Abstract
Two of the most commonly used substances by adolescents in the United States are cannabis and alcohol. Cannabis Use Disorder (CUD) and Alcohol Use Disorder (AUD) are associated with impairments in decision-making processes. One mechanism for impaired decision-making in these individuals is thought to be an inability to adequately represent future events during decision-making. In the current study involving 112 adolescents, we used a comparative optimism task to examine the relationship between relative severity of CUD/AUD (as indexed by the CUD/AUD Identification Tests [CUDIT/AUDIT]) and atypical function within neural systems underlying affect-based neural represenation future events. Greater CUDIT scores were negatively related to responses within subgenual anterior and posterior cingulate cortex when processing high-intensity potential future positive and negative events. There was also a particularly marked negative relationship between CUD symptoms and blood oxygen level-dependent (BOLD) responses within visual and premotor cortices to high-intensity, negatively valenced potential future events. However, AUD symptom severity was not associated with dysfunction within these brain regions. These data indicate that relative risk/severity of CUD is associated with reduced responsiveness to future high-intensity events. This may impair decision-making where future significant consequences should guide response choice.
Keywords: Adolescent, Cannabis, fMRI
Introduction
Cannabis use disorder (CUD) and Alcohol use disorder (AUD) have lifetime prevalence rates of 6% and 29%, respectively,1,2 making them two of the most common substance use disorders (SUDs) in the United States. Epidemiological evidence indicates that use of alcohol and/or cannabis during adolescence is associated with an increased risk for developing these SUDs in adulthood.3 Furthermore, individuals with AUD and/or CUD who initiated use of these substances during adolescence face a more severe disease course and a greater likelihood of relapse.4 This may be due in part to the putative deleterious neurodevelopmental effects of alcohol and cannabis on the adolescent brain.5,6
One characteristic of adolescents and adults with SUDs is that they show impairments in decision-making processes.7 One form of decision-making impairment implicated in individuals with SUDs is an inability to represent future events.8 This can manifest as episodic foresight.9,10 Episodic foresight refers to one’s ability to imagine future events with self-relevance when explicitly directed to do so, and impairments in episodic foresight have directly been associated with SUDs, such as CUD. For example, adults who reported regular cannabis use produced fewer details relating specifically to a given future event compared to recreational cannabis users and non-cannabis using controls.10 A similar effect has been found for adults with opiate use histories relative to controls.11 To summarize, adults with SUDs show impairments in episodic foresight, possibly reflecting a broader inability to represent future events.
An inability to represent future events/engage in episodic foresight may also manifest as temporal discounting impulsiveness [TDI].9,12 That is a greater propensity to choose smaller, immediate rewards rather than larger, delayed rewards. Greater TDI is seen in individuals with SUDs (i.e., “steeper” temporal discounting).13 For example, scores on the Alcohol Use Disorder Identification Test (AUDIT)14 in young adults were positively related to TDI.15 Similarly, with respect to CUD, greater TDI was seen in a cannabis-dependent adults relative to former cannabis-dependent and control adults16 and TDI has been positively associated with cannabis dependence symptoms in adults17 – though see Strickland and colleagues.18
Prior fMRI work with neurotypical populations has identified neural systems implicated in episodic foresight/representation of future events19 and temporal discounting.20 These include ventromedial prefrontal cortex (vmPFC), inferior frontal gyrus (iFG), precuneus/posterior cingulate cortex (PCC), and anterior cingulate cortex (ACC).19,20 Areas have demonstrated positive (vmPFC, PCC) and negative (iFG) responsiveness to magnitude of the future reward during temporal discounting.20 With respect to substance use disorders (SUDs), prior work has indicated that the roles of vmPFC/PCC in the representation of reward may be compromised in at least adults with CUD; adults with CUD showed reduced activity within vmPFC and PCC during feedback in the Iowa Gambling Task.21
It should be noted that most of the work considered above was conducted with adult participants. Yet, adolescence is a time when neuro-circuitries that underly decision-making are rapidly developing, and substance use during adolescents may be detrimental to long-term functioning of these neural systems/decision-making processes.22 For example, during resting state, adolescents with co-morbid SUD and Conduct Disorder (CD) showed reduced connectivity between mPFC and PCC compared to controls.23 However, to our knowledge, no previous task-based fMRI studies have investigated the neural representation of future events in adolescents as a function of CUD/AUD severity.
Importantly, there may be differential associations between AUD and CUD and dysfunctional representation of future events. Although AUD and CUD are highly co-morbid in adolescent populations,24 recent work has begun to delineate differences in the functional impairments associated with each within adolescents. For example, differential associations between AUD versus CUD symptoms in adolescents have been reported with respect to the functional integrity of neural systems engaged in reward processing,25 executive attention26 and emotional responsiveness.27
In the current study, we used a version of a comparative optimism (CO) task, developed by our group,28,29 to examine the relationship between AUD and/or CUD symptomatology and affect-based neural representation of future events in an adolescent population. In this task, participants rate how likely they believe positively and negatively high- and low-intensity valenced future events are to happen to them in the future (e.g, high intensity positive event: Winning the lottery; low intensity negative event: Getting teased). Previous work with adults with a version of this task has indicated that positive relative to negative future events are associated with greater activity in regions including vmPFC and PCC.28 However, this task has not previously been used with adolescents. Moreover, some of the future events used in our previous work with adults were less appropriate for an adolesecent sample (e.g., having a heart attack). For this reason, we conducted an initial study with an independent group of healthy participants with our novel adolescent CO task to establish the patterns of responsiveness in healthy adolescents and inform predictions for the clinical participant study. The full details of this study are reported in Supplemental Material (Table S1, Figures S1–S2). In brief, this study revealed that in adolescents both positive relative to negative and high intensity relative to low intensity future events were associated with greater activity within vmPFC and PCC (see Figure S2). Given these findings, and our main hypothesis that AUD/CUD are associated with compromised representation of future events within vmPFC and PCC, we predicted that greater scores on the Alcohol Use Disorder Identification Test (AUDIT)14 and/or Cannabis Use Disorder Identification Test (CUDIT)30 would be associated with (i) reduced responsiveness to positive relative to negative future events within vmPFC and/or PCC; and (ii) reduced responsiveness to high relative to low intensity future events within these regions.
Materials and Methods
Participants
Study participants included 112 adolescents aged 14–18 years (inclusive) from both a residential treatment program (Boys Town) and the surrounding community. They were recruited as part of a broader study aimed at determining associations between neural dysfunction and externalizing disorders (primarily CD), internalizing disorders (primarily anxiety and depression), and SUDs. Eight adolescents were excluded due to excessive movement (>20% censored volumes, at >0.5 mm root-mean-squared displacement across adjacent volumes) and/or low response rate (<40/48 responses) during fMRI scanning (details below). This resulted in a final sample of 104 adolescents (75 from the residential treatment program and 29 from the community); average age=16.3 years (SD=1.09), IQ=100.1 (SD=11.01), 59 males. Adolescents with significant SUD histories were recruited from the residential treatment program and were abstinent for at least four weeks prior to scanning. For details regarding recruitment, assent/consent, and exclusion criteria, see supplemental material.
Measures
Comparative Optimism (CO) Task
An adapted version of a task used in previous work was administered during fMRI scanning.28,29 The stimuli for this task consisted of 48 possible future events involving different levels of emotional valence (negative versus positive) and intensity (low versus high). Events consisted of 12 high-intensity negative stimuli (e.g., being hit by lighting), 12 low-intensity negative stimuli (e.g., being scratched by a cat), 12 low-intensity positive stimuli (e.g., finding $5 on the street), and 12 high-intensity positive stimuli (e.g., winning the lottery). The stimuli were adapted from a larger set of stimuli used by Blair et al.28,29
During the task, participants read these possible future events and rated the likelihood of the event happening to them across their lifetime, compared with other people of the same sex and age. Participants rated their likelihood on a scale of 1–4 where 1=much less likely; 2=less likely; 3=more likely; or 4=much more likely. Each event was presented for 6500 ms; after which a fixation cross was displayed for 500 ms. In addition, 48×3000 ms fixation points were presented randomly throughout the task to both serve as an implicit baseline and provide interstimulus jitters (Total task duration: 8.4 mins).
Substance Use Disorder (SUD) Assessments
All participants completed both the AUDIT and the CUDIT.14,30 These scales assess overall risk for/symptom severity of AUD and CUD, respectively, including overall quantity/frequency of use, abuse symptoms, and dependence symptoms. They show high validity, as elevated scores on these scales are associated with a high likelihood of an AUD and/or CUD diagnosis.14,30 Moreover, elevated scores on the CUDIT and the AUDIT, correspond to the presence of CUD and AUD diagnoses, respectively,14,31 indicating that these measures are valid measures of CUD and AUD symptoms. Smoking status was determined using the Monitoring the Future Survey.32
Functional MRI Parameters and Analysis
Whole-brain BOLD functional MRI data were acquired via a 3T MAGNETOM Skyra magnetic resonance imaging scanner (Siemens Medical Solutions; see supplemental materials for further details on MRI parameters and fMRI preprocessing). Data were analyzed with a random-effects general linear model using Analysis of Functional NeuroImages.33 Five indicator regressors were generated: one for low-intensity negatively valenced future events, one for high-intensity negatively valenced future events, one for low-intensity positively valenced future events, one for high-intensity positively valenced future events, and one for trials where the participant failed to respond to the stimulus. Conditions were modeled using a boxcar reference vector, which is convolved with the canonical hemodynamic response function. Both the duration of the events the event duration specification for fMRI analysis was set to 6500 ms. General Linear Model fitting was performed with the five regressors listed; six motion regressors, and a regressor modeling baseline drift (-polort 4). This produced a β-coefficient and an associated t-statistic for each voxel and regressor.
Statistical Analytic Plan
To reduce skewness and kurtosis, a Rankit Transformation was applied to participants’ AUDIT scores.34 The CUDIT score distribution did not show significant skewness or kurtosis, so a Rankit-Transformation was not applied to the CUDIT scores. The Rankit-Transformed AUDIT scores and the raw CUDIT scores were then z-scored, and these values were used as continuous covariates in all analyses.
To examine relationships between AUDIT/CUDIT scores and psychiatric symptom levels dimensionally, zero-order correlation analyses were run between AUDIT/CUDIT scores and psychiatric diagnosis. Steiger’s z-tests were performed to determine whether there were, or were not, significant differences in partial correlation coefficients between AUDIT and CUDIT scores and psychiatric symptom variables.
To examine relationships between AUDIT/CUDIT scores and behavioral data on the CO task, two (valence: negative, positive)-by-2 (intensity: high, low) repeated measures ANCOVAs were conducted on the rating and response time (RT) data respectively. These ratings were collected in the scanner as part of the CO task. In both cases, Rankit-transformed, z-scored AUDIT scores and z-scored CUDIT scores were used as continuous covariates.
To examine associations between AUDIT/CUDIT scores and dysfunction within brain regions that are responsive to the valence and intensity of future events, we ran a full factorial 2 (valence: negative, positive)-by-2 (intensity: high, low) repeated-measures ANCOVA on the BOLD response data with AUDIT scores, CUDIT scores, and the AUDIT-by-CUDIT interaction as continuous covariates. Follow-up partial correlations and Steiger-Z tests were performed within SPSS 22.0 and using freely available online tools.35 In order to facilitate future meta-analytic work, effect sizes for all clusters are reported.
Correction for multiple comparisons was performed using a spatial clustering operation in AFNI’s 3dClustSim utilizing the autocorrelation function (-acf) with 10,000 Monte Carlo simulations for the whole-brain analysis. Spatial autocorrelation was estimated from residuals from the individual-level GLMs. The F-statistics for each individual main and interaction effect were used to identify significant regions of interest (ROIs); the initial threshold was set at F(1,100)=11.40, p=.001.36 This process yielded an extent threshold of k=23 voxels at an initial threshold of p=.001 for the whole brain. Follow-up analyses were conducted on the percent signal change taken from all significant voxels within each functional ROI generated by AFNI to examine significant main effects and interactions with planned follow-up testing within SPSS 22.0.
Results
Clinical Data
Of the final sample of 104 participants, 83 endorsed past-year use of either alcohol and/or cannabis though all had been abstinent for at least 4 weeks prior to scanning. Of these 83 substance users, 10 were from the community and 73 were from the residential treatment program. AUDIT scores ranged from 0–34 (M=5.6, range=0–34, SD=7.50) and CUDIT scores from 0–32 (M=10.3, range=0–32, SD=9.72). Sixty-eight met the clinical cutoffs on the AUDIT and/or CUDIT suggestive of AUD (AUDIT≥4) or CUD (CUDIT≥6).14,31 Forty-five participants had an AUDIT score≥4 and 61 participants had a CUDIT score≥6. In line with prior reports of high rates of poly-substance use in adolescents,24 38 participants had both an AUDIT score≥4 and CUDIT score≥6.
Zero-order correlation analyses revealed a significant positive correlation between AUDIT and CUDIT scores [r=0.58, p<.001]. Additionally, AUDIT scores were highly correlated with the consumption subscale of the AUDIT (AUDIT-C; r=0.96, p<.001) and CUDIT scores were highly correlated with the consumption subscale of the CUDIT (CUDIT-C; r=0.88, p<.001). Both AUDIT and CUDIT scores were significantly positively associated with most psychiatric diagnoses and use of antidepressants (see Table 1). There were no significant correlations between age, IQ, AUDIT scores, and CUDIT scores (r’s<0.13, ns) and there were no significant differences between males and females on AUDIT scores or CUDIT scores (t’s<1.42, ns). Additionally, there were no significant differences in correlation strengths between AUDIT and CUDIT scores and levels of psychopathology [Steiger’s Z’s=0–1.24, ns]. There were also no significant differential correlations between AUDIT and CUDIT scores and level of smoking [Steiger’s Z=−0.88, ns]; See Table 1.
Table 1.
Zero-Order Correlations Across Demographic and Clinical Variables
| CD (N=52) | ADHD (N=48) | GAD (N=28) | MDD (N=27) | Stimulants | Antidepressants | Antipsychotics | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | ||||||||||||
| 2. IQ | 0.18 | |||||||||||
| 3. Gendera | 0.12 | 0.04 | ||||||||||
| 4. AUDIT | 0.40* | 0.37* | 0.31* | 0.16 | 0.10 | 0.40* | 0.03 | 0.04 | −0.18 | −0.14 | ||
| 5. CUDIT | 0.46* | 0.44* | 0.27* | 0.05 | 0.14 | 0.38* | 0.09 | −0.04 | −0.19 | 0.08 | 0.58* | |
| 6. Smoking | 0.15 | −0.06 | 0.14 | 0.64* | 0.61* |
Gender coded as Female=0, Male=1; CD=Conduct Disorder; ADHD=Attention Deficit Attention Disorder; GAD=Generalized Anxiety Disorder; MDD=Major Depressive Disorder
significant at p<0.05
significant at p<0.01
Behavioral Data
The rating ANCOVA revealed a significant main effect of valence [F(1, 101)=102.76, p<.001; η2=0.50]; participants rated positive events more likely to happen to them than negative events [MPos=2.78; MNeg=2.21]. Additionally, there was a valence-by-intensity interaction effect [F(1, 101)=104.66, p<.001; η2=0.51]; participants made significantly greater likelihood ratings for high-intensity-positive>low-intensity-positive>low-intensity-negative>high-intensity-negative events (see Figure S1). There were no significant effects of, or interactions with, AUDIT/CUDIT scores.
For the reaction time (RT) data, there was a significant main effect of intensity [F(1,101)=4.71, p<.05; η2=0.05]; participants had slower RTs for low-intensity future events [Mlow=2978.11 ms] relative to high-intensity future events [Mhigh=2922.67 ms]. There was a CUDIT-by-valence interaction that approached significance [F(1,101)=3.71, p=.06; η2=0.04], such that there was a stronger positive relationship between CUDIT scores and RT’s for positive future events relative to negative future events [Steiger’s Z=1.84, p=.07]. Futhermore, there was a significant AUDIT-by-valence-by-intensity interaction [F(1,101)=7.59, p<.01; η2=0.07]; greater AUDIT scores were associated with significantly greater RT’s for high-intensity, negative valence future events relative to all other future events [Steiger’s Z’s=2.56–3.32, ps<.02].
Movement Data
Participants, after removal of those excluded according to movement criteria (N=8), showed relatively little movement; mean censored volumes=1.3% [SD=2.5%] and mean average motion per volume=0.09mm [SD=0.04mm]. There were no significant correlations between AUDIT scores and CUDIT scores and censored volumes, average motion per volume, and maximum displacement during scanning within the final sample [r’s=−0.13-.12, p’s=0.18–0.22].
fMRI Results
We hypothesized that greater scores on the AUDIT and/or CUDIT would be associated with: (i) reduced responsiveness to positive relative to negative future events within vmPFC and/or PCC; and (ii) reduced differential responsivenes to high vs. low intensity events (potentially within iFG and rostral medial frontal cortex [rmFC]). Main effects of valence and intensity are reported in Supplemental Table S2 (these closely replicate the findings of our initial pilot study with an independent sample of 118 healthy participants; see Table S1, Figures S1 and S2). Our main analysis revealed the following interaction effects:
CUDIT-by-Intensity Interaction
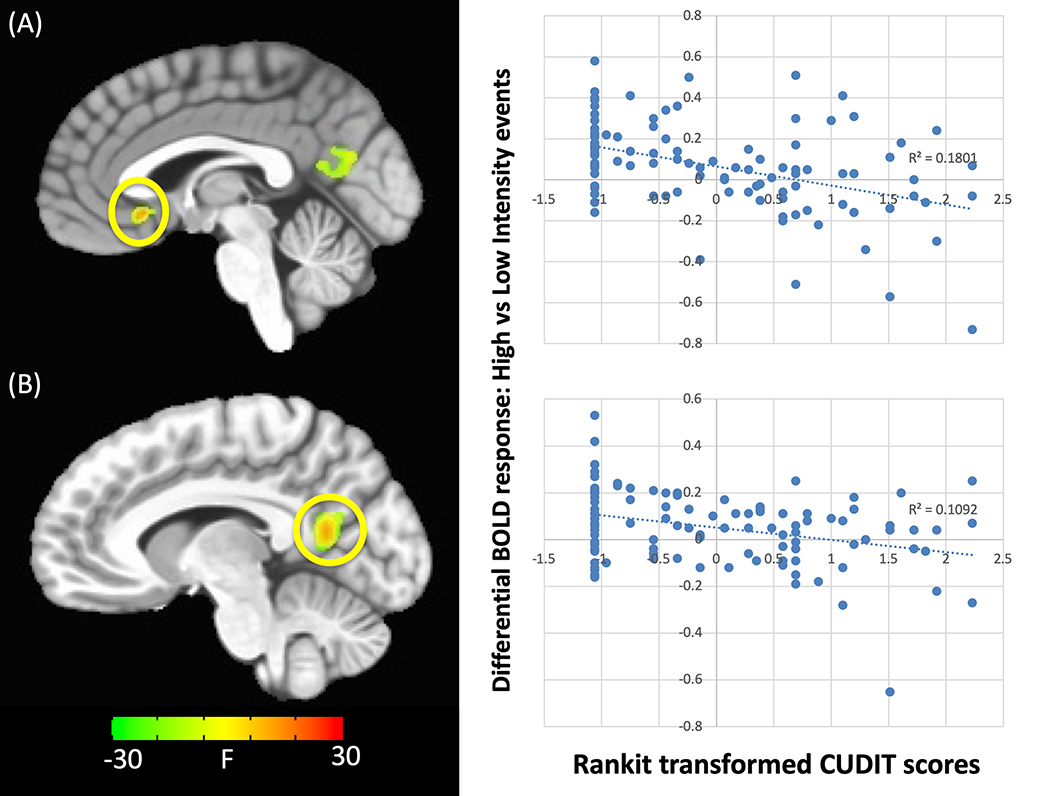
There was a significant two-way interaction between CUDIT and intensity within regions including subgenual ACC (sgACC), PCC, fusiform cortex and putamen (Table 2). In all brain regions there was a significant negative relationship between CUDIT scores and differential BOLD responsiveness to high-intensity relative to low-intensity future events (Figure 1). Within PCC and fusiform gyrus, greater CUDIT scores were associated with greater differential BOLD responsiveness to high- relative to low-intensity future events, although neither of the simple effects were individually significant. Within putamen, greater CUDIT scores were associated with reduced responsiveness to high-intensity future events (rp=−0.35, p<.001). Within sgACC, greater CUDIT scores were associated with greater responsiveness to low-intensity future events (rp’s=0.24–0.32, p’s<.02).
Table 2.
Brain regions demonstrating significant CUDIT-by-intensity, AUDIT-by-CUDIT-by-intensity, AUDIT-by-valence-by-intensity, and CUDIT-by-valence-by-intensity Interactions
| Coordinates of Peak Activationb | ||||||||
| Regiona | Hemisphere | BA | x | y | z | F | Partial η2 | Voxels |
| CUDIT-by-intensity | ||||||||
| sgACC | R/L | 25 | −1 | 20 | −4 | 31.53 | 0.238 | 26 |
| PCC | R/L | 23 | −7 | −55 | 14 | 20.57 | 0.169 | 85 |
| Superior Temporal Gyrus | R | 38 | 26 | 5 | −34 | 30.57 | 0.232 | 34 |
| Fusiform | L | 19 | −22 | −61 | −10 | 15.63 | 0.134 | 25 |
| Culmen | L | - | −22 | −43 | −13 | 20.89 | 0.173 | 24 |
| Putamen | R | - | 32 | 2 | −4 | 27.51 | 0.214 | 40 |
| CUDIT-by-valence-by-intensity | ||||||||
| Precentral Gyrus | L | 6 | −55 | 5 | 11 | 20.90 | 0.173 | 31 |
| Cuneus | R/L | 18 | 11 | −73 | 14 | 20.94 | 0.173 | 265 |
| Occipital Cortex | L | 17 | −19 | −91 | −7 | 15.63 | 0.135 | 26 |
| Culmen | R | - | 14 | −40 | −22 | 23.82 | 0.188 | 23 |
| AUDIT-by-CUDIT-by-intensity | ||||||||
| rmFC | R/L | 9/10 | 2 | 53 | 20 | 25.28 | 0.202 | 37 |
| AUDIT-by-valence-by-intensity | ||||||||
| Posterior Insula | R | 11/20 | 47 | −22 | 17 | 22.33 | 0.183 | 27 |
Note:
According to the Talairach Daemon Atlas (http://www.nitrc.org/projects/tal-daemon/)
Based on the Tournoux & Talairach standard brain template, BA= Brodmann’s Area
Figure 1. CUDIT-by-intensity interaction.
within the (A) sgACC and (B) PCC. Greater CUDIT scores were associated with reduced differential responses to high- relative to low-intensity future events. NOTE: Significant activation to this interaction is seen within both regions if the ANCOVA only includes CUDIT and not AUDIT as a covariate (p<0.001) but not if only AUDIT is included (even at p<0.05).
CUDIT-by-Valence-by-Intensity Interaction
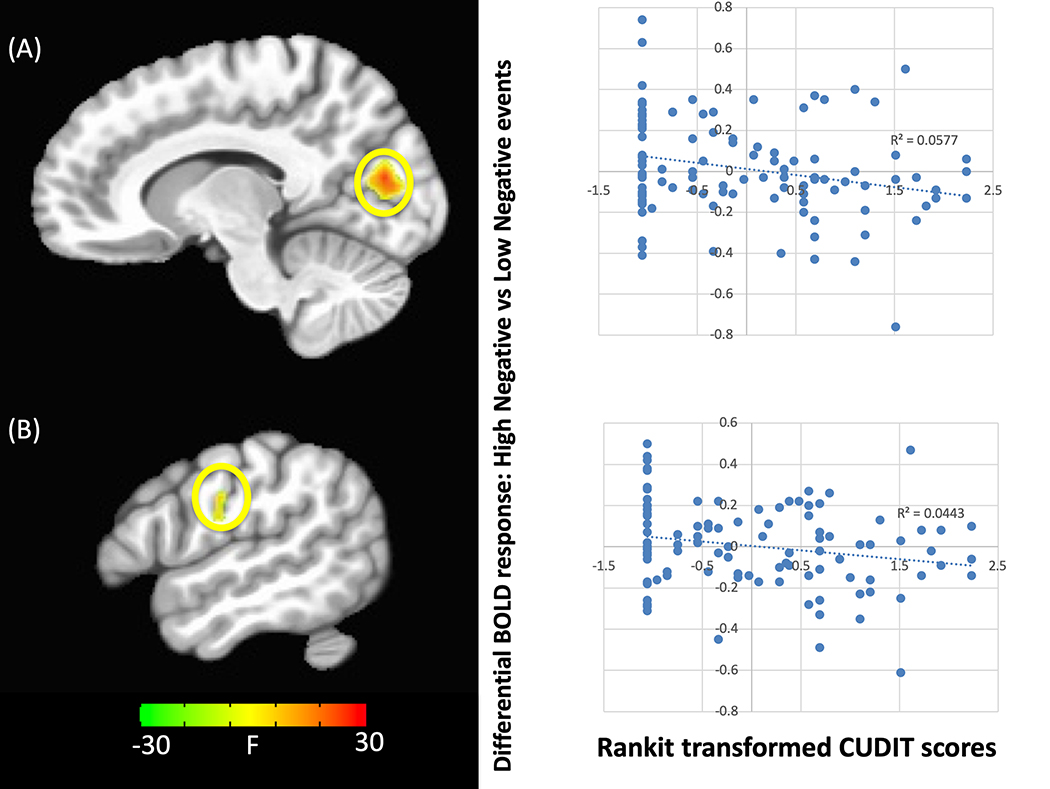
There was a significant three-way interaction between CUDIT, valence and intensity within regions including precentral gyrus, cuneus, and occipital cortex (Table 2). In all brain regions there was a significant negative relationship between CUDIT scores and differential BOLD responsiveness to high-intensity negative relative to low-intensity negative future events (Figure 2).
Figure 2. CUDIT-by-valence-by-intensity interaction.
within the (A) cuneus and (B) premotor gyrus. Greater CUDIT scores were associated with reduced differential responses to high-intensity negative valence relative to low-intensity negative valence future events. NOTE: Significant activation to this interaction is seen within both regions if the ANCOVA only includes CUDIT and not AUDIT as a covariate (p<0.001) but not if only AUDIT is included (even at p<0.05).
AUDIT-by-CUDIT-by-Intensity Interaction
There was a significant AUDIT-by-CUDIT-by-intensity interaction within rmFC (Table 2). There was a significant negative relationship between CUDIT scores and differential BOLD responsiveness to high-intensity relative to low-intensity future events within participants who did not report any past-year alcohol use (AUDIT=0). In participants who reported past-year alcohol use (AUDIT>0), the relationship between CUDIT scores and differential BOLD responsiveness to high-intensity relative to low-intensity future events was not significant.
AUDIT-by-Valence-by-Intensity Interaction
There was a significant three-way interaction between AUDIT, valence and intensity within posterior insula (Supplemental Figure S5, Table 2). Specifically, there was a significant positive relationship between AUDIT scores and differential BOLD responsiveness to high-intensity positive relative to high-intensity negative future events.
Potential Confounds
Calculation of Mahalanobis Distances revealed two multivariate outliers; therefore, the same analysis was repeated with these outliers removed from the dataset. This analysis revealed the PCC, although not the sgACC, region displaying the CUDIT-by-intensity interaction in the main analysis and largely replicated the results for the other three interactions of interest (Table S3).
Potential confounds included movement, recruitment source, age. Three additional covariates were conducted including average motion per volume, recruitment source (Community versus Boys Town) and age. All three ANCOVAs revealed similar findings to our main analysis (Tables S4–6). Note that the ANCOVA including age also revealed several regions showing significant Age-by-Valence interactions that survived comparisons for multiple comparisons.
To rule out the possibility that pathology related to psychiatric co-morbidities influenced our results, our main analysis was repeated with each of our most common co-morbid diagnoses as an additional covariate (i.e., ADHD, CD, Generalized Anxiety Disorder, or Major Depressive Disorder). All four of these ANCOVAs revealed results substantially similar to the findings from our main analysis (Tables S7 to S10). The only minor exception was the ANCOVA which included CD as a covariate (which revealed significant effects in the PCC, superior temporal gyrus, fusiform, and putamen, though not the sgACC).
Since participants using antidepressants were included in the sample, the same analysis was repeated with antidepressant use entered as a covariate. This analysis revealed a pattern of results highly similar to those reported in the main analysis (Table S11).
To rule out the possibility that smoking may have influenced our results, this analysis was repeated without participants who endorsed current regular smoking (N=16). This analysis largely replicated the results of our main analysis for most of the core interactions. However, the CUDIT-by-valence-by-intensity interaction was only seen within right cuneus (Table S12).
Finally, given the CUDIT and AUDIT include questions regarding frequency of use (which are included in the consumption subscales of these measures) and symptom severity (beyond frequency of use) we conducted two additional ANCOVAs. One included the sums of the consumption items of the AUDIT and CUDIT (AUDIT-C and CUDIT-C, respectively).30,37 The second, the sums of the symptom severity items (not including the consumption items; CUDIT-S and AUDIT-S). The consumption ANCOVA revealed the CUDIT-C-by-Valence-by-Intensity and the AUDIT-C-by-Valence-by-Intensity effects. The symptom severity ANCOVA revealed the CUDIT-S-by-Intensity and the CUDIT-S-by-Valence-by-Intensity effects (Table S13 & S14).
Discussion
The goal of the current study was to examine whether severity of AUD and/or CUD was related to dysfunction in the neural systems underlying responding to future events. In line with our hypotheses, CUDIT scores were negatively associated with responses to high intensity relative to low intensity future events within sgACC and PCC. However, there were no CUDIT-by-valence interactions and no regions showed either AUDIT-by-valence or AUDIT-by-intensity interactions.
Previous work has revealed greater responding within vmPFC and PCC as a function of reward level. This has been thought to represent the roles of these regions in the representation of subjective value.38 Previous work with individuals with CUD has indicated that these individuals may be compromised in recruiting these regions when responding to rewarding and emotional stimuli.21,39 Our pilot study revealed that typically developing adolescents show greater responding within vmPFC and PCC when performing the current task to both positive relative to negative future events as well as high-intensity relative to low-intensity future events. We therefore predicted that higher levels of CUD symptoms and/or AUD symptoms would be associated with (i) reduced responsiveness of vmPFC and/or PCC to positive relative to negative future events and/or (ii) reduced responsiveness of these brain regions to high-intensity relative to low-intensity future events. Consistent with our hypotheses, we observed that greater CUDIT scores were inversely related to differential activation in sgACC, PCC, and fusiform gyrus for high-intensity future events relative to low-intensity future events. However, inconsistent with our hypotheses, we did not find any association between AUDIT and/or CUDIT scores and differential activation to positive relative to negative future events.
The current data indicate some selectivity in the pathology associated with greater CUD severity (at least with respect to the functioning of sgACC, PCC, and fusiform gyrus). CUDIT scores were inversely associated with the capacity to differentiate high- relative to low-intensity future events but unrelated to the capacity to differentiate positive relative to negative future events. Previous work has shown that individuals who regularly use cannabis show impairments in episodic foresight relative to recreational users and controls.10 Moreover, it is worth noting that activity in sgACC/PCC during motivational interviewing has been shown to predict treatment outcomes in adolescents with SUDs.40 One critical component of MI is a patient’s ability to envision themselves in future events and discuss with a therapist how making changes with regard to a target behavior (e.g., alcohol/substance use) will improve future outcomes in these events.41 The current data indicate that participants with higher levels of CUD may be relatively insensitive to how bad or good a future outcome might be, even if they are representing that the outcome as bad/good. As such, treatments based around motivating change may require particular focus on differentiating the salience of particular positive/negative future events.40,41
No regions showed significant AUDIT-by-valence or AUDIT-by-intensity interactions, contrary to our hypotheses. There was a region of right posterior insula that showed a significant AUDIT-by-valence-by-intensity interaction. However, in contrast to hypotheses, this reflected a positive relationship between AUDIT scores and differential BOLD responsiveness to high-intensity positive relative to high-intensity negative future events (moreover, this was a region not identified as involved in task performance during our pilot study reinforcing our suspicion that this reflects a Type I error). In short, there were no indications that AUD severity relates to disruption in either the ability to represent the emotional intensity of future events or the emotional valence of future events. This is consistent with prior work from our group that has also shown that AUDIT scores were not associated with differential mPFC or PCC responsivity to emotional stimuli.26,27 However, as a caveat to this, it is worth remembering that there was a significant AUDIT-by-valence-by-intensity interaction in the behavioral RT data; greater AUDIT scores were associated with significantly longer RT’s for high-intensity, negative valence future events than all other future events. This suggests some association between greater AUD severity and issues processing at least some types of future event.
A region of rmFC showed a significant AUDIT-by-CUDIT-by-intensity interaction. Within this region, participants who had engaged in substance use, whether alcohol or cannabis, showed compromised differential BOLD responsiveness to high- relative to low-intensity future events relative to adolescents who had not engaged in substance use (see Supplemental Figure S4). The rmFC has been implicated in representation of subjective value, possibly through the generation of neural signals encoding salience and self-relevance to emotional stimuli42 and in the integration of subjective value information when engaging in decision-making.38,42,43 Indeed, recent work in a partially overlapping sample has shown that CUD symptom severity was negatively related to rmFC responsivity to looming emotional stimuli during a looming threat task.27 Additionally, dysfunction within rmFC has been implicated in increased risky decision-making.44 Risky decision-making is well documented in both adults and adolescents with SUDs.45–47 Furthermore, dysfunction in rmFC has been implicated in decision-making deficits in adults and adolescents with SUDs.21,48 In short, this finding might represent a more general consequence of substance use or a pre-existing general risk factor for the emergence of SUDs.
The results of this study must be viewed in light of several caveats. First, we did not conduct urine or breathalyzer testing for alcohol or cannabis use at the time of scanning. However, all adolescents with significant AUD and/or CUD histories were residents of a highly supervised residential treatment facility, which included random urine drug testing as part of treatment, for at least four weeks prior to scanning, mitigating this concern. A second caveat is that this study was cross-sectional in nature. As such, it is not possible to determine whether the relationships reported in the current study reflected the effects of alcohol/cannabis use on the developing brain or whether they reflected pre-existing risk factors for alcohol and/or cannabis use. Longitudinal neuroimaging work has shown that both alcohol and cannabis use alter neurodevelopment.5,6 However, it should be noted that it is unclear whether there are pre-existing neural risk factors that place adolescents at risk specifically for CUD rather than AUD (or vice-versa). Since there were differential relationships between AUDIT and CUDIT scores and brain function, the current results are more suggestive of the intensity of AUD and/or CUD on the developing brain. However, longitudinal work is needed to confirm this. A third significant caveat is that there was a high degree of psychiatric co-morbidity in the participant sample. As such, it could be argued that the findings presented here reflect psychiatric conditions related to psychiatric co-morbidities rather than AUD/CUD. Although it would be possible to test participants without co-morbidities, this would mean investigating a clinically atypical sample particularly since greater SUD symptoms compromise functions associated with a number of psychiatric conditions.49 Importantly, however, there were no significant differences in association strength between AUD and CUD symptom severity and the forms of psychiatric diagnosis examined. As such, the differential effects with respect to AUDIT/CUDIT scores could not reflect psychiatric comorbid conditions. Fourth, the task was shortened in the interests of constraining the amount of time the adolescent participants needed to be in the scanner. This may have compromised our power for individual level analyses. However, the covariate-by-intensity and covariate-by-valence interactions were based on 24 vs. 24 event comparisons; i.e., there was likely sufficient power for these interactions. Fifth, there was no association between CUD symptom severity and behavioral impairment on the CO task. However, the participants’ task was to rate the likelihood the event might happen to them. The principle dysfunction identified in the BOLD response data reflected inadequate differentiation of high vs. low intensity items. It is possible that if the participant’s task had been to rate level of positive/negative valenece of the future items, we would have observed behavioral effects also. Sixth, several of the future events were financially related. The salience of these events might be influenced by the participants’ socioeconomic status (SES). Future work could examine analyses with SES as a covariate. However, this information was only available for 63 of the participants in the current study. Finally, although we used a CUDIT score of 6 as a cutoff for possible CUD in our sample, it should be noted that this cutoff was validated in a sample of college students.31 While the AUDIT has been validated for use in an adolescent sample ranging from ages 13–17,14 similar validation studies for CUDIT have not been conducted in this specific age range in adolescents.
In summary, we found that CUDIT scores were related to reduced responsiveness to future high relative to low-intensity events within regions including sgACC, PCC, premotor and visual cortices (albeit the latter only for high-intensity negative relative to low-intensity negative future events). We hypothesize that this reflects a relationship between greater severity of CUD and disruption in the representation of the differential salience of future events and/or the use of this information within regions implicated in decision-making (particularly sgACC and PCC) and the organization of motor responding (premotor cortex). Such a relative disruption may further exaggerate the difficultes faced by adolescents with CUD, leading to a progressive failure to appropriately represent future consequences of their actions and interfere with interventions focusing on motivating change via future rewards.
Supplementary Material
Acknowledgements
We would like to thank Ron Copsey, Kim VanHorn, Michael Wright, Mark Timm, and Rhonda Tuel for their contributions to data collection. We would like to thank all subjects and their families for their participation. This work was supported by K22-MH109558 (RJB), R34-DA050286 (RJB), and Boys Town National Research Hospital. JA was supported by the AACAP Jeanne Spurlock Medical Student Research Fellowship in Substance Abuse and Addiction and a Program of Excellence Fellowship from the University of Nebraska Medical Center. KSB was supported by 5P20-GM109023; SFW was supported by K01-MH110643; KIC was supported by T32-MH018869 and U54-DA016511. The funders had no role in study design, data collection, data analysis, decision to publish, or manuscript preparation.
Footnotes
Conflicts of Interest
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder. JAMA Psychiatry. 2015;72(8):757. doi: 10.1001/jamapsychiatry.2015.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasin DS, Kerridge BT, Saha TD, et al. Prevalence and correlates of DSM-5 cannabis use disorder, 2012–2013: Findings from the national epidemiologic survey on alcohol and related conditions-III. Am J Psychiatry. 2016;173(6):588–599. doi: 10.1176/appi.ajp.2015.15070907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winters KC, Lee CYS. Likelihood of developing an alcohol and cannabis use disorder during youth: Association with recent use and age. Drug Alcohol Depend. 2008;92(1–3):239–247. doi: 10.1016/j.drugalcdep.2007.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babor TF, Hofmann M, DelBoca FK, et al. Types of alcoholics, I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry. 1992;49(8):599–608. doi: 10.1001/archpsyc.1992.01820080007002 [DOI] [PubMed] [Google Scholar]
- 5.Filbey FM, McQueeny T, DeWitt SJ, Mishra V. Preliminary findings demonstrating latent effects of early adolescent marijuana use onset on cortical architecture. Dev Cogn Neurosci. 2015;16:16–22. doi: 10.1016/j.dcn.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Squeglia LM, Tapert SF, Sullivan E V., et al. Brain Development in Heavy-Drinking Adolescents. Am J Psychiatry. 2015;172(6):531–542. doi: 10.1176/appi.ajp.2015.14101249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29(2):116–124. doi: 10.1016/j.tins.2005.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeWitt SJ, Ketcherside A, McQueeny TM, Dunlop JP, Filbey FM. The hyper-sentient addict: an exteroception model of addiction. Am J Drug Alcohol Abuse. 2015;41(5):374–381. doi: 10.3109/00952990.2015.1049701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulley A, Gullo MJ. The influence of episodic foresight on delay discounting and demand for alcohol. Addict Behav. 2017;66:1–6. doi: 10.1016/j.addbeh.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 10.Mercuri K, Terrett G, Henry JD, Curran HV, Elliott M, Rendell PG. Episodic foresight deficits in regular, but not recreational, cannabis users. J Psychopharmacol. 2018;32(8):876–882. doi: 10.1177/0269881118776672 [DOI] [PubMed] [Google Scholar]
- 11.Mercuri K, Terrett G, Henry JD, Bailey PE, Curran HV, Rendell PG. Episodic foresight deficits in long-term opiate users. Psychopharmacology (Berl). 2015;232(7):1337–1345. doi: 10.1007/s00213-014-3772-2 [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Feng T, Chen J, Li H. The value of emotion: How does episodic prospection modulate delay discounting? PLoS One. 2013;8(11). doi: 10.1371/journal.pone.0081717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1080/09652140020016978 [DOI] [PubMed] [Google Scholar]
- 14.Fairlie AM, Sindelar HA, Eaton CA, Spirito A. Utility of the AUDIT for screening adolescents for problematic alcohol use in the emergency department. Int J Adolesc Med Heal. 2006;18(1):115–122. doi: 10.1515/IJAMH.2006.18.1.115 [DOI] [PubMed] [Google Scholar]
- 15.Amlung M, MacKillop J. Delayed Reward Discounting and Alcohol Misuse: The Roles of Response Consistency and Reward Magnitude. J Exp Psychopathol. 2011;2(3):418–431. doi: 10.1055/s-0029-1237430.Imprinting [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson MW, Bickel WK, Baker F, Moore BA, Badger GJ, Budney AJ. Delay Discounting in Current and Former Marijuana-Dependent Individuals. Exp Clin Psychopharmacol. 2011;18(1):99–107. doi: 10.1037/a0018333.Delay [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aston ER, Metrik J, Amlung M, Kahler CW, MacKillop J. Interrelationships between marijuana demand and discounting of delayed rewards: Convergence in behavioral economic methods. Drug Alcohol Depend. 2016;169:141–147. doi: 10.1016/j.drugalcdep.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strickland JC, Lile JA, Stoops WW. Unique prediction of cannabis use severity and behaviors by delay discounting and behavioral economic demand. Behav Processes. 2017;140:33–40. doi: 10.1016/j.beproc.2017.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45(7):1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage. 2009;45(1):143–150. doi: 10.1016/j.neuroimage.2008.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wesley MJ, Hanlon CA, Porrino LJ. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res - Neuroimaging. 2011;191(1):51–59. doi: 10.1016/j.pscychresns.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: Possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72(1):114–123. doi: 10.1016/j.bandc.2009.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalwani MS, Tregellas JR, Andrews-Hanna JR, et al. Default mode network activity in male adolescents with conduct and substance use disorder. Drug Alcohol Depend. 2014;134(1):242–250. doi: 10.1016/j.drugalcdep.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason WA, Chmelka MB, Howard BK, Thompson RW. Comorbid alcohol and cannabis use disorders among high-risk youth at intake into residential care. J Adolesc Heal. 2013;53(3):350–355. doi: 10.1016/j.jadohealth.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 25.Aloi J, Meffert H, White SF, et al. Differential dysfunctions related to alcohol and cannabis use disorder symptoms in reward and error-processing neuro-circuitries in adolescents. Dev Cogn Neurosci. 2019;36. doi: 10.1016/j.dcn.2019.100618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aloi J, Blair KS, Crum KI, et al. Adolescents show differential dysfunctions related to Alcohol and Cannabis Use Disorder severity in emotion and executive attention neuro-circuitries. NeuroImage Clin. 2018;19:782–792. doi: 10.1016/j.nicl.2018.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blair RJ., White SF, Tyler PM, et al. Threat Responsiveness as a Function of Cannabis and Alcohol Use Disorder Severity. J Child Adolesc Psychopharmacol. 2019;XX(Xx):cap.2019.0004. doi: 10.1089/cap.2019.0004 [DOI] [PubMed] [Google Scholar]
- 28.Blair KS, Otero M, Teng C, et al. Dissociable roles of ventromedial prefrontal cortex (vmPFC) and rostral anterior cingulate cortex (rACC) in value representation and optimistic bias. Neuroimage. 2013;78:103–110. doi: 10.1016/j.neuroimage.2013.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blair KS, Otero M, Teng C, et al. Reduced optimism and a heightened neural response to everyday worries are specific to generalized anxiety disorder, and not seen in social anxiety. Psychol Med. 2017:1–10. doi: 10.1017/S0033291717000265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adamson SJ, Kay-Lambkin FJ, Baker AL, et al. An improved brief measure of cannabis misuse: The Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug Alcohol Depend. 2010;110(1–2):137–143. doi: 10.1016/j.drugalcdep.2010.02.017 [DOI] [PubMed] [Google Scholar]
- 31.Schultz NR, Bassett DT, Messina BG, Correia CJ. Evaluation of the psychometric properties of the cannabis use disorders identification test - revised among college students. Addict Behav. 2019;95(January):11–15. doi: 10.1016/j.addbeh.2019.02.016 [DOI] [PubMed] [Google Scholar]
- 32.Miech RA, Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use, 1975–2015: Volume I, Secondary School Students.; 2016. [Google Scholar]
- 33.Cox RW. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- 34.Bliss CI, Greenwood ML, White ES. A Rankit Analysis of Paired Comparisons for Measuring the Effect of Sprays on Flavor. Biometrics. 1956;12(4):381–403. [Google Scholar]
- 35.Lee IA, Preacher KJ. Calculation for the test of the difference between two dependent correlations with one variable in common. 2013. http://quantpsy.org/corrtest/corrtest2.htm.
- 36.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect. 2017;7(3):152–171. doi: 10.1089/brain.2016.0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): Validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163(7):821–829. doi: 10.1001/archinte.163.7.821 [DOI] [PubMed] [Google Scholar]
- 38.Clithero JA, Rangel A. Informatic parcellation of the network involved in the computation of subjective value. Soc Cogn Affect Neurosci. 2013;9(9):1289–1302. doi: 10.1093/scan/nst106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wesley MJ, Lile JA, Hanlon CA, Porrino LJ. Abnormal medial prefrontal cortex activity in heavy cannabis users during conscious emotional evaluation. Psychopharmacology (Berl). 2016;233(6):1035–1044. doi: 10.1007/s00213-015-4180-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldstein Ewing SW, Chung T, Caouette JD, Ketcherside A, Hudson KA, Filbey FM. Orbitofrontal cortex connectivity as a mechanism of adolescent behavior change. Neuroimage. 2017;151(December 2016):14–23. doi: 10.1016/j.neuroimage.2016.12.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: Guilford Press; 2013. doi: 10.1080/02615479.2014.894351 [DOI] [Google Scholar]
- 42.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Euston DR, Gruber AJ, McNaughton BL. The Role of Medial Prefrontal Cortex in Memory and Decision Making. Neuron. 2012;76(6):1057–1070. doi: 10.1016/j.neuron.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39(4):376–389. doi: 10.1016/S0028-3932(00)00136-6 [DOI] [PubMed] [Google Scholar]
- 45.Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180–1187. doi: 10.1016/S0028-3932(99)00158-X [DOI] [PubMed] [Google Scholar]
- 46.Whitlow CT, Liguori A, Brooke Livengood L, et al. Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend. 2004;76(1):107–111. doi: 10.1016/j.drugalcdep.2004.04.009 [DOI] [PubMed] [Google Scholar]
- 47.Schutter DJLG, Van Bokhoven I, Vanderschuren LJMJ, Lochman JE, Matthys W. Risky decision making in substance dependent adolescents with a disruptive behavior disorder. J Abnorm Child Psychol. 2011;39(3):333–339. doi: 10.1007/s10802-010-9475-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crowley TJ, Dalwani MS, Mikulich-Gilbertson SK, et al. Risky decisions and their consequences: neural processing by boys with Antisocial Substance Disorder. PLoS One. 2010;5(9):e12835. doi: 10.1371/journal.pone.0012835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med. 2016;374(4):363–371. doi: 10.1056/NEJMra1511480 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.