Abstract
Objective
Ejaculatory duct obstruction (EDO) is an uncommon but potentially treatable cause of male factor infertility. However, there are limited data on transurethral resection of the ejaculatory ducts (TURED) as a treatment option. A systematic review was therefore conducted to assess its efficacy and identify patient subgroups that benefit from the procedure.
Material and methods
A database search of PubMed, Embase, and Scopus (up to January 2019) and the World Health Organization trial registry was performed to identify all studies assessing infertile men with EDO undergoing TURED. The primary outcome measures included semen parameters and natural pregnancies. The secondary outcomes included complications, symptomatic improvement, and a change from in vitro fertilization to intrauterine insemination.
Results
Of 3,277 articles screened, 29 studies with 634 patients were included in the study. Although outcomes varied considerably among studies, a general increase in all semen parameters postoperatively was observed. Semen volume (n=23 studies) improved in a median of 83.0% of patients (interquartile range [IQR]: 37.5). Sperm motility and concentration (n=10 and n=21 studies) improved in a median of 63.0% (IQR: 15.0) and 62.5% (IQR: 16.5) of patients, respectively. The natural pregnancy rate across the studies was a median of 25.0% (IQR: 15.7). Improvements in both the outcomes were greater in patients with congenital etiologies and partial EDO. Differences in surgical technique did not appear to affect outcomes.
Conclusion
TURED is associated with improvements in semen parameters and offers a chance of restoring fertility in previously subfertile men. Although results are promising, the current evidence remains limited owing to predominantly retrospective studies with small sample sizes.
Keywords: Azoospermia, ejaculatory ducts, infertility, oligozoospermia
Introduction
Infertility affects 15% of couples, and male factor infertility contributes to 50% of all cases.[1] Ejaculatory duct obstruction (EDO) can be congenital or acquired and accounts for 1%–5% of cases of male factor infertility.[2] Congenital causes include cysts, atresia, or stenosis of the ejaculatory ducts, whereas acquired causes include infection, inflammation, trauma, or calculi.[3–5] Infertility is a common presentation, but EDO can also be symptomatic, causing hematospermia, peri-ejaculatory perineal, or testicular pain and dysuria.[2,5] The diagnosis is made by a semen analysis where typical findings include azoospermia with a low-volume ejaculate, followed by imaging to confirm the diagnosis. Vasography was the diagnostic test of choice, but owing to the risk of vasal injury and stricture[6], transrectal ultrasound (TRUS) and magnetic resonance imaging are now the preferred imaging modalities.[7] Once confirmed, EDO is amenable to surgical management, making it a vital differential to consider when diagnosing male factor infertility.[5] The mainstay treatment is transurethral resection of the ejaculatory ducts (TURED)[2,5,6,8–10], first described by Farley and Barnes in 1973.[11]
Although TURED is performed by urologists and recommended as a treatment for EDO by the European Association of Urology[12], its effectiveness remains poorly understood and the patient subgroups that are most likely to benefit from TURED require further clarification.
Objectives
This systematic review primarily aims to (1) provide a critical overview of the current literature on the use of TURED for treating obstructive infertility, (2) assess the effectiveness of TURED in EDO in terms of semen parameters and postoperative natural pregnancies, and (3) identify any predictors of the successful outcomes.
Material and methods
Protocol and registration
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement[13], and it was prospectively registered with PROSPERO (registration no.: CRD42019123024).
Study eligibility criteria
Primary research articles, including both experimental and observational studies, were included. All article types were included without any restriction on study size except for the case reports. Studies were included if participants were infertile men (confirmed by semen analysis results of azoospermia or oligozoospermia [<20×106 sperm/mL]) with EDO of any age, were treated with TURED, and outcomes regarding semen parameters or postoperative natural pregnancy were reported. The exclusion criteria included studies not documenting semen volume, sperm concentration, or natural pregnancy outcomes; animal studies; studies describing female factor infertility; and articles not in English.
Information sources and search
A systematic literature search was conducted using Embase, PubMed, and Scopus up to January 25, 2019. Only English language articles were searched with a combination of key search terms and medical subject headings (MeSH) terms. These included, but were not limited to, (ejaculatory duct), (infertility), and (surgery) for keywords and (azoospermia), (genital disease), and (surgical procedure) for MeSH terms. Search words were combined with duplicates removed (Appendix 1). A reference review of identified articles was subsequently conducted to identify any further pertinent articles. The gray literature was searched via abstracts on Embase and Scopus and authors of ongoing trials identified through searches of the World Health Organization (WHO) trial registry.
Study selection
Two reviewers (AM and MG) independently reviewed search results. Relevant titles were saved, and their abstracts were screened to determine whether they met the inclusion criteria. Subsequently, full texts were read to determine whether the paper can be included in the study. In case the same study was published in different journals or years, only the most recent paper was included. If only abstracts were available, they were included if sufficient information was present regarding the patient demographics, intervention, and outcomes.
Data collection and data items
Data extraction was conducted onto a predefined proforma. Specific data were extracted from each study, including author, publication year, study type, sample size, age, symptoms, diagnostic investigation, surgical technique, and follow-up period. The primary outcome measures extracted for the assessment of study aims included improvement in semen parameters postoperatively, looking specifically at semen volume, sperm concentration, sperm motility, and the number of natural pregnancies postoperatively. The secondary outcomes extracted included complications after treatment, resolution of symptoms postoperatively, and the conversion from an in vitro fertilization (IVF) program to intrauterine insemination (IUI) for assisted reproduction, given sufficient sperm concentration improvement and pregnancy outcomes.
Quality assessment
The risk of bias for each paper was conducted using the Methodological Index for Nonrandomized Studies tool[14], with a total score of 16 for noncomparative studies and 24 for comparative studies. No cutoff points for the total score were used, but individual strengths and weaknesses across domains were assessed. The Grading of Recommendations Assessment, Development and Evaluation (GRADE)[15] was also used to assess the overall quality of evidence for each outcome.
Results
Study selection
A total of 3,277 articles were identified, with 471 studies saved for abstract screening. Of these, 105 were assessed for eligibility by full-text screening, with 29 included in the final review (Figure 1). All studies were nonrandomized. Four of these were prospective single-arm studies, and two were double-armed prospective studies with fertile controls. The remaining studies were single-arm studies, of which 14 were retrospective, and the rest were not reported (Appendix 2).
Figure 1.
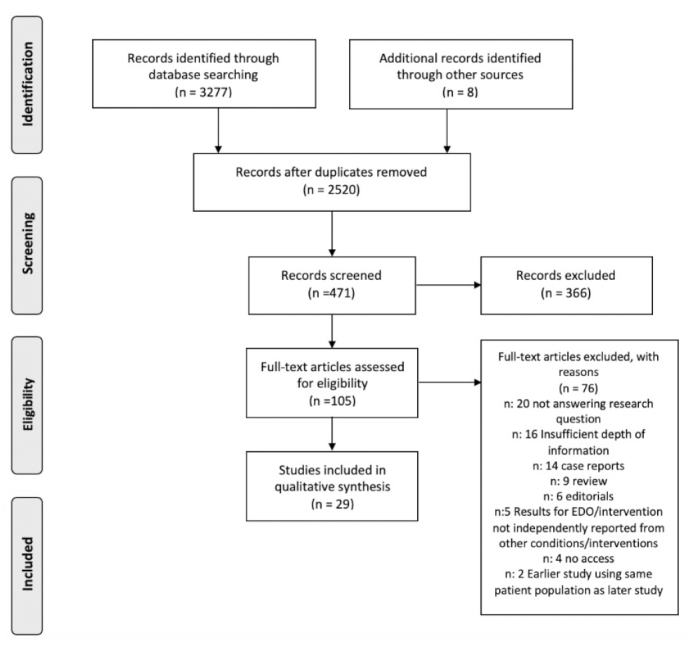
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowsheet for study selection[13]
Study characteristics and results synthesis
Medians with interquartile ranges (IQRs) across studies were calculated for each of the primary and secondary outcomes.
Patient demographics
The sample sizes were low, with a median of 12 for both the total number of patient with EDO and patients who had TURED. The number of males with EDO was 634, with 609 presenting with infertility, with a median age of 35 years. The ages of female partners were not documented. A total of 517 patients underwent TURED. The follow-up time was detailed in 21 studies, ranging from 2 to 78 months, with a median of 12 months postoperatively (Appendix 2).
EDO diagnosis
Infertility due to EDO was confirmed in most studies by a combination of at least two semen analyses, blood tests, and imaging techniques. Typical semen analysis results were a low semen volume (<1.5 mL) and azoospermia for complete EDO, and normal or low semen volume with oligozoospermia (<20×106 sperm/mL) or asthenozoospermia (<30% motility) for partial EDO. Other findings were low semen fructose levels and normal serum follicle-stimulating hormone and testosterone levels. A urinalysis was also performed to rule out retrograde ejaculation. The primary diagnostic imaging test was TRUS, which was reported in 25 of 29 studies, with typical findings including dilated seminal vesicles or ejaculatory ducts (Appendix 2).
Improvement in semen parameters
All 29 studies assessed the impact of TURED on semen parameters and reported improvements in at least one parameter. A total of 23 studies[5,8–10,16–34] reported semen volume outcomes and showed an increase from a median of 0.93 mL (IQR: 0.37) preoperatively to a median of 2.90 mL (IQR: 1.13) postoperatively. Across these studies, a median of 83.0% (IQR: 37.5) of patients demonstrated improvement in semen volume postoperatively. The improvements were less for sperm concentration and motility. Sperm concentration, reported in 21 studies[4,5,8–10,16–21,23,24,26–30,32,33,35], increased from a median of 3.2×106/mL (IQR: 12.0) to 17.0×106/mL (IQR: 19.6), but only a median of 62.5% (IQR: 16.5) of patients showed improvement. In one study, by Kuligowska et al.[32], none of the patients demonstrated improvements in sperm concentration. For sperm motility, a median of 63.0% (IQR: 15.0) of patients showed improvements with an increase from a median of 10.0% (IQR: 9.2) to 34.8% (IQR: 9.9) post-TURED (n=10 studies) (Table 1).[8,9,16,18,21,22,24,26,28,31]
Table 1.
Primary outcomes: results showing pre and postoperative semen variables and pregnancy outcomes for each study
| Preoperative semen variables, mean±SD (range) | Postoperative semen variables, mean±SD (range) | |||||||
|---|---|---|---|---|---|---|---|---|
| Study (author, year) | Preoperative semen analysis | Semen volume (mL) | Sperm concentration (×106/mL) | Sperm motility (%) | Numbers showing postoperatively, n (%)improvement | Semen volume (mL) | Sperm concentration (×106/mL) | Sperm motility (%) |
| Sangster et al. (2017)[16] | - | 0.9 | 12.5 (0–75)a | 10 (0–45)a | 16 (62) sperm concentration, motility, semen volume c** | 3.7a | 25 (4–137)a | 43 (14–68)a |
| Tu et al. (2013)[17] | 38 azoospermia, 4 severe oligozoospermia | 1.02 (0.2–3.5) | - | - | 38 (91) semen volume, 27 (68) sperm concentration | 3.68 (2.0–5.8) | - | - |
| Tu et al. (2012)[40] | Azoospermia | 1.1±0.76 | 0 | N/A | 51 (85) semen parameters | - | - | - |
| El-Assmy et al. (2012)[9] | 17 azoospermia, 3 severe oligozoospermia, 3 oligozoospermia | 0.54±0.24 | 1±0.2 | 3.5±1.4 | 12 (52) semen volume, 10 (43) sperm concentration and motility | 2.1±1.7, p=0.007 | 7±1, p=0.003 | 23±14, p<0.001 |
| Eisenberg et al. (2008)[18] | 3 azoospermia, 2 oligozoospermia | 0.56 (0.05–1.6) | 27.5 (0–125) | 5.7 (0–27) | 5 (83) semen volume and sperm concentration, 6 (100) sperm motility | 2.7 (1.0–5.6) | 52.5 (4–126) | 29.8 (1–57) |
| Yurdakul et al. (2008)[5] | 12 azoospermia | <1.5 | 0 | - | 12 (100) semen volume, 11 (92) sperm concentration | 1.5–5 | >20: 42%, 5–20: 25%, <5: 33% | - |
| Pace et al. (2008)[19] | 1±0.62 | 3.2±1.3 | - | 7 (100) semen volume, sperm concentration | 3.1±0.7 | 10.7±10.1 | - | |
| Johnson et al. (2005)[41] | - | 1.1 | 8.1d | - | - | 2.3 | 38.1d | - |
| Apaydin et al. (2004)[20] | - | - | - | - | 8 (80) semen volume, 6 (60) sperm concentration | - | - | - |
| Purohit et al. (2004)[21] | 2 azoospermia | 0.89 (0.03–2.9)b | 37.9 (0–214)b | 24 (1–64) | 8 (100) semen volume, 4 (50) sperm concentration, 5 (63) sperm motility | 3.4 (1.5–6.3) | 50.8 (1–183) | 39.2 (3.5–65) |
| Kadioglu et al. (2001)[37] | 22 azoospermia, 3 oligozoospermia, 3 asthenozoospermia, 10 oligoasthenozoospermia | 1±0.5 | 4±3.6 | 9.7±6.5 | 28 (74) >50% improvement semen variables | 2.2±1.2, p=0.000 | 17±12, p=0.000 | 32±27, p=0.000 |
| Ozgok et al. (2001)[22] | - | - | 1.66 | - | 15 (63) semen volume, 14 (58) sperm motility | - | 25.4, p=0.001 | - |
| Schroeder-Printzen et al. (2000)[23] | 12 azoospermia, 4 severe oligozoospermia | 0.95 (0.5–1.6) | 0.07 | - | 16 (100) semen volume, 8 (50) sperm concentration | Increased | 3.97 | - |
| Paick et al. (2000)[39] | 45: azoospermia or severe oligozoospermia | <1 in 45, 1.5–4 mL in 5 | <1 in 45, 2–240 in 5 | <30% | 18 (69) semen quality | - | - | - |
| Aggour et al. (1998)[35] | 11 azoospermia | - | 0 | N/A | Of 10 with intraoperative patency: 7 (70) increase sperm concentration | - | - | - |
| Popken et al. (1998)[10] | 8 azoospermia | 1.15±0.35 | 0 | N/A | 6 (75) semen volume and sperm concentration | 3.5±0.7 (successful TURED: n=6), 1.05±0.4 (unsuccessful TURED: n=2) | 12.6±10.7 (successful TURED) 0 (unsuccessful TURED) |
- |
| Netto et al. (1998)[24] | 0 azoospermia | 1.7 (0.4–3.1) | 6.99 (0.1–22) | 18.9 (0–65) | 11 (79) semen volume and sperm motility, 10 (71) sperm concentration | 2.8 (1.1–6.2) | 19.6 (0–70.5) | 44. 6 (0–78) |
| Turek et al. (1996)[25] | 23 azoospermia | - | 0.89 (0–3.9) | - | In 36 with low volume: 17 (46) normalized | - | 70.2 (21.8–78.8) | - |
| Vazquez-Levin et al. (1994)[26] | 2 azoospermia, 3 severe oligoasthenozoospermia, 2 normospermia plus asthenozoospermia, 1 oligoasthenozoospermia | 2.3 (0.5–5.1) | 14.79 (0–55.5) | 17.8 (10–26) | 6 (75) semen volume, 5 (63) sperm concentration and motility | 4.7 (1.4–7.2) | 33.4 (0.25–129) | 32.8 (19–47) |
| Jarow (1994)[27] | 1 azoospermia | - | - | - | 2 (100) increase in semen volume and sperm concentration | 2.35 (0.2–4.5) | 2.4 (0.8–4) | - |
| Hall and Oates (1993)[28] | 3 azoospermia | 0.64 (0.3–0.9) | 6.84 (0–34) | - | 4 (100) semen volumec, (60) sperm concentration and motility | 1.33 (0.8–2)c | 11.8 (0–25) | 20 (0–55) |
| Weintraub et al. (1993)[29] | 1 azoospermia | 0.8 (0.2–1) | - | - | 5 (100) semen volume, 3 (60) sperm concentration | 2.8 (1.2–7 | 17.6 (0–40) | - |
| Worischeck and Parra (1993)[30] | 3 azoospermia | 0.62 (0.4–0.9) | 0.26 (0–0.8) | - | 5 (100) semen volume, 3 (60) sperm concentration | 3.3 (1.6–4.4) | 16.4 (0–38) | - |
| Meacham et al. (1993)[31] | 13 azoospermia, 11 oligozoospermia | Not reported | 17a | 20a | 20 (83) semen volume, 12 (50) sperm motility | 126a | 60a | - |
| Kuligowska et al. (1992)[32] | 4 azoospermia, 2 oligozoospermia | <1 | - | - | 2 (50) semen volume | - | - | - |
| Pryor and Hendry (1991)[4] | 67 azoospermia, 17 severe oligozoospermia, 1 oligozoospermia, 2 normal sperm concentration | 0 in 67, <1 in 17, 1–10 in 1, >10 in 2 | - | - | 4 (31) increase sperm concentration | - | - | - |
| Goldwasser et al. (1985)[33] | 4 oligozoospermia, 1 azoospermia | <1.5 | <5 | - | 3 (60) semen volume, 4 (80) sperm concentration | - | - | - |
| Carson (1984)[8] | 3 azoospermia, 1 oligozoospermia | 0.75 (0.5–1) | 0.5 (0–2) | 10c | 4 (100) semen volume, 3 (75) sperm concentration plus motility | 2.9 (1.5–4.5) | 12 (0–22) | 34 (22–55) |
| Silber (1980)[34] | 5 azoospermia | 0.7 (0.5–1)c | 0c | N/A | 2 (50) semen volume | 1.75 (0.5–3.75) - | - | - |
Only in patients who showed postoperative improvement;
Only in patients who had TURED;
Not reported in all patients;
Different unit—×106/ejaculate.
N/A: not applicable
In the studies that documented individual semen parameter outcomes, it was possible to identify the percentage of patients who met the WHO normal semen criteria (Table 2).[36] In 14 studies[5,8,17–19,21,24,26–30,33,34], a median of 83.1% (IQR: 38.3) of patients had a semen volume of greater than 1.5 mL. A median of 40.9% (IQR: 12.3) of patients had a normal sperm concentration of more than 15×106/mL (n=14 studies)[5,8,17,18,21,23,24,26–30,33,34], and only a median of 29.2% (IQR: 18.5) of patients achieved the normal sperm motility of at least 40% (n=7 studies).[8,17,18,23,24,26,28]
Table 2.
Number of patients meeting normal World Health Organization* 2010 semen criteria[36] after TURED
| Study (author, year) | Sperm concentration: >15×106/mL, n (%) | Semen volume: >1.5 mL, n (%) | Motility: >40%, n (%) |
|---|---|---|---|
| Tu et al. (2013)[17] | 16 (38.1)a | 38 (90.5) | 16 (38.1)b |
| Yurdakul et al. (2008)[5] | 5 (41.7) | 12 (100) | - |
| Pace et al. (2008)[19] | - | 7 (100) | - |
| Eisenberg et al. (2008)[18] | 3 (50) | 4 (66.7) | 2 (33.3) |
| Purohit et al. (2004)[21] | 7 (87.5) | 8 (100) | 6 (75) |
| Schroeder-Printzen et al. (2000)[23] | 1 (6.3) | - | - |
| Netto, et al. (1998)[24] | 8 (57.1) | 11 (78.6) | 8 (57.1) |
| Jarow (1994)[27] | 0 | 1 (50.0) | - |
| Vazquez-Levin et al. (1994)[26] | 3 (37.5) | 7 (87.5) | 3 (37.5) |
| Worischeck and Parra (1993)[30] | 2 (40.0) | 5 (100) | - |
| Weintraub et al. (1993)[29] | 3 (60.0) | 3 (60.0) | - |
| Hall and Oates (1993)[28] | 2 (40.0) | 2 (40.0) | 1 (20.0) |
| Goldwasser et al. (1985)[33] | 2 (40.0) | 3 (60.0) | - |
| Carson (1984)[8] | 2 (50.0) | 4 (100) | 1 (25.0) |
| Silber (1980)[34] | 0 | 2 (66.7) | - |
Used 20×106/mL;
Used 35%.
Five studies[9,25,30,37,38] noted that some patients went from having low-volume azoospermia to normal-volume azoospermia post-TURED (median: 27.3% of patients with azoospermia, IQR: 35.5). A potential cause mentioned was a secondary epididymal obstruction and a recommendation was made to assess and treat this. In five other studies[4,22,29,34,39], epididymal obstruction was confirmed in a median of three patients (IQR: 1) and treated by an epididymovasostomy in a median of one patient (IQR: 0) showing improvement in sperm concentration.
Three studies performed statistical analyses comparing preoperative to postoperative semen parameters. El-Assmy et al.[9] (n=23 patients) found a significant improvement in all semen parameters, with semen volume increasing from an average of 0.54 mL to 2.10 mL (p=0.007), sperm concentration rising from 1 to 7×106/mL (p=0.03), and sperm motility increasing from 3.5% to 23% (p<0.001). Improvement in this study was defined as a 50% increase in any semen variable. Kadioglu et al.[37] (n=38) reported similar results, with semen volume rising from 1 to 2.2 mL, sperm concentration from 4 to 17×106/mL, and motility from 9.7% to 32% (p=0.000 in all). Improvement here was defined as at least a 50% increase in sperm concentration or motility. Ozgok et al.[22] (n=24) reported results only for sperm concentration but also showed a statistically significant rise from 1.66 to 25.4×106/mL (p=0.001) postoperatively, and no criteria were used to define improvement.
Pregnancy outcomes
Natural pregnancies
A total of 23 studies[4,5,8–10,17,19–27,29,31,33,35,37,39–41] assessed the number of natural pregnancies postoperatively, which occurred in 0%–67% of patients with a median pregnancy rate of 25% (IQR: 15.7) (Table 3). Subsequent live birth rates were not reported in any of these studies.
Table 3.
Pregnancy outcomes following TUREDa
| Study (authors, year) | Natural pregnancy, n (%) | Qualified for IUIa, TMCa >5×106, n (%) | Referred for IUI from IVFa (%) | IVF/ICSIa with ejaculated sperm (%) | Time of pregnancy (mean months following TURED±range) |
|---|---|---|---|---|---|
| Tu et al. (2013)[17] | 13(31.0) | - | - | - | 18 |
| Tu et al. (2012)[40] | 16 (26.7) | 6–78a | |||
| El-Assmy et al. (2012)[9] | 3 (13.0) | - | - | - | - |
| Eisenberg et al. (2008)[18] | - | 4(80.0) | - | - | - |
| Yurdakul et al. (2008)[5] | 3 (25.0) | - | 3 (25.0) 1 pregnancy | 3 (25.0) 1 pregnancy | - |
| Pace et al. (2008)[19] | 1 (14.0) | - | - | - | <6a |
| Johnson et al. (2005)[41] | 4 (66.7)b | - | - | - | - |
| Apaydin et al. (2004)[20] | 2 (20.0) | - | 3 (30.0) | - | 15 (13–20)a |
| Purohit et al. (2004)[21] | 2/8 (25.0) | 7 (87.5) | - | - | - |
| Kadioglu et al. (2001)[37] | 5 (13.2) | - | 15 (39.5) | 8 (21.1) | 10.5±3.5 |
| Ozgok et al (2001)[22] | 6 (25.0) | - | - | - | 9 (6–18) |
| Schroeder-Printzen et al. (2000)[23] | 2 (13.3) | - | - | - | <12a |
| Paick et al. (2000)[39] | 8 (30.8) | - | - | - | - |
| Aggour et al. (1998)[35] | 2 (28.6)b | - | - | - | <24a |
| Popken et al. (1998)[10] | 0 | - | - | - | - |
| Netto et al. (1998)[24] | 4 (28.6) | 9 (64.3) | 1 pregnancy | - | 5.8 (4–8) |
| Turek et al. (1996)[25] | 9 (19.6) | - | - | - | 6.1 |
| Vazquez-Levin et al. (1994)[26] | 1 (12.5) | 7 (87.5) | - | - | - |
| Jarow (1994)[27] | 1 (9.0) | - | - | - | - |
| Hall and Oates (1993)[28] | - | 1 (20.0) | - | - | - |
| Weintraub et al. (1993)[29] | 2 (40.0) | - | - | - | <12a |
| Worischeck and Parra (1993)[30] | - | - | - | - | - |
| Meacham et al. (1993)[31] | 7 (29.2) | - | - | - | - |
| Pryor and Hendry (1991)[4] | 1 (7.7) | - | 0 | - | - |
| Goldwasser et al. (1985)[33] | 2 (40.0) | - | 0 | - | 5 (2–8) |
| Carson (1984)[8] | 1 (25.0) | 3 (75.0) | - | - | 8 |
| Silber (1980)[34] | - | 0 | - | - | - |
Within the study follow-up period;
Only reported in patients available for long-term follow-up.
IUI: intrauterine insemination; ICSI: intracytoplasmic sperm injection; IVF: in vitro fertilization; TMC: total motile sperm count; TURED: transurethral resection of the ejaculatory ducts
Conversion from IVF to IUI
IUI is often offered to patients with a total motile sperm count (TMC) greater than 5×106.[42] The possibility of converting from IVF to IUI was assessed in six studies.[4,5,20,24,33,37] Of these, patients in only four studies[5,20,24,37] showed a sufficient increase in TMC to be converted to IUI, with 25%–40% of patients transferred (Table 3). Pregnancy rates were reported in two of these studies, ranging from 7% to 8% of patients.[5,24] Although the other studies did not assess this, with the cutoff for TMC alone, it was possible to identify the proportion of patients who would qualify for IUI in seven studies[8,18,21,24,26,28,34] where individual semen parameters were available. Across these studies, a median of 75.0% (IQR: 30.5) patients had a TMC of at least 5×106 postoperatively and could be considered for IUI. In two of these studies[5,37], where criteria for IUI were not met, IVF/intracytoplasmic sperm injection was possible with ejaculated sperm, and of the three patients referred by Yurdakul et al.[5], one patient became pregnant (Table 3).
Predictors of success
A total of 10 studies[4,9,10,23,24,27,31,33,37,39] reported the primary outcomes categorized into etiology and type of obstruction.
El-Assmy et al.[9] identified differences in outcomes between patients with partial obstruction (n=6), defined as oligozoospermia or asthenozoospermia with low-volume ejaculate, and complete (n=17) obstruction. In patients with complete obstruction, a significant improvement was only found in semen volume (p=0.023), whereas for the partial obstruction, there was a significant improvement across all variables (semen volume, p<0.001; sperm concentration, p=0.03; and sperm motility, p<0.001).
This study also compared outcomes based on etiology. In patients with a midline cyst (n=7), six (86%) showed improvement in semen volume and five (71%) in sperm concentration and motility. Conversely, in patients with noncystic causes (n=16), only six (46%) showed improvement in semen volume and five (38%) in both sperm concentration and motility.
Similar results were reported by Kadioglu et al.[37] Semen variables improved by only 59% in the complete obstruction group versus 94% in the partial group, defined as oligozoospermia or asthenozoospermia or both, with a statistically significant difference (p=0.04). Dividing the group into patients with cystic (n=27) and noncystic (n=11) etiologies, improvement in semen variables was found in 23 (85%) patients with cysts but in only five (45%) patients with calcification (noncystic).
Netto et al.[24] also found better outcomes in patients with congenital EDO. All patients with congenital causes (n=6) showed significant improvements in ejaculate volume and sperm motility (p<0.03), and five (83%) patients showed improvements in sperm concentration (p=0.01). Compared with the congenital group, there were significant differences in the acquired group (p<0.03), with only three of eight patients showing significant improvements in semen variables. The causes of acquired EDO included inflammation, which caused calcification of the ducts as well as trauma to the ejaculatory ducts following instrumentation and prolonged catheterization.
Pregnancy outcomes also varied depending on the etiology and type of EDO. Two of the three pregnancies reported by El-Assmy et al.[9] were in patients with a partial obstruction. Similarly, Kadioglu et al.[37] found that three of five pregnancies occurred in patients with partial obstruction. Regarding etiology, higher pregnancy rates were found in patients with congenital causes of obstruction by Netto et al.[24] (four of five pregnancies), Schroeder-Printzen et al.[23] (two of two pregnancies), and Paick et al.[39] (seven of eight pregnancies).
Surgical technique
TURED was performed to achieve a symptomatic improvement or improve fertility outcomes when EDO was confirmed. The standard technique reported involved using a 24F resectoscope and with a pure cutting current to resect the proximal verumontanum (Appendix 2). Differences in surgical technique were assessed to determine whether these differences had an impact on outcomes. Of note, eight studies[5,8,20,22,23,29,32,35] confirmed the obstruction intraoperatively by direct visualization before the resection was made, and postresection, 21 studies[4,5,8–10,17,19,20,22,29,33,35,37,39,41] confirmed patency of the ejaculatory ducts. Techniques to confirm patency included efflux of methylene blue dye, visualizing the ejaculatory duct openings with efflux of fluid, and efflux of fluid following a prostatic massage. No differences in outcomes were found based on these techniques.
Postoperative complications
The postoperative complication rate for TURED was reported in 18 studies (Table 4)[6,9,10,16,17,19–22,24–27,29,31,35,37,41], with a range of 0%–36% and a median of 10%. The total number of complications was 50, with the most common being epididymitis (26% complications), postoperative hematuria (22%), and watery ejaculate (20%) (Table 5). Two studies[9,21] reported epididymitis resolving with antibiotics, whereas in two other studies, the patients had chronic epididymitis requiring prolonged antibiotics and anti-inflammatory treatment.[25,37]
Table 4.
Secondary outcomes
| Study (author, year) | Symptomatic, n (%) | Symptom improvement (%) | Postop complications, n (%) |
|---|---|---|---|
| Sangster et al. (2017)[16] | 9 (31) | 68 | 7 (18) |
| Tu et al. (2013)[17] | 12 (29) | 100a | 2 (4.8) |
| Tu et al. (2012)[40] | - | - | - |
| El-Assmy et al. (2012)[9] | - | - | 6 (26) |
| Eisenberg et al. (2008)[18] | 2 | (22) 100 | - |
| Yurdakul et al. (2008)[5] | 7 (58) | 100 | - |
| Pace et al. 2008[19] | - | 0 (0) | |
| Johnson et al. (2005)[41] | Yes, no overall number | 100 for all, except pain with ejaculation in 50 | 0 (0) |
| Apaydin et al. (2004)[20] | - | - | 3 (30) |
| Purohit et al. (2004)[21] | 7 (28) | 100 | 3(25)b |
| Kadioglu et al. (2001)[37] | 17 (45) | 100 | 5 (13) |
| Ozgok et al. (2001)[22] | - | - | 1 (4) |
| Paick et al. (2000)[39] | None | - | 3 (12) |
| Aggour et al. (1998)[35] | - | - | 3 (30) |
| Popken et al. (1998)[10] | Yes but not reported | 100 | 0 (0) |
| Netto et al. (1998)[24] | 8 (57) | - | 5 (36) |
| Turek et al. (1996)[25] | - | - | 10 (22) |
| Vazquez-Levin et al. (1994)[26] | - | - | 0 (0) |
| Jarow (1994)[27] | - | - | 0 (0) |
| Weintraub et al. (1993)[29] | 2 (40) | 100 | 0 (0) |
| Meacham et al. (1993)[31] | - | - | 2 (8) |
| Pryor and Hendry (1991)[4] | - | - | - |
| Goldwasser et al. (1985)[33] | - | - | - |
Only improvement in haematospermia assessed;
Assessed in only 12 of the patients who had TURED
Table 5.
Number of times each complication occurred across the studies
| Complication | Number of patients, n | Percentage of total complications |
|---|---|---|
| Epididymitis | 13 | 26 |
| Hematuria | 11 | 22 |
| Watery ejaculate | 10 | 20 |
| Urinary tract infection | 4 | 8 |
| Urinary retention | 4 | 8 |
| Azoospermia | 3 | 6 |
| Retrograde ejaculation | 2 | 4 |
| Postvoid dribbling | 1 | 2 |
| Premature ejaculation | 1 | 2 |
| Pelvic pain | 1 | 2 |
| Urethral stricture | 0 | 0 |
| Total | 50 |
Symptom improvement
Symptoms were evaluated in 11 studies[5,10,16–18,21,24,29,37,39,41], and relief postoperatively was assessed in nine studies (Table 4).[5,10,16–18,21,29,37,41] The common symptoms were hematospermia and perineal or testicular pain, but others included dysuria, peri-ejaculatory pain, and a reduced orgasmic sensation. Relief of symptoms was reported in 50%–100% of patients, with 100% improvement observed in seven studies[5,10,17,18,21,29,37], particularly for hematospermia.
Quality assessment
The risk of bias assessment demonstrated a low quality of the included studies (Appendix 3), with several weaknesses across all studies. The predominant categories of concern were a lack of prospective calculation of study size and an unbiased endpoint assessment, which was postoperative semen variables and pregnancy rates. The overall scores ranged from 2 to 10 for the 27 noncomparative studies, and 15 to 17 for the two comparative studies, with no studies fulfilling all criteria. Only 13 of 29 (45%) studies had a clear description of their endpoint used to assess the success of TURED. GRADE assessment of the individual outcomes found serious levels of risk of bias and publication bias across most outcomes. Some outcomes were also imprecise and inconsistent, but all outcomes were directly measured. Nevertheless, the resultant quality of evidence for all outcomes was very low, with a weak recommendation supporting its use (Appendix 4).
Discussion
Summary of evidence
This systematic review demonstrated that TURED improves semen variables postoperatively. Improvement was most frequently seen in semen volume, with better outcomes in patients with partial EDO or congenital etiologies. Along with the improvements in semen variables, a median rate of 25% was reported for natural pregnancy postoperatively. In symptomatic patients, the treatment of symptoms by TURED was highly successful with a median of 100% of patients reporting relief. TURED is generally safe, with only a 10% median complication rate reported, all of which were non-life-threatening. Only a small number of papers looked at converting cases from IVF to IUI post-TURED with poor pregnancy outcomes reported, suggesting it is not commonly performed.
All studies demonstrated improvement in semen variables, but there was a marked variation in outcomes. One explanation is the nonstandardized definition of improvement among the studies. Some had a predefined percentage increase in semen variables[9,25,37], whereas others considered any increase as an improvement. The differences can also be attributed to the small sample sizes. This heterogeneity in outcomes limits the benefit of comparing the studies to determine the success of TURED.
Despite more than half of the patients showing improvement in semen outcomes, there was still a significant proportion who did not benefit from TURED. Several explanations can account for this; first, many studies used only TRUS as a diagnostic test. This has low specificity, and EDO may be misdiagnosed as a result.[18,21] Another reason is the possibility of having a functional obstruction, in which case TURED would not provide a benefit.[18,25] All studies showed a more significant improvement in semen volume compared with other variables. This could be secondary to a concomitant obstruction of the epididymis or vas deferens, and thus, an epididymovasostomy would additionally be needed to improve the other semen variables.[25,30,39] An epididymal obstruction can also be a consequence of long-standing EDO, which causes high intratubular pressures and subsequently, an epididymal blowout.[25,34] As a result, patients may have two obstructions that require treatment, which limits the success of TURED.[8] Finally, the success of TURED depends on the surgical technique and surgeon expertise. It is essential that a wide opening in the verumontanum is created to prevent restenosis[23], but also that patency is checked intraoperatively once the resection is performed. The latter can be checked by several methods, including observing semen or injecting methylene blue into the seminal vesicles and observing efflux from the ejaculatory ducts.[35] The use of diathermy coagulation should be limited, as this is associated with iatrogenic obstruction.[31] Using the resectoscope can be technically demanding, and experience is needed to do this successfully and safely, and therefore, imaging techniques such as TRUS should be used to guide the resection more accurately and safely.[20,34]
Regarding natural pregnancies postoperatively, the median rate of 25% was a modest improvement, given that most patients presented with infertility. Many of the patients were patients with azoospermia and may not have otherwise had a chance of conceiving naturally. When comparing pregnancy outcomes following TURED with the treatment of similar conditions causing subfertility, such as varicoceles, the results are very comparable, with a pregnancy rate of 235 per 1,000 patients undergoing varicocelectomy cited in a Cochrane review.[43] An important factor to consider when evaluating the pregnancy rate is the follow-up time. The included studies had a median of 12-months follow-up, with several only following up their patients for 6 months or less, which may have limited the pregnancy outcomes reported.
Finally, two main factors have been identified as predictors of TURED success: type of EDO and the etiology. Several studies showed improved outcomes preferentially in patients with partial obstruction[9,37,39], which could be due to having an earlier stage of, and therefore less severe, EDO. Varying outcomes were also found based on the etiology of EDO, with the congenital causes, especially cystic, responding better to TURED versus the acquired causes.[9,23,24,37] One explanation is that obstruction is longer and more proximal in the ejaculatory ducts or even extracapsular in patients with the acquired causes, making the operation more difficult or there is an inability to reach the level of stenosis.[10] However, one study paradoxically showed significant improvements in patients with stenosis due to infection causing EDO[29]; therefore, the cause of these differences is still a subject of discussion. Interestingly, although the surgical technique is imperative to achieve a successful procedure, differences in technique did not seem to affect outcomes. As previously mentioned, some patients develop an epididymal obstruction secondary to EDO. A combined treatment for these has been shown to be less successful at restoring fertility[8,34], so this should also be taken into account when determining who is best suited for TURED.
The findings in this systematic review are supported by another recent review looking at surgical treatment options for EDO. The article also highlighted the importance of discussing alternative fertility options with patients, such as artificial reproductive techniques (ARTs). This is particularly important in cases where TURED is less likely to be successful, such as with a secondary epididymal obstruction.[44] Despite the similarities to this review, by utilizing a systematic methodology, and stricter inclusion criteria whereby individual case reports were excluded, this review offers a more comprehensive and less heterogeneous overview of the current evidence for TURED outcomes.
Limitations
This systematic review has several limitations. Only English studies were included, and therefore, pertinent articles addressing this topic could have been missed. The studies considered have a poor design, with all of them being nonrandomized, 27 of 29 studies having no control arm, and the majority of studies being retrospective. This significantly limits the quality of evidence, with most of the studies falling into level four in the Oxford level of Evidence tool for interventional studies.[45] With no control, the effect of TURED in improving semen variables is difficult to ascertain because improvements could be part of the natural history of the disease. All studies had a high risk of bias with particularly significant weaknesses in sample size calculations and having an unbiased endpoint assessment, where no studies fulfilled these criteria. Sample sizes were small in several studies; hence, the effect size of outcomes across different variables could be inaccurate. The small sample sizes also meant heterogeneity between studies was high, precluding the possibility of conducting a meta-analysis. Finally, the very low certainty measured by the GRADE for all outcomes means that there is high uncertainty of the effect of TURED, limiting its ability to be recommended as a successful treatment.
Despite the limitations, there is a consensus among the studies that TURED is a successful treatment option for EDO to improve semen parameters. This systematic review will provide useful insight into the benefits of TURED in patients with EDO, and the data can be used to guide clinicians when recommending treatment. To further validate these outcomes, more robust study designs such as prospective studies comparing semen variables pre and post-TURED with healthy fertile controls over the same period are needed. Sufficient follow-up periods of at least 12 months should also be present so that pregnancy rates can be more accurately determined. This will reduce the bias in results and increase the level of evidence, hence enabling the results to be robust for everyday use in clinical practice.
In conclusion, the EDO treatment by TURED improves semen parameters and can result in symptomatic relief. It is an important diagnosis to consider because it is amenable to surgical management, with the potential of restoring fertility and allowing natural pregnancies in a quarter of patients who may not otherwise have been able to conceive naturally. By improving semen parameters, TURED also enables the utilization of less invasive ARTs, where natural pregnancy is not possible. A combination of making an accurate diagnosis and optimizing surgical technique is needed to achieve positive outcomes. However, these results should be interpreted with caution owing to the limited quality and retrospective nature of the included studies, as well as the low certainty of the effect of the outcomes, which all limit the evidence.
Main Points:
Transurethral resection of the ejaculatory ducts (TURED) has been shown to improve semen parameters, with the greatest improvement observed in semen volume, where a median of 83% of patients showed improvement postoperatively.
A median of one in four patients achieved natural pregnancy post-TURED.
Partial ejaculatory duct obstruction (EDO) and congenital etiologies of EDO (cysts, atresia) were associated with greater improvements in semen parameters and higher natural pregnancy rates.
The median complication rate post-TURED was 10%, with the most common complication being epididymitis followed by hematuria.
TURED is successful at improving semen parameters and results in modest improvements in natural pregnancy rates. The improvements in semen parameters could also allow for the possibility of utilizing less invasive artificial reproductive techniques for cases where natural pregnancies are not successful.
Appendix 1
Search strategy- Databases searched 20–25th December 2018 and reviewed again in January 2019
A different combination of the following terms was used in PubMed, EMBASE, Scopus:
“diagnosis or diagnostics or “diagnostic imaging” or radiology or examination or imaging or investigations”
“treatment outcome” or outcome or success or “success predictors” or “predictors of success”
“ejaculatory duct*” or “seminal vesicle” or “Mullerian duct cyst” or “ejaculatory duct obstruction” or “seminal vesicle cyst”
“surgical procedure” or surgery or operation or “transurethral resection” or “transurethral treatment” or transurethral
(infertility or infertile or subfertility or subfertile or oligospermia or azoospermia or “obstructive azoospermia” or “genital disease”
“ejaculatory duct*” or “seminal vesicle” or “Mullerian duct cyst” or “vas deferens” or “ductus deferens” or “vasa deferentia” or “ejaculatory duct obstruction”
“surgical procedure” or surgery or operation or “transurethral resection” or “transurethral treatment” or transurethral or treatment or “disease management”
Combinations included:
1 and 2 and 3 and 4 and 5
1 and 2 and 3 and 5
1 and 2 and 3 and 6
1 and 2 and 5 and 6 and 7
1 and 3 and 4 and 5
1 and 2 and 3 and 4
1 and 3 and 5
3 and 4 and 5
Also, the following combined searches were done:
- ((transurethral OR surgical OR operation or surgery) and (ejaculatory duct OR ejaculatory ducts or vasa deferentia or ductus deferens OR vas deferens OR seminal vesicle) and (infertility OR infertile OR sterile OR subfertility))
- (transurethral OR surgical OR operation) and (resection OR removal) of and (ejaculatory duct OR ductus deferens OR vas deferens OR seminal vesicle) for and (infertility OR infertile OR sterile OR subfertility
Appendix 2: General study characteristics
| Age (years) | Follow Up (months) | |||||||
|---|---|---|---|---|---|---|---|---|
| Study Author, Year | Study type | EDO patients (n) | Mean +/−SD (range) | TURED (n) | Resection technique | Success of Procedure | Mean +/− SD (Range) | Diagnostic investigation |
| Sangster, Kalejaiye et al. 2017 16 | Retrospective | 54 (29 infertile) | 38 (24–64) | 54 | - | - | - | TRUS |
| Tu, Zhuang et al. 2013 17 | Prospective | 42 | 34 (27–56) | 42 | Spinal anesthesia, lithotomy Bipolar plasma kinetic resectoscope, plasma loop electrode- pure cutting current, no coagulation Resect proximal verumontanum in midline followed by prostatic massage Digital compression of SV followed |
Prostatic massage demonstrating flow of fluid through the ED | 2–24 | TRUS +/− MRI/Vasography |
| Tu, Zhao et al. 2012 40 | Retrospective | 60 | - | 60 | - | - | 6–78 | TRUS +/− Vasography |
| El-Assmy, El-Tholoth et al. 2012 9 | Retrospective | 23 | 23–47 | 23 | Spinal anesthesia, lithotomy 24F resectoscope, electrocauterization used gently Resect proximal verumontanum If had midline cyst: Deepen resection until cyst unroofed |
Visualizing the openings of the ED or efflux of copious cloudy material Retrograde vasography in 3 cases to document vas patency |
34 +/− 16 9–60 |
TRUS +/− MRI |
| Eisenberg, Walsh et al. 200818 | Prospective | 9 | 35 +/− 8.6 | 6 | - | - | 2–6 | TRUS + Seminal vesicle (SV) chromotubation +/− SV aspiration |
| Yurdakul, Gokce et al. 2008 5 | Retrospective | 12 | 32 (24–40) | 12 | Surgically confirmed obstruction by visualizing obstructed ostia of ED cystoscopically first Avoided electrocauterization Resect verumontanum |
Efflux of methylene blue from the ED ostia | 12 (4–36) | TRUS |
| Pace, Galatioto et al. 2008 19 | Retrospective | 7 | 32 | 7 | Resectoscope using a cautery loop: pure cutting current, no coagulation Resect proximal verumontanum and dorsal part of prostate gland |
Efflux of methylene blue | 6 | TRUS + SV aspiration |
| Johnson, Bingham et al. 2005 41 | Retrospective | 15 | - | 15 | Resectoscope loop Resection of proximal verumontanum in midline followed by prostatic massage |
Improved flow through the ED following prostatic massage | 2 | TRUS |
| Apaydin, Killi et al. 200420 | - | 10 | 32 (25–47) | 10 | Spinal anesthesia, lithotomy Confirm obstruction first by TRUS guided contrast fluid observation: no efflux 24F Resectoscope loop Resect strip of tissue on floor of prostate proximally including part of the verumontanum |
Visualizing contrast agent on TRUS and methylene blue efflux from ED endoscopically | 15 (13–20) | TRUS and TRUS guided seminal vesiculography |
| Purohit, Wu et al. 2004 21 | Prospective | 25 (18 infertile) | 36 (16–52) | 12 (8 infertile) | - | - | - | TRUS, Seminal vesiculography, seminal vesicle chromotubation |
| Kadioglu, Cayan et al. 2001 37 | Retrospective | 38 | 35 +/− 4/26–51) | 38 | 24F Resectoscope loop: electrocauterisation only in cases of excessive bleeding Resection of proximal verumontanum |
Fluid efflux from the ED and sperm detection in bladder irrigation fluid | 26 +/− 8.5 (12–63) | TRUS +/− MRI |
| Ozgok, Tan et al. 2001 22 | - | 24 | 29 (20–40) | 24 | Lithotomy Initially: small scrotal incision and do hemivasotomy: cannulate and instill dye, cystoscopy to confirm obstruction by lack of efflux Limited electrocoagulation Resection of the verumontanum |
Efflux of methyleneblue Then vasotomy closed under magnification |
9 (6–18) | TRUS + open vasotomy vasography |
| Schroeder-Printzen, Ludwig et al. 2000 23 | - | 16 | 35 (27–42) | 15 | General anesthesia Initially supine: Microsurgical vasotomy performed, obstruction of ED identified using a catheter inserted into abdominal end of vas and vasography performed Then positioned in dorsal lithotomy Lateral area of the lesion resected As far as the prostate wall with the Turner-Warwick hook; resectoscope used to extend opening Extended cysts: Complete roof resected to the level of the verumontanum |
Methylene blue dye efflux from ED openings and vasography demonstrating distal filling of urethra Then vas incision closed microsurgically |
12 | TRUS |
| Paick, Kim et al. 2000 39 | - | 50 | 35 (27–43) | 26 | Resection between bladder neck and verumontanum, digitally compress SV to aid dye efflux +/− unroofing cysts | Methylene blue efflux from ED openings | >12 | TRUS+ vasography (open vasotomy or percutaneous cannulation)/transperineal seminal vesiculography |
| Aggour, Mostafa et al. 1998 35 | - | 11 | - | 11 | General or spinal anesthesia, dorsal lithotomy Vas exposed through small scrotal incision and cannulated 16CH urethrocystoscope with 30-degree lens to visualized ED first Resectoscope: Resect proximal and lateral to the verumontanum, slightly posterior and directed towards the bladder neck |
Efflux of semen from the ED opening observed, if not seen methylene blue efflux assessed | 24 * | TRUS |
| Popken, Wetterauer et al. 1998 10 | - | 8 | - | 8 | Pure cutting current, gentle coagulation if bleeding occurred Resection of ED in the dorsal part of the prostate and proximal part of the verumontanum in the midline Extended towards bladder neck and posteriorly until ED opening seen |
ED openings seen and semen efflux from ED openings observed | 12 | TRUS |
| Netto, Esteves et al. 1998 24 | - | 14 | 30 (22–35) | 14 | Cutting current and coagulation for excessive bleeding only Resect strip of tissue on floor of prostate proximal to and including part of the verumontanum, digitally compress the SV to aid dye efflux |
Visualizing the openings of the ED and methylene blue dye efflux seen (instilled via initial intraoperative vasotomy) | 24 (8–60) | TRUS + open vasotomy vasography |
| Turek, Magana et al. 1996 25 | Retrospective | 46 | 35 (25–45) | 46 | Transurethral Endoscope and electrocautery loop Resection of verumontanum in the midline |
Fluid efflux from the ED seen | 45 (5–84)- pregnancy 8 (2–48) for complications | TRUS |
| Vazquez-Levin, Dressler et al. 1994 26 | Retrospective | 8 | 36 (28–57) | 8 | Resection of proximal verumontanum towards the bladder neck and posteriorly until the EDs are seen, compression of SV digitally to aid efflux | Visualizing ED opening and efflux of seminal fluid seen | TRUS | |
| Jarow 1994 27 | Prospective | 11 | 37 (30–50) | 2 | Resectoscope using cautery loop: pure cutting current Proximal half of the verumontanum resected, digital compression of the SV |
Efflux of methylene blue through the ED (instilled at start of operation via cannulation of SV through peroneal approach under TRUS guidance) | - | TRUS + open vasotomy Vasography + Seminal Vesicle Aspiration |
| Hall, Oates 1993 28 | Prospective | 5 | - | 5 | Resection of the prostatic floor lateral to the verumontanum | Semen and indigo carmine dye efflux visualized from ED openings | 3 | TRUS +/− vasography |
| Weintraub, De Mouy et al. 1993 29 | - | 5 | 37 (27–39 | 5 | Prior obstruction visually confirmed by lack of dye efflux cystoscopically during vasography Resectoscope loop: cutting current, minimal electrocautery Resection of lateral part of verumontanum and lateral bladder neck +/− unroofing midline cysts |
Efflux of methylene blue dye from ED openings | 12 | TRUS +/− MRI/Open vasotomy Vasography |
| Worischeck, Parra 1993 30 | - | 5 | 29 (25–33) | 5 | Pure cutting current Resect proximal and lateral to the verumontanum If cystic: Limited resection of the verumontanum and posterior prostate until cyst entered and decompressed |
Engorgement of ED lumen seen | - | TRUS +/− open vasotomy vasography |
| Meacham, Hellerstein et al. 1993 31 | Retrospective | 24 | 33 | 24 | Resectoscope loop: Sparing use of electrocoagulation Deep resection of the verumontanum +/− unroofing of cyst |
- | - | TRUS in 22 |
| Kuligowska, Baker et al. 1992 32 | prospective | 11 | - | 9 | - | - | - | TRUS+/− open vasotomy vasography |
| Pryor, Hendry 1991 4 | Retrospective | 87 | - | 13 | General anesthesia, Lithotomy Resection of the verumontanum followed by compression of SV digitally to aid efflux |
Efflux of methylene blue | - | Open vasotomy/direct puncture Vasography |
| Goldwasser Weinerth et al. 1985 33 | Retrospective | 5 | 33 (24–40) | 5 | Resection of small area of the prostate lateral to the verumontanum | Contrast material/indigo carmine seen owing through ED opening and confirmed radiographically by repeat vasogram | - | Vasography |
| Carson 1984 8 | Retrospective | 4 | 35 (28–40) | 4 | Inject indigo carmine to visualize obstruction cystoscopically first Resectoscope Resection of small area of the prostate lateral to the verumontanum |
Dye flowing through resected ED and then confirmed by radiogram | 6–12months | Fine needle cannulation of vas: Vasography |
| Silber 1980 34 | Retrospective | 5 | 32 (28–41) | 4 | Resectoscope Resect floor of prostate, proximal to the external sphincter and distal to the bladder neck left of the verumontanum +/− cyst unroofing |
6 *(0–12) | Vasography | |
ED= Ejaculatory duct, SV= seminal vesicles
Appendix 3: Risk of Bias across different domains for all studies using the MINORS tool 14
| Study Author, Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sangster, Kalejaiye et al. 201716 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | n/a | n/a | n/a | n/a | 3 |
| Tu, Zhuang et al. 2013 17 | 1 | 2 | 1 | 0 | 0 | 2 | 2 | 0 | n/a | n/a | n/a | n/a | 8 |
| Tu, Zhao et al. 2012 40 | 1 | 1 | 0 | 1 | 0 | 2 | 1 | 0 | n/a | n/a | n/a | n/a | 6 |
| El-Assmy, El-Tholoth et al. 2012 9 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | n/a | n/a | n/a | n/a | 10 |
| Eisenberg, Walsh et al. 200818 | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 17 |
| Yurdakul, Gokce et al. 2008 5 | 1 | 2 | 0 | 0 | 0 | 2 | 2 | 0 | n/a | n/a | n/a | n/a | 7 |
| Pace, Galatioto et al. 2008 19 | 1 | 2 | 0 | 0 | 0 | 2 | 2 | 0 | n/a | n/a | n/a | n/a | 7 |
| Johnson, Bingham et al. 2005 41 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 0 | n/a | n/a | n/a | n/a | 8 |
| Apaydin, Killi et al. 200420 | 2 | 2 | 0 | 1 | 0 | 2 | 2 | 0 | n/a | n/a | n/a | n/a | 9 |
| Purohit, Wu et al. 2004 21 | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 0 | n/a | n/a | n/a | n/a | 10 |
| Kadioglu, Cayan et al. 2001 37 | 2 | 2 | 0 | 2 | 0 | 1 | 2 | 0 | n/a | n/a | n/a | n/a | 9 |
| Ozgok, Tan et al. 2001 22 | 1 | 2 | 0 | 1 | 0 | 2 | 2 | 0 | n/a | n/a | n/a | n/a | 8 |
| Schroeder-Printzen, Ludwig et al. 2000 23 | 1 | 2 | 0 | 1 | 0 | 2 | 2 | 0 | n/a | n/a | n/a | n/a | 7 |
| Paick, Kim et al. 2000 39 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 0 | n/a | n/a | n/a | n/a | 8 |
| Aggour, Mostafa et al. 1998 35 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | n/a | n/a | n/a | n/a | 4 |
| Popken, Wetterauer et al. 1998 10 | 1 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | n/a | n/a | n/a | n/a | 5 |
| Netto, Esteves et al. 1998 24 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | n/a | n/a | n/a | n/a | 10 |
| Turek, Magana et al. 1996 25 | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | n/a | n/a | n/a | n/a | 10 |
| Vazquez-Levin, Dressler et al. 1994 26 | 1 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 15 |
| Jarow 1994 27 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | n/a | n/a | n/a | n/a | 4 |
| Hall, Oates 1993 28 | 2 | 1 | 1 | 0 | 0 | 2 | 2 | 0 | n/a | n/a | n/a | n/a | 8 |
| Weintraub, De Mouy et al. 1993 29 | 0 | 1 | 0 | 0 | 0 | 2 | 2 | 0 | n/a | n/a | n/a | n/a | 5 |
| Worischeck, Parra 1993 30 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | n/a | n/a | n/a | n/a | 2 |
| Meacham, Hellerstein et al. 1993 31 | 1 | 2 | 0 | 1 | 0 | 0 | 2 | 0 | n/a | n/a | n/a | n/a | 6 |
| Kuligowska, Baker et al. 1992 32 | 2 | 1 | 2 | 1 | 0 | 1 | 2 | 0 | n/a | n/a | n/a | n/a | 9 |
| Pryor, Hendry 1991 4 | 2 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | n/a | n/a | n/a | n/a | 6 |
| Goldwasser Weinerth et al. 1985 33 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | n/a | n/a | n/a | n/a | 4 |
| Carson 1984 8 | 1 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | n/a | n/a | n/a | n/a | 5 |
| Silber 1980 34 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | n/a | n/a | n/a | n/a | 2 |
Questions 1–12 assess different domains:
1–8 for comparative and non-comparative studies
1- Was there a clear aim?
2- Were consecutive eligible patients included?
3-Was it prospective?
4- Clear and unambiguous endpoint?
5- Unbiased assessment of endpoint?
6- Appropriate follow-up?
7- Loss to follow up <5%?
8- Prospective study size calculation
Questions 9–12: comparative studies only
9- Adequate control
10- Contemporary control
11- Baseline between control and cases similar
12- Adequate statistical analysis
Score: 0- not reported, 1- reported but inadequate, 2- Reported adequately
Appendix 4: Transurethral resection of the ejaculatory ducts for Ejaculatory duct obstruction Generated using the GRADEPro online tool 15
| Certainty assessment | Summary of findings | ||||||
|---|---|---|---|---|---|---|---|
| № of participants (studies) Follow-up |
Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence | Impact |
| Semen parameters improvement postoperatively (assessed with: sperm count/motility/semen volume) | |||||||
| 463 (28 observational studies) | serious a | serious b | not serious c | not serious d | strong association | ⊕○○○ VERY LOW |
Median proportion of patients that showed improvement: - Semen volume: 83 % (46–100%) -Sperm count: 63% (0 100%) - Sperm motility: 62.5 (43–100%) |
| Spontaneous pregnancy postoperatively | |||||||
| 395 (23 observational studies) | very serious e | serious f | not serious c | serious g | none | ⊕○○○ VERY LOW |
Median pregnancy rate postoperatively was 25% (0–67%) |
| Postoperative complications | |||||||
| 369 (18 observational studies) | very serious e | not serious h | not serious c | not serious d | strong association | ⊕○○○ VERY LOW |
Median complication rate postoperatively was 10% (0–36%) |
| Symptom improvement postoperatively | |||||||
| 192 (9 observational studies) | very serious i | not serious h | not serious c | serious j | very strong association | ⊕○○○ VERY LOW |
Median percentage of patients showing symptomatic improvement was 100% (50–100%) IVF to IUI postoperatively |
| 92 (6 observational studies) | very serious k | very serious l | not serious c | very serious m | publication bias strongly uspected n | ⊕○○○ VERY LOW |
Patients were only eligible in 4 of the studies, with a range of 25–40% of patients transferred to IUI with pregnancy from 7–8% in 2 reported studies. |
CI: Confidence interval; MD: Mean difference
Explanations
Patients were not randomized or blinded, and the majority of studies were single arm retrospective studies. Only 6 of the studies reporting outcomes were prospective, including 2 with a control arm.
Different definitions of improvement in semen outcomes were used, so results varied between the studies. However, all studies reporting semen parameters showed improvement in at least one parameter post-operatively.
Results were measured directly for all of the outcomes.
Multiple studies reported similar outcomes, a large number of studies and reasonable study sizes.
Patients were not randomized or blinded, and the majority of studies were single arm retrospective studies. Only 3 of the studies reporting outcomes were prospective.
Studies had different follow-up periods, making a consistency for comparison of pregnancy rates between studies difficult. There was also a wide range of results.
A wide range of results between the studies and relatively small study sizes.
Similar findings were found across all of the studies.
Patients were not randomized or blinded, and the majority of studies were single arm retrospective studies. Only 2 of the studies reporting outcomes were prospective.
Only 9 studies were reporting this outcome, difficult to be sure of the effect. 5/9 of the studies had a study size under 20.
Patients were not randomized or blinded, and all the studies were a single arm, with no prospective studies documenting this outcome.
Definition of changing from IVF to IUI was not consistent between studies- lack of clarity of who was eligible.
Poorly documented outcome and the study sizes of papers reporting this outcome were under 15 for 5/6 of the studies.
Only 6 studies reported this outcome and had small study sizes.
Footnotes
You can reach the questionnaire of this article at https://doi.org/10.5152/tud.2020.20228
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.M., O.B., M.G., H.M.A., K.A.; Design – A.M., M.G., O.B., D.R., A.M., K.A.; Supervision – O.B., H.M.A., D.R., A.M., K.A.; Materials – A.M., M.G.; Data Collection and/or Processing – A.M., M.G.; Analysis and/or Interpretation – A.M., M.G., O.B., H.M.A., D.R., A.M., K.A.; Literature Search – A.M., M.G.; Writing Manuscript – A.M., M.G., O.B., H.M.A., D.R., A.M., K.A.; Critical Review – O.B., H.M.A., D.R., A.M., K.A.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: Oliver Brunckhorst acknowledges research support from the National Institute for Health Research (NIHR) and the MRC Centre for Transplantation at King’s College London. Asif Muneer acknowledges support from the NIHR Biomedical Research Centre at University College London Hospitals NHS Foundation Trust and University College London. Kamran Ahmed acknowledges receiving research support from The Urology Foundation, the Royal College of Surgeons of England, the Pelican Group, and the MRC Centre for Transplantation at King’s College London.
References
- 1.Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, et al. Best practice policies for male infertility. Fertil Steril. 2002;77:873–82. doi: 10.1016/S0015-0282(02)03105-9. [DOI] [PubMed] [Google Scholar]
- 2.Modgil V, Rai S, Ralph DJ, Muneer A. An update on the diagnosis and management of ejaculatory duct obstruction. Nat Rev Urol. 2016;13:13–20. doi: 10.1038/nrurol.2015.276. [DOI] [PubMed] [Google Scholar]
- 3.Goluboff ET, Stifelman MD, Fisch H. Ejaculatory duct obstruction in the infertile male. Urology. 1995;45:925–31. doi: 10.1016/S0090-4295(99)80109-5. [DOI] [PubMed] [Google Scholar]
- 4.Pryor JP, Hendry WF. Ejaculatory duct obstruction in subfertile males: analysis of 87 patients. Fertil Steril. 1991;56:725–30. doi: 10.1016/S0015-0282(16)54606-8. [DOI] [PubMed] [Google Scholar]
- 5.Yurdakul T, Gokce G, Kilic O, Piskin MM. Transurethral resection of ejaculatory ducts in the treatment of complete ejaculatory duct obstruction. Int Urol Nephrol. 2008;40:369–72. doi: 10.1007/s11255-007-9273-z. [DOI] [PubMed] [Google Scholar]
- 6.Paick JS. Transurethral resection of the ejaculatory duct. Int J Urol. 2000;7(Suppl):S42–7. doi: 10.1046/j.1442-2042.2000.00170.x. [DOI] [PubMed] [Google Scholar]
- 7.Belker AM, Steinbock GS. Transrectal prostate ultrasonography as a diagnostic and therapeutic aid for ejaculatory duct obstruction. J Urol. 1990;144:356–8. doi: 10.1016/S0022-5347(17)39455-7. [DOI] [PubMed] [Google Scholar]
- 8.Carson CC. Transurethral resection for ejaculatory duct stenosis and oligospermia. Fertil Steril. 1984;41:482–4. doi: 10.1016/S0015-0282(16)47734-4. [DOI] [PubMed] [Google Scholar]
- 9.El-Assmy A, El-Tholoth H, Abouelkheir RT, Abou-El-Ghar ME. Transurethral resection of ejaculatory duct in infertile men: outcome and predictors of success. Int Urol Nephrol. 2012;44:1623–30. doi: 10.1007/s11255-012-0253-6. [DOI] [PubMed] [Google Scholar]
- 10.Popken G, Wetterauer U, Schultze-Seemann W, Deckart A, Sommerkamp H. Transurethral resection of cystic and non-cystic ejaculatory duct obstructions. Int J Androl. 1998;21:196–200. doi: 10.1046/j.1365-2605.1998.00111.x. [DOI] [PubMed] [Google Scholar]
- 11.Farley S, Barnes R. Stenosis of ejaculatory ducts treated by endoscopic resection. J Urol. 1973;109:664–6. doi: 10.1016/S0022-5347(17)60510-X. [DOI] [PubMed] [Google Scholar]
- 12.Jungwirth A, Diemer T, ZK, Krausz C, Minhas S, Tournaye H. EAU Guidelines on Male Infertility European Association of Urology 2017. 2017. [Accessed 20-February-2019]. Available from: https://uroweb.org/wp-content/uploads/17-Male-Infertility_2017_web.pdf.
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 15.Schünemann HBJ, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. Available from: guidelinedevelopment.org/handbook.
- 16.Sangster P, Kalejaiye A, Chiriaco G, Raheem A, Muneer A, Ralph D. Presentation and treatment outcomes of ejaculatory duct obstruction. Journal of Urology. Conference: 112th Annual Meeting of the American Urological Association, AUA 2017; United States. Elsevier Inc; 2017. p. e1207. Availab from : https://doi.org/10.1016/j.juro.2017.02.2811 https://doi.org/10.1016/j.juro.2017.02.2811. [Google Scholar]
- 17.Tu XA, Zhuang JT, Zhao L, Zhao LY, Zhao JQ, Lu KL, et al. Transurethral bipolar plasma kinetic resection of ejaculatory duct for treatment of ejaculatory duct obstruction. J Xray Sci Technol. 2013;21:293–302. doi: 10.3233/XST-130377. [DOI] [PubMed] [Google Scholar]
- 18.Eisenberg ML, Walsh TJ, Garcia MM, Shinohara K, Turek PJ. Ejaculatory duct manometry in normal men and in patients with ejaculatory duct obstruction. J Urol. 2008;180:255–60. doi: 10.1016/j.juro.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Pace G, Galatioto GP, Guala L, Ranieri G, Vicentini C. Ejaculatory duct obstruction caused by a right giant seminal vesicle with an ipsilateral upper urinary tract agenesia: an embryologic malformation. Fertil Steril. 2008;89:390–4. doi: 10.1016/j.fertnstert.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Apaydin E, Killi RM, Turna B, Semerci B, Nazli O. Transrectal ultrasonography-guided echo-enhanced seminal vesiculography in combination with transurethral resection of the ejaculatory ducts. BJU Int. 2004;93:1110–2. doi: 10.1111/j.1464-410X.2003.04790.x. [DOI] [PubMed] [Google Scholar]
- 21.Purohit RS, Wu DS, Shinohara K, Turek PJ. A prospective comparison of 3 diagnostic methods to evaluate ejaculatory duct obstruction. J Urol. 2004;171:232–5. doi: 10.1097/01.ju.0000101909.70651.d1. [DOI] [PubMed] [Google Scholar]
- 22.Ozgok Y, Tan MO, Kilciler M, Tahmaz L, Kibar Y. Diagnosis and treatment of ejaculatory duct obstruction in male infertility. Eur Urol. 2001;39:24–9. doi: 10.1159/000052408. [DOI] [PubMed] [Google Scholar]
- 23.Schroeder-Printzen I, Ludwig M, Kohn F, Weidner W. Surgical therapy in infertile men with ejaculatory duct obstruction: technique and outcome of a standardized surgical approach. Hum Reprod. 2000;15:1364–8. doi: 10.1093/humrep/15.6.1364. [DOI] [PubMed] [Google Scholar]
- 24.Netto NR, Jr, Esteves SC, Neves PA. Transurethral resection of partially obstructed ejaculatory ducts: seminal parameters and pregnancy outcomes according to the etiology of obstruction. J Urol. 1998;159:2048–53. doi: 10.1016/S0022-5347(01)63243-9. [DOI] [PubMed] [Google Scholar]
- 25.Turek PJ, Magana JO, Lipshultz LI. Semen parameters before and after transurethral surgery for ejaculatory duct obstruction. J Urol. 1996;155:1291–3. doi: 10.1016/S0022-5347(01)66246-3. [DOI] [PubMed] [Google Scholar]
- 26.Vazquez-Levin MH, Dressler KP, Nagler HM. Urine contamination of seminal fluid after transurethral resection of the ejaculatory ducts. J Urol. 1994;152:2049–52. doi: 10.1016/S0022-5347(17)32303-0. [DOI] [PubMed] [Google Scholar]
- 27.Jarow JP. Seminal vesicle aspiration in the management of patients with ejaculatory duct obstruction. J Urol. 1994;152:899–901. doi: 10.1016/S0022-5347(17)32603-4. [DOI] [PubMed] [Google Scholar]
- 28.Hall S, Oates RD. Unilateral absence of the scrotal vas deferens associated with contralateral mesonephric duct anomalies resulting in infertility: laboratory, physical and radiographic findings, and therapeutic alternatives. J Urol. 1993;150:1161–4. doi: 10.1016/S0022-5347(17)35714-2. [DOI] [PubMed] [Google Scholar]
- 29.Weintraub MP, De Mouy E, Hellstrom WJ. Newer modalities in the diagnosis and treatment of ejaculatory duct obstruction. J Urol. 1993;150:1150–4. doi: 10.1016/S0022-5347(17)35711-7. [DOI] [PubMed] [Google Scholar]
- 30.Worischeck JH, Parra RO. Transrectal ultrasound in the evaluation of men with low volume azoospermia. J Urol. 1993;149:1341–4. doi: 10.1016/S0022-5347(17)36387-5. [DOI] [PubMed] [Google Scholar]
- 31.Meacham RB, Hellerstein DK, Lipshultz LI. Evaluation and treatment of ejaculatory duct obstruction in the infertile male. Fertil Steril. 1993;59:393–7. doi: 10.1016/S0015-0282(16)55683-0. [DOI] [PubMed] [Google Scholar]
- 32.Kuligowska E, Baker CE, Oates RD. Male infertility: role of transrectal US in diagnosis and management. Radiology. 1992;185:353–60. doi: 10.1148/radiology.185.2.1410338. [DOI] [PubMed] [Google Scholar]
- 33.Goldwasser BZ, Weinerth JL, Carson CC., 3rd Ejaculatory duct obstruction: the case for aggressive diagnosis and treatment. J Urol. 1985;134:964–6. doi: 10.1016/S0022-5347(17)47550-1. [DOI] [PubMed] [Google Scholar]
- 34.Silber SJ. Ejaculatory duct obstruction. J Urol. 1980;124:294–7. doi: 10.1016/S0022-5347(17)55415-4. [DOI] [PubMed] [Google Scholar]
- 35.Aggour A, Mostafa H, Maged W. Endoscopic management of ejaculatory duct obstruction. Int Urol Nephrol. 1998;30:481–5. doi: 10.1007/BF02550229. [DOI] [PubMed] [Google Scholar]
- 36.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 37.Kadioglu A, Cayan S, Tefekli A, Orhan I, Engin G, Turek PJ. Does response to treatment of ejaculatory duct obstruction in infertile men vary with pathology? Fertil Steril. 2001;76:138–42. doi: 10.1016/S0015-0282(01)01817-9. [DOI] [PubMed] [Google Scholar]
- 38.Meacham RB, Townsend RR, Drose JA. Ejaculatory duct obstruction: diagnosis and treatment with transrectal sonography. AJR Am J Roentgenol. 1995;165:1463–6. doi: 10.2214/ajr.165.6.7484587. [DOI] [PubMed] [Google Scholar]
- 39.Paick J, Kim SH, Kim SW. Ejaculatory duct obstruction in infertile men. BJU Int. 2000;85:720–4. doi: 10.1046/j.1464-410x.2000.00600.x. [DOI] [PubMed] [Google Scholar]
- 40.Tu X, Zhao L, Zheo L, Wang W, Deng L, Chen Y, et al. Effect of transurethral resection of ejaculatory duct for treatment of ejaculatory duct obstruction (report of 60 cases). Journal of Sexual Medicine. Conference: 13th Biennial Meeting of the Asia-Pacific Society for Sexual Medicine. Kaohsiung Taiwan (Republic of China). Conference Publication: (var.pagings); 2012; Wiley Blackwell; 2012. pp. 147–8. [Google Scholar]
- 41.Johnson CW, Bingham JB, Goluboff ET, Fisch H. Transurethral resection of the ejaculatory ducts for treating ejaculatory symptoms. BJU Int. 2005;95:117–9. doi: 10.1111/j.1464-410X.2004.05261.x. [DOI] [PubMed] [Google Scholar]
- 42.Dickey RP, Pyrzak R, Lu PY, Taylor SN, Rye PH. Comparison of the sperm quality necessary for successful intrauterine insemination with World Health Organization threshold values for normal sperm. Fertil Steril. 1999;71:684–9. doi: 10.1016/S0015-0282(98)00519-6. [DOI] [PubMed] [Google Scholar]
- 43.Kroese ACJ, de Lange NM, Collins J, Evers JLH. Surgery or embolization for varicoceles in subfertile men. Cochrane Database Syst Rev. 2012;10:CD000479. doi: 10.1002/14651858.CD000479.pub5. [DOI] [PubMed] [Google Scholar]
- 44.Avellino GJ, Lipshultz LI, Sigman M, Hwang K. Transurethral resection of the ejaculatory ducts: etiology of obstruction and surgical treatment options. Fertil Steril. 2019;111:427–43. doi: 10.1016/j.fertnstert.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Group* OLoEW. The Oxford Levels of Evidence 2 Oxford Centre for Evidence-Based Medicine. 2011. [Accessed 07-February-2019]. Available from: https://www.cebm.net/index.aspx?o=5653.


