Abstract
Purpose:
Assess follow-up treatment and clinical outcomes at 5 years in eyes initially treated with anti-VEGF therapy for center involved diabetic macular edema (CI-DME) in a 2-year randomized clinical trial.
Design:
Multicenter cohort study.
Participants:
Participants with DME and visual acuity (VA) 20/32 to 20/320 enrolled in DRCR.net Protocol T with visits 5 years after randomization (3 years after Protocol T completion).
Methods:
Participants were assigned randomly to aflibercept, bevacizumab, or ranibizumab with protocol-defined follow-up and retreatment for 2 years. Thereafter, participants were managed at clinician discretion and recalled for a 5-year visit.
Main Outcome Measures:
Anti-VEGF treatment, VA letter score, and central subfield thickness.
Results:
Sixty-eight percent (317 of 463) of eligible participants completed the 5-year visit. Between years 2 and 5, 68% (217 of 317) of study eyes received at least one anti-VEGF treatment (median [interquartile range] 4 [0, 12]). At 5 years, mean VA improved from baseline by 7.4 letters (95% confidence interval [CI]: 5.9 to 9.0), but decreased by 4.7 letters (95%CI: 3.3 to 6.0) between 2 and 5 years. When baseline VA was 20/50 to 20/320, mean 5-year VA was 11.9 letters (95%CI: 9.3 to 14.5) better than baseline, but 4.8 letters (95% CI: 2.5 to 7.0) worse than 2 years. When baseline VA was 20/32 to 20/40, mean 5-year VA was 3.2 letters (95% CI: 1.4 to 5.0) better than baseline, but 4.6 letters (95% CI: 3.1 to 6.1) worse than 2 years. Mean central subfield thickness decreased from baseline to 5 years by 154μm (95% CI: 142 to 166) and was stable between 2 and 5 years (−1 μm [95% CI: −12 to 9]).
Conclusions:
Among the two-thirds of eligible Protocol T participants who completed a 5-year visit, mean VA improved from baseline to 5 years without protocol-defined treatment after follow-up ended at 2 years. Although mean retinal thickness was similar at 2 and 5 years, mean VA worsened during this period. Additional investigation into strategies to improve long-term outcomes in eyes with DME seems warranted to determine if VA can be better maintained with different management approaches.
Précis:
Five-year VA improved from baseline after randomization to anti-VEGF for DME in Protocol T. During standard clinical care for 3 years after protocol end (at Year 2), VA decreased without a change in retinal thickness.
Introduction
Randomized clinical trials have established intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy as first-line treatment for visual impairment from center-involved diabetic macular edema (CI-DME).1-3 However, limited data exist on long-term outcomes once study participants discontinue protocol-defined retreatment and are placed into standard clinical care. A large retrospective study of electronic medical records suggests outcomes with standard care are worse than those reported in clinical trials for DME.4 Long-term visual acuity (VA), retinal thickness and treatment burden are relevant to understanding the course of DME.
The DRCR Retina Network conducted a randomized clinical trial (Protocol T) comparing aflibercept (Eylea), bevacizumab (Avastin), and ranibizumab (Lucentis) for eyes with visual impairment from CI-DME. A protocol retreatment regimen was followed for 2 years; results demonstrated improved mean VA using each agent. When baseline VA impairment was mild (20/32-20/40), there were no apparent differences between agents for mean change in VA from baseline at 1 year (primary outcome) or 2 years. However, when baseline VA impairment was more severe (20/50-20/320), aflibercept was superior to both bevacizumab and ranibizumab at 1 year and to bevacizumab at 2 years.5,6
Protocol T ended after 2 years of protocol-defined retreatment regimens. Participants were asked to return at 5 years from randomization to assess clinical outcomes and characterize follow-up treatments for DME and diabetic retinopathy (DR).. .
Methods
Protocol T Randomized Clinical Trial
Methods for Protocol T have been published elsewhere with the complete protocol available online.5 The study adhered to the tenets of the Declaration of Helsinki. Study participants provided written informed consent. Institutional review boards for all participating sites approved the protocol and Health Insurance Portability and Accountability Act-compliant informed consent forms. Eighty-eight sites enrolled 660 adults with diabetes mellitus between August 2012 and August 2013. One eye per participant was enrolled with best corrected electronic-Early Treatment Diabetic Retinopathy Study VA letter score of 78 to 24 (Snellen equivalent 20/32 to 20/320), CI-DME on optical coherence tomography (OCT) and clinical examination, and no anti-VEGF treatment within 1 year of randomization. Eyes were randomly assigned with equal probability to intravitreous injections of aflibercept (2.0 mg), bevacizumab (1.25 mg), or ranibizumab (0.3 mg). Each agent was administered based on the same treatment algorithm.
During Protocol T, visits were scheduled every 4 weeks in year 1 and every 4 to 16 weeks in year 2, depending on treatment response. At each visit, eyes were assessed for retreatment based on VA and OCT criteria. After Protocol T participation concluded at 2 years, follow-up and treatment were performed as standard care. Participants were notified of the primary study results and their individual treatment allocation after the primary results were published in February 2015.
Five-Year Follow-up Methods
The follow-up study was conducted between August 2017 and April 2019 with 76% (67 of 88) of the Protocol T sites. Seven of the non-participating sites were no longer part of the DRCR Retina Network and other reasons for non-participation included lack of personnel or interest. Participants who were not known to be deceased at the end of 2 years were targeted for participation. Informed consent was obtained from participants who could be contacted and were interested in participating. At the 5-year visit, medical and ocular history since the 2-year visit were collected from chart reviews. Certified examiners obtained best-corrected VA, OCT, eye exams, and color fundus photographs using the same procedures as in the randomized trial. To identify deceased participants, name, birthdate, state, and date of last contact for participants who could not be located were submitted to the National Death Index.
Statistical Methods
Descriptive statistics were computed for the number of clinic visits and treatments after Protocol T and clinical outcomes at 5 years. Statistical analyses included comparisons between the original treatment group assignments. Continuous outcomes were analyzed with general linear models. Binary outcomes were analyzed using logistic regression; the risk difference was estimated via conditional standardization with confidence intervals calculated with the delta method.7 Baseline value of the outcome variable was included as a covariate in regression models. Within each outcome, the Hochberg step-up procedure was used to control the Type 1 error in multiple treatment group comparisons.8 To minimize the influence of potential outliers, changes in VA and CST from baseline and 2 years were truncated to ± 3 standard deviations from the mean change from baseline. Analyses were conducted in SAS 9.4. All tests were two-sided and adjusted P values of less than .05 were considered statistically significant.
Results
At the 67 participating sites, there were 588 participants not known to have died prior to the 2-year visit. Ninety-five (17%) participants died between 2 and 5 years [12 identified from National Death Index search], leaving 463 eligible. Of these individuals, 317 (68%) completed the follow-up study between August 2017 and April 2019 (Figure S1), representing 48% (317 of 660) of the original Protocol T cohort. The time from the start of Protocol T to completion of the follow-up study ranged from 4.5 to 6.0 years (mean 5.1). Among 5-year completers, 115 (36%), 96 (30%), and 106 (33%) had been assigned to the aflibercept, bevacizumab, and ranibizumab treatment arms, respectively, in the randomized trial.
Among the 317 individuals who participated in the follow-up study versus the 146 who did not participate (and were not known to be deceased), mean baseline VA letter scores were 65.6 (Snellen equivalent 20/50) and 61.5 (Snellen equivalent 20/63), respectively (P<.001). Change in VA from baseline to 2 years was similar between participants (12.1 letters) and non-participants (12.5 letters, P=0.32) although mean 2-year VA letter score was higher among participants than non-participants (77.7 [Snellen equivalent 20/32] versus 73.6 [Snellen equivalent 20/40], P=.03). Mean number of anti-VEGF injections through 2 years was also similar among participants and non-participants (14.8 injections versus 14.6 injections; P=.74). Supplemental Table 1 shows baseline and 2-year demographics according to 5-year participation status.
Clinical Care after Randomized Trial
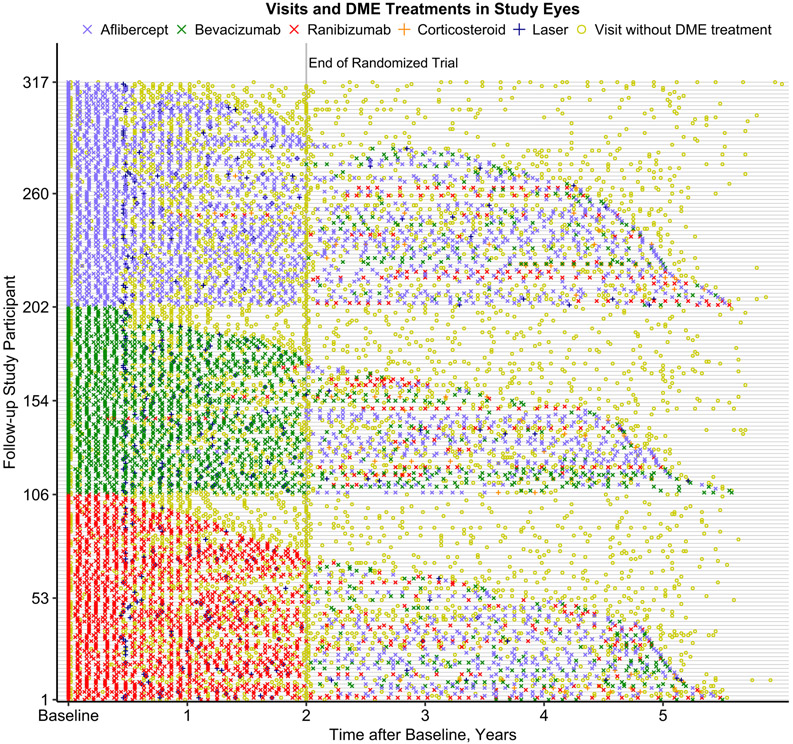
Among 5-year participants, 301 (95%) had at least one office visit with a retina specialist after Protocol T. The median (interquartile range [IQR]) number of office visits with a retina specialist between 2 and 5 years was 12 (5-21); 4 (2-7) in year 3, 4 (1-7) in year 4, and 4 (1-7) in year 5 (Table 1). Eighteen participants (6%) had at least one visit with a retina specialist at a non-Protocol T clinical site. Only 2% (104 of 4299) of visits with a retina specialist were completed somewhere other than a Protocol T clinical site. Figure 1 depicts the 5-year DME management for each participant. The figure illustrates the reduced injection frequency post 2 years, the frequency of visits in which DME treatment was not performed, and the eyes in each treatment group that received a non-randomized anti-VEGF agent.
Table 1.
Visits and Treatments in Years 3 to 5 by Randomized Treatment Group
| Visits and treatments in the study eye | All | Aflibercept | Bevacizumab | Ranibizumab |
|---|---|---|---|---|
| N | 317 | 115 | 96 | 106 |
| Visits in years 3 to 5, median (IQR) | 12 (5, 21) | 12 (5, 21) | 13 (7, 20) | 12 (5, 21) |
| Visits by year, median (IQR) | ||||
| Year 3 | 4 (2, 7) | 4 (1, 7) | 4 (2, 8) | 3 (1, 8) |
| Year 4 | 4 (1, 7) | 4 (1, 7) | 4 (2, 7) | 4 (1, 7) |
| Year 5 | 4 (1, 7) | 4 (1, 7) | 3 (1, 7) | 4 (1, 7) |
| Any treatments in years 3 to 5 for | ||||
| Diabetic macular edema, N (%) | 208 (66%) | 80 (70%) | 62 (65%) | 66 (62%) |
| Diabetic retinopathy, N (%) | 62 (20%) | 25 (22%) | 15 (16%) | 22 (21%) |
| DME or DR, N (%) | 221 (70%) | 83 (72%) | 65 (68%) | 73 (69%) |
| Any anti-VEGF injections in years 3 to 5, N (%) | 217 (68%) | 81 (70%) | 65 (68%) | 71 (67%) |
| Anti-VEGF injections in years 3 to 5 | ||||
| Mean (SD) | 6.7 (7.5) | 7.1 (7.5) | 6.5 (7.8) | 6.5 (7.3) |
| Median (IQR) | 4 (0, 12) | 4 (0, 13) | 2 (0, 13) | 4 (0, 11) |
| Anti-VEGF injections by year, median (IQR) | ||||
| Year 3 | 1 (0, 4) | 1 (0, 4) | 1 (0, 4) | 1 (0, 3) |
| Year 4 | 1 (0, 4) | 1 (0, 4) | 0 (0, 3) | 1 (0, 4) |
| Year 5 | 0 (0, 4) | 1 (0, 4) | 0 (0, 3) | 0 (0, 4) |
| Anti-VEGF treatment in years 3 to 5, N (%) | ||||
| None | 100 (32%) | 34 (30%) | 31 (32%) | 35 (33%) |
| Aflibercept only | 70 (22%) | 36 (31%) | 21 (22%) | 13 (12%) |
| Bevacizumab only | 56 (18%) | 19 (17%) | 18 (19%) | 19 (18%) |
| Ranibizumab only | 31 (10%) | 7 (6%) | 11 (11%) | 13 (12%) |
| More than one drug | 60 (19%) | 19 (17%) | 15 (16%) | 26 (25%) |
| Aflibercept injections in years 3 to 5, median (IQR) | 0 (0, 6) | 0 (0, 8) | 0 (0, 4) | 0 (0, 6) |
| Bevacizumab injections in years 3 to 5, median (IQR) | 0 (0, 2) | 0 (0, 2) | 0 (0, 1) | 0 (0, 2) |
| Ranibizumab injections in years 3 to 5, median (IQR) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 1) |
| Any Corticosteroid injections in years 3 to 5, N (%) | 32 (10%) | 12 (10%) | 13 (14%) | 7 (7%) |
| Any PRP treatments in years 3 to 5, N (%) | 31 (10%) | 10 (9%) | 9 (9%) | 12 (11%) |
| Any Focal/grid laser treatments in years 3 to 5, N (%) | 25 (8%) | 12 (10%) | 6 (6%) | 7 (7%) |
Abbreviations: DME = diabetic macular edema; DR = diabetic retinopathy; IQR = interquartile range; PRP = panretinal photocoagulation SD = standard deviation; VEGF = vascular endothelial growth factor.
Figure 1.
Distribution of office visits and diabetic macular edema (DME) treatments for follow-up study participants from baseline to 5 years after initial randomization into a 2-year clinical trial on anti-vascular endothelial growth factor (VEGF) treatment for eyes with DME (Protocol T). Each row along the vertical axis represents a timeline for a follow-up study participant. Symbols indicate office visits and DME treatments in the study eye (only one study eye per participant). Participants are ordered along the vertical axis by randomized treatment group assignment and the time of the last observed DME treatment.
From years 2 to 5, 221 (70%) eyes received treatment for DR with or without DME with 208 (66%) receiving DME treatment and 62 (20%) receiving treatment for just DR (Table 1). Two hundred seventeen (68%) eyes received at least one anti-VEGF treatment, with 24 (8%) receiving 20 or more injections (Figure 2). The median (IQR) number of anti-VEGF treatments over the 3 years following the 2-year visit was 4 (0, 12) with 2097 of 2123 (99%) administered at a Protocol T clinical site. Seventy (22%) eyes received aflibercept only, 56 (18%) received bevacizumab only, 31 (10%) received ranibizumab only, 60 (19%) received multiple types of anti-VEGF agents, and 100 (32%) received no anti-VEGF treatment. Among all 2123 anti-VEGF injections, 1212 (57%) were aflibercept, 517 (24%) were bevacizumab, 381 (18%) were ranibizumab, and 13 (<1%) were within a clinical trial of an investigational drug. Overall, 122 (39%) eyes received at least one aflibercept injection (46%, 34%, 34% in the original aflibercept, bevacizumab, and ranibizumab groups respectively); 103 (33%) eyes received at least one bevacizumab injection (30%, 32%, 35% in the original aflibercept, bevacizumab, and ranibizumab groups); and 59 (19%) eyes received at least one ranibizumab injection (11%, 20%, 26% in the original aflibercept, bevacizumab, and ranibizumab groups). Focal/grid laser photocoagulation for DME was administered in 25 (8%) eyes and intravitreous corticosteroids were given in 32 (10%) eyes.
Figure 2.
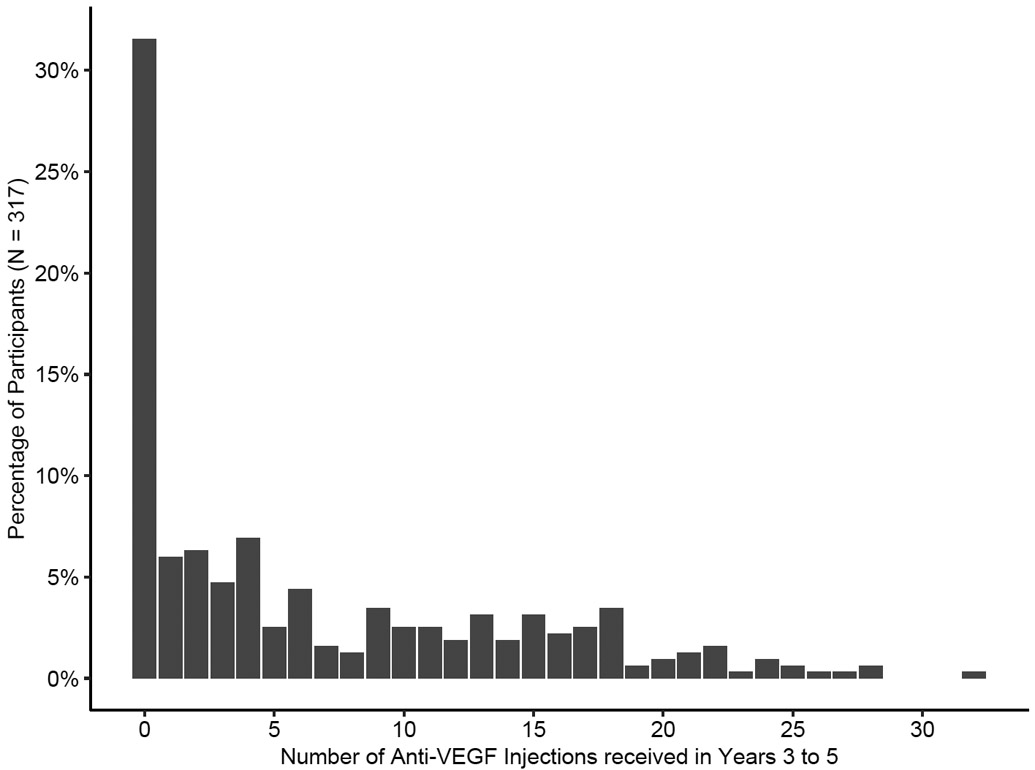
Distribution of the number of anti-vascular endothelial growth factor (VEGF) injections received between 3 to 5 years after initial randomization into a 2-year clinical trial on anti-VEGF treatment for diabetic macular edema (Protocol T).
Visual Acuity
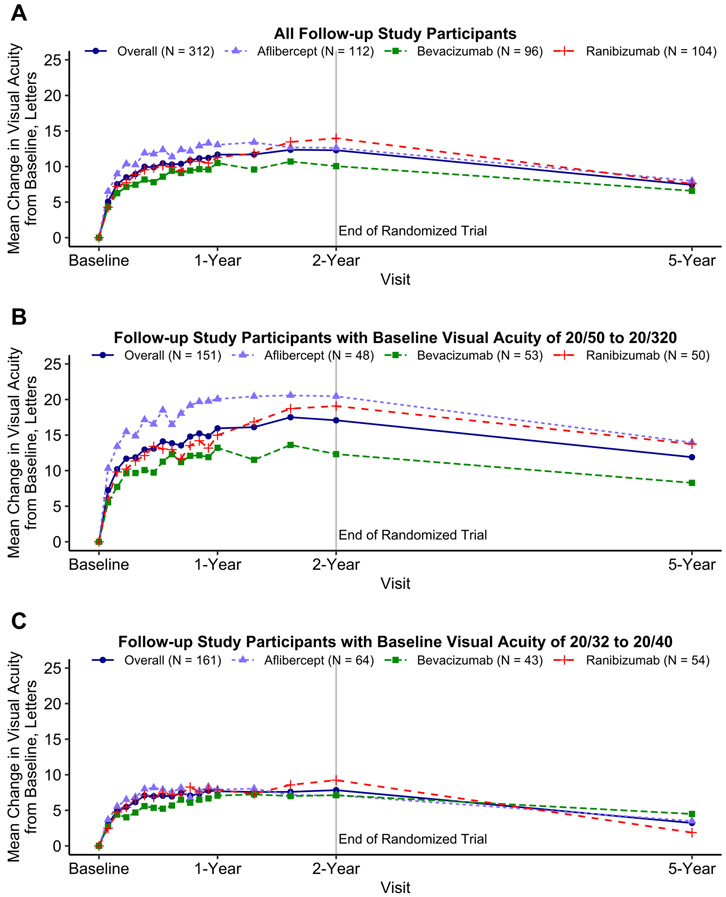
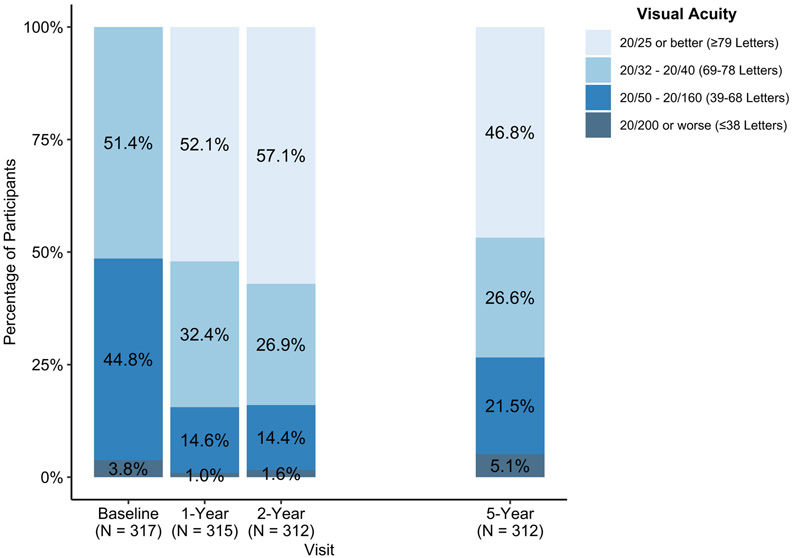
Among all participants, mean 5-year VA improved from baseline by 7.4 letters (95% confidence interval (CI): 5.9 to 9.0; Table 2, Figure 3A), but worsened from 2 years by 4.7 (95% CI: 3.3 to 6.0) letters. Among eyes with baseline VA 20/50 to 20/320, mean 5-year VA improved from baseline by 11.9 (95% CI: 9.3 to 14.5) letters, but worsened from 2 years by 4.8 (95% CI: 2.5 to 7.0) letters (Figure 3B). Among eyes with baseline VA 20/32 to 20/40, mean 5-year VA improved from baseline by 3.2 (95% CI: 1.4 to 5.0) letters, but worsened from 2 years by 4.6 (95% CI: 3.1 to 6.1) letters (Figure 3C). Among 312 eyes at 5 years, VA was 20/25 or better in 146 (47%), 20/40 or better in 229 (73%), and 20/200 or worse in 16 (5%) (Table 3, Figure 4). One hundred forty-eight (47%) eyes gained at least 10 letters from baseline and 31 (10%) lost 10 or more letters from baseline. Visual acuity outcomes by baseline VA subgroup are shown in Supplemental Table 2
Table 2.
Visual Acuity Change by Randomized Treatment Group and Stratified by Baseline Visual Acuity Subgroups
| Observed Data | Treatment Group Comparisons Differences in Mean Change (Adjusted 95% Confidence Interval) and Adjusted P-value |
||||||
|---|---|---|---|---|---|---|---|
| Visual Acuity | All | Aflibercept | Bevacizumab | Ranibizumab | Aflibercept vs. Bevacizumab |
Aflibercept vs. Ranibizumab |
Bevacizumab vs. Ranibizumab |
| All Participants | |||||||
| N | 312 | 112 | 96 | 104 | |||
| VA change, letters, mean (SD) | |||||||
| Baseline to 2 years* | 12.2 (12.3) | 12.4 (13.1) | 10.0 (13.8) | 14.0 (9.6) | 3.3 (0.0, 6.6) p=0.05 |
−1.5 (−4.3, 1.4) p=0.31 |
−4.8 (−8.4, −1.2) p=0.004 |
| Baseline to 5 years | 7.4 (14.9) | 8.0 (16.1) | 6.6 (15.2) | 7.6 (13.3) | 2.2 (−1.6, 6.0) p=0.79 |
0.5 (−3.2, 4.2) p=0.79 |
−1.7 (−5.6, 2.2) p=0.79 |
| Years 2 to 5* | −4.7 (12.1) | −4.5 (11.9) | −3.2 (13.5) | −6.2 (10.9) | −1.4 (−4.7, 1.9) p=0.40 |
1.7 (−1.5, 4.9) p=0.40 |
3.1 (−1.0, 7.2) p=0.20 |
| Participants with Baseline VA 20/50 to 20/320 (Letter Score 68 to 24) | |||||||
| N | 151 | 48 | 53 | 50 | |||
| VA change, letters, mean (SD) | |||||||
| Baseline to 2 years* | 16.8 (14.0) | 19.4 (13.7) | 12.3 (16.6) | 19.1 (9.8) | 6.3 (0.7, 11.9) p=0.02 |
−0.3 (−5.3, 4.6) p=0.90 |
−6.6 (−12.5, −0.7) p=0.02 |
| Baseline to 5 years | 11.9 (16.6) | 14.0 (19.0) | 8.3 (17.7) | 13.7 (11.9) | 5.1 (−2.0, 12.2) p=0.22 |
−0.3 (−6.6, 6.0) p=0.93 |
−5.4 (−12.4, 1.7) p=0.22 |
| Years 2 to 5* | −4.8 (14.1) | −5.6 (14.1) | −3.6 (16.2) | −5.2 (11.8) | −1.8 (−7.2, 3.7) p=0.94 |
−0.2 (−5.8, 5.3) p=0.94 |
1.5 (−3.9, 7.0) p=0.94 |
| Participants with Baseline VA 20/32 to 20/40 (Letter Score 78 to 69) | |||||||
| N | 161 | 64 | 43 | 54 | |||
| VA change, letters, mean (SD) | |||||||
| Baseline to 2 years* | 7.8 (8.5) | 7.1 (9.7) | 7.2 (8.6) | 9.2 (6.7) | 0.8 (−2.3, 3.9) p=0.62 |
−2.2 (−5.5, 1.1) p=0.27 |
−3.0 (−7.0, 0.9) p=0.20 |
| Baseline to 5 years | 3.2 (11.7) | 3.5 (11.9) | 4.5 (11.1) | 1.9 (12.0) | −0.9 (−5.4, 3.7) p=0.71 |
1.6 (−2.6, 5.8) p=0.71 |
2.5 (−2.2, 7.2) p=0.71 |
| Years 2 to 5* | −4.6 (10.0) | −3.7 (10.0) | −2.7 (9.4) | −7.1 (10.0) | −1.7 (−5.4, 2.0) p=0.37 |
3.5 (−0.4, 7.5) p=0.09 |
5.2 (0.5, 9.9) p=0.02 |
VA data at the last available Protocol T visit were used for 5 participants that were missing VA at the 2-year visit. VA change was truncated to the mean ± 3 standard deviations of VA change from baseline to 5 years (7.12 ± 3 × 16.04). Differences in Va change between treatment groups were adjusted for baseline VA. All p-values and confidence intervals for pairwise treatment group comparisons were adjusted using the Hochberg approach to control the type 1 error. Abbreviations: SD = standard deviation; VA = visual acuity.
Figure 3.
Mean change in visual acuity over time for all eyes (A) and stratified by baseline visual acuity of 68 to 24 letters (approximate Snellen equivalent 20/50 to 20/320) (B) and 78 to 69 letters (approximal Snellen equivalent 20/32 to 20/40) (C) among follow-up study participants from a 2-year clinical trial on anti-vascular endothelial growth factor treatment for eyes with diabetic macular edema (Protocol T). Change in visual acuity was truncated to the mean ± 3 standard deviations of the change from baseline to 5 years (7.12 ± 3 × 16.04). Eyes missing visual acuity at the 5-year visit were excluded.
Table 3.
Visual Acuity Outcomes by Randomized Treatment Group
| Visual Acuity | All | Aflibercept | Bevacizumab | Ranibizumab |
|---|---|---|---|---|
| N | 312 | 112 | 96 | 104 |
| VA, letters, mean (SD) | ||||
| Baseline | 65.7 (11.0) | 66.3 (11.5) | 64.6 (10.8) | 66.1 (10.8) |
| Year 2* | 77.8 (12.2) | 78.7 (10.7) | 74.4 (15.3) | 80.0 (9.5) |
| Year 5 | 72.8 (16.4) | 74.1 (15.3) | 70.7 (18.7) | 73.4 (15.0) |
| 5-Year VA Snellen group, N (%) | ||||
| 20/25 or better (≥79 letters) | 146 (47%) | 58 (52%) | 37 (39%) | 51 (49%) |
| 20/32 - 20/40 (69-78 letters) | 83 (27%) | 28 (25%) | 31 (32%) | 24 (23%) |
| 20/50 - 20/160 (39-68 letters) | 67 (21%) | 20 (18%) | 22 (23%) | 25 (24%) |
| 20/200 or worse (≤38 letters) | 16 (5%) | 6 (5%) | 6 (6%) | 4 (4%) |
| VA change from baseline to 5 years, N (%) | ||||
| ≥15 letter improvement | 94 (30%) | 34 (30%) | 27 (28%) | 33 (32%) |
| 10-14 letter improvement | 54 (17%) | 19 (17%) | 19 (20%) | 16 (15%) |
| 5-9 letter improvement | 50 (16%) | 17 (15%) | 14 (15%) | 19 (18%) |
| ±4 letter difference | 66 (21%) | 25 (22%) | 20 (21%) | 21 (20%) |
| 5-9 letter worsening | 17 (5%) | 5 (4%) | 6 (6%) | 6 (6%) |
| 10-14 letter worsening | 8 (3%) | 2 (2%) | 3 (3%) | 3 (3%) |
| ≥15 letter worsening | 23 (7%) | 10 (9%) | 7 (7%) | 6 (6%) |
VA data at the last available Protocol T visit were used for 5 participants that were missing VA at the 2-year visit. Abbreviations: SD = standard deviation; VA = visual acuity.
Figure 4.
Distribution of visual acuity (approximate Snellen equivalent) categories at annual visits among follow-up study participants from a 2-year clinical trial on anti-vascular endothelial growth factor treatment for eyes with diabetic macular edema (Protocol T).
The mean (SD) change in VA between baseline and 5 years was 8.0 (16.1), 6.6 (15.2) and 7.6 (13.3) letters in the original aflibercept, bevacizumab, and ranibizumab groups, respectively (mean difference [95% CI]: aflibercept vs. bevacizumab, 2.2 [−1.6 to 6.0]; aflibercept vs. ranibizumab, 0.5 [−3.2 to 4.2]; bevacizumab vs. ranibizumab −1.7 [−5.6 to 2.2], Table 2). The amount of decrease in VA between years 2 and 5 did not differ between the 3 original treatment groups.
A post hoc analysis evaluated outcomes among eyes that were pseudophakic at baseline for which changes in cataract and cataract surgery would not affect VA. Among 76 eyes pseudophakic at baseline, mean (SD) change in VA from baseline to 5 years was 2.1 (17.2) letters and mean change from 2 to 5 years was −8.2 (12.5) letters (Supplemental Table 3, Supplemental Figure 2).
Central Subfield Thickness
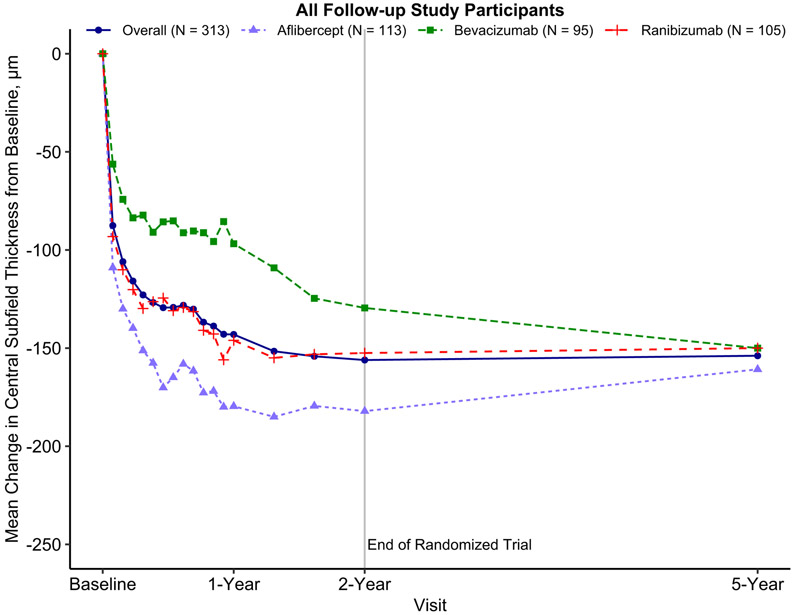
Among all participants, mean 5-year CST decreased from baseline by 154 μm (95% CI: 142 to 166, Supplemental Table 4, Figure 5) and decreased from 2 years by 1 μm (95% CI: −12 to 9). Among eyes with baseline VA 20/50 to 20/320, mean 5-year CST decreased from baseline by 188 μm (95% CI: 168 to 209), and increased from 2 years by 2 μm (95% CI: −16, 20) (Supplemental Figure 3). Among eyes with baseline VA 20/32 to 20/40, mean 5-year CST decreased from baseline by 121 μm (95% CI: 109 to 134), and decreased from 2 years by 5 μm (95% CI: −16 to 7) (Supplemental Figure 4).
Figure 5.
Mean change in optical coherence tomography central subfield thickness over time among follow-up study participants from a 2-year clinical trial on anti-vascular endothelial growth factor treatment for eyes with diabetic macular edema (Protocol T). Change in central subfield thickness was truncated to the mean ± 3 standard deviations of central subfield thickness change from baseline to 5 years (−154.5 ± 3 × 154.81). Eyes missing central subfield thickness at the 5-year visit were excluded.
The mean change in CST from baseline to 5 years was −161, −150, and −150 μm in the original aflibercept, bevacizumab, and ranibizumab groups, respectively (mean difference [95% CI]: aflibercept vs. bevacizumab, −15 μm [CI −49 to 19]; aflibercept vs. ranibizumab, 6 μm [−23 to 35]; bevacizumab vs. ranibizumab, 21 μm [−16 to 57], Supplemental Table 4).
Among 313 eyes at 5 years, 195 (62%) had a CST below the study-defined cutoff for definite CI-DME and only 25 (8%) had a Stratus equivalent thickness of 400 μm or more (Supplemental Table 4, Supplemental Figure 5). The correlation between 5-year VA and CST was −0.23 (95% CI: −0.33, −0.12). The correlation between 5-year change in VA and CST from baseline was −0.26 (95% CI: −0.37, −0.16).
Diabetic Retinopathy Severity Level on Fundus Photographs
At 5 years, 17 of 277 (6%) eyes with gradable photos had no retinopathy or less than mild nonproliferative DR, 162 (58%) had mild to severe nonproliferative DR, 57 (21%) had prior panretinal photocoagulation without other evidence of proliferative DR, and 41 (15%) had mild to high-risk proliferative DR (Supplemental Table 5, Supplemental Figure 6).9 Among eligible eyes at 5 years, 59 of 241 (24%) improved 2 or more steps in DR severity from baseline and 52 of 274 eyes (19%) worsened 2 or more steps from baseline; 20 of 195 (10%) improved 2 or more steps from 2 years and 50 of 254 (20%) worsened 2 or more steps from 2 years. Cross-tabulations of DR severity at 2 and 5 years are in Supplemental Table 6.
Adverse Events
Among 586 participants at sites participating in the follow-up study, 123 (21%) died over 5 years; 28 (5%) died within the first 2 years of Protocol T and an additional 95 (16%) died between 2 and 5 years. Causes of death and frequency of heart attacks and strokes occurring after 2 years are displayed in Supplemental Tables 7 and 8.
Discussion
The Protocol T 5-year follow-up study evaluated approximately two-thirds of the eligible Protocol T participants and explored standard care treatment distributions over time along with functional and anatomic outcomes three years after Protocol T participation ended. During this time, most patients continued to receive retinal care at DRCR Retina Network clinical sites and 68% of study eyes received anti-VEGF treatment with a median of 4 injections. No clear patterns were present in the subsequent choice of anti-VEGF agents relative to participants’ original treatment group assignment. Mean VA at 5 years was 7.4 letters (1.5 lines) better than baseline but had decreased between 2 and 5 years by 4.7 letters (1 line). Unlike VA, mean OCT CST was similar at 2 and 5 years.
It is not possible to compare pure treatment groups through 5 years since follow-up and treatment outside the study were not standardized and about half of the eyes received an anti-VEGF agent that was different from the randomized treatment. However, the anti-VEGF agent received during the first 2 years did not lead to any statistically significant treatment group differences in VA at 5 years.
Prior clinical trials have shown that long-term VA improvements from baseline are maintained with structured anti-VEGF retreatment. In a previous DRCR study (Protocol I), structured protocol retreatment with ranibizumab for DME continued through 5 years, and mean VA in the ranibizumab plus deferred focal/grid laser treatment group was 10 letters better than baseline at 5 years.2 Combined, the original 0.3-mg ranibizumab groups in RISE and RIDE had mean VA improvement of approximately 12 letters through 4 years after an open-label extension with ranibizumab.10 Similarly, VISTA and VIVID demonstrated that mean VA in the aflibercept group (2q8) was 10.5 (VISTA) and 11.7 (VIVID) letters higher than baseline at 3 years.11
Results from this study support the conclusion that anti-VEGF therapy is associated with better visual results than no treatment or focal/grid laser treatment, even 3 years after clinical trial completion. At 5 years, 30% of Protocol T eyes gained 3 or more lines (≥ 15 letters) of vision compared with baseline, while only 7% lost 3 or more lines (≥ 15 letters). In contrast, the natural history of eyes with DME is to experience vision loss over time. Unpublished data (personal communication, A. Glassman, October 22, 2019) from eyes in the ETDRS study demonstrate that among eyes with similar enrollment criteria as Protocol T, 4% and 11% of eyes in the observation and laser groups, respectively, gained 3 or more lines of vision at 5 years, while 43% and 30% had lost 3 or more lines.
In the current study, mean VA worsened by approximately one line between 2 and 5 years, although 47% of the eyes were 20/25 or better and only 5% were 20/200 or worse at 5 years. This mean VA decline during standard care differs from the stability seen in long-term studies wherein anti-VEGF agents for DME continued to be given within a protocol-defined regimen. RISE and RIDE demonstrated VA outcomes with ranibizumab that were consistent between 3 and 4 years, among the 66% of eyes available at 4 years.12 The VISTA and VIVID studies also demonstrated that participants’ 148-week visual outcomes were consistent with the 52-week visual outcomes.11 In the ranibizumab plus deferred laser group in Protocol I, the mean change in VA between 2 and 5 years was −0.3 letters (personal communication, K. Josic), October 22, 2019). The reasons for the difference in VA trajectories from years 2 to 5 in Protocols I and T are unknown. On average, there were more visits in Protocol I in years 3 through 5 (20) than after Protocol T completion (14), which may indicate that closer monitoring is needed to maintain vision gains. Although the number of injections in Protocol I between years 2 and 5 was similar to those seen in this study over the same time period (median of 4), individuals selected for treatment in the context of standard care versus clinical research may have been different, potentially impacting VA results. A retrospective study of electronic medical records reported a similar decline in visual outcomes after an initial period of rapid visual gain in eyes treated with anti-VEGF for DME in standard care.4
Although mean VA worsened from years 2 to 5, CST did not show a corresponding increase. Previous reports have established relatively modest correlations between changes in VA and CST over time in eyes with DME.13 The discordance between mean changes in VA and CST does not appear to be explained by cataract formation because post hoc analyses including only eyes that were pseudophakic at baseline, and therefore not at risk of VA loss due to cataract formation or extraction, produced similar results.
Limitations of this study include results based only on 68% of the potential cohort and only 48% of the original Protocol T cohort. Participants who completed the follow-up study may be different from the full cohort because participants who had 5-year visits entered the study with better baseline VA and had better 2-year VA than participants who did not enroll in the follow-up study. In addition, because follow-up visit and treatment data after the two-year visit were collected from chart reviews, some information may be incomplete; however, since most visits were conducted at Protocol T sites it is likely that most data were captured.
In conclusion, among the approximately two-thirds of eligible Protocol T participants who completed a 5-year visit, mean VA improved from baseline to 5 years with no protocol defined visit schedule or treatment protocol after study follow-up ended at 2 years. Although there were no substantial differences in mean retinal thickness at 2 and 5 years, mean VA decreased during this time. Additional investigation into strategies to improve long-term outcomes in eyes with DME seems warranted to determine if VA can be better maintained with different management approaches. .
Supplementary Material
Acknowledgments
Financial Disclosures: A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net.
Funding/Support: Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, U.S. Department of Health and Human Services EY14231, EY14229, EY18817.
Role of the Sponsor: The funding organization (National Institutes of Health) participated in oversight of the conduct of the study and review of the manuscript, but not directly in the design or conduct of the study, nor in the collection, management, analysis, or interpretation of the data, or in the preparation of the manuscript.
Appendix
A published list of the DRCR Retina Network investigators and staff participating in this protocol can be found in: Diabetic Retinopathy Clinical Research N, Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193-203. A current list of DRCR.net investigators is available at www.drcr.net.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Adam R. Glassman, Jaeb Center for Health Research, Tampa, Florida.
John A. Wells, III, Palmetto Retina Center, Columbia, South Carolina.
Kristin Josic, Jaeb Center for Health Research, Tampa, Florida.
Maureen G. Maguire, Department of Ophthalmology, University of Pennsylvania.
Andrew N. Antoszyk, Charlotte Eye, Ear, Nose and Throat Associates, PA.
Carl Baker, Paducah Retinal Center.
Wesley T. Beaulieu, Jaeb Center for Health Research, Tampa, Florida.
Michael J. Elman, Elman Retina Group, P.A.
Lee M. Jampol, Feinberg School of Medicine, Northwestern University Medical School, Chicago, Illinois.
Jennifer K. Sun, Joslin Diabetes Center, Beetham Eye Institute, Harvard Department of Ophthalmology, Boston, Massachusetts.
References
- 1.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: Results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. [DOI] [PubMed] [Google Scholar]
- 2.Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–1077 e1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(6):2247–2254. [DOI] [PubMed] [Google Scholar]
- 4.Ciulla TA, Bracha P, Pollack J, Williams DF. Real-world Outcomes of Anti-Vascular Endothelial Growth Factor Therapy in Diabetic Macular Edema in the United States. Ophthalmol Retina. 2018;2(12):1179–1187. [DOI] [PubMed] [Google Scholar]
- 5.Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13): 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Localio AR, Margolis DJ, Berlin JA. Relative risks and confidence intervals were easily computed indirectly from multivariable logistic regression. J Clin Epidemiol. 2007;60(9):874–882. [DOI] [PubMed] [Google Scholar]
- 8.Hochberg Y A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75(4):800–802. [Google Scholar]
- 9.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98:823–833. [PubMed] [Google Scholar]
- 10.Boyer DS, Nguyen QD, Brown DM, Basu K, Ehrlich JS. Outcomes with As-Needed Ranibizumab after Initial Monthly Therapy: Long-Term Outcomes of the Phase III RIDE and RISE Trials. Ophthalmology. 2015;122(12):2504–2513.e2501. [DOI] [PubMed] [Google Scholar]
- 11.Heier JS, Korobelnik JF, Brown DM, et al. Intravitreal Aflibercept for Diabetic Macular Edema: 148-Week Results from the VISTA and VIVID Studies. Ophthalmology. 2016;123(11):2376–2385. [DOI] [PubMed] [Google Scholar]
- 12.Sun JK, Wang PW, Taylor S, Haskova Z. Durability of Diabetic Retinopathy Improvement with As-Needed Ranibizumab: Open-Label Extension of RIDE and RISE Studies. Ophthalmology. 2019;126(5):712–720. [DOI] [PubMed] [Google Scholar]
- 13.Bressler NM, Odia I, Maguire M, et al. Association Between Change in Visual Acuity and Change in Central Subfield Thickness During Treatment of Diabetic Macular Edema in Participants Randomized to Aflibercept, Bevacizumab, or Ranibizumab: A Post Hoc Analysis of the Protocol T Randomized Clinical Trial. JAMA Ophthalmol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.