and Nusbaum M: Sexual health inquiry and support is a primary care priority. J. Sex. Med 2006; 3: 3–11. [DOI] [PubMed] [Google Scholar]
- 21.Paduch DA, Bolyakov A, Beardsworth A, et al. : Factors associated with ejaculatory and orgasmic dysfunction in men with erectile dysfunction: Analysis of clinical trials involving the phosphodiesterase type 5 inhibitor tadalafil. BJU Int. 2012; 109: 1060–1067. [DOI] [PubMed] [Google Scholar]
- 22.Waldinger MD and Olivier B: Selective serotonin reuptake inhibitor-induced sexual dysfunction: Clinical and research considerations. Int. Clin. Psychopharmacol 1998; 13 Suppl 6. [DOI] [PubMed] [Google Scholar]
- 23.Malatesta VJ, Pollack RH, Wilbanks WA, et al. : Alcohol Effects on the Orgasmic-Ejaculatory Response in Human Males. J. Sex Res 1979; 15: 101–107. [DOI] [PubMed] [Google Scholar]
- 24.Rosen RC: Prevalence and risk factors of sexual dysfunction in men and women. Curr. Psychiatry Rep 2000; 2: 189–195. [DOI] [PubMed] [Google Scholar]
- 25.Morley JE: Testosterone and behavior. Clin. Geriatr. Med 2003; 19: 605–616. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto AM: ‘Andropause’--are reduced androgen levels in aging men physiologically important? [editorial; comment]. West. J. Med 1993; 159: 618–620. [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen R, Altwein J, Boyle P, et al. : Lower Urinary Tract Symptoms and Male Sexual Dysfunction: The Multinational Survey of the Aging Male (MSAM-7). Eur. Urol 2003; 44: 637–649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


