Abstract
Purpose:
To evaluate trends in oncologic characteristics and outcomes, as well as perioperative management, among patients undergoing radical cystectomy at Memorial Sloan Kettering from 1995 to 2015.
Materials and Methods:
We retrospectively reviewed our institutional database to analyze changes in disease recurrence probability, cancer-specific and all-cause mortality, incidence of muscle-invasive bladder cancer, use of perioperative chemotherapy, rate of positive soft-tissue surgical margins, and lymph node yield.
Results:
In 2,740 patients with non-metastatic urothelial carcinoma undergoing radical cystectomy from 1995 to 2015, the 5-year probability of disease recurrence decreased from a peak of 42% in 1997 to 34% in 2013 (p=0.045), while 5-year probability of cancer-specific mortality likewise declined from 36% in 1997 to 24% in 2013 (p=0.009). Incidence of non-muscle-invasive disease before radical cystectomy did not change, comprising 30%–35% of patients across the study period. Use of neoadjuvant chemotherapy rose significantly: 57% of patients with muscle-invasive bladder cancer from 2010 to 2015 received it. We observed a corresponding rise in complete pathologic response (pT0) at radical cystectomy, as well as decreasing positive soft-tissue surgical margins (10% to 2.5%) and rising lymph node yield (7 to 24) from 1995 to 2015.
Conclusions:
Over a 21-year period, outcomes after radical cystectomy at our institution improved significantly, as probability of recurrence and cancer-specific mortality decreased. Increasing utilization of neoadjuvant chemotherapy, rising pT0 rates, decreased positive soft-tissue surgical margins, and increasing lymph node yields likely contributed, suggesting that optimized surgical and perioperative care led to improved cancer outcomes in patients undergoing radical cystectomy.
Keywords: cystectomy, neoadjuvant therapy, urinary bladder neoplasms, disease-free survival, lymph node excision
INTRODUCTION
Epidemiologic studies have demonstrated marginal improvements in cancer-specific and overall survival among patients with localized bladder cancer from the 1970s into the 2000s.1, 2 During this period, neoadjuvant chemotherapy (NAC), with its associated survival benefit,3 has been increasingly utilized for patients with muscle-invasive bladder cancer (MIBC) undergoing radical cystectomy (RC).4 In the United States, RC has become centralized at high-volume centers,5 which has been associated with improved perioperative outcomes and overall survival in multiple studies.6, 7 Optimization of surgical performance may play a similarly important role, with US population-based studies demonstrating increasing use of pelvic lymphadenectomy and higher lymph node (LN) yields over time,8 a surgical performance metric that has been associated with better outcomes among patients with MIBC in several observational studies.9–13
Conflicting evidence exists as to whether any improvement in cancer-specific outcomes has been achieved in the last two to three decades. Therefore, we sought to evaluate trends in oncologic outcomes over a 21-year span among patients undergoing RC at Memorial Sloan Kettering Cancer Center (MSK). We assessed the relationship over time between oncologic outcomes and disease characteristics, perioperative management, and pathologic and surgical outcomes, including surgical margin status and LN yield.
MATERIALS AND METHODS
Patients and Outcomes
We identified patients with urothelial carcinoma (UC) who underwent RC at MSK between January 1995 and December 2015, to examine how patient characteristics, treatment practices, and oncologic outcomes changed at our institution during this 21-year period. This study was performed with MSK Institutional Review Board approval (IRB No. 16–364).
We investigated whether the patient population changed over time by evaluating trends in patient age, sex, variant histology rates (any variant histology or no variant histology), and consensus tumor stage (defined as the higher of the clinical and pathologic stage)14. We then investigated trends in the use of perioperative chemotherapy and changes in surgical practice. For chemotherapy, we chose the outcomes of any NAC among patients with MIBC, any adjuvant chemotherapy use, and any use of neoadjuvant gemcitabine and cisplatin. To assess changes in surgical practice, we examined the rate of positive soft-tissue surgical margins (STSMs) and total number of LNs removed.
To assess surgical and oncologic outcomes, we examined the rate of pathologic T0 disease (pT0) after RC in patients with MIBC. We analyzed trends in 3-year and 5-year probability of disease recurrence and bladder cancer-specific mortality; patients who had nodal or visceral metastatic disease before RC were excluded from the survival analyses.
Statistical Analyses
To test for a change in outcome over time, we created univariate models for the association between surgery date and outcome for all outcomes. Linear and logistic regression models were used for continuous outcomes and binary outcomes, respectively. For all linear and logistic regression models, surgery date was entered into the models as a non-linear term using restricted cubic splines with knots at the quartiles. For survival outcomes, locally-weighted Cox proportional hazards models were used to account for possible non-linearity between rates and time.
To graphically present the change in outcome over time, locally-weighted scatterplot smoothing was used for continuous outcomes, and generalized additive models were used for binary outcomes. For survival outcomes, the previously created locally-weighted Cox regression models were used to predict 3-year and 5-year probability of recurrence or death over the range of surgery dates, and these predictions were then graphed by surgery date. Analyses were performed using Stata 15 (StataCorp, College Station, TX) and R 3.5.1.
RESULTS
Our cohort consisted of 2,911 patients with UC who underwent RC between 1995 and 2015. For the survival analyses, 171 patients with nodal or visceral metastatic disease before RC were excluded, leaving 2,740 patients for survival analysis. Three-quarters of patients were male (77%) and median age was 68 (IQR 61–75) (table 1). Median follow-up time among survivors was 5.7 years (IQR 3.5–9.9). All but 56 patients in this cohort underwent an open surgical approach, as robotic RC was only conducted as part of a randomized trial at our institution during this study period.15
Table 1.
Patient and disease characteristics.
| N=2911 | |
|---|---|
| No. male (%) | 2246 (77) |
| Median age at RC (IQR) | 68 (61–75) |
| No. consensus tumor stage (%) (N=2910) | |
| Ta | 41 (1.4) |
| TIS | 113 (3.9) |
| T1 | 686 (24) |
| T2 | 946 (33) |
| T3 | 744 (26) |
| T4 | 380 (13) |
| No. high-grade tumor (%) (N=2501) | 2455 (98) |
| No. variant histology (%) | |
| None | 1725 (59) |
| Mixed | 348 (12) |
| Squamous | 298 (10) |
| Glandular | 149 (5.1) |
| Micropapillary | 108 (3.7) |
| Nested | 80 (2.7) |
| Plasmacytoid | 60 (2.1) |
| Other | 52 (1.8) |
| Sarcomatoid | 52 (1.8) |
| Small cell | 34 (1.2) |
| Clear cell | 2 (<0.1) |
| Neuroendocrine | 2 (<0.1) |
| Type of urinary diversion, no. (%) | |
| Ileal conduit | 1797 (62) |
| Orthotopic neobladder | 962 (33) |
| Continent cutaneous diversion | 147 (5.0) |
| None | 5 (0.2) |
| No. presence of CIS (%) (N=2819) | 1804 (64) |
| No. multifocal disease (%) (N=2636) | 1226 (47) |
| No. lymph node involvement (%) | 570 (20) |
| No. metastatic disease (%) | 171 (5.9) |
| No. positive soft tissue margins (%) (N=2908) | 149 (5.1) |
| No. positive urothelial margins (%) (N=2909) | 324 (11) |
| No. MIBC patients receiving NAC (%) (N=1926) | 647 (34) |
| No. adjuvant chemotherapy (%) | 246 (8.5) |
| No. preoperative chemotherapy (%) | |
| Neoadjuvant | 655 (22.5) |
| Consolidative | 161 (5.5) |
| Other preoperative | 12 (0.4) |
Only non-metastatic patients (N=2740) were included in the survival analyses (recurrence, cancer-specific mortality, and all-cause mortality). All patients (N=2911) were included in all other analyses.
We observed improvement in oncologic outcomes over the study period, with significant decrease in the 3- and 5-year probabilities of disease recurrence, death from bladder cancer, and death from any cause (p=0.045, p=0.009, and p<0.001, respectively) (figure 1). For example, the probability of recurrence at 5 years decreased from a peak of 42% (95% CI 37%–47%) for patients undergoing RC in 1997 to 34% (95% CI 30%–38%) for patients undergoing RC in 2013. Similarly, the probability of cancer-specific death at 5 years decreased from a peak of 36% (95% CI 31%–40%) for 1997 patients to 24% (95% CI 20%–27%) for 2013 patients. This improvement in oncologic outcomes coincided with several important trends in disease characteristics and surgical outcomes.
Figure 1.
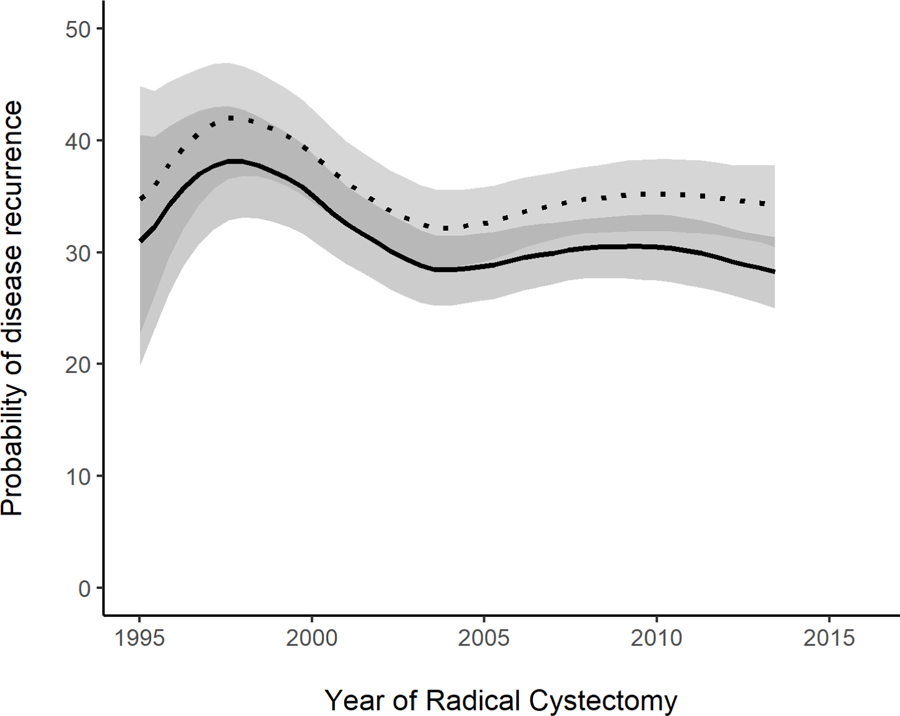
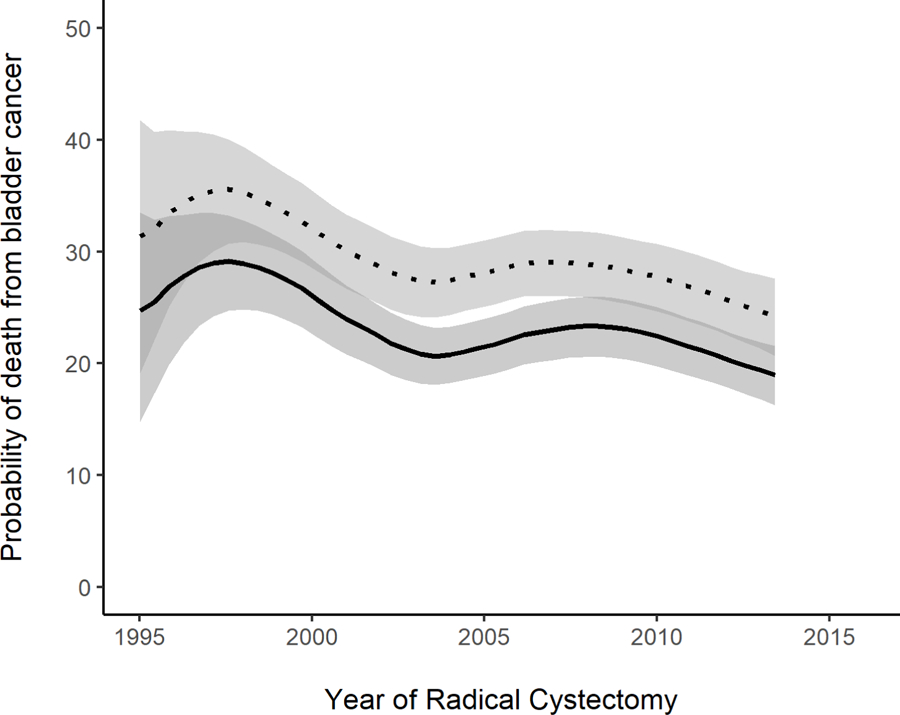

Probability of a) disease recurrence, b) death from bladder cancer, and c) death from any cause, among patients who did not have metastatic disease preoperatively, within 3 years (solid line) and 5 years (dotted line), by year of RC (p=0.045, 0.009, and <0.001, respectively) (N=2740). Estimates generated by univariable locally-weighted Cox regression models.
Twenty-nine percent of the study cohort had clinical stage ≤T1 disease at the time of RC (table 1). Although there was variation over time, a similar proportion of patients (≈28%) had clinical ≤T1 disease at the beginning and end of the study period. Similarly, the incidence of variant histology varied over time, ranging from 34% to 46% of patients. Rates of consensus stage T2 or higher disease decreased slightly over the study period, from 78% in 1995 to 69% by 2015 (p=0.01; figure 2). The incidence of LN involvement changed significantly over time, with an overall decrease in LN involvement from the year 2000 (19% pN+) to 2015 (8.7% pN+) (p=0.007). Rates of pT0 disease in patients with MIBC increased from 3.6% in 1995 to 18% by 2015 (p<0.001; figure 3). This trend coincided with a corresponding increase in use of NAC among patients with MIBC, from less than 5% before 2000 and reaching a plateau around 2010, with 57% of patients with MIBC who underwent RC from 2010 to 2015 receiving NAC (p<0.001; figure 4). Neoadjuvant gemcitabine-cisplatin was used in the vast majority of patients receiving NAC, an institutional practice based on level 1 evidence in the metastatic setting,16 and its use increased similarly to overall NAC use (p<0.001). Among all patients receiving NAC, 22% demonstrated a complete pathologic response (pT0N0). Use of adjuvant chemotherapy was highest between 2000 and 2005, peaking at 18% then declining (p<0.001; figure 4).
Figure 2.
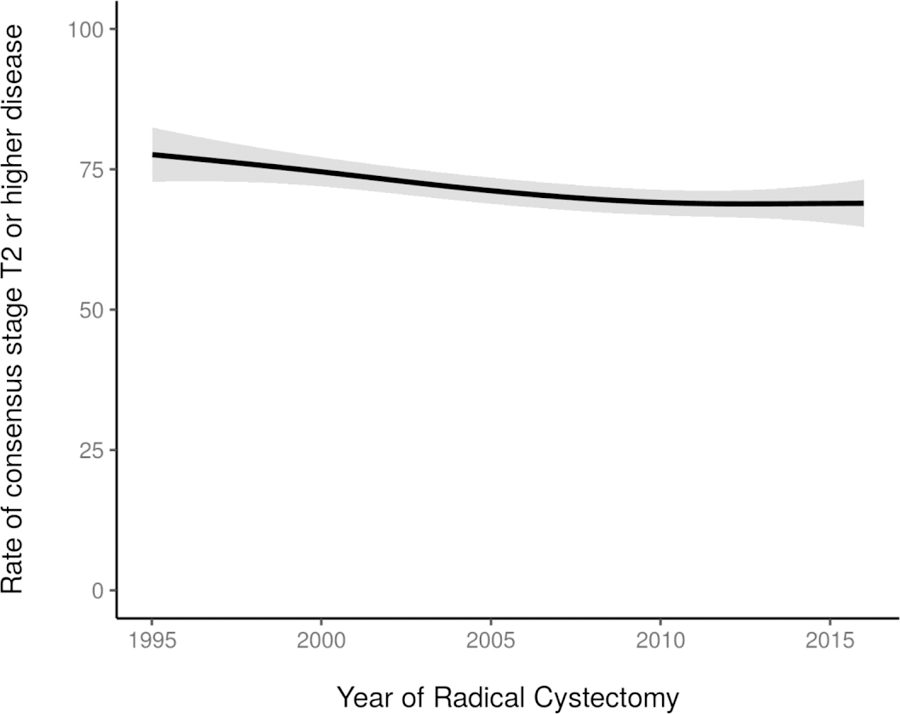
Rate of consensus stage T2 or higher disease, by year of RC (p=0.01) (N=2911). Estimates generated using univariable generalized additive models.
Figure 3.

Rate of pathologic stage pT0 in the 1926 patients with MIBC (p<0.001), by year of RC. Estimates generated using univariable generalized additive models.
Figure 4.
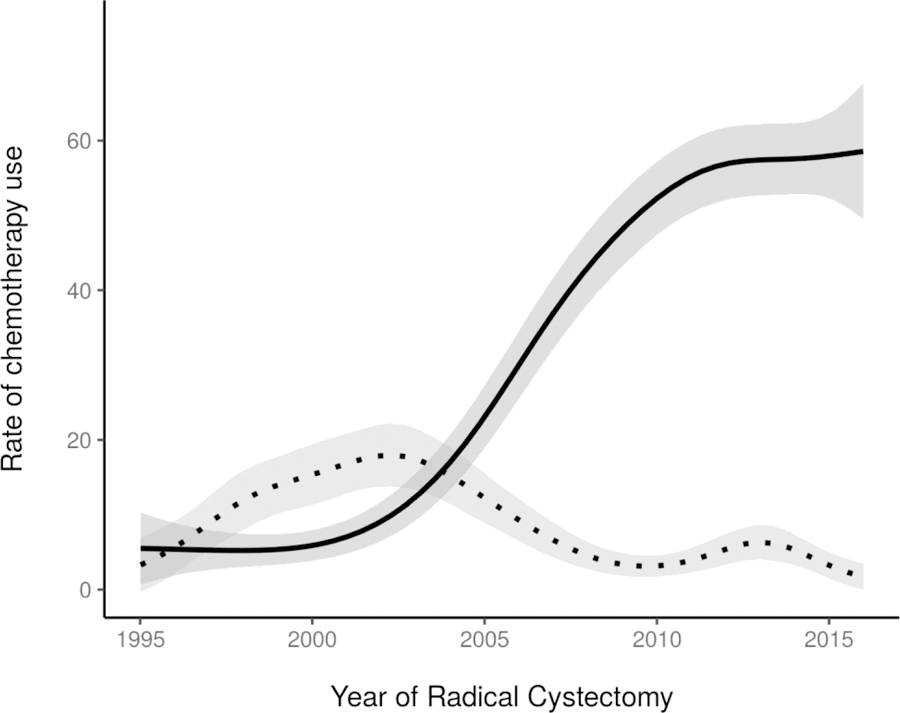
Rate of use of NAC among the 1926 patients with MIBC (solid line, p<0.001) and adjuvant chemotherapy among all 2911 patients (dotted line, p<0.001), by year of RC. Estimates generated using univariable generalized additive models.
Changes in surgical outcomes were observed during the study period. The incidence of positive STSMs decreased significantly over time (p<0.001; figure 5). In 1995, incidence of positive STSMs was about 10%, decreasing to approximately 2.5% by 2015. Of 149 patients with positive STSMs, 144 (97%) had ≥pT3 disease. We observed an increasing number of total reported LNs, from an average of 7 LNs in 1995 compared to 24 in 2015 (p<0.001). Importantly, the method of LN submission at our institution was modified in 2000 from en bloc LN submission to submission of discrete LN packets, the latter having been shown to be associated with a greater number of reported nodes on final surgical pathology.17 Our method of LN submission has remained consistent since the early 2000s, yet we still observed a subsequent steady increase in the mean number of LNs identified on surgical pathology, suggesting that the continued rise in reported LNs is due in part to a greater number removed at lymphadenectomy rather than method of submission alone. Despite the greater number of reported LNs, the rate of LN involvement decreased over time likely due to greater NAC use; among patients with MIBC, LN involvement was less common among patients receiving NAC compared to those who did not (18% vs 28%, p<0.001).
Figure 5.
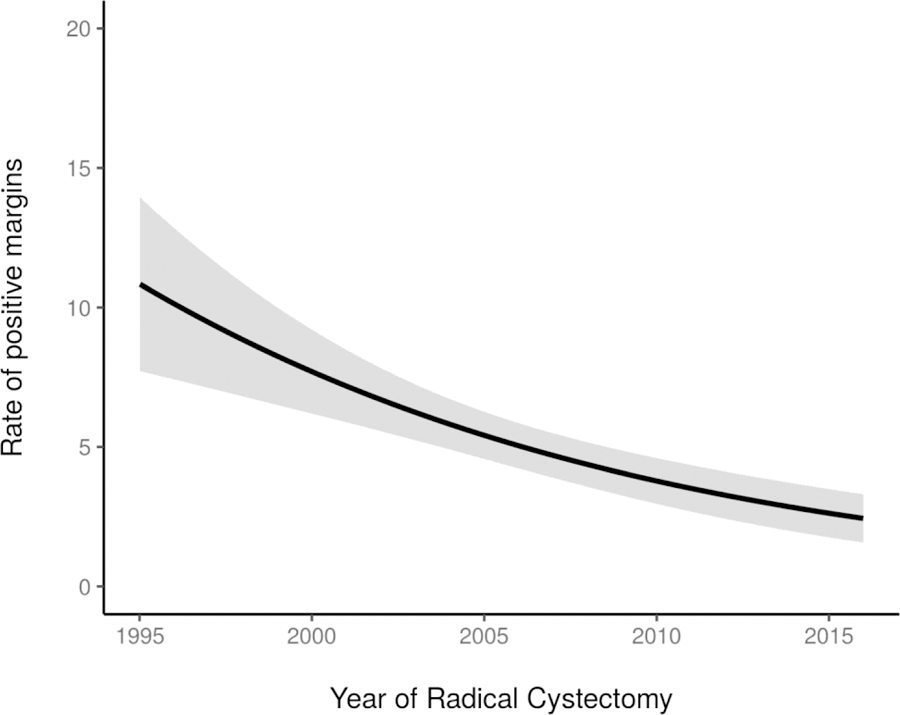
Rate of positive soft-tissue surgical margins (p<0.001), by year of RC (N=2911). Estimates generated using univariable generalized additive models.
DISCUSSION
We examined a cohort of over 2900 patients with UC undergoing RC at MSK over a 21-year span, observing trends in several important disease and treatment-related characteristics with corresponding improvements in oncologic outcomes. The 5-year probability of disease recurrence decreased from a peak of 42% for patients undergoing RC in 1997 to 34% for patients undergoing RC in 2013; the 5-year probability of death from bladder cancer similarly decreased from a peak of 36% in 1997 to 24% by 2013. These trends coincided with a significant increase in the use of NAC among patients with MIBC, with a corresponding rise in the incidence of pathologic complete response. Notably, 57% of patients with MIBC in this analysis received NAC from 2010 to 2015, significantly greater than the 21% utilization reported among patients with MIBC nationally at that time.4 Furthermore, the significant rise in pathologic complete response that we observed occurred over a period in which there was limited change in consensus ≥T2 disease. The incidence of positive STSMs, which occurred almost exclusively among patients with locally-advanced disease, decreased significantly over time, likely the result of multiple factors including increasing use of NAC, rising incidence of pathologic complete response, and possibly case selection or improvements in surgical technique. The strong association between positive STSM and risk of disease recurrence and cancer-specific death demonstrated in prior studies18, 19 highlights the clinical significance of this observation. A rise in LN yield, previously shown to be associated with improved survival in several observational studies,9, 12 was observed. Although packeted LN submission was instituted in the early 2000s, we observed continued rise in the number of LNs reported on surgical pathology, suggesting a greater extent of lymphadenectomy over time. These findings demonstrate important changes over time in the perioperative management, pathologic, and surgical outcomes of patients undergoing RC; together, these changes have likely contributed to the improved oncologic outcomes observed over time in this cohort.
Our observations differ from those reported in the University of Southern California (USC) RC experience from 1980 to 2005. In this study, the investigators grouped patients based on the decade in which RC was performed and observed similar oncologic outcomes across decades.20 Age, pathologic stage, positive surgical margins, and the receipt of NAC were independent predictors of disease recurrence and death. Their cohort differed from ours in several important ways. First, these investigators examined patients undergoing RC from 1980 to 2005, predating the widespread adoption of NAC. Only 6% of patients in this cohort received preoperative chemotherapy. In contrast, 34% of all patients with MIBC in our cohort received NAC, including 57% of patients with MIBC from 2010 to 2015. Based on prior studies of cisplatin eligibility in patients undergoing RC,21 approximately 60% of eligible patients (by pathology, ie, ≥T2) represents the upper limit of tolerability for cisplatin-based NAC in this patient population. Additionally, the authors note that the surgical technique of RC and pelvic lymphadenectomy at USC did not meaningfully change over the course of the study and included the routine use of extended pelvic lymphadenectomy. The incidence of positive STSMs or LN yield over time was not reported. In contrast, in the current study we demonstrated a significant decrease in the rate of positive STSMs, an independent factor associated with an elevated risk of disease recurrence and death.19 Additionally, we observed an increasing LN yield over time, which has been shown to be associated with improved outcomes in several prior studies.9–13 Improvement in these surgical outcomes combined with increasing utilization of NAC and corresponding pT0 rates over time coincided with significant improvements in cancer-specific outcomes in our cohort.
Prior observational studies have demonstrated an association between surgical and oncologic outcomes among patients undergoing RC. In a comparison of standard versus extended pelvic lymphadenectomy at two high-volume centers of excellence, Dhar and colleagues demonstrated improved survival among patients undergoing extended versus standard pelvic lymphadenectomy in the setting of node-positive disease (5-year RFS 35% versus 7%, p<0.001) and locally-advanced, node-negative disease (5-year RFS 57% versus 23%, p<0.001).9 Koppie and colleagues similarly demonstrated therapeutic benefit of more extensive lymphadenectomy in an analysis of over 1100 patients undergoing RC. In a multivariable model, the number of lymph nodes removed was a significant predictor of overall survival, irrespective of lymph node involvement. Furthermore, the probability of survival continued to increase with a greater number of lymph nodes removed.12 These data support the therapeutic benefit of a more extensive pelvic lymphadenectomy, highlighting the clinical significance of the rising LN yield observed over time in this study. In contrast to these studies, a recently-reported German randomized controlled trial failed to demonstrate a statistically significant survival advantage of extended over standard pelvic lymphadenectomy. In this trial, 401 patients were randomized to limited versus extended pelvic lymphadenectomy at RC. Powered to detect a 15% difference in 5-year recurrence-free survival (RFS), this trial demonstrated a 5% greater 5-year RFS and 11% greater cancer-specific survival in patients undergoing extended lymphadenectomy, differences that were not statistically significant. Only one patient in this trial, however, was found to have N3 (common iliac nodes) involvement.22 We and others have previously estimated that the improvement in RFS for the extended pelvic lymphadenectomy is approximately 5% for bladder cancer patients undergoing RC,14 similar to the finding in the German randomized trial.22 It is unlikely that a 15% benefit could be achieved. It is clear that the trial was not powered to detect a more realistic and clinically relevant improvement in cancer-specific survival. Results of the SWOG S-1011 trial, randomizing patients with MIBC to extended versus standard lymphadenectomy at RC, are pending; however, this trial was also powered to see a large 13% improvement in RFS.23
Our study has important limitations that warrant consideration. Factors known to impact RC survival outcomes, such as medical comorbidities and performance status, were not fully captured in this retrospective study and are not included in the analysis. LN yield is an imperfect surgical performance metric, varying not only by extent of lymphadenectomy but also methods of submission for pathologic analysis.24 With the objective of reporting changes in disease characteristics, perioperative management, and oncologic outcomes over time at our tertiary referral center, oncologic outcomes are presented in univariate models. The effect of a given variable on oncologic outcomes is not assessed in this analysis. The strength of this study lies in the observations it affords over a 21-year experience during which time several aspects of perioperative management and surgical performance were refined and standardized. This allowed us to observe trends in disease characteristics, perioperative management, surgical performance and oncologic outcomes among patients undergoing RC. The use of NAC among 57% of patients with MIBC treated from 2010 to 2015 is significantly greater than that reported nationally,4,25 appears to approach the upper limit of patients eligible for cisplatin chemotherapy,21 and may serve as a benchmark for other centers. Taken together, these findings suggest that high utilization of NAC among eligible patients, improved pathologic outcomes, higher LN yield and decreased positive STSMs have contributed to the improved oncologic outcomes observed over time among patients undergoing RC at our center.
CONCLUSIONS
Among patients with UC undergoing RC at MSK, we observed improved oncologic outcomes over a 21-year span, with improvement in the probability of disease recurrence, cancer-specific survival, and overall survival. This coincided with increasing utilization of NAC among patients with MIBC, increasing complete pathologic response rates, decreasing positive STSMs, and increasing LN yield over time. These findings suggest that improvement in surgical outcomes and use of neoadjuvant systemic therapy have led to improved oncologic outcomes in patients undergoing RC.
ACKNOWLEDGEMENTS
This work was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers and by National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748. N.A. was supported by National Institutes of Health T32 Ruth L. Kirschstein Institutional National Research Service Award. The authors thank Amy Plofker for editorial support.
ABBREVIATIONS AND ACRONYMS
- CIS
carcinoma in situ
- LN
lymph node
- MIBC
muscle-invasive bladder cancer
- MSK
Memorial Sloan Kettering Cancer Center
- NAC
neoadjuvant chemotherapy
- NMIBC
non-muscle invasive bladder cancer
- pT0
pathologic T0 disease
- RC
radical cystectomy
- RFS
recurrence-free survival
- UC
urothelial carcinoma
Footnotes
COI/DISCLOSURES
Nima Almassi, Eugene K. Cha, Emily A. Vertosick, Chun Huang, Nathan Wong, Shawn Dason, Victor McPherson, Lucas Dean, Nicole Benfante, Daniel D. Sjoberg, S. Machele Donat, Harry W. Herr, and Guido Dalbagni have no competing interests to disclose.
Jonathan E. Rosenberg reports:
• Stock and Other Ownership Interests: Merck, Illumina;
• Honoraria: UpToDate, Bristol-Myers Squibb, AstraZeneca, Medscape, Vindico, Peerview, Chugai Pharma;
• Consulting or Advisory Role: Lilly, Merck, Agensys, Roche/Genentech, Sanofi, AstraZeneca/MedImmune, Bristol-Myers Squibb, EMD Serono, Seattle Genetic, Bayer, Inovio Pharmaceuticals, BioClin Therapeutics, QED Therapeutic, Adicet Bio, Sensei Biotherapeutics;
• Patents, Royalties, Other Intellectual Property: Predictor of platinum sensitivity;
• Research Funding: Genentech, Oncogenex, Agensys, Mirati Therapeutics, Novartis, Viralytics, Genentech/Roche, Incyte, Seattle Genetics, Bayer;
• Travel, Accommodations, Expenses: Genentech/Roche, Bristol-Myers Squibb.
Dean F. Bajorin reports honoraria from the speaker’s bureau of Merck; lecture fees and travel support from Merck; and Consultant/advisory board: Merck, Pfizer, Bristol-Myers Squibb, Urogen, Genentech, Eli Lilly.
Bernard H. Bochner serves as Consultant: Olympus.
REFERENCES
- 1.Abdollah F, Gandaglia G, Thuret R, et al. : Incidence, survival and mortality rates of stage-specific bladder cancer in United States: a trend analysis. Cancer Epidemiol 2013; 37: 219. [DOI] [PubMed] [Google Scholar]
- 2.Bosetti C, Bertuccio P, Chatenoud L, et al. : Trends in mortality from urologic cancers in Europe, 1970–2008. Eur Urol 2011; 60: 1. [DOI] [PubMed] [Google Scholar]
- 3.Grossman HB, Natale RB, Tangen CM, et al. : Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003; 349: 859. [DOI] [PubMed] [Google Scholar]
- 4.Zaid HB, Patel SG, Stimson CJ, et al. : Trends in the utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer: results from the National Cancer Database. Urology 2014; 83: 75. [DOI] [PubMed] [Google Scholar]
- 5.Anderson CB, Gennarelli R, Herr HW, et al. : Regionalization of radical cystectomy in the United States. Urol Oncol 2017; 35: 528.e7 [DOI] [PubMed] [Google Scholar]
- 6.Hounsome LS, Verne J, McGrath JS, et al. : Trends in operative caseload and mortality rates after radical cystectomy for bladder cancer in England for 1998–2010. Eur Urol 2015; 67: 1056. [DOI] [PubMed] [Google Scholar]
- 7.Morgan TM, Barocas DA, Keegan KA, et al. : Volume outcomes of cystectomy--is it the surgeon or the setting? J Urol 2012; 188: 2139. [DOI] [PubMed] [Google Scholar]
- 8.Hellenthal NJ, Ramirez ML, Evans CP, et al. : Trends in pelvic lymphadenectomy at the time of radical cystectomy: 1988 to 2004. J Urol 2009; 181: 2490. [DOI] [PubMed] [Google Scholar]
- 9.Dhar NB, Klein EA, Reuther AM, et al. : Outcome after radical cystectomy with limited or extended pelvic lymph node dissection. J Urol 2008; 179: 873. [DOI] [PubMed] [Google Scholar]
- 10.Herr HW, Bochner BH, Dalbagni G, et al. : Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol 2002; 167: 1295. [PubMed] [Google Scholar]
- 11.Herr HW, Faulkner JR, Grossman HB, et al. : Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol 2004; 22: 2781. [DOI] [PubMed] [Google Scholar]
- 12.Koppie TM, Vickers AJ, Vora K, et al. : Standardization of pelvic lymphadenectomy performed at radical cystectomy: can we establish a minimum number of lymph nodes that should be removed? Cancer 2006; 107: 2368. [DOI] [PubMed] [Google Scholar]
- 13.Wright JL, Lin DW, Porter MP: The association between extent of lymphadenectomy and survival among patients with lymph node metastases undergoing radical cystectomy. Cancer 2008; 112: 2401. [DOI] [PubMed] [Google Scholar]
- 14.Tarin TV, Power NE, Ehdaie B, et al. : Lymph node-positive bladder cancer treated with radical cystectomy and lymphadenectomy: effect of the level of node positivity. Eur Urol 2012; 61: 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bochner BH, Dalbagni G, Sjoberg DD, et al. : Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: A randomized clinical trial. Eur Urol 2015; 67: 1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von der Maase H, Hansen SW, Roberts JT, et al. : Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000; 18: 3068. [DOI] [PubMed] [Google Scholar]
- 17.Bochner BH, Herr HW, Reuter VE: Impact of separate versus en bloc pelvic lymph node dissection on the number of lymph nodes retrieved in cystectomy specimens. J Urol 2001; 166: 2295. [PubMed] [Google Scholar]
- 18.Dotan ZA, Kavanagh K, Yossepowitch O, et al. : Positive surgical margins in soft tissue following radical cystectomy for bladder cancer and cancer specific survival. J Urol 2007; 178: 2308. [DOI] [PubMed] [Google Scholar]
- 19.Novara G, Svatek RS, Karakiewicz PI, et al. : Soft tissue surgical margin status is a powerful predictor of outcomes after radical cystectomy: a multicenter study of more than 4,400 patients. J Urol 2010; 183: 2165. [DOI] [PubMed] [Google Scholar]
- 20.Zehnder P, Studer UE, Skinner EC, et al. : Unaltered oncological outcomes of radical cystectomy with extended lymphadenectomy over three decades. BJU Int 2013; 112: E51. [DOI] [PubMed] [Google Scholar]
- 21.Thompson RH, Boorjian SA, Kim SP, et al. : Eligibility for neoadjuvant/adjuvant cisplatin-based chemotherapy among radical cystectomy patients. BJU Int 2014; 113: E17. [DOI] [PubMed] [Google Scholar]
- 22.Gschwend JE, Heck MM, Lehmann J, et al. : Extended versus limited lymph node dissection in bladder cancer patients undergoing radical cystectomy: Survival results from a prospective, randomized trial. Eur Urol 2019; 75: 604. [DOI] [PubMed] [Google Scholar]
- 23.Lerner SP, Svatek RS: What is the standard of care for pelvic lymphadenectomy performed at the time of radical cystectomy? Eur Urol 2019; 75: 612. [DOI] [PubMed] [Google Scholar]
- 24.Bochner BH, Cho D, Herr HW, et al. : Prospectively packaged lymph node dissections with radical cystectomy: evaluation of node count variability and node mapping. J Urol 2004; 172: 1286. [DOI] [PubMed] [Google Scholar]
- 25.Macleod LC, Yabes JG, Yu M, et al. : Trends and appropriateness of perioperative chemotherapy for muscle-invasive bladder cancer. Urol Oncol 2019; 37: 462. [DOI] [PubMed] [Google Scholar]


