Abstract
Objective
The prostate-specific antigen density (PSAD) is an accessory tool when suspecting prostate cancer. Multiparametric magnetic resonance imaging (mpMRI) of the prostate has a high rate of false negatives. The aim of this study is to evaluate the sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) when adding the PSAD and negative or equivocal mpMRI.
Material and methods
A retrospective study that included prostate biopsies performed using a transperineal approach and guided by ultrasound between 2015 and 2019 was conducted. Clinically significant prostate cancer (csPCa) was defined as Gleason score ≥3+4. The population was divided into groups according to the PSAD level-≤0.15 and >0.15. Sensitivity, specificity, NPV, and PPV of mpMRI were calculated.
Results
A total of 292 patients were included; 12.1% (4/33 patients) of the negative mpMRI group presented csPCa, and only 7 in the equivocal mpMRI group presented csPCa. NPV and sensitivity were 91.15% and 90.5%, respectively. In the positive mpMRI group, 53.7% (96/179) had csPCa, with a PPV of 53.6% and specificity of 55.3%. Of the patients with PSAD ≤0.15, 23 (16.54%) presented csPCa. All of them presented a positive mpMRI. All patients with a negative or equivocal mpMRI and a PSAD ≤0.15 presented a clinically non-significant tumor or benign result. The addition of this tool to mpMRI resulted in 100% sensitivity, 69% specificity, and 34.8% PPV.
Conclusion
In our series, PSAD ≤0.15 increased the NPV in negative or equivocal mpMRI, and through this unnecessary prostate biopsies could be avoided.
Keywords: Biopsy, prostate, prostate cancer, prostate-specific antigen
Introduction
Prostate cancer is the most common cancer in men and the second most common cause of cancer related deaths.[1] Early detection with prostate-specific antigen (PSA) screening can change the natural progression of the disease and reduce mortality.[2]
Every year, more than a million patients undergo a prostate biopsy in the United States. Overuse of this diagnostic method has resulted in an increase in the diagnosis of non-clinically significant prostate cancer (ncsPCa) leading to overtreatment.[3]
Furthermore, there is a substantial increase in financial costs, psychological consequences, and postoperative complications (pain, hematuria, urinary infection, acute urine retention, and sepsis).[4] For these reasons, the optimization of screening tests and a tool that can predict which patients could safely avoid an unnecessary prostate biopsy are sought.
The density of PSA (PSAD) obtained by dividing the total PSA by the prostate volume has been used as an accessory tool when suspecting prostate cancer and has demonstrated disparate results.[5,6] In the initial studies, it was found that adding PSAD to clinical risk prediction nomograms increased sensitivity and specificity.[7] However, other studies concluded that PSAD adds little diagnostic value to PSA and would only be useful in patients with abnormal PSA results or pathological/suspected digital rectal exam.[8] This bibliography is out of date, and the results are extrapolated from biopsies of two intakes per sextant, which differ from the current approaches (saturation biopsies, fusion-guided biopsies, and transperineal biopsies). In these, the estimated sensitivity of the PSAD with a cut-off point of 0.15 was 77%, with a negative predictive value (NPV) of 89%.[9]
In addition, the increasing availability of the multiparametric magnetic resonance imaging (mpMRI) of the prostate, the different functional imaging modalities, and the international standardization of these results (PI-RADS score) have led it to acquire a prominent place in the diagnosis of prostate cancer, and it is currently the recommended method in patients with previous biopsies with negative results.[10] A recent meta-analysis that evaluated mpMRI performance showed a sensitivity of 74% and NPV of 64–94%.[11]
The aim of this study is to evaluate the sensitivity and specificity when adding the PSAD in patients with negative mpMRI or with an equivocal result.
Material and methods
After ethics review board approval, a retrospective study that included all prostate biopsies performed at the Hospital Aleman of Buenos Aires between 2015 and 2019 was conducted. All patients gave informed consent. Biopsies were performed using a transperineal approach and guided by ultrasound. A cognitive or fusion biopsy was performed by taking three additional samples from the suspicious or positive area (target biopsy). Demographic data, PSA value, and prostate volume measured by mpMRI were recorded (to obtain the PSAD). Clinically significant prostate cancer (csPCa) was defined as Gleason score ≥3+4 or ≥International Society of Urologic Pathologists (ISUP) 2, according to the definition used in the PROMIS study.[12]
Prostate Imaging-Reporting and Data System (PI-RADS) v2.0 score was used as a reference and divided into three groups: PI-RADS 1 and 2 (negative), PI-RADS 3 (equivocal), and PI-RADS 4 and 5 (positive).[13] PI-RADS 3 is defined as focal mildly/moderately hypointense on apparent diffusion coefficient and isointense/mildly hyperintense on high b-value diffusion weighted image.[13] A PI-RADS 3 score was considered as negative to calculate the different variables. The population was divided into two groups according to the PSAD level-≤0.15 and >0.15. We calculated the sensitivity, specificity, NPV, and positive predictive value (PPV) of mpMRI for the detection of high-grade prostate cancer, alone and combined with PSAD groups.
Statistical analysis
Stata® version 13 was used to perform the analyses. Descriptive statistics of samples were expressed as mean±standard deviation. Frequencies were compared using chi-square test. A p<0.05 was considered as statistically significant.
Results
A total of 682 biopsies were performed, including 292 that had a previous mpMRI. Patients’ mean age was 65.3 years (SD±7.79) and mean PSA was 9.2 ng/dl (SD±6.17). The mean prostate volume estimated by mpMRI was 56.6 cc (SD±22.8). Thirty-three patients had a negative mpMRI result, 80 patients had an equivocal result, and 179 patients had a positive mpMRI.
A total of 12.12% (4/33) of the negative mpMRI group presented csPCa, and in the equivocal mpMRI group only 7.5% presented csPCa (6/80). The mpMRI NPV was 91.15%, and the sensitivity was 90.5%. In the positive mpMRI group, 53.7% (96/179) had csPCa with a PPV of 53.6% and specificity of 55.3% (Table 1).
Table 1.
Histopathology and PI-RADS score percentages of all patients (including PSAD; cut-off 0.15)
| PI-RADS | Benign or ncsPCa | csPCa | TOTAL | |
|---|---|---|---|---|
| All patients | 1–2 | 87.88 | 12.12 | 33 |
| 3 | 92.5 | 7.5 | 80 | |
| 4–5 | 46.3 | 53.7 | 179 | |
| TOTAL | 186 (63.7%) | 106 (36.3%) | 292 (100%) | |
|
| ||||
| PSAD >0.15 | 1–2 | 3 | 4 | 7 |
| 3 | 4 | 6 | 10 | |
| 4–5 | 40 | 73 | 113 | |
| TOTAL | 47 (36.15%) | 83 (63.85%) | 130 (100%) | |
|
| ||||
| PSAD ≤0.15 | 1–2 | 26 | 0 | 26 |
| 3 | 70 | 0 | 70 | |
| 4–5 | 43 | 23 | 66 | |
| TOTAL | 139 (85.8%) | 23 (14.2%) | 162 (100%) | |
ncsPCa: prostate cancer clinically non-significant; csPCa: prostate cancer clinically significant; PSAD: prostate-specific antigen density; PI-RADS: Prostate Imaging–Reporting and Data System
An analysis of prostate cancer detection in each group was performed according to the PI-RADS score, and the anatomopathological result was classified according to ISUP grades (Figure 1).
Figure 1.
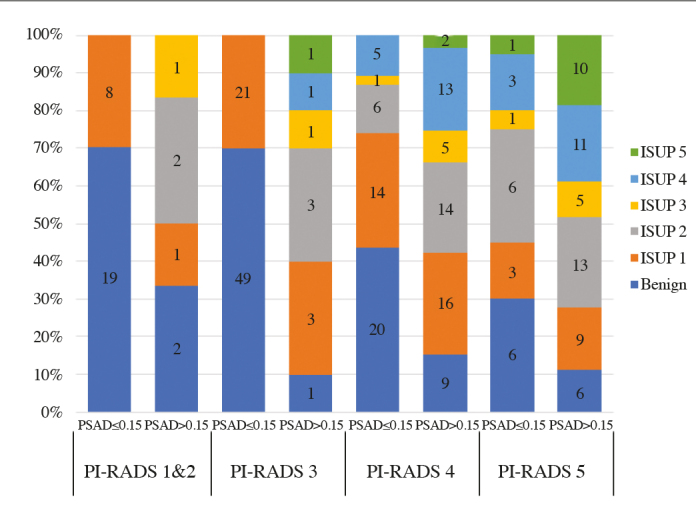
Outcomes stratified for the overall PI-RADS score and PSAD
PI-RADS: Prostate Imaging–Reporting and Data System; PSAD: prostate-specific antigen density
In the group with PSAD >0.15 (n=130), 83 patients (63.85%) presented csPCa. In this subgroup, 4 (4.82%) patients presented a negative mpMRI, 6 (7.23%) patients presented an equivocal mpMRI, and 73 (87.95%) patients presented a positive mpMRI (Table 1). The NPV was 41.1% and PPV was 64.6%.
When analyzing patients with PSAD ≤0.15 (n=162), 23 of them (16.54%) presented a diagnosis of csPCa. All of them presented a positive mpMRI. All the patients with a negative or equivocal mpMRI and a PSAD ≤0.15 presented a diagnosis of clinically non-significant tumor or benign result. The addition of this tool to mpMRI resulted in 100% sensitivity, 69% specificity, and 34.8% PPV (Table 1).
Using a PSAD cut-off of 0.15 and a positive or negative mpMRI (PI-RADS 1–3) yields four groups. (Table 2). A negative mpMRI with PSAD ≤0.15 presented a negative biopsy in 0% (0/96) of the cases; in the group of patients with a PSAD >0.15, there was no significant difference in the frequency of significant cancer detected. mpMRI was positive for 73 of 113 patients (64.6%) and mpMRI was negative for 10 of 17 patients (58.9%) (p=0.84); PPV value was 64% and NPV value was 41%. In the group of patients with a positive mpMRI and with a PSAD ≤0.15, the frequency of significant prostate cancer was 34.8%, which was significantly less than that of patients with a positive mpMRI and a PSAD >0.15 (64.6%) (p=0.002).
Table 2.
High-grade cases according to PSAD and mpMRI (including number of biopsies that could be avoided and significant cancers missed)
| mpMRI | PSAD ≤0.15 n=162 |
PSAD >0.15 n=130 |
||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| PI-RADS 1–3 | PI-RADS 4–5 | PI-RADS 1–3 | PI-RADS 4–5 | |||||
| 96 | 66 | 17 | 113 | |||||
|
| ||||||||
| csPCa n (%) | Yes | No | yes | no | Yes | no | yes | No |
| 0 (0) | 96 (100) | 23 (34.8) | 43 (65.2) | 10 (58.9) | 7 (41.1) | 73 (64.6) | 40 (35.4) | |
|
| ||||||||
| Biopsies could be avoided (n) | 96 | 0 | 0 | 0 | ||||
|
| ||||||||
| csPCa missed (n) | 0 | 0 | 0 | 0 | ||||
Key: Taking into account PI-RADS 4–5 or PSAD >0.15 as criteria for biopsy. PSAD: prostate-specific antigen density; csPCa: prostate cancer clinically significant; mpMRI, magnetic resonance imaging; PI-RADS: Prostate Imaging–Reporting and Data System.
Discussion
It is accepted that mpMRI is an independent factor for the diagnosis of prostate cancer and clinically significant disease and a reliable predictor of the pathological result of the prostate biopsy. A recent meta-analysis evaluated the performance of an mpMRI in detecting csPCa and showed a specificity of 88% and a sensitivity of 77% with an NPV ranging from 64–94%.[11] Panebianco et al.[14] reported an NPV of 94% in the diagnosis of csPCa in mpMRI reported and evaluated by experts.
In our experience, the NPV of the MRIs in 150 transrectal prostate biopsies was 84%.[15] In this study, when performing a transperineal approach, a higher NPV (91.15%) was obtained. Although the PI-RADS score predicts the outcome of a prostate biopsy, it is still difficult to decide which patients would safely avoid an unnecessary prostate biopsy with a negative or equivocal mpMRI result. This is mainly due to a discrepancy in the actual value of the real NPV.[16] In our study, 19.62% of the patients with negative or equivocal mpMRI (PI-RADS 1–3) would have lost the diagnosis of a clinically significant tumor had they avoided the prostate biopsy. In addition to the PSAD, we used total PSA for effective clinical management of the patient.
Lesions with a PI-RADS score of 3 have been controversial. These are radiographic lesions where the presence or absence of significant disease is uncertain, and there is no consensus on the immediate necessity of a biopsy. An alternative would be to perform a surveillance of these lesions. Some authors consider the lesion volume to manage category 3 lesions and consider using simplified PI-RADS and biparametric mpMRI for differentiating category 3a lesions (volume <0.5 mL) and category 3b lesions (volume ≥0.5 mL). They found csPCa in 2.8% and 27.6% of the cases, respectively and recommended clinical surveillance for category 3a lesions and target biopsy for category 3b lesions.[17–20] In a study by van de Sar et al.[21], prostate biopsies were performed only when there was an alteration in the PSA kinetics or an increase in the PI-RADS score, demonstrating a similar diagnostic risk profile when compared with the immediate biopsy group.
Multiple traditional clinical markers, biomarkers, and nomograms have been used to more safely decide which patients with PI-RADS 3 lesions can avoid an unnecessary prostate biopsy.[22] Among them, PSAD has been identified as a diagnostic tool that helps to predict csPCa. Therefore, PSAD also contributes to predicting the result of the prostate biopsy.[23] Recently, Jue et al.[24] described a prospective cohort from the United States and concluded that the PSAD had a higher performance in diagnosing prostate cancer when compared with the value of isolated PSA.
Furthermore, PSAD is a useful method in diagnosing clinically significant disease and evaluating the aggressiveness of prostate cancer. A negative mpMRI with PSAD ≤0.15 may be good in predicting a negative biopsy, whereas the other group does not permit avoiding biopsies. Kosaka et al.[25] reported that PSAD was a significant predictor of prostate cancer in patients aged ≤50 years.
In an update on ncsPCs, Bastian et al.[26] included PSAD with a cut-off point ≤0.15, together with a Gleason score of ≤6, <3 positive cylinders, and <50% tumor involvement as one of the predictors. When the result of the surgical piece was compared after a radical prostatectomy, a high PSAD value correlated with higher pathological stages and with greater aggressiveness of the disease and thus being a factor that could be used to predict progression-free rate after the radical treatment.[27]
Corcoran et al.[28] report that PSAD is the best predictor of increased Gleason score gradation between prostate biopsy and radical prostatectomy. All this information indicates that PSAD can not only act as a predictor of the result of a prostate biopsy but also as a predictor of clinically significant disease and tumor aggressiveness.
Despite the fact that mpMRI with PI-RADS score and PSAD allow predicting the result of a prostate biopsy, each of these diagnostic methods have their disadvantages. This was observed in our study when the NPV of the mpMRI was 91.15%. However, when we associated negative or equivocal MRIs (PI-RADS 1–3) with PSAD ≤0.15, both sensitivity and NPV increased to 100%. This translates into the fact that no patient with negative or equivocal mpMRI and a PSAD ≤0.15 had a diagnosis of csPCa.
This study has certain limitations. First, it is a retrospective study carried out in a single center. Second, the PSAD value is directly related to the operator who performs the complementary study that measures prostate volume. A limitation of PSAD is the variable production of PSA by benign prostatic hyperplasia, resulting in a non-linear increase in larger prostates. This means that PSAD may become less sensitive in larger prostates or as its volume increases.[29]
In this series, PSAD (with a cut-off point of 0.15) increased the sensitivity and NPV in negative or equivocal MRIs (PI-RADS 1–3); through this, unnecessary prostate biopsies could be avoided in patients presenting a combination of these diagnostic methods
Main Points:
In 292 patients biopsied using a transperineal approach, we found that the prostate-specific antigen density (PSAD) ≤0.15 increased the negative predictive value in negative or equivocal magnetic resonance imagings (MRIs).
In patients with PSAD ≤0.15, 23 (16.5%) of them presented clinically significant prostate cancer. All of them had a positive mpMRI.
All the patients with a negative or equivocal mpMRI and a PSAD ≤0.15 presented a clinically non-significant tumor or benign result. The addition of this tool to mpMRI resulted in 100% sensitivity, 69% specificity, and 34.8% positive predictive value.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Hospital Alemán of Buenos Aires (protocol number 126-2016).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - L.R., L.B., P.C., H.R.P., G.V., C.A.; Design - L.R., L.B.; Supervision - L.R., L.B., P.C., H.R.P., G.V., C.A.; Materials - L.R., L.B., P.C., H.R.P., G.V., C.A.; Data Collection and/or Processing - L.R., L.B.; Analysis and/or Interpretation - L.R., L.B.; Literature Search - L.R., L.B.; Writing Manuscript - L.R., L.B.; Critical Review - L.B.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–92. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 2.Roobol MJ, Kranse R, Bangma CH, van Leenders AG, Blijenberg BG, van Schaik RH, et al. Screening for prostate cancer: results of the Rotterdam Section of the European Randomized Study of Screening for Prostate Cancer. Eur Urol. 2013;64:530–9. doi: 10.1016/j.eururo.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 3.Pokorny MR, De Rooij M, Duncan E, Schröder FH, Parkinson R, Barentsz JO, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol. 2014;66:22–9. doi: 10.1016/j.eururo.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Rosario DJ, Lane JA, Metcalfe C, Donovan JL, Doble A, Goodwin L, et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ. 2012;344:d7894. doi: 10.1136/bmj.d7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramon J, Boccon-Gibod L, Billebaud T, Astier L, Kobelinsky M, Meulemans A, et al. Prostate-specific antigen density: a means to enhance detection of prostate cancer. Eur Urol. 1994;25:288–94. doi: 10.1159/000475303. discussion 304. [DOI] [PubMed] [Google Scholar]
- 6.Mueller EJ, Coventry J, Desmond PM, Zeidman EJ, Thompson IM. Relative performance characteristics of prostate specific antigen and prostatic specific antigen density for the diagnosis of carcinoma of the prostate. Urol Oncol. 1995;1:84–7. doi: 10.1016/1078-1439(95)00022-A. [DOI] [PubMed] [Google Scholar]
- 7.Van den Bergh RCN, Roemeling S, Roobol MJ, Roobol W, Schröder FH, Bangma CH. Prospective validation of active surveillance in prostate cancer: the PRIAS study. Eur Urol. 2007;52:1560–3. doi: 10.1016/j.eururo.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Akdas A, Tarcan T, Türkeri L, Cevik I, Biren T, Ilker Y. The impact of prostate-specific antigen density in predicting prostate cancer when serum prostate-specific antigen levels are less than 10 ng/ml. Eur Urol. 1996;29:189–92. [PubMed] [Google Scholar]
- 9.De Castro HAS, Iared W, Santos JEM, Solha RS, Shigueoka DC, Ajzen SA. Contribution of PSA density in the prediction of prostate cancer in patients with PSA values between 2.6 and 10.0 ng/ml. Radiol Bras. 2011;44:205–9. doi: 10.1590/S0100-39842011000400003. [DOI] [Google Scholar]
- 10.Quentin M, Blondin D, Arsov C, Schimmöller L, Hiester A, Godehardt E, et al. Prospective evaluation of magnetic resonance imaging guided in-bore prostate biopsy versus systematic transrectal ultrasound guided prostate biopsy in biopsy naive men with elevated prostate specific antigen. J Urol. 2014;192:1374–9. doi: 10.1016/j.juro.2014.05.090. [DOI] [PubMed] [Google Scholar]
- 11.de Rooij M, Hamoen EH, Fütterer JJ, Barentsz JO, Rovers MM. Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. AJR Am J Roentgenol. 2014;202:343–51. doi: 10.2214/AJR.13.11046. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–22. doi: 10.1016/S0140-6736(16)32401-1. [DOI] [PubMed] [Google Scholar]
- 13.Renard-Penna R, Mozer P, Cornud F, Barry-Delongchamps N, Bruguière E, Portalez D, et al. Prostate Imaging Reporting and Data System and Likert Scoring System: Multiparametric MR Imaging Validation Study to Screen Patients for Initial Biopsy. Radiology. 2015;275:458–68. doi: 10.1148/radiol.14140184. [DOI] [PubMed] [Google Scholar]
- 14.Panebianco V, Barchetti G, Simone G, Del Monte M, Ciardi A, Grompone MD, et al. Negative multipara-metric magnetic resonance imaging for prostate cancer: what’s next? Eur Urol. 2018;74:48–54. doi: 10.1016/j.eururo.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Contreras P, Blas L, Mieggi M, Ríos Pita H, Vitagliano G, Amer Ci. Resonancia multiparamétrica y biopsia prostática por vía transrectal dirigida por estimación visual. Nuestra experiencia en 150 casos. Rev Arg de Urol. 2017;82:102–7. [Google Scholar]
- 16.Washino S, Okochi T, Saito K, Konishi T, Hirai M, Kobayashi Y, et al. Combination of prostate imaging reporting and data system (Pi-RADS) score and prostate-specific antigen (PSA) density predicts biopsy outcome in prostate biopsy naïve patients. BJU Int. 2017;119:225–33. doi: 10.1111/bju.13465. [DOI] [PubMed] [Google Scholar]
- 17.Martorana E, Pirola GM, Scialpi M, Micali S, Iseppi A, Bonetti LRG, et al. Lesion volume predicts prostate cancer risk and aggressiveness: validation of its value alone and matched with prostate imaging reporting and data system score. BJU Int. 2017;120:92–103. doi: 10.1111/bju.13649. [DOI] [PubMed] [Google Scholar]
- 18.Scialpi M, Martorana E, Aisa MC, Rondoni V, D’Andrea A, Bianchi G. Score 3 prostate lesions: a gray zone for PI-RADS v2. Turk J Urol. 2017;43:237–40. doi: 10.5152/tud.2017.01058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scialpi M, Martorana E, Scialpi P, D’Andrea A, Torre R, Di Blasi A, et al. Round table: arguments in supporting abbreviated or biparametric MRI of the prostata protocol. Abdom Radiol (NY) 2020. [DOI] [PubMed]
- 20.Scialpi M, Aisa MC, D’Andrea A, Martorana E. Simplified Prostate Imaging Reporting and Data System for Biparametric Prostate MRI: A Proposal. Am J Roentgenol. 2018;211:379–82. doi: 10.2214/AJR.17.19014. [DOI] [PubMed] [Google Scholar]
- 21.van der Sar ECA, Kasivisvanathan V, Brizmohun M, Freeman A, Punwani S, Hamoudi R, et al. Management of radiologically indeterminate magnetic resonance imaging signals in men at risk of prostate cancer. Eur Urol Focus. 2019;5:62–8. doi: 10.1016/j.euf.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Gómez Rivas J, Giganti F, Álvarez-Maestro M, Freire MJ, Kasivisvanathan V, Martinez-Piñeiro L, et al. Prostate inde- terminate lesions on magnetic resonance imaging-biopsy versus surveillance: a literature review. Eur Urol Focus. 2018;5:799–806. doi: 10.1016/j.euf.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Veneziano S, Pavlica P, Compagnone G, Martorana G. Usefulness of the (F/T)/PSA density ratio to detect prostate cancer. Urol Int. 2005;74:13–8. doi: 10.1159/000082702. [DOI] [PubMed] [Google Scholar]
- 24.Jue JS, Barboza MP, Prakash NS, Venkatramani V, Sinha VR, Pavan N, et al. Re-examining Prostate-specific Antigen (PSA) density: defining the optimal psa range and patients for using psa density to predict prostate cancer using extended template biopsy. Urology. 2017;105:123–8. doi: 10.1016/j.urology.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Kosaka T, Mizuno R, Shinojima T, Miyajima A, Kikuchi E, Tanaka N, et al. The implications of prostate-specific antigen density to predict clinically significant prostate cancer in men ≤ 50 years. Am J Clin Exp Urol. 2014;2:332–6. [PMC free article] [PubMed] [Google Scholar]
- 26.Bastian PJ, Mangold LA, Epstein JI, Partin AW. Characteristics of insignificant clinical T1c prostate tumors - A contemporary analysis. Cancer. 2004;101:2001–5. doi: 10.1002/cncr.20586. [DOI] [PubMed] [Google Scholar]
- 27.Kundu SD, Roehl KA, Yu X, Antenor JAV, Suarez BK, Catalona WJ. Prostate specific antigen density correlates with features of prostate cancer aggressiveness. J Urol. 2007;177:505–9. doi: 10.1016/j.juro.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 28.Corcoran NM, Casey RG, Hong MK, Pedersen J, Connolly S, Peters J, et al. The ability of prostate-specific antigen (PSA) density to predict an upgrade in Gleason score between initial prostate biopsy and prostatectomy diminishes with increasing tumour grade due to reduced PSA secretion per unit tumour volume. BJU Int. 2012;110:36–42. doi: 10.1111/j.1464-410X.2011.10681.x. [DOI] [PubMed] [Google Scholar]
- 29.Partin AW, Carter HB, Chan DW, Epstein JI, Oesterling JE, Rock RC, et al. Prostate specific antigen in the staging of localized prostate cancer: influence of tumor differentiation, tumor volume and benign hyperplasia. J Urol. 1990;143:747. doi: 10.1016/S0022-5347(17)40079-6. [DOI] [PubMed] [Google Scholar]


