Abstract
The corneal wound healing response is typically initiated by injuries to the epithelium and/or endothelium that may also involve the stroma. However, it can also be triggered by immune or infectious processes that enter the stroma via the limbal blood vessels. For mild injuries or infections, such as epithelial abrasions or mild controlled microbial infections, limited keratocyte apoptosis occurs and the epithelium or endothelium regenerates, the epithelial basement membrane (EBM) and/or Descemet’s basement membrane (DBM) is repaired, and keratocyte- or fibrocyte-derived myofibroblast precursors either undergo apoptosis or revert to the parent cell types. For more severe injuries with extensive damage to EBM and/or DBM, delayed regeneration of the basement membranes leads to ongoing penetration of the pro-fibrotic cytokines transforming growth factor (TGF) β1, TGFβ2 and platelet-derived growth factor (PDGF) that drive the development of mature alpha-smooth muscle actin (SMA)+ myofibroblasts that secrete large amounts of disordered extracellular matrix (ECM) components to produce scarring stromal fibrosis. Fibrosis is dynamic with ongoing mitosis and development of SMA+ myofibroblasts and continued autocrine-or paracrine interleukin (IL)-1-mediated apoptosis of myofibroblasts and their precursors. Eventual repair of the EBM and/or DBM can lead to at least partial resolution of scarring fibrosis.
Keywords: cornea, injury, infection, wound healing, keratocyte apoptosis, corneal fibroblasts, fibrocytes, myofibroblasts, fibrosis, scarring. corneal, TGF beta, PDGF, interleukin-1, stromal-epithelial interactions
1. Introduction
The cornea is a unique organ for study of wound healing responses and interactions such as stromal-epithelial, stromal-endothelial, and epithelial-stromal-bone marrow-derived interactions due to its transparency and accessibility for manipulations and imaging. Because of the consistent anatomy of the normal cornea with well-defined epithelium, stroma, endothelium, basement membranes, innervation and limbal blood vessels, precise injuries can be performed and accurately monitored. In addition, many markers for cells involved in the corneal wound healing response—such as keratocytes, myofibroblasts, nerves, bone marrow-derived cells of different types—have been developed that allow specific interactions to be observed. In addition, many methodologies such as multiplex immunohistochemistry, quantitative real-time polymerase chain reaction (RT-PCR), laser capture RT-PCR, and chimeric animals have facilitated complex investigations of the numerous interactions that comprise the corneal wound healing response.
The complexity of the wound healing response did not begin to be well-appreciated until approximately 25 years ago. Prior to 1995, keratocytes were thought to be relatively quiescent cells that only monitored, maintained and repairing collagen and other extracellular matrix materials that made up the corneal stroma to preserve corneal transparency. Several investigators (Dohlman et al., 1968; Nakayasu, 1988; Crosson, 1989; Campos et al., 1994), apparently beginning with Claes Dohlman and colleagues in 1968, noted that when the corneal epithelium had a scrape injury, the keratocytes beneath the epithelium disappeared when the tissue was examined histologically. However, the mechanism of this disappearance was not appreciated and was attributed to artifact or other non-physiological factors. In 1995, when our findings were first presented at the Association for Research in Vision and Ophthalmology (ARVO; Wilson et al. 1995)) demonstrating that corneal epithelial scrape injuries in mice triggered anterior keratocyte apoptosis (Fig. 1) (Wilson et al., 1996), David M. Maurice, PhD, a giant in corneal physiology, approached the microphone: “Young man,” he began with his authoritative British accent, “are you also suggesting that keratocytes have the capacity to undergo cell division?” “Yes, Dr. Maurice,” I replied confidently, “after twenty to thirty percent of the cells in the stroma die, I don’t see how the keratocyte density could otherwise return to normal two weeks later.” “Hmm,” he muttered, yielding the microphone. It was be several years before James D. Zieske, PhD, and his colleagues, demonstrated conclusively that many of the remaining keratocytes in the posterior and peripheral stroma surrounding the decellularized zone begin to proliferate twelve to twenty-four hours after the original epithelial injury (Zieske et al., 2001). Surprisingly, it was nearly 25 years later before my lab demonstrated that corneal endothelial scrape injury produced similar apoptosis of posterior keratocytes underlying the endothelium that involves endothelial-stromal interactions (Medeiros, et al., 2018a). During that 25 intervening years, however, the work of numerous investigators has contributed to an explosive increase in our understanding of the remarkable regulatory, cellular, matrix, nerve and bone marrow-derived cell interactions involved in the varying responses to traumatic, surgical, infectious and disease-related corneal injuries. The discoveries are far from finished, and investigators for generations to come will continue to expand our understanding of the intricate details of the corneal wound healing response. In the paragraphs to follow, however, a detailed description of our current understanding of corneal wound healing will hopefully serve as a foundation for better appreciation of the physiology and pathophysiology of the corneal wound healing response that will be helpful to clinicians involved in clinical care and researchers striving to unravel still-uncertain details of the corneal responses to injuries and diseases. Also, see the article on the biology of keratorefractive surgery later in this Special Issue of Experimental Eye Research.
Fig. 1.

The original images illustrating keratocyte apoptosis in an adult Balb/C mouse cornea after epithelial scrape injury. A. Terminal deoxynucleotidyl transferase-dUTP nick end labeling (TUNEL) assay performed to detect anterior keratocytes with fragmented DNA characteristic of apoptosis (arrowheads) at four hours after epithelial debridement using a peroxidase-based TUNEL assay and darkfield light microscopy. 200X mag. B. Confirmatory transmission electron microscopy at one hour after epithelial debridement shows a keratocyte with chromatin condensation (C) and cytoplasmic contents of the cell in apoptotic bodies (arrowheads) that disperse into the surrounding stroma. 25,000X. Reprinted with permission from Wilson SE, et al. Exp. Eye Res. 1996;62:325–8.
2. Initiation of the corneal wound healing response
Many corneal injuries involve damage to the epithelium and epithelial basement membrane (EBM). However, some injuries also involve the endothelium and Descemet’s basement membrane (DBM)—which in some cases can be the localized site of original corneal injury—for example, in cytomegalovirus (CMV) endotheliitis (Koizumi et al., 2006). In still other cases, neither the epithelium or endothelium are initially injured but can subsequently be damaged—for example in infectious or immune-mediated disorders in the corneal periphery such as staph marginal keratitis (Thygeson, 1947) or early Mooren’s corneal ulcers (Wilson et al., 1993). Much of the following discussion also applies to corneal endothelial-stromal injuries, but will concentrate on epithelial-stromal injuries, since most of the published research has focused on these types of injuries.
When the corneal epithelium (and/or endothelium) is injured by trauma, surgery or infection, interleukin (IL)-1α and IL-1β (that are constitutively produced by corneal epithelial and endothelial cells) are released by the injured or dead cells, penetrate into the corneal stroma and bind to IL-1 receptors expressed by keratocytes (Wilson and Lloyd, 1991; Wilson et al., 1992; Wilson et al., 1994b; Weng et al., 1997). The first detectable change in the stroma is keratocyte apoptosis in cells adjacent to the injury that is modulated by released IL-1α and IL-1β—likely depending on the localized concentration of the cytokines—via the Fas-Fas ligand apoptosis regulatory system expressed in the cornea (Wilson et al., 1996; Weng et al., 1997). This keratocyte apoptosis response occurs within a few minutes, in that it is detectible by TEM (Fig. 1) in anterior keratocytes even if an intact corneoscleral segment has its epithelium (or endothelium) scrapped with a scalpel blade and is quickly plunged into TEM fixative (Wilson SE, unpublished data, 2001). It also doesn’t require the presence of tears (Mohan et al., 2002), although IL-1 is detectible in tears (Solomon et al., 2001). Importantly, the more extensive the injury to the corneal epithelium and underlying stroma, the greater the level of apoptosis of keratocytes (Mohan et al., 2003). Keratocyte apoptosis in response to epithelial injury occurs in every species studied thus far, including humans (Ambrósio et al, 2009).
Why does a system that triggers this fairly rapid keratocyte apoptosis response exist? We hypothesized it is a rapid response defense system to limit the spread of viruses that can infect the corneal epithelium, spread to keratocytes and the endothelial cells, and into the eye (and even the brain) prior to mobilization of an immune response to the virus. Viruses such as herpes simplex virus (HSV) or smallpox virus have the potential for this type of spreading from surface epithelium to other structures in the eye and brain. This hypothesis was supported by experiments in rabbits performed with the late virologist James M. Hill, PhD where the corneal epithelium was infected with herpes simplex virus-1 strain 17 Syn+ without scarification and the underlying keratocytes underwent apoptosis detected with the TUNEL assay and TEM (Wilson et al., 1997). This apoptosis system was subsequently shown in collaboration with George R. Stark, PhD to be defective in STAT1 null mice (Mohan et al., 2000) and when mice had a cornea infected with HSV more than 70% of the STAT1 knockout mice, but 0% of the control mice, died within a few days from HSV encephalitis (Hill JM, Wilson SE, unpublished data, 2001).
After the injury or infection of the corneal epithelium, surviving deeper and/or peripheral keratocytes (and their progeny corneal fibroblasts) that do not undergo apoptosis (presumably due to the IL-1 concentration being lower the further the cells are from the injured epithelium) are also modulated by IL-1 that enters the stroma. IL-1 triggers keratocytes and corneal fibroblasts to produce many chemokines, including monocyte chemotactic and activating factor (MCAF), granulocyte colony-stimulating factor (G-CSF), neutrophil-activating peptide (ENA-78), and monocyte-derived neutrophil chemotactic factor (MDNCF) (Hong et al., 2001). The IL-1 released by the epithelial injury, along with other cytokines and chemokines released by the injured epithelial cells, as well as chemokines produced by keratocytes in response to IL-1, attract tens of thousands of bone marrow-derived cells (Fig. 2) into the corneal stroma beginning a few hours after the injury and continuing for at least 10 days (Wilson et al., 2004; Barbosa et al., 2010a; Lassance et al., 2018). These bone marrow-derived cells include monocytes, macrophages, lymphocytes, fibrocytes and other cells. IL-1α and IL-1β also upregulate production of hepatocyte growth factor (HGF) and keratinocyte growth factor (KGF) by keratocytes and corneal fibroblasts (Wilson et al., 1993; Li et al., 1996; Weng et al., 1997; Li and Tseng, 1997). HGF and KGF are classical modulators of stromal-epithelial interactions that, along with autocrine epidermal growth factor (EGF), transforming growth factor (TGF) α and other growth factors produced by the epithelium and lacrimal glands (Tervo et al., 1997; Wilson et al., 1999), regulate the proliferation, motility, differentiation, and apoptosis of the healing epithelial cells (Wilson et al., 1994a).
Fig. 2.

The influx of large numbers of bone marrow-derived cells into the corneas of chimeric mice with GFP-labeled bone marrow-derived cells after epithelial scrape injury. Few GFP+ cells were detected in control corneas that did not have epithelial scrape injuries (Con 0 is unwounded, Con 24 and Con 72 are unwounded at 24 and 72 hours, respectively). Similarly, few GFP+ cells are detected immediately following epithelial scrape (SCR 0 min). However, by one day after epithelial scrape injury (SCR 24 hrs), and continuing at 3 days after epithelial scrape injury (SCR 72 hrs), thousands of bone marrow-derived cells entered the cornea. Some GFP+ bone marrow-derived cells were detected in the corneas up to the final observation point of 10 days. Mag 10X. Reprinted with permission from Wilson et al. Invest. Ophthalmol. Vis. Sci, 2004;45:2201–2211.
IL-1α and IL-1β released by the injured epithelium also upregulate the production of metalloproteinases and collagenases by surviving keratocytes, corneal fibroblasts and myofibroblasts (Girard et al., 1991; West-Mays et al., 1995; Li et al., 2001). These metalloproteinases and collagenases have a critical role in the stromal wound healing response since they are involved in the reorganization of the stromal ECM and degradation of disordered extracellular matrix that may be deposited in the stroma after injury, infections and surgeries (Girard et al., 1991; West-Mays et al., 1995; Li et al., 2001). Interleukin-1α (and tumor necrosis factor alpha) upregulates the expression of metalloproteinases and collagenases by corneal stromal cells via an autocrine IL-1 loop and also regulates expression in corneal epithelial cells (Girard et al., 1991; West-Mays et al., 1995; Li et al., 2001). IL-1 receptor antagonist produced by corneal epithelial cells downregulates MMP-2 produced by corneal fibroblasts (Ko et al., 2010).
3. Healing of the corneal epithelium
During homeostasis, the adult corneal epithelium is sustained by an integrated process of cell proliferation, migration, differentiation, and apoptosis that maintains a layer of basal cells and four to five cell layers of non-keratinized, stratified squamous epithelial cells that continuously desquamate at the surface (Liu and Kao., 2015). After traumatic, microbial or surgical injury, the epithelium must heal in a timely manner to re-establish barrier function (see article by Steven D. Klyce, PhD in this Special Issue of EER) and regenerate the normal EBM so that the fibrotic stromal wound healing response can be terminated in favor of regenerative repair. Tightly-regulated interactive signaling cascades involve the healing corneal epithelium, tears, lacrimal glands, corneal nerves, keratocytes, corneal fibroblasts and bone marrow-derived cells, along with cytokines, growth factors and other modulators they produce (Chi and Trinkaus-Randall, 2013; Yu et al., 2010). The leading edge of the healing corneal epithelium involves a single layer of flattened epithelial cells that maintain contact with neighboring epithelial cells and the underlying matrix. Actin ‘purse string’ filaments are anchored by E-cadherin-mediated adherens junctions and coordinate movement of the advancing epithelial margin (Danjo and Gipson, 1998). As the epithelial defect is closed, the basal epithelial cells begin regeneration of the EBM through the production of self-assembling laminins 511 and 521 that subsequently initiates the generation of a mature EBM through the coordinated actions of the epithelium and underlying keratocytes producing EBM components such as other laminins, perlecan, nidogens, and collagen type IV (Yurchenco et al., 1986; Wilson et al., 2017). The closure of the epithelial defect can be impeded by disorders such as limbal stem cell deficiency, viral infections, systemic diseases like diabetes mellitus and many other disorders and diseases of the cornea (Ljubimov and Saghizadeh, 2015). If a persistent epithelial defect develops, then fibrotic stromal repair associated with the generation of myofibroblasts occurs in the area of the cornea devoid of epithelium (Wilson et al, 2018). Even if the epithelium fully closes, repair of the mature EBM with lamina lucida and lamina densa may be impeded mechanically by a rough stromal surface and/or extensive loss of keratocytes that contribute to EBM regeneration (Wilson et al., 2017). More detailed information regarding the healing of the epithelium can be found in articles in this EER Special Issue series devoted to the corneal epithelium, corneal limbus, tears and epithelial basement membrane.
4. The fate of myofibroblast progenitors
Simultaneous with the release of IL-1α and IL-1β by injured corneal epithelial or endothelial cells, other growth factors, importantly including transforming growth factor (TGF)β1 and TGFβ2 (Wilson et al., 1991; Wilson et al., 1992a,b; Wilson et al., 1994a; Nishida et al., 1995; Huh et al., 2009), and platelet-derived growth factor PDGF (Kim et al., 1999) are upregulated, released and activated by corneal epithelial (and/or endothelial cells) and matrix, and enter into the corneal stroma adjacent to where the EBM (and/or DBM) was damaged or removed. TGFβ (used henceforth as a generic term for TGFβ1 and TGFβ2) is also present in the tears (Gupta et al., 1996; Vesaluoma et al., 1997) and aqueous humor (Granstein et al., 1990; Cousins et al., 1991; Bilgihan et al., 1997).
The EBM and/or DBM are critical regulators of TGFβ and PDGF activation and localization. Specific components of EMB and DBM that bind these profibrotic growth factors and prevent their passage into the stroma include perlecan (TGF β1, TGF β2, PDGF AA, and PDGF BB), collagen IV (TGF β1 and TGF β2), and nidogens (PDGF AA and PDGF BB) (Yurchenco et al., 1986; Paralkar et al., 1991; Gohring et al., 1998; Shibuya et al., 2006; Iozzo et al., 2009; Behrens et al., 2012; Lecler et al., 2018). Perlecan, a major component in both corneal basement membranes, also produces a high negative charge related to its three heparan sulfate side chains (Dowd et al., 1999; Fannon et al., 2003; Gohring et al., 1998; Iozzo et al., 2009). Thus, perlecan also provides a non-specific barrier to TGFβ penetration through either EBM or DBM into the corneal stroma. For sufficiently high and sustained levels of TGFβ1 and TGFβ2 needed to drive myofibroblast development from keratocytes (via corneal fibroblasts) and bone marrow-derived fibrocytes to enter the stroma, the injury usually must include the EBM and/or DBM—although in some disorders adequate TGFβ may be delivered via the limbal blood vessels or stromal cells themselves. TGFβ expression and localization patterns in the corneal epithelium and stromal cells are very different in unwounded compared to wounded corneas, and injury to the corneal epithelium results in upregulation of TGFβ production in these cells (Wilson et al., 1994b; Nishida et al., 1995). Changes in TGFβ expression in corneal endothelial cells (Wilson and Lloyd, 1991) after injury have not been well-studied.
Thus, in the normal uninjured cornea, epithelial TGFβ and PDGF production and/or activation is relatively low and these growth factors are blocked from entry into the corneal stroma by the EBM and DBM. When injury to the epithelium-EBM and/or endothelium-DBM occurs, TGFβ and PDGF (and likely other growth factors and cytokines) enter the stroma at higher levels sufficient to trigger surviving keratocytes to transform into corneal fibroblasts, and then both corneal fibroblasts and fibrocytes are modulated to begin development into mature myofibroblasts (Stramer et al., 2003; Masur et al., 1996; Jester et al., 1996; Nakamura et al., 2002; Jester et al., 2002; Kaur et al., 2009; Chaurasia et al., 2011; Singh et al., 2011). Extracellular vesicles could mediate transfer of growth factors from the epithelium or endothelium into the stroma (Zieske et al., 2020). The development of mature vimentin+, alpha smooth muscle actin+, desmin+ (VAD+) corneal myofibroblasts in rabbits in vivo after epithelial-stromal injury that triggers stromal fibrosis takes two to four weeks (Chaurasia et al., 2011; Lassance et al., 2018). In humans, based on the time to appearance of scarring after corneal lacerations or high-correction photorefractive keratectomy, this development is prolonged to one to four months (Lipshitz et al., 1997). This delay in the development of mature SMA+ myofibroblasts that produce prodigious amounts of disordered extracellular matrix likely evolved to prevent the unnecessary development of these cells that produce scarring fibrosis when they are not needed, and their development after relatively minor injuries, such as photorefractive keratectomy, is probably a fibrotic disorder related to defects in EBM repair and/or myofibroblast developmental regulatory pathways.
In order to complete their development into mature myofibroblasts, corneal fibroblasts or fibrocytes must continue to have sufficient levels of TGFβ (and likely PDGF) or they will undergo apoptosis (Marino, et al., 2017a; Lassance et al., 2018; Medeiros et al., 2019). After a corneal abrasion (that removes EBM) or injury such as low-correction photorefractive keratectomy (PRK), the EBM is fully-regenerated in rabbits in eight to ten days (Santhanam, et al., 2017). Early after epithelial injury, stromal cells produce TGFβ1 and TGFβ2, but this expression progressively declines beginning a few days after a mild to moderate injury such as PRK (G.L. Tye, R.C. de Oliveira, S.E. Wilson, unpublished data, 2019). Once the EBM is fully regenerated, TGFβ and PDGF entry into the corneal stroma is curtailed and the myofibroblast precursors, or any mature myofibroblasts that have developed, that are both dependent on ongoing sufficient levels of TGFβ, undergo apoptosis. A similar process occurs after injury to the endothelium and DBM, but there is less tendency for DBM to be regenerated, even in animals where the endothelium can proliferate (Marino et al., 2017b; Medeiros et al., 2019). Thus, in the case of mild corneal injuries that heal normally, corneal fibroblasts and fibrocytes begin, but don’t complete, their development into mature myofibroblasts. Transient stroma opacity is often seen, for example after normal PRK without fibrosis, because corneal fibroblasts downregulate their production of crystallins relative to keratocytes and produce limited amounts of disordered extracellular matrix (Jester et al., 1999; Marino et al., 2017a). In contrast, after severe trauma, microbial keratitis or some surgeries—for example, at the donor-recipient interface after penetrating keratoplasty (Fig. 3), regeneration of the EBM (or replacement of the DBM with surgery) may take months or years, or may never be completed (Hassell and Birk, 2010; Marino et al., 2017b; Wilson et al., 2017). When this occurs, large numbers of mature myofibroblasts develop from both corneal fibroblasts and fibrocytes to produce stromal fibrosis (Fig. 3)—with the localization depending on the site and level of injury— as well as whether the EBM or DBM or both fail to regenerate (Jester et al., 1999; Hassell and Birk, 2010; Torricelli et al. 2013a,b; Marino et al., 2017a,b). These myofibroblasts are opaque (Jester et al., 1999 and produce large amounts of disordered extracellular matrix (Marino et al., 2017a,b) that produces corneal fibrosis (also termed scarring or severe haze) (Fig. 3) (Jester et al., 1999; Hassell and Birk, 2010; Torricelli et al. 2013a,b; Marino et al., 2017a,b). If the EBM and/or DBM is eventually regenerated or replaced, then stromal levels of TGFβ and PDGF drop, myofibroblasts undergo apoptosis, and keratocytes repopulate the affected stroma—where they function to remove the disordered ECM and restore normal stromal structure that decreases or eliminates corneal opacity (Torricelli et al., 2013a,b; Wilson et al., 2017).44,76,77 This process can take months or years (for example after high-correction PRK or corneal laceration) or may never occur in the case of severe trauma, infections, or burns—in which case the corneal scarring (fibrosis) is permanent. The DBM rarely regenerates after severe injury (Marino et al., 2017b; Medeiros et al., 2019) and usually must be replaced by penetrating keratoplasty, DSAEK or DMEK for posterior corneal fibrosis to decrease or resolve.
Fig. 3.
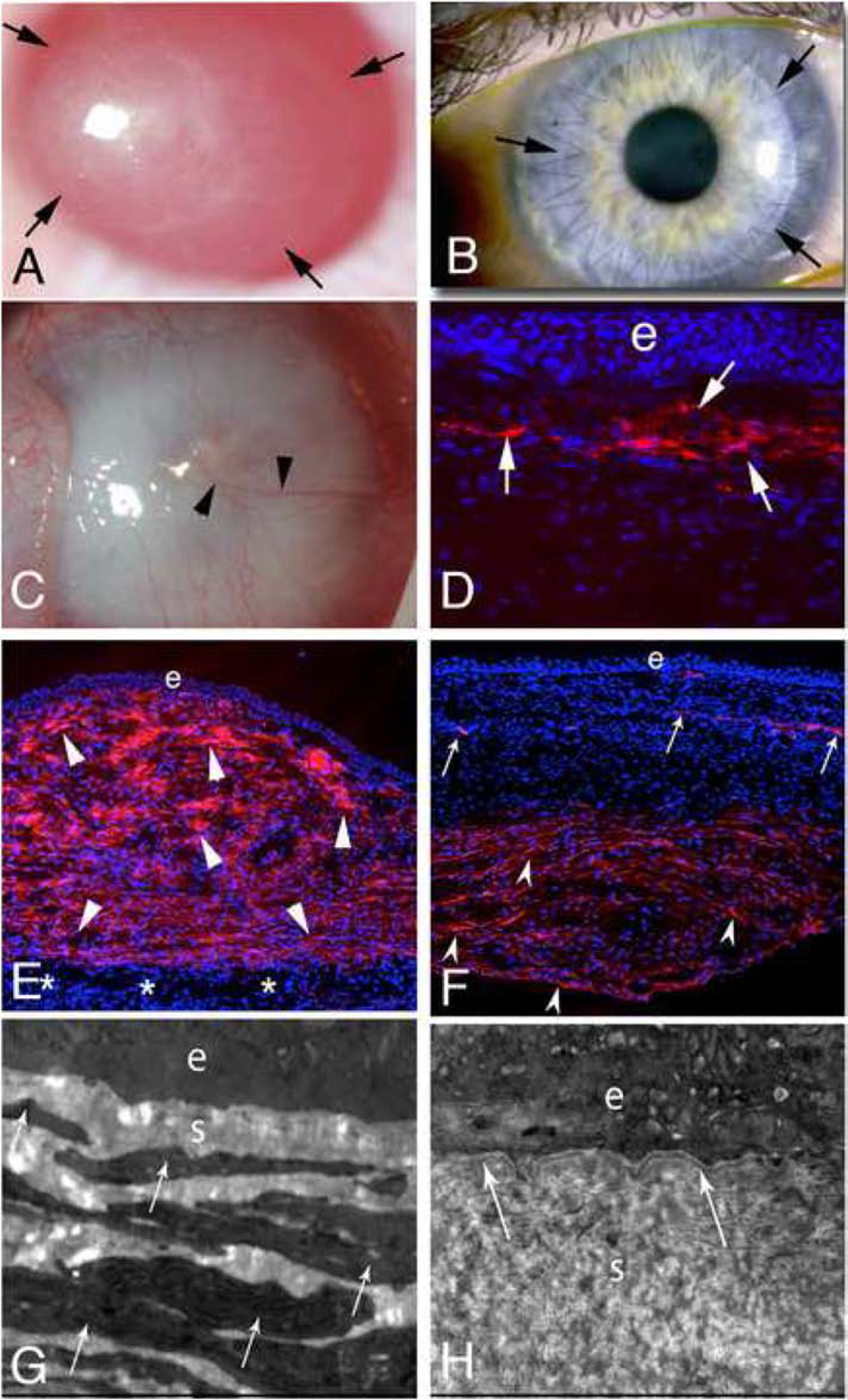
Fibrosis and myofibroblasts. A. At one month after −9 diopter PRK in the rabbit fibrosis (scarring or “late haze”) is present within the arrows, and is most dense on the left side of the cornea. B. At six months after penetrating keratoplasty in the human, fibrosis (arrows) is present within the donor-recipient interface and helps to hold the transplant in position once the sutures are removed. Immunohistochemistry performed on such corneas reveals myofibroblasts in the donor-recipient interface (not shown). C. At one month after severe Pseudomonas aeruginosa keratitis in the rabbit there is dense fibrosis (scarring) causing opacity of the entire cornea. Neovascularization (arrowheads) of the stroma can be noted. D. Immunohistochemistry for the myofibroblast marker α-smooth muscle actin (SMA) in a rabbit cornea at one month after −9D PRK shows a layer of myofibroblasts (arrows) in the anterior stroma. e is epithelium. Note there is artifactual dissociation of the epithelium from the myofibroblast-filled anterior stroma that occurred during sectioning of the cornea since the mature EBM had not regenerated. Mag. 200X. E. At one month after severe antibiotic-treated Pseudomonas aeruginosa keratitis in a rabbit, approximately 80% of the stroma is filled with SMA+ myofibroblasts (arrowheads). The infection did not spread completely through the stroma and there is normal stroma (*) with keratocytes just anterior to the endothelium. e is epithelium. Mag. 200X. F. In another rabbit cornea that had severe antibiotic-treated Pseudomonas aeruginosa keratitis at two months after infection, the anterior half of the stroma has very few SMA+ myofibroblasts, with the SMA present at neovascularization (arrows) being pericytes. In the posterior stroma, however, in this eye where the infection extended through the cornea and destroyed DBM and the endothelium, SMA+ myofibroblasts (arrowheads) fill the posterior stroma. e is epithelium. Mag 200X. G. Transmission electron microscopy (TEM) of the same cornea shown in E shows no epithelial basement membrane (EBM) lamina lucida or lamina densa beneath the epithelium (e). The anterior stroma is packed with stacked myofibroblasts (arrows) filled with rough endoplasmic reticulum. The stroma (s) between the myofibroblasts is disorganized. Mag. 25,000X. H. TEM of the same cornea shown in F shows EBM (arrows) with lamina lucida and lamina densa beneath the epithelium (e). Myofibroblasts have disappeared from the anterior stroma and the stromal matrix (s) is more organized, but not yet normal. Mag. 25,000X. C and E to H reprinted with permission from Marino GK, et al. Exp. Eye Res. 2017;161:101–105.
Corneal fibrosis can also occur after a minor injury—such as a corneal abrasion or low-correction PRK—if a persistent epithelial defect develops and the epithelium doesn’t heal within two weeks to a month—because the EBM cannot begin normal regeneration without contributions from the healed basal epithelial cells (Yurchenco et al., 1986; Wilson et al., 2018). These persistent epithelial defects can be due to factors such as ongoing infections, eyelid or lash abnormalities, neurotrophic disorders, systemic disease-related, or they can be idiopathic.
Thus, a drop in activated TGFβ entry into the stroma by a return to normal production by epithelial (and/or endothelial cells), as well as regeneration of EBM (and regeneration or replacement of DBM) is needed for resolution of fibrosis. Alternatively, changes in receptor expression or avidity could have a role in modulating TGFβ. Importantly, after injuries to the cornea, some stromal cells—keratocytes, corneal fibroblasts, myofibroblasts, monocytes, fibrocytes and other bone marrow-derived cells—may produce TGFβ during the early response to injury (Hassell et al., 1992; Meltendorf et al., 2009; Santhanam et al., 2019). However, the TGFβ produced by these cells isn’t of sufficient magnitude, duration or localization to drive myofibroblast generation and persistence in the absence of damage and defective regeneration of the EBM and/or Descemet’s basement membrane (Meltendorf et al., 2009; Marino et al., 2017a; Medeiros et al., 2019; Torricelli et al., 2013a,b).
Recent studies have demonstrated that regeneration of the EBM (and likely regeneration of DBM, if it occurs but usually only with small posterior injuries to endothelium and DBM) requires coordination between the healed epithelium and keratocytes (or endothelium and keratocytes in the case of DBM) (Hassell et al., 1992; Santhanam et al., 2015; Santhanam et al., 2017; Torricelli et al., 2015; Medeiros et al., 2019; Saikia et al., 2018). Thus, the epithelium and keratocytes produce perlecan, nidogen-1, nidogen-2, laminins and other components (Hassell et al., 1992; Santhanam et al., 2015; Santhanam et al., 2017; Torricelli et al., 2015; Medeiros et al., 2018a; Saikia et al., 2018). Factors that are known to interfere with EBM regeneration include stromal surface irregularity (Netto et al., 2006) and extensive loss of keratocytes that occurs with major traumatic, infectious or surgical injuries to the cornea (Mohan et al., 2003; Wilson, et al., 2017).
5. Resolution of the corneal wound healing response
The processes leading to resolution of the corneal wound healing response begin at the moment of injury—with the outcome determined by the interplay and competition between the numerous regenerative and fibrotic mechanisms—depending on the type, extent and duration of the insult, and possibly genetic factors. Thus, immediately after an acute traumatic or infectious injury to the epithelium and EBM, for example, IL-1α and possible other cytokines, released from the epithelium and chemokines produced by keratocytes and corneal fibroblasts draw bone marrow-derived cells, such as monocytes, macrophages, fibrocytes, and lymphocytes, into the corneal stroma, and many of these cells produce their own cytokines and chemokines that amplify the response (Shi and Pamer, 2011; Galligan and Fish, 2013). However, many of these cells, including fibrocyte myofibroblast progenitors, undergo apoptosis in an ongoing wave to control the level of inflammation and possible fibrosis (Lassance et al., 2018; Marino et al, 2017a,b). Keratocyte-derived progenitors, fibrocyte-derived progenitors, (and possibly other myofibroblast progenitors) begin development into mature myofibroblasts, but many of these cells abort their development and die—likely due to insufficient TGFβ, regulatory checks or other factors. If the injured EBM regenerates in a timely manner and the penetration of high levels of IL-1α, TGFβ, PDGF, and other cytokines, into the stroma is diminished, the regenerative processes become dominant and the cornea is restored to its normal homeostatic state within days (Santhanam, et al., 2017). If, however, the injury is extensive, such as a severe infection or injury, or there is delayed closure of the epithelium for whatever reason (Wilson et al., 2018), then the EBM does not regenerate—at least at some locations in the cornea—and growth factors and cytokines continue to penetrate, with the balance of processes tipped to the development of mature myofibroblasts from precursor cells, leading to fibrosis (Wilson et al., 2017; Marino et al., 2017b, Medeiros et al., 2019). If the injury includes the DBM, then the fibrotic response in the posterior cornea tends to be prolonged because there is less tendency to regenerate the DBM—even in species where the corneal endothelial cells can proliferate (Marino et al., 2017b, Medeiros et al., 2019). Less study has been devoted to corneal wound healing in chronic injuries associated with dystrophies, degenerations, and systemic diseases affecting the cornea, but presumably ongoing, low-grade profibrotic processes, perhaps involving injury to EBM or DBM, or which directly trigger alterations in the normal stromal collagen lamellae or lead to deposition of excess or defective extracellular matrix components underlie the pathophysiology of these diseases.
In the case of ongoing development versus apoptotic disappearance of myofibroblasts and their precursors, a balance between extra-stromal TGFβ (epithelial, tear film, endothelial and aqueous humor) and paracrine/autocrine IL-1 appears to be a key determinate of the fate of individual myofibroblasts (Kaur et al, 2009; Barbosa et al., 2010b; Barbosa et al., 2012; Wilson and Esposito, 2009). Corneal epithelial and stromal cells also produce interleukin-1 receptor antagonist (IL-1RA) that controls IL-1-modulated processes (Torres et al., 1994; Huer et al., 2009; Ko et al., 2010), but how this IL-1RA production is regulated in coordination with the production of the cytokines themselves by these cells is poorly understood. Thus, while an individual myofibroblast receives adequate TGFβ1 and/or TGFβ2, the effects of IL-1 in triggering apoptosis are checked, whether that IL-1 is produced by the myofibroblast itself (autocrine) or surrounding (paracrine) stromal cells (Fig. 4) (Kaur et al, 2009; Barbosa et al., 2010b; Barbosa et al., 2012; Wilson and Esposito, 2009). Once the EBM (and/or DBM) is regenerated or replaced, then the IL-1 effect predominates and the cell undergoes apoptosis.
Fig. 4.

IL-1α expression in stromal cells at one month after −9D PRK in rabbits. A. DAPI staining to show nuclei in all cells at one month after PRK in a rabbit. B. IHC for the myofibroblasts marker SMA. Arrows indicate SMA+ myofibroblasts in the anterior stroma. C. IHC for IL-1α. Many anterior stromal cells produce IL-1α (arrows). D. Overlay of B and C shows that some of the stromal cells producing IL-1α are SMA+ myofibroblasts (arrows) but many surrounding cells that could be keratocytes, corneal fibroblasts or bone marrow-derived cells are also producing IL-1α. Mag 200X.
6. Other processes associated with corneal wound healing
Immune related cells, such as monocytes, macrophages, fibrocytes and lymphocytes, are drawn into the cornea by cytokines such as IL-1 released from injured epithelial or endothelial cells and chemokines produced by keratocytes and corneal fibroblasts (Hong et al., 2001; Lassance et al., 2018). Some of these cells die by apoptosis as soon as they enter to stroma (Lassance et al., 2018), but others persist until the offending organisms are eliminated, in the case of infection, or once the disease process resolves.
After injuries to the cornea such as microbial infections, PRK or LASIK, the corneal nerves that innervate the corneal epithelium are commonly damaged and reinnervation usually occurs over months (Latvala et al., 1996; Calvillo et al., 2004; Medeiros et al., 2018b). Myofibroblasts, and the fibrosis they produce, can retard the innervation process (Jeon et al., 2018). Damage to the trigeminal nerve that innervates the cornea leads to diminished sensation and is often associated with corneal epithelial breakdown, non-healing epithelial defects and associated corneal pathology (Mastropasqua et al, 2017).
Corneal neovascularization often occurs with severe corneal infections (Fig. 3), corneal transplantation, especially in high risk cases, chronic trauma to the cornea caused by lashes or contact lenses, corneal burns, or limbal stem cell deficiency (Marino et al., 2017b; Sharif and Sharif, 2019).
7. Conclusions
After any traumatic, infectious, surgical, or disease-related injury to the cornea—even a minor abrasion that damages the EBM—the complex corneal stromal wound healing process is initiated with the development of corneal fibroblasts from keratocyte in proximity to the injury and entry of fibrocytes from the limbal blood vessels. How the wound healing process ensues from this point depends on the extent of injury, and especially whether or not the EBM (and/or DBM) regenerates to normal. If the epithelium heals and the EBM is restored, then the developing myofibroblast precursors are eliminated and transparency is maintained or can be reestablished (Torricelli et al., 2013a, _S1_Reference47Marino et al., 2017a,b; Lassance et al., 2018). If the EBM doesn’t heal in a timely manner, then mature myofibroblasts complete their development and fibrosis (scarring) is generated (_S1_Reference17Torricelli et al., 2013a, Marino et al., 2017a,b). Such fibrosis will persist until the EBM (and/or DBM if it is injured) is restored—likely because normal keratocytes eventually make their way through the fibrosis to cooperate with the epithelium in regenerating the EBM, resulting in a decrease in stromal TGFβ and PDGF levels to the point the mature myofibroblasts undergo apoptosis (Wilson et al., 2017). Subsequently, the keratocytes can completely repopulate the stroma and reabsorb disordered extracellular matrix and restore corneal transparency (Hassell and Birk, 2010; _S1_Reference78Torricelli et al., 2013b).
The complexity of the interactions between growth factors, cytokines and chemokines produced by corneal epithelial cells, keratocytes, corneal fibroblasts, fibrocytes, myofibroblasts, nerves, other infiltrating bone marrow-derived cells, the lacrimal glands and conjunctiva is indeed prodigious. In some ways, our current understanding is analogous to the proverbial group of blind men touching and describing different parts of an elephant. Generations of researchers will need to assimilate these interactions before the wound healing response and its modulation is fully-appreciated.
Keratocyte apoptosis is the first observable stromal event after anterior corneal injury.
Normal or defective regeneration of the EBM controls regenerative vs. fibrotic healing
Descemet’s membrane modulates regenerative vs. fibrotic healing for posterior injuries
Fibrosis is dynamic with ongoing mitosis, development and apoptosis of myofibroblasts
Funding
Supported in part by US Public Health Service grants RO1EY10056 (SEW) and P30-EY025585 from the National Eye Institute, National Institutes of Health, Bethesda, MD, Department of Defense grant VR180066, and Research to Prevent Blindness, New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Proprietary interest statement
The author doesn’t have any commercial or proprietary interests in this study.
References
- Ambrósio R Jr., Kara-José N, Wilson SE, 2009. Early keratocyte apoptosis after epithelial scrape injury in the human cornea. Exp. Eye Res 89, 597–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa FL, Chaurasia S, Cutler A, Asosingh K, Kaur H, de Medeiros F, Agrawal V, Wilson SE, 2010a. Corneal myofibroblast generation from bone marrow-derived cells. Exp. Eye Res 91, 92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa FL, Chaurasia S, Kaur H, de Medeiros FW, Agrawal V, Wilson SE 2010b. Stromal interleukin-1 expression in the cornea after haze-associated injury. Exp. Eye Res 91, 456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa FL, Lin M, Santhiago MR, Singh V, Agrawal V, Wilson SE, 2012. Interleukin-1 receptor role in the viability of corneal myofibroblasts. Exp. Eye Res 96, 65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens DT, Villone D, Koch M, Brunner G, Sorokin L, Robenek H, Bruckner-Tuderman L, Bruckner P, Hansen U, 2012. The epidermal basement membrane is a composite of separate laminin- or collagen IV-containing networks connected by aggregated perlecan, but not by nidogens. J. Biol. Chem 287, 18700–18709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgihan K, Gurelik G, Okur H, Bilgihan A, Hasanreisoglu B, Imir T, 1997. Aqueous transforming growth factor-beta-I levels in rabbit eyes after excimer laser photoablation. Ophthalmologica 211, 380–3. [DOI] [PubMed] [Google Scholar]
- Calvillo MP, McLaren JW, Hodge DO, Bourne WM, 2004. Corneal Reinnervation After LASIK: Prospective 3-year Longitudinal Study. Invest. Ophthalmol. Vis. Sci 45, 3991–6. [DOI] [PubMed] [Google Scholar]
- Campos M, Szerenyi K, Lee M, McDonnell JM, Lopez PF, McDonnell PJ, 1994. Keratocyte loss after corneal de-epithelialization in primates and rabbits. Arch. Ophthalmol 112, 254–260. [DOI] [PubMed] [Google Scholar]
- Chaurasia SS, Kaur H, Medeiros FW, Smith SD, Wilson SE, 2009. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp. Eye Res 89, 133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi C, Trinkaus-Randall V, 2013. New insights in wound response and repair of epithelium. J. Cell Physiol 228, 925–9. [DOI] [PubMed] [Google Scholar]
- Cousins SW, McCabe MM, Danielpour D, Streilein JW, 1991. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest. Ophthalmol. Vis. Sci 32, 2201–2211. [PubMed] [Google Scholar]
- Crosson CE, 1989. Cellular changes following epithelial abrasion in: Beuerman RW, Crosson CE, Kaufman HE, (eds), Healing Processes in the Cornea. Gulf Publishing, Houston, TX, pp. 3–14. [Google Scholar]
- Danjo Y, Gipson IK, 1998. Actin ‘purse string’ filaments are anchored by E-cadherin-mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. J. Cell Sci 111, 3323–32. [DOI] [PubMed] [Google Scholar]
- Dohlman CH, Gasset AR, Rose J, 1968. The effect of the absence of corneal epithelium or endothelium on stromal keratocytes. Invest. Ophthalmol 7, 520–534. [PubMed] [Google Scholar]
- Dowd CJ, Cooney CL, Nugent MA, 1999. Heparan sulfate mediates bFGF transport through basement membrane by diffusion with rapid reversible binding. J. Biol. Chem 274, 5236–5244. [DOI] [PubMed] [Google Scholar]
- Fannon M, Forsten-Williams K, Dowd CJ, Freedman DA, Folkman J, Nugent MA, 2003. Binding inhibition of angiogenic factors by heparan sulfate proteoglycans in aqueous humor: Potential mechanism for maintenance of an avascular environment. FASEB J. 17, 902–904. [DOI] [PubMed] [Google Scholar]
- Galligan CL, Fish EN, 2013. The role of circulating fibrocytes in inflammation and autoimmunity. J. Leukoc. Biol 93, 45–50. [DOI] [PubMed] [Google Scholar]
- Girard MT, Matsubara M, Fini ME, 1991. Transforming growth factor-beta and interleukin-1 modulate metalloproteinase expression by corneal stromal cells. Invest. Ophthalmol. Vis. Sci 32, 2441–54. [PubMed] [Google Scholar]
- Gohring W, Sasaki T, Heldin CH, Timpl R, 1998. Mapping of the binding of platelet-derived growth factor to distinct domains of the basement membrane proteins BM-40 and perlecan and distinction from the BM-40 collagen-binding epitope. Eur. J. Biochem 255, 60–66. [DOI] [PubMed] [Google Scholar]
- Granstein RD, Staszewski R, Knisely TL, Zeira E, Nazareno R, Latina M, Albert DM, 1990. Aqueous humor contains transforming growth factor-beta and a small (less than 3500 daltons) inhibitor of thymocyte proliferation. J. Immunol 144, 3021–3027. [PubMed] [Google Scholar]
- Gupta A, Monroy D, Ji Z, Yoshino K, Huang A, Pflugfelder SC, 1996. Transforming growth factor beta-1 and beta-2 in human tear fluid. Curr. Eye Res 15, 605–14. [DOI] [PubMed] [Google Scholar]
- Hassell JR, Birk DE, 2010. The molecular basis of corneal transparency. Exp. Eye Res 91, 326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell JR, Schrecengost PK, Rada JA, SundarRaj N, Sossi G, Thoft RA, 1992. Biosynthesis of stromal matrix proteoglycans and basement membrane components by human corneal fibroblasts. Invest. Ophthalmol. Vis. Sci 33, 547–57. [PubMed] [Google Scholar]
- Hong JW, Liu JJ, Lee J,S, Mohan RR, Mohan RR, Woods DJ, He YG, Wilson SE, 2001. Proinflammatory chemokine induction in keratocytes and inflammatory cell infiltration into the cornea. Invest. Ophthalmol. Vis. Sci 42, 2795–803. [PubMed] [Google Scholar]
- Huer M, Chaurasia SC, Wilson SE, 2009. Expression of interleukin-1 receptor antagonist in the cornea. Exp. Eye Res 88, 992–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh MI, Chang Y, Jung JC, 2009. Temporal and spatial distribution of TGF-beta isoforms and signaling intermediates in corneal regenerative wound repair. Histol. Histopathol 24, 1405–1416. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Zoeller JJ, Nystrom A, 2009. Basement membrane proteoglycans: modulators par excellence of cancer growth and angiogenesis. Mol. Cells 27, 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon KI, Hindman HB, Bubel T, McDaniel T, DeMagistris M, Callan C, Huxlin KR, 2018. Corneal myofibroblasts inhibit regenerating nerves during wound healing. Sci. Rep 8, 12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM, 1996. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea 15, 505–16. [PubMed] [Google Scholar]
- Jester JV, Huang J, Petroll WM, Cavanagh HD, 2002. TGF beta induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGF beta, PDGF and integrin signaling. Exp. Eye Res 75, 645–57. [DOI] [PubMed] [Google Scholar]
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J, 1999. The cellular basis of corneal transparency: evidence for ‘corneal crystallins’. J. Cell Sci 112, 613–22. [DOI] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, Agrawal V, Wilson SE, 2009. Corneal myofibroblast viability: Opposing effects of IL-1 and TGF beta-1. Exp. Eye Res 89, 152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, Medeiros FW, Agrawal V, Salomao MQ, Singh N, Ambati BK, Wilson SE, 2009. Corneal stroma PDGF blockade and myofibroblast development. Exp. Eye Res 88, 960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W-J, Mohan RR, Mohan RR, Wilson SE, 1999. Effect of PDGF, IL-1 alpha, and BMP2/4 on corneal fibroblast chemotaxis: expression of the platelet-derived growth factor system in the cornea. Invest. Ophthalmol. Vis. Sci 40, 1364–72. [PubMed] [Google Scholar]
- Ko JA, Yanai R, Chikama T, Nishida T, 2010. Downregulation of matrix metalloproteinase-2 in corneal fibroblasts by interleukin-1 receptor antagonist released from corneal epithelial cells. Invest. Ophthalmol. Vis. Sci 51, 6286–93. [DOI] [PubMed] [Google Scholar]
- Koizumi N, Yamasaki K, Kawasaki S, Sotozono C, Inatomi T, Mochida C, Kinoshita S, 2006. Cytomegalovirus in aqueous humor from an eye with corneal endotheliitis. Am. J. Ophthalmol 141, 564–5. [DOI] [PubMed] [Google Scholar]
- Lassance L, Marino GK, Medeiros CS, Thangavadivel S, Wilson SE, 2018. Fibrocyte migration, differentiation and apoptosis during the corneal wound healing response to injury. Exp. Eye Res 170, 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latvala T, Linna T, Tervo T, 1996. Corneal nerve recovery after photorefractive keratectomy and laser in situ keratomileusis. Int. Ophthalmol. Clin 36, 21–7. [DOI] [PubMed] [Google Scholar]
- Lecler VB, Roy O, Santerre K, Proulx S, 2018. TGF-β1 promotes cell barrier function upon maturation of corneal endothelial cells. Sci. Reports 8, 4438–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DQ, Lokeshwar BL, Solomon A, Monroy D, Ji Z, Pflugfelder SC, 2001. Regulation of MMP-9 production by human corneal epithelial cells. Exp. Eye Res 73, 449–59. [DOI] [PubMed] [Google Scholar]
- Li DQ, Tseng SC, 1997. Differential regulation of keratinocyte growth factor and hepatocyte growth factor/scatter factor by different cytokines in human corneal and limbal fibroblasts. J. Cell Physiol 172, 361–72. [DOI] [PubMed] [Google Scholar]
- Li Q, Weng J, Mohan RR, Bennett GL, Schwall R, Wang ZF, Tabor K, Kim J, Hargrave S, Cuevas KH, Wilson SE, 1996. Hepatocyte growth factor and hepatocyte growth factor receptor in the lacrimal gland, tears, and cornea. Invest. Ophthalmol. Vis. Sci 37, 727–39. [PubMed] [Google Scholar]
- Lipshitz I, Loewenstein A, Varssano D, Lazar M, 1997. Late onset corneal haze after photorefractive keratectomy for moderate and high myopia. Ophthalmology 104, 369–73. [DOI] [PubMed] [Google Scholar]
- Liu CY, Kao WW, 2015. Corneal epithelial wound healing. Prog. Mol. Biol. Transl. Sci 134, 61–71. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Saghizadeh M, 2015. Progress in corneal wound healing. Prog. Retin. Eye Res 49, 17–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino GK, Santhiago MR, Santhanam A, Lassance L, Thangavadivel S, Medeiros CS, Bose K, Tam KP, Wilson SE, 2017b. Epithelial basement membrane injury and regeneration modulates corneal fibrosis after pseudomonas corneal ulcers in rabbits. Exp. Eye Res 161, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino GK, Santhiago MR, Santhanam A, Lassance L, Thangavadivel S, Medeiros CS, Torricelli AAM, Wilson SE, 2017a. Regeneration of defective epithelial basement membrane and restoration of corneal transparency after photorefractive keratectomy. J. Ref. Surg 33, 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastropasqua L, Massaro-Giordano G, Nubile M, Sacchetti M, 2017. Understanding the pathogenesis of neurotrophic keratitis: The role of corneal nerves. J. Cell Physiol 232, 717–724. [DOI] [PubMed] [Google Scholar]
- Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S, 1996. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc. Natl. Acad. Sci. USA 93, 4219–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros CS, Lassance L, Saikia P, Wilson SE, 2018a. Posterior stromal keratocyte apoptosis triggered by mechanical endothelial injury and nidogen-1 production in the cornea. Exp. Eye Res 172, 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros CS, Marino G, Lassance L, Thangavadivel S, Santhiago MR, Wilson SE, 2018b. The impact of photorefractive keratectomy (PRK) and mitomycin C (MMC) on corneal nerves and their regeneration. J. Ref. Surg 34, 790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros CS, Saikia P, de Oliveira RC, Lassance L, Santhiago MR, Wilson SE, 2019. Descemet’s membrane modulation of posterior corneal fibrosis. Invest. Ophth. Vis. Sci 60, 1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltendorf C, Burbach GJ, Ohrloff C, Ghebremedhin E, Deller T, 2009. Intrastromal keratotomy with femtosecond laser avoids profibrotic TGF-beta1 induction. Invest. Ophthalmol. Vis. Sci 50, 3688–95. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AEK, Choi R, Hong J-W, Lee J-S., Mohan RR, Ambrósio R, Zieske JD., Wilson SE, 2003. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp. Eye Res 76, 71–87. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Liang Q, Kim W-J., Helena MC, Baerveldt F, Wilson SE, 1997. Apoptosis in the cornea: further characterization of Fas-Fas ligand system. Exp. Eye Res 65, 575–89. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Mohan RR, Ambrósio R Jr., Wilson SE, 2002. Activation of keratocyte apoptosis in response to epithelial scrape injury does not require tears. Invest. Ophthal. Vis. Sci (suppl). 43, 1679. [Google Scholar]
- Mohan RR, Mohan RR, Kim WJ, Stark GR, Wilson SE, 2000. Defective keratocyte apoptosis in response to epithelial injury in STAT1 null mice. Exp. Eye Res 70, 485–91. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kurosaka D, Yoshino M, Oshima T, Kurosaka H, 2002. Injured corneal epithelial cells promote myodifferentiation of corneal fibroblasts. Invest. Ophthalmol. Vis. Sci 43, 2603–8. [PubMed] [Google Scholar]
- Nakayasu K, 1988. Stromal changes following removal of epithelium in rat cornea. Jpn. J. Ophthalmol 32, 113–125. [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE, 2006. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp. Eye Res 82, 788–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K, Sotozono C, Adachi W, Yamamoto S, Yokoi N, Kinoshita S, 1995. Transforming growth factor-beta 1, -beta 2 and -beta 3 mRNA expression in human cornea. Curr. Eye Res 14, 235–41. [DOI] [PubMed] [Google Scholar]
- Paralkar VM, Vukicevic S, Reddi AH, 1991. Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: implications for development. Dev. Biol 143, 303–8. [DOI] [PubMed] [Google Scholar]
- Saikia P, Thangavadivel S, Lassance L, Medeiros CS, Wilson SE, 2018. IL-1 and TGFβ modulation of epithelial basement membrane components perlecan and nidogen production by corneal stromal cells. Invest. Ophth. Vis. Sci 59, 5589–5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam A, Marino GK, Torricelli AAM, Wilson SE, 2017. Epithelial basement membrane (EBM) regeneration and changes in EBM component mRNA expression in the anterior stroma after corneal injury. Mol. Vision 23, 39–51. [PMC free article] [PubMed] [Google Scholar]
- Santhanam A, Torricelli AAM, Wu J, Marino GK, Wilson SE, 2015. Differential expression of epithelial basement membrane components nidogens-1 and 2 and perlecan in corneal stromal cells in vitro. Mol. Vision 21, 1318–27. [PMC free article] [PubMed] [Google Scholar]
- Sharif Z, Sharif W, 2019. Corneal neovascularization: updates on pathophysiology, investigations & management. Rom. J. Ophthalmol 63, 15–22. [PMC free article] [PubMed] [Google Scholar]
- Shi C, Pamer EG, 2011. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol 11, 762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya H, Okamoto O, Fujiwara S, 2006. The bioactivity of transforming growth factor-beta1 can be regulated via binding to dermal collagens in mink lung epithelial cells. J. Dermatol. Sci 41, 187–195. [DOI] [PubMed] [Google Scholar]
- Singh V, Barbosa FL, Torricelli AAM, Santhiago MR, Wilson SE, 2014. Transforming growth factor β and platelet-derived growth factor modulation of myofibroblast development from corneal fibroblasts in vitro. Exp. Eye Res 120, 152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Santhiago MR, Barbosa FL, Agrawal V, Ambati BK, Singh N, Wilson SE, 2011. Effect of TGFβ and PDGF-B blockade on corneal myofibroblast development in mice. Exp. Eye Res 93, 810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC, 2001. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest. Ophthalmol. Vis. Sci 42, 2283–92. [PubMed] [Google Scholar]
- Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME, 2003. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Investigative ophthalmology & visual science 44, 4237–4246. [DOI] [PubMed] [Google Scholar]
- Tervo T, Vesaluoma M, Bennett GL, Schwall R, Helena M, Liang Q, Wilson SE, 1997. Tear hepatocyte growth factor (HGF) availability increases markedly after excimer laser surface ablation. Exp. Eye Res 64, 501–4. [DOI] [PubMed] [Google Scholar]
- Thygeson P, 1947. Marginal corneal infiltrates and ulcers. Trans. Amer. Acad. Ophthal. Otolaryng 51, I98–209. [PubMed] [Google Scholar]
- Torres P, de Vos AF, van der Gaag R, Kijlstra A, 1994. Expression of the interleukin 1 receptor antagonist in the normal human cornea. Ocul. Immunol. Inflamm 2, 217–22. [DOI] [PubMed] [Google Scholar]
- Torricelli AAM, Marino GK, Santhanam A, Wu J, Singh A, Wilson SE, 2015. Epithelial basement membrane proteins perlecan and nidogen-2 are up-regulated in stromal cells after epithelial injury in human corneas. Exp. Eye Res 134, 33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AAM, Singh V, Agrawal V, Santhiago MR, Wilson SE, 2013a. Transmission electron microscopy analysis of epithelial basement membrane repair in rabbit corneas with haze. Invest. Ophth. Vis. Sci 54, 4026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AAM, Singh V, Santhiago MR, Wilson SE, 2013b. The corneal epithelial basement membrane: Structure, function and disease. Invest. Ophth. Vis. Sci 54, 6390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesaluoma M, Teppo AM, Gronhangen-Riska C, Tervo T, 1997. Release of TGF-beta 1 and VEGF in tears following photorefractive keratectomy. Curr. Eye Res 16, 19–25. [DOI] [PubMed] [Google Scholar]
- Weng J, Mohan RR, Li Q, Wilson SE, 1997. IL-1 upregulates keratinocyte growth factor and hepatocyte growth factor mRNA and protein production by cultured stromal fibroblast cells: Interleukin-1 beta expression in the cornea. Cornea 16, 465–71. [PubMed] [Google Scholar]
- West-Mays JA, Strissel KJ, Sadow PM, Fini ME, 1995. Competence for collagenase gene expression by tissue fibroblasts requires activation of an interleukin 1 alpha autocrine loop. Proc. Natl. Aca.d Sci. USA 92, 6768–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Esposito A, 2009. Interleukin-1: A master regulator of the corneal response to injury. Exp. Eye Res 89, 124–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, He YG, Lloyd SA, 1992a. EGF, EGF receptor, basic FGF, TGF beta-1, and IL-1 alpha mRNA in human corneal epithelial cells and stromal fibroblasts. Invest. Ophthalmol. Vis. Sci 33, 1756–65. [PubMed] [Google Scholar]
- Wilson SE, He Y-G, Weng J, Li Q, McDowall AW, Vital M, Chwang EL, 1996. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp. Eye Res 62, 325–8. [DOI] [PubMed] [Google Scholar]
- Wilson SE, He Y-G, Weng J, Li Q, Vital M, Chwang EL, 1995. Epithelial- and endothelial-derived interleukin-1 (IL-1) modulates corneal tissue organization and wound healing response through induction of keratocyte apoptosis. Invest. Ophthalmol. Vis. Sci (Suppl) 36, S866 (#3969). [Google Scholar]
- Wilson SE, He YG, Weng J, Zieske JD, Jester JV, Schultz GS, 1994a. Effect of epidermal growth factor, hepatocyte growth factor, and keratinocyte growth factor, on proliferation, motility and differentiation of human corneal epithelial cells. Exp. Eye Res 59, 665–78. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Lee WM, Murakami C, Weng J, Moninger GA, 1993. Mooren’s corneal ulcers and hepatitis C virus infection. N. Engl. J. Med 329, 62. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Li Q, Weng J, Barry-Lane PA, Jester JV, Liang Q, Wordinger RJ, 1996. The Fas/Fas ligand system and other modulators of apoptosis in the cornea. Invest. Ophthalmol. Vis. Sci 37, 1582–92. [PubMed] [Google Scholar]
- Wilson SE, Liang Q, Kim W-J, 1999. Lacrimal gland HGF, KGF, and EGF mRNA levels increase after corneal epithelial wounding. Invest. Ophthalmol. Vis. Sci 40, 2185–90. [PubMed] [Google Scholar]
- Wilson SE, Lloyd SA, 1991. Epidermal growth factor and its receptor, basic fibroblast growth factor, transforming growth factor beta-1, and interleukin-1 alpha messenger RNA production in human corneal endothelial cells. Invest. Ophthalmol. Vis. Sci 32, 2747–56. [PubMed] [Google Scholar]
- Wilson SE, Lloyd SA, He YG, 1992b. EGF, basic FGF, and TGF beta-1 messenger RNA production in rabbit corneal epithelial cells. Invest. Ophthalmol. Vis. Sci 33, 1987–95. [PubMed] [Google Scholar]
- Wilson SE, Marino GK, Torricelli AAM, Medeiros CS, 2017. Corneal fibrosis: injury and defective regeneration of the epithelial basement membrane. A paradigm for fibrosis in other organs? Matrix Biology 64, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Medeiros CS, Santhiago MR, 2018. Pathophysiology of corneal scarring in persistent epithelial defects after PRK and other corneal injuries. J. Ref. Surg 34, 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Netto MV, Perez V, Possin D, Huang J, Kwon R, Alekseev A, 2004. RANK, RANKL, OPG, and M-CSF expression in stromal cells during corneal wound healing. Invest. Ophthalmol. Vis. Sci 45, 2201–2211. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Pedroza L, Beuerman R, Hill JM, 1997. Herpes simplex virus type-1 infection of corneal epithelial cells induces apoptosis of the underlying keratocytes. Exp. Eye Res 64, 775–9. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Schultz GS, Chegini N, Weng J, He YG, 1994b. Epidermal growth factor, transforming growth factor alpha, transforming growth factor beta, acidic fibroblast growth factor, basic fibroblast growth factor, and interleukin-1 proteins in the cornea. Exp. Eye Res 59, 63–71. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Walker JW, Chwang EL, He YG, 1993. Hepatocyte growth factor, keratinocyte growth factor, their receptors, fibroblast growth factor receptor-2, and the cells of the cornea. Invest. Ophthalmol. Vis. Sci 34, 2544–61. [PubMed] [Google Scholar]
- Yu FS, Yin J, Xu K, Huang J, 2010. Growth factors and corneal epithelial wound healing. Brain Res Bull. 81, 229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, Tsilibary EC, Charonis AS, Furthmayr H, 1986. Models for the self-assembly of basement membrane. J Histochem Cytochem. 34, 93–102. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Guimarães SR, Hutcheon AE, 2001. Kinetics of keratocyte proliferation in response to epithelial debridement. Exp. Eye Res 72, 33–9. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Hutcheon AEK, Guo X, 2020. Extracellular vesicles and cell-cell communications in the cornea. Anatomical Record. 303, 1727–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]


