Abstract
OBJECTIVE
Individuals with obesity suffer from an increased susceptibility to severe respiratory viral infections and respond poorly to vaccinations, making it imperative to identify interventions. Recent evidence suggesting that obesity leads to tissue-specific vitamin A deficiency (VAD) led us to investigate whether high-dose oral vitamin A, a strategy used for remediating VAD in developing countries, could correct obesity-associated tissue deficits.
METHODS
Adult, C57BL/6, diet-induced obese (DIO) mice were supplemented with vitamin A for four weeks. A subset of mice were then vaccinated with inactivated influenza and challenged. Following supplementation, tissue vitamin A levels, lung immune cell composition, blood inflammatory cytokines, antibody responses and viral clearance were evaluated.
RESULTS
Supplementation significantly improved vitamin A levels in lung and adipose tissues in DIO mice. Additionally, supplementation decreased inflammatory cytokines in the blood and altered the lung immune environment. Importantly, vaccinated, vitamin A-treated DIO mice exhibited improved antibody responses and significantly reduced viral loads post-challenge compared to PBS-treated mice.
CONCLUSIONS
Results demonstrate a low-cost intervention that may correct vitamin A tissue deficits and help control respiratory viral infections in individuals with obesity.
Keywords: Immunology, infections, inflammation, vitamins, vaccines
INTRODUCTION
Today, obesity affects approximately 13% of individuals worldwide and more than 1 in 3 adults in the United States. Obesity is associated with poor immune responses toward vaccines and pathogens, including influenza virus and tetanus (1), and a heightened risk for complications due to infectious disease (2, 3). In particular, following influenza virus infection individuals with obesity exhibit high virus loads and prolonged virus shedding, and are more vulnerable to hospitalization, ICU admission, and mortality (4, 5, 6).
The diets of individuals with obesity tend to favor high calorie, nutrient-poor foods, and thus vitamin deficiencies are common in this population. Recently, Trasino et. al. (7) demonstrated that obese mice fail to properly store vitamin A, resulting in tissue deficiency, despite elevated levels of vitamins in the blood. It is likely that these deficiencies are also present in humans, as non-alcoholic fatty acid disease, which alters vitamin A storage and metabolism (8), is common in individuals with obesity. The extent of tissue deficiency in individuals with obesity is unknown, because vitamin A status is routinely only measured using serum assays or is not assessed at all.
The interplay between malnutrition and immunity has been well established in the literature, particularly in the context of undernutrition. Notably, vitamin A is an essential micronutrient that regulates antibody production, immune cell (T, B and innate cell) residence, and the integrity of mucosal epithelial cells (9, 10, 11). Weaknesses in immune responses and natural barriers result in an increased vulnerability of vitamin A deficient (VAD) populations to infectious diseases (12, 13, 14). We and others have shown that vitamin A supplements benefit individuals with VAD or vitamin A insufficiencies to improve immune responses and protect against infectious diseases (14, 15, 16). Based on these findings, we asked if high-dose oral vitamin A supplementation could be beneficial in the context of obesity. Here we show that supplementation corrected vitamin A tissue deficits in obese mice, improved responses to influenza vaccination and protected against infection following challenge in vaccinated mice.
MATERIALS AND METHODS
Mice
Animal experiments followed AAALAC guidelines and were in accordance with Institutional Animal Care and Use Committee (IACUC) protocols. Three-week-old male C57BL/6 (B6) mice (Jackson Laboratories, Bar harbor, ME) were fed ad libitum a 60% high fat diet (cat #58Y1, LabDiets) for 15–19 weeks before experimentation, at which time mice weighed ~45–55 g. Age-matched B6 mice fed a standard rodent diet (cat #5001, LabDiets) were lean controls. Euthanasia was by CO2 inhalation and cervical dislocation.
Vaccination and Vitamin A Supplementation
Mice received vitamin A supplements on days (d.) 0,3,7,21,24, and 28 by oral gavage with 100 µL containing 600 IU vitamin A (retinyl palmitate; Nutrisorb A, Interplexus Inc.). Mice were vaccinated intramuscularly on d.0 and d.21 with a sucrose purified, betapropiolactone (BPL)-inactivated A/California/04/2009 pdmH1N1(CA09) H1N1 virus (1.2 μg hemagglutinin [HA], 50 µL PBS). Control mice received PBS, vitamin A, or vaccine only.
Vitamin A measurements in tissues
DIO and lean mice were euthanized on d.35 for blood collection by cardiac puncture. Liver, lung and white adipose tissues were frozen on dry ice. Serum retinol was quantified as described previously (16).
Virus challenge and lung titers
Two weeks following the second dose of vaccine, mice were anesthetized with isoflurane followed by intranasal inoculations (30 μL) with egg-grown A/California/04/2009 pdmH1N1 (106 TCID50 on MDCK cells). Mice were sacrificed on d.3. Lungs were homogenized in 2 ml PBS and serially diluted in DMEM/0.1% BSA + 1 µg/ml acetylated trypsin. Dilutions were plated on MDCK cells in duplicate. Plates were incubated at 37°C for 4 days. 50 µL from each well were mixed with 50 µL 5% turkey red blood cells for 30 min at room temperature (RT) to test hemagglutination. TCID50 values were calculated using the Reed-Muench formula.
Enzyme linked immunosorbent assays (ELISAs)
Sera were collected 10–14 days after the second dose of vaccine and tested for influenza-specific IgG and IgM. Plates were coated with 5 µg/mL of sucrose-purified pdmH1N1virus (overnight, 4°C), washed 3x with PBS and blocked with 1% BSA in PBS overnight at 4°C. Sera were diluted 1:100, 1:500 and 1:2500 in dilution buffer (PBS +1%BSA +0.05% Tween), and added to plates (1 hour, RT). Plates were washed 4x with PBS +0.05% Tween. Anti-IgG or anti-IgM (cat #1030-04 and 1020-04, Southern Biotech, 1:1000) were added (1 hour, RT). Plates were washed 4x and developed with pNPP (1mg/mL in diethanolamine buffer; cat #20-106, Sigma Aldrich), and read at 405 nm (VersaMax microplate reader).
Cytokine assays
Unvaccinated animals were euthanized and blood was collected by cardiac puncture one week following administration of the last dose of vitamin A. Thirty-two cytokines/chemokines were measured using a Milliplex MAP Kit (cat #: MCYTOMA-70K-PX32, Millipore). Samples (diluted 1:2 in PBS) were evaluated using a Luminex 200 Multiplexing Instrument and xPonent software.
Flow Cytometry
Lungs were collected (d.35) into gentleMACS C Tubes (cat #130-093-237, Miltenyi Biotech) and were processed with a Mouse Lung Dissociation Kit (cat #130-095-927, Miltenyi). Cells were suspended (70 μM strainer) and pelleted and red blood cells were lysed (red cell lysis buffer, cat #07850, Stem Cell Technologies). Cells were counted (Biorad TC20 automated counter), stained, and analyzed using an LSRFortessa X-20 (BD Biosciences) and FCS Express Software. All cells were stained with 7AAD (cat #A1310, Invitrogen) for live gating. Additional stains from Biolegend included: anti-CD3 (cat #100349), anti-CD4 (cat #100443), anti-CD8 (cat #100730), anti-CD45 (cat #103112), anti-F4/80 (cat #123133), anti-CD11b (cat #101206), anti-CD11c (cat #117301), anti-SiglecF (cat #155506).
Statistics
Analyses were performed with GraphPad Prism. Data were evaluated for normality and equality of variance. Unpaired T or Mann Whitney U tests and two-way ANOVA were performed as appropriate.
RESULTS AND DISCUSSION
Vitamin A supplements correct deficits in lung, liver and adipose tissues in animals with obesity
To test the corrective potential of vitamin A supplements, we administered 600 IU vitamin A or PBS orally to obese and control mice on d.0, 3, 7, 21, 24, and 28. Vitamin A levels, measured in the form of retinol, were evaluated on d.35 (Figure 1). Similar to previous reports (7), we observed elevated levels of retinol in the sera and reduced levels in the tissues of obese mice compared to lean controls. Vitamin A supplementation significantly improved vitamin A levels in the lung and adipose tissues, but not in the sera or liver, of obese mice. Importantly, vitamin A levels in the lungs of supplemented DIO mice were closely matched to those of lean controls.
Figure 1. High-dose vitamin A supplementation improves retinol levels in tissues of obese mice.
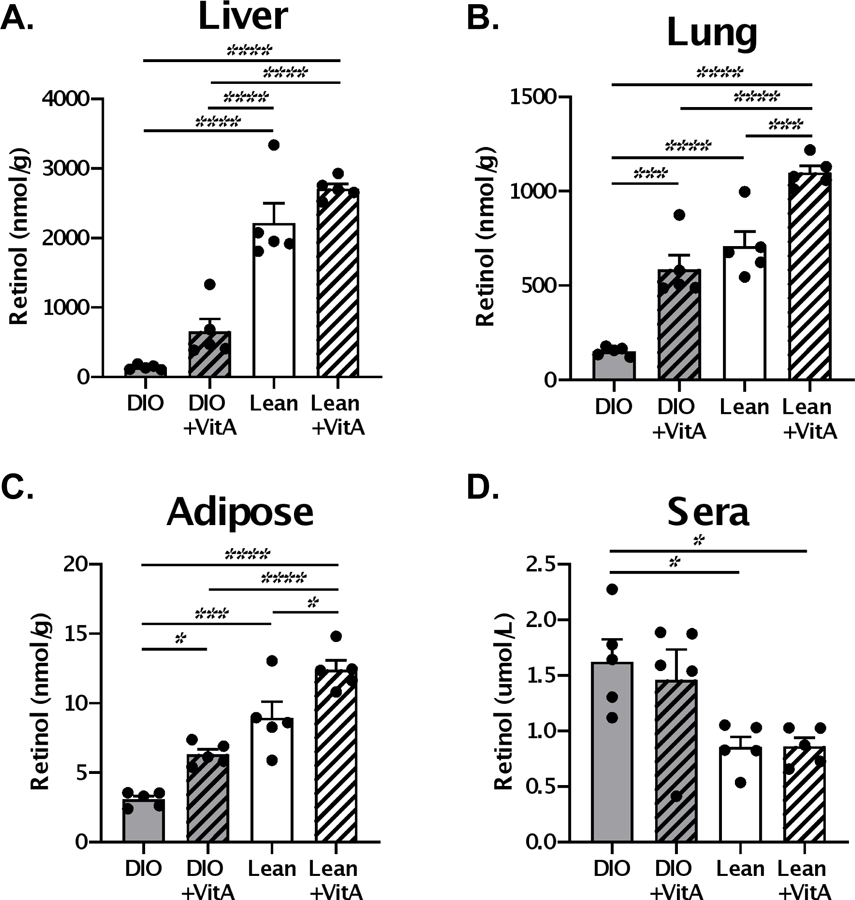
Mice received 600 IU vitamin A or PBS orally on d.0,3,7,21, 24 and 28. Retinol levels were evaluated in the (A) liver, (B) lung, (C) adipose tissue and (D) of lean and diet-induced obese (DIO) mice one week after administration of last vitamin A dose (d.35). Each dot represents a single mouse with 5 mice per group. A representative experiment is shown for two independent experiments, each with 5 mice per group. Significant differences were determined by two-way ANOVA followed by Tukey’s multiple comparisons test. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Supportive of our previous findings with non-obese mice (17), we found that vitamin A supplementation associated with marginally higher CD4+ and CD8+ T cell percentages among CD45+ cells in the respiratory tract (Figures 2A–B). Additionally, the percentages of CD3NEGB220NEGF4/80+CD11cHI/MEDCD11bLOWSiglecF+ cells (inclusive of alveolar macrophages) were marginally improved and the percentages of CD3NEGB220NEGF4/80+CD11cHI/MEDCD11bHISiglecFNEG cells (inclusive of interstitial macrophages and termed interstitial macrophages for simplicity (18)) were significantly reduced among CD45+ cells in the lung (Figures 2C–D).
Figure 2. Vitamin A supplementation improves lung cell composition and blood cytokines in obese mice.
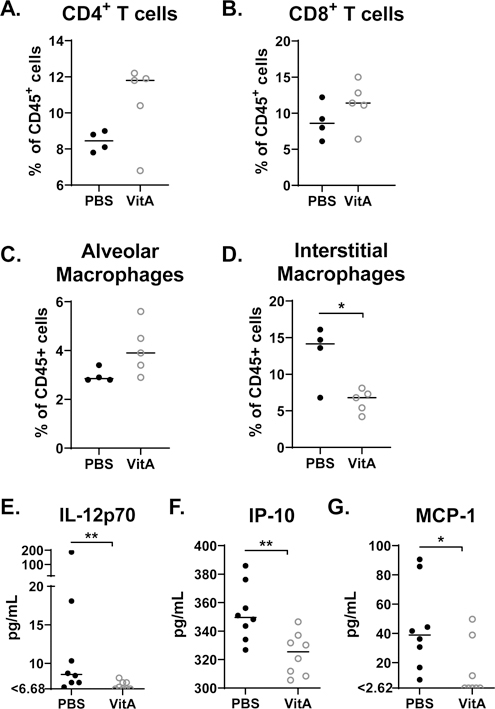
Lungs were analyzed by flow cytometry for percentages of (A) CD8+ and CD4+ T cells, and (B) cell populations inclusive of alveolar macrophages and interstitial macrophages. (C) Blood was analyzed for IL-12p70, IP-10, and MCP-1. For the cellular analyses, a representative experiment is shown for two independent experiments, each with 3–5 mice per group. Each dot represents a single mouse. For cytokine analyses, combined results are shown from two independent experiments, which included 3 mice per group and 5 mice per group, respectively. Significant differences were determined by Mann Whitney U tests. *p<0.05, **p<0.01, ***p<0.001. Cell percentages were determined as percent of live CD45+ cells. Populations were defined as follows: CD8+ T cells: CD3+CD4NEGCD8+; CD4+ T cells: CD3+CD4+CD8NEG; population inclusive of alveolar macrophages: CD3NEGB220NEGF4/80+CD11cHI/MEDCD11bLOWSiglecF+; population inclusive of interstitial macrophages: CD3NEGB220NEGF4/80+CD11cHI/MEDCD11bHISiglecFNEG (termed interstitial macrophages for simplicity).
Vitamin A supplements improve virus control in vaccinated mice following challenge
Mice were vaccinated via a prime-boost regimen with an inactivated influenza virus (pdmH1N1) and vitamin A or PBS was administered orally on d.0, 3 and 7 post-vaccination. Two weeks post-boost serum was evaluated for virus-specific antibodies. Overall, obese mice demonstrated a poor antibody response to vaccination compared to control mice (Figure 3). Only when vaccine was co-delivered with vitamin A were influenza-specific IgG antibody responses significantly above background (Figure 3B). Supplementation also increased IgG/IgM ratios, indicating enhanced antibody class switching (Figure 3C).
Figure 3. Virus-specific antibodies post-vaccination and virus load post-challenge in diet-induced obese (DIO) mice.
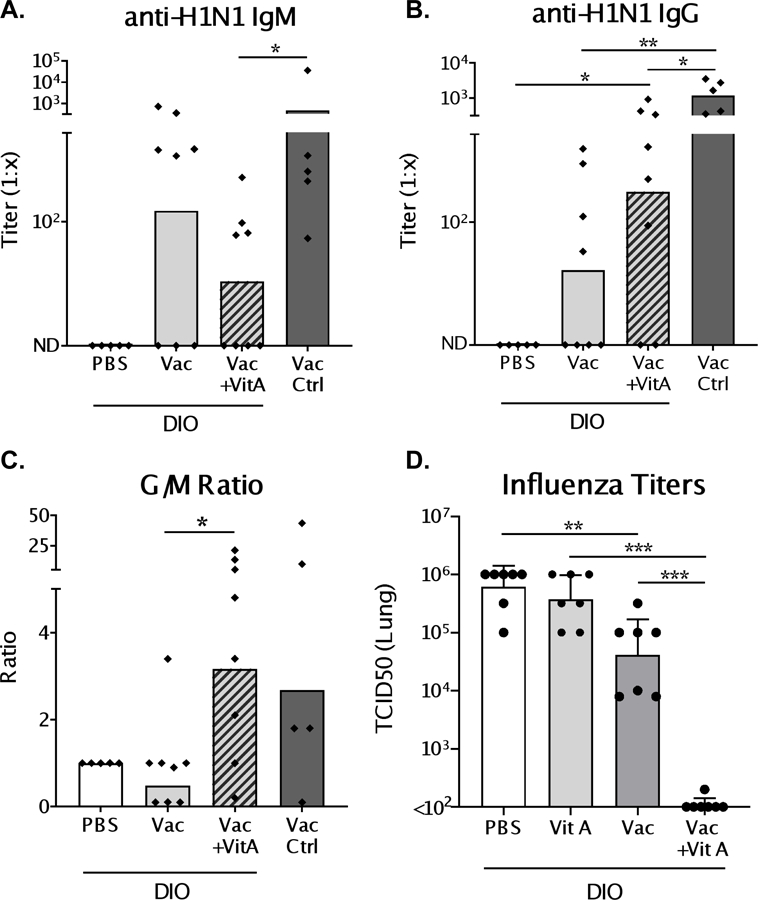
Mice were vaccinated with inactivated pdmH1N1 virus in a prime-boost scheme. At the time of vaccination, and d.3 and d.7 post-vaccination, mice received either 600 IU vitamin A or PBS. One week after boosting, sera were collected and tested by ELISA for IgM (A) and IgG (B) virus-specific antibody responses. (C) Ratio of IgG to IgM (G/M) titers were determined to evaluate the amount of antibody isotype switching. Mice were challenged intranasally with homologous virus (106 TCID50) two weeks after the last dose of vaccine. Viral titers in the lungs (D) were measured in both vaccinated and unvaccinated DIO mice treated with vitamin A or PBS on d.3 post-infection. Each dot represents a single mouse. For antibody results, a representative experiment is shown for 4 independent experiments with 5–8 mice per group [PBS/C57BL/6 control mice: n=5; vaccinated/vaccinated+vitamin A test groups: n=8]. For influenza virus titers, a representative experiment is shown for two experiments, each with 7 mice per group. Significant differences were determined by Mann Whitney U tests. *p<0.05, **p<0.01, ***p<0.001.
Two weeks after the second dose of vaccine, animals were challenged with a non-lethal dose (106 TCID50) of homologous influenza virus. There was transient, mild, weight loss after infection that was more pronounced in vitamin A-treated versus PBS-treated animals (Supplementary Figure 1), although absolute weights were similar between test and control groups throughout the time course. Weight loss may have reflected virus-specific immune responses (19), but significant correlations between weight loss and virus-specific antibodies were not observed (data not shown). Vaccination alone provided some protection against infection. Importantly, on d.3 post-infection vaccinated mice that received vitamin A exhibited a profound reduction in viral lung titers compared to vaccinated, unsupplemented animals (Figure 3D, p < 0.001).
CONCLUSION
Vitamin A tissue deficits may be common in individuals with obesity, but masked and under-reported due to normal or above-normal serum retinol levels. Vitamin A is pleiotropic in function and influences virtually every mammalian cell, including cells involved in adaptive and innate immune responses. We show that vitamin A supplements provide benefit to individuals with obesity by correcting tissue vitamin deficits, altering lung immune cell composition, improving vaccine responses and assisting in respiratory virus clearance. These findings are particularly relevant as the world scrambles to deal with the SARS-CoV-2 pandemic. Individuals with underlying health conditions, including obesity (20), are particularly vulnerable to serious disease (COVID-19) caused by SARS-CoV-2. Our demonstration that vitamin A supplements provide protection against a respiratory virus infection may inform healthcare recommendations for prophylaxis against severe disease from respiratory viral infection in individuals with obesity.
Supplementary Material
Supplementary Figure 1. Weight loss in vaccinated, challenged animals
Transient weight loss was measured following virus challenge. Each line represents an individual mouse. Significant differences between groups were evaluated by day using Mann Whitney U tests. *p<0.05, **p<0.01, ***p<0.001.
What is already known about this subject?
Individuals with obesity are highly susceptible to increased morbidity and mortality to respiratory viral infections
Tissue-specific vitamin A deficiencies have been demonstrated in mouse models of obesity
What are the new findings in your manuscript?
Vitamin A supplementation corrects tissue-specific deficiencies and improves immune responses in obese mice
Following challenge, vitamin A supplementation accelerates viral clearance in vaccinated obese mice
How might your results change the direction of research or the focus of clinical practice?
Results identify a low-cost intervention that may correct vitamin A tissue deficits, improve vaccine-induced immune responses, and control respiratory viral infections in individuals with obesity
Funding:
This study was supported in part by NIH NCI P30CA21765, NIH HD006982 [ACR], NIH NIAID Collaborative Influenza Vaccine Innovation Centers (CIVICs) contract 75N93019C00052, and ALSAC.
Abbreviations:
- TCID50
tissue culture infectious doses-50
- d
day
- VAD
vitamin A deficient
Footnotes
Disclosure: Authors declare no conflict of interest
REFERENCES
- 1.Painter SD, Ovsyannikova IG, Poland GA. The weight of obesity on the human immune response to vaccination. Vaccine 2015;33: 4422–4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond) 2012;36: 1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neidich SD, Green WD, Rebeles J, Karlsson EA, Schultz-Cherry S, Noah TL, et al. Increased risk of influenza among vaccinated adults who are obese. Int J Obes (Lond) 2017;41: 1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maier HE, Lopez R, Sanchez N, Ng S, Gresh L, Ojeda S, et al. Obesity Increases the Duration of Influenza A Virus Shedding in Adults. J Infect Dis 2018;218: 1378–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan OW, Bramley A, Fowlkes A, Freedman DS, Taylor TH, Gargiullo P, et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS One 2010;5: e9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cocoros NM, Lash TL, DeMaria A Jr., Klompas M. Obesity as a risk factor for severe influenza-like illness. Influenza Other Respir Viruses 2014;8: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trasino SE, Tang XH, Jessurun J, Gudas LJ. Obesity Leads to Tissue, but not Serum Vitamin A Deficiency. Sci Rep 2015;5: 15893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pettinelli P, Arendt BM, Teterina A, McGilvray I, Comelli EM, Fung SK, et al. Altered hepatic genes related to retinol metabolism and plasma retinol in patients with non-alcoholic fatty liver disease. PLoS One 2018;13: e0205747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi Y, Miura T, Takahashi K. Vitamin A is involved in maintenance of epithelial cells on the bronchioles and cells in the alveoli of rats. J Nutr 1993;123: 634–641. [DOI] [PubMed] [Google Scholar]
- 10.Rudraraju R, Surman SL, Jones BG, Sealy R, Woodland DL, Hurwitz JL. Reduced frequencies and heightened CD103 expression among virus-induced CD8(+) T cells in the respiratory tract airways of vitamin A-deficient mice. Clin Vaccine Immunol 2012;19: 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia OP. Effect of vitamin A deficiency on the immune response in obesity. Proc Nutr Soc 2012;71: 290–297. [DOI] [PubMed] [Google Scholar]
- 12.Hurwitz JL, Jones BG, Penkert RR, Gansebom S, Sun Y, Tang L, et al. Low Retinol-Binding Protein and Vitamin D Levels Are Associated with Severe Outcomes in Children Hospitalized with Lower Respiratory Tract Infection and Respiratory Syncytial Virus or Human Metapneumovirus Detection. J Pediatr 2017;187: 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mora JR, Iwata M, Von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. NatRevImmunol 2008;8: 685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sommer A Vitamin A, infectious disease, and childhood mortality: a 2 solution? J Infect Dis 1993;167: 1003–1007. [DOI] [PubMed] [Google Scholar]
- 15.Surman SL, Jones BG, Sealy RE, Rudraraju R, Hurwitz JL. Oral retinyl palmitate or retinoic acid corrects mucosal IgA responses toward an intranasal influenza virus vaccine in vitamin A deficient mice. Vaccine 2014;32: 2521–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel N, Penkert RR, Jones BG, Sealy RE, Surman SL, Sun Y, et al. Baseline Serum Vitamin A and D Levels Determine Benefit of Oral Vitamin A&D Supplements to Humoral Immune Responses Following Pediatric Influenza Vaccination. Viruses 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surman SL, Penkert RR, Jones BG, Sealy RE, Hurwitz JL. Vitamin Supplementation at the Time of Immunization with a Cold-Adapted Influenza Virus Vaccine Corrects Poor Mucosal Antibody Responses in Mice Deficient for Vitamins A and D. Clin Vaccine Immunol 2016;23: 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibbings SL, Thomas SM, Atif SM, McCubbrey AL, Desch AN, Danhorn T, et al. Three Unique Interstitial Macrophages in the Murine Lung at Steady State. American journal of respiratory cell and molecular biology 2017;57: 66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francesconi W, Sanchez-Alavez M, Berton F, Alboni S, Benatti C, Mori S, et al. The Proinflammatory Cytokine Interleukin 18 Regulates Feeding by Acting on the Bed Nucleus of the Stria Terminalis. J Neurosci 2016;36: 5170–5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan DH, Ravussin E, Heymsfield S. COVID 19 and the Patient with Obesity - The Editors Speak Out. Obesity (Silver Spring) 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Weight loss in vaccinated, challenged animals
Transient weight loss was measured following virus challenge. Each line represents an individual mouse. Significant differences between groups were evaluated by day using Mann Whitney U tests. *p<0.05, **p<0.01, ***p<0.001.


