Abstract
Rationale and objectives
Post-transplant membranous nephropathy (MN) represents a rare complication of kidney transplantation that can be classified as recurrent or de novo. The clinical, pathological, and immunogenetic characteristics of post-transplant MN and the differences between de novo and recurrent MN are not well understood.
Study Design
Multicenter case series.
Setting and Participants
We included 77 patients from five North American and European Medical Centers with post-kidney transplant MN (27 de novo and 50 recurrent). Patients with MN in the native kidney who received kidney allografts but did not develop recurrent MN were used as non-recurrent controls (n=43). To improve understanding of post-transplant MN, we compared de novo MN with recurrent MN and then contrasted recurrent MN with non-recurrent controls.
Findings
Compared to recurrent MN, de novo MN was less likely to be classified as primary MN (OR, 0.04; P<0.001), and had more concurrent antibody-mediated rejection (OR, 12.0; P<0.001) and inferior allograft survival (HR for allograft failure, 3.2; P=0.007). HLA-DQ2 and HLA-DR17 antigens were more common in recipients with recurrent MN compared to those with de novo MN; however, the frequency of these recipient antigens in recurrent MN was similar to that in non-recurrent MN controls. Among the 93 kidney transplant patients with native kidney failure attributed to MN, older recipient age (HR per each year older, 1.03; P=0.02), recipient HLA-A3 antigen (HR, 2.5; P=0.003), steroid-free immunosuppressive regimens (HR, 2.84; P<0.001) and living-related allograft (HR, 1.94; P=0.03), were predictors of MN recurrence.
Limitations
Retrospective case series, limited sample size due to rarity of the disease, non-standardized nature of data collection and biopsies.
Conclusions
De novo and recurrent MN likely represent separate diseases. De novo MN is associated with humoral alloimmunity and guarded outcome. Potential predisposing factors for recurrent MN include recipients who are older, recipient HLA-A3 antigen, steroid-free immunosuppressive regimen, and living-related donor kidney.
Keywords: Membranous nephropathy (MN); pathology; allograft; HLA, HLA-A3, recurrent glomerulonephritis, de novo glomerulonephritis; allograft biopsy; phospholipase A2 receptor (PLA2R); antibody-mediated rejection (AMR); humoral alloimmunity; renal transplantation; allograft survival; steroid-free immunosuppression; case series
Introduction
Membranous nephropathy (MN) is an immune complex-mediated glomerulopathy characterized by subepithelial electron dense deposits. It is the most common cause of nephrotic syndrome in adult individuals of European ancestry and progresses to kidney failure in at least one third of patients 1. Most cases (75%) of MN lack an identifiable cause (primary MN) 2, and the majority of primary MN cases are associated with antibodies against phospholipase A2 receptor (PLA2R) detectable in the serum and in the subepithelial deposits 3. In those of European ancestry, several studies have shown an association of primary MN with specific HLA types, such as HLA-DR3 (HLA-DR17) and the closely linked HLA-DQA1*05:01-DQB1*02:01 haplotype, which is detected by serologic typing as HLA-DQ2 4–7 In addition to inherited factors, MN in the native kidney may be associated with immune triggers 8.
Post-kidney transplantation MN can manifest as a recurrent or de novo disease. Our understanding of the pathogenesis and natural history of post-KTx MN is incomplete. Most, but not all, studies have found post-KTx MN (both de novo and recurrent) to be associated with inferior allograft survival 9–15. With regard to pathogenesis, a few reports have suggested that de novo MN is linked to alloimmunity 13,16,17 Data on genetic predisposing factors for recurrent MN are very limited. While there is some evidence that recipient HLA-DQA1*05:01 (DQ2 by serology) 18 and donor HLA-A3 19 may predispose to recurrent MN, these findings have not been confirmed. Importantly, although HLA-DQ2, HLA-DR17, and HLA-A3 antigens are common in the general population [20–30% 20,21, 25% 22, and 20–25% 23–25, respectively], a systematic evaluation of the association of recurrent MN with each of these antigens in the donors and recipients has not been performed.
This multicenter case series examined the risk factors and natural history in 27 de novo and 50 recurrent MN cases, with particular focus on the role of alloimmunity and HLA serotypes. We sought first to compare de novo MN and recurrent MN and then to contrast recurrent MN with non-recurrent controls.
Methods
The data for the case series were collected across 5 medical centers: Columbia University Irving Medical Center (CUIMC, USA), Cornell University (USA), Oregon Health & Science University (OHSU, USA), Laval University (Canada), and Necker Hospital (France). Each center obtained demographic, clinical, laboratory and pathology data under approval by their Institutional Review Boards (IRB) and de-identified data were shared with CUIMC. The reported clinical and research activities are consistent with the Principles of the Declaration of Istanbul. In this pathology-based retrospective case series, the requirement for informed consent was waived by the institutional review board.
Allograft biopsies with MN were identified retrospectively from 2005 to 2018. Membranous lupus nephritis, monoclonal MN, and MN that developed in the kidney allograft of patients whose kidney failure in the native kidney was of unknown etiology were excluded. From a total of 33,119 allograft biopsies interpreted at the 5 medical centers between 2005 to 2018, the final cohort comprised 77 patients with one or more biopsies showing post-KTx MN [CUIMC (n=38), OHSU (n=15), Cornell University (n=9), Laval University (n=8), and Necker Hospital (n=7)]. The first post-transplant biopsy with MN was considered as the “index biopsy”. At all centers, allograft biopsies were performed for graft dysfunction and/or proteinuria; in addition, all recipients at OHSU and Necker Hospital underwent protocol biopsies at 3 and 12 months post-transplantation. Post-KTx MN was classified as de novo MN or recurrent MN, based on the native kidney disease. The primary outcome was death-censored graft failure, defined as re-initiation of dialysis or re-transplantation. Steroid-free regimens were defined as steroid-free maintenance immunosuppression from the time of initial hospital discharge after kidney transplantation, without re-introducing maintenance steroids at any time prior to the diagnosis of post-KTx MN (for cases of de novo or recurrent MN), or the end of follow-up (for non-recurrent controls).
HLA Typing and Assessment of Alloimmunity
Recipients and donors were serologically typed for HLA-A, -B, -DR, and -DQ. Circulating donor-specific antibody (DSA) were assessed by Luminex single antigen beads (One Lambda, Canoga Park, CA) and considered positive if at least one of the HLA antibodies was directed against donor antigens [according to each center’s positive cutoff of mean immunofluorescence intensity values].
Pathologic assessment
Allograft biopsies were stained with hematoxylin and eosin, periodic acid–Schiff, Masson trichrome, and Jones methenamine silver. Immunofluorescence staining for IgG, IgM, IgA, C3, C1q, albumin, fibrin, kappa and lambda light chains, and C4d was performed. Electron microscopy was available for 35 patients. Data on PLA2R glomerular staining was available for 39 patients and serum antibody was available for 2 additional patients. Data on glomerular staining for IgG subclasses with clearly dominant or less intense staining for IgG4 in comparison to other subclasses was available for 10 additional patients [IgG4-dominant (n=3), weaker IgG4 staining (n=7)]. A diagnosis of primary MN was suggested based on either PLA2R positivity, or dominance of IgG4 subclass when data on PLA2R were not available.
Histologic parameters were evaluated according to Banff criteria for kidney allograft pathology 26–28. Antibody-mediated rejection (AMR) was defined according to Banff 2017 criteria while acute T-cell-mediated rejection (TCMR) was defined by the presence of borderline changes (as defined by Banff 1997 criteria) or greater changes (grades IA-III).
Controls
To identify variables predictive of recurrent MN, all patients who could be identified from all five participant centers to have kidney failure attributed to MN, transplanted between 2005–2017, and did not have evidence of recurrent MN (either clinically and confirmed by a dysfunction biopsy or incidentally detected on a protocol biopsy) were recorded. Only patients who were followed-up for longer than 2 standard deviation (SD) from the mean time to recurrent MN (≥2512 days post-transplantation) were included in the control group [n=43, CUIMC (n=13), Laval University (n=10), Necker Hospital (n=10), OHSU (n=9), and Cornell University (n=1)]. This cutoff value was selected to avoid misclassifying patients as not having recurrent MN merely because of inadequate follow-up.
Statistical analysis
Statistical analysis was performed using Prism 5 2007 (GraphPad Inc., San Diego CA) and SPSS Statistics 24 (IBM, Armonk, NY). Continuous variables were compared using Mann-Whitney test while categorical variables were compared using Fisher’s exact test. Allograft survival was assessed by the Kaplan-Meier method and univariate comparisons were performed using log rank test. Univariate Cox proportional hazards (PH) analyses for several demographic and clinico-pathologic variables were used to guide the selection of variables for multivariable analysis whenever these results were significant. P values <0.05 with two-sided hypothesis testing were considered statistically significant.
RESULTS
Demographic, clinical, and pathological characteristics
The case series was composed of 77 patients with post-KTx MN that was classified as de novo (n=27) or recurrent (n=50). As demonstrated in Table 1, recipients had a median age of 47 years and included 22% women, 13% self-reported blacks, 54% recipients of grafts from living donors, and 53% who received induction therapy with thymoglobulin. Index biopsies were obtained a median of 291 days post-transplantation, with median serum creatinine of 1.8 mg/d, and median proteinuria of 1.3 g/d. Detailed pathologic features are presented in Table 2. Only a minority of post-KTx MN cases (9%) were detected in protocol biopsies. Post-KTx MN was classified as primary in 21 of 51 (41%) patients [the vast majority of which (n=20) were recurrent MN], based on PLA2R positivity (n=18) or dominance of IgG4 staining (n=3) in cases where PLA2R staining was not available. Concurrent rejection was present in 23 (30%) index biopsies, and was characterized as TCMR (n=12, including 3 characterized as borderline changes), AMR (n=6), and mixed rejection (n=5). In addition to subepithelial deposits, among the individuals for whom electron microscopy data were available, sparse subendothelial or mesangial deposits were detected in 23% of cases, and diffuse foot process effacement was observed in 68%.
Table 1:
Demographic and clinical characteristics of posttransplantation MN
| Characteristics | Total post-Tx MN (n=77) | De novo MN (n=27) | Recurrent MN (n=50) | De novo vs. recurrent |
|---|---|---|---|---|
| Recipient age (years) | 47 (40, 61) | 40 (35, 52) | 56 (44, 67) | P<0.001 |
| Recipient female sex | 17 (22%) | 9 (33%) | 8 (16%) | P= 0.09 |
| Recipient black race | 10 (13%) | 5 (19%) | 5 (10%) | P= 0.3 |
| Donor black race 1 | 5 (8%) | 2 (10%) | 3 (7%) | P= 0.7 |
| Graft from living donor 2 | 41 (54%) | 13 (48%) | 28 (57%) | P= 0.5 |
| Graft from living-related donor 3 | 25 (34%) | 10 (38%) | 15 (31%) | P=0.6 |
| HCV-positive recipient 4 | 1 (1%) | 1 (4%) | 0 (0%) | P=0.4 |
| HLA mismatch 5 | 3 (2, 5) | 4 (3, 5) | 3 (2, 5) | P= 0.7 |
| Induction therapy 6 - Thymoglobulin - IL2 receptor inhibitors - Alemtuzumab - OKT3 - No induction |
37 (53%) 19 (27%) 4 (6%) 1 (1%) 9 (13%) |
14 (56%) 5 (20%) 1 (4%) 1 (4%) 4 (16%) |
23 (51%) 13 (29%) 3 (7%) 0 (0%) 5 (11%) |
P= 0.8 P= 0.6 P= 0.9 P= 0.4 P= 0.7 |
| Steroid-free regimen 7 | 31 (41 %) | 9 (35%) | 22 (44%) | P= 0.5 |
| Post-Tx interval to biopsy (days) | 291 (60, 2174) | 2174 (249, 3385) | 163 (58, 908) | P= 0.001 |
| Serum creatinine at biopsy (mg/dL) 8 | 1.8 (1.3, 2.5) | 2.0 (1.4, 3.2) | 1.6 (1.3, 2.0) | P= 0.09 |
| Proteinuria at biopsy (g/d or g/g) 9 | 1.3 (0.4, 2.5) | 1.3 (0.5, 2.2) | 1.3 (0.4, 2.7) | P= 0.9 |
| Donor-specific antibody 10 | 14 (24%) | 12 (60%) | 2 (5%) | P<0.001 |
Values for categorical variables given as count (%); for continuous variables as median [interquartile range]. Only 1 patient (with recurrent MN) received Rituximab before transplant. Therefore, the significance of pre-transplant treatment with Rituximab could not be assessed
Tx, transplantation
Information on donor race was not available for 14 recipients (7 de novo and 7 recurrent)
Information on graft type (living vs. deceased) was not available for 1 recipient (recurrent)
Information on living-related donor was not available for 3 recipients (1 de novo and 2 recurrent)
Information on HCV status in the recipient was not available for 5 recipients (1 de novo and 4 recurrent)
HLA mismatch is calculated based on A, B, and DR antigens; Information on HLA mismatch was not available for 3 recipients (all de novo)
Information on induction therapy was not available for 7 recipients (2 de novo and 5 recurrent)
Information on steroids maintenance was not available for 1 recipients (de novo)
Serum creatinine at biopsy was not available for 3 recipients (1 de novo and 2 recurrent)
Proteinuria value at biopsy was not available for 8 recipients (4 de novo and 4 recurrent)
Information on concurrent donor-specific antibody was not available for 19 patients (7 de novo and 12 recurrent)
Table 2:
Histologic characteristics of post-KTx MN
| Characteristics | Total post-Tx MN (n=77) | De novo MN (n=27) | Recurrent MN (n=50) | De novo vs. recurrent |
|---|---|---|---|---|
| Concurrent acute rejection 1 - AMR - TCMR |
23 (30%) 11 (14%) 17 (22%) |
13 (48%) 9 (33%) 8 (30%) |
10 (20%) 2 (4%) 9 (18%) |
P=0.02 P<0.001 P=0.3 |
| Protocol biopsies | 7 (9%) | 2 (7%) | 5 (10%) | P=0.9 |
| Likely primary MN 2 | 21 (41%) | 1 (6%) | 20 (61 %) | P<0.001 |
| Global glomerulosclerosis 3 | 5% (0%−14%) | 7% (0%−14%) | 4% (0%−13%) | P=0.4 |
| Segmental glomerulosclerosis 3 | 4 (5%) | 1 (4%) | 3 (6%) | P=0.9 |
| Interstitial inflammation (i: 0–3) | 0.4 ±0.7 | 0.5 ±0.9 | 0.3 ±0.7 | P=0.3 |
| Tubulitis (t: 0–3) | 0.5 ±0.8 | 0.6 ±0.8 | 0.4 ±0.7 | P=0.9 |
| Arteritis (v: 0–3) | 0.1 ±0.3 | 0.1 ±0.4 | 0.1 ±0.2 | P=0.8 |
| Transplant glomerulitis (g: 0–3) | 0.2 ±0.6 | 0.1 ±0.4 | 0.2 ±0.7 | P=0.6 |
| Peritubular capillaritis (ptc: 0–3) | 0.3 ±0.7 | 0.5 ±0.8 | 0.2 ±0.7 | P=0.9 |
| Transplant glomerulopathy (cg: 0–3) | 0.2 ±0.6 | 0.5 ±0.9 | 0.0 ±0.1 | P<0.001 |
| Interstitial fibrosis (ci: 0–3) | 0.9 ±0.8 | 1.0 ±0.9 | 0.8 ±0.8 | P=0.2 |
| Tubular atrophy (ct: 0–3) | 0.9 ±0.8 | 1.1 ±0.9 | 0.8 ±0.8 | P=0.2 |
| Arteriosclerosis (cv: 0–3) | 1.1 ±0.9 | 0.9 ±0.8 | 1.2 ±1.0 | P=0.3 |
| Arteriolar hyalinosis (ah: 0–3) | 1.2 ±1.0 | 1.1 ±1.1 | 1.2 ±1.0 | P=0.8 |
| C4d in peritubular capillary (C4d0–3) | 0.5 ±1.0 | 0.9 ±1.2 | 0.2 ±0.7 | P=0.003 |
| Subendothelial or mesangial deposits 4 | 8 (23%) | 5 (38%) | 3 (14%) | P=0.1 |
| Foot process effacement 4 - Focal (≤50%) - Diffuse (>50%) |
80% (50%−95%) 11 (32%) 23 (68%) |
70% (40%−70%) 6 (42%) 7 (58%) |
90% (75%−100%) 5 (24%) 16 (76%) |
P=0.04 P=0.3 P=0.3 |
Semi-quantitative values are presented as mean ±SD; values in parentheses for global glomerulosclerosis and foot-process effacement are ______. Abbreviations: AMR, antibody-mediated rejection; TCMR, acute T cell mediated rejection.
Five patients had mixed (AMR and TCMR) rejection (4 de novo and 1 recurrent)
Information on PLA2R and clear dominance of one IgG subclass was not available for 26 patients (9 de novo and 17 recurrent). Primary MN was defined by PLA2R positivity and if PLA2R is not available, then by the dominance of IgG4 subclass in the deposits.
Histologic scoring were not available for 1 patient (recurrent MN) and information regarding glomerular sclerosis were absent for 3 patients (all recurrent MN)
Electron microscopic evaluation was not available for 42 patients (14 de novo and 28 recurrent) and foot process effacement could not be precisely assessed in an additional patient (recurrent)
Features of De novo MN
Information regarding PLA2R status or IgG subtyping was available for 18 patients with de novo MN, who were classified as PLA2R positive (n=1), PLA2R negative (n=14), IgG1-dominant (n=2), and IgG3-dominant (n=1). Thus, only 1 of 18 (6%) of de novo MN cases was suggestive of primary MN, compared to 20/33 (61%) cases of recurrent MN (Table 2); the OR for de novo MN being classified as primary MN was 0.04 (95% CI, 0.005 – 0.3; P<0.001). Only a single recipient in the de novo MN group was HCV-positive versus none in the recurrent MN group (Table 1).
Compared to recurrent MN, de novo MN was encountered in younger recipients, occurred later in the course of transplantation, and had less foot process effacement, despite similar proteinuria levels (Tables 1 and 2). Furthermore, de novo MN was associated with individual features often encountered in AMR, including detectable DSA (Table 1), and higher scores for C4d staining in peritubular capillaries and for transplant glomerulopathy (Table 2). Indeed, as shown in Table 2, the diagnosis of overt AMR was more frequent in de novo MN compared to recurrent MN [9/27 (33%) vs. 2/50 (4%); OR, 12.0; 95%CI, 2.4–61; P<0.001].
Associations of HLA Serotype with Recurrent MN and Kidney Failure
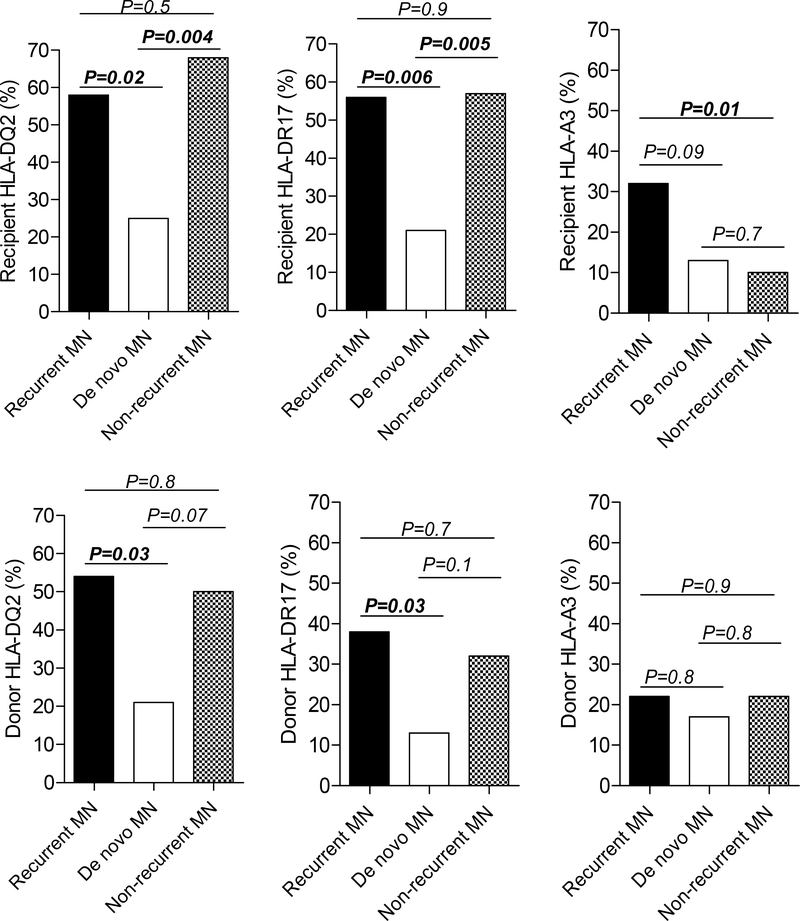
To test whether HLA-DQ2, HLA-DR17, and/or HLA-A3 are associated with recurrent MN, we compared the frequencies of these HLA antigens in both recipients and donors with recurrent MN, de novo MN, and non-recurrent MN controls (whose characteristics are presented in Table S1). Recipients with recurrent MN had higher frequency of HLA-DQ2 antigen (P=0.02) and HLA-DR17 antigen (P=0.006) compared to those with de novo MN, but not compared to recipients classified as non-recurrent MN controls (Figure 1 and Table S2). In contrast, recipients with recurrent MN had higher frequency of HLA-A3 antigen compared to recipients with non-recurrent MN (P=0.01) and nominally more frequent HLA-A3 antigen compared to recipients with de novo MN, but this was not statistically significant (P=0.09) (Figure 1).
Figure 1: Comparison of recipient and donor HLA-DQ2, HLA-DR17, and HLA-A3 antigens between recurrent MN, de novo MN, and patients with kidney failure secondary to MN in the native kidney who did not develop recurrent MN post-transplantation.
For recipient typing, HLA-DQ2 typing was not available for 5 recurrent MN, 7 de novo MN, and 12 non-recurrent MN. Molecular typing for DQA1*05 was available in 9 DQ2+ recipients with recurrent MN (all positive), 2 DQ2+ recipients with de novo MN (both positive), and 5 DQ2+ recipients with non-recurrent MN (all positive). HLA-DR17 and HLA-A3 typing were not available for 3 de novo MN, and 1 non-recurrent MN. For donor typing, HLA-DQ2 typing was not available for 9 recurrent MN, 8 de novo and 13 non-recurrent MN. Molecular typing for DQA1*05 was available in in 6 DQ2+ donors with recurrent MN (5 of 6 positive), 2 DQ2+ donors with de novo MN (1 of 2 positive), and 2 DQ2+ donors with non-recurrent MN (none positive). HLA-DR17 and HLA-A3 typing were not available for 3 de novo MN, and 2 non-recurrent MN.
With regard to donor HLA, patients with recurrent MN were more likely to have received grafts from donors with HLA-DQ2 antigen (P=0.03) and HLA-DR17 antigen (P=0.03) compared to patients with de novo MN but these were similar to those with non-recurrent MN. In contrast, the frequencies of donor HLA-A3 were similar amongst patients with recurrent MN, de novo MN, and non-recurrent MN (Figure 1).
Factors Associated with Recurrent MN
To identify variables predictive of recurrent MN, all patients from the 5 participating centers with native kidney failure attributed to MN regardless of the recurrent disease status were combined (n=93) and MN recurrence was considered as the outcome of interest. On univariate analysis, older recipient age (HR per 1 year older, 1.03; P=0.02), recipient HLA-A3 antigen (HR, 2.5; P=0.003), steroid-free regimen (HR, 2.84; P<0.001), and receiving a living-related allograft (HR, 1.94; P=0.03) were associated with recurrent MN (Table 3). Other demographic variables or induction therapy regimens were not significantly associated with recurrent MN. On multivariable analysis, older recipient age, recipient HLA-A3 antigen, and steroid-free regimens were significantly associated with recurrent MN while receiving an allograft from a living-related donor was numerically similar to the HR for recurrent MN from the univariate analysis, though it was no longer statistically significant (P=0.07) (Table 3). These results should be interpreted with caution due to the non-standardized collection of data and biopsies.
Table 3:
Univariate and multivariable analyses of the association of different variables with recurrence of membranous nephropathy
| Variables | Univariate (n=93) | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
| Recipient age, per 1-y older | 1.03 (1.01 – 1.05) | 0.02 | 1.04 (1.01 – 1.06) | 0.006 |
| Recipient female sex | 0.51 (0.24 – 1.09) | 0.08 | ||
| Recipient black race | 1.22 (0.49 – 3.19) | 0.7 | ||
| Donor black race | 1.63 (0.50 – 5.30) | 0.4 | ||
| Allograft from deceased donor | 0.57 (0.33 – 1.01) | 0.05 | ||
| Allograft from living-related donor | 1.94 (1.05 – 3.58) | 0.03 | 1.86 (0.96 – 3.60) | 0.07 |
| Allograft from living-unrelated donor | 1.06 (0.55 – 2.04) | 0.9 | ||
| Centers that perform protocol biopsies | 0.82 (0.46 – 1.46) | 0.5 | ||
| HLA mismatch,* per each additional mismatch | 0.94 (0.80 – 1.10) | 0.4 | ||
| Induction with Thymoglobulin | 1.69 (0.94 – 3.05) | 0.08 | ||
| Induction with IL-2 receptor inhibitor | 0.81 (0.43 – 1.52) | 0.5 | ||
| No induction therapy | 0.44 (0.17 – 1.11) | 0.08 | ||
| Steroid-free regimen | 2.84 (1.61 – 4.99) | <0.001 | 2.66 (1.47 – 4.83) | 0.001 |
| Recipient HLA-DQ2 | 0.82 (0.45 – 1.48) | 0.5 | ||
| Recipient HLA-DR17 | 1.05 (0.60 – 1.83) | 0.9 | ||
| Recipient HLA-A3 | 2.50 (1.37 – 4.55) | 0.003 | 2.89 (1.53 – 5.41) | 0.001 |
| Donor HLA-DQ2 | 1.17 (0.63 – 2.16) | 0.6 | ||
| Donor HLA-DR17 | 1.24 (0.70 – 2.19) | 0.5 | ||
| Donor HLA-A3 | 1.07 (0.55 – 2.08) | 0.9 | ||
Four variables with univariate P values <0.05, namely recipient age, allograft from living-related donor, steroid-free regimen, and recipient HLA-A3, were included in the multivariable analysis
HLA mismatch is calculated based on A, B, and DR antigens
HLA antigens of donor-recipient pairs from the 15 patients with recurrent MN who received an allograft from a living-related donor were analyzed separately (Table S3). The most frequently shared antigens in each class were HLA-A1 [6 of 15 pairs (40%)], B18 [5 of 15 pairs (33%)], DR11 [6 of 15 pairs (40%)], and DQ2 [6 of 12 pairs (50%)]. Notably, the most frequent shared antigen (HLA-DQ2) was not a significant predictor for recurrent MN (Table 3).
Treatment, outcome, and prognostic indicators of post-KTx MN
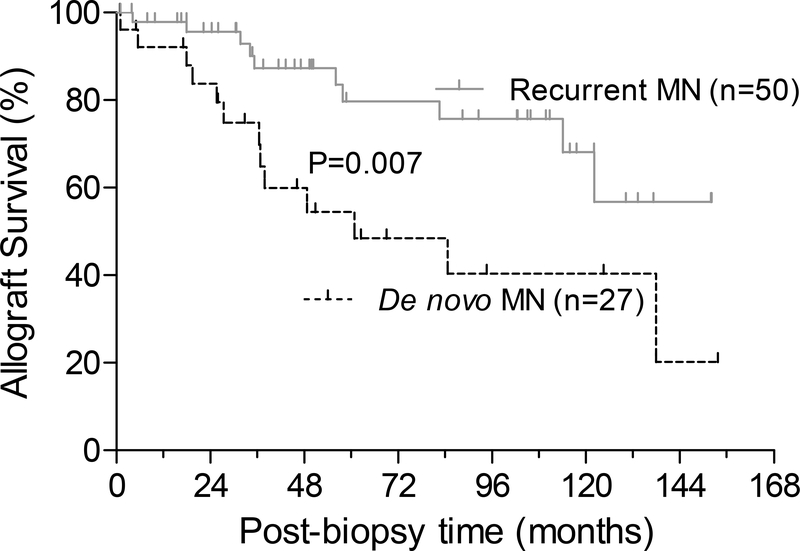
Data on treatment was available for 72 patients (Table S4). Thirty-two (44%) patients were managed conservatively (12 de novo and 20 recurrent). Corticosteroids was used in 15 (21%) patients (7 de novo and 8 recurrent) while rituximab was administered in 22 (31%) patients (5 de novo and 17 recurrent). Plasmapheresis and/or IVIG were used for the remaining 3 (4%) patients (all with de novo MN). Graft failure developed in 23 (30%) patients at a median of 36 (IQR, 23–59) months after the index biopsy. When classified according to MN types, we found that graft failure occurred at an event rate of 11.7 per 100 person-years of follow-up in de novo MN compared to 3.7 per 100 person-years of follow-up in recurrent MN (Figure 2; HR, 3.2; 95%CI, 1.3 – 7.7; P=0.007 by Log rank test).
Figure 2: Allograft survival in de novo and recurrent MN.
Kaplan–Meier curves for post-biopsy cumulative kidney allograft survival in patients with de novo vs. recurrent MN
Variables associated with allograft outcome were then analyzed. In de novo MN, serum creatinine at biopsy, transplant glomerulopathy scores, and C4d scores were associated with inferior allograft survival on univariate analysis although none of them remained significant on multivariable analysis (Table S5). In recurrent MN, the level of proteinuria and transplant glomerulitis scores were associated with inferior allograft survival and both remained significant on multivariable analysis (Table S6).
Discussion
This report represents the largest clinico-pathologic case series of post-KTx MN, which is a rare and understudied condition. Typically, MN occurs as de novo or recurrent disease. Our findings confirm previous reports that de novo MN is usually PLA2R negative 29, unlike MN in the native kidney or recurrent MN. Based largely on immunostaining for PLA2R, and to a lesser extent on the dominant IgG subclass, only 6% of de novo cases were classified as primary, compared to 61% of recurrent MN. Furthermore, our findings show that, compared to recurrent MN, de novo MN is encountered in younger recipients, occurs later in the course of transplantation, is associated with less severe foot-process effacement and is not associated with increased frequency of HLA-DQ2 or DR17 antigens in the recipients. Taken together, these results support the idea that de novo MN has different pathogenetic mechanisms. Some studies have suggested a relation between de novo MN and HCV 30,31, but in our case series, only 1 of 26 recipients of de novo MN had evidence of HCV infection. Some 16,17, but not all 29, more recent studies have suggested that de novo MN is associated with AMR. We found that 33% of index biopsies with de novo MN were diagnostic of overt AMR. This high incidence of AMR is significantly more than that encountered in recurrent MN (4%, P<0.001).
The association of subsets of MN with alloimmunity is backed by several lines of evidence: First, the occurrence of MN in neonates following production of IgG antibodies in mothers who are deficient in neutral endopeptidase 32, and in stem cell transplant recipients following graft vs. host disease 33,34. Second, the strikingly higher incidence of de novo MN in kidney allograft recipients (≥1%) 35–37 compared to MN’s annual incidence of 0.0012% in the general population 38.
Similar to MN in the native kidney, recurrent MN is usually PLA2R positive 29,39 and recurrence after kidney transplantation occurs in 15–50% of patients with kidney failure secondary to MN 40,41. Risk factors for recurrent MN are not well understood. A few studies have suggested that high titers of anti-PLA2R antibodies prior to or at the time of transplantation can predict recurrent disease 18,42. Receiving grafts from living-related and identical donors has been considered a potential risk factor for recurrent MN in some 14,19,43 but not all 44 studies. With regard to HLA antigens, the results are even more conflicting. While Quintana et al. showed that 6 of 7 (86%) recipients with PLA2R-positive recurrent MN were carriers of the HLA allele DQA1*05:0118, other studies have failed to replicate these findings 45,46. Notably, these studies did not specifically address the role of donor HLA-DQ2/DQA1*05:01-DQB1*02:01 in recurrent MN. Andrésdóttir and Wetzels found that 6/8 (75%) of patients who developed recurrent MN received grafts from donors with HLA-A3 antigen compared to 2/15 (13%) of patients without recurrent disease 19. In addition to the small sample size, the Andrésdóttir and Wetzels study did not present data on HLA-A3 antigen in the recipients to address the potential confounding effects of HLA-A3 recipient-donor matching.
In our case series, both recipients with recurrent MN or with native kidney MN without recurrent disease had higher frequencies of HLA-DQ2 and HLA-DR17 compared to those with de novo MN and to the general population. These results suggest that HLA-DQ2 and HLA-DR17 are associated with MN in the native kidney irrespective of recurrent disease. Similar findings, albeit to a lesser extent, were observed for HLA-DQ2 and HLA-DR17 in the donors, probably reflecting considerable efforts to match class-II HLA in recipient-donor pairs.
In contrast to HLA-DQ2 and HLA-DR17, the rate of HLA-A3 positivity in recipients with recurrent MN was considerably higher than in control recipients without recurrent MN, and was nominally higher than that of de novo MN. Furthermore, recipient HLA-A3 antigen was a predictor for recurrent MN, whereas donor HLA-A3, donor or recipient HLA-DQ2, and donor or recipient HLA-DR17 were not (Table 3). While intriguing, these findings require replication in other cohorts. A steroid-free regimen also emerged as a predictor of MN recurrence, suggesting that steroids may work as immune modulators to mitigate potential immunologic triggers for recurrent MN. However, these results should be interpreted with caution, given the longer follow-up (and thus, the greater potential for introducing steroid maintenance over the long-term) in non-recurrent controls compared to recurrent MN. Other predictors for MN recurrence included older recipient age and receiving grafts from living-related donors; the latter may reflect intrafamilial genetic predisposition. Assessing HLA of donor-recipient pairs from the patients with recurrent MN who received allografts from living-related donors could not identify a single HLA antigen that was overwhelmingly shared in the majority of these patients. These findings support further search for additional risk factors in the donor, including non-HLA markers, which may help in recipient-donor matching to reduce the incidence of recurrent MN.
With regard to prognosis, de novo MN had worse allograft survival than recurrent MN. Given the small sample size for de novo MN, it is difficult to tease out the prognostic effects of de novo MN per se vs. AMR. Indeed, in univariate analysis, poor outcome in de novo MN was associated with transplant glomerulopathy, C4d staining in peritubular capillaries, and serum creatinine, all of which may well be related to AMR. In contrast to de novo MN, worse allograft survival in recurrent MN was associated with higher proteinuria and transplant glomerulitis scores. Given the lack of other features supporting AMR in recurrent MN, this glomerulitis may reflect non-specific margination of leukocytes within the glomerular capillaries. In the native kidney, similar leukocyte margination has been described in some patients with renal vein thrombosis, and has been linked to worse outcome 47.
Limitations of our case series include its retrospective nature and incomplete data, notably precluding assessment of anti-PLA2R antibody levels in predicting recurrent MN. Additionally, due to retrospective analysis of the HLA data, we are unable to control for potential population stratification in our HLA analysis. Given the lack of information on haplotype blocks that are in high linkage disequilibrium, and taking into account that HLA-A3 is differentially distributed across worldwide populations, there is a possibility that our association of HLA-A3 with MN recurrence is confounded by the aforementioned factors. Thus, a genetic study that properly controls for ancestry and other HLA antigens is needed to confirm the role of recipient HLA-A3 in predicting recurrent MN.
In summary, although de novo MN and recurrent MN share similar pathologic features (subepithelial deposits), they appear to represent separate diseases. Recurrent MN is frequently PLA2R-mediated, often encountered in older recipients, and occurs early post-transplantation whereas de novo MN is PLA2R-negative, encountered in younger patients, and occurs later in the course of transplantation. The association of de novo MN with overt AMR, as defined by Banff criteria, supports that de novo MN may represent a manifestation of humoral alloimmunity. While our data support the association of HLA-DQ2 and HLA-DR17 antigens with MN in the native kidney, the presence of these HLA antigens, in either the donor or recipient, were not predictive of recurrence. Our findings suggest that both intra-renal donor factors (e.g., living-related allograft), extra-renal recipient factors (including older age and the presence of HLA-A3 antigen) and immune modulators (e.g. steroid-free immunosuppressive regimen) are important contributors in the development of recurrent MN. Genetic studies in donors and recipients will be required to dissect more precisely intra-renal from extra-renal predisposing effects. Exploring the potential interactions between PLA2R polymorphisms, HLA antigens, non-HLA genetic risk factors, and antigen presenting cells in the recipient and donor are potentially rewarding avenues for future research.
Supplementary Material
Acknowledgements
We thank Dr. David J. Salant and Megan Troxell for their valuable insights. We thank Yuancheng Wang, Patricia Maldonado, Hope Dzameshie, and Maria Lourdes Diaz Belvis of CUIMC and Lori Fletcher from Oregon Health & Science University for their excellent technical assistance.
Support: IB is supported by a grant from the American Society of Transplantation (AST) Research Network. KK is supported by the National Institute for Diabetes and Digestive Kidney Diseases (NIDDK) grant numbers RC2-DK116690 and R01-DK105124. SM is supported by the NIH (NIDDK/NIHMD/NIAID) (R01 DK114893 and U01 DK116066). SAH is supported by a Young Investigator award from the National Kidney Foundation. The funders and the authors’ institutions had no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ronco P, Debiec H. Molecular pathomechanisms of membranous nephropathy: from Heymann nephritis to alloimmunization. J Am Soc Nephrol. 2005;16(5):1205–1213. [DOI] [PubMed] [Google Scholar]
- 2.Glassock RJ. Secondary membranous glomerulonephritis. Nephrol Dial Transplant. 1992;7 Suppl 1:64–71. [PubMed] [Google Scholar]
- 3.Beck LH Jr., Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1): 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klouda PT, Manos J, Acheson EJ, et al. Strong association between idiopathic membranous nephropathy and HLA-DRW3. Lancet. 1979;2(8146):770–771. [DOI] [PubMed] [Google Scholar]
- 5.Vaughan RW, Demaine AG, Welsh KI. A DQA1 allele is strongly associated with idiopathic membranous nephropathy. Tissue Antigens. 1989;34(5):261–269. [DOI] [PubMed] [Google Scholar]
- 6.Vaughan RW, Tighe MR, Boki K, et al. An analysis of HLA class II gene polymorphism in British and Greek idiopathic membranous nephropathy patients. Eur J Immunogenet. 1995;22(2):179–186. [DOI] [PubMed] [Google Scholar]
- 7.Stanescu HC, Arcos-Burgos M, Medlar A, et al. Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med. 2011;364(7):616–626. [DOI] [PubMed] [Google Scholar]
- 8.van de Logt AE, Fresquet M, Wetzels JF, Brenchley P. The anti-PLA2R antibody in membranous nephropathy: what we know and what remains a decade after its discovery. Kidney Int. 2019;96(6):1292–1302. [DOI] [PubMed] [Google Scholar]
- 9.Cosyns JP, Couchoud C, Pouteil-Noble C, Squifflet JP, Pirson Y. Recurrence of membranous nephropathy after renal transplantation: probability, outcome and risk factors. Clin Nephrol. 1998;50(3):144–153. [PubMed] [Google Scholar]
- 10.El-Zoghby ZM, Grande JP, Fraile MG, Norby SM, Fervenza FC, Cosio FG. Recurrent idiopathic membranous nephropathy: early diagnosis by protocol biopsies and treatment with anti-CD20 monoclonal antibodies. Am J Transplant. 2009;9(12):2800–2807. [DOI] [PubMed] [Google Scholar]
- 11.Pippias M, Stel VS, Areste-Fosalba N, et al. Long-term Kidney Transplant Outcomes in Primary Glomerulonephritis: Analysis From the ERA-EDTA Registry. Transplantation. 2016;100(9): 1955–1962. [DOI] [PubMed] [Google Scholar]
- 12.Pruthi R, McClure M, Casula A, et al. Long-term graft outcomes and patient survival are lower posttransplant in patients with a primary renal diagnosis of glomerulonephritis. Kidney Int. 2016;89(4):918–926. [DOI] [PubMed] [Google Scholar]
- 13.Truong L, Gelfand J, D’Agati V, et al. De novo membranous glomerulonephropathy in renal allografts: a report of ten cases and review of the literature. Am J Kidney Dis. 1989;14(2):131–144. [DOI] [PubMed] [Google Scholar]
- 14.Berger BE, Vincenti F, Biava C, Amend WJ, Jr., Feduska N, Salvatierra O, Jr. De novo and recurrent membranous glomerulopathy following kidney transplantation. Transplantation. 1983;35(4):315–319. [DOI] [PubMed] [Google Scholar]
- 15.Couchoud C, Pouteil-Noble C, Colon S, Touraine JL. Recurrence of membranous nephropathy after renal transplantation. Incidence and risk factors in 1614 patients. Transplantation. 1995;59(9):1275–1279. [PubMed] [Google Scholar]
- 16.Honda K, Horita S, Toki D, et al. De novo membranous nephropathy and antibody-mediated rejection in transplanted kidney. Clin Transplant. 2011;25(2):191–200. [DOI] [PubMed] [Google Scholar]
- 17.Wen J, Xie K, Zhang M, et al. HLA-DR, and not PLA2R, is expressed on the podocytes in kidney allografts in de novo membranous nephropathy. Medicine (Baltimore). 2016;95(37):e4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quintana LF, Blasco M, Seras M, et al. Antiphospholipase A2 Receptor Antibody Levels Predict the Risk of Posttransplantation Recurrence of Membranous Nephropathy. Transplantation. 2015;99(8):1709–1714. [DOI] [PubMed] [Google Scholar]
- 19.Andresdottir MB, Wetzels JF. Increased risk of recurrence of membranous nephropathy after related donor kidney transplantation. Am J Transplant. 2012;12(1):265–266. [DOI] [PubMed] [Google Scholar]
- 20.Karhus LL, Thuesen BH, Skaaby T, Rumessen JJ, Linneberg A. The distribution of HLA DQ2 and DQ8 haplotypes and their association with health indicators in a general Danish population. United European Gastroenterol J. 2018;6(6):866–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummins AG, Roberts-Thomson IC. Prevalence of celiac disease in the Asia-Pacific region. J Gastroenterol Hepatol. 2009;24(8):1347–1351. [DOI] [PubMed] [Google Scholar]
- 22.Tjernstrom F, Hellmer G, Nived O, Truedsson L, Sturfelt G. Synergetic effect between interleukin-1 receptor antagonist allele (IL1RN*2) and MHC class II (DR17,DQ2) in determining susceptibility to systemic lupus erythematosus. Lupus. 1999;8(2):103–108. [DOI] [PubMed] [Google Scholar]
- 23.Edwards CQ, Griffen LM, Kushner JP. Increased frequency of HLA-A3 in subjects with sporadic porphyria cutanea tarda. Tissue Antigens. 1988;31(5):250–253. [DOI] [PubMed] [Google Scholar]
- 24.Fogdell-Hahn A, Ligers A, Gronning M, Hillert J, Olerup O. Multiple sclerosis: a modifying influence of HLA class I genes in an HLA class II associated autoimmune disease. Tissue Antigens. 2000;55(2):140–148. [DOI] [PubMed] [Google Scholar]
- 25.Posthuma EF, Falkenburg JH, Apperley JF, et al. HLA-B8 and HLA-A3 coexpressed with HLA-B8 are associated with a reduced risk of the development of chronic myeloid leukemia. The Chronic Leukemia Working Party of the EBMT. Blood. 1999;93(11):3863–3865. [PubMed] [Google Scholar]
- 26.Loupy A, Haas M, Solez K, et al. The Banff 2015 Kidney Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting Molecular Pathology. Am J Transplant. 2017;17(1):28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713–723. [DOI] [PubMed] [Google Scholar]
- 28.Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18(2):293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsen CP, Walker PD. Phospholipase A2 receptor (PLA2R) staining is useful in the determination of de novo versus recurrent membranous glomerulopathy. Transplantation. 2013;95(10):1259–1262. [DOI] [PubMed] [Google Scholar]
- 30.Morales JM, Pascual-Capdevila J, Campistol JM, et al. Membranous glomerulonephritis associated with hepatitis C virus infection in renal transplant patients. Transplantation. 1997;63(11):1634–1639. [DOI] [PubMed] [Google Scholar]
- 31.Cruzado JM, Carrera M, Torras J, Grinyo JM. Hepatitis C virus infection and de novo glomerular lesions in renal allografts. Am J Transplant. 2001;1(2):171–178. [PubMed] [Google Scholar]
- 32.Debiec H, Guigonis V, Mougenot B, et al. Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med. 2002;346(26):2053–2060. [DOI] [PubMed] [Google Scholar]
- 33.Byrne-Dugan CJ, Collins AB, Lam AQ, Batal I. Membranous nephropathy as a manifestation of graft-versus-host disease: association with HLA antigen typing, phospholipase A2 receptor, and C4d. Am J Kidney Dis. 2014;64(6):987–993. [DOI] [PubMed] [Google Scholar]
- 34.Reddy P, Johnson K, Uberti JP, et al. Nephrotic syndrome associated with chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;38(5):351–357. [DOI] [PubMed] [Google Scholar]
- 35.Charpentier B, Levy M. [Cooperative study of de novo extramembranous glomerulonephritis in renal allografts in humans: report of 19 new cases in 1550 renal transplant patients of the transplantation group of the Ile de France]. Nephrologie. 1982;3(4):158–166. [PubMed] [Google Scholar]
- 36.Schwarz A, Krause PH, Offermann G, Keller F. Impact of de novo membranous glomerulonephritis on the clinical course after kidney transplantation. Transplantation. 1994;58(6):650–654. [PubMed] [Google Scholar]
- 37.Antignac C, Hinglais N, Gubler MC, Gagnadoux MF, Broyer M, Habib R. De novo membranous glomerulonephritis in renal allografts in children. Clin Nephrol. 1988;30(1):1–7. [PubMed] [Google Scholar]
- 38.McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26(2):414–430. [DOI] [PubMed] [Google Scholar]
- 39.Debiec H, Martin L, Jouanneau C, et al. Autoantibodies specific for the phospholipase A2 receptor in recurrent and De Novo membranous nephropathy. Am J Transplant. 2011;11(10):2144–2152. [DOI] [PubMed] [Google Scholar]
- 40.Allen PJ, Chadban SJ, Craig JC, et al. Recurrent glomerulonephritis after kidney transplantation: risk factors and allograft outcomes. Kidney Int. 2017;92(2):461–469. [DOI] [PubMed] [Google Scholar]
- 41.Grupper A, Cornell LD, Fervenza FC, Beck LH, Jr., Lorenz E, Cosio FG. Recurrent Membranous Nephropathy After Kidney Transplantation: Treatment and Long-Term Implications. Transplantation. 2016;100(12):2710–2716. [DOI] [PubMed] [Google Scholar]
- 42.Kattah A, Ayalon R, Beck LH Jr., et al. Anti-phospholipase A(2) receptor antibodies in recurrent membranous nephropathy. Am J Transplant. 2015;15(5):1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agarwal SK, Dash SC, Mehta SN, Bhuyan UN. Recurrence of idiopathic membranous nephropathy in HLA-identical allograft. Nephron. 1992;60(3):366. [DOI] [PubMed] [Google Scholar]
- 44.Kennard AL, Jiang SH, Walters GD. Increased glomerulonephritis recurrence after living related donation. BMC Nephrol. 2017;18(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cosio FG, Cattran DC. Recent advances in our understanding of recurrent primary glomerulonephritis after kidney transplantation. Kidney Int. 2017;91(2):304–314. [DOI] [PubMed] [Google Scholar]
- 46.Gupta G, Fattah H, Ayalon R, et al. Pre-transplant phospholipase A2 receptor autoantibody concentration is associated with clinically significant recurrence of membranous nephropathy post-kidney transplantation. Clin Transplant. 2016;30(4):461–469. [DOI] [PubMed] [Google Scholar]
- 47.Saito T, Yusa A, Soma J, Ootaka T, Sato H, Ito S. Significance of leukocyte infiltration in membranous nephropathy with segmental glomerulosclerosis. Nephron. 1998;80(4):414–420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.