Abstract
Focused ultrasound (FUS) is a promising technology for facilitating treatment of brain diseases including chronic pain. Focused ultrasound is a unique modality for delivering therapeutic levels of energy into the body, including the central nervous system (CNS). It is non-invasive and can target spatially localized effects through the intact skull to cortical or subcortical regions of the brain. FUS can achieve three different mechanisms of action in the brain that are relevant for chronic pain treatment: (1) localized thermal ablation of neural tissue; (2) localized and transient disruption of the blood-brain barrier for targeted drug delivery to CNS structures; and (3) inhibition or stimulation of neuronal activity in targeted regions. This review provides an in-depth look at the technology of FUS with emphasis placed on applications to CNS-based treatments of chronic pain. While still in the early stages of clinical translation and with some technical challenges remaining, we suggest that FUS has great potential as a novel approach for manipulating CNS networks involved in pain treatment.
Keywords: brain, pain, focused ultrasound, neural circuits, neuromodulation, neurosurgical ablation, targeted drug delivery, analgesia
Introduction
The experience of pain results from a remarkably complex set of actions and interactions. Under normal conditions a nociceptive stimulus detected at the periphery of the nervous system initiates a signal that is transmitted and modulated through systems in the spinal cord and ultimately integrated into a conscious experience in the brain (Dubin and Patapoutian, 2010; Garland, 2012; Woolf and Ma, 2007). However, in chronic pain, abnormalities in brain function, structure and chemistry may result in persistent pathological pain (Borsook et al., 2018; May, 2008; Vania Apkarian et al., 2013). Many current treatments for chronic pain use drugs targeted to the central nervous system (CNS) in an attempt to either produce symptomatic relief, or as disease modifiers to normalize the pain connectome (i.e., back to the pre-pain state) (Ashburn and Staats, 1999). As many drugs have serious side effects or limited efficacy for certain chronic pain conditions, the use of medical devices is often integrated into patient care. Devices such as Transcranial Magnetic Stimulation (TMS; (Klein et al., 2015; Young et al., 2014)), transcutaneous electrical nerve stimulation (TNS; (DeSantana et al., 2008)), transcranial direct current stimulation (tDCS; (Pinto et al., 2018)); or deep brain stimulation (DBS; (Farrell et al., 2018)) may produce their effects through alteration in brain connectivity, neurochemistry or neuronal activity (Dossantos et al., 2018; Hanlon et al., 2018; Lozano et al., 2019).
Focused ultrasound (FUS) is an emerging technology that has great potential to be added to the set of medical devices used in the treatment of chronic pain. The underlying principle of the technique is contained in the name – a convergence of ultrasound energy to create an amplified effect at one location while leaving the surrounding region unaffected. FUS devices can perform non-invasive and targeted delivery of energy into the brain, even through the intact skull. Ultrasound parameters can be set to achieve one of three primary mechanisms of action in the brain: (1) thermal ablation of tissue; (2) transient disruption of the blood-brain barrier; or (3) excitation/inhibition of neuronal activity. Focused ultrasound treatments in the brain have now entered the clinic (Krishna et al., 2018). The technology is being used successfully for non-invasive neurosurgical ablative treatments (Elias et al., 2013); clinical trials for targeted drug delivery through the disrupted blood-brain barrier have begun (Lipsman et al., 2018; Mainprize et al., 2019); and the first human studies on neuromodulation of brain activity have been published (Lee et al., 2015; Legon et al., 2018). However, applications to chronic pain treatment remain largely unexplored and represent fertile ground for researchers and clinicians to investigate.
A thematic summary of this review is presented in Figure 1. The chronic pain state is depicted as aberrant functioning within, and connections between, particular regions of the brain involved in processing the experience of pain. Focused ultrasound can target these regions for different treatments that attempt to restore their proper functioning and connectivity. In this review, we start a broad overview of chronic pain and provide The Rational for focused ultrasound as a therapeutic strategy to treat chronic pain. We then summarize the Physical Principles of Focused Ultrasound and give a brief perspective on the Historical Development of Focused Ultrasound. Development of the technology culminated in the recent clinical successes of using focused ultrasound for neurosurgical applications, described in the section, Into the Clinic: Transcranial MR-Guided Focused Ultrasound Ablation. Building on the above sections we then move to more recent applications of focused ultrasound: Targeted Delivery of Pharmaceuticals: Focused Ultrasound Disruption of the Blood-Brain Barrier andAltering Neural Circuitry: Focused Ultrasound Neuromodulation. Finally, in the section onClinical Translation and Future Directions for Pain Applications we summarize the current hurdles the technology faces in maximizing its clinical utility and discuss how the technology could be applied to pain applications for symptomatic treatment or disease modification today and in the future. Throughout the review we emphasize the few studies that have specifically looked at applications to pain conditions and other studies that have not been pain specific but have targeted their therapy to regions of the brain known to be a part of the pain network.
Figure 1:
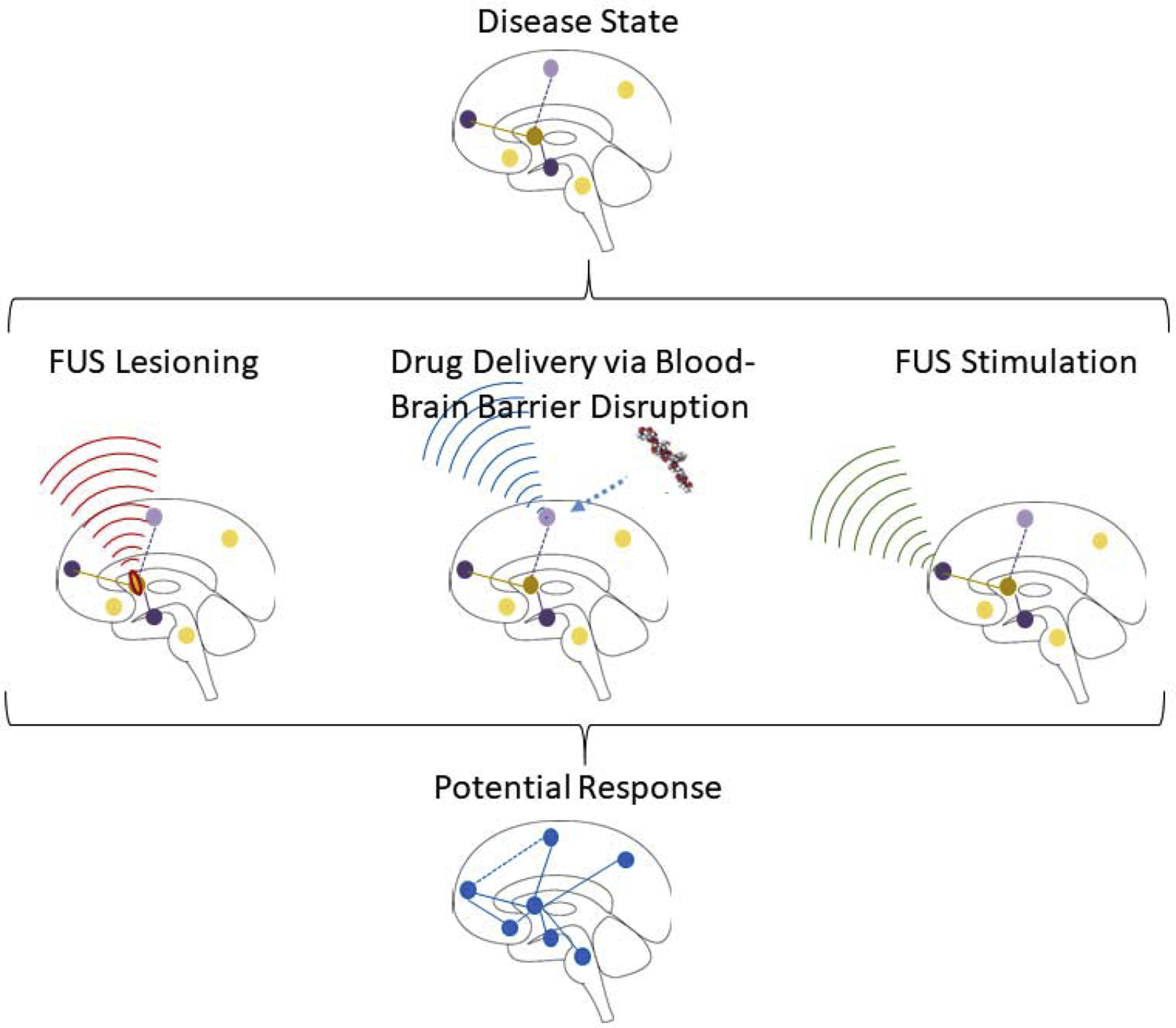
Thematic overview of focused ultrasound treatments for chronic pain. Chronic pain can result in aberrant activity within, and connections between, brain regions that collectively give rise to the experience of pain. Focused ultrasound is uniquely suited to selectively target any of these pain network nodes to deliver a therapy with the goal of normalizing the network back to the pre-pain state.
1. The Rational for focused ultrasound as a therapeutic strategy to treat chronic pain
In 2015, a task force from the International Association for the Study of Pain converged on a definition for chronic pain as a condition with “persistent or recurrent pain lasting longer than 3 months” (Treede et al., 2015). This simple definition reflects the fact that pain enduring past the normal time of healing is no longer performing the necessary function of alerting the body to potential acute harm, but is rather a pathological state that serves no protective purpose. Chronic pain can be classified according to the underlying cause (e.g., cancer), the physiological mechanism (e.g., neuropathic pain), or the perceived location in the body (e.g., headache). The International Association for the Study of Pain task force identified seven classifications of chronic pain: Chronic primary pain, chronic cancer pain, chronic postsurgical and posttraumatic pain, chronic neuropathic pain, chronic headache and orofacial pain, chronic visceral pain, and chronic musculoskeletal pain (Treede et al., 2015). It is estimated that 20% of the global adult population suffers from some form of chronic pain (Goldberg and McGee, 2011), including 100 million Americans (Simon, 2012).
While an initial painful injury can occur anywhere in the body, the transition to chronic pain involves changes to the CNS (Harte et al., 2018; Woolf, 2011). This evolution from acute to chronic pain is considered to be a process where an initial sensory barrage on normal nociceptive circuits evolves into driving changes in other brain circuits, including reward, salience and cognitive networks (Simons et al., 2014). Changes to these various brain networks, collectively referred to as the pain connectome, can manifest as patient behaviors that may include depression, enhanced anxiety or fear of pain (Borsook et al., 2007). It is therefore not always sufficient to treat only the initial injury site in order to resolve chronic pain; the altered connections and chemistry of the affected brain networks must also be brought back to the pre-pain state. The brain regions and pathways identified as being a part of the perception and processing of the pain experience are detailed in Figure 2.
Figure 2:
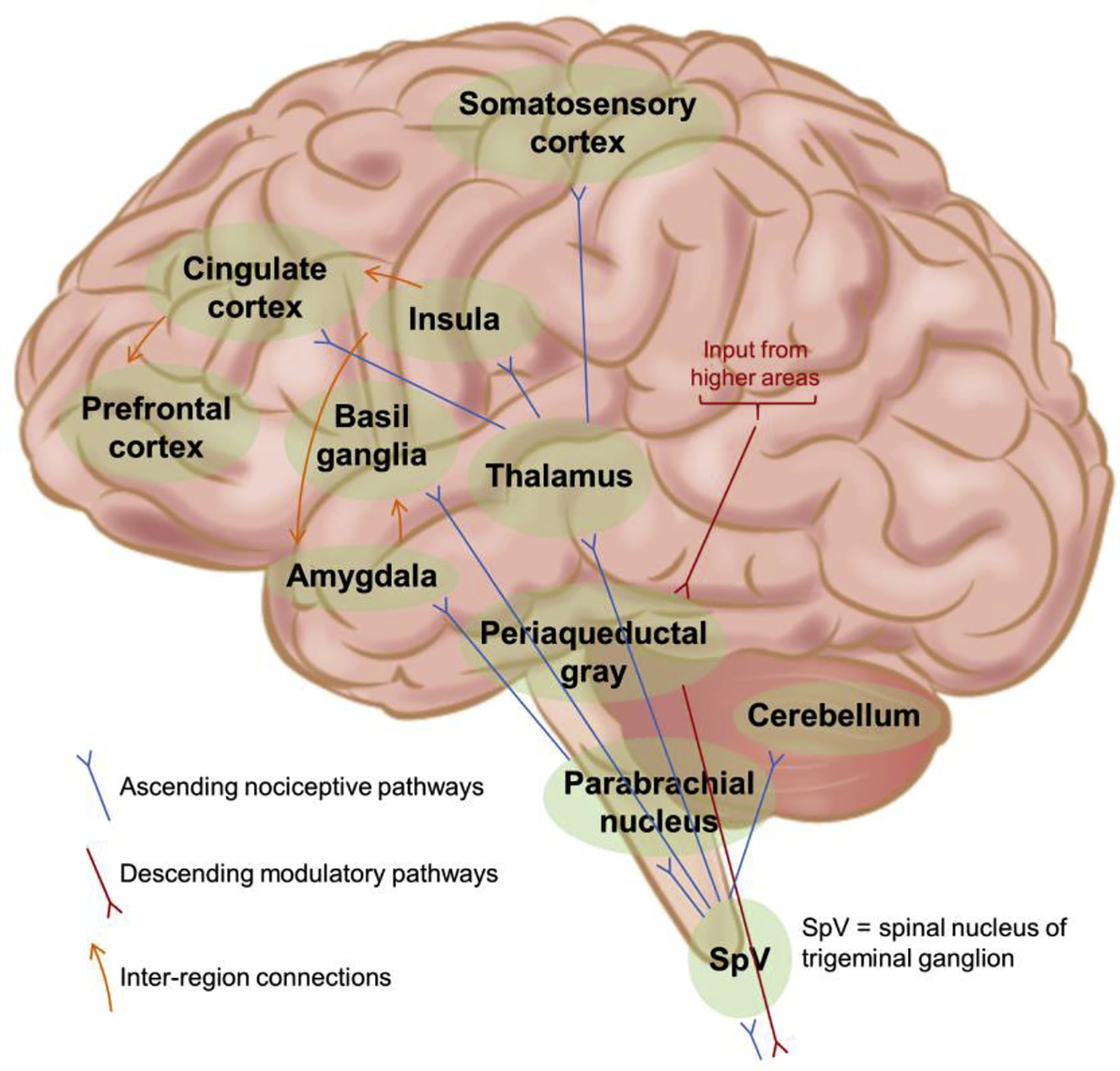
Brain regions and pathways involved in the perception and experience of pain. Nociceptive signals from the periphery travel from the spinal cord into the brain along pathways that include the spinothalamic, spinoparabrachio–amygdaloid and spinoreticulo–thalamic tracts. Information entering the thalamus is relayed to cortical areas that include the primary somatosensory cortex, insula and cingulate cortex. Further connections within the brain to regions such as prefrontal cortex and the amygdala allow for attentional and emotional factors to be incorporated in the experience of pain. Integrated signals from various higher order areas of the brain are transmitted via the periaqueductal gray to the spinal cord to act as descending modulatory influences on the incoming nociceptive signals. Figure adapted from (Simons et al., 2014).
Treating chronic pain has proven to be very difficult (Borsook et al., 2014). To date for most chronic pain patients, treatments are psychological, pharmacological or interventional. Several recent meta-analyses from the Cochrane Database of Systematic Reviews and other sources illustrate that these treatments have varying, and often limited, efficacy. See, for example, reviews on the evidence for gabapentin for neuropathic pain (Wiffen et al., 2017), topical analgesics for the treatment of pain (Derry et al., 2017), opioids for noncancer pain (Busse et al., 2018), various neurostimulation techniques for the management of chronic pain (Hofmeister et al., 2020), or nonpharmacologic therapies for back pain (Chou et al., 2017). Perhaps the issue of needing new and more effective treatments is best considered in the context of the efficacy of opioids and the current opioid crisis that in part has emanated from the lack of alternative treatments available for pain.
This lack of evidence for effective treatment outcomes extends to device-based interventions. Another paper from the Cochrane Database of Systematic Reviews considered the evidence for “non-invasive brain stimulation techniques aim to induce an electrical stimulation of the brain in an attempt to reduce chronic pain by directly altering brain activity” (O’Connell et al., 2014). The technologies considered were repetitive transcranial magnetic stimulation (rTMS), cranial electrotherapy stimulation (CES), transcranial direct current stimulation (tDCS), transcranial random noise stimulation (tRNS) and reduced impedance non-invasive cortical electrostimulation (RINCE). Overall, the review concluded that there was a lack of high-quality evidence to support, or refute, the use of these modalities for effectively treating chronic pain (O’Connell et al., 2014).
Focused ultrasound falls into the domain of manipulation of the CNS from a device-based therapy. While other device-based treatments aimed at normalizing pain-related networks in the brain have yet to demonstrate long lasting success in the treatment of chronic pain, focused ultrasound has several unique features that make it a promising candidate for continued research. As this review will detail, focused ultrasound can deliver energy into the brain that is both localized and deeply penetrating, allowing targeting of pain nodes in cortical and subcortical structures. It further has the flexibility to harness this energy delivery for different mechanisms of action that are not available to existing modalities. Very little research has been done with focused ultrasound on the specific application of pain so it remains to be seen whether these attributes can be translated into successful treatments. There is enough initial promise to make further research into pain applications a worthwhile endeavor.
2. Physical Principles of Focused Ultrasound
A brief overview of the physical principles of ultrasound is given to explain several concepts that appear throughout this review: how FUS energy propagates through tissue to a small volume focused deep in the body and how the FUS energy interacts with biological tissues to produce several different bioeffects. These basic principles are important in the context of utilizing this approach safely in patients. While each of the three methods have been safely used in humans, they all carry potential concerns that need to be carefully deployed as the technology advances for pain treatment.
Ultrasound is a mechanical longitudinal wave in soft tissue, displacing particles in the direction of wave propagation. The particles in the medium oscillate back and forth creating microscopic and rapidly switching regions of compression (high pressure) and rarefaction (low pressure). By definition, an ultrasound wave can have any frequency above 20 kHz, however all of the applications considered here use a frequency between 200 kHz and 3 MHz. The user has control over the center frequency, physical geometry of the ultrasound transducer, input power, pulse duration, pulse repetition frequency, and number of pulses applied. These parameters combine with the physical properties of the tissue to determine the spatiotemporal distribution of energy deposition in the body and the bioeffects on the tissue.
2.1. Absorption and Reflection
As the ultrasound wave travels through tissue it is affected by the phenomena of absorption, reflection, refraction and scattering. Absorption is the process of vibrational energy being converted into heat, which raises the temperature of the tissue. The combination of absorption and scattering attenuates the ultrasound energy as it propagates through the tissue and is the primary factor in determining how deeply the energy can be delivered into the body. Attenuation is highly dependent on frequency and tissue properties. In brain tissue, the intensity of an ultrasound wave at 1 MHz will be attenuated by 50% for each 5 cm of distance traveled (Goss et al., 1979). For comparison, 50% attenuation of visible light in the brain occurs over less than 1 mm (Jacques, 2013). The attenuation of ultrasound energy in skull bone is much more severe, to the extent that only approximately 10% of the original energy remains after traversing the roughly 8 mm thick human skull. Reflections occur when the wave encounters a boundary between two tissue types with differing acoustic impedances (the tissue’s speed of sound multiplied by its density). At soft tissue layers, the ultrasound tissue properties are similar enough that the amount of reflection is only a few percent. At tissue-bone interfaces, the reflected energy is closer to 30% when the wave is normal to the surface and even higher for oblique incidences. The highest reflectance occurs at tissue-gas interfaces, where essentially all of the energy is reflected. Such an interface could be at the skin/air surface, or inside the body if the lungs or other gas-filled regions are encountered.
2.2. Focusing
The focusing of the ultrasound beam is most often achieved by using a transducer, or array of transducers, with a curved geometry. For a spherically curved transducer, the geometric focus of the beam will lie at a distance equal to the radius of curvature. Modern transducers for human transcranial applications consist of many (up to ~1,000) small individual piezoelectric sources arrayed onto a hemispherically shaped surface. The size of the focus is determined by the transducer geometry and operating frequency, with smaller focusing achievable at higher frequencies. A focal diameter of ~2 mm can be achieved for the frequency range used in transcranial FUS applications (roughly 200 kHz to 1.0 MHz). The length of the focus depends on the ratio of the transducer diameter to radius of curvature and can be twice to upwards of 20 times larger than the focal diameter. As an example, the ExAblate 4000 system from InSightec, a transcranial focused ultrasound system with FDA approval, is a hemispherical design with 1,024 elements, 30 cm diameter and 15 cm radius of curvature. It operates at 670 MHz and quotes a focal size of 1.5 × 1.5 × 3.0 mm.
Figure 3 provides a conceptual approach to demonstrating the benefits of focusing. Ultrasound intensity at a particular location is the most important property for eliciting bioeffects. The important point is that intensity is a per area value, specifically quantifying power per unit area (W/cm2) at a given location. The schematic shows a hemispherical transcranial FUS system with a 30 cm diameter and a 15 cm radius of curvature. An assumed power on concentric hemispheres (W) is plotted as a function of radial distance from the transducer, showing attenuation of the energy as it propagates through the water bath, skull and brain tissue. The next plot shows the corresponding hemispherical surface area (cm2) as a function of radial distance from the transducer face, with a minimum surface area at the focus based on the ExAblate 4000 focal diameter of 1.5 mm. The intensity (W/cm2) plot is then obtained as the ratio of these two plots. At the skull surface, the total power in the ultrasound beam is 468 W, but this is spread out over a surface area of 443 cm2 and therefore the intensity impinging on the skull at any location is only 1.06 W/cm2. For reference, the American Institute of Ultrasound in Medicine (AIUM) has proposed 1.0 W/cm2 as a limit below which heating from ultrasonic exposure is not a concern (Fowlkes, 2011). When the ultrasound beam reaches the focus, it’s total power has been attenuated down to only 17.3 W, however this is concentrated over a surface area of only 0.04 cm2 and therefore the intensity at the focus is the much stronger value of 432 W/cm2. It is the ratio of surface areas (approximately 10,000 to 1 for skull to focus in this example) that allows the transmitted ultrasound energy to pass through intervening tissue without having an effect until it reaches the focus where a large effect can be generated.
Figure 3:
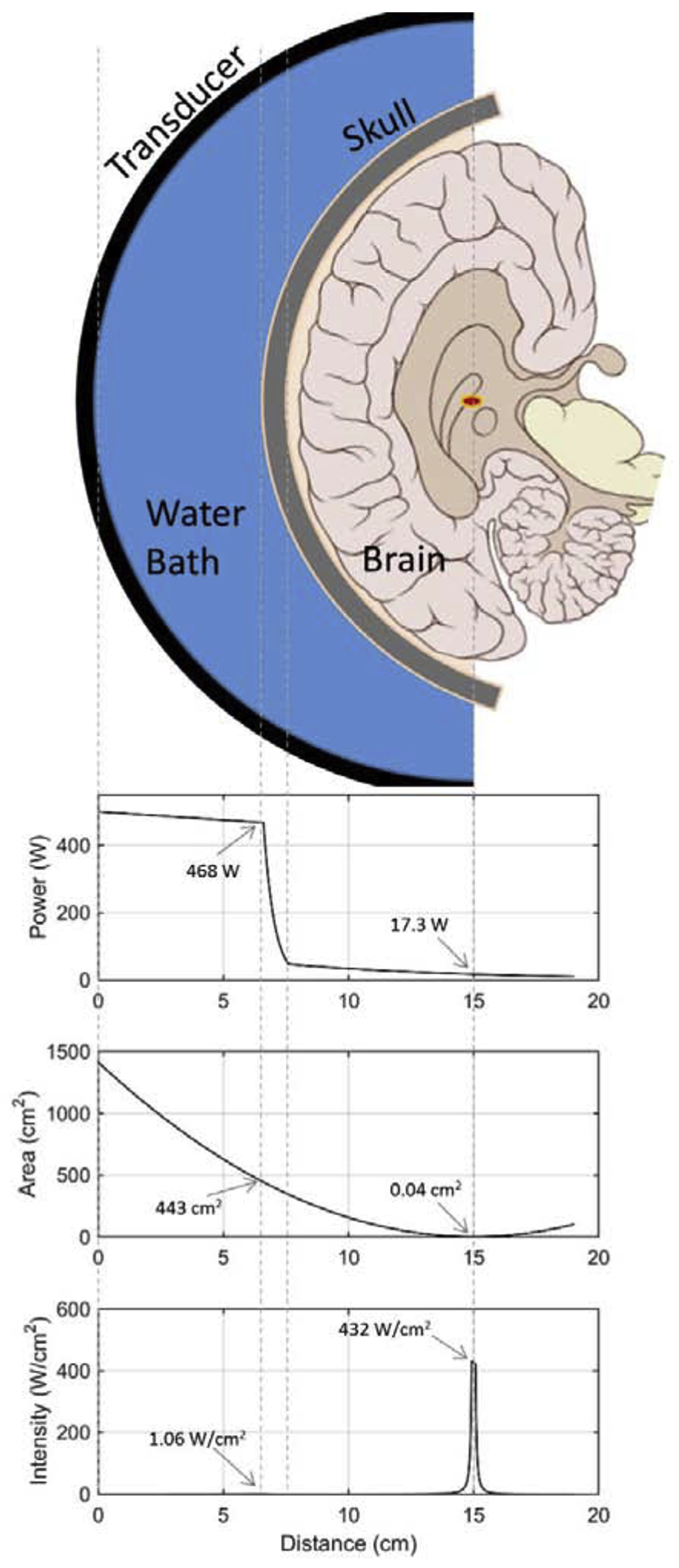
A conceptual demonstration of ultrasound focusing in the brain. The ultrasound energy should be considered along concentric hemispheric surfaces starting at the transducer face and propagating with decreasing radius towards the focus. The first plot shows the total power (W) on these surfaces as a function of radial distance from the transducer where the power gets attenuated sharply by the skull and mildly by the brain tissue. The second plot shows the corresponding surface areas (cm2) of the hemispheres. The bottom plot of ultrasound intensity is obtained by taking the ratio of the power over the area (W/cm2).
2.3. Bioeffects
Ultrasound affects tissue primarily in two ways: the absorbed energy being converted into heat and the pressure wave interacting with the microenvironment to induce local mechanical forces. Heating is the much slower process of the two, occurring on the order of seconds to tens of seconds. The rate of heating depends on the ultrasound intensity, the absorption properties of the tissue (which is frequency dependent), and the thermal properties of the tissue (which are independent of any ultrasound parameters). It is highly influenced by the dissipative effects of the surrounding vasculature. Heating rates in brain tissue can be as high as 1.8 °C/s, achieving a temperature rise from 37°C to 55°C in 10 seconds. The damage done to cells by heating can be quantified by thermal dose (Sapareto and Dewey, 1984), an integrative metric that accounts for both the temperature rise and the duration of elevated temperature. Thermal coagulative necrosis of cells occurs if the temperature reaches ~55–60 °C. Irreversible damage can also occur at lower temperatures held for a longer time.
The mechanical forces most relevant for this review are those that are produced when the ultrasound field interacts with gas-filled microbubbles in the tissue. Microbubbles can be purposefully injected into the blood stream, as is done in imaging applications to improve signal contrast or can be created spontaneously in the tissue when a region of large amplitude negative pressure causes an existing gas pocket to grow into a microbubble. The oscillating pressure field of the ultrasound wave causes the microbubble to expand and contract in size, a process referred to as stable cavitation. The changing size of the microbubble is what creates the forces on the adjacent tissue. If the ultrasound pressure amplitude is too large, the compression cycle of the pressure wave can cause the microbubble to collapse, a much more violent phenomenon known as inertial cavitation that causes shock waves strong enough to damage local tissue. The chain of nucleation, inertial cavitation and cell damage happens at much higher intensities than stable cavitation and is frequency dependent. For example, empirical results in dog muscle show a cavitation threshold of 1,000 W/cm2 at 1 MHz and 2,800 W/cm2 at 3 MHz (Hynynen, 1991).
Mechanical forces can also be exerted on tissue without the presence of microbubbles. Focused ultrasound is known to produce a measurable radiation force at the focus, a push on tissue acting in the direction of propagation that is maintained throughout the sonication duration. Another type of force, acoustic streaming, is the phenomenon whereby ultrasound forces cause a liquid medium to circulate. These forces also act in the direction of propagation and can create movement of free floating small molecules and organelles (Johns, 2002).
3. Historical Development of Focused Ultrasound
Adoption of new technologies across disease entities can proceed in fits and starts. The feasibility of delivering energy to the brain was realized early in the development of FUS, but only very recently has it begun to be applied transcranially for functional brain diseases. It can target superficial or deep regions of the brain to alter behavior (Kubanek, 2018), but still little work has been done to date in chronic pain. The modern study of ultrasonics dates back to World War I when acoustic methods were being developed for underwater detection and communication purposes. Early investigations into possible medical applications soon followed as ultrasound had potential advantages over electromagnetic radiation for the non-invasive delivery of energy into the body. At the wavelengths needed for suitable focusing of the energy (~1 mm), electromagnetic radiation has a very shallow penetration depth and cannot reach deep lying tissue. Much higher electromagnetic frequencies can penetrate deeply, but these fall into the realm of ionizing radiation that would potentially affect all tissues in the beam path, not just at the focus. Ultrasound was seen as having the advantageous combination of being a nonionizing energy source with the capability to achieve deep penetration at the 1 mm wavelength scale necessary for tight focusing of the energy. The early development work was driven by thermal ablation applications as other mechanisms of action were not discovered until much more recently.
Applications of focused ultrasound in the brain faced two major challenges: focusing the energy through the skull and receiving some form of feedback that could aid in targeting and monitoring the treatment. Initially both problems were addressed by performing a craniotomy to create a window for unimpeded propagation of the focused ultrasound and through which to perform ultrasound imaging. The first study to demonstrate that focused ultrasound could destroy brain tissue was done in animals in the early 1940s by Lynn and Putnam (Lynn et al., 1942). The technology was pushed forward during the 1950s through the 1970s by the brothers William and Francis Fry. They demonstrated focused ablation of brain tissue in a primate model and brought the technology into the clinic in an attempt to treat Parkinson’s disease and brain cancer patients (Fry and Fry, 1960; Fry and Meyers, 1962). By the 1970s, the treatments were still done with craniotomy, but the systems now incorporated multiple ultrasound transducers for better focusing, a stereotactic device to help with targeting and ultrasound b-mode imaging to help guide the treatment (Jagannathan et al., 2009). In these early years a handful of other groups also used focused ultrasound with craniotomy for neurosurgical applications, attempting to treat disorders such as brain tumors, depression, epilepsy, and cancer-related pain (Ballantine et al., 1960; Lele, 1967; Lindstrom, 1954; Meyers et al., 1959). The ability to selectively ablate brain tissue with focused ultrasound was established, but the treatments still required craniotomies and thus were far from the goal of complete non-invasiveness.
Two technical advances in the 1990s enabled fully non-invasive treatments: focused ultrasound systems were integrated with magnetic resonance imaging (MRI) scanners for much improved treatment guidance, and phased array transducers were developed to focus the energy through the intact skull. MRI is a much more challenging environment to work in than using ultrasound imaging, but the benefits proved to outweigh the difficulties as images of the brain could now be obtained without having to perform a craniotomy. MRI provides good spatial resolution and superior soft tissue contrast for identification of anatomical landmarks that can guide targeting, and, crucially, MRI is sensitive to temperature changes in tissue so it can provide real time temperature mapping of the targeted region to monitor thermal treatments for safety and efficacy (Poorter et al., 1995). The first demonstration of MR-guided focused ultrasound thermal ablation was performed under the leadership of Kullervo Hynynen and Ferenc Jolesz in the early 1990s (Cline et al., 1992).
The problem of focusing through the skull was solved by the combination of two technological advances. First, transducers were designed to be big enough to spread out the energy impinging on the skull over a large surface. As detailed in Figure 3, this would create relatively low ultrasound intensities on the skull surface even for a large amount of initial total energy. The second challenge, which is not captured in the simple schematic of Figure 3, is that the varying shape and thickness of the skull aberrates the ultrasound beam path such that coherent wave fronts leaving the transducer are no longer coincident and in phase at the focus. This leads to a less sharp and less intense pressure field at the intended focus. This problem was solved by designing transducers made up of hundreds of individual piezoelectric elements that could be independently driven and phased. By applying appropriate corrective phase offsets to the ultrasound field emanating from each element, a sharp focus could be recovered after propagation through the skull (Hynynen and Jolesz, 1998).
Figure 4 shows all of the components of a modern MR-guided transcranial focused ultrasound system. This includes the large phased array transducer, a skull CT image that is used for skull aberration correction, MRI temperature mapping for real-time monitoring and feedback, and high resolution anatomical MRI images for post-treatment evaluation. With the technical pieces in place, a commercial system for MR-guided transcranial focused ultrasound treatments was designed and built by InSightec in the early-2000s and clinical trials for truly non-invasive treatments were ready to commence.
Figure 4.
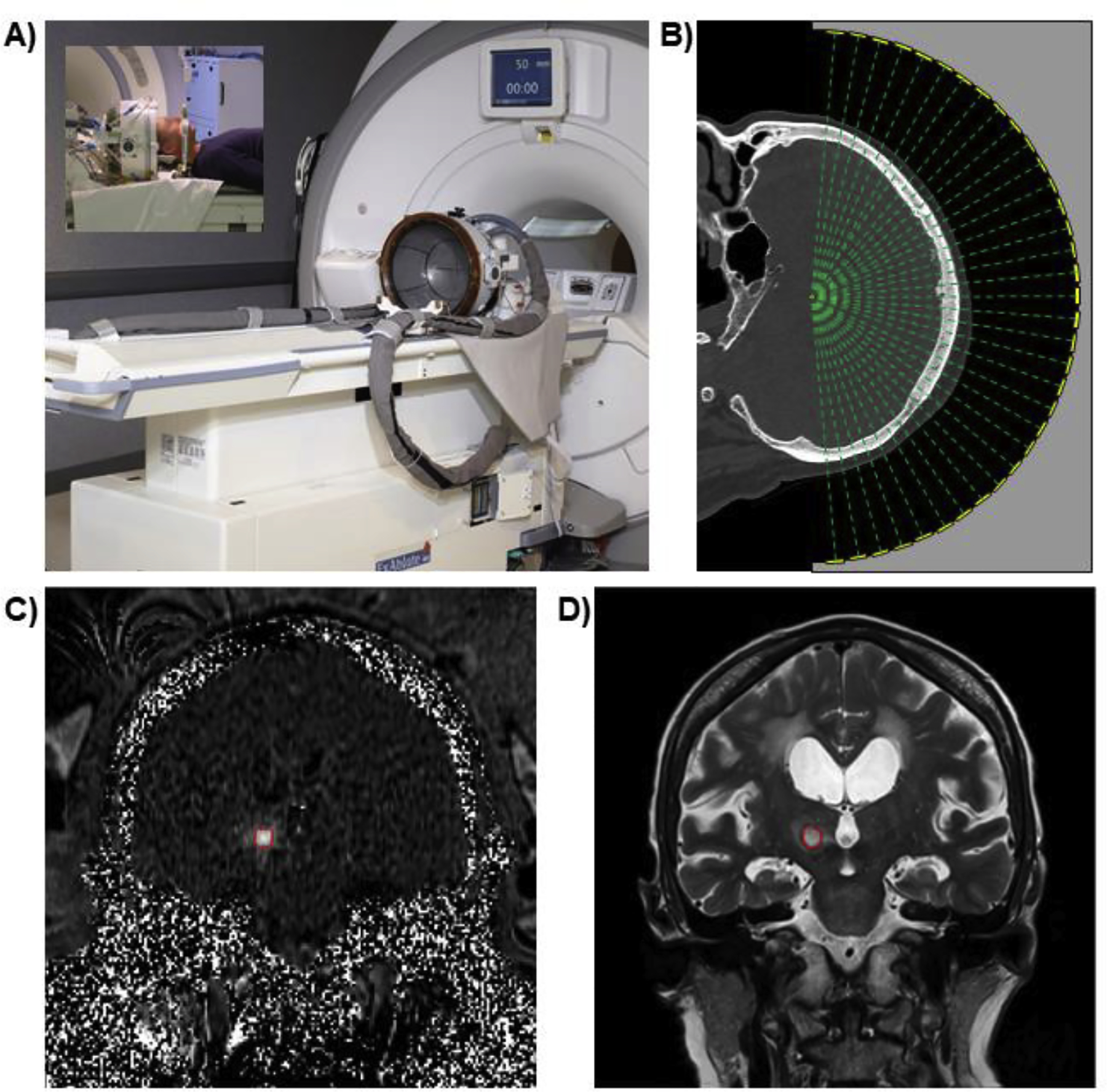
Elements of a modern MR-guided focused ultrasound brain tissue ablation procedure. A) The FDA-approved ExAblate Neuro MR-guided transcranial focused ultrasound system from InSightec. B) Treatment planning step using patient-specific CT for skull aberration correction. C) Real-time temperature mapping during the treatment. D) T2-weighted MRI evaluation of lesion at one day post-treatment.
For a more complete overview of the history of focused ultrasound, see the excellent reviews by Jagannathan et al. (Jagannathan et al., 2009), Harary et al. (Harary et al., 2018) and O’Brien et al. (O’Brien and Dunn, 2015).
4. Into the Clinic: Transcranial MR-Guided Focused Ultrasound Ablation
Of the three primary applications for focused ultrasound in the brain, ablation is the most well understand and furthest advanced for clinical use. The ultrasound is applied continuously at high power and can heat tissue at the focus to above 60 °C in under 30 seconds. It is a completely non-invasive approach to lesioning that could theoretically be used in place of invasive neurosurgical ablation methods.
4.1. Clinical trials
The first patients to undergo a transcranial MR-guided focused ultrasound ablation procedure were treated at Brigham and Women’s Hospital in 2005 (McDannold et al., 2010). The treatments were performed on three patients with inoperable recurrent glioblastoma tumors. The study established the feasibility of focusing energy through the human skull to a targeted location inside the brain while using MRI to measure the temperature rise at the focus and on the brain surface. The experience also revealed the challenges in sonicating a large volume of tissue that was not near the center of the brain (see Section 3.3, Limitations).
The experience in tumors led investigators to turn their attention to functional neurosurgical applications in which only a small volume of white matter near the center of the brain would be targeted for ablation. The first application was to neuropathic pain (see below), followed by treatment of essential tremor. For essential tremor patients, a single lesion was targeted unilaterally to the ventral intermediate nucleus of the thalamus. Results from the first 15-patient clinical trial were published in 2013 demonstrating the safety and efficacy of the procedure. Since the success of these first clinical trials, the scope of treatments has been expanded to include treatments for Parkinson’s disease, epilepsy, neuropsychiatric disorders such as obsessive compulsive disorder and depression, and a return to brain tumors. The treatment for essential tremor received FDA approval in 2016. See Table 2 for a full list of completed and ongoing clinical trials as of December 2019.
Table 2:
FUS Neurosurgery ablation clinical trials. Trials were obtained from clinicaltrials.gov using search for Other terms “ExAblate OR transcranial focused ultrasound OR transcranial MRgFUS”. Up to date through December 2019. VIM = ventral intermediate nucleus, ACC = anterior cingulate cortex.
| Disease | Trial Number | Dates | Patients | Target | Primary Outcome Measure |
|---|---|---|---|---|---|
| Essential Tremor | NCT01932463 | 2011 – 2012 | 6 | Thalamotomy | Accuracy of Thalamotomy lesion |
| NCT01304758 | 2011–2012 | 15 | VIM Thalamus | Adverse events | |
| NCT02037217 | 2011–2014 | 10 | VIM Thalamus | Adverse events | |
| NCT01698450 | 2012 – 2013 | 10 | Thalamus | Lesion size / Safety | |
| NCT01827904 | 2013 – 2017 | 72 | Thalamotomy | Upper limb CRST Subscore | |
| NCT03253991 | 2014 – 2023 | 100 | Thalamotomy | Device Related Complications / Effectiveness | |
| NCT02289560 | 2015 – 2017 | 50 | Thalamotomy | Adverse Events | |
| NCT02252380 | 2015 – 2017 | 10 | VIM Thalamus | Device and Procedure related complications | |
| NCT04074031 | 2019 – 2023 | 15 | VIM Thalamus | Contralateral upper limb tremor at 3-months | |
| NCT03935581 | 2019 – 2020 | 20 | VIM Thalamus | Adverse Events | |
| NCT03465761 | 2019 – 2021 | 30 | Bilateral Thalamotomy | Clinical Rating Scale for Tremor | |
| NCT02692183 | 2018 – 2019 | 100 | Thalamotomy | EEG/ ERP recordings | |
| NCT03560622 | 2018 – 2019 | 30 | Thalamotomy | Connectivity correlates of tremor | |
| Parkinson’s Disease | NCT01772693 | 2012 – 2017 | 27 | VIM Thalamus | Device and Procedure related complications |
| NCT02347254 | 2014 – 2017 | 6 | Pallidotomy | Adverse events | |
| NCT02246374 | 2014 – 2018 | 10 | Subthalamic Nucleus | Device and Procedure related complications | |
| NCT02263885 | 2015 – 2017 | 20 | Globus Pallidum | Device and Procedure related complications | |
| NCT02912871 | 2016 – 2017 | 10 | Subthalamic Nucleus | Adverse events | |
| NCT03454425 | 2018 – 2019 | 40 | Subthalomotomy | Motor MDS-UPDRS score; Adverse events | |
| NCT04002596 | 2017 – 2020 | 50 | Thalamotomy | Clinical Rating Scale for Tremor | |
| NCT03964272 | 2019 – 2020 | 10 | Bilateral subthalamotomy | Adverse events; Motor MDS-UPDRS score | |
| NCT04112381 | 2020 – 2021 | 10 | Bilateral thalamotomy | Adverse events | |
| Dyskinesia | NCT02003248 | 2013 – 2017 | 5 | Pallidotomy | Unified Dyskinesia Rating Scale |
| NCT03319485 | 2018 – 2020 | 116 | Pallidotomy | Unified Dyskinesia Rating Scale | |
| Pain | NCT01699477 | 2008 – 2013 | 30 | Thalamus | Precision of lesioning / Adverse events / Lesion size |
| NCT03309813 | 2017 – 2019 | 10 | Thalamotomy | Device and Procedure related complications | |
| NCT03111277 | 2017 – 2021 | 5 | Thalamus | Adverse events | |
| Brain Tumors | NCT01698437 | 2011 – 2016 | 3 | Brain tumors | Adverse events / Lesion size |
| NCT01473485 | 2011 – 2017 | 10 | Brain gliomas | Adverse events | |
| NCT00147056 | 2012 – 2017 | 10 | Brain tumors | Adverse events | |
| NCT03028246 | 2017 – 2019 | 10 | Benign tumors | Adverse events / Tumor volume | |
| Obsessive Compulsive Disorder | NCT01986296 | 2013 – 2019 | 15 | ACC, internal Capsule, Ventral Striatum or Subgenual Cingulate Cortex | Effectiveness of treatment |
| Depression | NCT02348411 | 2014 – 2017 | 5 | Bilateral Anterior Capsulotomy | Hamilton Depression Rating Scale-17 |
| NCT03421574 | 2018 – 2020 | 12 | Capsulotomy | Adverse events | |
| Epilepsy | NCT02804230 | 2016 – 2019 | 10 | Subcortical focal epileptic target | Device and procedure related complications |
| NCT03417297 | 2018 – 2020 | 10 | Anterior Nucleus | Feasibility; Safety | |
| Graves’ disease | NCT03013257 | 2017 – 2020 | 266 | Thyroid parenchyma | Rate of remission |
4.2. Ablation Pain studies
Chronic pain was among the first conditions that researchers aimed to treat with ablative transcranial MR-guided focused ultrasound. The first trial was carried in nine patients with chronic therapy-resistant neuropathic pain (Martin et al., 2009). The target was the central lateral thalamus, which was successfully heated to between 51 °C and 60 °C by continuous ultrasound sonications lasting 10 to 20 seconds. The sonications were performed under the guidance of real-time MR temperature monitoring and the patients remained awake throughout the procedure. Lesions at the targeted location of 3–5 mm in diameter could be seen on follow up MR imaging immediately after the treatment and at 48 hours later. No adverse events were reported at the time of procedure.
A second paper from the same group expanded their results to 12 patients and included follow up out to one year post treatment (Jeanmonod et al., 2012). They report successful lesioning in 9 out of the 12 patients; the first patient in the study was purposefully heated to only 42 °C and two other patients had lesions that were too small to fully cover the posterior part of the thalamic central lateral nucleus. Those 9 patients experienced clinical benefits from the treatments as measured by their visual analog scale score (49% improvement at 3 months, 57% improvement at 1 year), moderate reduction in drug intake, and some reports of significant somatosensory improvements around the pain area.
Two separate clinical trials using transcranial MR-guided focused ultrasound to treat neuropathic pain patients have begun at the University of Maryland and the University of Virginia in the United States (NCT03111277, NCT03309813). Both trials will enroll therapy-resistant neuropathic pain patients with the goal of lesioning the Central Lateral Thalamic nucleus, each trial aims to treat a total of 10 patients.
4.3. Limitations
One of the major limitations of focused ultrasound ablative treatments is that they are currently constrained to targets only in central regions of the brain. This small treatment envelope is due to the inherent tradeoffs in heating at the focus versus heating at the skull surface. As the target moves closer to the skull, the ratio of skull-to-focus surface areas decreases. The same amount of energy is getting to the focus, but it is passing through a smaller area of skull surface which leads to greater intensity and more skull heating. Expanding the treatment envelope is an area of on-going research (McDannold et al., 2013; Odéen et al., 2014).
Another challenge is treating large volumes of tissue. The dimensions of the focal region were given above as 1.5 × 1.5 × 3.0 mm, a volume of 6.75 mm3, which is ideal for precise lesioning and required in order to achieve the skull-to-focus surface area ratio necessary for reaching high temperatures. However, this volume is about 150 times smaller than the fairly modest volume of 1 cm3. Ablating a volume with that number of repeated sonications would be prohibitively long, primarily due to the time that is allocated to allow the skull to cool. For example, a case report of using focused ultrasound for brain tumor ablation reported using 25 high-power sonications with “cooling periods of several minutes” between sonications to achieve an ablation volume of 0.7 CC in 4 hours of treatment time (Coluccia et al., 2014). Current research is investigating beam steering and focus shaping strategies that can increase the volume treated in a clinically reasonable amount of time.
4. Targeted Delivery of Pharmaceuticals: Focused Ultrasound Disruption of the Blood-Brain Barrier
By modifying the ultrasound parameters towards lower power and shorter bursts repeated at a low duty cycle over a longer duration, it is possible to produce biophysical effects on tissue other than heating. In this section we describe how focused ultrasound can be combined with systemically circulating microbubbles to be used for transient disruption of the blood-brain barrier (BBB).
4.1. Mechanism of action
A seminal paper from 2001 demonstrated that pulsed focused ultrasound applied in the presence of systemically circulating gas-filled microbubbles could open the BBB to agents that normally do not cross from the vasculature into the brain (Hynynen et al., 2001). The hypothesis at the time was that the ultrasound pressure wave would induce the microbubbles into a state of stable cavitation and that this rapid physical oscillation in size would create forces on the vessel walls strong enough to disrupt the tight junctions between endothelial cells that help to form the BBB. Experimental evidence validated that focused ultrasound BBB opening did indeed make the BBB permeable to normally non-penetrant agents. Later studies investigating the underlying mechanisms implicated several changes at the cellular level including disruption of tight junctions, a reduction in drug efflux mechanisms, and an increase in the number of transcytotic vesicles (Aryal et al., 2014; Burgess et al., 2015; Hynynen et al., 2005; Sheikov et al., 2008, 2006, 2004). Figure 5 shows a schematic of a pre-clinical system for focused ultrasound BBB opening, a conceptual diagram of microbubbles oscillating in the blood stream, and MRI contrast imaging for confirmation of cortical BBB disruption in a rat.
Figure 5.
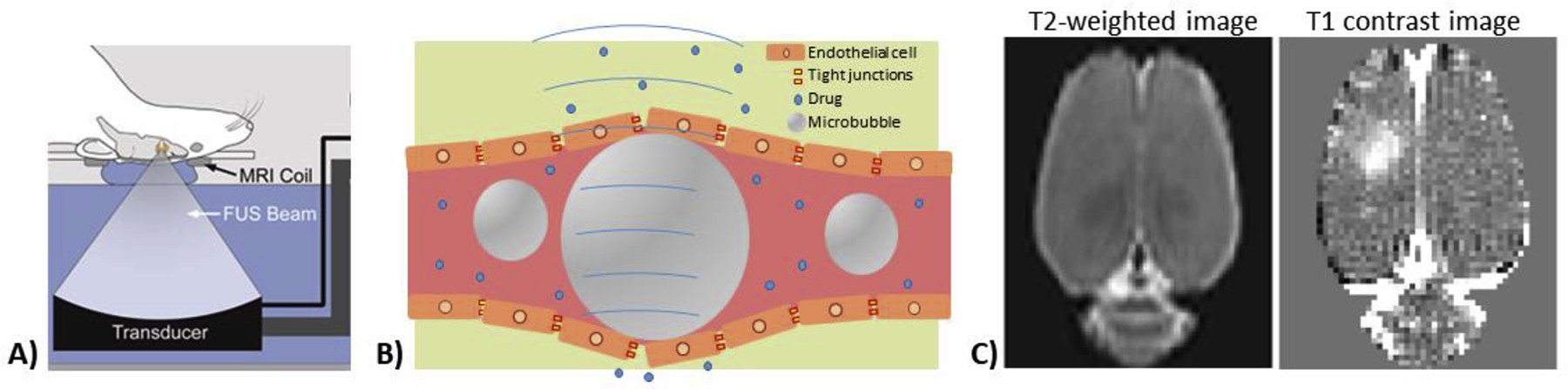
Focused ultrasound BBB opening. A) Pre-clinical MRI-compatible focused ultrasound system. B) Schematic of microbubbles undergoing stable cavitation within blood vessel to induce disruption of the blood-brain barrier. C) Gadolinium-based contrast MRI imaging for characterization of location and extent of BBB opening.
4.2. Extent of BBB Opening, Safety, and Clinical Trials
Focused ultrasound opening of the BBB has been used in pre-clinical studies to deliver a wide range of agents to the brain, from the chemotherapeutic agent Temozolomide with a molecular weight of 194 Da (Wei et al., 2013) up to antibodies as large as ~150 kDa (Aryal et al., 2014; Jordão et al., 2010). For comparison, molecules that are able to naturally cross the intact BBB have a molecular weight under 400 Da and high lipid solubility (Banks, 2009). The BBB remains permeable over several hours, with closing following an exponential decay curve (Conti et al., 2019; Marty et al., 2012; Park et al., 2012). Depending on the ultrasound parameters, MRI contrast agent molecular weight and animal model, BBB opening has been seen to persist for over 24 hours in a few cases (Marquet et al., 2014). The location and extent of BBB opening can be determined in vivo using contrast MRI. Following systemic injection of a gadolinium-based contrast agent, regions of hyperintense signal on a T1-weighted image correspond to locations where the agent has leaked out of the blood stream and into the brain parenchyma. The size of BBB opening matches the dimensions of the ultrasound focal zone. It therefore has the same trade-offs as ablation – very precise opening in a small targeted region can be achieved but opening larger volumes will be time consuming if done in a point-by-point fashion.
Numerous studies in pre-clinical animals have demonstrated the primary safety outcome that focused ultrasound BBB opening can make the BBB permeable without causing damage to vascular or neuronal tissues (Baseri et al., 2010; Downs et al., 2015; McDannold et al., 2012). This includes studies of focused ultrasound BBB opening at titrated ultrasound power levels (Sun et al., 2015), and studies of repeated focused ultrasound BBB opening in rodents (Tsai et al., 2018) and non-human primates (Downs et al., 2015; McDannold et al., 2012). Damage due to thermal heating is not a risk. The ultrasound is applied at lower power and a 1–2% duty cycle so that any heat generated would dissipate before building up to increase the temperature of the tissue. Because heating is not a concern, BBB opening can be targeted to both cortical and sub-cortical regions. The primary risk is inertial cavitation, the rapid collapse of microbubbles that can produce shock waves violent enough to damage tissue. The probability of cavitation occurring increases with higher ultrasound power and lower ultrasound frequency (Shin et al., 2018). It has also been shown recently that focused ultrasound BBB opening induces a local inflammatory response that is resolved after 24 hours (Kovacs et al., 2017). The extent, duration, variation across species, and dependence on ultrasound parameters of this response is still under debate (Kovacs et al., 2018; McMahon and Hynynen, 2018, 2017; Sinharay et al., 2019).
Clinical tests of focused ultrasound BBB opening using a transcranial device from InSightec that is similar to the one used for ablation have begun. The first clinical trials were in Alzheimer’s disease patients, brain tumor patients, and amyotrophic lateral sclerosis patients. In the case of Alzheimer’s disease, the BBB was opened but no agent was delivered. This approach was based on pre-clinical results that showed focused ultrasound disruption of the BBB alone helped to reduce plaque burden in an Alzheimer’s disease mouse model, most likely due to the initiation of an immune response at the site of opening (Burgess et al., 2014; Leinenga and Götz, 2015). The results published on the human studies for Alzheimer’s disease and ALS showed that the BBB could be opened without any adverse events (Abrahao et al., 2019; Lipsman et al., 2018). The study in tumor patients delivered the chemotherapy agent doxorubicin or temozolimide and similarly showed safe opening of the BBB was possible (Mainprize et al., 2019). Additional clinical studies on ultrasound-mediated BBB disruption have used a surgically implanted transducer (Carpentier et al., 2016). A list of completed and ongoing clinical trials using focused ultrasound BBB opening as of December 2019 is shown in Table 3.
Table 3: FUS Blood-Brain Barrier Opening clinical trials.
Trials were obtained from clinicaltrials.gov using search Other terms “(ExAblate AND Blood brain barrier) OR (transcranial focused ultrasound AND Blood brain barrier) OR (transcranial MRgFUS AND Blood brain barrier)”. Up to date through December 2019.
| Disease | Trial Number | Dates | Patients | Target | Primary Outcome Measure |
|---|---|---|---|---|---|
| Brain Tumor | NCT02343991 | 2014 – 2017 | 10 | Brain tumors | Adverse events |
| NCT03322813 | 2018 – 2020 | 15 | Glioblastoma | Adverse events | |
| NCT03551249 | 2019 – 2021 | 20 | Glioma | Adverse events; Feasibility of repeated BBB disruption | |
| NCT03616860 | 2018 – 2021 | 20 | Glioblastoma | Adverse events | |
| NCT03714243 | 2018 – 2020 | 10 | Breast cancer metastases in the brain | Adverse events | |
| NCT03712293 | 2018 – 2021 | 10 | Glioblastoma | Adverse events | |
| NCT03626896 | 2018 – 2019 | 9 | Glioblastoma | Adverse events | |
| NCT04063514 | 2019 – 2020 | 15 | Low grade gliomas | Adverse events | |
| Alzheimer Disease | NCT02986932 | 2016 – 2017 | 6 | Right frontal lobe | Change in contrast enhancement (intensity) following BBB disruption |
| NCT03739905 | 2018 – 2019 | 30 | Not specified | Adverse events | |
| NCT03119961 | 2017 – 2019 | 10 | Not specified | Florbetapir SUVr and Fluorodeoxyglucose MUV changes | |
| NCT03671889 | 2018 – 2020 | 10 | Not specified | Adverse events | |
| Amyotrophic Lateral Sclerosis | NCT03321487 | 2018 – 2019 | 8 | Primary motor cortex | Adverse events |
| Parkinson’s disease | NCT03608553 | 2019 – 2020 | 10 | Parieto-occipito-temporal cortex | Adverse Events |
4.3. Pain Studies
To our knowledge, no focused ultrasound BBB opening studies have been published that were carried out in animal models of chronic pain or with the goal of alleviating acute pain. Our group has investigated the possibility of delivering gamma aminobutyric acid (GABA) through the disrupted BBB to the rat brain somatosensory cortex as a way of suppressing neuronal activity (Todd et al., 2019). This was done is healthy rats undergoing non-noxious electrical hind paw stimulation. Functional MRI measurements demonstrated that the BBB opening alone had a suppressive effect on the functional response to stimulation and GABA delivery further attenuated the response. We are currently pursuing similar experiments in the spared nerve injury model of chronic pain in rats (Decosterd and Woolf, 2000) and plan to investigate the effects of GABA delivered to both cortical and thalamic targets. We hypothesize that GABA induced suppression of activity in a critical node of the pain network will work as an analgesic. Unlike focused ultrasound for thermal ablation, the application of BBB opening is not restricted to central areas of the brain. BBB opening can be achieved in deep sub-cortical structures as well as cortical regions.
There have been several studies applying focused ultrasound to open the blood-spinal cord barrier (Payne et al., 2017; Weber-Adrian et al., 2015). This means that agents could be targeted for delivery to essentially any point in the pain pathway in the CNS. This approach could allow for targeted delivery of an analgesic to a particular hub in the pain network. Another possibility would be the ability to give a lower systemic dose of a drug that has adverse side effects in the periphery while still achieving a therapeutic effect due to the increased delivery at the targeted region in the brain. Given the lack of use of FUS in pain studies, targeted delivery of analgesics and other chronic pain therapeutics by focused ultrasound BBB opening is an area of fertile research.
5. Altering Neural Circuitry: Focused Ultrasound Neuromodulation
It has been known for some time that ultrasound can be used to manipulate the activity of peripheral nerves. Ultrasound has been used for both peripheral nerve stimulation and peripheral nerve conduction block. Peripheral applications will not be covered here; see the excellent reviews by Bobola et al. and Blackmore et al. (Blackmore et al., 2019; Bobola et al., 2018). Accumulating evidence now suggests that focused ultrasound can also be used to directly affect the activity of neurons in the brain. Modulation of neuronal function is achieved by applying the focused ultrasound in repeated bursts at lower power, similar to the case of BBB opening but without the use of microbubbles. The phenomenon is caused by the direct action of ultrasound-induced mechanical forces on cells. This application is the newest and least well understood of the three, but a picture is starting to emerge of how focused ultrasound can be applied directly to alter brain function.
5.1. Mechanism of action
Reversible effects that ultrasound may have on neuronal function has fascinated neuroscientists for over 50 years (Ballantine et al., 1960; Fry et al., 1958), and this application has received renewed interest in the last decade. There is in vitro and in vivo empirical evidence that direct application of bursts of focused ultrasound can both excite and suppress neuronal activity, depending on the specific parameters used. The evidence includes in vitro work on brain slices and individual neurons (Choi et al., 2013; Khraiche et al., 2008; Kim et al., 2017; Tyler et al., 2008), in vivo work stimulating the motor cortex of rodents (King et al., 2013; Ye et al., 2016; Yoo et al., 2011), and in vivo work in non-human primates (Deffieux et al., 2013; Folloni et al., 2019; Kubanek et al., 2019; Verhagen et al., 2019; Yang et al., 2018) and humans (Lee et al., 2016b, 2016a, 2015; Legon et al., 2014). These studies were done using a wide range of ultrasound parameters and readout modalities, and although they each showed an effect, there is not yet a theoretical framework for understanding how the effect is generated. Most studies have been performed at a low ultrasound power level and duty cycle combination that does not heat the tissue appreciably and therefore excludes heating as a cause (Constans et al., 2018), however others have reported thermally-induced suppression (Darrow et al., 2019). Other recent studies have all but ruled out the possibility that the motor responses seen in rodents were due exclusively to a startle response through the auditory pathway (Mohammadjavadi et al., 2019).
It is known that ultrasound can impart a mechanical force on the lipid bilayer of cell membranes. Several papers have modelled the biophysics of this interaction to provide a theoretical framework for understanding how ultrasound may affect the electrical activity of neuronal cells (Krasovitski et al., 2011; Naor et al., 2016; Plaksin et al., 2016). These models predict that absorption of ultrasound mechanical energy leads to oscillations of the intramembrane space (Krasovitski et al., 2011). These deformations could lead to several effects that would explain neuronal excitation or inhibition, including changes to the membrane’s capacitance that could drive a current, the opening of pores in the membrane lipid bilayer through which ions could flow, or the activation of mechanosensitive ion channels (Blackmore et al., 2019). There is currently the most empirical evidence for the mechanism of activation of mechanosensitive ion channels. Studies have demonstrated the effect on voltage-gated sodium and calcium channels in an in vitro preparation (Tyler et al., 2008), sodium and potassium channels in xenopus oocytes (Kubanek et al., 2016), the MEC-4 ion channel in Caenorhabditis elegans (Kubanek et al., 2018), opening of TRPA1 channels in astrocytes that leads to release of glutamate and activation of nearby neurons (Oh et al., 2019) and TRP-4 channels expressed in ASH neurons of Caenorhabditis elegans (Ibsen et al., 2015). More work remains to be done to determine which of these is the primary mechanism of action and what are the optimal ultrasound parameters for initiating the effect.
5.2. Non-Human Primate and Human Studies
Based on the promising results from in vitro and rodent work, several groups have investigated using focused ultrasound neuromodulation in non-human primate and human studies. In one of the first studies to demonstrate a behavioral effect, Deffieux et al. showed that focused ultrasound could modulate the latency time of an antisaccade task in awake rhesus macaque monkeys (Deffieux et al., 2013). Non-human primate studies using functional MRI as their readout have demonstrated that focused ultrasound can induce a response in somatosensory cortex similar to what is produced by traditional tactile stimulation (Yang et al., 2018), and can modify brain functional connectivity by targeting areas such as the supplementary motor area, frontal polar cortex (Verhagen et al., 2019), anterior cingulate cortex, or amygdala (Folloni et al., 2019). Importantly, these last two papers reported that the effects of functional connectivity modulation lasted for tens of minutes after the ultrasound had been applied. Further work from this group demonstrated that stimulation of the anterior cingulate cortex could modulate the outcome of a counterfactual choice task (Fouragnan et al., 2019).
Thus far, studies in humans have mostly shown effects related to sensory stimulation and perception, with some reports of modulation of task performance. Legon et al. showed that focused ultrasound applied to the somatosensory cortex could modulate the evoked potential response to median nerve stimulation (Legon et al., 2014). This same study reported that the ultrasound sonications could alter performance on touch and frequency discrimination tasks. Separate studies reported that focused ultrasound applied to the primary somatosensory cortex could induce perceptions in the hand and finger such as numbness or tingling (Lee et al., 2015), while application to the visual cortex produced the phenomenon of visual phosphenes, the experience of seeing white light without light actually entering the eye (Lee et al., 2016b). Subcortical regions have also been targeted, where ultrasound applied to thalamic regions showed an effect on EEG and somatosensory evoked potential measurements of median nerve stimulation (Legon et al., 2018).
A summary of focused ultrasound neuromodulation applications currently in clinical trials is shown in Table 4. Epilepsy is the first neurological disorder that researchers are aiming to treat. Results have not yet been published on these studies. Other clinical treatment applications include Alzheimer’s disease, where patients with mild cognitive impairments will receive four successive low intensity pulsed ultrasound treatments while undergoing fMRI scans (NCT03347084); ultrasound targeted to the thalamus as a “neurorestorative stimulation” method for patients in conditions of coma, Vegetative State, or Minimally Conscious State (NCT02522429); and amygdala targeting for treatment of anxiety (NCT03782194).
Table 4: FUS neuromodulation clinical trials.
Trials were obtained from clinicaltrials.gov using search Other terms “Focused ultrasound AND (modulation OR neuromodulation OR stimulation)”. Up to date through December 2019.
| Disease | Trial Number | Dates | Patients | Target | Primary Outcome Measure |
|---|---|---|---|---|---|
| Epilepsy | NCT03868293 | 2019 – 2020 | 10 | Temporal lobe | Patient tolerability; Reducing seizure frequency |
| NCT03657056 | 2019 – 2021 | 3 | Temporal lobe | Change in BOLD fMRI signal during sonication | |
| NCT02151175 | 2014 – 2020 | 12 | Temporal lobe | Absence of histological changes | |
| NCT03860298 | 2019 – 2019 | 6 | Site of surgery | Safety and tolerability | |
| NCT04192149 | 2019 – 2021 | 300 | Various | Electrophysiological Changes as a result of Ultrasound | |
| Neuroscience | NCT03192436 | 2018 – 2021 | 80 | Motor/Somatosensory | Changes in EEG signal |
| NCT03634631 | 2017 – 2018 | 5 | Cortical/Subcortical | BOLD signal | |
| NCT03882931 | 2019 – 2020 | 90 | DL prefrontal cortex | Performance Error on Visuomotor Task | |
| NCT03717922 | 2019 – 2022 | 50 | Entorhinal cortex | Rey Auditory Verbal Learning Task | |
| NCT04168086 | 2019 – 2020 | 45 | Cerebellum | Performance Error on Visuomotor Task | |
| NCT04168762 | 2019 – 2020 | 80 | Motor cortex | Change in Motor Evoked Potential Amplitude | |
| NCT04190940 | 2019 – 2021 | 39 | Thalamus (ablation) | Performance Error on Visuomotor Task | |
| Alzheimer’s disease | NCT03347084 | 2018 – 2020 | 8 | Hippocampus | Change from baseline in fMRI brain scan |
| Acute Brain Injury | NCT02522429 | 2015 – 2019 | 15 | Thalamus | fMRI; Coma Recovery Scale Revised |
| Psychiatric Disorders | NCT03782194 | 2019 – 2022 | 50 | Amygdala | Emotion Reactivity |
| NCT02685488 | 2015 – 2016 | 26 | Right fronto-temporal | Depressive Symptoms Assessed With the Beck Depression Inventory-II |
5.3. Studies targeting pain-related CNS regions
Almost no work has been done using focused ultrasound to stimulate or suppress brain activity for the applications of alleviating acute or chronic pain. The closest study was one by Hameroff et al. in which a standard 8 MHz imaging ultrasound probe was used in B mode to try to target the frontal cerebral cortex through the temporal bone in chronic pain patients (Hameroff et al., 2013). The authors measured a subjective measure of pain, a rating for mood, and several physiological readouts after ultrasound and placebo trials, and report a few differences that just meet the significant threshold of p < 0.05 (uncorrected for multiple comparisons), including the rating for mood.
Despite not addressing the issue of pain directly, several of the studies reviewed above applied ultrasound stimulation to regions of the brain that are known to be involved in the experience of pain. This includes primary somatosensory cortex, frontal cortical regions, thalamic targets, and the amygdala. The ability of focused ultrasound to reach subcortical regions is one of its main advantages over other stimulation modalities. Stimulation or suppression of regions such as the habenula, nucleus accumbens or amygdala would have high relevance for pain studies. Also of note are the recent studies showing that modulation of functional connectivity between brain regions lasts for tens of minutes after the sonications were applied (Folloni et al., 2019; Verhagen et al., 2019). Most studies measure the effects of focused ultrasound stimulation as it is being applied, but a longer lasting effect indicates that we need further research into how the ultrasound sonications are altering the local environment and how these changes can be harnessed for treatments.
5.4. Indirect approaches for ultrasound neuromodulation
Beyond direct sonication, several groups are looking at ways to use focused ultrasound to indirectly modulate brain activity. One approach is to target neuromodulatory drugs to a specific brain region. This can be achieved by giving a systemic dose of a BBB-permeable drug packaged in a carrier and then using focused ultrasound to “uncage” it from the carrier at the desired location (Airan et al., 2017), or by a systemic dose of a free non-BBB permeable drug and using focused ultrasound to open the BBB at the targeted location (Todd et al., 2019). Another application is to use focused ultrasound BBB opening to deliver existing methods for neuromodulation such as optogenetics (Wang et al., 2017) or chemogenetics (Szablowski et al., 2018). Finally, based on the idea that ultrasound can activate mechanosensitive ion channels, several groups are investigating the possibility of “sonogenetics” where genes for optimally responsive mechanosensitive ion channels would be delivered to the brain and expressed in neurons (Huang et al., 2020; Maresca et al., 2018; Ye et al., 2018). This would allow their activation by ultrasound in the same way that optogenetics relies on light activation, with all of the pros and cons that come with ultrasound energy delivery versus light energy delivery.
6. Clinical Translation and Future Directions for Pain Applications
Each of the three ways discussed here that focused ultrasound is used in the CNS, ablation, BBB disruption and neuromodulation, has made it to human use. They can each be applied, under a specific set of parameters, in a safe manner that has not yet led to any adverse events. In this sense they have achieved clinical translation. The challenge now is to expand the range of ultrasound protocols that can be safely used and thereby unlock new applications for the technology. This includes more regions of the brain that can be targeted for ablation, transducers optimally designed for BBB opening and neuromodulation applications where improved ease of use is required for multiple repeated treatments, methods for targeting and monitoring of the ultrasound energy deposition that do not require MRI, and optimization of ultrasound parameters that can reliably induce neuronal stimulation and suppression.
While some of these improvements may be a few years away, there are still many ways in which the technology of focused ultrasound in its current state can be applied for the treatment of chronic pain. As noted above, there have only been a few ablation studies targeting pain, while BBB-opening and neuromodulation remain almost entirely unexplored. Of all the approaches to manipulating brain physiology, focused ultrasound provides the unique combination of being non-invasive, highly localized and able to reach deep lying structures. These attributes, combined with the flexible ways that it can be deployed to achieve different effects on brain tissue, make it a very attractive candidate for developing novel treatments of chronic pain.
Table 1:
FUS mechanisms of action in the brain.
| Acoustic Power | Pulse Duration / Repetition Frequency / Number Repeats | Mechanism of action | Duration of effect | |
|---|---|---|---|---|
| Ablation | 1000 W | 15 s / Continuous / 1 | Energy absorption converted into thermal heating | Permanent |
| BBB Opening | 10 – 50 W | 10 ms / 1 Hz / 100 | Mechanical forces due to expansion and contraction of microbubbles | 4 – 8 hours |
| Neuromodulation | 10 – 50 W | 1 ms / 500 Hz / 150 | Likely mechanical forces on cell membranes opening mechanical-sensitive ion channels. | 10s ms to minutes |
Highlights.
Focused ultrasound treatments in the brain have recently entered the clinic.
Applications to the treatment of chronic pain are currently understudied.
The technology can target aberrantly functioning nodes of the pain network.
A review of the pre-clinical and clinical literature suggests an opportunity.
Acknowledgements
This review was supported by The Hereditary Disease Foundation, The Brigham Research Institute, and NIH grant K01EB023983.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahao A, Meng Y, Llinas M, Huang Y, Hamani C, Mainprize T, Aubert I, Heyn C, Black SE, Hynynen K, Lipsman N, Zinman L, 2019. focused ultrasound. Nat. Commun 1–9. 10.1038/s41467-019-12426-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airan RD, Meyer RA, Ellens NPK, Rhodes KR, Farahani K, Pomper MG, Kadam SD, Green JJ, 2017. Noninvasive Targeted Transcranial Neuromodulation via Focused Ultrasound Gated Drug Release from Nanoemulsions. Nano Lett 10.1021/acs.nanolett.6b03517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal M, Arvanitis CD, Alexander PM, McDannold N, 2014. Ultrasound-mediated blood-brain barrier disruption for targeted drug delivery in the central nervous system. Adv. Drug Deliv. Rev 72, 94–109. 10.1016/j.addr.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburn MA, Staats PS, 1999. Management of chronic pain. Lancet. 10.1016/S0140-6736(99)04088-X [DOI] [PubMed] [Google Scholar]
- BALLANTINE HT, BELL E, MANLAPAZ J, 1960. Progress and problems in the neurological applications of focused ultrasound. J. Neurosurg 10.3171/jns.1960.17.5.0858 [DOI] [PubMed] [Google Scholar]
- Banks WA, 2009. Characteristics of compounds that cross the blood-brain barrier, in: BMC Neurology. 10.1186/1471-2377-9-S1-S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseri B, Choi JJ, Tung YS, Konofagou EE, 2010. Multi-modality safety assessment of blood-brain barrier opening using focused ultrasound and definity microbubbles: A short-term study. Ultrasound Med. Biol 10.1016/j.ultrasmedbio.2010.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore J, Shrivastava S, Sallet J, Butler CR, Cleveland RO, 2019. Ultrasound Neuromodulation: A Review of Results, Mechanisms and Safety. Ultrasound Med. Biol 10.1016/j.ultrasmedbio.2018.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobola MS, Chen L, Ezeokeke CK, Kuznetsova K, Lahti AC, Lou W, Myroniv AN, Schimek NW, Selby ML, Mourad PD, 2018. A Review of Recent Advances in Ultrasound, Placed in the Context of Pain Diagnosis and Treatment. Curr. Pain Headache Rep 10.1007/s11916-018-0711-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Carlezon WA, Shaw M, Renshaw P, Elman I, Levine J, 2007. Reward-aversion circuitry in analgesia and pain: Implications for psychiatric disorders. Eur. J. Pain 10.1016/j.ejpain.2005.12.005 [DOI] [PubMed] [Google Scholar]
- Borsook D, Hargreaves R, Bountra C, Porreca F, 2014. Lost but making progress - Where will new analgesic drugs come from? Sci. Transl. Med 10.1126/scitranslmed.3008320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Youssef AM, Simons L, Elman I, Eccleston C, 2018. When pain gets stuck: The evolution of pain chronification and treatment resistance. Pain. 10.1097/j.pain.0000000000001401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A, Dubey S, Yeung S, Hough O, Eterman N, Aubert I, Hynynen K, 2014. Alzheimer disease in a mouse model: Mr imaging-guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior. Radiology 10.1148/radiol.14140245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A, Shah K, Hough O, Hynynen K, 2015. Focused ultrasound-mediated drug delivery through the blood-brain barrier. Expert Rev. Neurother 10.1586/14737175.2015.1028369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse JW, Wang L, Kamaleldin M, Craigie S, Riva JJ, Montoya L, Mulla SM, Lopes LC, Vogel N, Chen E, Kirmayr K, De Oliveira K, Olivieri L, Kaushal A, Chaparro LE, Oyberman I, Agarwal A, Couban R, Tsoi L, Lam T, Vandvik PO, Hsu S, Bala MM, Schandelmaier S, Scheidecker A, Ebrahim S, Ashoorion V, Rehman Y, Hong PJ, Ross S, Johnston BC, Kunz R, Sun X, Buckley N, Sessler DI, Guyatt GH, 2018. Opioids for Chronic Noncancer Pain: A Systematic Review and Meta-analysis. JAMA - J. Am. Med. Assoc 10.1001/jama.2018.18472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C, Karachi C, Leclercq D, Lafon C, Chapelon JY, Capelle L, Cornu P, Sanson M, Hoang-Xuan K, Delattre JY, Idbaih A, 2016. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med 10.1126/scitranslmed.aaf6086 [DOI] [PubMed] [Google Scholar]
- Choi JB, Lim SH, Cho KW, Kim DH, Jang DP, Kim IY, 2013. The effect of focused ultrasonic stimulation on the activity of hippocampal neurons in multi-channel electrode, in: International IEEE/EMBS Conference on Neural Engineering, NER. 10.1109/NER.2013.6696038 [DOI] [Google Scholar]
- Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, Fu R, Dana T, Kraegel P, Griffin J, Grusing S, Brodt ED, 2017. Nonpharmacologic therapies for low back pain: A systematic review for an American College of physicians clinical practice guideline. Ann. Intern. Med 10.7326/M16-2459 [DOI] [PubMed] [Google Scholar]
- Cline HE, Schenck JF, Hynynen K, Watkins RD, Souza SP, Jolesz FA, 1992. Mr-guided focused ultrasound surgery. J. Comput. Assist. Tomogr [DOI] [PubMed] [Google Scholar]
- Coluccia D, Fandino J, Schwyzer L, O’Gorman R, Remonda L, Anon J, Martin E, Werner B, 2014. First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. J. Ther. Ultrasound 10.1186/2050-5736-2-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constans C, Mateo P, Tanter M, Aubry JF, 2018. Potential impact of thermal effects during ultrasonic neurostimulation: Retrospective numerical estimation of temperature elevation in seven rodent setups. Phys. Med. Biol 10.1088/1361-6560/aaa15c [DOI] [PubMed] [Google Scholar]
- Conti A, Mériaux S, Larrat B, 2019. About the Marty model of blood-brain barrier closure after its disruption using focused ultrasound. Phys. Med. Biol 10.1088/1361-6560/ab259d [DOI] [PubMed] [Google Scholar]
- Darrow DP, O’Brien P, Richner TJ, Netoff TI, Ebbini ES, 2019. Reversible neuroinhibition by focused ultrasound is mediated by a thermal mechanism. Brain Stimul 10.1016/j.brs.2019.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ, 2000. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain. 10.1016/S0304-3959(00)00276-1 [DOI] [PubMed] [Google Scholar]
- Deffieux T, Younan Y, Wattiez N, Tanter M, Pouget P, Aubry JF, 2013. Low-intensity focused ultrasound modulates monkey visuomotor behavior. Curr. Biol 10.1016/j.cub.2013.10.029 [DOI] [PubMed] [Google Scholar]
- Derry S, Wiffen PJ, Kalso EA, Bell RF, Aldington D, Phillips T, Gaskell H, Moore RA, 2017. Topical analgesics for acute and chronic pain in adults - an overview of Cochrane Reviews. Cochrane Database Syst. Rev 10.1002/14651858.CD008609.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA, 2008. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr. Rheumatol. Rep 10.1007/s11926-008-0080-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossantos MF, Oliveira AT, Ferreira NR, Carvalho ACP, Rosado De Castro PH, 2018. The Contribution of Endogenous Modulatory Systems to TMS- and tDCS-Induced Analgesia: Evidence from PET Studies. Pain Res. Manag 10.1155/2018/2368386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs ME, Buch A, Sierra C, Karakatsani ME, Chen S, Konofagou EE, Ferrera VP, 2015. Long-term safety of repeated blood-brain barrier opening via focused ultrasound with microbubbles in non-human primates performing a cognitive task. PLoS One. 10.1371/journal.pone.0125911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin AE, Patapoutian A, 2010. Nociceptors: The sensors of the pain pathway. J. Clin. Invest 10.1172/JCI42843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias WJ, Huss D, Voss T, Loomba J, Khaled M, Zadicario E, Frysinger RC, Sperling SA, Wylie S, Monteith SJ, Druzgal J, Shah BB, Harrison M, Wintermark M, 2013. A pilot study of focused ultrasound thalamotomy for essential tremor. N. Engl. J. Med 10.1056/NEJMoa1300962 [DOI] [PubMed] [Google Scholar]
- Farrell SM, Green A, Aziz T, 2018. The current state of deep brain stimulation for chronic pain and its context in other forms of neuromodulation. Brain Sci. 10.3390/brainsci8080158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folloni D, Verhagen L, Mars RB, Fouragnan E, Constans C, Aubry JF, Rushworth MFS, Sallet J, 2019. Manipulation of Subcortical and Deep Cortical Activity in the Primate Brain Using Transcranial Focused Ultrasound Stimulation. Neuron. 10.1016/j.neuron.2019.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouragnan EF, Chau BKH, Folloni D, Kolling N, Verhagen L, Klein-Flügge M, Tankelevitch L, Papageorgiou GK, Aubry JF, Sallet J, Rushworth MFS, 2019. The macaque anterior cingulate cortex translates counterfactual choice value into actual behavioral change. Nat. Neurosci 10.1038/s41593-019-0375-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes JB, 2011. American Institute of ultrasound in medicine consensus report on potential bioeffects of diagnostic ultrasound: Executive summary. J. Diagnostic Med. Sonogr 10.1177/8756479310394986 [DOI] [PubMed] [Google Scholar]
- Fry FJ, Ades HW, Fry WJ, 1958. Production of reversible changes in the central nervous system by ultrasound. Science (80-. ). 10.1126/science.127.3289.83 [DOI] [PubMed] [Google Scholar]
- Fry WJ, Fry FJ, 1960. Fundamental Neurological Research and Human Neurosurgery Using Intense Ultrasound. IRE Trans. Med. Electron 10.1109/IRET-ME.1960.5008041 [DOI] [PubMed] [Google Scholar]
- FRY WJ, MEYERS R, 1962. Ultrasonic method of modifying brain st ructures. Confin. Neurol [PubMed] [Google Scholar]
- Garland EL, 2012. Pain Processing in the Human Nervous System. A Selective Review of Nociceptive and Biobehavioral Pathways. Prim. Care - Clin. Off. Pract 10.1016/j.pop.2012.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg DS, McGee SJ, 2011. Pain as a global public health priority. BMC Public Health. 10.1186/1471-2458-11-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss SA, Frizzell LA, Dunn F, 1979. Ultrasonic absorption and attenuation in mammalian tissues. Ultrasound Med. Biol 10.1016/0301-5629(79)90086-3 [DOI] [PubMed] [Google Scholar]
- Hameroff S, Trakas M, Duffield C, Annabi E, Gerace MB, Boyle P, Lucas A, Amos Q, zuadu A, Badal JJ, 2013. Transcranial ultrasound (TUS) effects on mental states: A pilot study. Brain Stimul. 10.1016/j.brs.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Scott Henderson J, 2018. Modulating neural circuits with transcranial magnetic stimulation: Implications for addiction treatment development. Pharmacol. Rev 10.1124/pr.116.013649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harary M, Segar DJ, Huang KT, Tafel IJ, Valdes PA, Cosgrove GR, 2018. Focused ultrasound in neurosurgery: A historical perspective. Neurosurg. Focus 10.3171/2017.11.FOCUS17586 [DOI] [PubMed] [Google Scholar]
- Harte SE, Harris RE, Clauw DJ, 2018. The neurobiology of central sensitization. J. Appl. Biobehav. Res 10.1111/jabr.12137 [DOI] [Google Scholar]
- Hofmeister M, Memedovich A, Brown S, Saini M, Dowsett LE, Lorenzetti DL, McCarron TL, MacKean G, Clement F, 2020. Effectiveness of Neurostimulation Technologies for the Management of Chronic Pain: A Systematic Review. Neuromodulation. 10.1111/ner.13020 [DOI] [PubMed] [Google Scholar]
- Huang YS, Fan CH, Hsu N, Chiu NH, Wu CY, Chang CY, Wu BH, Hong SR, Chang YC, Yan-Tang Wu A, Guo V, Chiang YC, Hsu WC, Chen L, Pin-Kuang Lai C, Yeh CK, Lin YC, 2020. Sonogenetic Modulation of Cellular Activities Using an Engineered Auditory-Sensing Protein. Nano Lett 10.1021/acs.nanolett.9b04373 [DOI] [PubMed] [Google Scholar]
- Hynynen K, 1991. The threshold for thermally significant cavitation in dog’s thigh muscle in vivo. Ultrasound Med. Biol 10.1016/0301-5629(91)90123-E [DOI] [PubMed] [Google Scholar]
- Hynynen K, Jolesz FA, 1998. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med. Biol 10.1016/S0301-5629(97)00269-X [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N, 2005. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 10.1016/j.neuroimage.2004.06.046 [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA, 2001. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 220, 640–6. 10.1148/radiol.2202001804 [DOI] [PubMed] [Google Scholar]
- Ibsen S, Tong A, Schutt C, Esener S, Chalasani SH, 2015. Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans. Nat. Commun 10.1038/ncomms9264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques SL, 2013. Optical properties of biological tissues: A review. Phys. Med. Biol 10.1088/0031-9155/58/11/R37 [DOI] [PubMed] [Google Scholar]
- Jagannathan J, Sanghvi NT, Crum LA, Yen CP, Medel R, Dumont AS, Sheehan JP, Steiner L, Jolesz F, Kassell NF, 2009. High-intensity focused ultrasound surgery of the brain: Part 1-A historical perspective with modern applications. Neurosurgery. 10.1227/01.NEU.0000336766.18197.8E [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanmonod D, Werner B, Morel A, Michels L, Zadicario E, Schiff G, Martin E, 2012. Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg. Focus 10.3171/2011.10.FOCUS11248 [DOI] [PubMed] [Google Scholar]
- Johns LD, 2002. Nonthermal effects of therapeutic ultrasound: The frequency resonance hypothesis. J. Athl. Train [PMC free article] [PubMed] [Google Scholar]
- Jordão JF, Ayala-Grosso CA, Markham K, Huang Y, Chopra R, McLaurin JA, Hynynen K, Aubert I, 2010. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-β plaque load in the TgCRND8 mouse model of Alzheimer’s disease. PLoS One. 10.1371/journal.pone.0010549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khraiche ML, Phillips WB, Jackson N, Muthuswamy J, 2008. Ultrasound induced increase in excitability of single neurons, in: Proceedings of the 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS’08 - “Personalized Healthcare through Technology” [DOI] [PubMed] [Google Scholar]
- Kim HB, Swanberg KM, Han HS, Kim JC, Kim JW, Lee S, Lee CJ, Maeng S, Kim TS, Park JH, 2017. Prolonged stimulation with low-intensity ultrasound induces delayed increases in spontaneous hippocampal culture spiking activity. J. Neurosci. Res 10.1002/jnr.23845 [DOI] [PubMed] [Google Scholar]
- King RL, Brown JR, Newsome WT, Pauly KB, 2013. Effective parameters for ultrasound-induced in vivo neurostimulation. Ultrasound Med. Biol 10.1016/j.ultrasmedbio.2012.09.009 [DOI] [PubMed] [Google Scholar]
- Klein MM, Treister R, Raij T, Pascual-Leone A, Park L, Nurmikko T, Lenz F, Lefaucheur JP, Lang M, Hallett M, Fox M, Cudkowicz M, Costello A, Carr DB, Ayache SS, Oaklander AL, 2015. Transcranial magnetic stimulation of the brain: Guidelines for pain treatment research. Pain. 10.1097/j.pain.0000000000000210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs ZI, Burks SR, Frank JA, 2018. Focused ultrasound with microbubbles induces sterile inflammatory response proportional to the blood brain barrier opening: Attention to experimental conditions. Theranostics. 10.7150/thno.24181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs ZI, Kim S, Jikaria N, Qureshi F, Milo B, Lewis BK, Bresler M, Burks SR, Frank JA, 2017. Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation. Proc. Natl. Acad. Sci 114, E75–E84. 10.1073/pnas.1614777114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasovitski B, Frenkel V, Shoham S, Kimmel E, 2011. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc. Natl. Acad. Sci. U. S. A 10.1073/pnas.1015771108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna V, Sammartino F, Rezai A, 2018. A review of the current therapies, challenges, and future directions of transcranial focused ultrasound technology advances in diagnosis and treatment. JAMA Neurol 10.1001/jamaneurol.2017.3129 [DOI] [PubMed] [Google Scholar]
- Kubanek J, 2018. Neuromodulation with transcranial focused ultrasound. Neurosurg. Focus 10.3171/2017.11.FOCUS17621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubanek J, Brown J, Ye PP, Butts Pauly K, Newsome III WT, 2017. Transcranial ultrasound impacts monkey choice behaviour, in: Cell Symposia: Big Questions in Neuroscience. [Google Scholar]
- Kubanek J, Shi J, Marsh J, Chen D, Deng C, Cui J, 2016. Ultrasound modulates ion channel currents. Sci. Rep 10.1038/srep24170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubanek J, Shukla P, Das A, Baccus SA, Goodman MB, 2018. Ultrasound elicits behavioral responses through mechanical effects on neurons and ion channels in a simple nervous system. J. Neurosci 10.1523/JNEUROSCI.1458-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Chung YA, Jung Y, Song IU, Yoo SS, 2016a. Simultaneous acoustic stimulation of human primary and secondary somatosensory cortices using transcranial focused ultrasound. BMC Neurosci. 10.1186/s12868-016-0303-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Kim H, Jung Y, Song IU, Chung YA, Yoo SS, 2015. Image-guided transcranial focused ultrasound stimulates human primary somatosensory cortex. Sci. Rep 10.1038/srep08743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Kim HC, Jung Y, Chung YA, Song IU, Lee JH, Yoo SS, 2016b. Transcranial focused ultrasound stimulation of human primary visual cortex. Sci. Rep 10.1038/srep34026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon W, Bansal P, Tyshynsky R, Ai L, Mueller JK, 2018. Transcranial focused ultrasound neuromodulation of the human primary motor cortex. Sci. Rep 10.1038/s41598-018-28320-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon W, Sato TF, Opitz A, Mueller J, Barbour A, Williams A, Tyler WJ, 2014. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat. Neurosci 10.1038/nn.3620 [DOI] [PubMed] [Google Scholar]
- Leinenga G, Götz J, 2015. Scanning ultrasound removes amyloid-b and restores memory in an Alzheimer’s disease mouse model. Sci. Transl. Med 10.1126/scitranslmed.aaa2512 [DOI] [PubMed] [Google Scholar]
- Lele PP, 1967. Production of deep focal lesions by focused ultrasound-current status. Ultrasonics. 10.1016/S0041-624X(67)80011-8 [DOI] [PubMed] [Google Scholar]
- Lindstrom PA, 1954. Prefrontal ultrasonic irradiation—a substitute for lobotomy. Arch. Neurol. Psychiatry 10.1001/archneurpsyc.1954.02330040001001 [DOI] [PubMed] [Google Scholar]
- Lipsman N, Meng Y, Bethune AJ, Huang Y, Lam B, Masellis M, Herrmann N, Heyn C, Aubert I, Boutet A, Smith GS, Hynynen K, Black SE, 2018. Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun 9 10.1038/s41467-018-04529-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano AM, Lipsman N, Bergman H, Brown P, Chabardes S, Chang JW, Matthews K, McIntyre CC, Schlaepfer TE, Schulder M, Temel Y, Volkmann J, Krauss JK, 2019. Deep brain stimulation: current challenges and future directions. Nat. Rev. Neurol 10.1038/s41582-018-0128-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn JG, Zwemer RL, Chick AJ, Miller AE, 1942. A new method for the generation and use of focused ultrasound in experimental biology. J. Gen. Physiol 10.1085/jgp.26.2.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, 2019. Blood-Brain Barrier Opening in Primary Brain Tumors with Non- invasive MR-Guided Focused Ultrasound : A Clinical Safety and Feasibility Study. Sci. Rep 1–7. 10.1038/s41598-018-36340-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca D, Lakshmanan A, Abedi M, Bar-Zion A, Farhadi A, Lu GJ, Szablowski JO, Wu D, Yoo S, Shapiro MG, 2018. Biomolecular Ultrasound and Sonogenetics. Annu. Rev. Chem. Biomol. Eng 10.1146/annurev-chembioeng-060817-084034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquet F, Teichert T, Wu SY, Tung YS, Downs M, Wang S, Chen C, Ferrera V, Konofagou EE, 2014. Real-time, transcranial monitoring of safe blood-brain barrier opening in non-human primates. PLoS One. 10.1371/journal.pone.0084310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B, 2009. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann. Neurol 10.1002/ana.21801 [DOI] [PubMed] [Google Scholar]
- Marty B, Larrat B, Van Landeghem M, Robic C, Robert P, Port M, Le Bihan D, Pernot M, Tanter M, Lethimonnier F, Mériaux S, 2012. Dynamic study of blood-brain barrier closure after its disruption using ultrasound: A quantitative analysis. J. Cereb. Blood Flow Metab 32, 1948–1958. 10.1038/jcbfm.2012.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A, 2008. Chronic pain may change the structure of the brain. Pain. 10.1016/j.pain.2008.02.034 [DOI] [PubMed] [Google Scholar]
- McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS, 2012. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: Safety and efficacy evaluation in rhesus macaques. Cancer Res. 72, 3652–3663. 10.1158/0008-5472.CAN-12-0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Clement GT, Black P, Jolesz F, Hynynen K, 2010. Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: Initial findings in 3 patients. Neurosurgery. 10.1227/01.NEU.0000360379.95800.2F [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Zhang YZ, Power C, Jolesz F, Vykhodtseva N, 2013. Nonthermal ablation with microbubble-enhanced focused ultrasound close to the optic tract without affecting nerve function. J. Neurosurg 10.3171/2013.8.JNS122387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon D, Hynynen K, 2018. Reply to Kovacs et al.: Concerning acute inflammatory response following focused ultrasound and microbubbles in the brain. Theranostics. 10.7150/thno.25468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon D, Hynynen K, 2017. Acute inflammatory response following increased blood-brain barrier permeability induced by focused ultrasound is dependent on microbubble dose. Theranostics. 10.7150/thno.21630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYERS R, FRY WJ, FRY FJ, DREYER LL, SCHULTZ DF, NOYES RF, 1959. Early experiences with ultrasonic irradiation of the pallidofugal and nigral complexes in hyperkinetic and hypertonic disorders. J. Neurosurg 10.3171/jns.1959.16.1.0032 [DOI] [PubMed] [Google Scholar]
- Mohammadjavadi M, Ye PP, Xia A, Brown J, Popelka G, Pauly KB, 2019. Elimination of peripheral auditory pathway activation does not affect motor responses from ultrasound neuromodulation. Brain Stimul. 10.1016/j.brs.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor O, Krupa S, Shoham S, 2016. Ultrasonic neuromodulation. J. Neural Eng 10.1088/1741-2560/13/3/031003 [DOI] [PubMed] [Google Scholar]
- O’Brien WD, Dunn F, 2015. An early history of high-intensity focused ultrasound. Phys. Today 10.1063/PT.3.2947 [DOI] [Google Scholar]
- O’Connell NE, Wand BM, Marston L, Spencer S, Desouza LH, 2014. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst. Rev 10.1002/14651858.CD008208.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odéen H, de Bever J, Almquist S, Farrer A, Todd N, Payne A, Snell JW, Christensen DA, Parker DL, 2014. Treatment envelope evaluation in transcranial magnetic resonance-guided focused ultrasound utilizing 3D MR thermometry. J. Ther. Ultrasound 10.1186/2050-5736-2-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SJ, Lee JM, Kim HB, Lee J, Han S, Bae JY, Hong GS, Koh W, Kwon J, Hwang ES, Woo DH, Youn I, Cho IJ, Bae YC, Lee S, Shim JW, Park JH, Lee CJ, 2019. Ultrasonic Neuromodulation via Astrocytic TRPA1. Curr. Biol 10.1016/j.cub.2019.08.021 [DOI] [PubMed] [Google Scholar]
- Park J, Zhang Y, Vykhodtseva N, Jolesz FA, McDannold NJ, 2012. The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound. J. Control. Release 162, 134–42. 10.1016/j.jconrel.2012.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AH, Hawryluk GW, Anzai Y, Odéen H, Ostlie MA, Reichert EC, Stump AJ, Minoshima S, Cross DJ, 2017. Magnetic resonance imaging-guided focused ultrasound to increase localized blood-spinal cord barrier permeability. Neural Regen. Res 10.4103/1673-5374.221162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto CB, Teixeira Costa B, Duarte D, Fregni F, 2018. Transcranial Direct Current Stimulation as a Therapeutic Tool for Chronic Pain. J. ECT 10.1097/YCT.0000000000000518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaksin M, Kimmel E, Shoham S, 2016. Cell-type-selective effects of intramembrane cavitation as a unifying theoretical framework for ultrasonic neuromodulation. eNeuro. 10.1523/ENEURO.0136-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter J. De, Wagter C. De, Deene Y. De, Thomsen C, Ståhlberg F, Achten E, 1995. Noninvasive MRI Thermometry with the Proton Resonance Frequency (PRF) Method: In Vivo Results in Human Muscle. Magn. Reson. Med 10.1002/mrm.1910330111 [DOI] [PubMed] [Google Scholar]
- Sapareto SA, Dewey WC, 1984. Thermal dose determination in cancer therapy. Int. J. Radiat. Oncol. Biol. Phys 10.1016/0360-3016(84)90379-1 [DOI] [PubMed] [Google Scholar]
- Sheikov N, McDannold N, Jolesz F, Zhang YZ, Tam K, Hynynen K, 2006. Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood-brain barrier. Ultrasound Med. Biol 10.1016/j.ultrasmedbio.2006.05.015 [DOI] [PubMed] [Google Scholar]
- Sheikov N, McDannold N, Sharma S, Hynynen K, 2008. Effect of Focused Ultrasound Applied With an Ultrasound Contrast Agent on the Tight Junctional Integrity of the Brain Microvascular Endothelium. Ultrasound Med. Biol 10.1016/j.ultrasmedbio.2007.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K, 2004. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med. Biol 10.1016/j.ultrasmedbio.2004.04.010 [DOI] [PubMed] [Google Scholar]
- Shin J, Kong C, Cho JS, Lee J, Koh CS, Yoon MS, Na YC, Chang WS, Chang JW, 2018. Focused ultrasound-mediated noninvasive blood-brain barrier modulation: Preclinical examination of efficacy and safety in various sonication parameters. Neurosurg. Focus 10.3171/2017.11.FOCUS17627 [DOI] [PubMed] [Google Scholar]
- Simon LS, 2012. RELIEVING PAIN IN AMERICA: A BLUEPRINT FOR TRANSFORMING PREVENTION, CARE, EDUCATION, AND RESEARCH. J. Pain Palliat. Care Pharmacother 10.3109/15360288.2012.678473 [DOI] [Google Scholar]
- Simons LE, Elman I, Borsook D, 2014. Psychological processing in chronic pain: A neural systems approach. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinharay S, Tu T-W, Kovacs ZI, Schreiber-Stainthorp W, Sundby M, Zhang X, Papadakis GZ, Reid WC, Frank JA, Hammoud DA, 2019. In vivo imaging of sterile microglial activation in rat brain after disrupting the blood-brain barrier with pulsed focused ultrasound: [18F]DPA-714 PET study. J. Neuroinflammation 10.1186/s12974-019-1543-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Samiotaki G, Wang S, Acosta C, Chen CC, Konofagou EE, 2015. Acoustic cavitation-based monitoring of the reversibility and permeability of ultrasound-induced blood-brain barrier opening. Phys. Med. Biol 10.1088/0031-9155/60/23/9079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szablowski JO, Lee-Gosselin A, Lue B, Malounda D, Shapiro MG, 2018. Acoustically targeted chemogenetics for the non-invasive control of neural circuits. Nat. Biomed. Eng 10.1038/s41551-018-0258-2 [DOI] [PubMed] [Google Scholar]
- Todd N, Zhang Y, Power C, Becerra L, Borsook D, Livingstone M, McDannold N, 2019. Modulation of brain function by targeted delivery of GABA through the disrupted blood-brain barrier. Neuroimage. 10.1016/j.neuroimage.2019.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Kosek E, Lavand’homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ, 2015. A classification of chronic pain for ICD-11. Pain. 10.1097/j.pain.0000000000000160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Tsai CH, Chen WS, Inserra C, Wei KC, Liu HL, 2018. Safety evaluation of frequent application of microbubble-enhanced focused ultrasound blood-brain-barrier opening. Sci. Rep 10.1038/s41598-018-35677-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, Majestic C, 2008. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS One. 10.1371/journal.pone.0003511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vania Apkarian A, Baliki MN, Farmer MA, 2013. Predicting transition to chronic pain. Curr. Opin. Neurol 10.1097/WCO.0b013e32836336ad [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen L, Gallea C, Folloni D, Constans C, Jensen DEA, Ahnine H, Roumazeilles L, Santin M, Ahmed B, Lehericy S, Klein-Flügge MC, Krug K, Mars RB, Rushworth MFS, Pouget P, Aubry JF, Sallet J, 2019. Offline impact of transcranial focused ultrasound on cortical activation in primates. Elife. 10.7554/eLife.40541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Kugelman T, Buch A, Herman M, Han Y, Karakatsani ME, Hussaini SA, Duff K, Konofagou EE, 2017. Non-invasive, Focused Ultrasound-Facilitated Gene Delivery for Optogenetics. Sci. Rep 10.1038/srep39955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Adrian D, Thévenot E, O’Reilly MA, Oakden W, Akens MK, Ellens N, Markham-Coultes K, Burgess A, Finkelstein J, Yee AJM, Whyne CM, Foust KD, Kaspar BK, Stanisz GJ, Chopra R, Hynynen K, Aubert I, 2015. Gene delivery to the spinal cord using MRI-guided focused ultrasound. Gene Ther. 10.1038/gt.2015.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei KC, Chu PC, Wang HYJ, Huang CY, Chen PY, Tsai HC, Lu YJ, Lee PY, Tseng IC, Feng LY, Hsu PW, Yen TC, Liu HL, 2013. Focused Ultrasound-Induced Blood-Brain Barrier Opening to Enhance Temozolomide Delivery for Glioblastoma Treatment: A Preclinical Study. PLoS One. 10.1371/journal.pone.0058995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiffen PJ, Derry S, Bell RF, Rice ASC, Tölle TR, Phillips T, Moore RA, 2017. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst. Rev 10.1002/14651858.CD007938.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, 2011. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 10.1016/j.pain.2010.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Ma Q, 2007. Nociceptors-Noxious Stimulus Detectors. Neuron. 10.1016/j.neuron.2007.07.016 [DOI] [PubMed] [Google Scholar]
- Yang PF, Phipps MA, Newton AT, Chaplin V, Gore JC, Caskey CF, Chen LM, 2018. Neuromodulation of sensory networks in monkey brain by focused ultrasound with MRI guidance and detection. Sci. Rep 10.1038/s41598-018-26287-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Tang S, Meng L, Li Xia, Wen X, Chen S, Niu L, Xiangyao Li, Qiu W, Hu H, Jiang M, Shang S, Shu Q, Zheng H, Duan S, Li Y, 2018. Ultrasonic Control of Neural Activity through Activation of the Mechanosensitive Channel MscL. Nano Lett. 10.1021/acs.nanolett.8b00935 [DOI] [PubMed] [Google Scholar]
- Ye PP, Brown JR, Pauly KB, 2016. Frequency dependence of ultrasound neurostimulation in the mouse brain. Ultrasound Med. Biol 10.1016/j.ultrasmedbio.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SS, Bystritsky A, Lee JH, Zhang Y, Fischer K, Min BK, McDannold NJ, Pascual-Leone A, Jolesz FA, 2011. Focused ultrasound modulates region-specific brain activity. Neuroimage. 10.1016/j.neuroimage.2011.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young NA, Sharma M, Deogaonkar M, 2014. Transcranial magnetic stimulation for chronic pain. Neurosurg. Clin. N. Am 10.1016/j.nec.2014.07.007 [DOI] [PubMed] [Google Scholar]


