Abstract
Although hippocampal volume has served as a long-standing predictor of cognitive decline, diffusion MRI studies of white matter have shown similar relationships. Still, it remains unclear if gray and white matter interact to predict cognitive impairment and longitudinal decline. Here, we investigate whether FW and FW-corrected fractional anisotropy (FAT) within medial temporal lobe white matter tracts provides meaningful contribution to cognition and cognitive decline beyond hippocampal volume. Using data from the Vanderbilt Memory & Aging Project (n=319), we found that FW was associated with baseline memory and executive function beyond that of hippocampal volume and other comorbidities for memory loss and executive function. Longitudinal analyses demonstrated significant interactions of hippocampal volume and FAT within the inferior longitudinal fasciculus (p=0.043) and cingulum bundle (p=0.025) with decline in memory. For decline in executive function, we found significant interactions on hippocampal volume and FAT within fornix (p=0.025). Results suggest that free-water metrics of white matter have a unique role in cognitive decline and should be include in theoretical models of aging, cerebrovascular disease, and AD.
Introduction
Late-life cognitive impairment arises from a variety of mechanistic pathways of injury including cerebrovascular damage (van der Flier et al., 2018; Vemuri and Knopman, 2016), AD neuropathology (Jack et al., 2018; McKhann et al., 2011), and other age-related neuropathologies (Schneider et al., 2007), resulting in damage to both the grey and white matter. Cerebrovascular disease and AD are two of the most common neuropathologies in aging and frequently co-occur, with nearly 80% of autopsy confirmed AD cases showing substantial cerebrovascular disease both of which contribute to the clinical manifestation of disease (Arvanitakis et al., 2016). Interestingly, the hippocampus is particularly vulnerable to many of the age-related neuropathological changes, making hippocampal structure and function a key indicator of age-related disease. For example, the hippocampus is susceptible to the earliest AD neuropathological changes (Frankó et al., 2013), subtle alterations in cerebral hemodynamics over the course of aging (Okonkwo et al., 2014), blood-brain barrier breakdown (Montagne et al., 2015), and hippocampal sclerosis (Barker et al., 2002). Thus, it is not surprising that hippocampal volume is a strong predictor of concurrent (Besson et al., 2015; Seab et al., 1988) and future cognitive (Goukasian et al., 2019; Jack et al., 2000) impairment.
While hippocampal volume is a well-established biomarker of neurodegeneration, including being a recommended measure in the recent biomarker definitions of AD (Jack et al., 2018), there is less focus on the white matter tracts projecting out of the hippocampus. Like the hippocampus, the cerebral white matter sits at the intersection of many age-related disease processes including well-established associations between cerebrovascular disease and white matter integrity (Croall et al., 2017; Fuhrmann et al., 2019; Vemuri et al., 2018), and emerging evidence of direct associations of AD neuropathology with white matter integrity (Hoy et al., 2017). While some changes in the white matter tracts projecting from the hippocampus may be the result of neurodegeneration within the hippocampus, it is also possible that hippocampal atrophy and white matter damage in tracts projecting from the hippocampus have a synergistic relationship, through which individuals with both reduced gray matter volume and reduce white matter microstructure are predisposed to lower cognitive function and more rapid cognitive decline.
Diffusion MRI (dMRI) is a particularly useful neuroimaging technique as it allows for the in-vivo quantification of white matter tract microstructural deficits. The most well-established microstructural measure in dMRI is fractional anisotropy (FA), and FA reductions have been associated with demyelination and axonal degradation (Beaulieu, 2002). White matter FA has been found to contribute to cognitive decline in normal aging (Cremers et al., 2016; Kennedy and Raz, 2009), AD (Bozzali et al., 2012; Mielke et al., 2012; Yasmin et al., 2008), vascular risk factors (Vemuri et al., 2018), and overt cerebrovascular disease (Vernooij et al., 2009). While these finding have suggested a significant role of white matter microstructure in cognitive function, FA is susceptible to partial volume effects (i.e., both fluid and tissue are present within a voxel) and could therefore limit our findings in AD. Fortunately, post-processing techniques, such as free-water (FW) elimination, have allowed for the separation of the fluid (FW) and tissue (FW-corrected FA [FAT]) components, thus increasing the biological specificity of dMRI studies.
Recently, the FW elimination post-processing technique has been paired with tractography algorithms to quantify white matter tract alterations in a variety of neurodegenerative disorders, including Parkinsonism (Archer, Derek B. et al., 2019) and AD (Archer, D. B. et al., 2019; Hoy et al., 2017). A recent study conducted tractography to create individual-specific estimations of several white matter tracts, including the fornix, superior cingulum bundle, and transcallosal tracts. Higher FW within the superior cingulum was associated with higher CSF measures of neurofibrillary tangle load (CSF p-tau), thin unmyelinated axons (CSF t-tau), and soluble amyloid precursor protein beta (sAPPβ) (Hoy et al., 2017). Interestingly, however, tract microstructure was not associated with cognitive decline. One potential reason for this finding is that tractography results were individual-specific, limiting generalizability. Recent work has resolved this generalizability limitation by creating high-resolution, spatially precise tractography templates using Human Connectome Project (HCP) data (Archer, D. B. et al., 2019). Specifically, the transcallosal tract template (TCATT) includes 32 transcallosal tracts, including projections to the temporal lobe (e.g., tapetum). Widespread elevated FW was found within the TCATT in AD, and the tapetum was robustly associated with global cognition as measures by the Montreal Cognitive Assessment (MoCA). Still, it is unclear how white matter microstructure within the tapetum, as well as in other medial temporal lobe white matter tracts, interacts with gray matter on cognitive impairment and longitudinal cognitive decline.
The goal of the present study is to expand upon prior tractography research in AD to provide a detailed exploration of the cognitive consequences of medial temporal lobe changes in older adults free of dementia. Specifically, we leveraged high-resolution HCP data to create white matter tractography templates of the cingulum bundle, inferior longitudinal fasciculus, and uncinate fasciculus. These templates, in addition to freely available white matter tract templates of the fornix (Brown et al., 2017) and tapetum (Archer, D. B. et al., 2019), will be used to determine unique contributions of white matter FW and FAT in cognitive function in a longitudinal cohort of individuals with normal cognition and mild cognitive impairment (MCI). Furthermore, we will examine the synergistic relationship of gray matter atrophy (i.e., hippocampal volume) and medial temporal lobe white matter FW and FAT in cognitive decline. Our hypothesis is that medial temporal lobe tract microstructure will explain unique variance in baseline cognitive performance and that individuals with both reduced medial temporal lobe tract microstructure and smaller hippocampal volume will have a more rapid rate of cognitive decline over the follow-up period.
Methods
Study Cohort
The Vanderbilt Memory & Aging Project was launched in 2012 and is a longitudinal observational study. Cohort inclusion criteria required participants to be 60 years of age or older, speak English, have adequate auditory and visual acuity for testing, and have a reliable study partner. Full characterization of cohort has been described elsewhere (Jefferson et al., 2016). At study entry, participants completed a comprehensive neuropsychological examination and were placed into three categories – cognitively normal, early mild cognitive impairment (eMCI), and MCI (Kresge et al., 2018). Neuropsychological assessment was collected longitudinally up to five years and neuropsychological composites of executive function and memory were calculated (Kresge et al., 2018). Apolipoprotein E (APOE) haplotype status (ε2, ε3, ε4) was determined using single-nucleotide polymorphisms (SNPs) rs7412 and rs429358, which were genotyped using TaqMan on frozen whole blood. T1-weighted MRI and dMRI was also collected. The protocol was approved by the Vanderbilt University Medical Center Institutional Review Board, and written informed consent was obtained prior to data collection.
Neuropsychological Composites
The neuropsychological protocol was performed by experienced technicians who assessed several cognitive domains, including episodic memory and executive function. There has been a longstanding effort to minimize multiple comparisons in neuropsychological testing by creating psychometrically sound composites of executive function and memory (Crane et al., 2012). As previously described (Kresge et al., 2018), we created psychometrically sound composite measures, in which we based our item assignments of memory and executive function on expert opinion from 2 clinical neuropsychologists (K.A.G., A.L.J.). The executive functioning composite included the Delis-Kaplan Executive Function System (DKEFS) Tower Test, DKEFS Letter-Number Switching, DKEFS Color-Word Inhibition, and Letter Fluency (FAS). For the memory composite, the California Verbal Learning Test-Second Edition (CVLT-II) Total Learning, Interference Condition, Long Delay Free Recall, and Recognition components were used in addition to the identical components of the Biber Figure Learning Test (Gifford et al., 2018; Glosser et al., 2002).
Acquisition and Quantification of Hippocampal Volume
T1-weighted MRI images (TR/TE: 8.9/4.6 ms, resolution: 1mm isotropic) were collected from each participant on a 3T Philips Achieva system (Best, The Netherlands) using an 8-channel SENSE reception coil. Multi-Atlas segmentation was conducted to obtain hippocampal segmentations and calculate volumes (Asman and Landman, 2012). Hippocampal volume was quantified by summing the left and right hippocampal mask volumes. Multi-Atlas segmentation also estimated the total intracranial volume (TIV) and total white mater volume. The hippocampal volume measures was adjusted by TIV based on an established approach (Mormino et al., 2014), which allowed us to eliminate the need for a TIV covariate in our statistical analyses. Further, participants were classified into two groups based on this adjusted hippocampal volume measure – neurodegenerative negative (>6723 mm3) and neurodegenerative positive (<6723 mm3)
dMRI Acquisition and Preprocessing
dMRI images (resolution: 2mm isotropic, b-values: 0, 1,000 s/mm2) were collected from each participant using the same scanner described above. Images were collected along 32 diffusion gradient vectors and five non-diffusion (B0) weighted images. FSL 5.0.9 (fsl.fmrib.ox.ax.uk) was used for all dMRI preprocessing (Andersson and Sotiropoulos, 2016; Jenkinson et al., 2012). Quality assessment of all dMRI scans was performed manually. The data were first corrected for head motion and eddy currents using eddy_correct and the brain was then extracted from the skull (BET). This corrected image was used as input in two different procedures: (1) DTIFIT to calculate fractional anisotropy maps (FA), and (2) custom written MATLAB (R2019a, The Mathworks, Natick, MA, USA) code to calculate FW and FAT maps, as described in detail previously (Archer, D. B. et al., 2019; Pasternak et al., 2009). To obtain a standardized space representation of the FW and FAT maps, the FAT map was registered to an inhouse FAT template (1mm isotropic) by a nonlinear warp using the Advanced Normalization Tools (ANTs) package (Avants et al., 2008). This nonlinear warp was applied to the FW map.
White Matter Tractography Templates
HCP dMRI data (resolution: 1.25mm isotropic, b-values: 0, 1,000, 2,000, 3,000 s/mm2, 90 directions per shell) (Van Essen et al., 2013) was used to create white matter tract templates using an established approach (Archer et al., 2018). This approach performs probabilistic tractography in the white matter tract of interest in 100 HCP individuals. The results are transformed into the MNI space, averaged into a mean tract template, and thresholded using a novel slice-level thresholding approach. This approach allows higher threshold levels compared to conventional thresholding methods, thus reducing the likelihood of false positives in the resulting tract templates. This approach has been used to create white matter tract templates of the sensorimotor tracts (Archer et al., 2018), transcallosal tracts (Archer, D. B. et al., 2019), and subcortical tracts (Archer, Derek B. et al., 2019). White matter tract templates included tracts projecting from hippocampus (tapetum, fornix, cingulum bundle, uncinate fasciculus, and inferior longitudinal fasciculus). For the tapetum, we used a template tract from the Transcallosal Tract Template (TCATT)(Archer, D. B. et al., 2019). For the fornix, we used a well-established fornix template (Brown et al., 2017). For the cingulum bundle, uncinate fasciculus, and inferior longitudinal fasciculus, we conducted probabilistic tractography to create white matter tract templates as current tractography templates for these tracts were either incomplete or nonexistent. Consistent with prior work (Archer, D. B. et al., 2019; Archer et al., 2018), we conducted probabilistic tractography using the probtrackx2 program in FSL using default settings (samples: 5,000, curvature threshold: 0.2, FA threshold: 0.2) on 100 individuals from the HCP (Van Essen et al., 2013). For the cingulum bundle, we used a hippocampal mask as a seed and an anterior cingulate cortex mask as a waypoint. For the uncinate fasciculus, we used a hippocampal mask as a seed and a prefrontal cortex mask as a waypoint. For the inferior longitudinal fasciculus, we used a hippocampal mask as a seed and a posterior parietal cortex mask as a waypoint. FAT and FW were then calculated within all tracts for all participants.
Statistical Analyses
All statistical analyses were performed in R version 3.5.2 (http://www.r-project.org/). Covariates included age, sex, education, cognitive status, race/ethnicity, Framingham Stroke Risk Profile (FSRP) scores (D'Agostino et al., 1994; Wolf et al., 1991), total white matter volume, and APOE-ε4 carrier status. APOE-ε4 carrier status was defined as positive (ε2/ε4, ε3/ ε4, ε4/ ε4) or negative (ε2/ε2, ε2/ε3, ε3/ε3). A variance inflation factor was calculated for all covariates in all models to ensure that multicollinearity was not a limitation. Significance was set a priori as α=0.05 and correction for multiple corrections were made using the false discovery rate (FDR) method.
For all analyses, only one tract per model was considered. Baseline effects of all tracts (tapetum, fornix, cingulum, uncinate fasciculus, and inferior longitudinal fasciculus) for both measures (FW and FAT) were estimated using a general linear model for each of the 3 outcomes (hippocampal volume, memory composite, executive function composite). Further, cognitive status x white matter tract interaction terms (e.g., cognitive status x cingulum FAT) were evaluated for each tract and measure on the 3 outcomes. We then conducted an analysis of hippocampal volume x white matter tract interaction terms on memory and executive function composites. This analysis was followed with post-hoc competitive model analysis which assessed the unique variance white matter measures contributed to cognitive function beyond comorbidities and hippocampal volume.
Finally, we evaluated the interaction between baseline hippocampal volume and white matter tract FW and FAT on longitudinal memory and executive function composites using mixed-effects regression analyses. Time was modeled as years from baseline for each participant, and both time and the intercept were inputted as both fixed and random effects in the model. The three-way hippocampal volume x white matter tract x time term assessed baseline interaction effects on longitudinal change in cognition. All lower-order interactions were included in the model.
Results
Participant Characteristics
Demographic and clinical variables for each cognitive status group (cognitively normal, eMCI, MCI) are summarized in Table 1. Patients were mostly well-educated, elderly, non-Hispanic white individuals. There were no significant differences in age, sex distribution, or race distribution between groups. The cognitively normal group had more education than the MCI group. The MCI group had more APOE-ε4 positive individuals than the cognitively normal group. As expected, there were significant differences between the cognitively normal, eMCI, and MCI groups in hippocampal volume, total FSRP score, and neuropsychological composites.
Table 1 –
Demographic and Health Characteristics
| Measure | Cognitive Status | p-value | ||
|---|---|---|---|---|
| Cognitively Normal |
eMCI | MCI | ||
| Demographic and health characteristics | ||||
| Sample Size | 164 | 27 | 128 | |
| Age (yrs) | 72 (7) | 73 (6) | 73 (8) | 0.59 |
| Sex (% male) | 58 | 74 | 57 | 0.24 |
| Education (yrs) | 16 (3)c | 16 (3) | 15 (3)a | <0.001 |
| Race (% Non-hispanic white) | 87 | 85 | 87 | 0.98 |
| APOE-ε4 (% positive) | 30c | 22 | 45a | 0.008 |
| Hippocampal Volume (mm3) | 3079 (408)c | 3007 (438) | 2949 (470)a | <0.001 |
| FSRP (total score) | 12 (4)c | 14 (3) | 13 (4)a | 0.008 |
| Systolic blood pressure (mmHg) | 140 (18)b | 150 (18)a | 145 (19) | 0.009 |
| Antihypertensive medication usage (%) | 56 | 55 | 53 | 0.95 |
| Diabetes (%) | 22 | 20 | 15 | 0.42 |
| Current smoking (%) | 4 | 3 | 2 | 0.72 |
| Left ventricular hypertrophy (%) | 4 | 6 | 3 | 0.41 |
| Atrial fibrillation (%) | 11 | 7 | 6 | 0.63 |
| Prevalent CVD (%) | 4 | 3 | 6 | 0.48 |
| Neuropsychological Composites | ||||
| Memory Composite | 0.44 (0.61)b,c | 0.17 (0.42)a,c | −0.55 (0.92)a,b | <0.001 |
| Executive Function Composite | 0.57 (0.73)b,c | −0.06 (0.76)a,c | −0.74 (0.74)a,b | <0.001 |
Values denoted as mean (standard deviation) or frequency. Abbreviations: MCI, mild cognitive impairment; eMCI, early MCI; yrs, years; APOE-ε4, apolipoprotein E ε4; FSRP, Framingham Stroke Risk Profile; CVD, cardiovascular disease. p-values were generated using a one-way analysis of variance for continuous variables and a chi-square test was used for categorical variables. pFDR<0.05
versus Normal
versus eMCI
versus MCI.
Baseline Tract Microstructure Association with Baseline Hippocampal Volume and Memory/Executive Composites
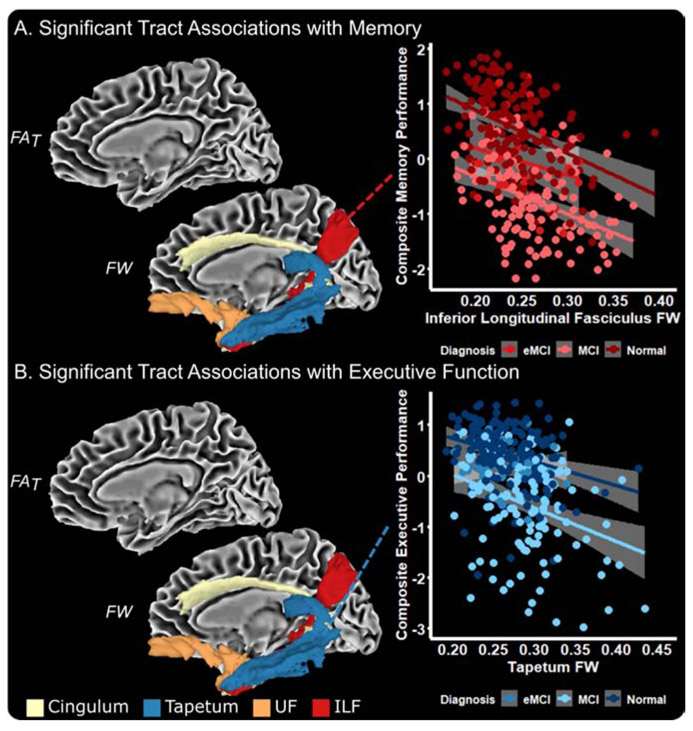
Baseline results are presented in Table 2 and graphically summarized in Figure 1. White matter associations with hippocampal volume included FAT in the tapetum (p=0.033) and fornix (p<0.001) and FW in all tracts (all p≤0.003). White matter associations with memory and executive function included FW in the inferior longitudinal fasciculus, tapetum, uncinate fasciculus, and cingulum (all p≤0.045), but no FAT associations with baseline cognitive performance were observed. As shown in Figure 1A, higher FW in the inferior longitudinal fasciculus was associated with lower composite memory performance in all cognitive status groups. Similarly, Figure 1B shows that higher FW in the tapetum is associated with lower composite executive performance in all cognitive status groups. We did not observe any diagnosis x white matter tract interactions (all p≥0.191). Further, variance inflation factor values were low (range: 1.21-2.04) in all models, demonstrating that multicollinearity was not present in our models.
Table 2 –
Baseline Tract Microstructure Association with Baseline Hippocampal Volume, Composite Memory Performance, and Composite Executive Function Performance
| Tract | FAT | FW | ||
|---|---|---|---|---|
| β (95% CI) | p-value | β (95% CI) | p-value | |
| Association with Baseline Hippocampal Volume | ||||
| Inferior Longitudinal Fasciculus | 3622 (−458 to 7701) | 0.155 | −4739 (−7086 to −2392) | 0.001 |
| Tapetum | 6771 (1457 to 12085) | 0.033 | −3824 (−6033 to −1615) | 0.003 |
| Uncinate Fasciculus | 2329 (−2370 to 7029) | 0.51 | −4040 (−6131 to −1949) | 0.001 |
| Cingulum Bundle | 2275 (−2394 to 6943) | 0.51 | −6722 (−9322 to −4123) | <0.001 |
| Fornix | 11223 (6702 to 15745) | <0.001 | −3068 (−4242 to −1894) | <0.001 |
| Association with Baseline Memory Composite | ||||
| Inferior Longitudinal Fasciculus | 2.82 (−1.27 to 6.92) | 0.314 | −4.84 (−7.24 to −2.44) | 0.001 |
| Tapetum | −1.32 (−6.73 to 4.09) | 0.730 | −2.70 (−4.96 to −0.43) | 0.045 |
| Uncinate Fasciculus | −0.14 (−4.84 to 4.56) | 0.954 | −2.97 (−5.13 to −0.82) | 0.020 |
| Cingulum Bundle | 2.00 (−2.66 to 6.66) | 0.547 | −4.01 (−6.79 to −1.24) | 0.015 |
| Fornix | 1.87 (−2.99 to 6.72) | 0.589 | −0.81 (−2.07 to 0.46) | 0.358 |
| Association with Baseline Executive Function Composite | ||||
| Inferior Longitudinal Fasciculus | 0.44 (−3.48 to 4.36) | 0.884 | −2.72 (−5.05 to −0.39) | 0.045 |
| Tapetum | 2.30 (−2.86 to 7.45) | 0.547 | −3.57 (−5.72 to −1.43) | 0.005 |
| Uncinate Fasciculus | −1.60 (−6.08 to 2.89) | 0.608 | −3.26 (−5.31 to −1.2) | 0.007 |
| Cingulum Bundle | −0.24 (−4.7 to 4.21) | 0.947 | −3.13 (−5.79 to −0.47) | 0.045 |
| Fornix | −1.22 (−5.86 to 3.42) | 0.728 | −0.18 (−1.39 to 1.04) | 0.860 |
Abbreviations: FAT, free-water corrected fractional anisotropy; FW, free-water. Boldface signifies p<0.05.
Figure 1 – Baseline Associations with Composite Memory and Executive Function Performance.
The medial temporal lobe tract measures which were associated with memory (A) and executive function (B) performance include FW in the cingulum bundle, tapetum, uncinate fasciculus (UF), and inferior longitudinal fasciculus (ILF). The association of ILF FW with memory performance (A) and tapetum FW with executive function performance (B) is shown.
We then conducted a competitive model analysis to determine the unique variance explained by hippocampal volume and white matter tract microstructure in addition to all covariates (age, sex, education, cognitive status, APOE-ε4 status, race, FSRP scores, total white matter volume) (Table 3). We found that covariates explained approximately 50% of variance in baseline memory performance (Radj2=50.54%). When adding hippocampal volume to this model, we found a small increase (Δ Radj2=0.91%) in the overall model. We then iteratively added tract FW and FAT measures to this model to determine if tract microstructure explained any unique variance beyond covariates and hippocampal volume. We found that tract FAT did not significantly contribute to the models; however, for FW, all tracts (aside from the fornix) were significant contributors to the model and provided increases in the Radj2 (range: 0.70%-2.24%). For executive function performance, there was a strong relationship with covariates along (Radj2=47.13%), and the addition of hippocampal volume to this model provided a small, insignificant increase (Δ Radj2=0.03%) in the overall model (Radj2=47.16%). We then added tract FW and FAT measures to this model to determine if tract microstructure explained any unique variance beyond covariates and hippocampal volume to executive function performance. For FAT, we found that no tracts were significant contributors to the model. For FW, we found that all tracts (aside from the fornix) were significant contributors to the model and provided increases in the Radj2 (range: 0.73%-1.63%).
Table 3 –
Comparison of Main Effect Models
| Memory | Executive Function | |||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | p-value | Δ Radj2 | β | SE | p-value | Δ Radj2 | |
| Covariates + | ||||||||
| Hippocampal Volume | 1.48x10−4 | 5.72x10−5 | 0.010 | 0.907 | −5.91x10−5 | 5.46x10−5 | 0.280 | 0.030 |
| Covariates + Hippocampal Volume + | ||||||||
| ILF FAT | 2.824 | 2.09 | 0.178 | 0.132 | 0.442 | 2.001 | 0.825 | −0.166 |
| Tapetum FAT | −1.32 | 2.759 | 0.633 | −0.124 | 2.298 | 2.63 | 0.383 | −0.041 |
| UF FAT | −0.14 | 2.399 | 0.954 | −0.160 | −1.6 | 2.288 | 0.486 | −0.090 |
| Cingulum Bundle FAT | 2.00 | 2.38 | 0.401 | −0.047 | −0.24 | 2.274 | 0.915 | −0.173 |
| Fornix FAT | 1.866 | 2.478 | 0.452 | −0.069 | −1.22 | 2.366 | 0.606 | −0.128 |
| ILF FW | −4.84 | 1.225 | <0.001 | 2.236 | −2.72 | 1.188 | 0.023 | 0.730 |
| Tapetum FW | −2.7 | 1.156 | 0.020 | 0.701 | −3.57 | 1.094 | 0.001 | 1.634 |
| UF FW | −2.97 | 1.101 | 0.007 | 0.988 | −3.26 | 1.047 | 0.002 | 1.470 |
| Cingulum Bundle FW | −4.01 | 1.416 | 0.005 | 1.100 | −3.13 | 1.357 | 0.022 | 0.743 |
| Fornix FW | −0.81 | 0.647 | 0.215 | 0.087 | −0.18 | 0.619 | 0.774 | −0.160 |
Abbreviations: FAT, free-water corrected fractional anisotropy; FW, free-water; ILF, inferior longitudinal fasciculus; UF, uncinate fasciculus. B, SE, and p-values represent the parameter estimate for each variable. Boldface signifies p<0.05. Covariates explained 50.54% and 47.13% for memory and executive function respectively. Covariates + hippocampal volume explained 51.45% and 47.16% for memory and executive function respectively.
We then determined if there were baseline hippocampal x white matter tract interactions on memory and executive performance. The results are presented in Supplemental Table 1, which shows there were no significant interactions between white matter tract FAT or FW and hippocampal volume on baseline memory or executive function performance.
Tract-by-hippocampal Volume Interaction on Longitudinal Memory/Executive Composite
For annual change in memory performance, we found significant interactions between hippocampal volume and FAT in the inferior longitudinal fasciculus (p=0.043) and cingulum (p=0.025). No significant interactions were found with FW. For annual change in executive function performance, the only significant interaction with hippocampal volume was with fornix FAT (p=0.025). Results for all models can be found in Table 4. Figure 2 provides a summary of our findings for the annual change in memory and executive function. As shown in Figure 2A, lower FAT in the cingulum is associated with a more rapid decline in memory performance, particularly among individuals with baseline neurodegeneration in the hippocampus. Figure 2B shows that higher FW in the fornix is associated with a more rapid decline in executive function, particularly among individuals with neurodegeneration in the hippocampus.
Table 4 –
Baseline Tract Microstructure x Hippocampal Interaction Associations with Longitudinal Decline in Memory and Executive Function
| Tract | FAT | FW | ||
|---|---|---|---|---|
| B*103 (95% CI) | p-value | B*103 (95% CI) | p-value | |
| Longitudinal Composite Memory Performance | ||||
| Inferior Longitudinal Fasciculus | −1.70 (−2.92 to −0.48) | 0.043 | 0.34 (−0.32 to 1.00) | 0.560 |
| Tapetum | −1.35 (−3.10 to 0.40) | 0.293 | −0.17 (−0.84 to 0.50) | 0.710 |
| Uncinate Fasciculus | −1.68 (−3.09 to −0.26) | 0.085 | −0.19 (−0.75 to 0.37) | 0.710 |
| Cingulum Bundle | −2.11 (−3.43 to −0.79) | 0.025 | −0.16 (−0.80 to 0.49) | 0.710 |
| Fornix | 0.00 (−1.31 to 1.30) | 0.995 | 0.09 (−0.25 to 0.43) | 0.710 |
| Longitudinal Composite Executive Function Performance | ||||
| Inferior Longitudinal Fasciculus | −0.69 (−1.94 to 0.55) | 0.552 | 0.21 (−0.46 to 0.88) | 0.710 |
| Tapetum | −1.61 (−3.38 to 0.16) | 0.213 | 0.25 (−0.44 to 0.93) | 0.710 |
| Uncinate Fasciculus | −1.21 (−2.67 to 0.24) | 0.258 | 0.02 (−0.58 to 0.61) | 0.995 |
| Cingulum Bundle | −0.44 (−1.82 to 0.93) | 0.710 | 0.76 (0.11 to 1.40) | 0.085 |
| Fornix | −1.97 (−3.24 to −0.70) | 0.025 | 0.35 (0.00 to 0.69) | 0.164 |
Abbreviations: FAT, free-water corrected fractional anisotropy; FW, free-water. Boldface signifies p<0.05.
Figure 2 – Baseline Tract x Hippocampal Interaction on Annual Change in Memory and Executive Function.
The medial temporal lobe tract measures which had a significant interaction with hippocampal volume for annual change in memory include FAT in the inferior longitudinal fasciculus (ILF) and cingulum bundle (A). For annual change in executive function performance, temporal tract measures which had a significant interaction with hippocampal volume include FAT in the fornix (B). Hippocampal neurodegeneration groups (negative, positive) are based on a previously identified cutoff value for hippocampal neurodegeneration (positive: volume ≤ 6723 mm3) (Mormino et al., 2014).
Discussion
The present study examined relationships between hippocampal volume and the microstructure of the medial temporal lobe white matter tracts to evaluate how these sensitive imaging metrics relate to cognitive performance and cognitive decline. Specifically, we used FW elimination, an innovative post-processing technique which overcomes the limitations of conventional dMRI, to quantify microstructural values (FW and FAT) in several medial temporal lobe projections, including the cingulum bundle, fornix, tapetum, inferior longitudinal fasciculus, and uncinate fasciculus. We report three main findings. First, we found that FW and FAT in medial temporal lobe tracts were strongly associated with hippocampal volume. Second, we found that baseline measures of medial temporal lobe white matter tract FW were robustly associated with baseline cognitive performance. Competitive model analyses determined that FW in the tapetum, cingulum, uncinate fasciculus, and inferior longitudinal fasciculus explained variance above and beyond hippocampal volume and covariates in cognitive performance. Third, we found significant interactions of hippocampal volume and medial temporal lobe white matter tract FAT on longitudinal cognitive trajectory, whereby individuals with lower hippocampal volume and lower white matter tract FAT exhibited greater longitudinal decline. This study therefore provides direct evidence that medial temporal lobe white matter projections are relevant to cognitive performance even when statistically accounting for hippocampal atrophy.
The medial temporal lobe white matter tract microstructure association with memory and executive function performance is consistent with previous reports (Ji et al., 2019; Mielke et al., 2012); however, the mechanisms by which medial temporal lobe microstructure is linked to memory and executive function is unclear. One prevailing hypothesis is that white matter microstructure reflects cerebrovascular damage, and thus would primarily serve as a metric of concomitant disease in the case of early AD (Vemuri et al., 2018). A second possibility is that white-matter abnormalities arise downstream of AD neuropathology or hippocampal sclerosis as a result of Wallerian-degeneration following hippocampal atrophy (Sachdev et al., 2013). Both scenarios seem plausible given previous research suggesting that white matter degeneration may precede noticeable hippocampal atrophy (Hoy et al., 2017; Metzler-Baddeley et al., 2019; Zhuang et al., 2013). While these hypothesized pathways of injury differ in the temporal ordering of white matter damage, the literature consistently reports strong associations between white matter measures and cognitive performance (Bozzali et al., 2012; Mielke et al., 2012). Our results add to this growing body of literature by demonstrating that hippocampal volume synergistically interacts with white matter microstructure to explain longitudinal decline in memory and executive function and highlights the unique contribution of white matter changes in explaining cognitive decline over the course of aging and disease. Future longitudinal studies should determine if white matter decline in aging is independent of neurpathological process, a consequence of hippocampal atrophy, or a combination of these processes.
One interesting observation is that while medial temporal lobe white matter FW is robustly associated with baseline memory and executive function performances, medial temporal lobe FAT interacts with hippocampal volume to explain longitudinal decline in memory and executive function. These two white matter metrics are thought to reflect different neurobiological processes. FW measures unbound water molecules in the white matter, and thus higher FW could reflect a neuroinflammatory or more general axonal damage process (Pasternak et al., 2012). Therefore, the finding that higher FW is associated with lower baseline cognitive performance may reflect a more advanced neurodegenerative state in which atrophy has already impacted the white matter. In contrast, FAT is a more direct measure of white matter microstructure, as it calculates intracellular white matter microstructure, with lower FAT indicating more white matter vulnerability. Thus, while we found that FAT does not appear to be as sensitive to baseline cognitive performance as FW, our findings indicate that individuals with higher white matter vulnerability (i.e., lower FAT) are predisposed to a more rapid disease progression.
The present study has several strengths, including a well-characterized longitudinal cohort and the application of free-water imaging, which overcomes the limitations of conventional dMRI techniques. An additional strength of this study is the incorporation of novel medial temporal lobe white matter tract templates. The use of white matter tract templates increases consistency between studies as the identical voxels are being evaluated in the MNI space. Accordingly, prior studies have implemented white matter tract templates. For example, the most predominantly used white matter tract template, the Johns Hopkins White Matter Tract Atlas, which is a white matter tract atlas based on 28 individuals (resolution: 2.5 x 2.5 x 2.5 mm) (Hua et al., 2008) and has been used to evaluate microstructural deficits in AD (Araque Caballero et al., 2018; Kantarci et al., 2017). Here, we conducted probabilistic tractography in 100 HCP participants (resolution: 1.25 x 1.25 x 1.25 mm) (Van Essen et al., 2013). Using well-established methods to create white matter tract templates (Archer et al., 2018), we have provided a newly available white matter tract atlas of the uncinate fasciculus, parietal component of the inferior longitudinal fasciculus, and cingulum. In addition to recently available templates of the fornix (Brown et al., 2017) and tapetum (Archer, D. B. et al., 2019), this atlas provides significantly more coverage of the brain compared to the aforementioned tractography template (see Supplemental Figure 1). Despite these strengths, this study used a cohort which is both highly educated and primarily non-Hispanic white individuals, thus limiting the generalizability to other cohorts. An additional potential limitation is that our dMRI acquisition was single-shell, and therefore, we could not perform advanced multi-compartment models such as NODDI; however, recent studies have shown that the extracellular compartment component derived from NODDI (i.e., VISO) are highly associated with FW. Further, while FW elimination is a novel post-processing technique to quantify both extracellular and intracellular microstructure in a dMRI image, it is still unclear what exact cellular processes contribute to each variable. Still, since our novel medial temporal lobe tract templates are freely available, we are confident that future studies can easily incorporate these into studies and begin to further elucidate these mechanisms.
In conclusion, this study provided compelling evidence that changes in hippocampal volume and FW white matter metrics in tracts projecting from the hippocampus co-occur and synergistically interact. Findings provide additional evidence that AD is a network-level disease, with white matter alterations tightly coupled with gray matter changes, and the downstream consequences of gray matter atrophy. White matter and gray matter metrics of damage are likely complementary, and both should be thoughtfully incorporated into theoretical models of aging and AD.
Supplementary Material
Highlights.
Evaluated free-water (FW) metrics in medial temporal lobe (MTL) white matter tracts
MTL FW is associated with hippocampal volume
MTL FW is associated with memory and executive function
MTL FW metrics have unique contribution to cognitive performance
MTL FW metrics interact with hippocampal volume to predict cognitive decline
Funding Acknowledgements:
R01-AG059716, K01-AG049164, IIRG-08-88733, R01-AG034962, R01-AG056534, K24-AG046373, F30-AG064847, T32-GM007347
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: No authors report a conflict of interest relevant to this research.
Literature Cited
- Andersson JLR, Sotiropoulos SN, 2016. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125, 1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque Caballero MA, Suarez-Calvet M, Duering M, Franzmeier N, Benzinger T, Fagan AM, Bateman RJ, Jack CR, Levin J, Dichgans M, Jucker M, Karch C, Masters CL, Morris JC, Weiner M, Rossor M, Fox NC, Lee JH, Salloway S, Danek A, Goate A, Yakushev I, Hassenstab J, Schofield PR, Haass C, Ewers M, 2018. White matter diffusion alterations precede symptom onset in autosomal dominant Alzheimer's disease. Brain 141(10), 3065–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer DB, Bricker JT, Chu WT, Burciu RG, McCracken JL, Lai S, Coombes SA, Fang R, Barmpoutis A, Corcos DM, Kurani AS, Mitchell T, Black ML, Herschel E, Simuni T, Parrish TB, Comella C, Xie T, Seppi K, Bohnen NI, Muller MLTM, Albin RL, Krismer F, Du G, Lewis MM, Huang X, Li H, Pasternak O, McFarland NR, Okun MS, Vaillancourt DE, 2019. Development and validation of the automated imaging differentiation in parkinsonism (AID-P): a multicentre machine learning study. The Lancet Digital Health 1(5), e222–e231. [DOI] [PubMed] [Google Scholar]
- Archer DB, Coombes SA, McFarland NR, DeKosky ST, Vaillancourt DE, 2019. Development of a transcallosal tractography template and its application to dementia. Neuroimage 200, 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer DB, Vaillancourt DE, Coombes SA, 2018. A Template and Probabilistic Atlas of the Human Sensorimotor Tracts using Diffusion MRI. Cereb Cortex 28(5), 1685–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA, 2016. Relation of cerebral vessel disease to Alzheimer's disease dementia and cognitive function in elderly people: a cross-sectional study. The Lancet. Neurology 15(9), 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asman AJ, Landman BA, 2012. Formulating spatially varying performance in the statistical fusion framework. Medical Imaging, IEEE Transactions on 31(6), 1326–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC, 2008. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12(1), 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, Waters C, Jimison P, Shepherd E, Sevush S, Graff-Radford N, Newland D, Todd M, Miller B, Gold M, Heilman K, Doty L, Goodman I, Robinson B, Pearl G, Dickson D, Duara R, 2002. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord 16(4), 203–212. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, 2002. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed 15(7-8), 435–455. [DOI] [PubMed] [Google Scholar]
- Besson FL, La Joie R, Doeuvre L, Gaubert M, Mezenge F, Egret S, Landeau B, Barre L, Abbas A, Ibazizene M, de La Sayette V, Desgranges B, Eustache F, Chetelat G, 2015. Cognitive and Brain Profiles Associated with Current Neuroimaging Biomarkers of Preclinical Alzheimer's Disease. J Neurosci 35(29), 10402–10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzali M, Giulietti G, Basile B, Serra L, Spano B, Perri R, Giubilei F, Marra C, Caltagirone C, Cercignani M, 2012. Damage to the cingulum contributes to Alzheimer's disease pathophysiology by deafferentation mechanism. Hum Brain Mapp 33(6), 1295–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CA, Johnson NF, Anderson-Mooney AJ, Jicha GA, Shaw LM, Trojanowski JQ, Van Eldik LJ, Schmitt FA, Smith CD, Gold BT, 2017. Development, validation and application of a new fornix template for studies of aging and preclinical Alzheimer's disease. Neuroimage Clin 13, 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, Jones RN, Mukherjee S, Curtis SMK, Harvey D, 2012. Development and assessment of a composite score for memory in the Alzheimer's Disease Neuroimaging Initiative (ADNI). Brain Imaging and Behavior 6(4), 502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers LG, de Groot M, Hofman A, Krestin GP, van der Lugt A, Niessen WJ, Vernooij MW, Ikram MA, 2016. Altered tract-specific white matter microstructure is related to poorer cognitive performance: The Rotterdam Study. Neurobiol Aging 39, 108–117. [DOI] [PubMed] [Google Scholar]
- Croall ID, Lohner V, Moynihan B, Khan U, Hassan A, O'Brien JT, Morris RG, Tozer DJ, Cambridge VC, Harkness K, Werring DJ, Blamire AM, Ford GA, Barrick TR, Markus HS, 2017. Using DTI to assess white matter microstructure in cerebral small vessel disease (SVD) in multicentre studies. Clin Sci (Lond) 131(12), 1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB, 1994. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke 25(1), 40–43. [DOI] [PubMed] [Google Scholar]
- Frankó E, Joly O, for the Alzheimer’s Disease Neuroimaging, I., 2013. Evaluating Alzheimer’s Disease Progression Using Rate of Regional Hippocampal Atrophy. PLOS ONE 8(8), e71354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann D, Nesbitt D, Shafto M, Rowe JB, Price D, Gadie A, Cam CAN, Kievit RA, 2019. Strong and specific associations between cardiovascular risk factors and white matter micro- and macrostructure in healthy aging. Neurobiol Aging 74, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford KA, Liu D, Neal JE, Acosta LMY, Bell SP, Wiggins ME, Wisniewski KM, Godfrey M, Logan LA, Hohman TJ, Pechman KR, Libon DJ, Blennow K, Zetterberg H, Jefferson AL, 2018. Validity and Normative Data for the Biber Figure Learning Test: A Visual Supraspan Memory Measure. Assessment, 1073191118773870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glosser G, Cole L, Khatri U, DellaPietra L, Kaplan E, 2002. Assessing nonverbal memory with the Biber Figure Learning Test-Extended in temporal lobe epilepsy patients. Arch Clin Neuropsychol 17(1), 25–35. [PubMed] [Google Scholar]
- Goukasian N, Porat S, Blanken A, Avila D, Zlatev D, Hurtz S, Hwang KS, Pierce J, Joshi SH, Woo E, Apostolova LG, 2019. Cognitive Correlates of Hippocampal Atrophy and Ventricular Enlargement in Adults with or without Mild Cognitive Impairment. Dement Geriatr Cogn Dis Extra 9(2), 281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy AR, Ly M, Carlsson CM, Okonkwo OC, Zetterberg H, Blennow K, Sager MA, Asthana S, Johnson SC, Alexander AL, Bendlin BB, 2017. Microstructural white matter alterations in preclinical Alzheimer's disease detected using free water elimination diffusion tensor imaging. PLoS One 12(3), e0173982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S, 2008. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 39(1), 336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, 2018. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement 14(4), 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Tangalos EG, Kokmen E, 2000. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology 55(4), 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Gifford KA, Acosta LMY, Bell SP, Donahue MJ, Taylor Davis L, Gottlieb J, Gupta DK, Hohman TJ, Lane EM, 2016. The Vanderbilt Memory & Aging Project: Study Design and Baseline Cohort Overview. Journal of Alzheimer's Disease(Preprint), 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM, 2012. FSL. Neuroimage 62(2), 782–790. [DOI] [PubMed] [Google Scholar]
- Ji F, Pasternak O, Ng KK, Chong JSX, Liu S, Zhang L, Shim HY, Loke YM, Tan BY, Venketasubramanian N, Chen CL, Zhou JH, 2019. White matter microstructural abnormalities and default network degeneration are associated with early memory deficit in Alzheimer's disease continuum. Sci Rep 9(1), 4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Murray ME, Schwarz CG, Reid RI, Przybelski SA, Lesnick T, Zuk SM, Raman MR, Senjem ML, Gunter JL, Boeve BF, Knopman DS, Parisi JE, Petersen RC, Jack CR Jr., Dickson DW, 2017. White-matter integrity on DTI and the pathologic staging of Alzheimer's disease. Neurobiol Aging 56, 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Raz N, 2009. Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia 47(3), 916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresge HA, Khan OA, Wagener MA, Liu D, Terry JG, Nair S, Cambronero FE, Gifford KA, Osborn KE, Hohman TJ, Pechman KR, Bell SP, Wang TJ, Carr JJ, Jefferson AL, 2018. Subclinical Compromise in Cardiac Strain Relates to Lower Cognitive Performances in Older Adults. Journal of the American Heart Association 7(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH, 2011. The diagnosis of dementia due to Alzheime's disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia: The Journal of the Alzheimer's Association 7(3), 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Mole JP, Sims R, Fasano F, Evans J, Jones DK, Aggleton JP, Baddeley RJ, 2019. Fornix white matter glia damage causes hippocampal gray matter damage during age-dependent limbic decline. Sci Rep 9(1), 1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Okonkwo OC, Oishi K, Mori S, Tighe S, Miller MI, Ceritoglu C, Brown T, Albert M, Lyketsos CG, 2012. Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer's disease. Alzheimers Dement 8(2), 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV, 2015. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85(2), 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Betensky RA, Hedden T, Schultz AP, Amariglio RE, Rentz DM, Johnson KA, Sperling RA, 2014. Synergistic effect of β-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA neurology 71(11), 1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo OC, Xu G, Oh JM, Dowling NM, Carlsson CM, Gallagher CL, Birdsill AC, Palotti M, Wharton W, Hermann BP, LaRue A, Bendlin BB, Rowley HA, Asthana S, Sager MA, Johnson SC, 2014. Cerebral blood flow is diminished in asymptomatic middle-aged adults with maternal history of Alzheimer's disease. Cereb Cortex 24(4), 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y, 2009. Free water elimination and mapping from diffusion MRI. Magn Reson Med 62(3), 717–730. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Westin CF, Bouix S, Seidman LJ, Goldstein JM, Woo TU, Petryshen TL, Mesholam-Gately RI, McCarley RW, Kikinis R, Shenton ME, Kubicki M, 2012. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci 32(48), 17365–17372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev PS, Zhuang L, Braidy N, Wen W, 2013. Is Alzheimer's a disease of the white matter? Current opinion in psychiatry 26(3), 244–251. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA, 2007. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69(24), 2197–2204. [DOI] [PubMed] [Google Scholar]
- Seab JP, Jagust WJ, Wong ST, Roos MS, Reed BR, Budinger TF, 1988. Quantitative NMR measurements of hippocampal atrophy in Alzheimer's disease. Magn Reson Med 8(2), 200–208. [DOI] [PubMed] [Google Scholar]
- van der Flier WM, Skoog I, Schneider JA, Pantoni L, Mok V, Chen CLH, Scheltens P, 2018. Vascular cognitive impairment. Nat Rev Dis Primers 4, 18003. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K, Consortium, W.U.-M.H., 2013. The WU-Minn Human Connectome Project: an overview. Neuroimage 80, 62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Knopman DS, 2016. The role of cerebrovascular disease when there is concomitant Alzheimer disease. Biochimica et biophysica acta 1862(5), 952–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Lesnick TG, Przybelski SA, Graff-Radford J, Reid RI, Lowe VJ, Zuk SM, Senjem ML, Schwarz CG, Gunter JL, Kantarci K, Machulda MM, Mielke MM, Petersen RC, Knopman DS, Jack CR Jr., 2018. Development of a cerebrovascular magnetic resonance imaging biomarker for cognitive aging. Ann Neurol 84(5), 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, Niessen WJ, Van der Lugt A, Breteler MM, 2009. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry 66(5), 545–553. [DOI] [PubMed] [Google Scholar]
- Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB, 1991. Probability of stroke: a risk profile from the Framingham Study. Stroke 22(3), 312–318. [DOI] [PubMed] [Google Scholar]
- Yasmin H, Nakata Y, Aoki S, Abe O, Sato N, Nemoto K, Arima K, Furuta N, Uno M, Hirai S, Masutani Y, Ohtomo K, 2008. Diffusion abnormalities of the uncinate fasciculus in Alzheimer's disease: diffusion tensor tract-specific analysis using a new method to measure the core of the tract. Neuroradiology 50(4), 293–299. [DOI] [PubMed] [Google Scholar]
- Zhuang L, Sachdev PS, Trollor JN, Reppermund S, Kochan NA, Brodaty H, Wen W, 2013. Microstructural white matter changes, not hippocampal atrophy, detect early amnestic mild cognitive impairment. PLoS One 8(3), e58887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.