Abstract
Alcohol use disorder affects millions of people each year. Currently approved pharmacotherapies have limited success in treating this disorder. Evidence suggests that this lack of success is partly due to how these pharmacotherapies are tested in preclinical settings. The vast majority of preclinical studies assessing the effects of pharmacotherapies on alcohol or drug self-administration are done in individually housed animals. However, it is known that alcohol and drug intake are heavily influenced by social settings. Here we adapted radiofrequency tracking technology to determine the effects of oxytocin, a potential therapy for alcohol use disorder, on alcohol consumption in socially housed male and female prairie voles. Voluntary alcohol consumption in these animals resulted in high daily alcohol intakes, blood ethanol concentrations that are considered intoxicating, and central changes in FosB immunoreactivity, indicative of changes in neural activity. Prairie voles that received oxytocin temporarily reduced alcohol consumption but not alcohol preference, compared to control prairie voles regardless whether their cagemates received a similar treatment or not. Our results demonstrate that oxytocin can decrease consummatory behaviors in the presence of peers that are not receiving this treatment, and therefore, its potential use in clinical trials is warranted. Moreover, effectiveness of other pharmacotherapies in preclinical studies can be tested in mixed treatment socially-housed animals similarly to clinical studies in humans.
Keywords: Oxytocin, alcoholism, alcohol use disorder, Microtus ochrogaster, social environment, Edinger-Westphal nucleus
Introduction
While alcohol use disorder (AUD) affects millions of people, the current pharmacotherapies approved for the treatment of this disorder in the United States (acamprosate, disulfiram, and naltrexone) are only modestly effective1,2. Therefore, development of novel medications to treat AUD is necessary.
Rational development of potential therapies requires animal models of high predictive validity. One complex factor that until recently has been insufficiently addressed in animal models on addiction is the social environment (for reviews on this caveat see3,4). In fact, in the majority of animal models of AUD, animals are single-housed during self-administration. These conditions are quite different from the situation in which a potential pharmacotherapy is expected to work in an addicted patient: the subject may be taking the medication in the presence and under the influence of surrounding individuals. This is an important omission as volitional access to social conditions can prevent signs of drug dependence in laboratory animals5, suggesting that testing pharmacotherapies in such conditions could prevent translational predictability. Recognizing this caveat, researchers attempted several approaches to take social influences on drug taking into account. Most common of these approaches to date were: 1) housing animals socially and calculating the total intake for an entire cage6,7; 2) housing animals socially, but testing drug intake in a probe trial in isolated condition8,9; 3) housing animals in semi-social conditions by separating them within a cage by a mesh divider10–12; 4) housing animals socially, measuring total cage intake and dividing it by the time each individual animal spends near a drinking spout or touches the spout through video tracking or lickometer approaches13,14. The latter approaches were a step forward in assessing social aspects of substance abuse, but either did not provide high resolution needed to measure individual substance consumption in social settings or introduce additional factors capable of affecting drug intake (for example, stress of mesh-separation). For additional discussion of these caveats see15.
In contrast to addiction research, ethological science has a long tradition in tracking behavior of individual animals within groups. The capabilities of such tracking have been greatly improved through the use of radio frequency detection16,17. This technology is gaining increased use in neuroscience. First studies to apply radio frequency tracking to addiction research suggested existence of complex effects of social interactions and hierarchies on alcohol drinking in rats18,19. However, these studies were hampered by imprecise measures of individual alcohol drinking and overall low drinking of alcohol. A more recent study used radio frequency tracking in the automated Intellicage®. Since this system is based on the lickometer approach, the study did not measure alcohol intake. However, it demonstrated very low preference for alcohol solution in C57BL/6 mice, which made it incompatible with future studies on medications to decrease alcohol intake20. The only investigation to date that used radio frequency to test effects of a potential medication adapted the “Herdsman” (HM) system, a novel apparatus developed to measure fluid or food intake in socially housed animals. The system consists of a large enclosure with protruding channels allowing access to a fluid or food source which is located on a precision balance. Radio frequency detection of individual animals in the channels allows tracking exact amounts of fluid (or food) consumed at any particular time of testing. Thomsen et al. (2017) used this system to demonstrate the effects of a glucagon-like peptide 1 receptor agonist (Exendin-4) on alcohol consumption in socially-housed male C57BL/6 mice21. While this development presented an important advancement to alcohol research, the study did not present data from individual animals and assigned the same condition to all animals in each individual cage (all animals in a cage were either treated or untreated). Therefore, the question whether a potential medication could be effective in the presence of peers not receiving the medication remained unanswered.
The goal of the present study, therefore, was to test the possibility of assessing the effects of one of the new promising medications to treat AUD in socially housed animals unrestrained in their ability to interact with other peers. Specifically, we adapted the HM system to explore effects of oxytocin on alcohol consumption. Oxytocin is a nine amino acid peptide playing a role in lactation, parturition and social behaviors. It is also implicated in the modulation of processes associated with drug use22–24. There has been a substantial amount of research in rodents and a growing interest of research in humans on the effects of oxytocin on substance use disorders. Preclinical studies showed that peripheral oxytocin treatment can reduce voluntary alcohol consumption and preference in single-housed and semisocially-housed rodents25–31. The majority of these studies used the intraperitoneal route (0.25–10 mg/kg) with larger rodents sensitive to smaller doses, but intracranial (3 microg) or intranasal (0.5–1 mg/kg) administration were also effective. Meanwhile, a limited number of clinical studies have shown that intranasal oxytocin decreases alcohol craving, cue reactivity and withdrawal symptoms30,32,33.
It is known that oxytocin plays an important role in regulating social behaviors and that alcohol use is heavily influenced by social environment in humans. However, a majority of studies exploring oxytocin’s effects on alcohol consumption in animal models of AUD have been completed in rodents which strongly differ in their social behaviors from humans. Therefore, we decided to explore the effect of oxytocin on alcohol consumption in a socially monogamous rodent model, the prairie vole (Microtus ochrogaster). Prairie voles display many similarities in social behaviors with humans, including the ability to form long-term emotionally-based attachments between adult male-female and same-sex individuals34,35. Moreover, the oxytocin system first identified as regulating partner preference in this species has been subsequently found to be involved in social behaviors in humans, demonstrating translational validity of the prairie vole model of social attachment and effects of oxytocin35,36. Prairie voles also freely consume substantial doses of unadulterated alcohol, when given the opportunity11. Oxytocin has been shown to inhibit alcohol consumption in prairie voles housed in semi-social conditions (separated by mesh dividers)29. Here we used radio frequency detection to investigate effects of oxytocin on alcohol consumption in socially housed prairie voles in two different experiments. In the first experiment (Across experiment), all animals in each cage were treated with saline or oxytocin. In the second experiment (Mix experiment), half of the animals in each cage were treated with oxytocin and the other half with saline to determine if the effectiveness of oxytocin was altered when animals receiving treatment were housed with animals not receiving oxytocin. Following the behavioral analysis, we also assessed whether alcohol consumption in this new system produced central effects.
Materials and Methods
Animals
Experimentally naïve adult female and male prairie voles (n = 91) ranging between 77 – 141 days old at the start of the experiment were used from our breeding colony at the VA Portland Health Care System (VAPORHCS) Veterinary Medical Unit. Voles were weaned at 21 days and housed in same-sex groups in standard housing cages (3–5 animals per 27×27×13 cm cages) under a 14:10-h light/dark cycle (lights on at 6am) until the start of the experiment. Before experiments occurred, females were housed in a separate room to prevent induction of ovulation because prairie voles are induced ovulators. All subjects had access to cotton nestlets and ab libitum access to water and a diet of mixed rabbit chow (LabDiet Hi-Fiber Rabbit; PMI Nutrition International, Richmond, IN), corn (Nutrena Cleaned Grains; Cargill, Inc., Minneapolis, MN) and oats (Grainland Select Grains; Grainland Cooperative, Eureka, IL) throughout the duration of the experiment. Experiments were approved by the Institutional Animal Care and Use Committee at the VAPORHCS and performed in accordance to the NIH Guidelines for the Care and Use of Laboratory Animals.
Apparatus
To monitor individual fluid consumption in socially housed animals we used an automated rodent HM-2 system (MBrose, Faaborg, Denmark). Our version of the HM-2 system was built to record fluid (but not food) consumption from two bottles at the level of individual animal, based on the use of radio frequency identification tags (RFID). Each bottle is at the end of a 11 cm channel, that was customized to allow only one prairie vole to drink from the bottles at a time. In each channel photocells are located at the beginning of the channel and near the bottle spout, to detect the presence of an animal. When the presence of an animal was detected, an RFID reader at the spout of the bottle determined which animal was drinking and the raw drinking data were recorded on a computer. Fluid consumption was based on the weight displacement when an animal was present at the spout. A drip tray was suspended under each fluid bottle to catch any spillage, which is automatically subtracted from the displacement drinking bout. Along with spillage, evaporation was not a factor because fluid displacement was only measured when an animal was present in the channel and consuming fluid from the spout. These channels were attached to a Techniplast 1500U eurostandard type IV S (48×37.5×21 cm) rodent housing cage. Each cage was custom modified to include a set of stairs leading to each channel, the same bedding as used in homecages, and a 10” Habitrail OVO tube (Habitrail, Hagen Inc.) to build a nest in (Supplementary Figure S1).
Training and Experimental Timeline
Animals were implanted with RFID chips (UNO MICRO ID/8, ISO Transponder 2.12 × 8 mm) under isoflurane anesthesia and then placed back into their homecage to recover for 2–3 days. After animals recovered from RFID chip implantation, 3–5 same-sex prairie voles were put into each HM-2 cage for 2 days to habituate and remained there for the rest of the experiment. Food access of ad libitum. Most animals assigned to a cage were sibling offspring from the same breeding pair. Some cages contained offspring from two breeding pairs. Follow-up comparison between these pairings of animals did not detect any difference in drinking behaviors. During the habituation period, access to the channels was closed to allow animals to establish nests in the cages and not the channels. Five 25mL sipper tubes were placed in the cage top to allow the animals access to water. All animals built nests in the cages during this two-day period. After nest building, sipper tubes were removed and animals were given access to the two channels with a bottle of water at the end of the channel for 5 days. The following 12 days, all cages received access to one bottle of 5% EtOH and one bottle of water. Bottles stayed on the same side throughout the experiment but were counterbalanced between cages. During this 12-day period, habituation injections of saline occurred on days 5, 6, and 7 between at 11am. During these three days, all animals were treated equally. Following habituation injections, animals received daily injections of either saline or oxytocin (3 mg/kg, i.p.) on days 8–12. In the first experiment (Across experiment: n = 9, Female/Saline; n = 10, Female/Oxytocin; n = 14, Male/Saline; n=15, Male/Oxytocin; 3–4 animals per cage), all the animals in a cage received the same treatment (saline or oxytocin). In the following experiment (Mix experiment: n = 9, Female/Saline; n = 10, Female/Oxytocin; n = 10, Male/Saline; n=10, Male/Oxytocin, 4–5 animals per cage), half of the animals in a cage received saline, while the other half received the oxytocin treatment. The assignment to the two treatment groups was based on drinking on the last baseline day (day 4) trying to match the drinking measures as much as possible between the treatment groups. In the Across experiment, the matching was done according to average drinking per cage, in the Mix experiment, the matching was done based on individual drinking. Drinking was analyzed at 1, 3, 6, 12 and 24 hours post-injection time. The 1-hour time point showed variability independent of treatment from day to day. This was most likely due to the fact that prairie voles distribute their drinking behaviors evenly throughout 24 hours without strong peaks at any particular time point11,36. Therefore, behavioral analysis of treatment’s effects was performed at 3, 6, 12 and 24 hours. All animals were euthanized 2 hours after the last treatment injection on day 12 and brains and blood were collected for later processing.
Drugs
Oxytocin acetate salt (3 mg/kg, i.p., Bachem, Torrence, CA, USA) was dissolved in 0.9% saline. This dose was chosen as the medium between 3 doses (1, 3 and 10 mg/kg) that decreased alcohol drinking in prairie voles in a previous study29. Since all three doses selectively reduced alcohol preference, and did not affect water drinking or locomotor activity, using the medium dose ensured that the observed effects were not due to non-specific (for example, sedative or diuretic) effects. The 5% alcohol solution was a dilution of 95% ethyl alcohol in water.
Additional Methods are in the online Supplementary Information.
Results
Oxytocin’s effects on alcohol intake when all animals in a cage are receiving the same treatment.
To determine if oxytocin treatment would affect alcohol consumption/preference, we peripherally treated socially-housed male and female prairie voles with oxytocin or saline. In the first experiment (Across experiment), all animals in a cage received the same treatment (oxytocin or saline) during experimental days 8 – 12. A repeated measures ANOVA performed over 12 experimental days revealed a main effect of sex (F1,48 = 8.73, P = 0.005), showing that female prairie voles in the first 3-hrs of each day consumed higher amounts of alcohol over the entire 12-day period compared to male prairie voles. Additionally, there was a significant day by treatment interaction (F6.5,311.7 = 2.57, P = 0.016). Post hoc analysis confirmed a significant difference in alcohol consumption between oxytocin- and saline-treated on the first day of oxytocin treatment in female (p= 0.02), but not male voles (Figure 1). However, this difference was difficult to interpret because of the higher intakes of ethanol on the last pre-treatment day. Analysis of the 6- and 12-hour time points revealed that females consumed more alcohol compared to males. However, there was no significant difference in the amount of alcohol consumed at the 24-hour time point (Table S1 and Figure S2). Additionally, there was no significant day × treatment interaction for the 6-, 12-, and 24-hour time points in both sexes.
Figure 1: Alcohol consumption and preference in the first three hours of alcohol access following oxytocin treatment when all animals in a cage receive the same treatment.
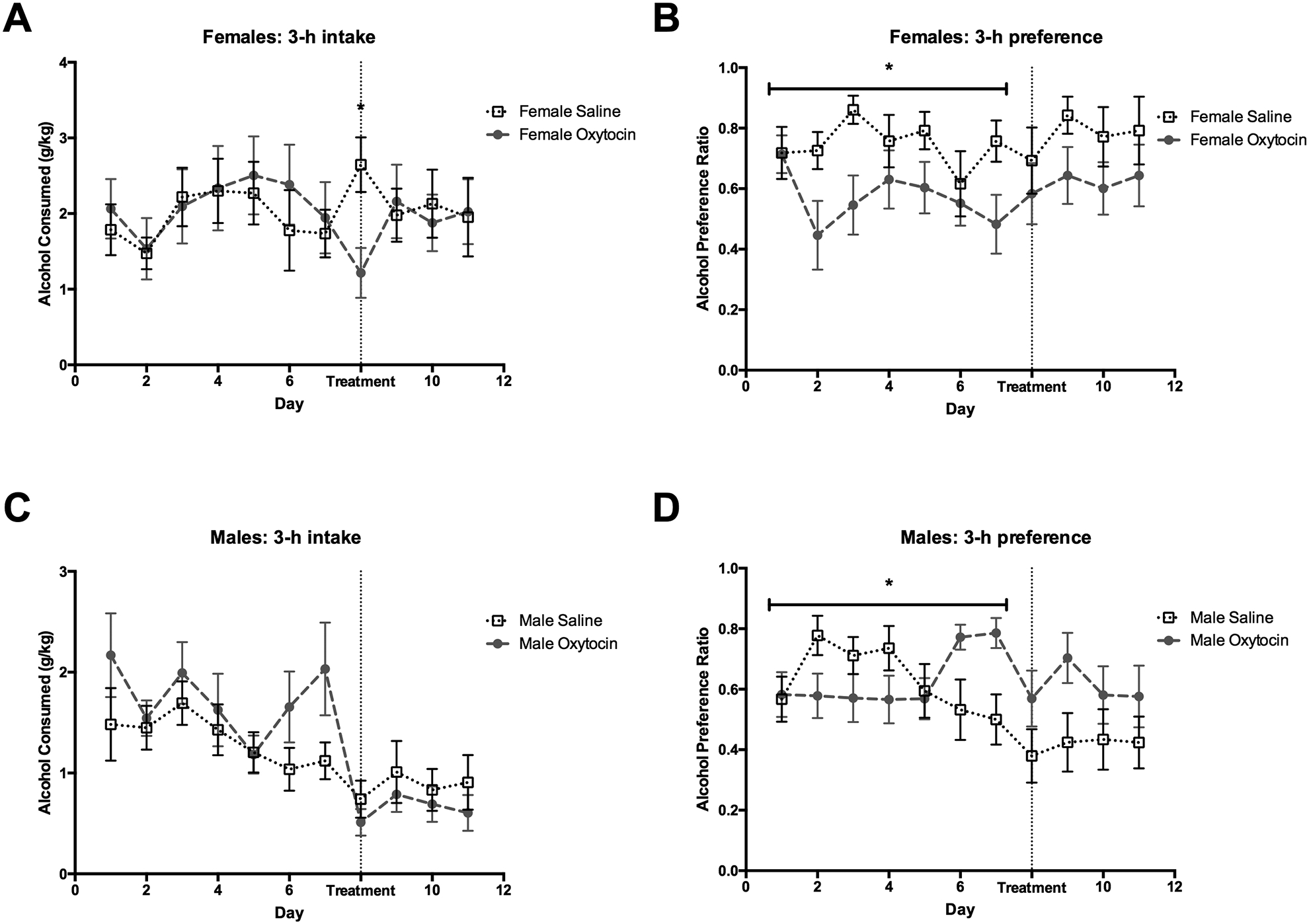
(A) Oxytocin reduces alcohol consumption in female prairie voles for the first day of treatment relative to control treated females; meanwhile, (C) there was no difference in alcohol consumption between saline and oxytocin treated males. There was a difference between treatment groups for alcohol preference during the baseline drinking in (B) female and (D) male prairie voles. Therefore, effects of treatment on alcohol preference could not be reliably inferred. *p<0.05. Error bars indicate mean ± SEM.
At the 3hr time point a repeated ANOVA found no main effect of sex (F1,48 = 2.43, P = 0.13) and treatment (F1,48 = 1.20, P = .28) on alcohol preference, but there was a significant interaction of sex by treatment (F1,48 = 6.19, P = 0.02). Additionally, we found a 2-way interaction of day by treatment (F6.0,289.5 = 2.20, P = 0.04). Post hoc analysis revealed that there were significant differences between treatment groups (in male and females) during the baseline period (Figure 1). Therefore, effects of oxytocin treatment on preference were difficult to interpret. Similarly, we saw group differences between treatment groups at the 6- and 12-hour time points regardless of the treatment (Table S2 and Figure S2), again making comparisons during the treatment period not reliable. Such observed differences in preference (and intake in males) preceding treatment were most likely due to the difficulty of experimental matching of these measures between cages. Such matching is easier to achieve when taking into account individual drinking of animals, as was done in the next experiment.
Oxytocin’s effects on alcohol intake when half of the animals in the same cage receive oxytocin treatment.
Treatments for substance use disorders are usually taken outside of the clinic and in social settings (i.e., around others who are not prescribed the same medication). The next (Mix) experiment modelled this condition. In a separate group of animals, half of the voles in each cage received the oxytocin treatment, while the other half received the saline treatment during days 8 – 12. A repeated ANOVA performed across 12 experimental days at the 3-hour time point identified no main effects of sex (F1,35 = 0.59, P = 0.46), treatment (F1,35 = 1.8, P = 0.19), and no sex × treatment interaction (F1,35 = 0.02, P = 0.90) for alcohol consumption. However, there was a significant 2-way interaction of day by treatment (F5.3,185.8 = 2.76, P = 0.02). Post hoc analysis revealed that oxytocin reduced alcohol consumption compared to saline controls for the first three days of treatment (p= 0.007 for the first day of treatment, p= 0.02 and p= 0.01 for subsequent two days, respectively). Post hoc analysis also indicated that alcohol consumption on all treatment days was significantly lower than on the last pretreatment day in the oxytocin group but not in the saline group (Figure 2). Similarly, at the 6-hour time point, there was no main effect of sex (F1,35 = 1.63, P = 0.21), treatment (F1,35 = 0.75, P = 0.39), and no sex by treatment interaction (F1,35 = 0.73, P = 0.40), but there was a significant 2-way interaction of day by treatment (F5.6,196.1 = 5.6, P = 0.02). Post hoc tests confirmed that oxytocin decreased alcohol consumption during the first day of treatment compared to saline controls. Alcohol consumption on all treatment days was significantly lower than on the last pretreatment day in the oxytocin group but not in the saline group (Figure 3). Oxytocin treatment did not significantly decrease alcohol consumption at the 12- and 24-hour time points (P > 0.05, Table S1 and Figure S3).
Figure 2: Alcohol consumption and preference in the first three hours of alcohol access following oxytocin treatment when animals in the same cage receive different treatments.
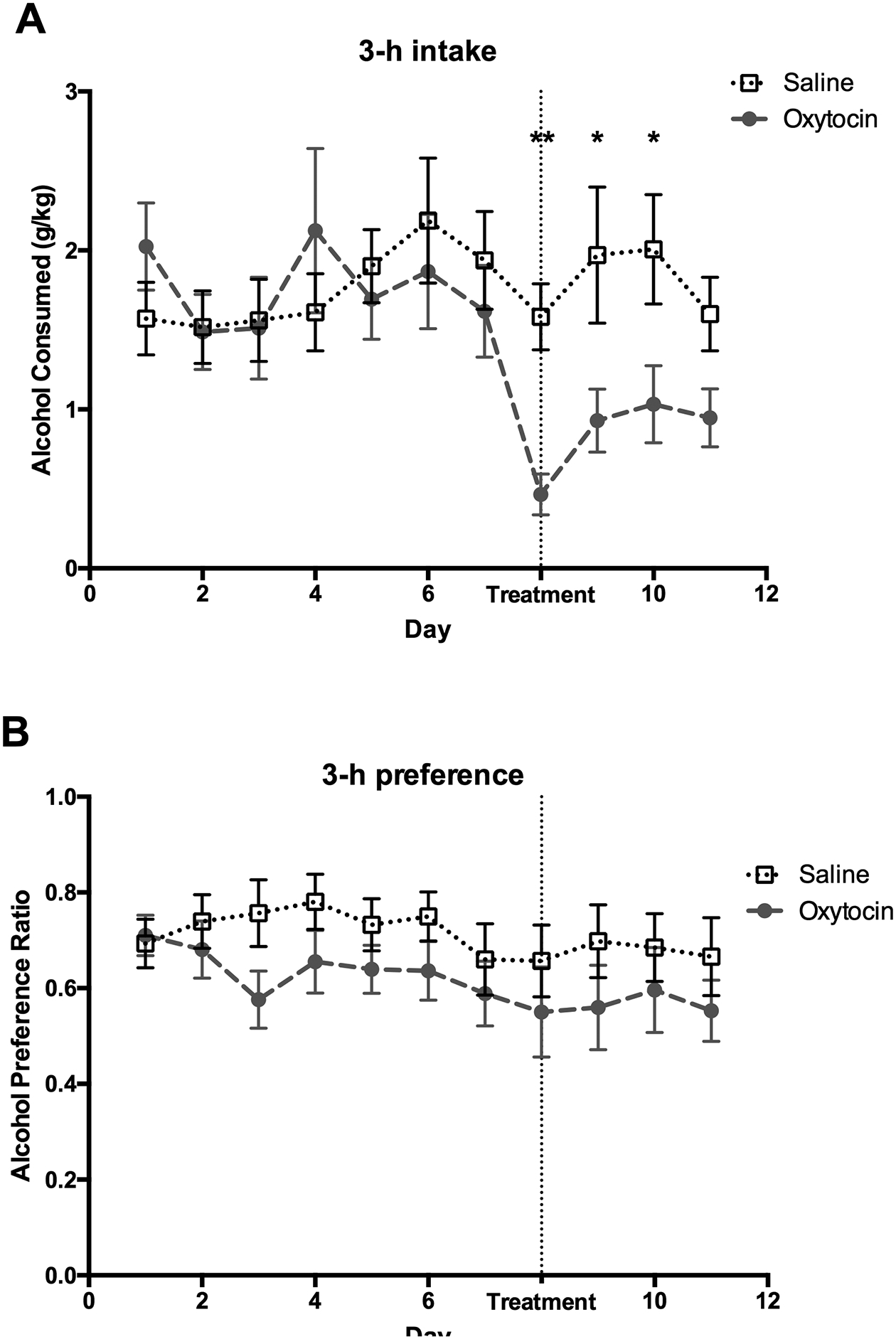
Data from animals were collapsed across sex for both (A) alcohol consumption and (B) preference as there was no sex difference or statistical interaction between treatment and sex for these measures. (A) Alcohol consumption during the first three days of treatment was lower in the oxytocin group versus the saline-treated animals. However, (B) oxytocin did not affect alcohol preference. *p<0.05, **p<0.01. Error bars indicate mean ± SEM.
Figure 3: Alcohol consumption and preference in the first six hours of alcohol access following oxytocin treatment when animals in the same cage receive different treatments.
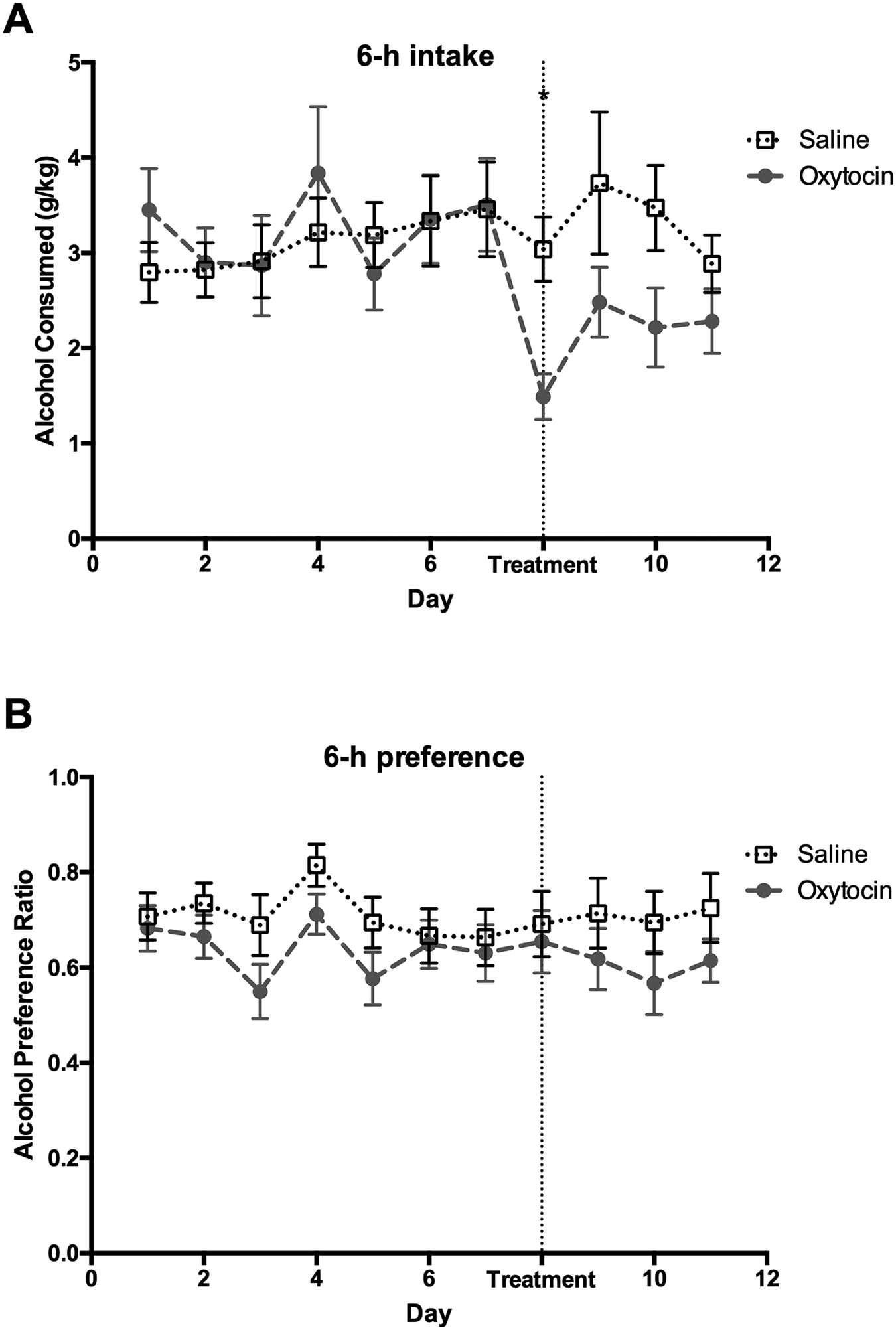
(A) Alcohol consumption during the first day of treatment was lower in the oxytocin group versus the saline-treated animals. (B) Alcohol preference did not differ between treatment groups during any day of alcohol access. *p<0.05. Error bars indicate mean ± SEM.
A repeated ANOVA found no significant effects of sex or treatment or any interactions between these factors on alcohol preference at any of the analyzed time points (P > 0.05). This lack of effect reflected that there were no visible differences in alcohol preference at either baseline or during treatment (Figure 3, Figure S3 and Table S2), revealing that oxytocin treatment decreased both alcohol and water consumption and the effect was not specific to alcohol.
To assess whether the effects of oxytocin on alcohol intake were independent of the cage arrangement of the oxytocin injection (in Across versus Mix experiment) we also have combined the 3 hr intake data from both experiments into an overall repeated ANOVA. This combined analysis was only performed on the 3 hr intake data to decrease the likelihood of false-positive results. The analysis revealed a significant effect of day (F7.25, 601.46= 5.9, P <0.001), significant effect of sex (F1,83=6.439, P= 0.013), and significant interaction of day and sex (F7.25, 601.46=3.253, P = 0.002), reflecting higher g/kg values in females versus males, but not on all days of experiment. Importantly, there was a significant day by treatment interaction (F7.25,601.46=4.371, P<0.001) and no other significant effects or interactions, including no interactions with experiment, indicating that oxytocin was effective in decreasing alcohol intake independently of the Across or Mix settings of administration.
Alcohol levels and neural activity.
To confirm that alcohol consumption in this paradigm could result in physiological effects, blood samples and brains from 16 untreated male and female voles from both experiments were subsequently analyzed for blood ethanol concentration (BEC) and FosB immunoreactivity, respectively. Alcohol metabolism in prairie voles is significantly faster than in humans11. Therefore, FosB levels were analyzed to assess whether alcohol intake affected neural activity in three brain regions known to respond to alcohol administration: nucleus accumbens (NAcc), central nucleus of amygdala (CeA) and the centrally-projecting Edinger-Westphal nucleus (EWcp)11,37. Untreated animals were used to avoid the complexity of oxytocin’s potential interference with activity in these regions. The BECs ranged between 1.8 and 132.1mg/dl (Figure 4C). There was no effect of sex (F1,10 = 1.93, P = 0.19) or experiment (F1,10 = 1.18, P = 0.30) on these BEC values.
Figure 4: Alcohol consumption is associated with neural activation in the centrally-projecting Edinger-Westphal nucleus (EWcp).
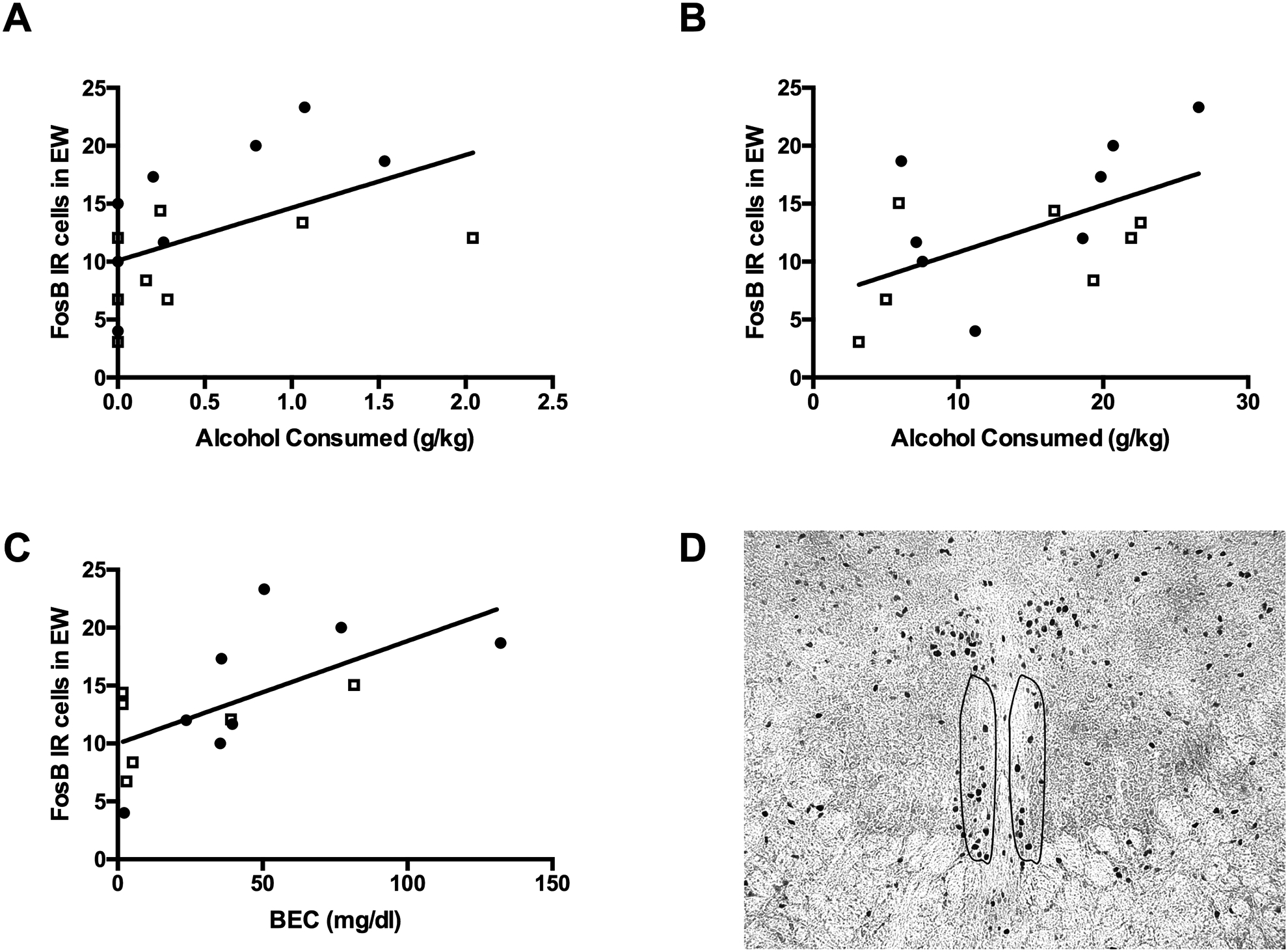
FosB activation in the EWcp was positively correlated with average alcohol intake during the (A) 1-hour and (B) 24-hours of alcohol consumption. (C) Similarly, blood ethanol concentration was positively correlated with FosB activation in the EWcp. (D) Representative photomicrograph of FosB immunoreactivity in the EWcp. Open squares represent male prairie voles and closed circles represent female prairie voles.
Alcohol consumption and BECs did not significantly correlate with FosB-ir in the NAcc core or shell and CeA (results not shown). However, FosB-ir in the EWcp (Figure 4) positively correlated with alcohol consumption at the 1 hr (R2 = 0.25, F1,14 = 4.7, P = 0.05) and 24 hr time points (R2 = 0.31, F1,14 = 6.4, P = 0.02). Similarly, FosB-ir in the EWcp positively correlated with BECs (R2 = 0.40, F1,12 = 8.1, P = 0.01). Thus, alcohol consumption in these experiments was associated with changes in neural activity, at least in the EWcp.
Discussion
Our study for the first time examined and observed significant effects of a promising anti-AUD treatment on alcohol consumption in animals housed together with untreated cagemates. These results not only provide additional evidence on potential effectiveness of oxytocin as a potential therapy for this disorder, but also demonstrate that other potential medications can be tested for effectiveness in social settings. Interestingly, the experiment in the mixed treatment settings produced clearer effects of oxytocin on alcohol intake than the experiment where all animals in each cage received similar treatments. This difference is likely due to a procedural caveat since it is easier to match baseline behavior across animal groups on individual basis than on a cage-by-cage basis. Nevertheless, this observation suggests that future studies using this technology can and should adapt mixed treatment social settings to assess the effects of pharmacotherapies.
Our study is also the first to examine alcohol consumption in individual prairie voles unconstrained in their interactions with their peers. Contrary to some of the previous observations of low alcohol consumption in rodents in such settings18–20, voles in our study consumed substantial amounts of alcohol with a few BEC samples reaching over 75 mg/dL. These levels were reached despite rapid ethanol elimination rates in this species11. Whether high intakes in social settings are a special feature of prairie voles or can be achieved in other rodent species would need to be examined in future studies, but this observation suggests advantages to using prairie voles in alcohol research. Alcohol consumption in our experiments was also associated with changes in central nervous activity as reflected in a significant correlation between BECs and FosB-ir in the EWcp. The FosB antibody used here measures levels of both the full length FosB protein and a truncated FosB protein, deltaFosB. Full length FosB is usually rapidly expressed after an acute stimulation, while deltaFosB gradually accumulates after repeated stimulations38. Therefore, our results suggest that both forms of FosB protein are present in the EWcp, where the increased deltaFosB is correlated with prolonged alcohol drinking (24hr) and the full length FosB correlated with acute (1hr) alcohol drinking. The EWcp is not only sensitive to alcohol, but also is involved in regulation of alcohol consumption39. However, the design of our study did not allow to distinguish whether activation of EWcp was regulating alcohol consumption or responding to consumed alcohol. Further studies on this subject are needed.
We have administered oxytocin via an intraperitoneal injection. This contrasts with the intranasal route of oxytocin administration in clinical studies29,31,32. Earlier reports have suggested that oxytocin is too large of a molecule to cross the blood-brain barrier40. On the other hand, peripheral oxytocin treatment rapidly increases oxytocin levels in brain dialysates and plasma during the first 30 minutes after treatment, which then subsequently return to baseline41. A recent study discovered that oxytocin is actively transported through the blood-brain barrier by the Receptor for Advanced Glycation End-products (RAGE)42. A search of the National Center for Biotechnology Information gene web database identified two predicted prairie vole transcripts of RAGE. Therefore, it seems possible that peripheral treatment with oxytocin in the current study decreased alcohol consumption through direct central effects. There could be differences in the organization of the oxytocin system between rodents and humans. However, studies in non-human primates showing that labeled oxytocin can cross the blood-brain barrier after both intranasal and intravenous routes of administration suggest that similar mechanisms could work across species43.
Our study expands the growing literature showing that oxytocin can be a potential treatment for AUD. In clinical studies, intranasal oxytocin treatment decreased withdrawal symptoms and alcohol cue-reactivity in AUD patients30,32,33. There is an even greater body of evidence on effects of oxytocin in rodent models. Specifically, McGregor and Bowen (2012) found that a single peripheral administration of oxytocin led to a long-lasting decrease in preference for a sweetened alcohol solution over the sweetener in male and female rats25. Since then, selective reduction of alcohol consumption and operant self-administration after oxytocin administration have been repeatedly observed in rats, including preferential suppression of dependence-induced drinking26,27,30,31. Additionally, peripheral oxytocin administration reduced alcohol consumption in male C57BL/6J mice in a no-choice binge-like alcohol self-administration and operant self-administration procedures for alcohol, with higher doses also being capable of decreasing sucrose preference28. Similarly, Stevenson et al. (2017) reported that peripheral oxytocin administration (1, 3 and 10 mg/kg, i.p.) decreased alcohol consumption and preference in an intermittent and continuous access two-bottle choice paradigms in male and female prairie voles semi-socially housed in mesh-separated cages29.
In the preclinical studies mentioned above, peripheral oxytocin treatment decreased consumption of alcohol and alcohol preference but did not affect consumption of non-alcohol containing solutions. In contrast, in the current study oxytocin did not alter alcohol preference. In the Across experiment, it was difficult to interpret the results because during the baseline period alcohol preference was significantly different between treatment groups. However, in the Mix experiment, alcohol preference between the treatment groups did not differ during the baseline and treatment periods, indicating that oxytocin reduced water consumption as well. The use a single dose could be considered as a limitation of our study, such that a lower dose could possibly produce a more alcohol-selective effect. It is theoretically possible that oxytocin decreased total fluid consumption due to anti-dipsogenic effects, especially since this peptide can interact with vasopressin receptors. However, the decrease in total fluid consumption seems unlikely to be due to non-specific effects of oxytocin since the same and a substantially higher dose of this peptide showed specific effects on alcohol preference in prairie voles in the previous vole study29. The decrease in total fluid consumption observed here could also be theorized to be similar to oxytocin’s reported effects on food consumption44. However, experiments that document suppression of food consumption following oxytocin administration in rodents were performed in individually-housed, and not socially-housed, animals45. Therefore, the difference between the current study demonstrating a decrease in alcohol and total fluid consumption versus the several studies demonstrating selective suppression of alcohol consumption is more likely due to effects of oxytocin that can only be observed in socially-housed animals, i.e. due to social interactions.
Indeed, oxytocin administration in mammals can increase their social interactions. For example, repeated peripheral oxytocin treatments increased partner preference formation in female prairie voles46. In naked mole rats, an eusocial rodent, a 1 mg/kg or 10 mg/kg IP injection of oxytocin also increased huddling behavior with familiar conspecifics47. It seems likely that alcohol preference was decreased in previous studies because oxytocin treatment was given to animals consuming alcohol housed in individual or in semi-social conditions (i.e. two animals in a cage divided by a mesh divider). In the current study, increased social interaction (i.e., huddling) could have competed with consumption of both alcohol and water. Increased social attachment is not likely to be of negative consequence as a side effect for the potential use of an AUD medication. Moreover, naltrexone, one of the currently approved medications for AUD, also does not always show exclusive effects on alcohol consumption over other fluids, or food, even in individually-housed rodents48,49. Further work investigating oxytocin’s ability to decrease fluid consumption moderated by an increase in social behaviors is warranted.
Oxytocin suppressed alcohol consumption at 3 and at 6 hours post treatment. Since the difference in alcohol intake between the oxytocin group and saline group in the Mix experiment was significant on the first days of treatment, but not on the last day of treatment, one could suspect decreasing efficacy with repeated treatment due to oxytocin receptor down-regulation or desensitization. Efforts towards designing small molecule agonist could overcome this difficulty. On the other hand, this interpretation is complicated by the tendency of cagemate prairie voles to match each other’s alcohol consumption11,50. This tendency could diminish the difference in intake between oxytocin-treated and saline controls over time. Importantly, despite this tendency, alcohol intake in oxytocin-treated prairie voles remained lower even on the last day of treatment versus on the last day of pretreatment, demonstrating remaining efficacy.
Importantly, this study to our knowledge is the first to explore the effects of a potential alcohol treatment in a mixed treatment group setting. We showed that the current procedure is sensitive enough to discover significant effects. In the future, these mixed treatment group setting procedures should be used when studying other treatments of AUD and other addictive drugs. In addition, while our study focused on same-sex groups of animals, this situation is not typical of natural conditions, and research should expand to male-female groups. Future studies should also assess more translationally relevant routes of administration of oxytocin (i.e., intranasal), test multiple doses of treatment and investigate whether it interferes prolonged alcohol consumption. These future studies could also provide a better understanding of involved neurocircuitry in individual animals, test effects of oxytocin on this neurocircuitry, as well as evaluate whether the effects of oxytocin administration depend on alcohol’s disruptive effects on the oxytocin system. The combination of mixed treatment group settings and radio frequency identification tracking will ultimately improve the translational value of animal studies on treatment for addiction in humans.
Supplementary Material
Acknowledgements
This work by NIH Grants: RO1 AA019793 and T32 AA007468. We thank Sheena Potretzke for the photograph of the HM-2 system.
Footnotes
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of Interest:
Authors declare have no competing financial interests in relation to the work described.
References
- 1.Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006; 111: 855–876. [DOI] [PubMed] [Google Scholar]
- 2.Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim MM, Shanahan E, Gass CE, Rowe CJ, Garbutt JC. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014; 311:1889–1900. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed SH, Badiani A, Miczek KA, Müller CP. Non-pharmacological factors that determine drug use and addiction. Neurosci Biobehav Rev. 2018. e-pub ahead of print 1 Sept 2018; doi: 10.1016/j.neubiorev.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heilig M, Epstein DH, Nader MA, Shaham Y. Time to connect: bringing social context into addiction neuroscience. Nat Rev Neurosci. 2016; 17: 592–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venniro M et al. Volitional social interaction prevents drug addiction in rat models. Nat Neurosci. 2018; 21: 1520–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deatherage G Effects of housing density on alcohol intake in the rat. Physiol Behav. 1972; 9: 55–57. [DOI] [PubMed] [Google Scholar]
- 7.Ehlers CL, Walker BM, Pian JP, Roth JL, Slawecki CJ. Increased alcohol drinking in isolate-housed alcohol-preferring rats. Behav Neurosci. 2007; 121: 111–19. [DOI] [PubMed] [Google Scholar]
- 8.Rockman GE, Gibson JE, Benarroch A. Effects of environmental enrichment on voluntary ethanol intake in rats. Pharmacol Biochem Behav. 1989; 34: 487–90. [DOI] [PubMed] [Google Scholar]
- 9.Rockman GE, Hall AM, Markert LE, Glavin GB. Influence of rearing conditions on voluntary ethanol intake and response to stress in rats. Behav Neural Biol. 1988; 49: 184–91. [DOI] [PubMed] [Google Scholar]
- 10.Tomie A, Lewis K, Curiotto J, Pohorecky LA. Intermittent exposure to a social stimulus enhances ethanol drinking in rats. Pharmacol Biochem Behav. 2007; 87: 341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anacker AMJ, Loftis JM, Kaur S, Ryabinin AE. Prairie voles as a novel model of socially facilitated excessive drinking. Addict Biol. 2011; 16: 92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walcott AT, Smith ML, Loftis JM, Ryabinin AE. Social transfer of alcohol withdrawal-induced hyperalgesia in female prairie voles. Soc Neurosci. 2018; 105: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logue S, Chein J, Gould T, Holliday E, Steinberg L. Adolescent mice, unlike adults, consume more alcohol in the presence of peers than alone. Dev Sci. 2014; 17: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varlinskaya EI, Truxell EM, Spear LP. Ethanol intake under social circumstances or alone in sprague-dawley rats: impact of age, sex, social activity, and social anxiety-like behavior. Alcohol Clin Exp Res. 2015; 39: 117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryabinin AE, Walcott AT. Assessing Social Alcohol Drinking in Rodent Models: Are We There Yet? Int Rev Neurobiol. 2018; 140: 33–51. [DOI] [PubMed] [Google Scholar]
- 16.Bonter DN, Bridge ES. Applications of radio frequency identification (RFID) in ornithological research: a review. Journal of Field Ornithology. 2011; 82: 1–10. [Google Scholar]
- 17.Schneider CW, Tautz J, Grunewald B, Fuchs S. RFID tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of Apis mellifera. PLoS One. 2012; 7: e30023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pohorecky LA. Housing and rank status of male Long-Evans rats modify ethanol’s effect on open-field behaviors. Psychopharmacology (Berl). 2006; 185: 289–97. [DOI] [PubMed] [Google Scholar]
- 19.Pohorecky LA. Acute novel stressors modify ethanol intake of psychosocially stressed rats. Pharmacol Biochem Behav. 2010; 95: 390–400. [DOI] [PubMed] [Google Scholar]
- 20.Holgate JY, Garcia H, Chatterjee S, Bartlett SE. Social and environmental enrichment has different effects on ethanol and sucrose consumption in mice. Brain Behav. 2017; 7: e00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomsen M, Dencker D, Wörtwein G, Weikop P, Egecioglu E, Jerlhag E, Fink-Jensen A, Molander A. The glucagon-like peptide 1 receptor agonist Exendin-4 decreases relapse-like drinking in socially housed mice. Pharmacol Biochem Behav. 2017; 160: 14–20 [DOI] [PubMed] [Google Scholar]
- 22.Lee H-J, Macbeth AH, Pagani JH, Young WS. Oxytocin: the great facilitator of life. Prog Neurobiol. 2009; 88: 127–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leong KC, Cox S, King C, Becker H, Reichel CM. Oxytocin and Rodent Models of Addiction. Int Rev Neurobiol. 2018; 140: 201–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee MR, Rohn MC, Tanda G, Leggio L. Targeting the Oxytocin System to Treat Addictive Disorders: Rationale and Progress to Date. CNS Drugs. 2016; 30: 109–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGregor IS, Bowen MT. Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm Behav. 2012; 61:331–39. [DOI] [PubMed] [Google Scholar]
- 26.Peters S, Slattery DA, Flor PJ, Neumann ID, Reber SO. Differential effects of baclofen and oxytocin on the increased ethanol consumption following chronic psychosocial stress in mice. Addict Biol. 2013; 18: 66–77. [DOI] [PubMed] [Google Scholar]
- 27.MacFadyen K, Loveless R, DeLucca B, Wardley K, Deogan S, Thomas C, Perris. Peripheral oxytocin administration reduces ethanol consumption in rats. Pharmacol Biochem Behav. 2016; 140: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King CE, Griffin WC, Luderman LN, Kates MM, McGinty JF, Becker HC. Oxytocin Reduces Ethanol Self-Administration in Mice. Alcohol Clin Exp Res. 2017; 41: 955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevenson JR, Wenner SM, Freestone DM, Romaine CC, Parian MC, Christian SM, Bohidar AE, Ndem JR, Vogel IR, O’Kane CM. Oxytocin reduces alcohol consumption in prairie voles. Physiol Behav. 2017; 179: 411–21. [DOI] [PubMed] [Google Scholar]
- 30.Hansson AC, Koopmann A, Uhrig S, Buhler S, Domi E, Kiessling E, Ciccocioppo R, Froemke RC, Grinevich V, Kiefer F, Sommer WH, Vollstad-Klein S, Spanagel R. Oxytocin Reduces Alcohol Cue-Reactivity in Alcohol-Dependent Rats and Humans. Neuropsychopharmacology. 2018; 43: 1235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tunstall BJ, Kirson D, Zallar LJ, McConnell SA, Vendruscolo JCM, Ho CP, Oleata CS, Khom S, Manning M, Lee MR, Leggio L, Koob GF, Roberto M, Vendruscolo LF. Oxytocin blocks enhanced motivation for alcohol in alcohol dependence and blocks alcohol effects on GABAergic transmission in the central amygdala. PLoS Biol. 2019; 17: e2006421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, Casey RL, Fender T, Garbutt JC. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcohol Clin Exp Res. 2013; 37: 484–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell JM, Arcuni PA, Weinstein D, Woolley JD. Intranasal Oxytocin Selectively Modulates Social Perception, Craving, and Approach Behavior in Subjects With Alcohol Use Disorder. J Addict Med. 2016; 10: 182–89. [DOI] [PubMed] [Google Scholar]
- 34.de Vries AC, Johnson CL, Carter CS. Familiarity and gender influence social preferences in prairie voles (Microtus ochrogaster). Can J Zool. 1997; 75: 295–301. [Google Scholar]
- 35.Young LJ, Wang Z. The neurobiology of pair bonding. Nature Neurosci. 2004; 7: 1048–1054de. [DOI] [PubMed] [Google Scholar]
- 36.Walum H, Young LJ. The neural mechanisms and circuitry of pair bond. Nature Rev Neurosci. 2018; 19:643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachtell RK, Wang YM, Freeman P, Risinger FO, Ryabinin AE Alcohol drinking produces brain region-selective changes in expression of inducible transcription factors. Brain Res. 1999; 847: 157–165 [DOI] [PubMed] [Google Scholar]
- 38.Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci USA. 2001; 98: 11042–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giardino WJ, Rodriguez ED, Smith ML, Ford MM, Galili D, Mitchell SH, Chen A, Ryabinin AE Control of chronic excessive alcohol drinking by genetic manipulations of the Edinger-Westphal nucleus urocortin-1 neuropeptide system. Trans Psychiatry; 2017; 7: e1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ermisch A, Ruhle HJ, Landgraf R, Hess J. Blood-brain barrier and peptides. J Cereb Blood Flow Metab. 1985; 5: 350–57. [DOI] [PubMed] [Google Scholar]
- 41.Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinol. 2013; 38: 1985–93. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto Y, Liang M, Munesue S, Deguchi K, Harashima A, Furuhara K, Yuhi T, Zhong J, Akther S, Goto H, Eguchi Y, Kitao Y, Hori O, Shiraishi Y, Ozaki N, Shimizu Y, Kamide T, Yoshikawa A, Hayashi Y, Nakada M, Lopatina O, Gerasimenko M, Komleva Y, Malinovskaya N, Salmina AB, Asano M, Nishimori K, Shoelson SE, Yamamoto H, Higashida H. Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice. Commun Biol. 2019; 2: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee MR, Scheidweiler KB, Diao XX, Akhlaghi F, Cummins A, Huestis MA, Leggio L, Averbeck BB. Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay. Mol Psychiatry. 2018; 23: 115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ott V, Finlayson G, Lehnert H, Heitmann B, Heinrichs M, Born J, Hallschmid M. Oxytocin reduces reward-driven food intake in humans. Diabetes. 2013; 62: 3418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides. 1989; 10: 89–93. [DOI] [PubMed] [Google Scholar]
- 46.Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000; 37: 49–56. [DOI] [PubMed] [Google Scholar]
- 47.Mooney SJ, Douglas NR, Holmes MM. Peripheral administration of oxytocin increases social affiliation in the naked mole-rat (Heterocephalus glaber). Horm Behav. 2014; 65: 380–85. [DOI] [PubMed] [Google Scholar]
- 48.Phillips TJ, Wenger CD, Dorrow JD. Naltrexone effects on ethanol drinking acquisition and on established ethanol consumption in C57BL/6J mice. Alcohol Clin Exp Res. 1997; 21: 691–702. [PubMed] [Google Scholar]
- 49.Middaugh LD, Bandy AL. Naltrexone effects on ethanol consumption and response to ethanol conditioned cues in C57BL/6 mice. Psychopharmacology (Berl). 2000; 151: 321–327. [DOI] [PubMed] [Google Scholar]
- 50.Anacker AMJ, Ryabinin AE. Identification of subpopulations of prairie voles differentially susceptible to peer influence to decrease high alcohol intake. Front Pharmacol. 2013; 4: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


