Abstract
Background
Hyperplastic Polyposis Protein 1 (HPP1) encodes a tumor suppressive transmembrane cleavable Epidermal Growth Factor (EGF)-like ligand. It is unclear as to whether cleavage and shedding of HPP1 are essential steps in achieving its tumor suppressive properties. ADAM proteins are key players in cellular ectodomain shedding processes with ADAM17 being well characterized and representing the most likely sheddase for HPP1. In this study, we explore the mechanisms and importance of ectodomain shedding in contributing to HPP1-mediated tumor suppression.
Methods
Baseline characterization of HPP1 ectodomain shedding and ADAM-family member expression was performed in HCT116 colon cancer cells with forced overexpression of HPP1 and controls. Subsequent impact of attenuation of ADAM expression by siRNA on HPP1 shedding was evaluated. Furthermore, we examined the functional impact of an uncleavable HPP1 mutant construct (HPP1-ΔStalk) generated by site-directed mutagenesis. Cellular growth potential functions were analyzed by MTT and soft agar assays.
Results
Select pro-inflammatory cytokines enhanced HPP1 ectodomain shedding while siRNA-mediated knockdown of ADAM17 resulted in abrogation of HPP1 ectodomain shedding. ADAM17 knockdown concomitantly resulted in increased cell proliferation and anchorage-independent growth. HPP1-ΔStalk-transfected cells exhibited significantly higher proliferation and reduced STAT1 activation relative to full-length HPP1 further suggesting a critical role for ectodomain shedding in HPP1-mediated tumor suppression.
Conclusions
The tumor suppressive properties of HPP1 in colorectal cancer require cleavage and shedding of its ectodomain which in turn, are mediated by ADAM17. Further investigations into the regulation of HPP1 may lead to a greater understanding of EGF-like ligand family biology and potential novel therapeutic strategies.
Keywords: Ectodomain shedding, EGF-like ligand, TMEFF2, Colorectal Cancer
Introduction
Hyperplastic polyposis protein-1 (HPP1; aka TMEFF2, TPEF) functions as a tumor suppressor that commonly undergoes epigenetic silencing in gastrointestinal cancers including those of the colon, rectum, stomach, esophagus, and gallbladder [1–3]. We have previously described HPP1’s tumor suppressive function, pro-apoptotic activities and contributory signaling pathways in both in vitro and in vivo experimental models of colon cancer [1]. Structurally, the HPP1 gene encodes a cleavable transmembrane protein with an EGF-like domain, two follistatin modules, and a cytosolic tail with a potential G-protein activating motif [4].
HPP1 is prominently expressed in normal human prostate tissue as well as in androgen-dependent prostate cancer. Paradoxically, in the latter, HPP1 appears to have oncogenic effects [5, 6]. HPP1 expression correlates with the onset of cellular proliferation following castration in prostate cancer xenograft models while silencing of HPP1 by a monoclonal antibody results in inhibition of prostate tumor growth in vivo [7, 8].
Based on the structure of HPP1, it has been postulated that its soluble ectodomain undergoes cleavage and functions as an EGF-like ligand. In gastric cancer cells, cleaved HPP1 ectodomain was found to activate the erbB-4 receptor [9]. Other known EGF-like ligands that bind to various EGF receptors include TGF-α, amphiregulin, heparin-binding EGF, betacellulin, and neureglin [10, 11]. It is well recognized that the physiologic responses elicitied by specific EGF-like ligands may vary based on differential expression and activation of associated erbB family receptors [12, 13]. Mechanistically, EGF-like ligands commonly undergo shedding events (i.e. cleavage from the cell surface) that are mediated by metalloproteinases; most commonly ADAM (a disintegrin and metalloproteinase) family members [14]. Notably, ADAM17, also known as tumor necrosis factor alpha converting enzyme (TACE) has recently been described as a mediator responsible for HPP1 shedding events in prostate cancer cells [6]. Neither the mechanism nor functional effects of HPP1 ectodomain shedding have been examined in colon cancer, particularly in the context of growth inhibition. Our overarching hypothesis is that the tumor suppressive properties of HPP1 require cleavage and shedding of its ectodomain. We thus sought to evaluate the importance and mechanims of HPP1 ectdomain shedding and to confirm its critical role in mediating its tumor suppressive function in a colon cancer.
Materials and Methods
Cell Culture and Transfection
Cells were cultured in RPMI 1640 (Invitrogen, Grand Island, NY) and supplemented with heat-inactivated 10% fetal bovine serum (Invitrogen) at 37°C in a humidified incubator containing 5% CO2. HCT116 cell lines natively demonstrated downregulation of HPP1. Stably-transfected full length HPP1- overexpressing HCT116 cells (HCT116-HPP1) and HCT116 cells were used for analysis as previously described [1]. For short-interfering RNA (siRNA) transfection, 2.5×105 cells were seeded into 6-well plates, incubated overnight and then transfected with control or targeted (ADAM 17, 19, Dharmacon RNA Technologies, Lafayette, CO) siRNAs using Lipofectamine 2000 (Invitrogen) at a final concentration of 100 nM according to the manufacturer’s instructions. Plasmid DNA transient transfections were also undertaken. For plasmid DNA, we have used both lipofectamine methodology from Life Technologies according to manufacturer’s protocol and polyethylenimine (PEI) transfection reagent with NaCl solution following in house protocol. For Plasmid transfection, 4–5 μg/well (6-well plates) of plasmid DNA was used for the transfection purposes. siRNA experiments were conducted according to the manufacturer’s protocol (Dharmacon).
Site directed mutagenesis
Using the QuickChange Lightning Site-Directed Mutagenesis kit (Agilent Technologies, La-Jolla, CA), we performed site-directed mutagenesis to alter a cloned target gene (HPP1) by the deletion of 54 bases (amino acid residues 303–320). We employed PCR-based overlap extension mutagenesis with the following primers:
Forward: 5’ACTGTGAAAAATTAATCGCAGC TGTGATTGGAACAATTCAGA-3’
Reverse: 5’-CAGCTGCGATTAATTTTTCA CAGTGTTGTCCAGTATAACCAG-3’.
Mutant deletion standard synthesis, Dpn1 digestion and transformation were performed following the manufacturer’s protocol. Plasmid DNA from 12 colonies was sent for sequencing to confirm that the HPP1 deletion construct lacked the juxtamembrane stalk sequence.
HPP1 Ectodomain Sheddase Analysis by Western Blot
Cells were seeded in 6-well plates and grown to 60% confluency at which time serum free media was added for 24 hours. Supernatants were recovered as conditioned media (CM) which were then treated with 1uM pepstatin and 1mM of phenylmethyl sulfonyl fluoride (PMSF) and concentrated approximately 80-fold using an Amicon concentrator (MilliporeSigma, Burlington, MA) and denatured in SDS sample buffer. Cell lysates were prepared in parallel. Cells were washed and lysed as previously described [6]. Protein concentrations of both the conditioned media and cell lysates were determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA). Aliquots of concentrated CM (75 μg/lane) were subjected to western blot analysis. A commercially-available HPP1 antibody was used to recognize the cleaved extracellular HPP1 domain as described above (R&D Systems, Minneapolis, MN).
The shedding of ADAM17 substrate can be stimulated by phorbol esters such as Phorbol 12-myristate 13-acetate (PMA) and Tumor Necrosis Factor - alpha (TNF-α)[15]. To investigate the effects of inducible HPP1 shedding by the pro-inflammatory cytokines TNF-α (100 ng/mL) (PeproTech EC LTD, UK) and PMA (100ng/mL) (R&D Systems), the cells were prepared as described above. TNF-α or PMA was added at 4, 8, and 12 hours and the conditioned media was collected and subjected to western blot analysis as described above. Also, the pan-metalloprotease inhibitor GM6001 (MilliporeSigma) at a concentration of 50 μM was used to investigate effects on shedding in the presence of pro-inflammatory cytokines. GM6001 was added 6 hours after seeding and incubated for 14 hours prior to collection with CM, DMSO was used in the control samples.
Cellular Proliferation Assays
1 × 103 cells/well were seeded in 10 wells per sample in 96 well plates. Cells were incubated for 72 hours and cell proliferation was assayed using the XTT Cell Proliferation Kit II (Roche, Mannheim, Germany) by measuring the spectrophotometrical absorbance of the samples using an ELISA reader.
Growth in soft agar
A bottom layer of 3% Bacto-agar (BD Biosciences, San Jose, CA) stock was made and sterilized. At 48°C, growth medium was mixed with 3% soft agar to make 0.6% and 2 ml were plated in each well of a 12-well tissue culture plate and solidified. 5 × 103 cells per well were suspended in agar medium. 1.5 ml of cell-agar mixture (0.3%) was dispensed on top of the base layer and was incubated for 3 weeks. Subsequent colony formation was quantitated.
Statistical Analysis
Comparisons were analyzed using 2-sided Student’s t-test with p < 0.05 being considered significant.
Results
HPP1 Ectodomain is Constitutively Shed from HCT116-HPP1 Transfectants and can be Augmented by TNF-α and PMA
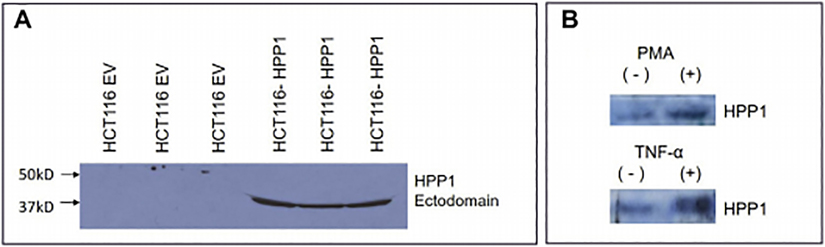
In an unstimulated baseline state, we were able to consistently detect expression of shed HPP1 ectodomain in conditioned media, confirming that HPP1 does undergo shedding similar to other EGF-like ligands.. (Figure 1a). Similar to what has been described in other cancer cell systems (e.g. prostate, glioma), HPP1 shedding from colon cancer cells into conditioned media was measured [6, 16]. Treating HCT116-HPP1 transfectants with inflammatory cytokines (TNF-α and PMA) resulted in a significant increase in HPP1 ectodomain shedding (Figure 1b).
Figure 1.
A) HPP1 ectodomain shedding detected at baseline. B) HPP1 shedding into conditioned media is induced by TNF-α and PMA.
ADAM-17 is the sheddase responsible for HPP1-ectodomain shedding in HPP1 cells
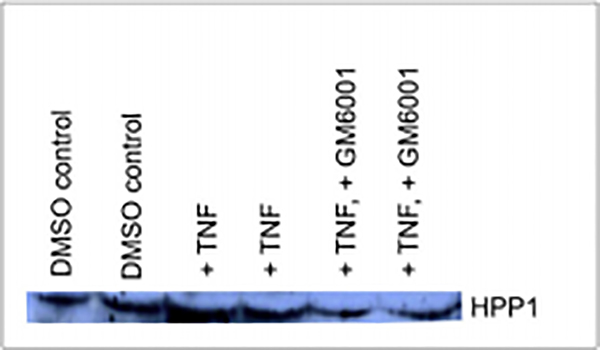
To investigate the potential involvement of a metalloproteinase in HPP1 ectodomain shedding, we treated our HCT116-HPP1 transfectants with GM6001 (a pan-metalloproteinase inhibitor). Even in the presence of pro-inflammatory cytokines, shedding was decreased relative to controls, suggesting that metalloproteinase activity is necessary for HPP1 shedding events in colon cancer cells (Figure 2).
Figure 2.
HPP1 Shedding in Conditioned Media is Induced by TNF-α and subsequently inhibited with addition of GM6001 (Pan-Metalloprotease Inhibitor).
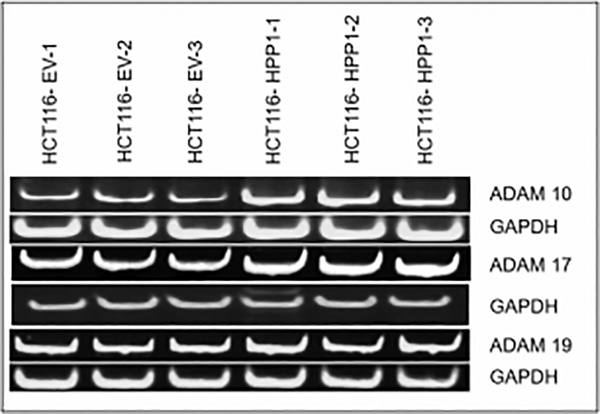
To further elucidate the sheddase(s) responsible for HPP1 cleavage, we investigated expression of different ADAM family members in both HCT116 and HCT116-HPP1 expressing cells. RT-PCR analysis confirmed expression of ADAMs 10, 17, and 19 in both cell lines. HCT116-HPP1 overexpressing cells demonstrated increased expression of ADAMs 10 and 17 but not ADAM19 relative to empty vector controls. (Figure 3).
Figure 3.
RT-PCR Assay Confirms higher expression of Adam 10,17 and 19 in HPP1 overexpressing cells (HCT116 EV-HPP1) as compared to HCT116 EV.
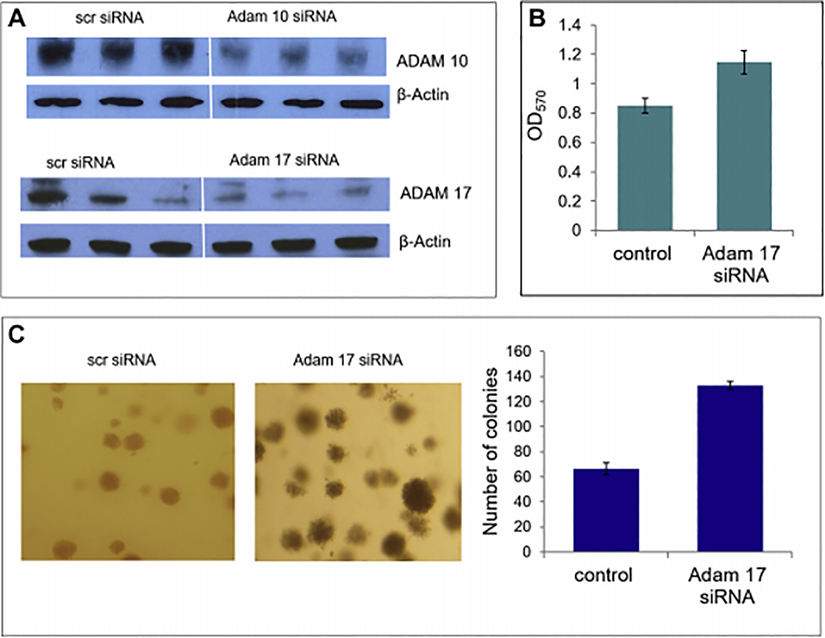
Following confirmation of expression of candidate sheddases (ADAM10, 17, 19), we sought to determine which of the ADAM family members was necessary for HPP1 gene function and shedding. Given that ADAMs 10 and 17 were upregulated in HPP1 overexpressing cells, we specifically targeted those candidate sheddases for silencing using siRNA in HCT116-HPP1 cells. Successful knockdown of both ADAM10 and ADAM17 was achieved and conditions were optimized (Figure 4a). HPP1 ectodomain shedding was decreased in the ADAM17 siRNA treated HCT116-HPP1 transfectants). Our findings implicate ADAM17 as the key sheddase responsible for HPP1’s ectodomain cleavage and was the focus of subsequent experiments (Figure 4b).
Figure 4.
A) Successful knockdown of both ADAM 10 and ADAM 17 were achieved at a concentration of 100nm using siRNA. B) MTT assay confirms a significant increase in cellular proliferative capacity with ADAM17 siRNA treated HCT116-HPP1 cells. C) ADAM17 siRNA treated cells also showed a significant increase in colony formation in soft agar.
ADAM-17 Mediated Cleavage is required for HPP1 Associated Tumor Suppression
To further investigate the role of ADAM17 in HPP1-associated tumor suppression, we investigated the functional consequences of siRNA-mediated abrogation of ADAM17 in HCT116-HPP1 cells. Cell proliferative capacity, as measured by MTT assay, was significantly increased in the ADAM17 knockdown cells (p=0.002 vs control). Anchorage-independent growth capacity in ADAM17 siRNA-treated cells was analyzed using soft agar assay. ADAM17 abrogation resulted in a significant increase in anchorage-independent growth capacity relative to controls (p=0.046), suggesting that HPP1’s tumor suppressive effects require ADAM17 (Figure 4c).
HPP1’s Stalk Sequence is Required for Ectodomain Shedding and Tumor Suppression
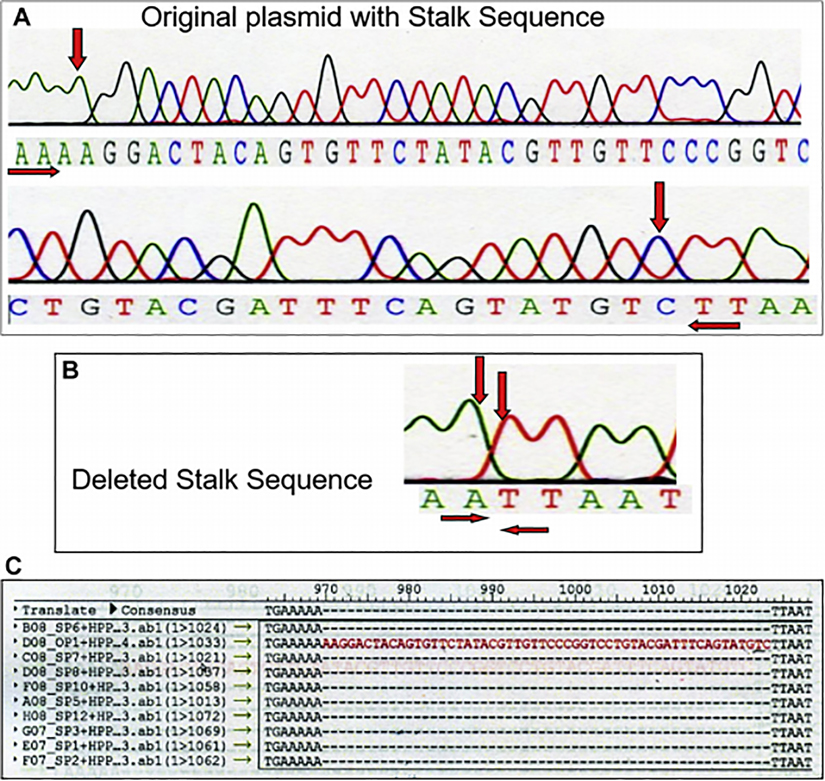
To further ascertain the importance of HPP1 cleavage and shedding, a deletion construct lacking HPP1’s juxtamembrane stalk sequence (HPP1-Δ stalk) was created. The juxtamembrane sequence is the cleavage site necessary for shedding. PCR-based overlap extension mutagenesis was successfully undertaken and confirmed with sequence analysis (Figure 5).
Figure 5.
A) Sequence analysis demonstrates the presence of juxtamembrane stalk sequence marked with red arrows. B) Sequence analysis confirms successful deletion of juxtamembrane stalk sequence. C) Stalk plasmid (SP) showing absence of juxtamembrane stalk sequence as compared to original plasmid (OP) shown in red.
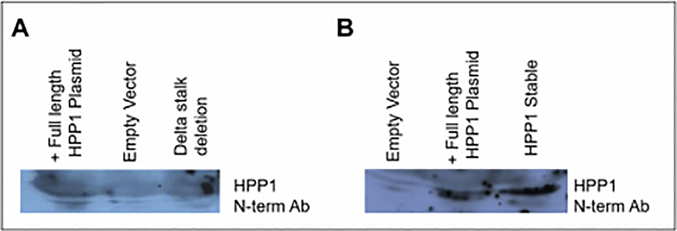
Transient PEI-based transfections were used to insert a plasmid containing either full length HPP1, HPP1-Δ stalk, or an empty vector into HCT116 cells. Cell lysates were analyzed by western blot to confirm successful transfection of both the HPP1 and HPP1-Δ stalk genes into HCT116 cells. After confirmation of successful transfection, media was collected and concentrated after a 24-hour incubation period and analyzed by western blotting for the HPP1 ectodomain. The HPP1 ectodomain was detected in media from cells transfected with the full-length HPP1 vector but was not detected in media from cells transfected with HPP1-Δ stalk or control vectors (Figure 6a). As an additional control, conditioned media from transiently-transfected HCT116-HPP1 cells were analyzed in parallel with stable HPPl-transfected HCT116 cells. Both HPPl-overexpressing transfectants demonstrated robust shedding of the HPP1 ectodomain into media (Figure 6b).
Figure 6.
A) HPP1 ectodomain was detected in the conditioned media of cells transiently transfected with full length HPP1 plasmid. The Δstalk mutant had very little detectable ectodomain in the media, which was similar to control B) Both full length HPP1 plasmid containing HCT116 and stable HPP1 overexpressing transfectants had robust HPP1 ectodomain shed into the media.
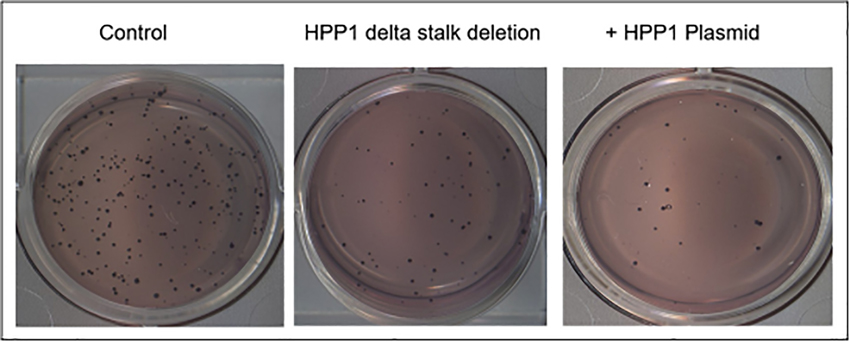
The HPP1-Δ stalk transfectants were then subjected to functional analysis including MTT assay and growth in soft agar. The HPP1-Δ stalk mutants exhibited an intermediate ability for growth in soft agar. The mutants formed a significantly higher number of colonies relative to HPP1-overexpressing cells (p<0.001) and similarly demonstrated higher capacity for cellular proliferation relative to control (p<0.001) implying a role for shedding in tumor suppression (Figure 7).
Figure 7.
The HPP1 Δ stalk mutants exhibited a significantly higher capacity for anchorage independent growth relative to cells transfected with full length HPP1. This growth advantage did not match that seen in the control cells, however.
STAT signaling in HPP1-Δ Stalk Mutants
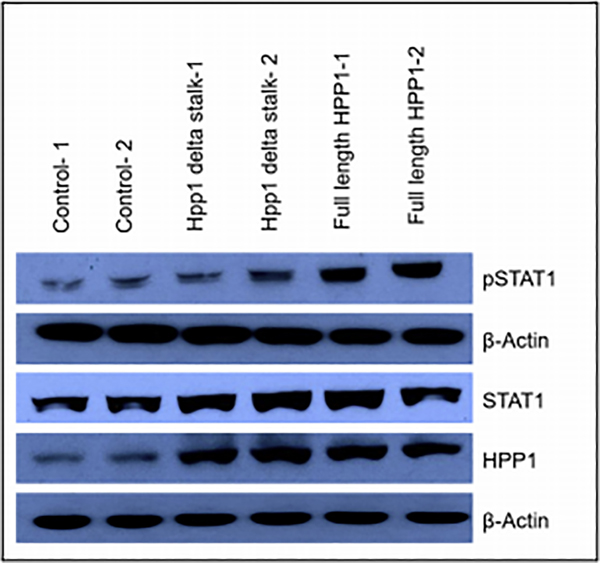
To investigate whether HPP1-associated tumor suppression is associated with activation of STAT1 signaling via ectodomain shedding, we examined total STAT1 and phosphorylated STAT1 (activated; pSTAT1) expression in the cells transfected with full-length HPP1, HPP1-Δ stalk mutation, and empty vector controls. The cells transfected with HPP1-Δ stalk mutation demonstrated an intermediate level of pSTAT1 expression. Total HPP1 and STAT1 protein expression were similar in the HPP1-treated cells and the HPP1-Δ stalk mutants. Also as expected, the control group had lower expression of HPP1, STAT1 and pSTAT1 (Figure 8). This level of expression parallels what was observed in the functional experiments and suggests that HPP1 signaling, via ectodomain shedding acts via STAT1 pathways.
Figure 8.
Transfected cells with HPP1- Δ stalk mutation demonstrated an intermediate level of pSTAT1 expression and a similar Total HPP1 and STAT1 protein expression when compared to full length HPP1-transfeted cells and empty vector controls.
Discussion
Although HPP1 may harbor tissue-specific biologic properties, there is strong evidence that HPP1 functions as a tumor suppressor gene in colon cancer [1, 2, 4]. We have elucidated novel information regarding the mechanisms of HPP1-associated tumor suppression in colon cancer. Specifically, we have confirmed that HPP1-overexpressing colon cancer cells demonstrate shedding of a soluble HPP1 ectodomain and have identified ADAM17 as the key responsible sheddase. We have further shown that HPP1’s tumor suppressive effects require shedding of this EGF-like ectodomain which in turn, is the responsible moiety that activates the growth-inhibiting STAT1 signaling axis.
As noted, HPP1 has a paradoxical oncogenic function and in prostate cancer, Ali et al. have hypothesized that ADAM17-mediated HPP1 ectodomain shedding events induce Erk1/2 phosphorylation via EGFR activation [6]. Given the similar processing mechanisms of HPP1 between disease types, a possible explanation for this biologic discrepancy may lie in the differential expression of and affinity of HPP1 for erbB family receptors.
Among EGF-like ligands, HPP1 has the most phylogenetic similarity to betacellulin which predominantly binds erbB1 and erbB4 [17] but does have an extended ability to bind all heterodimeric erbB receptor combinations [18]. In one of the earliest structure-function descriptions of HPP1, Uchida et al. indeed noted in gastric cancer cells that the soluble ectodomain functioned as a ligand for ErbB4 receptors [9]. Indeed, we have previously observed that forced expression of HPP1 results in activation of erbB4 in colon cancer cells. (data not shown). ErbB4’s role and mechanisms of action in carcinogenesis remains incompletely understood however, it functions as a tumor suppressor in a number of model systems [19, 20]. ErbB4 is the only erbB-family receptor for which expression is selectively lost in a number of aggressive tumor subtypes [21]. Moreover, ErbB4 expression is associated with a more favorable prognosis in a number of different cancers [19]. Notably, prostate cancer cell lines tend to lack expression of erbB4 [22], which may favor the binding of HPP1 to EGFR leading to the observed tissue-specific growth-promoting effects.
Chen et al. postulated that the HPP1 protein may have dual functionality (both oncogenic and tumor suppressive effects in the same tissue) with the full-length protein contributing to its tumor suppressive function and the ectodomain contributing to increased cellular proliferation [23]. Of note, their experiments were done in the embryonic kidney cell line, HEK293 and erbB receptor family expression and activation profiles were not examined or described for these cells. In our colon cancer cell line models, we have observed that cleavage and shedding of the soluble ectodomain is important for the full biological function of HPP1. In our HPP1-Δ stalk mutation experiments, we have observed that deletion of the HPP1 cleavage site resulted in an intermediate degree of tumor suppressive function and STAT1 activation between wild-type HPP1 and control. We did not observe any acquisition of growth promoting effects in the setting of HPP1 cleavage inhibition. However, the retention of minimal tumor suppressive function suggests that HPP1 may have a juxtacrine component to its signaling. Alternatively, HPP1 may be associated with other contributing mechanisms of action. Among such mechanisms may include the process of regulated intramembrane proteolysis (RIP) as has been described with other EGF-like ligands such as Notch, HB-EGF and neuregulin. For example, Notch undergoes proximal membrane shedding that leads to a second cleavage in the transmembrane portion allowing release of the cytoplasmic domain from the membrane. This fragment is able to enter the nucleus and contribute to transcriptional regulation [24]. This has yet to be described in HPP1 but may warrant additional investigation.
In summary, HPP1 functions as a tumor suppressor in colon cancer with its biologic effects requiring ADAM17-mediated cleavage and shedding of its soluble ectodomain. We have previously demonstrated that the effects of full-length wild-type HPP1 are associated with activation of a proposed erbB4-JAK-STAT signaling pathway and herein, have confirmed that the soluble ectodomain engages this same pathway. Further elucidation of HPP1’s mechanisms of action may have implications for a greater understanding of EGF-like ligand biology and for potential novel therapeutic strategies.
Synopsis.
HPP1 is a putative tumor suppressor gene that has generated interest as a serum- and stool-based biomarker for colorectal cancer detection. Elucidation of its role and mechanisms of regulation may have implications for a greater understanding of EGF-like ligand biology and for potential novel therapeutic strategies.
Highlights –ADAM 17 Manuscript.
HPP1 encodes a transmembrane protein and it is commonly methylated in colorectal polyps and cancer.
HPP1 tumor suppressive effects in colorectal cancer require cleavage and ectodomain shedding.
ADAM17 mediates HPP1’s cleavage and ectodomain shedding.
Acknowledgements
This work was supported by National Institutes of Health (NIH) grant number R01 CA131398 (DS).
Abbreviations
- HPP1
Hyperplastic Polyposis Protein 1
- EGF
Epidermal Growth Factor
- CRC
Colorectal cancer
- TACE
Tumor necrosis factor alpha converting enzyme
- CM
Conditioned media
- PMSF
Phenylmethyl Sulfonyl Fluoride
Footnotes
Declarations of Interests
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Elahi A, et al. , HPP1-mediated tumor suppression requires activation of STAT1 pathways. Int J Cancer, 2008. 122(7): p. 1567–72. [DOI] [PubMed] [Google Scholar]
- 2.Ebert MP, et al. , Hypermethylation of the TPEF/HPP1 gene in primary and metastatic colorectal cancers. Neoplasia, 2005. 7(8): p. 771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki M, et al. , DNA methylation-associated inactivation of TGFbeta-related genes DRM/Gremlin, RUNX3, and HPP1 in human cancers. Br J Cancer, 2005. 93(9): p. 1029–37. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Young J, et al. , HPP1: a transmembrane protein-encoding gene commonly methylated in colorectal polyps and cancers. Proc Natl Acad Sci U S A, 2001. 98(1): p. 265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gery S, et al. , TMEFF2 is an androgen-regulated gene exhibiting antiproliferative effects in prostate cancer cells. Oncogene, 2002. 21(31): p. 4739–46. [DOI] [PubMed] [Google Scholar]
- 6.Ali N and Knauper V, Phorbol ester-induced shedding of the prostate cancer marker transmembrane protein with epidermal growth factor and two follistatin motifs 2 is mediated by the disintegrin and metalloproteinase-17. J Biol Chem, 2007. 282(52): p. 37378–88. [DOI] [PubMed] [Google Scholar]
- 7.Afar DE, et al. , Preclinical validation of anti-TMEFF2-auristatin E-conjugated antibodies in the treatment of prostate cancer. Mol Cancer Ther, 2004. 3(8): p. 921–32. [PubMed] [Google Scholar]
- 8.Glynne-Jones E, et al. , TENB2, a proteoglycan identified in prostate cancer that is associated with disease progression and androgen independence. Int J Cancer, 2001. 94(2): p. 178–84. [DOI] [PubMed] [Google Scholar]
- 9.Uchida T, et al. , A novel epidermal growth factor-like molecule containing two follistatin modules stimulates tyrosine phosphorylation of erbB-4 in MKN28 gastric cancer cells. Biochem Biophys Res Commun, 1999. 266(2): p. 593–602. [DOI] [PubMed] [Google Scholar]
- 10.Singh B, Carpenter G, and Coffey RJ, EGF receptor ligands: recent advances. F1000Res, 2016. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macdonald-Obermann JL and Pike LJ , Different epidermal growth factor (EGF) receptor ligands show distinct kinetics and biased or partial agonism for homodimer and heterodimer formation. J Biol Chem, 2014. 289(38): p. 26178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hubbard SR and Miller WT, Receptor tyrosine kinases: mechanisms of activation and signaling. Curr Opin Cell Biol, 2007. 19(2): p. 117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amit I, Wides R, and Yarden Y, Evolvable signaling networks of receptor tyrosine kinases: relevance of robustness to malignancy and to cancer therapy. Mol Syst Biol, 2007. 3: p. 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blobel CP, ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol, 2005. 6(1): p. 32–43. [DOI] [PubMed] [Google Scholar]
- 15.Arribas J, et al. , Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J Biol Chem, 1996. 271(19): p. 11376–82. [DOI] [PubMed] [Google Scholar]
- 16.Lin H, et al. , Tomoregulin ectodomain shedding by proinflammatory cytokines. Life Sci, 2003. 73(13): p. 1617–27. [DOI] [PubMed] [Google Scholar]
- 17.Riese DJ, 2nd,, et al. , Betacellulin activates the epidermal growth factor receptor and erbB-4, and induces cellular response patterns distinct from those stimulated by epidermal growth factor or neuregulin-beta. Oncogene, 1996. 12(2): p. 345–53. [PubMed] [Google Scholar]
- 18.Dunbar AJ and Goddard C, Structure-function and biological role of betacellulin. Int J Biochem Cell Biol, 2000. 32(8): p. 805–15. [DOI] [PubMed] [Google Scholar]
- 19.Mill CP, Gettinger KL, and Riese DJ, 2nd, Ligand stimulation of ErbB4 and a constitutively-active ErbB4 mutant result in different biological responses in human pancreatic tumor cell lines. Exp Cell Res, 2011. 317(4): p. 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tvorogov D, et al. , Somatic mutations of ErbB4: selective loss-of-function phenotype affecting signal transduction pathways in cancer. J Biol Chem, 2009. 284(9): p. 5582–91. [DOI] [PubMed] [Google Scholar]
- 21.Mill CP, et al. , ErbB2 Is Necessary for ErbB4 Ligands to Stimulate Oncogenic Activities in Models of Human Breast Cancer. Genes Cancer, 2011. 2(8): p. 792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koumakpayi IH, et al. , Expression and nuclear localization of ErbB3 in prostate cancer. Clin Cancer Res, 2006. 12(9): p. 2730–7. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, et al. , The tumor suppressor activity of the transmembrane protein with epidermal growth factor and two follistatin motifs 2 (TMEFF2) correlates with its ability to modulate sarcosine levels. J Biol Chem, 2011. 286(18): p. 16091–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartmann D, et al. , The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum Mol Genet, 2002. 11(21): p. 2615–24. [DOI] [PubMed] [Google Scholar]