Abstract
Vesicouterine fistula (VUF) is a rare extra-anatomical communication developing between the uterus or cervix and the urinary bladder, most commonly after an iatrogenic injury during a cesarean section. Patients with VUF may have various clinical presentations, ranging from Youssef’s syndrome (vaginal urine leakage, amenorrhea, and menouria) to urinary tract infection and infertility. Quality of life for patients having this pathology is strongly affected owing to the psychological burden. Treatment is surgery based because low success rates have been reported for conservative or minimally invasive approaches. Herein, we present a case of a 35-year-old woman successfully treated by a minimally invasive endoscopic repair procedure with the injection of microfragmented autologous adipose tissue (Lipogems®).
Keywords: Endoscopic, fistula, genitourinary, lipogems, microfragmented autologous adipose tissue, vesicouterine
Introduction
Vesicouterine fistula (VUF) is a rare abnormal communication between the bladder and the uterus, accounting for approximately 1%–4% of the genitourinary fistulas.[1] It was first described in 1957 by Youssef, who defined the classic triad of presentation symptoms: vaginal urine leakage, amenorrhea, and cyclic hematuria during the menstrual cycle (menouria).[2] In addition to this, the clinical presentation may vary from incontinence and recurrent urinary tract infections to secondary infertility and first-trimester abortion.[1]
VUF is caused by an iatrogenic injury during cesarean section (CS) in 83%–93% of cases, and its treatment can be distinguished as conservative or surgical.[3]
The surgical treatment brings, as a complication, a risk of hysterectomy, raising several cultural and quality-of-life-related implications.
We present a case of a 35-year-old woman who underwent emergency CS and developed a VUF treated by a minimally invasive endoscopic repair procedure with the injection of microfragmented autologous adipose tissue (Lipogems®).
Case presentation
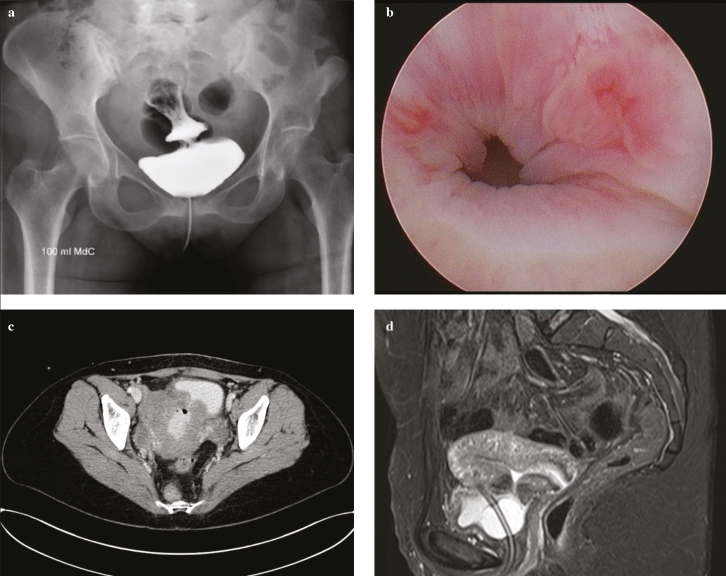
A 35-year-old, 37-week pregnant woman was referred to the emergency department for a CS because of premature rupture of the membranes. Her history was remarked by 4 previous CSs. Owing to a difficult dissection of lower uterine segment, methylene blue dye test was performed in a precautionary fashion that was negative. In the early postoperative hours, she presented with hematuria, requiring continuous bladder irrigation. She was discharged asymptomatic 5 days after surgery, without a urinary catheter. Three weeks later, she presented at the emergency department for vaginal urine leakage and Escherichia coli urinary tract infection; physical examination was unremarkble. A cystogram revealed a urinary leakage on the bladder dome suspected for VUF (Figure 1a). Contrast-enhanced computed tomography (CT), cystoscopy, and magnetic resonance imaging (MRI) confirmed a 1 cm fistula located between the bladder and the uterus (Figure 1).
Figure 1. a–d.
Diagnostic work-up before treatment: (a) cystogram; (b) cystoscopy; (c) abdominal computed tomography scan; (d) magnetic resonance imaging showing the presence of a vesicouterine fistula
At the time of diagnosis, a Foley catheter was placed as initial conservative management of VUF, leading to reduction of fistula at 3 months. After the refusal of a standard surgical excision, an endoscopic procedure for fistula repair consisting of transurethral resection of the fistulous tract and injection of microfragmented autologous adipose tissue (Lipogems®) at the bladder side of the fistula was performed.
The following is the step-by-step procedure.
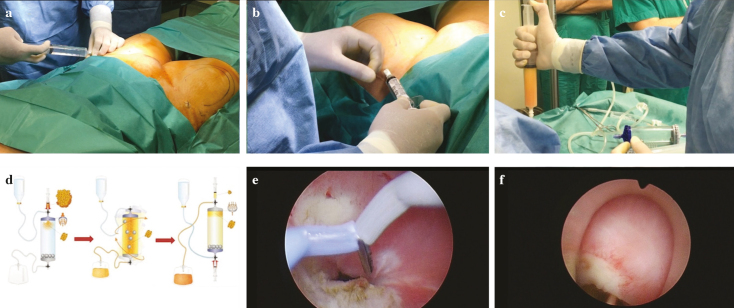
Local anesthesia: The thighs were used as the donor sites for adipose tissue harvesting. Before harvesting the fat, the thighs were injected with 360 mL (180 mL in each thigh) of modified Klein solution (500 mL of saline solution; 20 mL lidocaine, 20 mg/mL; and 1 mL adrenaline, 1 mg/mL) using a 17-G disposable cannula (Figure 2a).
Harvesting of the adipose tissue: The adipose tissue was harvested using a 13-G blunt cannula connected to a Vaclock® 20 mL syringe (Figure 2b).
The aspirated tissue was then collected in syringes and positioned for decanting (Figure 2c).
Processing of the adipose tissue with the dedicated Lipogems® device according to the instructions of the manufacturing company: The fat was then processed in the Lipogems® surgery kit, a disposable device that progressively reduces the size of the adipose tissue clusters while removing the substances with proinflammatory properties. Lipogems® device is a completely closed system. It works using gravity and saline flow. After making a complete filling with saline, the lipoaspirates are inserted in the device. Then, the surgeon manually shakes the device, which activates 5 stainless steel marbles. The marble movement allows oil/physiologic emulsion to be created and washed away by the flux fluid against the gravity. The continuous flow of the solution eliminates the waste products against gravity in a collection waste bag and the 2 filters retain the adipose clusters in the device. When the separation line between the saline solution and Lipogems® is stable, the clear watery solution can be removed obtaining the micronized adipose tissue. The product is then transferred to several 10 mL syringes with a 22-G and 30-mm-long needle to be injected into the patient (Figure 2d).
Resection of the fistula: Transurethral resection of the bladder side of the fistula was performed with a Collins knife. Resection of the fistula borders was performed to remove the unhealthy tissue and inject the Lipogems® into the normal mucosa, giving better chance to the tissue to heal properly. Collins knife was used because it allows more precise incision of the borders than a cutting loop.
Endoscopic injection of Lipogems®: The final product was injected into the mucosal and muscular layers at the bladder side of the fistula (Figure 2).
Figure 2. a–f.
Steps of the procedure: (a) anesthesia of the thighs; (b) harvesting of the adipose tissue; (c) collection of the harvested tissue; (d) processing the tissue with Lipogems® surgery kit; (e) transurethral resection of the bladder side of the fistula; (f) injection of microfragmented adipose tissue around the bladder side of the fistula
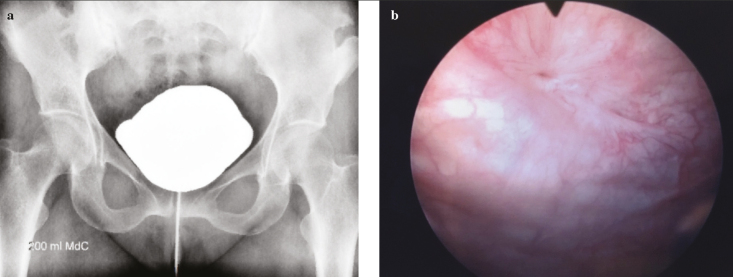
At the end of procedure, a pig-tail suprapubic catheter was placed. The patient was discharged on day 1 after surgery. Three months after surgery, she was asymptomatic; cystoscopy was performed, showing the scar tissue in the previous location of the fistula, and the cystogram revealed complete repair of VUF (Figure 3). At the 24-month follow-up, no recurrences were documented.
Figure 3. a, b.
Cystogram (a) and cystoscopy after treatment (b)
Discussion
VUF is the rarest type of urogenital fistula, and its cause is mainly iatrogenic.[1,3] Risk factors are the dissection of the lower uterine segment, excessive intra-operative bleeding, severe dystocia, forceps delivery, obstetrical vacuum, manual removal of the placenta, placenta percreta, uterine rupture, previous CS, and dilatation/curettage. Other less frequent causes include inflammatory bowel diseases, endometriosis, intrauterine device migration, and congenital lesions.[4] In addition, immunohistochemical studies proved that cells in the fistulous tract are similar to those in the endometrium, suggesting a possible role of endometriosis in some cases and hormonal manipulations as medical treatment of VUF, with the induction of amenorrhea.[5]
Cystogram is mandatory to make a diagnosis of VUF. Moreover, CT and MRI are useful to improve fistula typification. We performed contrast-enhanced CT to visualize the entire anatomy of the urinary system and evaluate, in particular, the presence of an ureteral injury and a consequent concomitant ureteral fistula, which cannot be detected by a cystogram alone. Moreover, cystogram was reported to be inconclusive in some series, with a sensitivity of 40%,[6] probably because of the higher intrauterine pressure against the intravesical pressure. MRI is usually performed because it is a noninvasive method that can help understand the exact location of the fistula (above or below the uterine isthmus) and the postsurgical fibrosis of the nearby soft tissue, which is important in the choice and planning of the treatment.[6–8]
Treatment is divided into conservative and surgical approaches. Conservative management consists mainly of bladder catheterization, whereas no consensus is given about modality (suprapubic versus urethral catheter) and timing. As a minimally invasive treatment, electrocauterization and endoscopic application of tissue sealants and fibrin glue have been proposed with a success rate of 5%.[1–3]
The surgical approach is considered the gold standard for VUF definitive treatment, but there is no consensus on timing, the diagnosis being intra-operatively elusive.[3] Nearly 100% success rate has been reported for the surgical treatment of VUF;[1] in contrast, it potentially involves a hysterectomy risk, raising the psychological concern for the patients. Regarding the surgical technique, fistula repair could be made by the transvesical-transperitoneal route with tissue flap interposition, usually omental or peritoneal tissue.
In the light of this, Lipogems® mimics a flap and helps the healing of the tissue with a regenerative stimulus. To date, the use of cells or tissues with regenerative properties, such as mesenchymal stem cells (MSCs), or fat with mesenchymal properties (adipose-derived mesenchymal stem cells [ASCs]) has become a wide field of research for the treatment of several medical and surgical conditions.[9–11] MSCs seem to be able to induce the secretion of mitogenic and immunomodulatory factors,[12] whereas ASCs either expanded or obtained by enzymatic modality have shown regenerative and immunomodulatory functions both in vitro and in vivo.[13] Nevertheless, the enzymatic modality or cell expansion has several constraints. Hence, a minimally manipulated autologous adipose tissue, such as Lipogems®, would be preferable as a therapeutic option. For these reasons, we think our technique may be an alternative to the surgical approach, especially for small-diameter VUFs and when the patient refuses a more invasive approach.
Follow-up depends on the kind of approach. Cystogram is not performed before 3–8 weeks in different conservative series, whereas it is usually performed 2 weeks after the surgery.[1] More recently, a systematic review compared shorter (<10 days) and longer (>10 days) durations of bladder catheterization after the surgical repair of urinary obstetric fistula, showing no statistically significant difference in terms of fistula repair breakdown, urinary tract infection, post-repair urinary incontinence, urinary retention, or extended hospital stay.[14]
Fertility and pregnancy after treatment could represent a substantial concern for women suffering from VUF. Pregnancy rates after VUF surgical treatment reported in the literature range from 25% to 37.5%, and spontaneous conception seems to be not adversely affected by VUF in the case series published by Rajamaheswari et al.[1,15] However, adequate counseling is mandatory in this condition.
In conclusion, the presented minimally invasive technique for repairing a VUF is safe, feasible, and effective especially for small-diameter fistulas (<1 cm) and is suitable as an intermediate treatment between conservative management and the more invasive surgical corrections. The use of autologous tissue in uterus-sparing fistula management should be considered as an alternative option in patients for whom maintenance of fertility is advocated.
Main Points:
Vesicouterine fistula is the rarest type of urogenital fistula, and its cause is mainly iatrogenic.
Surgical complications include hysterectomy, raising several cultural and quality-of-life–related implications.
Fragmented autologous adipose tissue mimics a flap in the surgical treatment of fistulas and helps the healing of the tissue with a regenerative stimulus.
As a minimally invasive treatment, endoscopic transurethral resection of the fistulous tract and injection of microfragmented autologous adipose tissue proved to be successful with no complication.
This technique is safe, feasible, and effective and should be considered for small-diameter fistulas (<1 cm) refractory to conservative therapy.
Footnotes
Informed Consent: Written informed consent was obtained from patient who participated in this case.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.G.S.; Design - M.G.S., F.P., C.T.; Supervision - P.G.D.O., E.M.; Resources - M.G.S.; Materials - V.L., M.M.; Data Collection and/or Processing - V.L.; Analysis and/or Interpretation - M.G.S., F.P., P.G.D.O.; Literature Search - V.L., M.M.; Writing Manuscript - V.L., F.P.; Critical Review - F.P., M.G.S.; E.M.; Other - F.P., C.T., E.M..
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Symeonidis EN, Sdralis E, Symeonidis A, Georgiadis C, Kalyvas V, Malioris A, et al. Vesicouterine Fistula (VUF) as a Rare Urogenital Complication Managed with Delayed Surgical Repair: A Case Report and Review of the Literature. Case Rep Obstet Gynecol. 2018;2018 doi: 10.1155/2018/2394896. 2394896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youssef AF. Menouria following lower segment cesarean section; a syndrome. Am J Obstet Gynecol. 1957;73:759–67. doi: 10.1016/0002-9378(57)90384-8. [DOI] [PubMed] [Google Scholar]
- 3.Hadzi-Djokic JB, Pejcic TP, Colovic VC. Vesico-uterine fistula: report of 14 cases. BJU Int. 2007;100:1361–3. doi: 10.1111/j.1464-410X.2007.07067.x. [DOI] [PubMed] [Google Scholar]
- 4.Bettez M, Breault G, Carr L, Tule M. Early versus delayed repair of vesicouterine fistula. Can Urol Assoc J. 2011;5:E52–5. doi: 10.5489/cuaj.10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokoyama M, Arisawa C, Ando M. Successful management of vesicouterine fistula by luteinizing hormone-releasing hormone analog. Int J Urol. 2006;13:457–9. doi: 10.1111/j.1442-2042.2006.01325.x. [DOI] [PubMed] [Google Scholar]
- 6.Abou-El-Ghar ME, El-Assmy AM, Refaie HF, El-Diasty TA. Radiological diagnosis of vesicouterine fistula: role of magnetic resonance imaging. J Magn Reson Imaging. 2012;36:438–42. doi: 10.1002/jmri.23667. [DOI] [PubMed] [Google Scholar]
- 7.Smayra T, Ghossain MA, Buy JN, Moukarzel M, Jacob D, Truc JB. Vesicouterine fistulas: imaging findings in three cases. AJR Am J Roentgenol. 2005;184:139–42. doi: 10.2214/ajr.184.1.01840139. [DOI] [PubMed] [Google Scholar]
- 8.Shanmugasundaram R, Gopalakrishnan G, Kekre NS. Youssef’s syndrome: Is there a better way to diagnose? Indian J Urol. 2008;24:269–70. doi: 10.4103/0970-1591.40631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gimble JM, Guilak F, Bunnell BA. Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther. 2010;1:19. doi: 10.1186/scrt19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.e02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 12.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–5. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–49. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 14.Torloni MR, Riera R, Rogozińska E3, Tunçalp Ö, Gülmezoglu AM, Widmer M. Systematic review of shorter versus longer duration of bladder catheterization after surgical repair of urinary obstetric fistula. Int J Gynaecol Obstet. 2018;142:15–22. doi: 10.1002/ijgo.12462. [DOI] [PubMed] [Google Scholar]
- 15.Rajamaheswari N, Chhikara AB. Vesicouterine fistulae: our experience of 17 cases and literature review. Int Urogynecol J. 2013;24:275–9. doi: 10.1007/s00192-012-1798-8. [DOI] [PubMed] [Google Scholar]