There is a Blood Commentary on this article in this issue.
Key Points
Patients with Myc-driven DLBCL had less favorable outcomes after EPOCH, and vorinostat had no impact on HIV or chemotherapy’s efficacy.
Permitting 1 cycle of systemic treatment before EPOCH-based chemotherapy facilitated protocol enrollment without worsening outcomes.
Abstract
EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) is a preferred regimen for HIV-non-Hodgkin lymphomas (HIV-NHLs), which are frequently Epstein-Barr virus (EBV) positive or human herpesvirus type-8 (HHV-8) positive. The histone deacetylase (HDAC) inhibitor vorinostat disrupts EBV/HHV-8 latency, enhances chemotherapy-induced cell death, and may clear HIV reservoirs. We performed a randomized phase 2 study in 90 patients (45 per study arm) with aggressive HIV-NHLs, using dose-adjusted EPOCH (plus rituximab if CD20+), alone or with 300 mg vorinostat, administered on days 1 to 5 of each cycle. Up to 1 prior cycle of systemic chemotherapy was allowed. The primary end point was complete response (CR). In 86 evaluable patients with diffuse large B-cell lymphoma (DLBCL; n = 61), plasmablastic lymphoma (n = 15), primary effusion lymphoma (n = 7), unclassifiable B-cell NHL (n = 2), and Burkitt lymphoma (n = 1), CR rates were 74% vs 68% for EPOCH vs EPOCH-vorinostat (P = .72). Patients with a CD4+ count <200 cells/mm3 had a lower CR rate. EPOCH-vorinostat did not eliminate HIV reservoirs, resulted in more frequent grade 4 neutropenia and thrombocytopenia, and did not affect survival. Overall, patients with Myc+ DLBCL had a significantly lower EFS. A low diagnosis-to-treatment interval (DTI) was also associated with inferior outcomes, whereas preprotocol therapy had no negative impact. In summary, EPOCH had broad efficacy against highly aggressive HIV-NHLs, whereas vorinostat had no benefit; patients with Myc-driven DLBCL, low CD4, and low DTI had less favorable outcomes. Permitting preprotocol therapy facilitated accruals without compromising outcomes. This trial was registered at www.clinicaltrials.gov as #NCT0119384.
Visual Abstract
Introduction
HIV infection is associated with an 11-fold higher risk of highly aggressive non-Hodgkin lymphoma (NHL), despite the use of combination antiretroviral therapy (ART).1 The clinical outcome in patients with HIV-NHL has improved, approaching that of the general population when standard-dose chemotherapy paradigms are used in conjunction with ART.2,3 Several phase 2 studies performed by the AIDS Malignancy Consortium (AMC) and National Cancer Institute (NCI) have demonstrated higher complete response (CR) rates (73% to 91%) when EPOCH-based chemotherapy (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) is administered as a continuous IV infusion over 96 hours, in combination with the anti-CD20 antibody rituximab (R), compared with the rates previously seen with conventional R-CHOP (cyclophosphamide, doxorubicin hydrochloride, vincristine, and prednisolone with rituximab; 57% in the AMC-010 trial).4-7 Improved CR rates and overall survival (OS) was also observed for EPOCH compared with CHOP in a pooled analysis of 1546 patients with HIV-NHL.2 Infusional regimens, such as EPOCH, may circumvent high expression of the multidrug resistance-1 gene by prolonged continuous drug exposure.8,9 EPOCH +/−R is currently listed as the preferred regimen for the treatment of patients with HIV-associated diffuse large B-cell lymphoma (HIV-DLBCL), primary effusion lymphoma (PEL), and plasmablastic lymphoma (PBL), under current National Comprehensive Cancer Network guidelines,10 although PBL, PEL, and the nongerminal center B-cell (non-GCB) subtype of DLBCL still have worse outcomes.6,11-13
Approximately 40% of DLBCLs and 80% of PBLs are Epstein-Barr virus positive (EBV+).14 PEL is caused by human herpesvirus type-8 (HHV-8) and coinfects with EBV in 80% of cases.14 These oncogenic viruses establish latency and therefore may be targeted therapeutically in lymphomas with lytic-inducing chemotherapy or drugs that disrupt viral latency, such as histone deacetylase (HDAC) inhibitors.15-17 Vorinostat, a potent HDAC inhibitor, was highly synergistic with rituximab, anthracyclines, and etoposide (drugs contained in EPOCH) in causing cell death in preclinical models.18-21 Further, vorinostat induced HHV-8 lytic gene expression and p53 acetylation, followed by apoptosis in PEL xenografts and increased survival in mice.22 Finally, vorinostat disrupts HIV latency, suggesting that it may eradicate latently infected reservoirs via HIV-cytopathic effects and immune-mediated mechanisms.23-25
Based on the above considerations, we performed a sequential phase 1/2 randomized trial of EPOCH (+/−R, depending on CD20 expression status), with and without vorinostat in HIV-NHL. The phase 1 portion of the study established vorinostat 300 mg given with EPOCH on days 1 to 5 of each cycle as the maximum tolerated dose and showed promising efficacy evidenced by a 1-year event-free survival (EFS) and OS rates of 83%.7 The primary objective of the phase 2 study was to determine whether vorinostat significantly improved CR rates when added to EPOCH. Secondary objectives included toxicity, survival, and the effect of vorinostat on HIV infection and latent reservoirs. The protocol allowed 1 cycle of systemic chemotherapy to reduce logistical barriers before trial enrollment, shorten the period between diagnosis and treatment, and facilitate accrual. As prior studies have shown, a diagnosis-to-treatment interval (DTI) of ≥15 days was associated with a better prognosis in an HIV− population treated with R-CHOP,26 we anticipated that allowing preprotocol therapy would facilitate recruitment of subjects more representative of real-world practice and reduce selection bias associated with longer DTI.
Patients and methods
Study design
This open-label, randomized, phase 2 study was designed to test and compare the efficacy of dose–adjusted (DA)-EPOCH+/−R, with or without vorinostat, in patients with highly aggressive HIV-NHL. Patients were stratified according to CD4 count (<100 cells/mm3) and age-adjusted International Prognostic Index (aa-IPI) scores (0-1 vs 2-3). Preprotocol treatment was not stratified. The primary end point driving the sample size of 90 patients was CR, the rates of which were compared by using the 1-sided, pooled-variance z-test of 2 proportions. Equal sample sizes of 45 subjects per treatment arm provide this planned comparison of CR rates, with ≥80% power at the 10% significance level to detect an odds ratio of 3.27, based on expected CR rates of 70% without vorinostat and 88.4% with vorinostat.
This study was approved by the local institutional review board at each of the participating sites: Miller School of Medicine and affiliated Jackson Memorial Hospital, University of Miami; Moores Cancer Center, University of California, San Diego; Montefiore Medical Center, Albert Einstein College of Medicine; Sidney Kimmel Cancer Center, John Hopkins University; Memorial Sloan Kettering Cancer Center; University of California, San Francisco, and affiliated Zuckerberg San Francisco General Hospital; John H. Stroger, Jr Hospital of Cook County; Virginia Mason Medical Center; Ohio State University; Siteman Cancer Center, Washington University School of Medicine; University of California, Los Angeles; Harborview Medical Center and Seattle Cancer Care Alliance, University of Washington; Baylor College of Medicine; University of North Carolina at Chapel Hill; Abramson Cancer Center, University of Pennsylvania; Beth Israel Deaconess Medical Center; and Boston Medical Center.
Eligibility
All patients provided written informed consent before enrollment. Eligible subjects included adult HIV+ patients of both sexes, with absolute CD4+ count ≥50 cells/mm3 who were required to be in treatment with ART before enrollment or starting after recovery from cycle 1. The protocol was amended to prohibit the ritonavir-based ART and strong CYP3A4 inhibitors after toxic death of 1 patient taking ART containing cobicistat (a potent CYP3A4 inhibitor). Patients with central nervous system involvement, identified by imaging or cerebrospinal fluid analysis by cytology or flow cytometry, were ineligible, as were patients with active viral hepatitis from hepatitis B (Hep B) or C. Those positive for the Hep B core antibody were eligible only so long as they received prophylactic treatment (tenofovir or lamivudine). One cycle of systemic chemotherapy (ie, CHOP-like or EPOCH) before protocol screening without evidence of disease progression was allowed. Patients were required to have adequate organ function, Eastern Cooperative Oncology Group performance status ≤2, and at least 1 of the following high-risk features: aa-IPI, 2 to 3; Ki-67, ≥80%; non-GCB–cell DLBCL; or any other aggressive non-DLBCL, non-Burkitt B-cell NHL, as categorized under the 2008 World Health Organization (WHO) classification of lymphoid tumors.
Central pathology review, immunohistochemistry, and EBV early RNA–in situ hybridization
Central pathology review was conducted as previously described.7 DLBCL cases were categorized as GCB vs non-GCB types by immunohistochemistry studies assessing Bcl-6, Bcl-10, and MUM-1 expression.27 Bcl-2 and Myc protein expression was assessed by immunohistochemistry, and EBV expression by EBV early RNA–in situ hybridization of diagnostic tumor specimens.7,28,29 For tissues exhausted at local sites or with uninterpretable confirmatory staining results, the histologic, immunophenotypic, and fluorescence in situ hybridization (FISH) data from the local institution’s pathology reports were reviewed and diagnoses made accordingly.
Treatment and supportive care
Treatment and supportive drug administration guidelines are summarized in supplemental Table 1, available on the Blood Web site. Treatment consisted of dose-adjusted EPOCH, plus rituximab if CD20+, alone or with vorinostat 300 mg administered on days 1 to 5 of each cycle. Cyclophosphamide was capped at 750 mg/m2 and doxorubicin or etoposide doses were not escalated, as they were in previous trials in patients with HIV-associated lymphoma.5,30 The starting cyclophosphamide dose was based on baseline CD4 count for cycle 1 and was further dose adjusted based on nadir absolute neutrophil and platelet counts (supplemental Tables 2 and 3). After cycle 1, dose adjustments of all chemotherapy drugs were made according to table guidelines. Rituximab was omitted in 6 patients with CD20− tumors (5 PBLs and 1 PEL).
Clinical and response assessments
The response was assessed by standard whole-body computed tomographic scan criteria31 after cycle 4 and serially, after treatment (4-8 weeks, and at months 6, 12, 18, and 24). Positron-emission tomography or computed tomography-positron emission tomography were necessary to confirm CR after treatment.32 A bone marrow biopsy was performed to confirm CR, if previously positive. Thereafter, subjects were followed up every 3 months for 2 years, and then every 6 months for years 3 to 5. OS and EFS were determined for each treatment arm by using Kaplan-Meier proportions with Greenwood standard errors, and the log-rank test was used to compare the underlying Kaplan-Meier curves between treatment arms.
Correlative studies
Plasma EBV DNA-load studies were conducted by quantitative polymerase chain reaction (qPCR), as previously reported.33 Standard immunoglobulin levels, T-cell (CD4+/CD8+) subsets, and HIV viral load (VL) levels by qPCR were measured at local institutional laboratories at baseline, after cycle 2, and after treatment (months 1, 6, and 12). Latent HIV reservoirs were investigated longitudinally in patients who had HIV VLs below the lower limit of detection, as measured by standard RNA qPCR at any point after treatment, using a quantification viral outgrowth assay.34 Post- and pretreatment values were compared by implementing a mixed-effects Bayesian model with an unconstrained treatment and temporal effect. Summary statistics describing and comparing all patient baseline characteristics and tumor features listed in Tables 1 and 2 are provided in the supplemental Methods.
Table 1.
Baseline demographics and characteristics of evaluable patients
| Characteristic | Vorinostat-EPOCH +/−R (n = 44) | EPOCH +/−R (n = 42) | All patients (N = 86) | P* | ||
|---|---|---|---|---|---|---|
| n (%) | Median (range) | n (%) | Median (range) | Total (%) | ||
| Sex | .056 | |||||
| Female | 5 (11) | 0 | 5 (6) | |||
| Male | 39 (89) | 42 (100) | 81 (94) | |||
| Race/ethnicity | .87 | |||||
| White/non-Hispanic | 16 (36) | 20 (48) | 36 (42) | |||
| White/Hispanic | 7 (16) | 8 (19) | 15 (17) | |||
| Other Hispanic | 6 (14) | 4 (10) | 10 (12) | |||
| African American | 12 (27) | 8 (19) | 20 (23) | |||
| Asian | 1 (2) | 1 (2) | 2 (2) | |||
| Unknown | 2 (5) | 1 (2) | 3 (3) | |||
| Age, y | 50 (26-70) | 46 (24-66) | .048 | |||
| CDC risk factors | ||||||
| Homosexual/bisexual contact | 18 (43) | 26 (62) | 44 (52) | .13 | ||
| Heterosexual contact | 22 (51) | 10 (24) | 32 (38) | .014 | ||
| IV drug use | 2 (5) | 4 (10) | 6 (7) | .43 | ||
| Transfusion recipient | 1 (2) | 2 (5) | 3 (4) | .62 | ||
| Other CDC risk factor | 2 (5) | 3 (7) | 5 (6) | .68 | ||
| More than 1 risk factor | 4 (9) | 4 (9) | 8 (9) | .99 | ||
| ART use (at baseline) | .27 | |||||
| Yes | 38 (86) | 40 (95) | — | |||
| No | 6 (14) | 2 (5) | — | |||
| Absolute CD4 count (cells/mm3) | ||||||
| Numeric values | 186 (63-1 137) | 195 (50-1 061) | .84 | |||
| <100 | 5 (11) | 6 (14) | 11 (13) | .87 | ||
| 100-200 | 19 (43) | 16 (38) | 35 (41) | |||
| >200 | 20 (45) | 20 (47) | 40 (46) | |||
| HIV VL (copies/mL) | ||||||
| Positive and above limit | 25 (57) | 27 (64) | 52 (60) | .51 | ||
| Undetected or below limit | 19 (43) | 15 (36) | 34 (40) | |||
| Values that were positive and above limit | 17 100 (36-1 712 000) | 934 (2-901 000) | .32 | |||
| Ann Arbor stage | .99 | |||||
| I-II | 8 (18) | 7 (17) | 15 (17) | |||
| III-IV | 36 (82) | 35 (83) | 71 (83) | |||
| aa-IPI risk | .65 | |||||
| 0-1 | 16 (36) | 13 (31) | 29 (34) | |||
| 2-3 | 28 (64) | 29 (69) | 57 (66) | |||
| LDH elevation | .81 | |||||
| Yes | 34 (77) | 30 (71) | 64 (74) | |||
| No | 10 (23) | 12 (29) | 32 (26) | |||
| Ki-67 expression | .08 | |||||
| ≥80% | 31 (70) | 37 (88) | 68 (79) | |||
| <80% | 6 (14) | 4 (10) | 10 (12) | |||
| Inconclusive or ND | 7 (16) | 1 (2) | 8 (9) | |||
| Pathologic diagnosis | .79 | |||||
| DLBCL | 30 (68) | 31 (73) | 61 (71) | |||
| PBL | 8 (18) | 7 (17) | 15 (17) | |||
| PEL | 5 (11) | 2 (5) | 7 (8) | |||
| BCL-U | 1 (2) | 1 (2) | 2 (2) | |||
| BL | 0 (0) | 1 (2) | 1 (1) | |||
| Prior chemotherapy | .51 | |||||
| 1 cycle | 16 (36) | 19 (45) | 35 (41) | |||
| None | 28 (64) | 23 (55) | 51 (59) | |||
| DTI | .39 | |||||
| <15 d | 25 (57) | 19 (45) | 44 (51) | |||
| 15 d | 19 (43) | 23 (55) | 42 (49) | |||
BCL-U, B-cell lymphoma, unclassifiable, with features between DLBCL and BL; LDH, lactate dehydrogenase.
P-values are from Wilcoxon rank-sum tests, on age, absolute CD4 counts, and HIV VL, and from Fisher’s exact test on all other patient characteristics. All P-values are 2-sided.
Table 2.
Lymphoma subtypes and tumor-associated features
| Pathologic diagnosis | Vorinostat-EPOCH +/−R (n = 44), n (%) | EPOCH +/−R (n = 42), n (%) | All patients (N = 86), n (%) | P* |
|---|---|---|---|---|
| DLBCL | 30 (68) | 31 (73) | 61 (71) | |
| Subtype | .52 | |||
| GCB | 15/30 (50) | 19/31 (61) | 34/61 (56) | |
| Non-GCB | 14/30 (47) | 12/31 (39) | 26/61 (43) | |
| Unclassified DLBCL | 1/30 (3) | 0/31 (0) | 1/61 (2) | |
| EBV status | .23 | |||
| Positive | 9/30 (30) | 7/31 (22) | 16/61 (26) | |
| Negative | 13/30 (43) | 20/31 (65) | 33/61 (54) | |
| Inconclusive or ND | 8/30 (27) | 4/31 (13) | 12/61 (20) | |
| Bcl-2 protein expression | .07 | |||
| Positive | 15/30 (50) | 17/31 (55) | 32/61 (52) | |
| Negative | 7/30 (23) | 12/31 (39) | 19/61 (31) | |
| Inconclusive or ND | 8/30 (27) | 2/31 (6) | 10/61 (17) | |
| Myc protein expression | .05 | |||
| Positive | 8/30 (27) | 10/31 (32) | 18/61 (30) | |
| Negative | 11/30 (37) | 7/31 (23) | 18/61 (30) | |
| Inconclusive or ND | 11/30 (37) | 14/31 (45) | 25/61 (40) | |
| PBL | 8 (18) | 7 (17) | 15 (17) | |
| EBV status | .47 | |||
| Positive | 6/8 (75) | 7/7 (100) | 13/15 (87) | |
| Negative | 2/8 (25) | 0/7 (0) | 2/15 (13) | |
| Bcl-2 protein expression | .99 | |||
| Positive | 2/8 (25) | 3/7 (43) | 5/15 (33) | |
| Negative | 2/8 (25) | 1/7 (14) | 3/15 (20) | |
| Inconclusive or ND | 4/8 (50) | 3/7 (43) | 7/15 (47) | |
| Myc protein expression | .99 | |||
| Positive | 5/8 (63) | 4/7 (57) | 9/15 (60) | |
| Inconclusive or ND | 3/8 (37) | 3/7 (43) | 6/15 (40) | |
| PEL | 5 (11) | 2 (5) | 7 (8) | |
| EBV status | .99 | |||
| Positive | 3/5 (60) | 2/2 (100) | 5/7 (71) | |
| Negative | 2/5 (40) | 0/2 (0) | 2/7(29) | |
| Bcl-2 protein expression | .99 | |||
| Negative | 2/5 (40) | 1/2 (50) | 3/7 (43) | |
| Inconclusive or ND | 3/5 (60) | 1/2 (50) | 4/7 (57) | |
| Myc protein expression | .99 | |||
| Negative | 2/5 (40) | 1/2 (50) | 3/7 (43) | |
| Inconclusive or ND | 3/5 (60) | 1/2 (50) | 4/7 (57) | |
| BCL-U | 1 (2) | 1 (2) | 2 (2) | |
| EBV status | — | |||
| Negative | 1/1 (100) | 1/1 (100) | 2/2 (100) | |
| Bcl-2 protein expression | — | |||
| Positive | 1/1 (100) | 1/1 (100) | 2/2 (100) | |
| Myc protein expression | — | |||
| Positive | 1/1 (100) | 1/1 (100) | 2/2 (100) | |
| BL | 0 | 1 (2) | 1 (1) | |
| EBV status | — | |||
| Negative | 0/0 | 1/1 (100) | 1/1 (100) | |
| Bcl-2 protein expression | — | |||
| Negative | 0/0 | 1/1 (100) | 1/1 (100) | |
| Myc protein expression | — | |||
| Positive | 0/0 | 1/1 (100) | 1/1 (100) |
BCL-U, B-cell lymphoma, unclassifiable, with features between DLBCL and BL; ND, not done.
P-values by 2-sided Fisher’s exact test.
Exploratory analysis of impact by preprotocol therapy and diagnosis-to-treatment interval
We also conducted exploratory analyses evaluating the clinical characteristics and outcomes for (1) patients who received 1 cycle of preprotocol therapy compared with those who did not, and (2) those with short (<15 days) vs long (≥15 days) diagnosis-to-treatment intervals (DTIs). DTI was defined as time elapsed from diagnosis to the start of cycle 1 of treatment, whether during the study or before protocol enrollment. Summary statistics used to analyze the patient subgroups are provided in supplemental Methods.
Results
Patient characteristics, tumor features, and associated risks
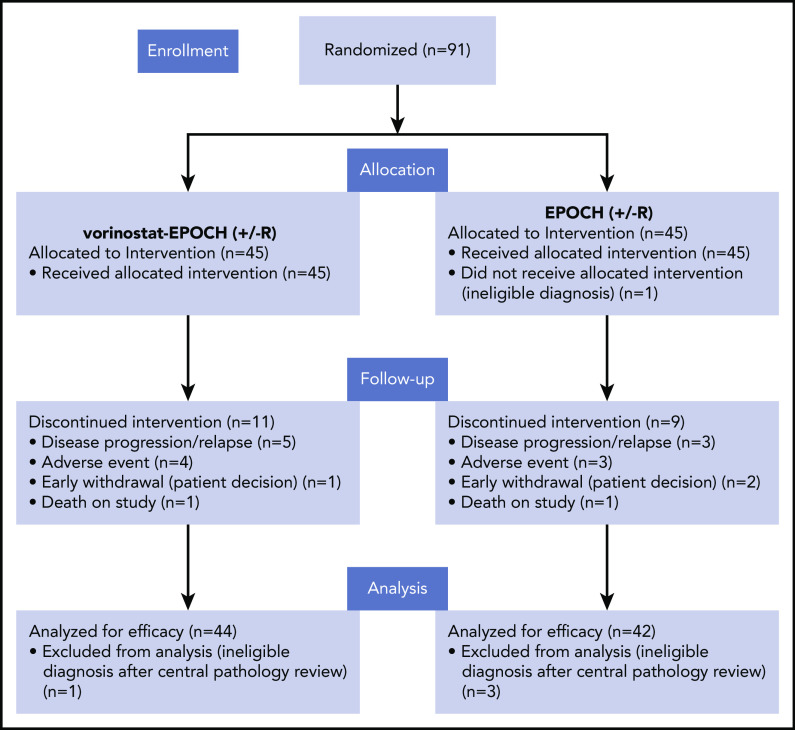
From January 2012 through June 2017, 91 patients were enrolled and randomized at 21 AMC sites; 5 were excluded based on central pathology review (n = 4) or were deemed to have an ineligible diagnosis before enrollment (n = 1; Figure 1). Baseline characteristics of the remaining 86 response-evaluable subjects are summarized in Table 1. Lymphoma types consisted of 61 DLBCLs (71%), 15 PBLs (17%), 7 PELs (8%), and 2 B-cell lymphomas, unclassifiable, with features between DLBCL and Burkitt lymphoma (BL), as categorized under the 2008 WHO classification. One BL was included in the analysis, though initially classified as GCB DLBCL and later confirmed to have Myc rearrangement by FISH, together with morphologic features typical of BL. Sixteen (26%) of the DLBCLs were EBV+, 33 (54%) tested negative, and 12 (20%) were either not tested or had inconclusive results (Table 2). Thirteen of 15 (87%) PBLs were EBV+, whereas 5 of 7 (71%) HHV-8+ PELs were coinfected with EBV (Table 2). The median baseline CD4+ cell count was 190 cells/mm3 (range, 50-1137), with 11 participants (13%) having CD4+ cell counts between 50 and 99 CD4+ cells/mm3. As of 30 July 2019, when data were frozen for analysis, after a median follow-up of 40 months (range, 0.4-65 months), 33 of 86 patients had experienced at least 1 EFS-defining event: relapse or progression (n = 27) and/or death (n = 24).
Figure 1.
Consort diagram. Patients with aggressive HIV-related B-cell non-Hodgkin lymphomas enrolled in AMC-075.
Treatment-related adverse events
Seven patients discontinued protocol treatment after experiencing adverse events (AEs; 4 from the vorinostat-EPOCH group and 3 from the EPOCH-only group). Two patients (1 from each arm) died early of possible treatment-related toxicities: 1 patient who was receiving cobicistat-containing ART and was in the R-EPOCH (control arm) treatment group died unexpectedly at home with partial gastrointestinal obstruction, leading to protocol amendment; and 1 patient in the vorinostat-R-EPOCH treatment group died of unknown cause. The vorinostat arm was more myelosuppressive; the most frequently reported grade 4 treatment-related hematologic AEs at the participant level were neutropenia (47% and 20%), lymphopenia (20% and 7%), and thrombocytopenia (29% and 2%) for vorinostat-EPOCH and EPOCH alone, respectively (supplemental Table 4). Febrile neutropenia (18% and 16%) and grade 3 infections and infestations (9% and 7%) were similar for vorinostat-EPOCH and EPOCH alone, respectively; grade 4 sepsis occurred in 1 patient treated with EPOCH alone. Of the nonhematologic toxicities, peripheral neuropathy occurred at grades 1 and 2 in 29% and 44% of patients treated with vorinostat-EPOCH and EPOCH alone, respectively, and at grade 3 in 4% in the EPOCH arm. Other rare severe or life-threatening events included thromboembolisms (n = 2), respiratory failure (n = 1), left ventricular dysfunction (n = 1), atrial fibrillation (n = 1), and gastric hemorrhage from lymphomatous involvement (n = 1) in the vorinostat-EPOCH arm.
Efficacy
CR rates were similar in both treatment arms, resulting in 68% (95% confidence interval [CI], 54-82) for vorinostat-EPOCH and 74% (95% CI, 61-87) for EPOCH (1-sided P = .72) (Table 3). CR rates for all patients in both arms were 74% for DLBCL, 67% for PBL, and 71% for PEL, with similar rates irrespective of DLBCL subtype or EBV status. There was a correlation between CR rate and CD4+ cell count: CR rates improved from 45% in patients with absolute CD4+ cell counts of 50 to 99 cells/mm3 to 85% in patients with >200 cells/mm3, respectively (trend P = .0038; Table 3).
Table 3.
Clinical outcomes according to baseline characteristics
| Patient subgroups | CR rates | P | ||
|---|---|---|---|---|
| Vorinostat-EPOCH +/− R (%) | EPOCH +/− R (%) | Total (%) | ||
| All patients, n (% [95% CI])* | 30/44 (8 [54-82]) | 31/42 (74 [61-87]) | 61/86 (71 [61-81]) | .72† |
| DLBCL | 21/30 (70) | 24/31 (77) | 45/61 (74) | .57 |
| GCB type | 10/15 (67) | 15/19 (79) | 25/34 (74) | .46 |
| Non-GCB type | 11/14 (79) | 9/12 (75) | 20/26 (77) | .99 |
| Unclassified DLBCL | 0/1 (0) | — | 0/1 (0) | — |
| PBL | 5/8 (63) | 5/7 (71) | 10/15 (67) | .99 |
| PEL | 3/5 (60) | 2/2 (100) | 5/7 (71) | .99 |
| BL | — | 0/1 (0) | 0/1 (0) | — |
| BCL-U, with features b/w DLBCL and BL | 1/1 (100) | 0/1 (0) | 1/2 (50) | .99 |
| EBV status | ||||
| All NHL types | ||||
| Positive | 13/18 (72) | 12/16 (75) | 25/34 (74) | .99 |
| Negative | 12/18 (67) | 16/22 (73) | 28/40 (70) | .74 |
| Inconclusive or ND | 5/8 (62) | 3/4 (75) | 8/12 (67) | .99 |
| Bcl-2 protein | ||||
| All NHL types | ||||
| Positive | 13/18 (72) | 16/21 (76) | 29/39 (74) | .99 |
| Negative | 6/11 (55) | 10/14 (71) | 16/25 (64) | .43 |
| Inconclusive or ND | 11/15 (73) | 5/7 (71) | 16/22 (72) | .99 |
| DLBCL | ||||
| Positive | 10/15 (67) | 14/17 (82) | 24/32 (75) | .42 |
| Negative | 5/7 (71) | 9/12 (75) | 14/19 (74) | .99 |
| Inconclusive or ND | 6/8 (75) | 1/2 (50) | 7/10 (70) | .99 |
| Myc protein | ||||
| All NHL types | ||||
| Positive | 9/14 (64) | 10/16 (63) | 19/30 (63) | .99 |
| Negative | 10/13 (77) | 6/8 (75) | 16/21 (76) | .99 |
| Inconclusive or ND | 11/17 (65) | 15/18 (83) | 26/35 (74) | .26 |
| DLBCL | ||||
| Positive | 5/8 (63) | 7/10 (70) | 12/18 (67) | .99 |
| Negative | 9/11 (82) | 5/7 (71) | 14/18 (78) | .99 |
| Inconclusive or ND | 7/11 (64) | 12/14 (86) | 19/25 (76) | .35 |
| Absolute CD4 count (cells/mm3) | ||||
| <100 | 2/5 (40) | 3/6 (50) | 5/11(45) | .99 |
| 100-199 | 12/19 (63) | 10/16 (63) | 22/35 (63) | .99 |
| >200 (range, 224-1137) | 16/20 (80) | 18/20 (90) | 34/40 (85) | .66 |
| Preprotocol chemotherapy | ||||
| 1 cycle | 9/16 (56) | 13/19 (68) | 22/35 (63) | .50 |
| None | 21/28 (75) | 18/23 (78) | 39/51 (76) | .99 |
| DTI | ||||
| <15 d | 15/25 (60) | 10/19 (53) | 25/44 (57) | .76 |
| ≥15 d | 15/19 (79) | 21/23 (91) | 36/42 (86) | .38 |
| Time from enrollment | EFS (standard error) | P | ||
| 12 mo | 70 (6.9) | 74 (6.8) | 72 (4.8) | .32 |
| 24 mo | 63 (7.4) | 71 (7.0) | 67 (5.1) | |
| 36 mo | 63 (7.4) | 69 (7.2) | 66 (5.2) | |
| Time from enrollment | OS (standard error) | P | ||
| 12 mo | 77 (6.4) | 85 (5.5) | 81 (4.3) | .39 |
| 24 mo | 70 (7.1) | 83 (5.9) | 76 (4.7) | |
| 36 mo | 70 (7.1) | 77 (6.8) | 73 (5.0) | |
Unless otherwise stated, data are number of patients/total patients analyzed (% of total analyzed). Shown are CR and EFS and OS rates after intention to treat according to NHL subtypes, EBV status, Bcl-2 and Myc protein expression, absolute CD4 counts, preprotocol therapy, and DTI.
ND, not done.
95% confidence interval on CR rate. Data are in percentage units.
P value from a 1-sided z-test to detect increase on the VOR-EPOCH arm. All other P-values are from 2-sided tests that used Fisher’s exact test for CR rates and the log-rank test for EFS and OS.
Survival
The 1-, 2-, and 3-year EFS and OS rates were not significantly different between study arms (Table 3; Figure 2; supplemental Figure 1). Notably, the 3-year EFS rates (standard errors) were 63% (7.4%) for vorinostat-EPOCH (20 subjects still at risk) vs 69% (7.2%) for EPOCH (24 subjects still at risk; Table 3). Likewise, the 3-year OS rates were 70% (7.1%) for vorinostat-EPOCH (21 subjects still at risk) vs 77% (6.8%) for EPOCH (26 subjects still at risk; Table 3). The Kaplan-Meier EFS and OS curves for all 86 patients with NHL and those with DLBCL histologic subtypes are shown in Figure 2 and supplemental Figure 1.
Figure 2.
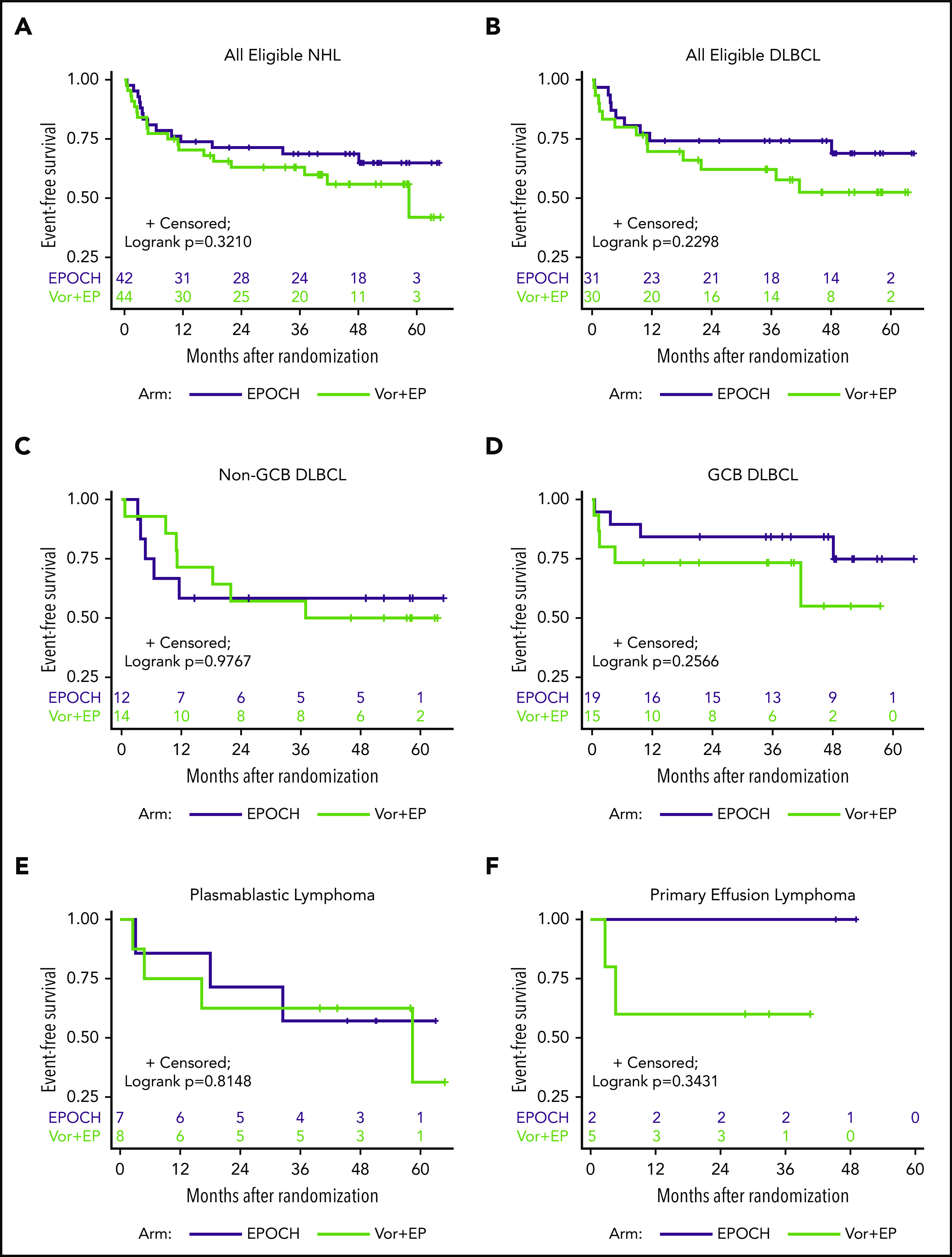
EFS analysis. Kaplan-Meier curves of EFS after treatment with vorinostat-R-EPOCH and R-EPOCH alone for all study-eligible patients with aggressive HIV-NHL (n = 86) (A); all DLBCL (n = 61) (B); non-GCB (n = 26) (C) and GGB (n = 34) DLBCL subtypes (1 case was not classified) (D); and PBL (n = 15) (E) and PEL (n = 7) (F).
Prognostic effects of Myc and Bcl-2 and evaluation of BCL2, BCL6, and MYC gene rearrangements
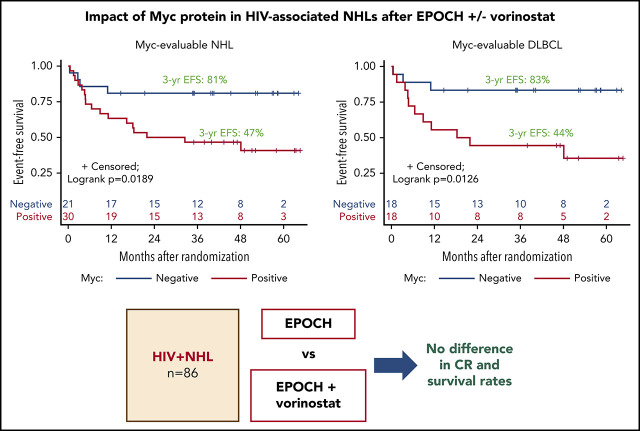
Only Myc protein expression was significantly associated with inferior outcomes. Patients with Myc+ tumors, particularly DLBCL (n = 18), tended to have lower CR rates, lower DTIs, and significantly inferior 3-year EFS, as compared with Myc− patients (n = 18; 47% vs 81% for all NHL [log-rank P = .0189] and 44% vs 83% for DLBCL [log-rank P = .0126]; Table 3; Figure 3A-B; supplemental Figure 2). All 9 PBL patients were Myc+, whereas 3 PEL patients were Myc−. Eight of 29 (28%) DLBCL cases analyzed had MYC rearrangement (-R) (supplemental Table 5). BCL-2 expression was found in 63% of those with DLBCLs, but it had no significant impact on response or survival rates (Table 3; supplemental Table 5; supplemental Figures 3 and 4). Patients with Myc+/Bcl-2+ DLBCL (n = 9; double expressers) and Myc+/Bcl-2− patients (n = 15) had similar survival outcomes (supplemental Figure 5). BCL2-R was present in only 1 of 24 DLBCL patients, and 1 showed equivocal results (supplemental Table 5). Nine of 24 (38%) patients with DLBCL had BCL6-R, and 1 patient showed equivocal results (supplemental Table 5). Finally, only 1 in 24 DLBCLs with fully available FISH panels had a double hit (BCL-6R/MYC-R); another patient had MYC-R with equivocal results for BCL6-R (supplemental Table 5).
Figure 3.
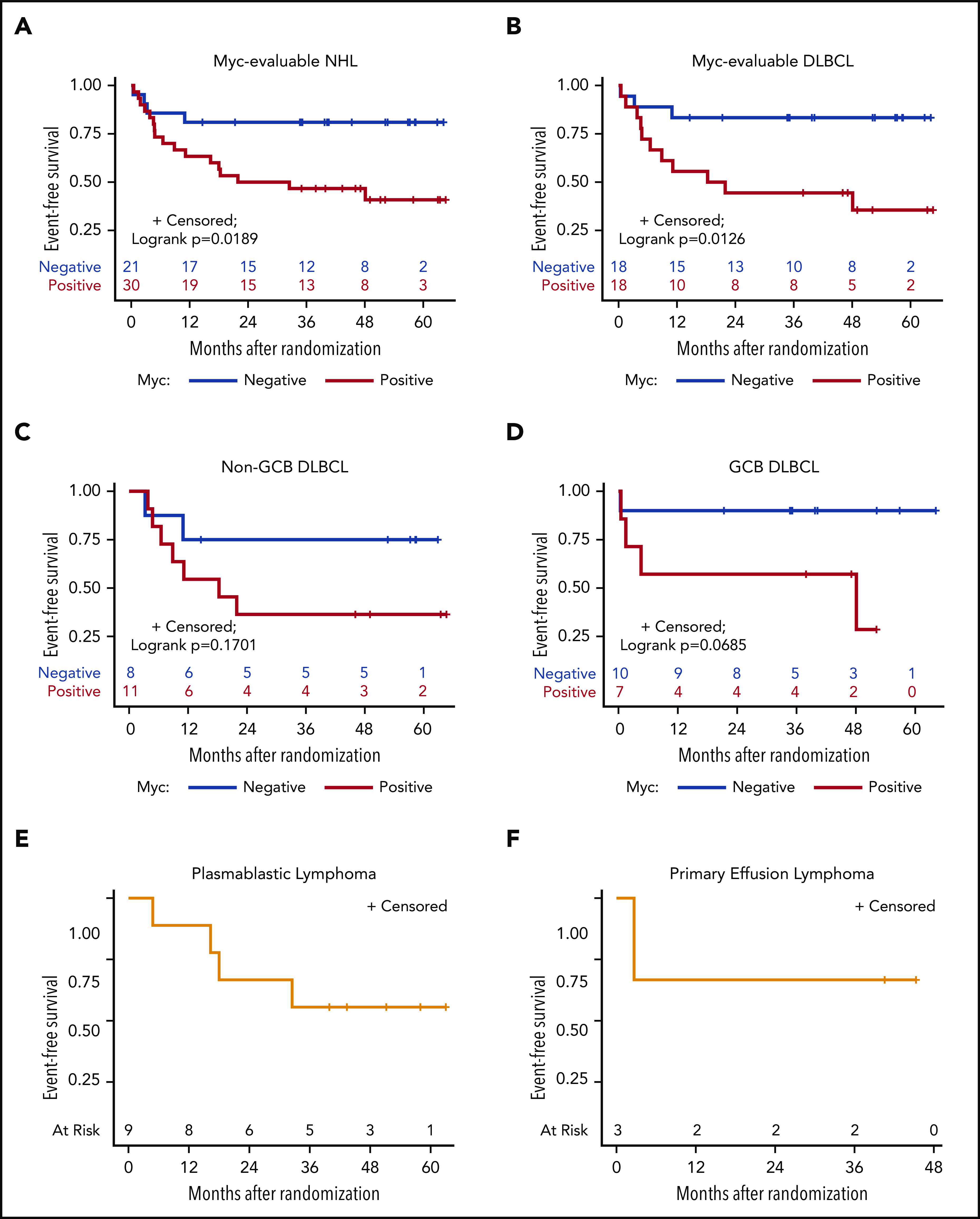
EFS, according to Myc protein expression. Kaplan-Meier curves of EFS in evaluable Myc+ vs Myc− cases in all study-eligible patients with aggressive HIV-NHL (n = 51) (A); all DLBCL (n = 36) (B); non-GCB (n = 19) (C) and GGB (n = 17) (D) DLBCL subtypes; and PBL (9 of 9) (E) and PEL (3 of 3) (F).
Effect of vorinostat on T-cell subsets, HIV and EBV VLs, and immunoglobulin levels
No significant difference between CD4+ and CD8+ cell counts was observed between the treatment arms. In both arms, mean and median CD4+ cell counts decreased from baseline to treatment discontinuation, then rebounded to attain statistical significance above baseline by 12 months (supplemental Figure 6). Most patients with HIV viremia at baseline achieved HIV VLs below the limit of detection at various time points during treatment, with transient increases (“blips”), but no significant differences between arms in terms of HIV control rates or ART failure (supplemental Figure 7). The latent HIV reservoir was investigated longitudinally in patients with undetectable HIV VLs after standard RNA quantitative assays at any point after treatment, using a quantitative viral outgrowth assay; both treatment arms had similar effects on the HIV reservoirs in the small sample of participants who had longitudinal measurements available (n = 7 per arm; supplemental Figure 8). Plasma EBV VLs generally decreased and correlated with tumor response in patients with EBV+ NHL, with transient increases in some cases suggestive of lytic reactivation regardless of treatment (vorinostat or not; data not shown). Mean and median plasma immunoglobulin levels decreased significantly from baseline during treatment in both arms and did not recover fully at 1 year (data not shown).
Impact of preprotocol therapy and DTI on clinical outcomes
We evaluated the association of preprotocol therapy on patient characteristics and clinical outcomes. Of the 86 patients analyzed, 51 (59%) were treatment naive, and 35 (41%) had been treated with 1 cycle of chemotherapy (CHOP-like or EPOCH) +/−R before enrollment. Patient characteristics were largely similar in the 2 groups, although the proportion of patients with short DTI (<15 days) was somewhat higher (22/35; 63%) in the pretreated group than in the treatment-naive group (22/51; 43%; P = .083; supplemental Table 6). When analyzed independently, preprotocol therapy made no difference in CR rates, according to any of the baseline characteristics (Table 3; supplemental Table 7).
We also evaluated separately the association between DTI and clinical outcomes. Baseline patient characteristics and disease-associated features were evenly distributed between short- and long-DTI groups (supplemental Table 6). The proportion of patients with more advanced disease (Ann Arbor stage III-IV) tended to be higher in the short-DTI group than in the long-DTI group (89% vs 76%; χ2 P = .13; supplemental Table 6). A short DTI was associated with a lower CR rate (57% vs 86%; P = .0032), particularly in patients with DLBCL (57% vs 90%; P = .0036; supplemental Table 8). Further, a shorter DTI was associated with lower EFS rates in all patients, and in those with DLBCL, particularly the GCB type (Figure 4A,C,G; supplemental Table 8).
Figure 4.
Survival analysis according to short vs long DTI in all subtype groups. Kaplan-Meier curves of EFS (A,C,E) and OS (B,D,F) in patients with a DTI of <15 days vs ≥15 days for all study-eligible patients with aggressive HIV-NHL (n = 86) (A-B)B); all DLBCL (n = 61) (C-D); non-GCB (n = 26) (E-F); and GGB (n = 34) (G-H) DLBCL subtypes (1 case was not classified).
Discussion
We report results from a randomized phase 2 trial of R-EPOCH, alone or in combination with the oral HDAC inhibitor vorinostat in patients with aggressive HIV-NHL and high aa-IPI score,2,3 high Ki-67 proliferative index ≥80%, or non-GCB DLBCL histologic subtype. The addition of vorinostat to EPOCH +/−R had no effect on CR rates, the primary trial end point, or on HIV viral reservoir size, a secondary trial end point. In the overall population, including both treatment arms, 61 patients had CD20+ DLBCL (43% with non-GCB subtype), 15 had PBL, and 7 had PEL. The CR rates were 74% for DLBCL, 67% for PBL, and 71% for PEL. Between both arms collectively, CR rates and 3-year EFS rates compare favorably with results from previous studies involving noninfusional chemotherapy regimens30 and support EPOCH-based chemotherapy as an effective treatment option for these highly aggressive lymphomas occurring in the context of HIV infection. An earlier study, in which 3 to 6 cycles of EPOCH with dose-dense rituximab were used in patients with HIV and DLBCL, demonstrated significantly lower 1-year OS and progression-free-survival (PFS) of nearly 40% in the non-GCB type, compared with >90% in GCB type.6 By contrast, the histologic type of DLBCL did not have significant impact on survival end points in our study. Notably, the 3-year OS rates on both arms between non-GCB and GCB DLBCL subtypes were 73% (with vorinostat) and 81% (control) vs 73% (with vorinostat) and 89% (control), respectively (supplemental Figure 1C-D). In addition, the 3-year EFS rates showed only modest differences: 57% (with vorinostat) and 58% (control) in non-GCB type DLBCL vs 73% (with vorinostat) and 84% (Control) in GCB-type DLBCL (Figure 2C-D). Regardless, combining R-CHOP or R-EPOCH with drugs that can block non-GCB DLBCL and B-cell receptor signaling, such as Bruton’s tyrosine kinase inhibitors and lenalidomide, represent good clinical trial options to be tested in patients with HIV-NHL. The AMC is currently conducting a pilot trial (AMC-101) using the Bruton’s tyrosine kinase inhibitor ibrutinib with R-EPOCH in patients with HIV-DLBCL. Finally, our trial (AMC-075) included the largest prospective cohorts to date of HIV patients with PBL and PEL with 3-year EFS rates averaging 60% and 71%, respectively, comparing favorably with the 3-year OS rates of ≤40% reported in large retrospective studies.11,35
A recent study demonstrated that Bcl-2 and Myc double expression is associated with prognosis in DLBCL from large cohorts treated with R-CHOP or R-CHOP+etoposide (R-CHOEP).36 Another study that included patients from various clinics and hospitals demonstrated that Myc expression is significantly higher in HIV-related vs HIV-unrelated DLBCL (64% vs 32%) and that Myc positivity increases mortality at 2 years.37 Our study showed that Myc expression was the main driver of poor outcome in patients with HIV-DLBCL treated with EPOCH-based chemotherapy with 3-year EFS of only 44%, compared with 83% in Myc− cases. Perhaps future clinical trials for HIV-DLBCL should be designed to improve outcomes in Myc+ tumors. Likewise, in our study, 63% of DLBCLs expressed Bcl-2, which also showed a consistent trend toward lower survival (supplemental Figures 3 and 4). The availability of Bcl-2 inhibitors, such as venetoclax, may warrant future clinical trials combining these agents with R-EPOCH or standard R-CHOP, as was done in a recent phase 1b trial.38 Such an approach could improve the outcome of DLBCL double expressers in patients with HIV-DLBCL, and the efficacy of R-CHOP, which is a simpler regimen to administer than infusional R-EPOCH.
Based on prior reports of increased toxicity and risk of death caused by infectious complications in patients with CD4+ counts <50 cells/mm,3,5,30 patients were required to have a CD4 count of at least 50 cell/mm3 to be eligible, and the cyclophosphamide dose in the first cycle was tailored to the baseline CD4 count (375 mg/m2 for CD4+ count <200 cells/mm3; 750 mg/m2 for CD4 count ≥200/mm3). Cyclophosphamide and chemotherapy doses were escalated or de-escalated on subsequent cycles based on nadir counts and toxicities from the previous cycle, according to protocol guidelines (supplemental Tables 2 and 3). CR rates were significantly lower in the 46 subjects with CD4+ counts <200 cells/mm3 than in the 40 subjects with higher CD4+ cell counts (59% vs 85%; Fisher’s exact test P = .0091). Patients with lower CD4+ counts received fewer cumulative doses of chemotherapy than did those with higher CD4+ counts, according to protocol guidelines. Given this fact, it is plausible that the inferior outcomes observed in the low CD4+ counts were attributable to the lower chemotherapy administered.
Aside from baseline characteristics and tumor features at the time of diagnosis, other factors may contribute to therapy outcomes in aggressive NHL. Patients with highly aggressive NHL often cannot be enrolled in clinical trials because of logistical challenges, such as immediate treatment needs. Our study allowed 1 cycle of systemic chemotherapy given before study enrollment. Forty-one percent of patients received 1 cycle of chemotherapy without evidence of progression between treatment and registration and had similar baseline characteristics and outcomes, indicating that this strategy was successful in enhancing accrual and increasing the applicability of the outcomes observed in this study with EPOCH-based chemotherapy to routine clinical practice.
A recent large study evaluating the outcomes of HIV− patients with DLBCL treated with R-CHOP demonstrated that a DTI ≥15 days was associated with a better prognosis.26 Similarly, our study showed that a longer DTI was associated with higher CR and EFS rates. There were no substantive differences in the patient characteristics by DTI, although patients with a short DTI (<15 days) were more likely to receive preprotocol therapy (50% vs 31%; P = .072). Future reports should include information on DTI in addition to IPI and other clinical variables, which can be used as metrics to evaluate whether favorable results could be attributable to selection of more favorable patients.
We evaluated combining vorinostat with EPOCH in patients with HIV-NHL, to augment the antineoplastic effects of both rituximab and cytotoxic chemotherapy and induce lytic reactivation of EBV and HHV-8, as shown in preclinical models.13,18-20 In this study, EBV VLs generally decreased and correlated with tumor response in EBV+ NHL with transient increases in some cases, but CR rates did not differ significantly between EBV+ and EBV− NHL groups. HDAC inhibitors have been considered to be promising “shock-and-kill” agents capable of activating HIV transcription leading to viral cytopathic effects and inducing immune-mediated cell death.39-41 In this study, reduction of HIV VLs from baseline occurred in several patients receiving ART, regardless of treatment arm. Subjects who had HIV VLs below the limits of detection at baseline generally continued to have suppressed virus, whereas transient “blips” were observed in some patients in both arms. Analysis of changes in the latent HIV reservoir in resting CD4 memory T cells with a quantitative viral outgrowth assay in several patients revealed no significant effect by either arm, although the sample size was small. Our findings are consistent with those of other studies of vorinostat that have demonstrated activation of HIV gene expression but limited impact on reducing long-lived HIV reservoirs.39,40
In summary, this prospective trial demonstrated that EPOCH-based chemotherapy is broadly efficacious against highly aggressive HIV-NHLs, including non-GCB type DLBCL, PBL, and PEL, each of which have been considered to have relative poor prognosis. However, patients with Myc-driven DLBCL and those with low DTI had the worse outcomes. A 5-day course of vorinostat during each chemotherapy cycle added some toxicity and failed to improve efficacy. Allowing 1 cycle of preprotocol chemotherapy facilitated accrual without affecting the overall results and could be more broadly applied in clinical trials, which may ultimately result in outcomes that are more reflective of the efficacy of novel regimens in real-world practice.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The study was coordinated by the AIDS Malignancy Consortium’s Lymphoma Working Group (A.N., Chair).
This work was supported by National Institutes of Health (NIH), National Cancer Institute (NCI) grant UM1CA121947 and by the NIH, NCI award UM1CA181255. Research reported in this publication was also supported by NIH, NCI award UM1CA181255 to the AMC Biorepository, and by NIH, NCI award P30CA240139 to the Univerity of Miami-Sylvester Comprehensive Cancer Center. All authors are supported by the NCI-sponsored AIDS Malignancy Consortium.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Original data are available from the AIDS Malignancy Consortium upon request to amcpm@emmes.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.C.R. and J.A.S., as protocol chairs, designed and oversaw conduct of the protocol, assisted in the data analysis, and prepared the manuscript; A.N., as the lymphoma working group chair, contributed to the protocol design, conduct, and manuscript preparation; E.G.R., R.F.A., P.G.R., D.A., R.B., L.R., L.K., and R.M. performed additional trial conduct and manuscript preparation; R.F.A., C.M.D., and A.A.C. performed the correlative studies and also contributed to manuscript preparation; A.C. and E.C. performed additional correlative studies, the central pathology review, and contributed to manuscript preparation; and E.R.S., P.C.M., and J.Y.L. performed the statistical analysis and contributed to manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Juan C. Ramos, Hematology Division, Sylvester Comprehensive Cancer Center, 1475 NW 12th Ave, D8-4, Miami, FL 33136; e-mail: jramos2@med.miami.edu.
REFERENCES
- 1.Shiels MS, Islam JY, Rosenberg PS, Hall HI, Jacobson E, Engels EA. Projected cancer incidence rates and burden of incident cancer cases in HIV-infected adults in the United States through 2030. Ann Intern Med. 2018;168(12):866-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barta SK, Samuel MS, Xue X, et al. . Changes in the influence of lymphoma- and HIV-specific factors on outcomes in AIDS-related non-Hodgkin lymphoma. Ann Oncol. 2015;26(5):958-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barta SK, Xue X, Wang D, et al. . Treatment factors affecting outcomes in HIV-associated non-Hodgkin lymphomas: a pooled analysis of 1546 patients. Blood. 2013;122(19):3251-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Little RF, Pittaluga S, Grant N, et al. . Highly effective treatment of acquired immunodeficiency syndrome-related lymphoma with dose-adjusted EPOCH: impact of antiretroviral therapy suspension and tumor biology. Blood. 2003;101(12):4653-4659. [DOI] [PubMed] [Google Scholar]
- 5.Sparano JA, Lee JY, Kaplan LD, et al. ; AIDS Malignancy Consortium . Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood. 2010;115(15):3008-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunleavy K, Little RF, Pittaluga S, et al. . The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood. 2010;115(15):3017-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramos JC, Sparano JA, Rudek MA, et al. . Safety and Preliminary Efficacy of Vorinostat With R-EPOCH in High-risk HIV-associated Non-Hodgkin’s Lymphoma (AMC-075). Clin Lymphoma Myeloma Leuk. 2018;18(3):180-190.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tulpule A, Sherrod A, Dharmapala D, et al. . Multidrug resistance (MDR-1) expression in AIDS-related lymphomas. Leuk Res. 2002;26(2):121-127. [DOI] [PubMed] [Google Scholar]
- 9.Moore SM, Cannon JS, Tanhehco YC, Hamzeh FM, Ambinder RF. Induction of Epstein-Barr virus kinases to sensitize tumor cells to nucleoside analogues. Antimicrob Agents Chemother. 2001;45(7):2082-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid E, Suneja G, Ambinder RF, et al. . Cancer in People Living With HIV, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(8):986-1017. [DOI] [PubMed] [Google Scholar]
- 11.Castillo JJ, Furman M, Beltrán BE, et al. . Human immunodeficiency virus–associated plasmablastic lymphoma: poor prognosis in the era of highly active antiretroviral therapy. Cancer. 2012;118(21):5270-5277. [DOI] [PubMed] [Google Scholar]
- 12.Bayraktar UD, Ramos JC, Petrich A, et al. . Outcome of patients with relapsed/refractory acquired immune deficiency syndrome-related lymphoma diagnosed 1999-2008 and treated with curative intent in the AIDS Malignancy Consortium. Leuk Lymphoma. 2012;53(12):2383-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster WR, Bischin A, Dorer R, Aboulafia DM. Human herpesvirus type 8-associated large B-cell lymphoma: a nonserous extracavitary variant of primary effusion lymphoma in an HIV-infected man: a case report and review of the literature. Clin Lymphoma Myeloma Leuk. 2016;16(6):311-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbone A, Cesarman E, Spina M, Gloghini A, Schulz TF. HIV-associated lymphomas and gamma-herpesviruses. Blood. 2009;113(6):1213-1224. [DOI] [PubMed] [Google Scholar]
- 15.Lima RT, Seca H, Brás S, Nascimento MS, Vasconcelos MH. Treatment of Akata EBV-positive cells with doxorubicin causes more EBV reactivation than treatment with etoposide. Chemotherapy. 2011;57(3):195-203. [DOI] [PubMed] [Google Scholar]
- 16.Seo JS, Cho NY, Kim HR, et al. . Cell cycle arrest and lytic induction of EBV-transformed B lymphoblastoid cells by a histone deacetylase inhibitor, Trichostatin A. Oncol Rep. 2008;19(1):93-98. [PubMed] [Google Scholar]
- 17.Klass CM, Krug LT, Pozharskaya VP, Offermann MK. The targeting of primary effusion lymphoma cells for apoptosis by inducing lytic replication of human herpesvirus 8 while blocking virus production. Blood. 2005;105(10):4028-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nolan L, Crabb S, Beers S, et al. . Synergistic cell death elicited with CD20 monoclonal antibodies and vorinostat [abstract]. Cancer Res AACR 2009;69(9). Abstract 3241. [Google Scholar]
- 19.Sanchez-Gonzalez B, Yang H, Bueso-Ramos C, et al. . Antileukemia activity of the combination of an anthracycline with a histone deacetylase inhibitor. Blood. 2006;108(4):1174-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiozawa K, Nakanishi T, Tan M, et al. . Preclinical studies of vorinostat (suberoylanilide hydroxamic acid) combined with cytosine arabinoside and etoposide for treatment of acute leukemias. Clin Cancer Res. 2009;15(5):1698-1707. [DOI] [PubMed] [Google Scholar]
- 21.Lindemann RK, Newbold A, Whitecross KF, et al. . Analysis of the apoptotic and therapeutic activities of histone deacetylase inhibitors by using a mouse model of B cell lymphoma. Proc Natl Acad Sci USA. 2007;104(19):8071-8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatt S, Ashlock BM, Toomey NL, et al. . Efficacious proteasome/HDAC inhibitor combination therapy for primary effusion lymphoma. J Clin Invest. 2013;123(6):2616-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Archin NM, Espeseth A, Parker D, Cheema M, Hazuda D, Margolis DM. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res Hum Retroviruses. 2009;25(2):207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Contreras X, Schweneker M, Chen CS, et al. . Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol Chem. 2009;284(11):6782-6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edelstein LC, Micheva-Viteva S, Phelan BD, Dougherty JP. Short communication: activation of latent HIV type 1 gene expression by suberoylanilide hydroxamic acid (SAHA), an HDAC inhibitor approved for use to treat cutaneous T cell lymphoma. AIDS Res Hum Retroviruses. 2009;25(9):883-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurer MJ, Ghesquières H, Link BK, et al. . Diagnosis-to-treatment interval is an important clinical factor in newly diagnosed diffuse large B-cell lymphoma and has implication for bias in clinical trials. J Clin Oncol. 2018;36(16):1603-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hans CP, Weisenburger DD, Greiner TC, et al. . Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275-282. [DOI] [PubMed] [Google Scholar]
- 28.Chadburn A, Chiu A, Lee JY, et al. . Immunophenotypic analysis of AIDS-related diffuse large B-cell lymphoma and clinical implications in patients from AIDS Malignancies Consortium clinical trials 010 and 034. J Clin Oncol. 2009;27(30):5039-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chadburn A, Hyjek E, Mathew S, Cesarman E, Said J, Knowles DM. KSHV-positive solid lymphomas represent an extra-cavitary variant of primary effusion lymphoma. Am J Surg Pathol. 2004;28(11):1401-1416. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan LD, Lee JY, Ambinder RF, et al. . Rituximab does not improve clinical outcome in a randomized phase 3 trial of CHOP with or without rituximab in patients with HIV-associated non-Hodgkin lymphoma: AIDS-Malignancies Consortium Trial 010. Blood. 2005;106(5):1538-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheson BD, Pfistner B, Juweid ME, et al. ; International Harmonization Project on Lymphoma . Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586. [DOI] [PubMed] [Google Scholar]
- 32.Van Heertum RL, Scarimbolo R, Wolodzko JG, et al. . Lugano 2014 criteria for assessing FDG-PET/CT in lymphoma: an operational approach for clinical trials. Drug Des Devel Ther. 2017;11:1719-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ambinder RF. Plasma Epstein-Barr virus DNA for screening. N Engl J Med. 2017;377(6):584-585. [DOI] [PubMed] [Google Scholar]
- 34.Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol. 2005;304:3-15. [DOI] [PubMed] [Google Scholar]
- 35.Qunaj L, Castillo JJ, Olszewski AJ. Survival of patients with CD20-negative variants of large B-cell lymphoma: an analysis of the National Cancer Data Base. Leuk Lymphoma. 2018;59(6):1375-1383. [DOI] [PubMed] [Google Scholar]
- 36.Staiger AM, Ziepert M, Horn H, et al. ; German High-Grade Lymphoma Study Group . Clinical impact of the cell-of-origin classification and the MYC/BCL2 dual expresser status in diffuse large B-cell lymphoma treated within prospective clinical trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2017;35(22):2515-2526. [DOI] [PubMed] [Google Scholar]
- 37.Chao C, Silverberg MJ, Xu L, et al. . A comparative study of molecular characteristics of diffuse large B-cell lymphoma from patients with and without human immunodeficiency virus infection. Clin Cancer Res. 2015;21(6):1429-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zelenetz AD, Salles G, Mason KD, et al. . Venetoclax plus R- or G-CHOP in non-Hodgkin lymphoma: results from the CAVALLI phase 1b trial. Blood. 2019;133(18):1964-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Archin NM, Liberty AL, Kashuba AD, et al. . Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy [published correction appears in Nature. 2012;489(7416):460]. Nature. 2012;487(7408):482-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elliott JH, Wightman F, Solomon A, et al. . Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014;10(10):e1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deeks SG. HIV: shock and kill. Nature. 2012;487(7408):439-440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.