Abstract
BACKGROUND:
Compared with adults, phenotypic characterization of children with asthma is still limited and it remains difficult to predict which children with asthma are at highest risk for poor outcomes.
OBJECTIVE:
To identify latent classes in a large population of treatment-adherent children with mild to moderate asthma enrolled in clinical trials and determine whether latent class assignment predicts future lung function abnormalities and exacerbation rate.
METHODS:
Latent class analysis was performed on 2593 children with mild to moderate asthma aged 5 18 years, with 19 variables encompassing demographic characteristics, medical history, symptoms, lung function, allergic sensitization, and type 2 inflammation. Outcomes included lung function and the annualized exacerbation rate at 12 months of follow-up.
RESULTS:
Five latent classes were identified with differing demographic features, asthma control, sensitization, type 2 inflammatory markers, and lung function. Exacerbation rates were 1.30 ± 0.12 for class 1 (multiple sensitization with partially reversible airflow limitation), 0.90 ± 0.05 for class 2 (multiple sensitization with reversible airflow limitation), 0.87 ± 0.08 for class 3 (lesser sensitization with reversible airflow limitation), 0.87 ± 0.05 for class 4 (multiple sensitization with normal lung function), and 0.71 ± 0.06 for class 5 (lesser sensitization with normal lung function). Lung function abnormalities persisted in class 1 at 12 months.
CONCLUSIONS:
Children with mild to moderate asthma are a heterogeneous group. Allergic sensitization and lung function may be particularly useful in identifying children at the greatest risk for future exacerbation. Additional studies are needed to determine whether latent classes correspond to meaningful phenotypes for the purpose of personalized treatment.
Keywords: Asthma in children, Phenotype, Asthma exacerbation, Asthma control, Asthma outcomes, Latent class analysis, Lung function, Type 2 inflammation, Aeroallergen sensitization
INTRODUCTION
Asthma currently affects 8.4% of all children in the United States.1 Yet despite widespread availability of inhaled corticosteroids (ICSs) and standardization of asthma treatment guidelines, asthma control remains suboptimal in most children.2,3 Consequently, more than 50% of all children with asthma experience at least 1 exacerbation each year,1 including children with nonsevere asthma who have less troublesome day-to-day symptoms4 and normal lung function.5 The morbidity from exacerbations of asthma in children is significant and contributes to missed school/work days,6–8 impaired caregiver functional status,9 and a growing personal10 and societal11 economic burden estimated at more than $80 billion annually.12
Although the factors responsible for poor asthma control and asthma exacerbations in children are complex,13 there is also growing recognition that children with asthma are a heterogeneous group, with many underlying biological pathways or “endotypes” that contribute to differing phenotypic disease presentations, differential responses to asthma treatments, and varied clinical outcomes.14–21 Mandates for “personalized” versus “one size fits all” treatment of children with asthma have therefore been issued,22 but several challenges persist. First, compared with adults, phenotypes of childhood asthma are understudied and still unclear. There are also notable differences in the clinical manifestations of asthma between adults and children such as the magnitude of lung function deficits23 and exacerbation frequency24 that prohibit extrapolation of phenotypic findings between age groups. Second, most previous phenotypic analyses in children have focused on difficult-to-treat or severe asthma populations,25 which are not the predominant group encountered in most clinical practice settings. Therefore, phenotypic characterization of children with asthma is still limited and it remains difficult to predict which children with asthma are at the highest risk for poor outcomes (such as recurrent exacerbations) across the spectrum of disease severity.
Given these knowledge gaps, we applied latent class analysis (LCA) to a cohort of more than 2500 well-characterized children with mild to moderate persistent asthma aged 5 to 18 years with documented adherence to asthma controller therapies enrolled in previous National Heart, Lung and Blood Institute asthma network phase 3 clinical trials. The purpose was to (1) identify latent classes and (2) determine whether latent class assignment predicts subsequent lung function abnormalities and exacerbation rate at 12 months of follow-up. We hypothesized that a latent class distinguished by underlying type 2 eosinophilic inflammation and airflow limitation despite nonsevere disease would be identified and would have the lowest lung function and highest exacerbation rate by 1 year of follow-up.
METHODS
LCA was performed on 8 National Heart, Lung and Blood Institute clinical trials involving 2593 children with mild to moderate asthma aged 5 to 18 years: the Childhood Asthma Management Research Program (CAMP, NCT00000575),26,27 Characterizing the Response to a Leukotriene Receptor Antagonist and an Inhaled Corticosteroid (no NCT),28–30 Pediatric Asthma Controller Trial (NCT00272506),31,32 Best Add-On Therapy Giving Effective Response (NCT00395304),20,33 Treating Children to Prevent Exacerbations of Asthma (NCT00394329),34 Step-Up Yellow Zone Inhaled Corticosteroids to Prevent Exacerbations (NCT02066129),35 Best African American Response to Asthma Drugs (NCT01967173),21 and Steroids in Eosinophil Negative Asthma (NCT02066298).36 Details of these studies are presented in Table E1 in this article’s Online Repository at www.jaci-inpractice. org. All studies were overseen by dedicated quality control committees and data coordinating centers and used similar intake questionnaires. Paper case report forms were entered electronically and mailed to the data coordinating center for review and accuracy upon completion. Each center maintained staff and site certification and used the same manual of procedures for characterization. Written informed consent was obtained from all caregivers, and written or verbal assent was obtained from all participants for trial participation and secondary analyses. CAMP data were obtained from BioLINCC through a material transfer agreement (A.M.F.). Other study data were used with the permission of network principal investigators (coauthors).
Participants
All participants with persistent asthma with documented adherence to paper or electronic diaries were included in this analysis (N = 2593). Thresholds for acceptable adherence were defined as more than 75% to 80% of expected diaries completed during the study run-in periods. In each study, asthma was physician-diagnosed and was confirmed by symptom thresholds (ie, symptoms more than twice weekly off therapy or well controlled with daily asthma therapy). Most of the studies also had 12% or more absolute reversibility in the FEV1 after bronchodilator administration or airway hyper-reponsiveness to methacholine (ie, provocative concentration causing a 20% decline in FEV1 [PC20] <12.5 or <16 mg/mL) as criteria for study entry.
Participant characterization procedures
Participants completed questionnaires pertaining to demographic characteristics, family history, child allergy and respiratory symptoms, and treatment of symptoms including medications and health care utilization. A subset of participants (n = 1551 [59.8%]) also completed the Asthma Control Test (ACT)37,38 and the 6-question Asthma Control Questionnaire (ACQ).39 Peripheral blood eosinophils and total serum IgE were quantified in clinical laboratories. Spirometry with bronchodilator reversibility was performed at baseline and after receipt of 2 to 4 inhalations of albuterol sulfate (90 mg per actuation) delivered by metered-dose inhaler. Spirometry was conducted according to published standards at the time of the test.40 FEV1, forced vital capacity (FVC), and the ratio of FEV1/FVC were recorded from the best of 3 attempts. Sensitization was assessed by skin testing or specific IgE testing for the following aeroallergens common to each study: dust mite (mix), cockroach (mix), cat dander, dog dander, mold (mix), grass (mix), tree (mix), and weed (mix). Skin testing was performed using the Multi-test II (Lincoln Diagnostics, Decatur, Ill) prick technique. Test results were considered positive if the prick resulted in a wheal with a mean diameter (mean of maximum and 90° midpoint diameters) that was at least 3 mm greater than that produced by the saline control. Specific IgE levels were quantified at centralized laboratories. Tests with levels more than 0.34 IU/mL were considered positive. Exhaled nitric oxide was also measured by online methods in a subset of participants.41
Variable selection and handling
Variable selection is detailed in the Online Repository and in Table E2 in this article’s Online Repository at www.jaci-inpractice. org. Dichotomous variables included (1) age group (6–11 vs >11 years), (2) sex, (3) hospitalization in the past year, (4) intensive care unit admission for asthma (ever), (5) blood eosinophil group (<4% or ≥4%), (6) sibling with asthma, (7) parent with asthma, (8) sensitization to pets, (9) sensitization to other aeroallergens, (10) indoor pet, and (11) tobacco smoke exposure. Categorical variables included (1) race (white/Caucasian, black/African American, and other/mixed), (2) number of unscheduled visits for asthma in the past year (0, 1, ≥ ≥2), (3) prebronchodilator FEV1 z score (< −1.64, −1.64 to 0, >0), (4) prebronchodilator FEV1/FVC z score (< −1.64, −1.64 to 0, >0), (5) postbronchodilator FEV1 z score (< −1.64, −1.64 to 0, >0), (6) IgE level in kU/L (<100, 100–500, 500–1000, >1000), (7) body mass index (BMI) percentile (<60%, 60%−90%, >90%), and (8) asthma control quartile (lowest = worst control; highest = control) obtained from either the ACT or the best ACQ score. Lung function data were expressed as z scores and interpreted according to lower limit of normal (LLN) values established by the Global Lung Function Initiative prediction equations.42
Outcomes
Outcomes included lung function (pre- and postbronchodilator FEV1, FVC, FEV1/FVC) and the annualized rate of exacerbation at the end of follow-up. For the CAMP study, outcomes were assessed over the first year only. The definition of exacerbation used was proposed by a National Institutes of Health Working Group43 and was defined as escalation of symptoms resulting in treatment with systemic corticosteroids (ie, dexamethasone, prednisone, or prednisolone equivalent to 2 mg/kg/d for 2 days followed by 1 mg/kg/d for 2 days). Two courses of systemic corticosteroids had to be separated by at least 1 week to count as 2 exacerbations.
Latent class analyses
LCA was performed with SAS software version 9.4 (SAS Institute, Cary, NC) using the PROC LCA procedure44 and a maximum likelihood model algorithm that allows missing data under the missing completely at random assumption, which was tested and evaluated. Models of 1 to 8 latent classes were repeatedly fitted with the number of latent classes in a stepwise fashion. Models were freely estimated with no specified parameter restrictions. Conditional probabilities (probability of selected characteristics within a class) and posterior probabilities (probability of latent class membership for each participant) were calculated. Best fit was assessed by comparison of the bootstrapped P values for the likelihood ratio test and the Bayesian information criterion test. Each participant was assigned to the latent class with the highest membership probability.
Outcome analyses
End of follow-up lung function and the annualized rate of exacerbations were assessed in each latent class irrespective of treatment assignment with generalized linear models with adjustment for study. Exploratory analyses were performed on parallel-arm treatment studies (CAMP, Pediatric Asthma Controller Trial, Treating Children to Prevent Exacerbations of Asthma, and Step-Up Yellow Zone Inhaled Corticosteroids to Prevent Exacerbations) with asthma treatment assignment as follows: (1) placebo, (2) other asthma medication (leukotriene receptor antagonist), nedocromil, or rescue ICS), or (3) daily ICS. Analyses used a significance level of .05 without adjustment for multiple testing.
RESULTS
A total of 2593 children were included in the LCA. Evaluations were done for 4-, 5-, and 6-class solutions; the 5-class solution was chosen as best fit and yielded a high class membership probability for all participants with acceptable fit statistics (adjusted Bayesian information criterion: 26,930.16; entropy: 0.88). The distribution of study variables by latent class assignment is presented in Table I. By design, each LCA variable was significantly different between latent classes. The distribution of studies within each latent class is shown in Figure 1. Other features of the study participants are presented in Table II. To simplify the discussion, latent classes were assigned a summary label. Key features of the 5 latent classes are detailed herein.
TABLE I.
Distribution of study variables by latent class assignment
| Feature | All participants | Class 1 (multiple sensitization with partially reversible airflow limitation) | Class 2 (multiple sensitization with reversible airflow limitation) | Class 3 (lesser sensitization with reversible airflow limitation) | Class 4 (multiple sensitization with normal lung function) | Class 5 (lesser sensitization with normal lung function) |
|---|---|---|---|---|---|---|
| N | 2593 | 244 | 926 | 315 | 718 | 390 |
| Age group | 9.3 (7.4, 11.4) | 10.3 (8.0, 11.8) | 9.9 (8.0, 11.9) | 8.9 (7.0, 11.1) | 8.9 (7.4, 10.9) | 8.2 (7.0, 10.2) |
| 6–11 y | 2108 (81.3) | 189 (77.5) | 708 (76.5) | 257 (81.6) | 610 (85.0) | 344 (88.2) |
| >11 y | 485 (18.7) | 55 (22.5) | 218 (23.5) | 58 (18.4) | 108 (15.0) | 46 (11.8) |
| Sex | ||||||
| Male | 1554 (59.9) | 156 (63.9) | 587 (63.4) | 168 (53.3) | 459 (63.9) | 184 (47.2) |
| Female | 1039 (40.1) | 88 (36.1) | 339 (36.6) | 147 (46.7) | 259 (36.1) | 206 (52.8) |
| Race | ||||||
| White/Caucasian | 1637 (63.1) | 164 (67.2) | 600 (64.8) | 203 (64.4) | 399 (55.6) | 271 (69.5) |
| Black/African American | 692 (26.7) | 72 (29.5) | 238 (25.7) | 90 (28.6) | 216 (30.1) | 76 (19.5) |
| Other/mixed | 264 (10.2) | 8 (3.3) | 88 (9.5) | 22 (7) | 103 (14.3) | 43 (11.0) |
| BMI percentile | 74 (47, 92) | 58 (34, 86) | 71 (44, 91) | 76 (46, 94) | 77 (53, 94) | 79 (54, 94) |
| <60% | 917 (35.6) | 125 (51.2) | 348 (38.1) | 110 (34.9) | 221 (30.9) | 113 (29.4) |
| 60%−90% | 893 (34.7) | 70 (28.7) | 325 (35.6) | 95 (30.2) | 261 (36.5) | 142 (37.0) |
| >90% | 763 (29.7) | 49 (20.1) | 241 (26.4) | 110 (34.9) | 234 (32.7) | 129 (33.6) |
| Parent with asthma | 1220 (47.0) | 134 (54.9) | 425 (45.9) | 134 (42.5) | 337 (46.9) | 190 (48.7) |
| Sibling with asthma | 1080 (41.7) | 85 (34.8) | 376 (40.6) | 155 (49.2) | 294 (40.9) | 170 (43.6) |
| Unscheduled asthma visits | ||||||
| 0 visits (past year) | 493 (19.0) | 41 (16.8) | 175 (18.9) | 72 (22.9) | 134 (18.7) | 71 (18.2) |
| 1 visit (past year) | 422 (16.3) | 42 (17.2) | 148 (16.0) | 37 (11.7) | 137 (19.1) | 58 (14.9) |
| ≥2 visits (past year) | 1678 (64.7) | 161 (66.0) | 603 (65.1) | 206 (65.4) | 447 (62.3) | 261 (66.9) |
| Hospitalization for asthma (ever) | 173 (6.7) | 28 (11.5) | 64 (6.9) | 7 (2.2) | 68 (9.5) | 6 (1.5) |
| Intensive care unit admission for asthma (ever) | 156 (6.0) | 21 (8.6) | 60 (6.5) | 14 (4.4) | 47 (6.5) | 14 (3.6) |
| Indoor pet | 1208 (46.6) | 109 (44.7) | 411 (44.4) | 163 (51.7) | 305 (42.5) | 22 (56.4) |
| Indoor cat | 479 (18.5) | 60 (24.6) | 158 (17.1) | 71 (22.5) | 114 (15.9) | 76 (19.5) |
| Indoor dog | 952 (36.7) | 74 (30.3) | 329 (25.5) | 120 (38.1) | 238 (33.1) | 191 (49.0) |
| Tobacco smoke exposure | 789 (30.4) | 81 (33.2) | 243 (26.2) | 121 (38.4) | 232 (32.3) | 112 (28.7) |
| Blood eosinophils (%) | 5.0 (2.4, 8.0) | 7.0 (4.6, 10.0) | 6.0 (4.0, 9.0) | 2.0 (1.1, 3.8) | 5.3 (3.4, 8.1) | 2.0 (1.0, 3.0) |
| Missing | 54 (2.1) | 6 (2.5) | 16 (1.7) | 6 (1.9) | 20 (2.8) | 6 (1.5) |
| <4% | 993 (39.1) | 36 (15.1) | 216 (23.7) | 234 (75.7) | 185 (26.5) | 322 (83.9) |
| ≥4% | 1546 (60.9) | 202 (84.9) | 694 (76.3) | 75 (24.3) | 513 (73.5) | 62 (16.1) |
| IgE (kU/L) | 210 (66, 516) | 347 (208, 556) | 363 (182, 779) | 41 (17, 70) | 371 (190, 744) | 45 (15, 83) |
| Missing | 469 (18.1) | 26 (10.7) | 168 (18.1) | 57 (18.1) | 143 (7.9) | 75 (19.2) |
| <100 | 508 (23.9) | 6 (2.8) | 24 (3.2) | 194 (75.2) | 29 (5.0) | 255 (81.0) |
| 100–500 | 820 (38.6) | 87 (39.9) | 349 (46.0) | 61 (23.6) | 266 (46.3) | 57 (18.1) |
| 500–1000 | 345 (16.2) | 46 (21.1) | 162 (21.4) | 3 (1.2) | 131 (22.8) | 3 (1.0) |
| >1000 | 451 (21.2) | 79 (36.2) | 223 (29.4) | — | 149 (25.9) | — |
| Sensitization to pets | 1409 (54.3) | 180 (74.4) | 663 (72.4) | 21 (6.7) | 509 (72.6) | 36 (9.4) |
| Missing | 38 (1.5) | 2 (0.8) | 10(1.1) | 1 (0.3) | 17 (2.4) | 8 (2.1) |
| Sensitization to cat | 1263 (48.7) | 163 (66.8) | 595 (64.3) | 17 (5.4) | 455 (63.4) | 33 (8.5) |
| Sensitization to dog | 738 (28.5) | 104 (42.6) | 346 (37.4) | 7 (2.2) | 270 (37.6) | 11 (2.8) |
| Sensitization to other aeroallergens | 1888 (72.8) | 223 (92.1) | 840 (91.6) | 79 (25.2) | 645 (92.0) | 101 (26.4) |
| Missing | 37 (1.4) | 2 (0.8) | 9 (1.0) | 1 (0.3) | 17 (2.4) | 8 (2.1) |
| Prebronchodilator FEV1 z score | −0.2 (−1.0, 0.5) | −2.1 (−2.7, −1.8) | −0.7 (−1.1, −0.3) | −0.8 (−1.3, −0.4) | 0.7 (0.3, 1.2) | 0.6 (0.2, 1.1) |
| Missing | 21 (0.8) | — | 13 (1.4) | — | 2 (0.3) | 6 (1.5) |
| <−1.64 | 284 (11.0) | 244 (100) | — | 40 (12.7) | — | — |
| −1.64 to 0 | 1216 (46.9) | — | 913 (100) | 273 (86.7) | — | 30 (7.8) |
| >0 | 1072 (41.3) | — | — | 2 (0.6) | 716 (100) | 354 (92.2) |
| Prebronchodilator FEV1/FVC z score | −0.2 (−1.9, −0.4) | −0.4 (−2.9, −1.8) | −0.5 (−2.1, −0.8) | −1.4 (−2.0, −0.8) | −0.8 (−1.4, −0.2) | −0.4 (−1.0, −0.2) |
| Missing | 21 (0.8) | — | 13 (1.4) | — | 2 (0.3) | 6 (1.5) |
| <−1.64 | 866 (33.4) | 197 (80.7) | 399 (43.7) | 134 (42.5) | 119 (16.6) | 17 (4.4) |
| −1.64 to 0 | 1346 (51.9) | 40 (16.4) | 444 (48.6) | 156 (49.5) | 445 (62.2) | 261 (68) |
| >0 | 360 (13.9) | 7 (2.9) | 70 (7.7) | 25 (7.9) | 152 (21.2) | 106 (27.6) |
| Postbronchodilator FEV1 z score | 0.5 (−0.3, 1.2) | −1.0 (−1.5, −0.4) | 0.1 (−0.4, 0.6) | −0.3 (−0.8, 0.1) | 1.3 (0.9, 1.8) | 1.1 (0.7, 1.6) |
| Missing | 52 (2.0) | 3 (1.2) | 23 (2.4) | 7 (2.2) | 9 (1.3) | 10 (2.6) |
| <−1.64 | 58 (2.2) | 38 (15.8) | 7 (0.8) | 13 (4.2) | — | — |
| −1.64 to 0 | 756 (29.2) | 176 (73.0) | 361 (40.0) | 207 (67.2) | 8 (1.1) | 4 (1.1) |
| >0 | 1727 (66.6) | 27 (11.2) | 535 (59.2) | 88 (28.6) | 701 (98.9) | 376 (98.9) |
| Asthma control quartile | ||||||
| Missing | 1042 (40.2) | 141 (57.8) | 422 (45.6) | 120 (38.1) | 243 (33.8) | 116 (29.7) |
| Lowest (worst control) | 404 (15.6) | 36 (35.0) | 138 (27.4) | 42 (21.5) | 137 (28.8) | 51 (18.6) |
| Low | 371 (14.3) | 31 (30.1) | 130 (25.8) | 39 (20.0) | 111 (23.4) | 60 (21.9) |
| Higher | 377 (14.5) | 21 (20.4) | 116 (23.0) | 58 (29.7) | 100 (21.1) | 82 (29.9) |
| Highest (best control) | 399 (15.4) | 15 (14.6) | 120 (23.8) | 56 (28.7) | 127 (26.7) | 81 (29.6) |
Note. By design, all variables were significantly different between latent classes, so P values are not shown. Data represent the median (25th, 95th percentile) or the number of participants (%).
FIGURE 1.
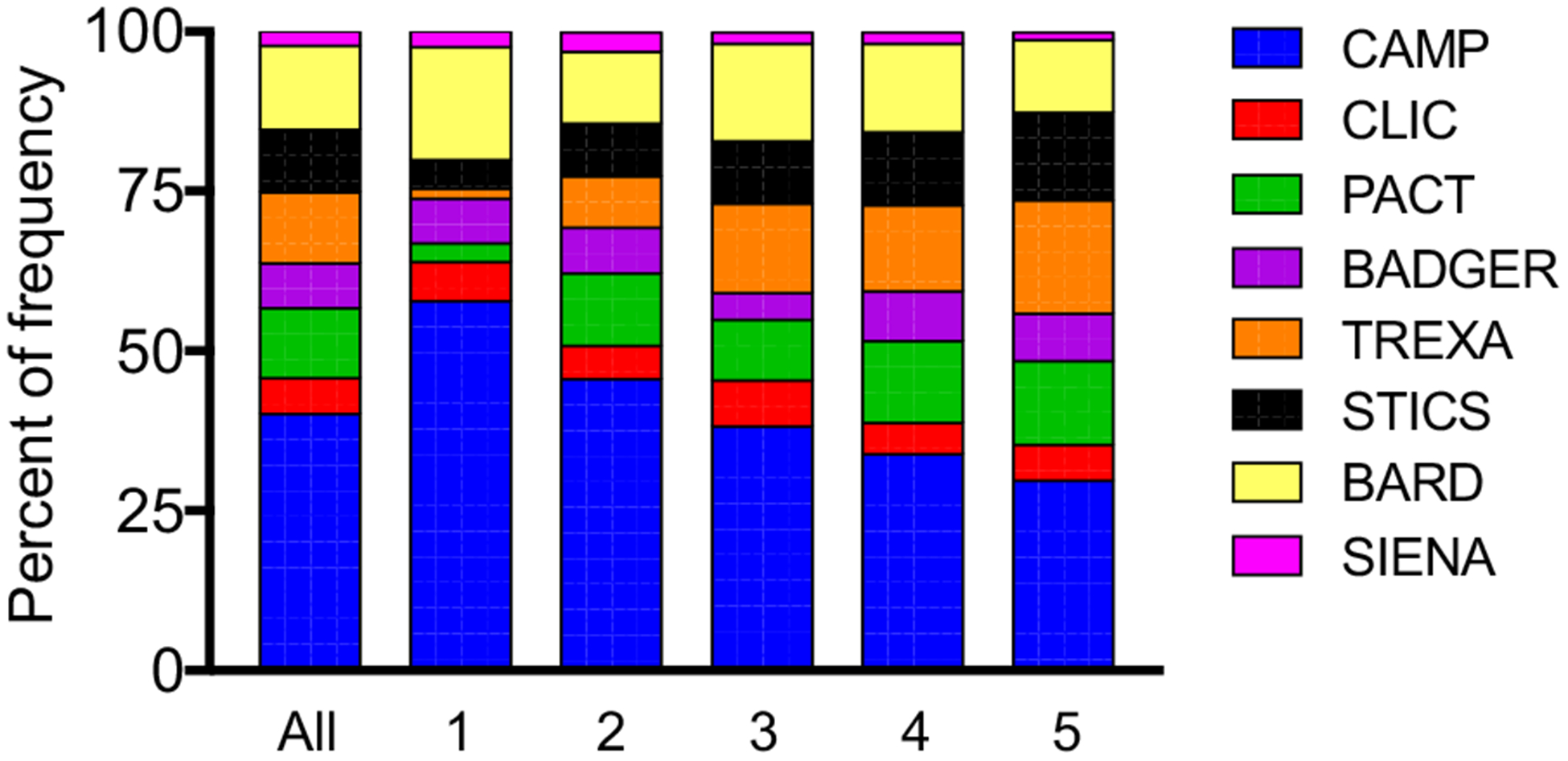
Distribution of studies within each latent class. BADGER, Best Add-On Therapy Giving Effective Response; BARD, Best African American Response to Asthma Drugs; CAMP, Childhood Asthma Management Research Program; CLIC, Characterizing the Response to a Leukotriene Receptor Antagonist and an Inhaled Corticosteroid; PACT, Pediatric Asthma Controller Trial; SIENA, Steroids in Eosinophil Negative Asthma; STICS, Step-Up Yellow Zone Inhaled Corticosteroids to Prevent Exacerbations; TREXA, Treating Children to Prevent Exacerbations of Asthma.
TABLE II.
Other features of the participants
| Feature | Class 1 (multiple sensitization with partially reversible airflow limitation) | Class 2 (multiple sensitization with reversible airflow limitation) | Class 3 (lesser sensitization with reversible airflow limitation) | Class 4 (multiple sensitization with normal lung function) | Class 5 (lesser sensitization with normal lung function) | P value |
|---|---|---|---|---|---|---|
| N | 244 | 926 | 315 | 718 | 390 | |
| Asthma symptom duration (y) | 6.9 (4.9, 9.7) | 6.7 (4.5, 9.2) | 6.4 (4.6, 8.5) | 6.2 (4.1, 8.1) | 6.1 (4.1, 7.8) | <.001 |
| Hispanic ethnicity | 52 (21.3) | 155 (16.7) | 38 (12.1) | 81 (11.3) | 39 (10.0) | <.001 |
| Obesity (BMI ≥95%) | 244 (13.5) | 926 (17.7) | 315 (23.8) | 718 (23.4) | 390 (24.9) | <.001 |
| Parent with allergies | 149 (61.1) | 571 (61.7) | 181 (57.5) | 446 (62.1) | 235 (60.3) | .681 |
| Sibling with allergies | 86 (35.2) | 396 (42.8) | 135 (42.9) | 322 (44.8) | 160 (41.0) | .123 |
| Physician-diagnosed eczema | 80 (32.8) | 366 (39.5) | 90 (28.6) | 323 (45.0) | 139 (35.6) | <.001 |
| Asthma controller medications | <.001 | |||||
| None | 101 (41.4) | 367 (39.6) | 117 (37.1) | 207 (28.8) | 114 (29.2) | |
| Non-ICS | 11 (4.5) | 29 (3.1) | 18 (5.7) | 31 (4.3) | 19 (4.9) | |
| ICS monotherapy | 81 (33.2) | 359 (38.8) | 111 (35.2) | 324 (45.1) | 166 (42.6) | |
| ICS plus additional | 51 (20.9) | 171 (18.5) | 69 (21.9) | 156 (21.7) | 91 (23.3) | |
| Oral corticosteroid bursts, n (past year) | <.001 | |||||
| 0 | 137 (56.1) | 556 (60.0) | 197 (62.5) | 375 (52.2) | 231 (59.2) | |
| 1 | 28 (11.5) | 136 (14.7) | 54 (17.1) | 159 (22.1) | 76 (19.5) | |
| 2 | 46 (18.9) | 126 (13.6) | 24 (7.6) | 106 (14.8) | 45 (11.5) | |
| ≥3 | 33 (13.5) | 108 (11.7) | 40 (12.7) | 78 (10.9) | 38 (9.7) | |
| Sensitization* | <.001 | |||||
| No sensitization | 2 (0.8) | 14 (1.5) | 206 (65.6) | 241 (63.1) | ||
| Monosensitization | 24 (9.9) | 120 (13.1) | 69 (22.0) | 12 (1.7) | 70 (18.3) | |
| Multiple sensitization | 216 (89.3) | 783 (85.4) | 39 (12.4) | 71 (10.1) | 71 (18.6) | |
| Percentage of positive aeroallergens (of 8) | 50 (28.6, 75.0) | 42.9 (28.6, 71.4) | 0 (0, 12.5) | 618 (88.2)42.9 (25.0, 62.5) | 0 (0, 12.5) | |
| Blood eosinophils (per microliter)† | 476 (305, 722) | 425 (260, 637) | 151 (89, 248) | 426 (230, 594) | 146 (82, 229) | <.001 |
| Exhaled nitric oxide (ppb)‡ | 13.7 (9.5, 31.5) | 15.9 (9.0, 27.7) | 9.3 (6.4, 12.9) | 13.0 (7.0, 22.5) | 7.4 (5.8, 11.0) | <.001 |
| Prebronchodilator§ | ||||||
| FVC (% predicted) | 89 (83, 96) | 103 (96, 109) | 99 (93, 105) | 116 (109, 123) | 111 (105, 118) | <.001 |
| FEV1 (% predicted) | 74 (67, 77) | 92 (87, 96) | 90 (84, 95) | 108 (104, 114) | 107 (103, 114) | <.001 |
| FEV1/FVC (% predicted) | 80 (74, 86) | 89 (83, 95) | 90 (84, 95) | 94 (90, 99) | 97 (93, 100) | <.001 |
| Postbronchodilator | ||||||
| FVC (% predicted)∥ | 97 (88, 105) | 105 (98, 113) | 100 (93, 107) | 117 (110, 123) | 113 (106, 119) | <.001 |
| FEV1 (% predicted)¶ | 88 (92, 95) | 102 (96, 107) | 96 (90, 102) | 116 (111, 123) | 113 (108, 119) | <.001 |
| FEV1/FVC (% predicted)∥ | 91 (86, 96) | 96 (92, 101) | 96 (90, 100) | 100 (96, 103) | 101 (97, 104) | <.001 |
| Methacholine PC20# | 0.5 (0.2, 1.0) | 0.8 (0.4, 2.3) | 1.6 (0.6, 3.0) | 1.4 (0.6, 3.9) | 2.8 (1.2, 6.3) | <.001 |
Note. Data represent the median (25th, 95th percentile) or the number of participants (%).
Class 1, n = 242; class 2, n = 917; class 3, n = 314; class 4, n = 701; class 5, n = 382.
Class 1, n = 236; class 2, n = 910; class 3, n = 309; class 4, n = 698; class 5, n = 385.
Class 1, n = 55; class 2, n = 391; class 3, n = 144; class 4, n = 370; class 5, n = 224.
Class 1, n = 244; class 2, n = 913; class 3, n = 315; class 4, n = 716; class 5, n = 384.
Class 1, n = 219; class 2, n = 751; class 3, n = 255; class 4, n = 584; class 5, n = 309.
Class 1, n = 241; class 2, n = 903; class 3, n = 308; class 4, n = 709; class 5, n = 380.
Class 1, n = 205; class 2, n = 821; class 3, n = 258; class 4, n = 636; class 5, n = 322.
Latent class 1: Multiple sensitization with partially reversible airflow limitation
Latent class 1, titled “multiple sensitization with partially reversible airflow limitation,” included 244 children (9.4%). Children in this latent class were predominantly white/Caucasian males and had the poorest overall asthma control. This class also had the highest proportion of Hispanics. In addition, this class was characterized by multiple sensitization to pets and other aeroallergens, the highest blood eosinophils, and high total serum IgE concentrations, with 36% of children having concentrations more than 1000 kU/L. Forty-one percent of children in this latent class were not receiving controller therapy at study enrollment and 11.5% had been hospitalized for asthma in the previous year. Lung function values were also the lowest in this group, and 100% of children in this class had prebronchodilator FEV1 z scores below the LLN (ie, < −1.64), respectively. Lung function values remained the lowest in this latent class after bronchodilation and 16% of children had postbronchodilator FEV1 z scores below the LLN. Airway hyperresponsiveness to methacholine was also the greatest in this latent class.
Latent class 2: Multiple sensitization with reversible airflow limitation
This was the largest latent class, with 926 children (35.7%), and was titled “multiple sensitization with reversible airflow limitation.” Children in this latent class were similar to those in latent class 1 with regard to demographic characteristics, sensitization patterns, and markers of type 2 inflammation, but had fewer historical severe exacerbations requiring hospitalization and lesser exposure to tobacco smoke. All children in this latent class had baseline FEV1 z scores above the LLN (ie, > −1.64). Lung function values improved further with albuterol in nearly all children and only 0.8% of children had postbronchodilator FEV1 z scores below the LLN.
Latent class 3: Lesser sensitization with reversible airflow limitation
Latent class 3 included 315 children (12.1%) and was titled “lesser sensitization with reversible airflow limitation.” This latent class included more females and more obese children with fewer historical severe exacerbations requiring hospitalization than classes 1 and 2. Tobacco smoke exposure was also the greatest in this latent class, and most of the children had either no sensitization (66%) or monosensitization (22%). Blood eosinophil percentages and total serum IgE concentrations were also low in this group compared with those in classes 1 and 2. Approximately 13% of children in this class had prebronchodilator FEV1 z scores below the LLN, respectively. Lung function values improved significantly with albuterol in most of the children and only 4.2% of children had postbronchodilator FEV1 z scores below the LLN.
Latent class 4: Multiple sensitization with normal lung function
Latent class 4 included 718 children (27.6%) and was titled “multiple sensitization with normal lung function.” This class was similar to classes 1 and 2 but included more nonwhite/non-Caucasian children with higher BMI percentiles. Like classes 1 and 2, this class was characterized by multiple sensitization, elevated blood eosinophils, and elevated IgE, but had a higher proportion of children (71.2%) who were treated with asthma controller medications. Lung function values were also higher in this class compared with those in classes 1 and 2, and 100% of children had baseline FEV1 z scores above the LLN.
Latent class 5: Lesser sensitization with normal lung function
Latent class 5 included 390 children (15.0%) and was titled “lesser sensitization with normal lung function.” This latent class was younger and included the highest proportion of females and obese children with BMI percentiles of 95% or higher. Asthma control was also the greatest in this group, although 18.6% of children still had asthma control values in the lowest (ie, poorest) quartile. Sensitization patterns were similar to those in latent class 3, with most of the children having either no sensitization (63%) or monosensitization (18%) to allergens. Blood eosinophils and total serum IgE concentrations were also low and were similar to concentrations observed in latent class 3. However, lung function was higher than in class 3, and FEV1 z scores were above the LLN in 100% of children in this class. Airway hyperresponsiveness to methacholine was still noted in this class, but methacholine PC20 values were higher than in the other classes.
Sensitivity analyses
Given that the asthma control variable had a relatively large amount of missing data, sensitivity analyses were performed excluding this variable from the LCA. As presented in Table E3 in this article’s Online Repository at www.jaci-inpractice.org, exclusion of the asthma control variable did not significantly change the results.
Outcome analyses
Days to end of follow-up were 316 ± 95 for all participants (class 1, 328 ± 97; class 2, 322 ± 91; class 3, 308 ± 104; class 4, 316 ± 91; class 5, 302 ± 103). Pre- and postbronchodilator lung function outcomes are presented in Table III. At the end of follow-up, prebronchodilator lung function values improved in each latent class compared with study entry, although children in latent class 1 still had the highest proportion of values below the LLN. After bronchodilator administration, lung function values remained the lowest in class 1. In exploratory analyses, the change in FEV1 at the end of follow-up was not due to asthma treatment (see Table E4 in this article’s Online Repository at www.jaci-inpractice.org). Study treatment assignment also did not change the proportion of participants with prebronchodilator FEV1 or FEV1/FVC z scores below the LLN in class 1 or 3 (Figure 2). However, children in class 2 had a greater lung function response with any asthma medication (ie, ICS or other asthma controller treatments such as leukotriene receptor antagonist, nedocromil, or rescue ICS), whereas children in classes 4 and 5 had greater lung function responses with ICSs (Figure 2).
TABLE III.
Lung function values in the 5 latent classes at the end of follow-up
| Feature | Class 1 (multiple sensitization with partially reversible airflow limitation) | Class 2 (multiple sensitization with reversible airflow limitation) | Class 3 (lesser sensitization with reversible airflow limitation) | Class 4 (multiple sensitization with normal lung function) | Class 5 (lesser sensitization with normal lung function) | P value |
|---|---|---|---|---|---|---|
| N (pre-, postbronchodilator) | 211, 190 | 803, 639 | 258, 206 | 622, 456 | 318, 234 | |
| Prebronchodilator | ||||||
| FVC (% predicted) | 111 (102, 121) | 116 (107, 124) | 115 (106, 122) | 127 (119, 137) | 124 (116, 134) | <.001 |
| FEV1 (% predicted) | 94 (84, 107) | 104 (96, 112) | 102 (94, 110) | 119 (111, 127) | 119 (112, 127) | <.001 |
| z score | −0.4 (−1.3, 0.5) | 0.3 (−0.3, 1.0) | 0.2 (−0.4, 0.8) | 1.6 (0.9, 2.2) | 1.6 (1.0, 2.2) | <.001 |
| z score <LLN* | 37 (17.5) | 36 (4.5) | 8 (3.1) | 8 (1.3) | — | <.001 |
| FEV1/FVC (% predicted) | 85 (78, 92) | 90 (83, 95) | 90 (85, 96) | 94 (88, 98) | 96 (92, 100) | <.001 |
| z score | −1.9 (−2.5, −1.0) | −1.4 (−2.1, −0.8) | −1.3 (−1.9, −0.6) | −0.9 (−1.6, −0.3) | −0.7 (−1.2, −0.1) | <.001 |
| z score <LLN | 126 (59.7) | 321 (40.0) | 101 (39.1) | 143 (23.0) | 35 (11.0) | <.001 |
| Postbronchodilator | ||||||
| FVC (% predicted) | 115 (107, 125) | 118 (109, 127) | 115 (107, 123) | 128 (119, 137) | 125 (117, 133) | <.001 |
| FEV1 (% predicted) | 107 (96, 116) | 113 (106, 121) | 109 (102, 118) | 126 (118, 135) | 124 (116, 133) | <.001 |
| z score | 0.6 (−0.3, 1.3) | 1.1 (0.5, 1.7) | 0.7 (0.1, 1.5) | 2.1 (1.5, 2.8) | 2.0 (1.4, 2.7) | <.001 |
| z score <LLN | 10 (5.2) | 7 (1.1) | 2 (1.0) | 3 (0.7) | — | <.001 |
| FEV1/FVC (% predicted) | 93 (87, 99) | 96 (91, 100) | 96 (91, 99) | 99 (94, 102) | 99 (96, 103) | <.001 |
| z score | −1.0 (−1.7, −0.2) | −0.6 (−1.3, 0.1) | −0.6 (−1.2, −0.1) | −0.2 (−0.8, 0.4) | −0.1 (−0.6, 0.5) | <.001 |
| z score <LLN | 52 (27.4) | 85 (13.3) | 30 (14.6) | 29 (6.4) | 7 (3.0) | <.001 |
Note. Data represent the median (25th, 95th percentile) or the number of participants (%).
LLN equivalent to a z score of ≤1.64.
FIGURE 2.
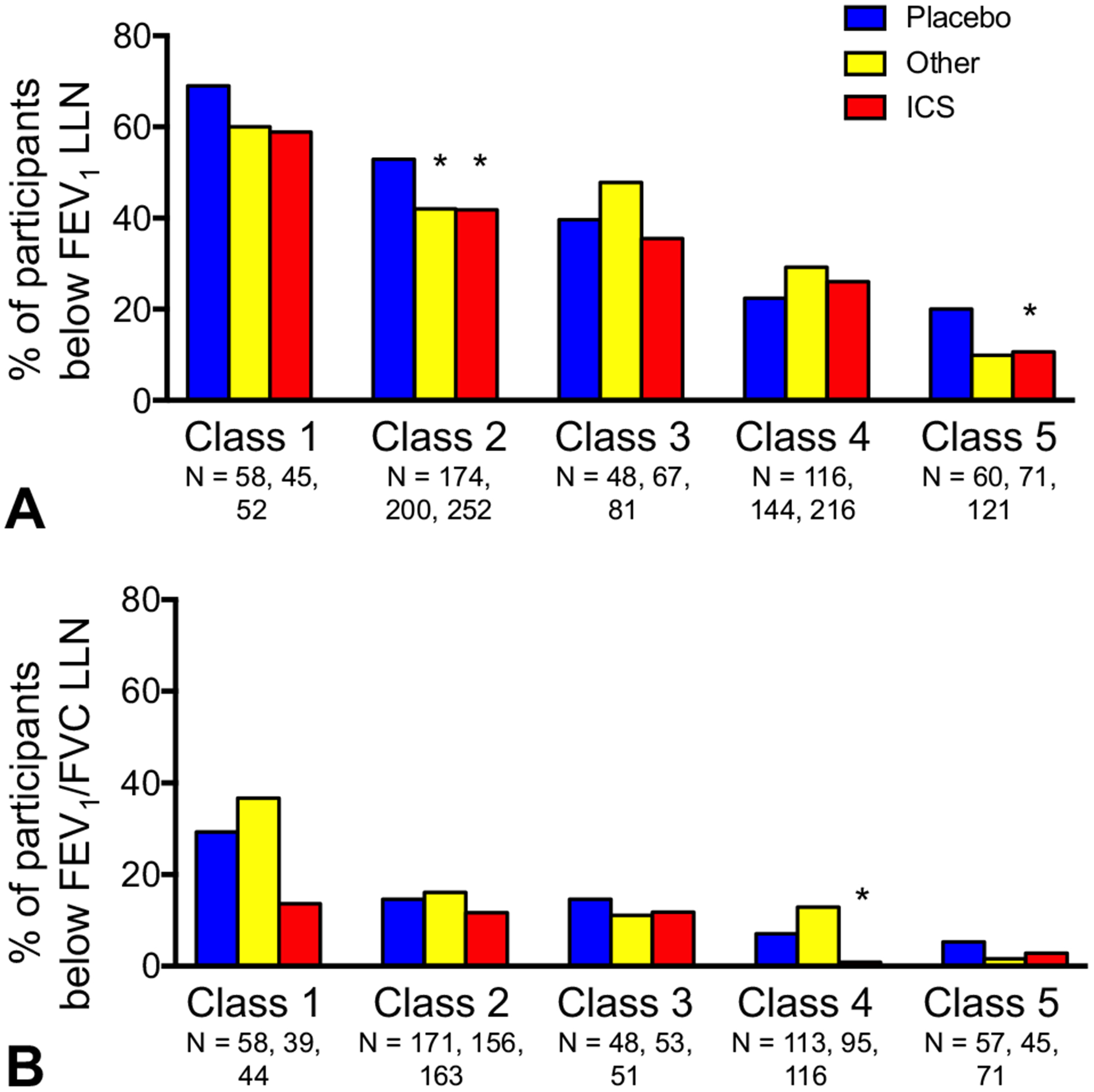
Participants with (A) FEV1 and (B) FEV1/FVC below the LLN at the end of follow-up, stratified by treatment assignment and latent class 1 (multiple sensitization with partially reversible airflow limitation), 2 (multiple sensitization with reversible airflow limitation), 3 (lesser sensitization with reversible airflow limitation), 4 (multiple sensitization with normal lung function), and 5 (lesser sensitization with normal lung function). *P <.05 vs placebo.
Exacerbations occurred by the end of follow-up in 41.3% of participants (class 1, 52.5%; class 2, 41.6%; class 3, 36.8%; class 4, 43.0%; class 5, 34.1%; P < .001). Latent class 1, compared with the other classes, had more cumulative exacerbations and a significantly higher annualized exacerbation rate (class 1, 1.30 ± 0.12; class 2, 0.90 ± 0.05; class 3, 0.87 ± 0.08; class 4,0.87 ± 0.05; class 5, 0.71 ± 0.07; P < .001) (Figure 3). In exploratory analyses, ICS treatment significantly reduced exacerbation occurrence and the annualized exacerbation rate by the end of follow-up in classes 1, 2, and 4 but not in classes 3 and 5 (Figure 4).
FIGURE 3.
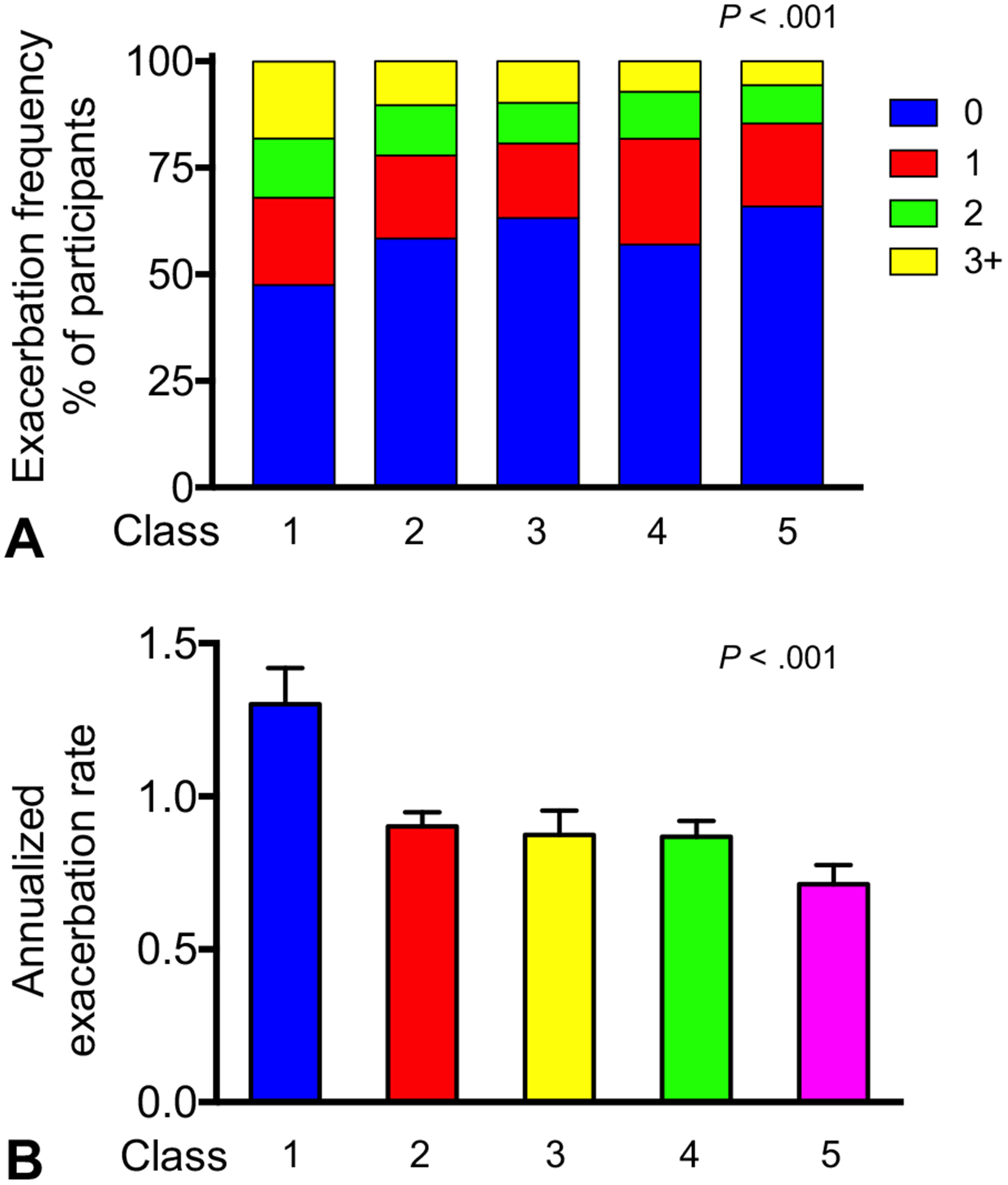
(A) Exacerbation frequency and (B) annualized rate of exacerbations (mean ± SEM) at the end of follow-up in latent classes 1 (multiple sensitization with partially reversible airflow limitation), 2 (multiple sensitization with reversible airflow limitation), 3 (lesser sensitization with reversible airflow limitation), 4 (multiple sensitization with normal lung function), and 5 (lesser sensitization with normal lung function).
FIGURE 4.
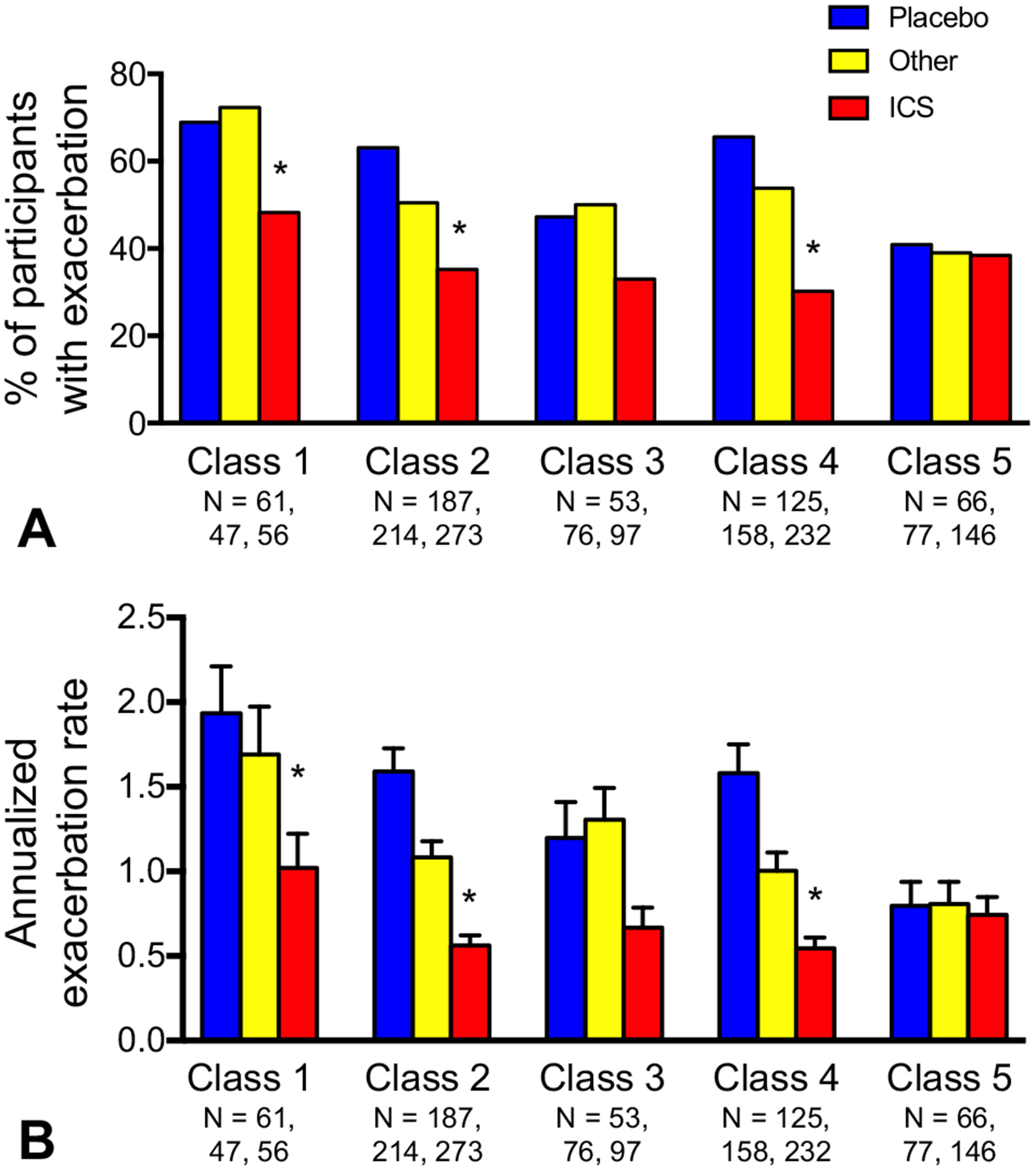
(A) Exacerbation occurrence and (B) annualized exacerbation rate at the end of follow-up, stratified by treatment assignment and latent class 1 (multiple sensitization with partially reversible airflow limitation), 2 (multiple sensitization with reversible airflow limitation), 3 (lesser sensitization with reversible airflow limitation), 4 (multiple sensitization with normal lung function), and 5 (lesser sensitization with normal lung function). *P < .05 vs placebo.
DISCUSSION
Although it is well recognized that children with asthma are a heterogeneous group, personalized medicine for these children is not common practice. Instead, treatment guidelines for children with asthma are still based on a “one size fits all” approach, with ICS as the cornerstone of therapy, in part due to limited understanding of pediatric phenotypes and their association with clinical outcomes. We therefore used LCA to uncover previously unobservable patterns in a well-characterized, large study population of children with mild to moderate persistent asthma enrolled in clinical trials. LCA has foundations in the social sciences and is particularly useful for identifying class membership among participants with multivariate categorical data. Although multivariable regression analyses could have been used to identify factors associated with 1-year asthma outcomes in this population, the present study sought to extend the existing body of hypothesis-directed literature through consideration of multiple variables simultaneously. However, we recognize that this approach is exploratory and hypothesis-generating; thus, the results should be interpreted in the context of clinical and biological plausibility.
Using LCA, we identified 5 latent classes of children with mild to moderate asthma who differed primarily in sensitization and lung function patterns. Consistent with our hypothesis, we identified a latent class with markers of type 2 inflammation and persistent airflow limitation (despite having mild to moderate disease) who also had the highest exacerbation rate by 1 year of follow-up. However, exacerbations were noted in each of the identified latent classes, albeit to a lesser extent. We therefore cannot conclude that our latent classes reflect distinct phenotypes of mild to moderate asthma in children, but these latent classes do provide some insight into potential (and differing) mechanisms of asthma in children that could be probed in future analyses.
Our findings also have plausibility and are supported by the results of other studies. Previous unsupervised analyses of children with severe asthma have also identified “clusters” with more prominent type 2 inflammatory markers (ie, sensitization and eosinophils) and features of greater asthma severity, including greater airflow limitation.45–48 Other independent cluster analyses of children with nonsevere asthma or children across the severity spectrum have noted similar results.49–53 However, few studies have attempted to validate the utility of the identified groupings with prospective outcomes. Although 1 study of predominantly African American, inner-city children across the asthma severity spectrum identified a cluster with prominent type 2 inflammation, multiple aeroallergen sensitization, significant airflow limitation, and the highest proportion of exacerbations requiring treatment with systemic corticosteroids, exacerbations were included as a variable in the clustering algorithm and were not assessed independently of cluster assignment.52 Schatz et al46 attempted to validate their cluster assignments in children aged 6 to 11 years with difficult-to-treat or severe asthma enrolled in the Natural History of Asthma: Outcomes and Treatment Regimens study, but found no association with exacerbation occurrence, asthma control, or quality of life at month 12. However, the clustering algorithm used in that study included only 8 variables, which may have been inadequate to discriminate subtle differences between groups. Furthermore, the percentage of children with exacerbations by 12 months was also relatively high in each cluster given the difficult-to-treat nature of the patients and ranged from 37% to 53%.46 Most relevant to the present study, a previous cluster analysis of the CAMP study (which excludes severe asthma) involving 18 variables identified a highly atopic cluster of children with airflow limitation that also had the shortest time to systemic corticosteroids for asthma exacerbation over 4 years of follow-up.50 However, in contrast to the present study, exacerbation rates and follow-up lung function data were not reported in that analysis.43
Although our observed associations between multiple sensitization, greater airflow limitation, and exacerbations are not particularly novel, the magnitude of airflow limitation and previous health care utilization observed in this population of children with mild to moderate asthma is somewhat surprising. However, a recent review of asthma-related deaths in the United Kingdom found that only 39% of asthma deaths occurred in patients with severe asthma.54 Instead, 9% and 46% of deaths occurred in patients treated for mild and moderate asthma, respectively, although that report also concluded that most of these nonsevere patients likely had poorly controlled under-treated asthma as opposed to truly mild or moderate disease.54 Although it is certainly possible that the inclusion criteria for our included studies were insufficient in capturing mild to moderate asthma, it is also recognized that, as a construct, asthma severity reflects the level of treatment required to control symptoms and exacerbations at the present time55 and is not a static feature.56 Indeed, other analyses of children with mild to moderate asthma in the CAMP study noted abnormal patterns of lung function growth in 75% of children, including markedly impaired lung function growth in 11% of participants who subsequently met criteria for advanced chronic obstructive pulmonary disease.23
This study has several strengths, including the large sample size of diverse and representative children across the United States. Because variable prescription of (and adherence to) asthma controller medications such as ICS can complicate outcome assessment, our focus on clinical trials with criteria for protocol adherence also increases the likelihood that children were compliant with prescribed therapies. The close follow-up and standardization of care in these clinical trials also helped to mitigate the impact of limited access to primary care, which is an important determinant of asthma outcomes in general populations.57 The clinical trials included in this analysis also assessed asthma exacerbations in a standardized way and each used a consistent definition of exacerbation in accordance with current recommendations for asthma outcomes research.43 This is also one of the few studies intent on asthma phenotype discovery in children that attempted to validate outcomes prospectively.
Nonetheless, there are some limitations with our approach. First, we acknowledge that model selection for LCA can be subjective. Although LCA models do allow for statistical comparisons of model fit, the outputs (and patient groupings) are ultimately dependent on the variables that are entered and the study population. Indeed, a study of different unsupervised statistical learning methods found that different variable sets led to inconsistent groupings of asthma that were not necessarily associated with severity.58 There are also no criterion standard variables for the purpose of phenotype discovery. For example, one study affirmed the importance of medication usage, current symptoms, lung function, parental asthma, BMI, and age of asthma onset in the prediction asthma outcomes,58 whereas another found that only 4 features identified by clinical experts (ie, age of onset, allergic sensitization, severity, and recent exacerbations) were meaningful.49 Because many of the variables used in our LCA model were assessed only at study entry, we were also unable to assess the temporal stability of the identified latent classes and transition over time. The relatively short, 1-year follow-up period and the inclusion of both school-age and adolescent participants were also insufficient for examination of the role of puberty and sex hormones, which have been shown to impact airway responsiveness and asthma prevalence on a population level.59 The study interventions may have also impacted the outcome measures selected for this analysis. For example, it is possible that the 12-month observations reflect suboptimal treatment of certain subsets of participants. Furthermore, the CAMP cohort may have impacted the findings because it represented most of the study population. There are also potential limitations with the generalization of our findings. Although most of the children in the present study were adherent, poor adherence to controller medications is prevalent in general populations.13,60 Furthermore, economic hardships, limited access to primary care,57 and ongoing exposures to environmental allergens and irritants61–63 are known factors associated with poorer asthma outcomes in general populations of children that may not have been well represented in this study.
CONCLUSIONS
Using LCA, we identified 5 latent classes of children with mild to moderate asthma that differed with regard to multiple variables, most notably allergic sensitization and other features of type 2 inflammation and lung function patterns. Although exacerbations were noted in each latent class, exacerbation rates were the highest in children with multiple sensitization and partially reversible airflow limitation, suggestive of more advanced disease. These lung function deficits persisted at 1 year despite intervention with asthma controller medications. Sensitization and lung function measures in children with mild to moderate asthma may therefore be useful for predicting future risk in clinical settings. However, additional studies are needed to determine whether our identified latent classes correspond to meaningful phenotypes for the purpose of personalized treatment.
Supplementary Material
What is already known about this topic?
In contrast to children with difficult-to-treat or severe asthma, phenotypic characterization of children with mild to moderate persistent asthma is still limited and it remains unclear which of these children are at the highest risk for poor outcomes.
What does this article add to our knowledge?
Five latent classes were identified. At 1 year, lung function deficits and exacerbations were the greatest in the latent class with multiple sensitization and partially reversible airflow limitation despite intervention with asthma controller therapy.
How does this study impact current management guidelines?
Latent class analysis is useful for identifying risk factors in children with mild to moderate asthma. Children with multiple sensitization and partially reversible airflow limitation are a particularly vulnerable group that may warrant more aggressive treatment.
Acknowledgments
This work was supported by NIH/NCATS UL1TR002378 and the Children’s Healthcare of Atlanta Pediatric Research Alliance Center for Clinical Outcomes and Public Health Research.
Abbreviations used
- ACQ
Asthma Control Questionnaire
- ACT
Asthma Control Test
- BMI
Body mass index
- CAMP
Childhood Asthma Management Research Program
- FVC
Forced vital capacity
- ICS
Inhaled corticosteroid
- LCA
Latent class analysis
- LLN
Lower limit of normal
Footnotes
Conflicts of interest: L. B. Bacharier reports personal fees from GlaxoSmithKline (GSK), Genentech/Novartis, Merck, DBV Technologies, Teva, Boehringer Ingelheim, Sanofi/Regeneron, Vectura, Circassia, and AstraZeneca, outside the submitted work. D. J. Jackson reports personal fees from Vectura, Boehringer Ingelheim, Pfizer, Sanofi/Regeneron, AstraZeneca, Vifor, GSK, and Novartis, outside the submitted work. S. J. Szefler reports consultancy fees from Boehringer Ingelheim, Genentech, GSK, Aerocrine, Novartis, AstraZeneca, Daiichi Sankyo, Propeller Health, Roche, and Teva; and grants from GSK, outside the submitted work. M. Cabana reports personal fees from Merck, Thermo Fisher, Genentech, and Novartis, outside the submitted work. R. Covar reports grants from Roche and AstraZeneca; and nonfinancial support from GSK, outside the submitted work. T. Guilbert reports personal fees from the American Board of Pediatrics, Pediatric Pulmonary Sub-board, GSK, Regeneron Pharmaceuticals, Merck, Novartis/Regeneron, Aviragen, GSK/Regeneron, Sanofi/Regeneron, and Teva; grants from Sanofi/Regeneron, AstraZeneca, and the National Institutes of Health; and personal fees from UpToDate, outside the submitted work. R. F. Lemanske reports grants from Pharmaxis; and personal fees from Elsevier and UpToDate, outside the submitted work. F. D. Martinez reports grants from the National Institutes of Health/Office of the Director and Johnson & Johnson; and personal fees from Copeval as well as Commense, Inc, outside the submitted work. W. Morgan reports grants from the Cystic Fibrosis Foundation; and personal fees from the Cystic Fibrosis Foundation, Genentech, the American Thoracic Society, and the American College of Chest Physicians, outside the submitted work. W. Phipatanakul reports consulting fees from Regeneron, Novartis, GSK, and Genentech; and clinical trials support and medication/supplies for trial support from Genentech, Novartis, Thermo Fisher, ALK-Abelló, and Monaghan. J. A. Pongracic reports grants from Northwestern University, during the conduct of the study; and research grants from Boehringer Ingelheim, GSK, Teva, and Merck, outside the submitted work. R. S. Zeiger reports grants from Aerocrine, Genentech, MedI-mmune/AstraZeneca, Merck, GSK, and ALK Pharma; personal fees from AstraZeneca, Genentech, Novartis, Teva, GSK, Theravance BioPharma, Regeneron Pharmaceuticals, and Patara Pharma, outside the submitted work. D. T. Mauger reports nonfinancial support from Merck, Boehringer Ingelheim, GSK, Teva, and Vifor, outside the submitted work. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Centers for Disease Control and Prevention, US Department of Health and Human Services. Most recent national asthma data. Available from: https://www.cdc.gov/asthma/most_recent_data.htm. Accessed September 16, 2019.
- 2.Sullivan PW, Ghushchyan V, Kavati A, Navaratnam P, Friedman HS, Ortiz B. Trends in asthma control, treatment, health care utilization, and expenditures among children in the United States by place of residence: 2003–2014. J Allergy Clin Immunol Pract 2019;7:1835–1842.e2. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan PW, Ghushchyan V, Navaratnam P, Friedman HS, Kavati A, Ortiz B, et al. National prevalence of poor asthma control and associated outcomes among school-aged children in the United States. J Allergy Clin Immunol Pract 2018;6:536–544.e1. [DOI] [PubMed] [Google Scholar]
- 4.Reddel HK, Busse WW, Pedersen S, Tan WC, Chen YZ, Jorup C, et al. Should recommendations about starting inhaled corticosteroid treatment for mild asthma be based on symptom frequency: a post-hoc efficacy analysis of the START study. Lancet 2017;389:157–66. [DOI] [PubMed] [Google Scholar]
- 5.Romagnoli M, Caramori G, Braccioni F, Ravenna F, Barreiro E, Siafakas NM, et al. Near-fatal asthma phenotype in the ENFUMOSA cohort. Clin Exp Allergy 2007;37:552–7. [DOI] [PubMed] [Google Scholar]
- 6.Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol 2012;129: 1229–35. [DOI] [PubMed] [Google Scholar]
- 7.Szefler SJ, Zeiger RS, Haselkorn T, Mink DR, Kamath TV, Fish JE, et al. Economic burden of impairment in children with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol 2011;107:110–119.e1. [DOI] [PubMed] [Google Scholar]
- 8.Fleming M, Fitton CA, Steiner MFC, McLay JS, Clark D, King A, et al. Educational and health outcomes of children treated for asthma: Scotland-wide record linkage study of 683 716 children. Eur Respir J 2019;54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen ME, Mendelson MJ, Desplats E, Zhang X, Platt R, Ducharme FM. Caregiver’s functional status during a young child’s asthma exacerbation: a validated instrument. J Allergy Clin Immunol 2016;137:782–788.e6. [DOI] [PubMed] [Google Scholar]
- 10.Bui AL, Dieleman JL, Hamavid H, Birger M, Chapin A, Duber HC, et al. Spending on children’s personal health care in the United States, 1996–2013. JAMA Pediatr 2017;171:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dieleman JL, Squires E, Bui AL, Campbell M, Chapin A, Hamavid H, et al. Factors associated with increases in US health care spending, 1996–2013. JAMA 2017;318:1668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008–2013. Ann Am Thorac Soc 2018;15:348–56. [DOI] [PubMed] [Google Scholar]
- 13.Slejko JF, Ghushchyan VH, Sucher B, Globe DR, Lin SL, Globe G, et al. Asthma control in the United States, 2008–2010: indicators of poor asthma control. J Allergy Clin Immunol 2014;133:1579–87. [DOI] [PubMed] [Google Scholar]
- 14.Lodge CJ, Zaloumis S, Lowe AJ, Gurrin LC, Matheson MC, Axelrad C, et al. Early-life risk factors for childhood wheeze phenotypes in a high-risk birth cohort. J Pediatr 2014;164:289–294.e1–294.e2. [DOI] [PubMed] [Google Scholar]
- 15.Spycher BD, Silverman M, Brooke AM, Minder CE, Kuehni CE. Distinguishing phenotypes of childhood wheeze and cough using latent class analysis. Eur Respir J 2008;31:974–81. [DOI] [PubMed] [Google Scholar]
- 16.Savenije OE, Granell R, Caudri D, Koppelman GH, Smit HA, Wijga A, et al. Comparison of childhood wheezing phenotypes in 2 birth cohorts: ALSPAC and PIAMA. J Allergy Clin Immunol 2011;127:1505–1512.e14. [DOI] [PubMed] [Google Scholar]
- 17.Panico L, Stuart B, Bartley M, Kelly Y. Asthma trajectories in early childhood: identifying modifiable factors. PLoS One 2014;9:e111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med 1995;332:133–8. [DOI] [PubMed] [Google Scholar]
- 19.Fitzpatrick AM, Jackson DJ, Mauger DT, Boehmer SJ, Phipatanakul W, Sheehan WJ, et al. Individualized therapy for persistent asthma in young children. J Allergy Clin Immunol 2016;138:1608–1618.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemanske RF Jr, Mauger DT, Sorkness CA, Jackson DJ, Boehmer SJ, Martinez FD, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med 2010;362:975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wechsler ME, Szefler SJ, Ortega VE, Pongracic JA, Chinchilli V, Lima JJ, et al. Step-up therapy in black children and adults with poorly controlled asthma. N Engl J Med 2019;381:1227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung KF. Personalised medicine in asthma: time for action: number 1 in the series “Personalised medicine in respiratory diseases” edited by Renaud Louis and Nicolas Roche. Eur Respir Rev 2017;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med 2016;374:1842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szefler SJ, Chmiel JF, Fitzpatrick AM, Giacoia G, Green TP, Jackson DJ, et al. Asthma across the ages: knowledge gaps in childhood asthma. J Allergy Clin Immunol 2014;133:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitzpatrick AM, Moore WC. Severe asthma phenotypes—how should they guide evaluation and treatment? J Allergy Clin Immunol Pract 2017;5:901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Childhood Asthma Management Program Research Group. Control Clin Trials 1999;20:91–120. [PubMed] [Google Scholar]
- 27.Childhood Asthma Management Program Research Group; Szefler S Weiss S, Tonascia J, Adkinson NF, Bender B, Cherniack R, et al. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000;343:1054–63. [DOI] [PubMed] [Google Scholar]
- 28.Strunk RC, Szefler SJ, Phillips BR, Zeiger RS, Chinchilli VM, Larsen G, et al. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol 2003;112:883–92. [DOI] [PubMed] [Google Scholar]
- 29.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol 2005;115:233–42. [DOI] [PubMed] [Google Scholar]
- 30.Zeiger RS, Szefler SJ, Phillips BR, Schatz M, Martinez FD, Chinchilli VM, et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol 2006;117:45–52. [DOI] [PubMed] [Google Scholar]
- 31.Sorkness CA, Lemanske RF Jr, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol 2007;119:64–72. [DOI] [PubMed] [Google Scholar]
- 32.Knuffman JE, Sorkness CA, Lemanske RF Jr, Mauger DT, Boehmer SJ, Martinez FD, et al. Phenotypic predictors of long-term response to inhaled corticosteroid and leukotriene modifier therapies in pediatric asthma. J Allergy Clin Immunol 2009;123:411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malka J, Mauger DT, Covar R, Rabinovitch N, Lemanske RF Jr, Spahn JD, et al. Eczema and race as combined determinants for differential response to step-up asthma therapy. J Allergy Clin Immunol 2014;134:483–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF Jr, Mauger DT, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet 2011;377:650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson DJ, Bacharier LB, Mauger DT, Boehmer S, Beigelman A, Chmiel JF, et al. Quintupling inhaled glucocorticoids to prevent childhood asthma exacerbations. N Engl J Med 2018;378:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazarus SC, Krishnan JA, King TS, Lang JE, Blake KV, Covar R, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med 2019;380:2009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 2004;113:59–65. [DOI] [PubMed] [Google Scholar]
- 38.Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol 2007;119:817–25. [DOI] [PubMed] [Google Scholar]
- 39.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999;14:902–7. [DOI] [PubMed] [Google Scholar]
- 40.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–38. [DOI] [PubMed] [Google Scholar]
- 41.American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005;171:912–30. [DOI] [PubMed] [Google Scholar]
- 42.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40:1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA Jr, Gern J, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol 2012;129:S34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lanza ST, Collins LM, Lemmon DR, Schafer JL. PROC LCA: a SAS procedure for latent class analysis. Struct Equ Modeling 2007;14:671–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol 2011;127: 382–389.e1–389.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol 2014;133:1549–56. [DOI] [PubMed] [Google Scholar]
- 47.Just J, Gouvis-Echraghi R, Rouve S, Wanin S, Moreau D, Annesi-Maesano I. Two novel, severe asthma phenotypes identified during childhood using a clustering approach. Eur Respir J 2012;40:55–60. [DOI] [PubMed] [Google Scholar]
- 48.Guiddir T, Saint-Pierre P, Purenne-Denis E, Lambert N, Laoudi Y, Couderc R, et al. Neutrophilic steroid-refractory recurrent wheeze and eosinophilic steroid-refractory asthma in children. J Allergy Clin Immunol Pract 2017;5:1351–1361.e2. [DOI] [PubMed] [Google Scholar]
- 49.Deliu M, Yavuz TS, Sperrin M, Belgrave D, Sahiner UM, Sackesen C, et al. Features of asthma which provide meaningful insights for understanding the disease heterogeneity. Clin Exp Allergy 2018;48:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howrylak JA, Fuhlbrigge AL, Strunk RC, Zeiger RS, Weiss ST, Raby BA, et al. Classification of childhood asthma phenotypes and long-term clinical responses to inhaled anti-inflammatory medications. J Allergy Clin Immunol 2014;133: 1289–1300.e1–1300.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Just J, Saint-Pierre P, Gouvis-Echraghi R, Laoudi Y, Roufai L, Momas I, et al. Childhood allergic asthma is not a single phenotype. J Pediatr 2014; 164:815–20. [DOI] [PubMed] [Google Scholar]
- 52.Zoratti EM, Krouse RZ, Babineau DC, Pongracic JA, O’Connor GT, Wood RA, et al. Asthma phenotypes in inner-city children. J Allergy Clin Immunol 2016; 138:1016–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cabral AL, Sousa AW, Mendes FA, Carvalho CR. Phenotypes of asthma in low-income children and adolescents: cluster analysis. J Bras Pneumol 2017;43: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Royal College of Physicians. Why asthma still kills: the National Review of Asthma Deaths (NRAD) confidential enquiry report; 2014. Available from: http://www.rcplondon.ac.uk/projects/outputs/why-asthma-still-kills. Accessed September 19, 2019.
- 55.Global Initiative for Asthma. Global strategy for asthma management and prevention; 2019. Available from: www.ginasthma.org. Accessed October 1, 2019.
- 56.Newby C, Heaney LG, Menzies-Gow A, Niven RM, Mansur A, Bucknall C, et al. Statistical cluster analysis of the British Thoracic Society Severe refractory Asthma Registry: clinical outcomes and phenotype stability. PLoS One 2014;9: e102987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kodjebacheva GD, Sabo T, Parker S. Influences of asthma on reported health indicators and access to health care among children. Ann Allergy Asthma Immunol 2016;116:126–33. [DOI] [PubMed] [Google Scholar]
- 58.Prosperi MC, Sahiner UM, Belgrave D, Sackesen C, Buchan IE, Simpson A, et al. Challenges in identifying asthma subgroups using unsupervised statistical learning techniques. Am J Respir Crit Care Med 2013;188:1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tantisira KG, Colvin R, Tonascia J, Strunk RC, Weiss ST, Fuhlbrigge AL, et al. Airway responsiveness in mild to moderate childhood asthma: sex influences on the natural history. Am J Respir Crit Care Med 2008;178:325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kit BK, Simon AE, Ogden CL, Akinbami LJ. Trends in preventive asthma medication use among children and adolescents, 1988–2008. Pediatrics 2012; 129:62–9. [DOI] [PubMed] [Google Scholar]
- 61.Wang Z, May SM, Charoenlap S, Pyle R, Ott NL, Mohammed K, et al. Effects of secondhand smoke exposure on asthma morbidity and health care utilization in children: a systematic review and meta-analysis. Ann Allergy Asthma Immunol 2015;115:396–401.e2. [DOI] [PubMed] [Google Scholar]
- 62.Sala KA, Carroll CL, Tang YS, Aglio T, Dressler AM, Schramm CM. Factors associated with the development of severe asthma exacerbations in children. J Asthma 2011;48:558–64. [DOI] [PubMed] [Google Scholar]
- 63.Dick S, Doust E, Cowie H, Ayres JG, Turner S. Associations between environmental exposures and asthma control and exacerbations in young children: a systematic review. BMJ Open 2014;4:e003827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


