Abstract
Despite the great breakthroughs in the past few decades in illuminating the pathological mechanisms of cancer and in developing new anticancer drugs, it remains extremely challenging to cure most cancers. Therefore, it is imperative to develop more sophisticated and more biomimetic preclinical cancer models. 3D models combined with dynamic culture techniques show great potential to accurately emulate the volumetric tumor microenvironment (TME). Here we introduce advances in bioprinting technologies for in vitro cancer modeling and their applications. Finally, we look ahead to the remaining challenges associated with current bioprinting strategies and further requirements for standardization.
Introduction
President Richard Nixon along with the US Congress declared a ‘war on cancer’ in 1971 [1]. The goal was to make the public aware that this devastating disease is a dangerous threat with massive economic costs to societies, and to gather several forces and resources to win the war. Since the late 1990s, specialists in cancer medicine have been expecting treatments to disrupt molecular mechanisms that drive tumor formation, growth, and progression [1]. Despite the tremendous breakthroughs in uncovering the pathological mechanisms of cancer and the development of new anticancer drugs during the past few decades, it is widely recognized that cures are still rare for most types of cancer [1]. The tumor microenvironment (TME, see Glossary) is identified as a principal factor in orchestrating the tumor initiation, progression, and reaction to various therapeutic regimens [2]. It has been a challenge to uncover the specific interactions with the stroma that induce tumor growth in vivo in general, and therefore in vitro cell culture systems have been instrumental for studies of cancer [3].
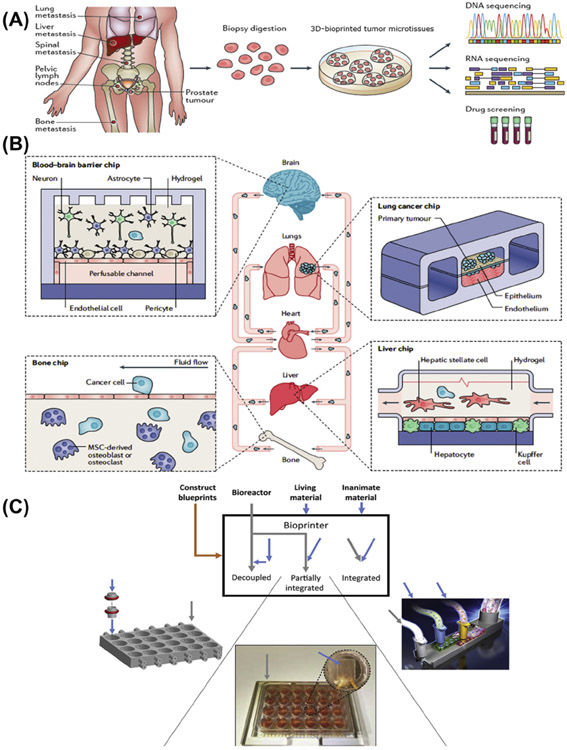
Bioprinting makes it possible to establish 3D structures with precise spatial arrangement of ECM materials, cells, and various biologically active factors [4]. These techniques represent better biomimetics by reproducing the complexity of tissues and organs and can be potentially used in manufacturing and production at scale. For example, bioprinting has helped the achievement of more advanced microvessel models as perfusable channels to mimic the native angiogenesis process more precisely than traditional preparation process [5]. 3D models allow better control of key microenvironmental parameters and architectures than 2D models, such as mechanical stiffness or softness that can be patterned in gradients, growth factors that can be released at a specific time or location from the ECM, and regulated vascular prefusion [6]. These are essential, due to the fact that cancer is a pathology that evolves with time and space. Furthermore, a combination of biofabrication and 3D-bioprinting techniques can be utilized to develop even more advanced in vitro cancer models. One of the most prominent biofabrication techniques is organ-on-a-chip technology. An organ on a chip is a microfluidic cell culture device that has continuously perfused chambers, which are inhabited by living cells arranged to mimic tissue- and organ-level physiology [7]. Recently, glioblastoma (GBM) samples from patients during tumor excision surgery were dissociated into single cells using papain digestion [8]. These patient-derived cancer cells were 3D bioprinted using the extrusion technique to produce a patient-specific GBM on a chip. Afterwards, total RNA was isolated and the transcriptomic profiles were identified to select various candidate drug combinations that target the specific mutations observed. Various candidate drug combinations were tested using the chip and prioritized according to their efficiencies to identify the best drug combination. Potentially, the physician could use those test results to design a treatment plan for that patient. For individual patients this would be only the first step in the realization of personalized medicine that identifies therapeutic targets and brings maximum benefits to patients based on genetic changes specific to individual cancers (Figure 1A) [9].
Figure 1.
(A) A biopsy can be performed on a specific patient and used to 3D bioprint the patient-specific cancer model using autologous cells. The microtissues will reflect the same genetic mutations as are in the neoplastic cells of that patient and will be particularly useful to generate therapeutic selections towards personalized medicine. Adapted, with permission, from [9]. Copyright 2017 Nature Publishing Group. (B) Schematic diagrams showing the biofabrication of a body on a chip to study systemic metastasis. This will help various organ chips, such as lung, liver, bone, and brain, to be fluidically linked to explore the mechanisms of preferential tumor cell targeting to specific organs (organotropism) as well as to identify potential pharmacological strategies to block metastatic spread in the future. Adapted, with permission, from [10]. Copyright 2019 Nature Publishing Group. (C) Degree of integration between tissue construction and bioreactor production including separation, partial integration, and integration methods. Adapted, with permission, from [11]. Copyright 2019 Elsevier Ltd. Abbreviations : MSC, mesenchymal stem cell.
Organ-on-a-chip technology provides engineered tissue models with the capacity to recapitulate physicochemical microenvironments and the vascular perfusion of the body to analyze the genetic, biochemical, and metabolic activities of living cells in a functional tissue context [7]. In the future, metastasis and invasion might also be modeled using a platform comprising multiple fluid-connected organ/cancer-on-chip modules (Figure 1B) [10]. For example, metastatic spread could be detected by observing lung cancer cells entering the bloodstream and invading liver, bone, or brain chips. Growth of metastatic lesions could be studied with the potential to explore preferential tumor cell targeting to specific organs and pharmacological strategies to block metastatic spread [10]. Integration of 3D bioprinting of tissue structures with the design of microfluidic bioreactors may allow greater flexibility to meet experimental and clinical needs (Figure 1C) [11]. Importantly, bioprinting will add the volumetric cues present in the native TME in addition to the dynamics provided by the microfluidic chip devices [7].
In this opinion article, we present an overview of the state of the art of existing bioprinting techniques to simulate volumetric TME as well as their applications. We conclude with future perspectives on the remaining challenges associated with the current bioprinting strategies and the further need for standardization.
Bioink Selection
Several engineering strategies to construct in vitro cancer models have made use of scaffolds as matrices to load cells. Scaffolds are 3D templates that support cells to attach, proliferate, and expand throughout the entire structure before they develop their own ECM. These scaffolds are bioprinted using bioinks in the form of hydrogels [12]. Hydrogels are a class of 3D networks formed by stabilizing polymer chains in a water-rich environment through mechanisms including physical entanglement, ionic interactions, and/or chemical crosslinking, among others [13]. A bioink is a solution of a hydrogel biomaterial or a mixture of several hydrogel biomaterials that encapsulate cells and other biologically active components that are suitable for processing by the bioprinting technology to create tissue constructs [14]. The main properties of a bioink include biocompatibility, rheology, mechanics, and crosslinking capabilities [15]. These properties can affect the printability and bioprinting fidelity, cell survival, propagation, and differentiation post-bioprinting. It is sometimes not straightforward to create a bioink that has a stable structure to enable bioprinting and provides protection and support to encapsulated cells.
Existing bioinks include both synthetic and natural origins. Natural biomaterials are derived from living sources. Some of the most commonly used natural biomaterials include alginate, collagens, hyaluronic acid, fibrin, and gelatin [16,17]. Synthetic biomaterials are artificial materials that are either biocompatible or can be modified by adding bioactive components to mimic the natural ECM properties. Some synthetic hydrogel biomaterials are pluronics, poly(ethylene glycol) (PEG), and poly(N-isopropylacrylamide) (pNIPAAm) [16,17]. Along with bioactivity, stiff hydrogels bioprinted from bioink might exert stress on encapsulated cells and hinder their spreading and migration [18]. Matrix stiffness is involved in the regulation of tumorigenesis, evolution, metastasis, and chemoresistance [19]. Thus, a balanced combination of synthetic and natural biomaterials can perhaps best achieve recapitulation of the pathological and morphological properties that exist in bioprinted cancer models. Stiffness can also be adjusted through control of the compositions, densities, and crosslinking conditions of synthetic or natural materials [20].
Shear stress during bioprinting affects cell morphology and metabolic activity and has the potential to cause cell death [21]. One approach to this challenge, developed by us, was to directly bioprint cell-laden gelatin methacryloyl (GelMA) constructs through the extrusion of bioinks of GelMA physical gels (GPGs) attained over a cooling process [22]. The GPG bioinks have self-healing and shear-thinning properties. This allows GPG bioinks to maintain shape and create integral structures following deposition, where chemical stabilization can be achieved through subsequent photocrosslinking. Constructs that were bioprinted with inferior concentrations of the GPG bioinks were demonstrated to permit cell survival as well as promote cell proliferation plus spreading. We also developed a pore-forming GelMA-based bioink relying on an aqueous two-phase emulsion [23]. This mixture, formed by two aqueous phases of cells and GelMA, encapsulates droplets of poly(ethylene oxide) (PEO) and is photocrosslinked to construct a cell-laden hydrogel. The pores are formed by subsequently removal of the PEO phase from the GelMA hydrogel throughout the time of incubation in cell culture medium. The pore distribution can be controlled by adjusting the ratio of the PEO:GelMA solution. Results showed that cells in the 3D-bioprinted porous cell-laden hydrogel showed superior rates of cell viability and spread compared with nonporous GelMA constructs. Moreover, tumor-derived decellularized ECM (dECM)-based bioinks have the potential to maintain biochemical cues of the TMEs. Their use is also anticipated in the development of future cancer models, due to the unique TME composition and strong heterogeneity among patients [24].
Common Bioprinting Techniques
Bioprinting is a 3D fabrication technology by which bioinks are typically processed layer by layer to precisely patterned into cell-laden constructs for the generation of complex volumetric living tissues from computer-aided designs [25]. This technology holds great potential for applications in cancer research. To obtain a better understanding of cancer genesis and progression, cancer models should mimic the in vivo TME. The TME comprises the ECM, myofibroblasts, fibroblasts, neuroendocrine cells, adipose cells, immune-inflammatory cells, and the blood and lymphatic vascular networks [26]. Bioprinting offers the possibility to recreate 3D complexity with controlled spatial distributions that enable physiologically relevant cell–cell and cell–matrix interactions existent in the TME.
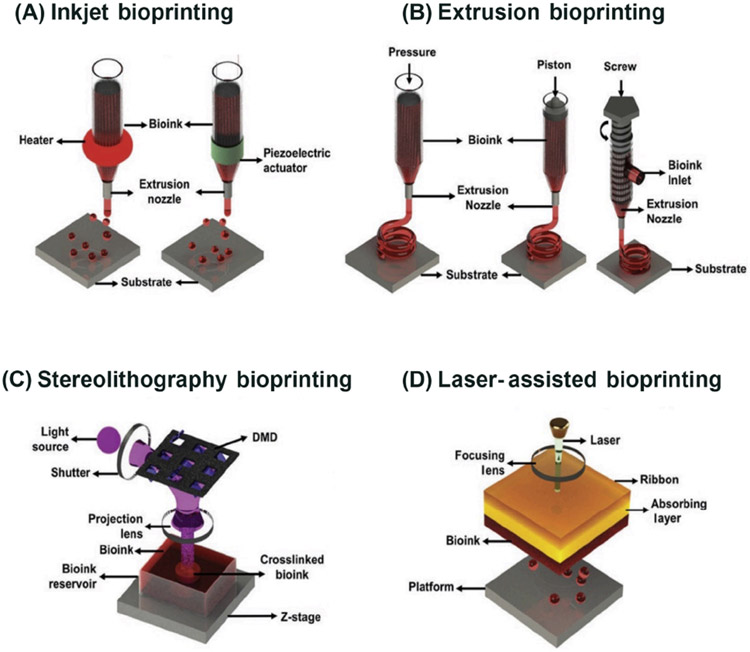
While the choice of bioinks impacts the activities of cells in the bioprinted constructs, the different techniques will determine the selection of the bioinks usable and the structural complexity achievable with bioprinting. The two methods that inkjet bioprinting uses to deliver small droplets of bioink are piezoelectric and thermal (Figure 2A) [24]. The piezoelectric inkjet printer forces liquid through its nozzle through acoustic waves produced by piezoelectric crystals [27]. The thermal inkjet system expels droplets out of its printhead by using pulses of pressure to vaporize the bioink around the heating element [27]. Through cell-loaded bioinks, this technique allows precise positioning of the cells. An average cell viability of over 85% is observed with most inkjet bioprinters [28]. Each nozzle found in thermal inkjet printers raises the local temperature to 300°C for a few microseconds and therefore ejected cells are likely to undergo a temperature rise of 4-−10°C above ambient for only 2 μs [29]. Studies have demonstrated that the rise in temperature caused by thermal inkjet bioprinters has minimal impact on the stability of DNA for such a short duration of time [30]; however, potential risks remain when exposing cells and tissue constructs to heat and mechanical stress [31]. Interestingly, cancer cells have shown more resistance to shear stress than non-cancerous cells [32]. A limiting factor regarding inkjet bioprinting is the need to use low-viscosity solutions to achieve the ejection of a droplet [17]. Some of the advantages that inkjet bioprinting provides are bioprinting speed, low cost, and broad availability; the downsides lie in restricted droplet directional patterns and defective cell encapsulation from low bioink viscosity.
Figure 2. Schematic Diagrams Showing the Common Bioprinting Techniques.
(A) Inkjet bioprinting utilizes air-pressure pulses through a thermal heater or mechanical pulses through a piezoelectric actuator to eject tiny droplets of the bioink. (B) Extrusion bioprinting extrudes the bioink through a nozzle, driven by either pneumatic or mechanical pressure. (C) Stereolithography (SLA)/digital light processing (DLP) bioprinting uses UV or visible light to cure photosensitive bioink in a layer-by-layer fashion. (D) Laser-assisted bioprinting comprises three parts: a donor slide, a laser pulse, and a receiver slide. The bioink is transferred from the ribbon onto the receiver slide when the bioink under the metal layer is vaporized by a laser pulse. Adapted, with permission, from [24]. Copyright 2019 John Wiley & Sons. Abbreviations: DMD, digital micromirror array device.
In extrusion bioprinting, pneumatic or mechanical pressure is utilized to extrude the bioink through a nozzle (Figure 2B) [24,33]. This is the most feasible technique to bioprint with very high cell densities, although it is reported to have from 40 to 80% cell viability [28]. Nozzles can be added to build multimaterial extrusion bioprinters to alternate between building stiff materials and layers with softer conformations for encapsulated cells. Our group, for instance, developed a single-printhead setup that eliminates the need for physical nozzle switches and allows simultaneous or sequential deposition of multiple different bioinks [34]. This can also facilitate the spatial patterning of multiple cell types in the same cancer model. These advantages offered by extrusion-based bioprinting result in enhanced differentiation of diverse cell types, shown by the expression of diverse markers [16]. A key point is that cancer research will be greatly benefited by the identification of chemical ligands and mechanical stiffness that correlate with metastasis development. This is especially important because it will contribute to the discovery of new therapeutic targets that can inhibit these specific interactions. Freeform structures can be constructed through extrusion using a technique termed embedded bioprinting, which allows bioprinting in the true 3D space in a supporting hydrogel without necessarily having to fight against the gravity as is the case when extruding in air, as is conventionally done [35-38].
Stereolithography (SLA) or digital light processing (DLP) is a bioprinting technique that utilizes patterned light to cure photosensitive bioinks layer by layer (Figure 2C) [24]. Compared with other 3D bioprinting technologies, SLA/DLP offers a relatively fast bioprinting speed. The bioprinting time using SLA for a final layered 3D structure of approximately 500 μm [39] resulted in a longitudinal log of 20 μm wide and ~1800 μm long. The spacing between the logs was 100 μm and the supporting transverse logs were 50 μm wide and ~1730 μm long. Therefore, SLA/DLP can significantly improve bioprinting resolution for cell-laden structures, while also providing protection to cells by limiting their exposure to conditions that are not physiological. Mechanical stiffness can be patterned in gradients through SLA/DLP; this can be used as a tool to study local invasion by neoplastic cells and its association with the mechanical properties of the ECM [16]. Through SLA/DLP, cells are not inflicted with shear stress. Nonetheless, designs with hollow structures, such as vessels, might be obstructed by the remnant of the precursor hydrogel in the bioprinted constructs [18], requiring meticulous optimization of the bioink and photoabsorber formulations. UV-based SLA/DLP systems have a cell viability of over 90% [40], but the light source and photoinitiators used for SLA/DLP bioprinting (and most other bioprinting techniques where relevant) can damage the cells. Researchers are beginning to look at alternatives, such as materials without photoinitiators or photoinitiators that can absorb visible light [41].
Laser-assisted bioprinting has three main components: a laser pulse, a donor slide or ribbon, and a receiver slide (Figure 2D) [24,41]. First, a laser pulse vaporizes the metal layer under the hydrogel; then, the bioink moves from the ribbon onto the receiver slide. This bioprinting technique can position one cell per droplet or several cell types while maintaining accuracy [42], but it is a costly technique [43]. This technique has a cell viability of over 95% [28]. Additionally, concentric cylinders of multiple cell types can be constructed through the interchange of ‘ribbons’ coated with bioinks of different compositions [16].
Recently, 4D bioprinting has emerged; the fourth dimension refers to time. 4D bioprinting endows patterned structures with the programmable ability to transform by exploiting the shape-changing features of various types of stimulus-responsive biomaterials and by using advanced 3D-bioprinting strategies [13]. 4D bioprinting provides the capacity to manipulate variables such as timed and localized release of growth factors from the surrounding matrix, patterned mechanical softening or stiffening, and controlled perfusion into vasculature [44]. Through 4D bioprinting, singular variables can be controlled and tested to establish the modulators of cancer cells or key environmental factors that influence cancer behaviors.
Future developments should combine techniques to complement each other and optimize the process of bioprinting tissue models, including those of tumors. Bioprinting of cancer models could allow the rapid creation of prototypes to study the precise molecular signaling interactions in a neoplasm and select the best chemotherapeutic for a specific patient. However, the lack of standardized 3D bioprinters for applications is a major obstacle for the adoption of 3D bioprinting by nonspecialists. There is a need to develop standardized equipment as well as commercially available bioinks and bioprinting toolkits to overcome the limited interlaboratory reproducibility in the near future [16]. There is also a possibility to lower costs and enable greater access across scientists by enabling open-source 3D bioprinting. More publications that describe 3D bioprinting methods will continue to push boundaries in the field, but this requires a transdisciplinary effort between engineers, biologists, and clinicians to allow these techniques to break into mainstream research.
Applications of Bioprinting in Cancer Tissue Engineering
3D-bioprinted cancer models expose more pathologically relevant features regarding cell–cell and cell–ECM interactions than 2D cultures. Differences in many aspects, such as gene and protein expression, phenotypes, and drug responses, have been observed between 3D and 2D cancer models, as well. Several 3D cancer models have already shown better clinical translational outcomes [45], as illustrated chronologically below.
Recently, a metastatic model was developed using the extrusion-based 3D-bioprinting technique to build tumor constructs via precise positioning of cells, functional biomaterials, and programmable release capsules that generate chemical cues and regulate cellular behaviors at a local level [46]. First, 3D-bioprinted stimulus-responsive microcapsules that contained epidermal growth factor (EGF) were programmed to be released by the hydrogels. Then, vascular endothelial growth factor (VEGF) was introduced to promote the formation of a perfusable vascular network from a pre-existing vessel with the intention to learn more about tumor cell intravasation through an endothelial barrier. Finally, chemotactic pathways were programmed to guide tumor cell invasion and angiogenesis. Through this metastatic model, mechanisms of tumor progression and metastasis can be researched.
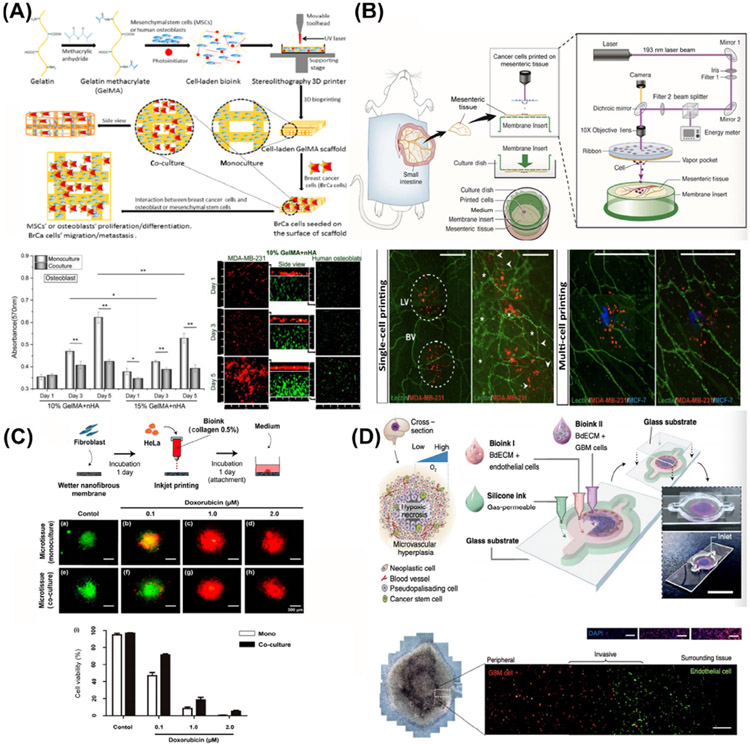
A cell-laden bone matrix to study breast cancer invasion and metastasis was generated using SLA technology (Figure 3A) [47]. Bone matrices were fabricated with GelMA and osteoblasts or mesenchymal stem cells (MSCs) with nanocrystalline hydroxyapatite; breast cancer cells were then seeded into the cell-laden matrices. In the co-culture model, the growth of breast cancer cells was enhanced whereas the proliferation of the osteoblasts or MSCs was inhibited. This illustrates the application of SLA-based bioprinting in tumor modeling, which provides an important advantage in the study of tumor invasion and metastasis through the production of complex structures.
Figure 3.
(A) Schematic Illustration of a 3D-Bioprinted Breast Cancer Metastasis Model. Breast cancer cells and osteoblasts were mono- and co-cultured in the 3D-bioprinted platforms. Adapted, with permission, from [47]. Copyright 2016 American Chemical Society. (B) Schematic diagram of laser-assisted bioprinting of breast cancer cells on cultured ex vivo rat mesentery tissue. Adapted, with permission, from [45]. Copyright 2016 John Wiley & Sons. (C) Schematic representation of the cervical cancer fabrication process with inkjet bioprinting. Drug tests were performed on day 7 with both monocultured and co-cultured bioprinted cancer microtissues. Adapted, with permission, from [51]. Copyright 2017 MDPI. (D) Schematic diagram of the bioprinting process for a human glioblastoma-on-a-chip model. Adapted, with permission, from [8]. Copyright 2019 Nature Publishing Group. Abbreviations: GBM, glioblastoma; MSC, mesenchymal stem cell.
To emulate the TME, breast cancer cells were directly written on cultured rat mesentery tissues with laser-assisted bioprinting technology to quantitatively study cancer cell activity and its effects on angiogenesis and lymphangiogenesis (Figure 3B) [48]. This technique of bioprinting directly on living tissues better simulates the situation of tumors in living organisms, and this ex vivo/in vitro model may be used to study tumor invasion, metastasis, and neovascularization.
A 3D-bioprinted human GBM model was established using a gelatin, alginate, and fibrinogen-based porous hydrogel to mimic the ECM [49]. The cell survival rate was more than 80% with high cellular activity. This example shows the potential of 3D bioprinting in the modeling of neurological tumors. During the culture period, GBM cells exhibited high expression of proteins related to differentiation and angiogenesis. In the drug-sensitivity test, the 3D tumor model revealed resistance to temozolomide. Furthermore, high-resolution tomographic analysis of the bioprinted GBM model after long-term drug treatment provides a potential for noninvasive evaluation of neurological tumor therapeutics compared with previous approaches [50].
In addition, inkjet bioprinting with type I collagen has been used to fabricate a cervical cancer microtissue array on a nanofibrous membrane in co-culture with fibroblasts (Figure 3C) [51]. Tumor cells, ECM, and stromal cells were combined and, on day 7 after bioprinting, tumor cells were found to have higher matrix metalloproteinase (MMP) expression (indicating that the tumor was highly metastatic; MMP degrades the ECM to allow tumor invasion [1,52]) and greater resistance to doxorubicin with to 2D culture (indicating its chemoresistant nature). This demonstrates the feasibility of the inkjet bioprinting technology in forming 3D tumor models that possess characteristics similar to their counterparts, showing its potential for applications in tumor biology and pharmacology.
Extrusion bioprinting has also been used to fabricate a compartmentalized concentric ring structure with patient-derived GBM cells, vascular endothelial cells, and porcine brain dECM (BdECM)-based bioink (Figure 3D) [8]. This direct 3D bioprinting of patient-derived cancer cells mimics the genetic characteristics of the native tumor to a good extend in vitro, where invasion into the ECM by the GBM cells was observed and the resistance during treatment with temozolomide was reproduced. This study indicated that a bioprinted model can be used to investigate tumor progression and transformation. Moreover, a sacrificial extrusion bioprinting strategy has been used to fabricate a mammary ductal carcinoma model, creating a mammary duct-like structure in a GelMA matrix to model the genesis of ductal carcinoma and subsequent progression [53]. This technique can simulate tumor tissues with tubular structures such as mammary ductal carcinoma and other adenocarcinomas, as well as the vasculature. Lymphangiogenesis was also studied in another breast tumor model, generated using the same sacrificial bioprinting strategy, that, differently, contained a microchannel seeded with lymphatic endothelial cells [54]. Combining these two concepts, a tumor-on-a-chip model was created with a bioprinted blood and lymphatic vessel pair that exhibited a drug transport profile that could better recapitulate the in vivo tumor microcirculation [55].
3D-bioprinted tumor models also provide several advantages when studying interactions between cancer cells and cells of the immune system. A GelMA-based 3D-bioprinted mini-brain was used to explore cellular interactions between GBM cells and macrophages [56]. In the co-culture model, the macrophages were polarized into a glioma-associated macrophage (GAM)-specific phenotype, while the GBM cells showed more relevance to clinical transcriptomic and patient survival data. This model demonstrated the inhibitory interactions between tumor cells and macrophages since the co-culture showed a reduction in tumor growth and more sensitivity to chemotherapy.
These diverse research findings exemplify the application of four widely used 3D-bioprinting technologies (inkjet, extrusion, SLA/DLP, and laser-assisted bioprinting) in several cancer models. Each showed advantages over traditional 2D cultures in TME replication and towards the exploration of tumor biology at a gene or protein level. Bioprinting technology can also potentially minimize costs as a platform to identify novel cancer therapeutics through high(er)-throughput screening.
Concluding Remarks
We have outlined the latest progress in various bioprinting techniques used to create in vitro cancer models to unveil the pathological mechanisms of cancer and to develop new therapeutic strategies. However, to fully reconstruct the complex TME, detailed biological characterizations of tumor-specific cell populations and phenotypes are needed, including cell–cell and cell–ECM interactions and morphogenesis (see Outstanding Questions). In addition, the immune system is an important part of the interactions between tumor cells and the TME that cannot be ignored. At the same time, immunotherapy for cancer has become popular in recent years, and many immunomodulators, such as programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) antibodies, are gradually approaching tumor treatment. However, research on immunomodulators has many limitations in traditional 2D cell culture methods, and in vivo experiments have the disadvantages of long experimental periods and high costs. As an inclusive model, 3D bioprinting can bring different types of cells together in the same system. It can enable research on immune–tumor interactions as well as the detection of the immune biomarkers and preclinical tests of immunomodulators. If the in vitro tumor models can more closely emulate the in vivo pathophysiology, the efficacy and toxicity of candidate drugs can be more accurately measured and therapeutics more precisely selected. Another critical factor for the applicability of in vitro bioprinted cancer models is the obtention of patient-derived cells and the construction of reliable primary cell-derived cell lines for high(er)-throughput screening. We also anticipate the use of dECM bioinks to take off in modeling true TMEs that contain maximums of their native compositional cues.
Outstanding Questions.
How far are we from achieving true emulation of in vivo pathophysiology in in vitro tumor models using 3D bioprinting?
Can the combination of different 3D bioprinting techniques be adopted to optimize tumor model biofabrication and cancer research?
Can the integration of tumor-on-chip platforms into bioprinted tumor models be revolutionary in emulating the TME?
What would be the impact of standardizing the bioprinting techniques and bioinks in modeling tumors in vitro?
Is it feasible to obtain patient-derived cells to construct reliable primary cell-derived cell lines for the construction of tumor models for high(er)-throughput drug screening?
Further work, such as combining bioprinting technology with microfluidic chips or the development of dynamic materials, is desired to better mimic the dynamic physiological and biochemical characteristics of the TME to construct fully integrated tumor-on-a-chip platforms that involve both volumetric features and dynamics [11]. With the development of these technologies, multiple miniaturized organ systems could be connected through a microfluidic network and the detection of antitumor drugs would be revolutionary via the integration of biosensors to study the real-time interdependent effects on blood flow, pH, and metabolism in each organ [57].
New 3D-bioprinting strategies, such as freeform bioprinting, have been emerging in recent years. This technique extrudes the bioink into support hydrogel baths to act against the influence of gravity and allows the fabrication of discrete patterns in space and constructs with sophisticated architectures [35]. Complex physiological structures, such as blood vessels, bile ducts, mammary ducts, and tumors in some junction areas, can be potentially fabricated using freeform bioprinting [4]. Among the future challenges for bioprinting are the size-matched resolutions necessary to achieve the architectural resemblance of the bioprinted constructs to their native counterparts [58]. Bioprinting resolution is partly determined by the minimum feature size that allows structural fidelity of a bioprinted construct [40]. Generally, precise patterns in cell-laden constructs can be achieved with bioprinting resolutions under 100 μm [40]. Currently, inkjet and extrusion bioprinting have resolutions in the range 100–500 μm, laser-assisted bioprinting has a resolution >100 μm, and SLA/DLP bioprinting has a resolution >50 μm [40]. However, it is necessary to supply in vitro cancer models with a hierarchical network of blood vessels in which the smallest capillaries have a diameter of 5–10 μm [59] and serve as an exchange point for oxygen, nutrients, and waste products. We anticipate that the bioprinting field might overcome this challenge by creatively combining bioprinting technology with other techniques. Our recent work demonstrated that shrinking a 3D-bioprinted hydrogel construct resulted in a reduction of its original dimensions [60]. Through this phenomenon, hydrogel-based 3D-bioprinted vasculature of higher-resolution could be generated. Another example of this would be to incorporate pores of controlled size and growth factors (e.g., VEGF) to promote cellular proliferation, infiltration, and vascularization in in vitro cancer models. Microscale tumor models can potentially be fabricated by multiscale materials in combination with large-area projection microstereolithography, and the addressable spatial light modulator (SLM) technique [61]. Along with the proper bioprinter setup, such as an advanced hardware/software interface to reduce bioprinter platform inaccuracies such as positioning or vibration, bioprinting resolution can be potentially enhanced while also preserving cell viability and minimizing operative costs.
In summary, numerous new strategies are being implemented in the 3D bioprinting and biofabrication of cancer. It is hoped that in the near future they will help us overcome the predicament of cancer.
Highlights.
It has generally been a challenge to uncover the specific interactions within the stroma that induce tumor growth in vivo, and therefore in vitro cell culture systems have been instrumental for studies of the biology and pathology of cancer.
Several distinctions have been demonstrated between in vitro 3D models and 2D platforms used in drug screening studies, including cell morphology, adhesion, mechanics, proliferation, apoptosis, and drug response, among others.
Bioprinting makes it possible to establish 3D structures with precise spatial arrangements of extracellular matrix materials, cells, and/or various biologically active factors, also with reproducibility.
Precisely controlled TMEs can be established by further integrating advanced tumor-on-a-chip models and bioprinting technologies, which can then be used to probe tumor pathological mechanisms and personalized phenotypic drug screening.
Acknowledgments
The authors gratefully acknowledge funding from the National Institutes of Health (K99CA201603, R00CA201603, R21EB025270, R21EB026175, R01EB028143, R01GM134036) and the Brigham Research Institute.
Glossary
- Bioink
solution of a hydrogel biomaterial or a mixture of several hydrogel biomaterials that encapsulate cells and/or other biologically active components that are suitable for processing by bioprinting technology to create tissue-like constructs.
- Bioprinting
3D biofabrication technology by which bioinks are typically processed layer by layer to precisely pattern cell-laden constructs for the construction of complex volumetric living tissues from computer-aided designs.
- Bioprinting resolution
the minimum feature size that allows structural fidelity during bioprinting.
- Cell encapsulation
embedment of cells within biocompatible materials.
- dECM bioink
a bioink devoid of cells yet maintaining the key ECM components, usually obtained through decellularization.
- Hydrogel
a class of 3D networks formed by stabilized polymer chains in a water-rich environment through mechanisms such as physical entanglement, ionic interactions, and/or chemical crosslinking.
- Scaffold
3D ECM-mimicking structures that support cells to attach, proliferate, expand, and/or differentiate.
- Shear thinning
non-Newtonian behavior of fluids whose viscosity decreases under shear stress.
- Tumor microenvironment (TME)
the complex microenvironment featuring hierarchically assembled structures, ECM molecules, and cells within and surrounding a tumorous tissue.
- Tumor on a chip
a system that integrates microfabrication and microfluidics to replicate the cell–cell interactions and extracellular environment of a living tumor.
References
- 1.Hanahan D (2014) Rethinking the war on cancer. Lancet 383, 558–563 [DOI] [PubMed] [Google Scholar]
- 2.Leonard F and Godin B (2016) 3D in vitro model for breast cancer research using magnetic levitation and bioprinting method. Methods Mol. Biol 1406, 239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Ali J et al. (2006) Cells on chips. Nature 442, 403–411 [DOI] [PubMed] [Google Scholar]
- 4.Moroni L et al. (2018) Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater 3, 21–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song HH et al. (2014) Hydrogels to model 3D in vitro microenvironment of tumor vascularization. Adv. Drug Deliv. Rev 79–80, 19–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eduati F et al. (2018) A microfluidics platform for combinatorial drug screening on cancer biopsies. Nat. Commun 9, 2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia SN and Ingber DE (2014) Microfluidic organs-on-chips. Nat. Biotechnol 32, 760–772 [DOI] [PubMed] [Google Scholar]
- 8.Yi HG et al. (2019) A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat. Biomed. Eng 3, 509–519 [DOI] [PubMed] [Google Scholar]
- 9.Wang S et al. (2017) The potential of organoids in urological cancer research. Nat. Rev. Urol 14, 401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sontheimer-Phelps A et al. (2019) Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 19, 65–81 [DOI] [PubMed] [Google Scholar]
- 11.Parrish J et al. (2019) New frontiers for biofabrication and bioreactor design in microphysiological system development. Trends Biotechnol. 37, 1327–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang YS et al. (2017) 3D bioprinting for tissue and organ fabrication. Ann. Biomed. Eng 45, 148–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang YS and Khademhosseini A (2017) Advances in engineering hydrogels. Science 356, eaaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gungor-Ozkerim PS et al. (2018) Bioinks for 3D bioprinting: an overview. Biomater. Sci 6, 915–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groll J et al. (2018) A definition of bioinks and their distinction from biomaterial inks. Biofabrication 11, 013001. [DOI] [PubMed] [Google Scholar]
- 16.Albritton JL and Miller JS (2017) 3D bioprinting: improving in vitro models of metastasis with heterogeneous tumor microenvironments. Dis. Model. Mech 10, 3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy SV and Atala A (2014) 3D bioprinting of tissues and organs. Nat. Biotechnol 32, 773–785 [DOI] [PubMed] [Google Scholar]
- 18.Miri AK et al. (2018) Microfluidics-enabled multimaterial maskless stereolithographic bioprinting. Adv. Mater 30, e1800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butcher DT et al. (2009) A tense situation: forcing tumour progression. Nat. Rev. Cancer 9, 108–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill BJ et al. (2012) A synthetic matrix with independently tunable biochemistry and mechanical properties to study epithelial morphogenesis and EMT in a lung adenocarcinoma model. Cancer Res. 72, 6013–6023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bishop ES et al. (2017) 3-D bioprinting technologies in tissue engineering and regenerative medicine: current and future trends. Genes Dis. 4, 185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W et al. (2017) Extrusion bioprinting of shear-thinning gelatin methacryloyl bioinks. Adv. Healthc. Mater 6, 1601451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ying GL et al. (2018) Aqueous two-phase emulsion bioink-enabled 3D bioprinting of porous hydrogels. Adv. Mater 30, e1805460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinrich MA et al. (2019) 3D bioprinting: from benches to translational applications. Small 15, e1805510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandrycky C et al. (2016) 3D bioprinting for engineering complex tissues. Biotechnol. Adv 34, 422–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen F et al. (2015) New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med. 13, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jammalamadaka U and Tappa K (2018) Recent advances in biomaterials for 3D printing and tissue engineering. J. Funct. Biomater 9, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowlton S et al. (2015) Bioprinting for cancer research. Trends Biotechnol. 33, 504–513 [DOI] [PubMed] [Google Scholar]
- 29.Cui X et al. (2012) Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat. Drug Deliv. Formul 6, 149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldmann T and Gonzalez JS (2000) DNA-printing: utilization of a standard inkjet printer for the transfer of nucleic acids to solid supports. J. Biochem. Biophys. Methods 42, 105–110 [DOI] [PubMed] [Google Scholar]
- 31.Roti JL (2008) Cellular responses to hyperthermia (40–46 degrees C): cell killing and molecular events. Int J. Hyperth 24, 3–15 [DOI] [PubMed] [Google Scholar]
- 32.Mitchell MJ and King MR (2013) Computational and experimental models of cancer cell response to fluid shear stress. Front. Oncol 3, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia Z et al. (2018) Tissue and organ 3D bioprinting. SLAS Technol. 23, 301–314 [DOI] [PubMed] [Google Scholar]
- 34.Liu W et al. (2017) Rapid continuous multimaterial extrusion bioprinting. Adv. Mater 29, 1606630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinton TJ et al. (2015) Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv 1, e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Highley CB et al. (2016) Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol 40, 35–40 [DOI] [PubMed] [Google Scholar]
- 37.Domian IJ et al. (2009) Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science 326, 426–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Bryan CS et al. (2017) Self-assembled micro-organogels for 3D printing silicone structures. Sci. Adv 3, e1602800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grogan SP et al. (2013) Digital micromirror device projection printing system for meniscus tissue engineering. Acta Biomater. 9 7218–7126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miri AK et al. (2019) Effective bioprinting resolution in tissue model fabrication. Lab Chip 19, 2019–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann A et al. (2017) New stereolithographic resin providing functional surfaces for biocompatible three-dimensional printing. J. Tissue Eng 8, 2041731417744485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kacarevic ZP et al. (2018) An introduction to 3D bioprinting: possibilities, challenges and future aspects. Materials (Basel) 11, 2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawecki F et al. (2018) Self-assembled human osseous cell sheets as living biopapers for the laser-assisted bioprinting of human endothelial cells. Biofabrication 10, 035006. [DOI] [PubMed] [Google Scholar]
- 44.Gladman AS et al. (2016) Biomimetic 4D printing. Nat. Mater 15, 413–418 [DOI] [PubMed] [Google Scholar]
- 45.Wang C et al. (2014) Three-dimensional in vitro cancer models: a short review. Biofabrication 6, 022001. [DOI] [PubMed] [Google Scholar]
- 46.Meng F et al. (2019) 3D bioprinted in vitro metastatic models via reconstruction of tumor microenvironments. Adv. Mater 31, e1806899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou X et al. (2016) 3D bioprinting a cell-laden bone matrix for breast cancer metastasis study. ACS Appl. Mater. Interfaces 8, 30017–30026 [DOI] [PubMed] [Google Scholar]
- 48.Burks HE et al. (2016) Laser direct-write onto live tissues: a novel model for studying cancer cell migration. J. Cell. Physiol 231, 2333–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai X et al. (2016) 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication 8, 045005. [DOI] [PubMed] [Google Scholar]
- 50.Ozturk MS et al. (2020) High-resolution tomographic analysis of in vitro 3D glioblastoma tumor model under long-term drug treatment. Sci. Adv 6, eaay7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park TM et al. (2017) Fabrication of in vitro cancer microtissue array on fibroblast-layered nanofibrous membrane by inkjet printing. Int. J. Mol. Sci 18, 2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Kempen LC and Coussens LM (2002) MMP9 potentiates pulmonary metastasis formation. Cancer Cell 2, 251–252 [DOI] [PubMed] [Google Scholar]
- 53.Duchamp M et al. (2019) Sacrificial bioprinting of a mammary ductal carcinoma model. Biotechnol. J 14, e1700703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu T et al. (2020) Investigating lymphangiogenesis in a sacrificially bioprinted volumetric model of breast tumor tissue. Methods Published online April 8, 2020. 10.1016/j.ymeth.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao X et al. (2019) A tumor-on-a-chip system with bioprinted blood and lymphatic vessel pair. Adv. Funct. Mater 29, 1807173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heinrich MA et al. (2019) 3D-bioprinted mini-brain: a glioblastoma model to study cellular interactions and therapeutics. Adv. Mater 31, e1806590. [DOI] [PubMed] [Google Scholar]
- 57.Eisenstein M (2015) Artificial organs: honey, I shrunk the lungs. Nature 519, S16–S18 [DOI] [PubMed] [Google Scholar]
- 58.Mao M et al. (2017) The emerging frontiers and applications of high-resolution 3D printing. Micromachines 8, 113 [Google Scholar]
- 59.Corbett DC et al. (2019) A FRESH take on resolution in 3D bioprinting. Trends Biotechnol. 37, 1153–1155 [DOI] [PubMed] [Google Scholar]
- 60.Gong J et al. (2020) Complexation-induced resolution enhancement of 3D-printed hydrogel constructs. Nat. Commun 11, 1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng X et al. (2016) Multiscale metallic metamaterials. Nat. Mater 15, 1100–1106 [DOI] [PubMed] [Google Scholar]