Abstract
Proper regulation of centromeric cohesion is required for faithful chromosome segregation that prevents chromosomal instability. Extensive studies have identified and established the conserved protein Shugoshin (Sgo1/2) as an essential protector for centromeric cohesion. In this review, we summarize the current understanding of how Shugoshin-1 (Sgo1) protects centromeric cohesion at the molecular level. Targeting of Sgo1 to inner centromeres is required for its proper function of cohesion protection. We therefore discuss about the molecular mechanisms that install Sgo1 onto inner centromeres. At metaphase-to-anaphase transition, Sgo1 at inner centromeres needs to be disabled for the subsequent sister-chromatid segregation. A few recent studies suggest interesting models to explain how it is achieved. These models are discussed as well.
Introduction
Faithful chromosome segregation is essential for the maintenance of chromosomal stability. To ensure faithful chromosome segregation, various mechanisms have been evolved. Among these mechanisms is proper regulation of sister chromatid cohesion. Sister chromatid cohesion is usually established at S-phase when DNA replication occurs, and subsequently resolved at mitosis and meiosis when chromosome segregation occurs. At early mitosis and meiosis, cohesin on chromosome arms is released but cohesin at centromeres is preserved until anaphase onset. Protection of centromeric cohesion is carried out by the Shugoshin/MEI-S332 family proteins that are conserved across species [1,2]. In vertebrates, Shugoshin (Sgo1) depletion results in massive loss of cohesin and severe chromosome missegregation during mitosis, demonstrating its essential role in cohesion protection [3–6]. In yeast and plant, Shugoshin (Sgo1/2) is not required for centromeric cohesion protection at mitosis but plays an essential role in centromeric cohesion protection at meiosis [7–14]. These important findings have established Shugoshin as the central hub to orchestrate centromeric cohesion protection albeit its exact roles vary among distinct species. In addition to this critical function, Shugoshin has also been shown to regulate chromosome attachments and spindle checkpoint response [15–29]. In this review, we mainly focus on the functioning mechanisms of Shugoshin in cohesion protection during mitosis. Its meiotic functions were summarized previously [30]. Specifically, we discuss in great details about how human Shugoshin-1 (Sgo1) protects centromeric cohesion, how it is installed to centromeres and how it is disabled.
Molecular mechanisms whereby Sgo1 protects centromeric cohesion
Drosophila melanogaster Mei-S332 was the first member identified in the Shugoshin family [31]. It plays an essential role in sister chromatid cohesion in meiosis, but not in mitosis [32,33]. A following study suggested that instead of Mei-S332, Dalmatian, a functional equivalent to Sororin and Shugoshin, is the one that functions to protect mitotic cohesion in D. melanogaster [34]. The other members in Shugoshin family were subsequently identified from a wide spectrum of organisms [7]. They were found to localize at centromeric regions to protect centromeric cohesion in both mitosis and meiosis. Since its discovery, a great deal of attention has been paid to the molecular mechanisms whereby Shugoshin protects centromeric cohesion. In vertebrates, cohesin on chromosome arms is first removed in a Wapl-dependent manner at early mitosis [35–38]. This process is facilitated by phosphorylation of the core cohesin subunits and its regulator Sororin by multiple mitotic kinases, including Aurora B, Plk1 and Cdk1 [38–43]. Although the majority of cohesin is released from chromosome arms, centromeric cohesin is protected, implying that signals exist at centromeres to antagonize the functions of these kinases. Subsequently, several important studies demonstrated that Shugoshin forms a complex with phosphatase 2A (PP2A-B56) to protect centromeric cohesion [44–46]. Ectopic expression of non-phosphorylatable cohesin or Sororin largely rescued the loss of centromeric cohesion by Sgo1 depletion [41,43,47]. Thus, the tug of war between kinases and phosphatases at centromeres determines the status of centromeric cohesion. The main antagonistic force that combats the multiple mitotic kinase activities at centromeres is Sgo1-bound PP2A.
How is the Sgo1–PP2A complex regulated to protect centromeric cohesion? Extensive studies have shown that Bub1, a mitotic kinase, enriches Sgo1 to centromeres, revealing a plausibly important pathway for centromeric cohesion regulation. However, although Bub1 knockdown decreased Sgo1 localization at centromeres [5–7,48,49], no significant centromeric cohesion defects were observed in human cells [5,6,50], suggesting that other critical regulatory pathway(s) exist at centromeres to protect cohesion. We have previously found that Cdk1 phosphorylation of Sgo1 at Thr346 is essential for Sgo1 function of cohesion protection [47] (Figure 1). Mechanistically, the phosphorylation promotes the direct binding of Sgo1 to cohesin, which in turn brings PP2A in close proximity to cohesin to prevent cohesin and Sororin from being phosphorylated by mitotic kinases. Hypo-phosphorylated cohesin and Sororin antagonize Wapl to maintain centromeric cohesion [38,41]. Interestingly, Cdk1 phosphorylation-enabled binding of Sgo1 to cohesin not only presents PP2A to cohesin, but also blocks Wapl from directly binding to cohesin [51], which provides an additional dimension for centromeric cohesion protection. Thus, Cdk1 phosphorylation-enabled direct binding of Sgo1 to cohesin is a critical step toward centromeric cohesion protection. This process is facilitated by Bub1-mediated Sgo1 enrichment to centromeres albeit it is not essential. Of note, although Sgo1-bound PP2A can combat the action of multiple mitotic kinases on cohesin and Sororin, it appears to have no effects on Sgo1 phosphorylation at Thr346. The underlying reason remains unknown.
Figure 1. Mechanisms explaining how Sgo1 regulates centromeric cohesion during mitosis.
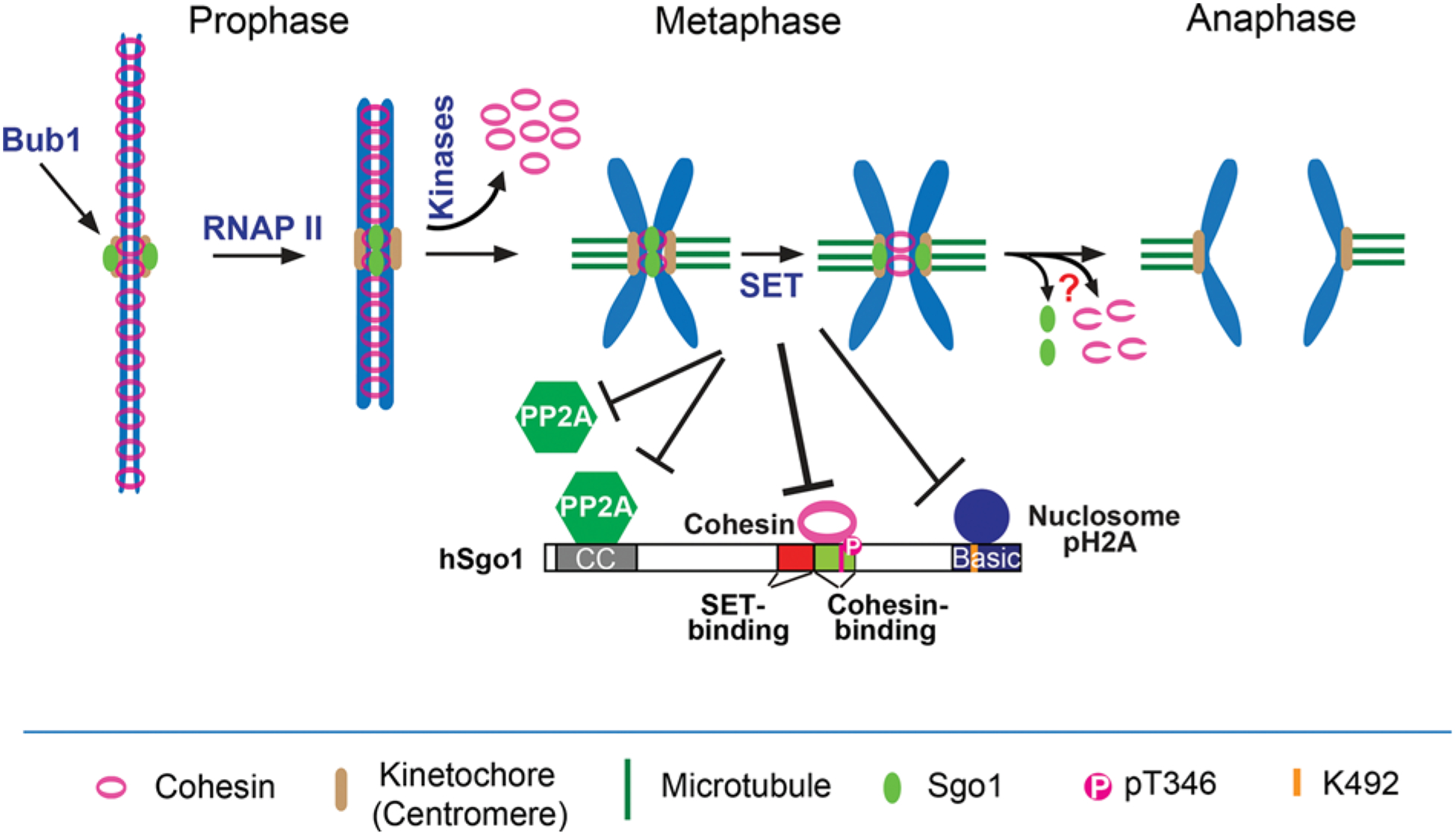
In prophase, Bub1-phosphorylated H2A recruits Sgo1 to kinetochores. RNA Polymerase (RNAP) II transcription promotes the relocation of kinetochore Sgo1 to the inner centromere, where Cdk1-phosphorylated Sgo1 binds to cohesin to protect centromeric cohesion. Cohesin on chromosome arms is released with the aid of multiple mitotic kinases. At metaphase-to-anaphase transition, SET removes Sgo1 from inner centromeres to de-protect centromeric cohesion by disrupting either the Sgo1–cohesin or Sgo1-nuclosome binding or both. SET may also inhibit PP2A activity to de-protect centromeric cohesion. It is unknown whether Separase cleavage of centromeric cohesin requires Sgo1 removal from inner centromeres (marked by “?”).
Localization of Sgo1 at centromeres
Shugoshin is predominantly enriched at centromeres and the centromeric localization is important for its function of centromeric cohesion protection. Extensive studies have demonstrated that Bub1 plays a critical role in enriching Shugoshin to centromeres [5–7,48,49] (Figure 1). A subsequent study determined the underlying mechanism whereby Bub1 phosphorylates histone H2A to recruit Sgo1 to centromeres [49]. The binding between Sgo1 and histone is direct and through the “Shugoshin” domain located in the C-terminus of Sgo1 [49,52]. Truncation of the “C-terminus” of human Sgo1 (ΔC) or mutation of a key amino acid residue Lys492 to a negatively charged residue glutamic acid (K492E) completely abolished the Sgo1-histone binding in vitro [52]. Accordingly, these two Sgo1 mutants (ΔC and K492E) completely lost their localization to centromeres and chromosomes. These results indicated that the C-terminus of Sgo1 is absolutely required for its localization to chromosomes and its enrichment to centromeres. Surprisingly, mutation of human Sgo1 Lys492 to a neutral alanine (K492A) largely decreased, but did not abolish the Sgo1-histone binding in vitro [52], suggesting that a basal binding exists between unphosphorylated histone and Sgo1. The basal binding was sufficient to recruit Sgo1 to centromeres albeit at a reduced level and largely rescued centromeric cohesion defects caused by Sgo1 depletion [50]. Moreover, the mutant K492A was also shown to localize to chromosome arms and cause ectopic cohesion there, recapitulating the phenotype of Bub1 depletion [50,53]. Interestingly, endogenous Sgo1 can also localize to chromosome arms at very early mitosis under unperturbed conditions, as suggested by our preliminary data. The chromosome-arm localization is predominantly dependent on cohesin [50]. Thus, Bub1 phosphorylation on histone increases the binding affinity between Sgo1 and histone, which greatly facilitates but is not required for Sgo1 enrichment to centromeres. Other critical pathways must exist to regulate Sgo1 localization at centromeres.
Cdk1 phosphorylation of human Sgo1 at Thr346 promotes Sgo1 binding to cohesin [50]. Mutation of this site to non-phosphorylatable alanine (T346A) or truncation of the cohesin-binding domain (Δcohesin) altered Sgo1 localization on chromosomes [47]. Precise analyses on the cellular localization of Sgo1 demonstrated that Sgo1 is actually located to the inner centromere, the place between two sister centromeres [5,6]. The Sgo1 T346A and Δcohesin mutants lost their localization at inner centromeres; instead, they were enriched to a place in closer proximity to outer kinetochores, termed kinetochore-proximal region [47]. Interestingly, Bub1 phosphorylated histone H2A (pH2A) is also enriched at the similar region [50,54] and Bub1 depletion abolished the localization of Sgo1 T346A on chromosomes [50]. All these findings strongly support that notion that Sgo1 localization is mainly controlled by two pathways: Bub1-pH2A recruits Sgo1 to the kinetochore-proximal region and cohesin installs Sgo1 to the inner centromere (Figure 1). The Sgo1–cohesin interaction installs Sgo1 into inner centromeres, which is essential for Sgo1 function of cohesion protection; and the pH2A–Sgo1 interaction enriches Sgo1 at the kinetochore-proximal region, which facilitates Sgo1 installment to the inner centromere.
Notably, Sgo1 localization at centromeres is far more complicated because of multi-layer regulation. First, the chromosome passenger complex (CPC) and Sgo1 can mutually regulate the centromeric localization of each other [55–61]. The mutual regulation could offer a positive feedback loop to enhance their individual functions. Second, Sgo1 localization at centromeres is also balanced by Plk1 and PP2A [46]. Finally, Aurora A-dependent phosphorylation of CENP-A may promote Sgo1 localization to centromeres in prolonged-metaphase cells, thus antagonizing against cohesion fatigue [62]. Thus, a complex regulatory network of Sgo1 ensures a tight and timely control of its functions in coordination with mitotic progression and chromosome segregation.
Transcription promotes Sgo1 installment to inner centromeres
We have shown that Sgo1 can localize to two places: the inner centromere and kinetochore-proximal region [50]. Mutation of cohesin-binding domain in Sgo1 retained Sgo1 at the kinetochore-proximal region [50], suggesting that Sgo1 is likely recruited to the kinetochore-proximal region and then relocated to the inner centromere, where Sgo1 binds and protects centromeric cohesin (Figure 1). Then an interesting question was asked how the kinetochore-proximal pool of Sgo1 was relocated to the inner centromere. Although much of RNA Polymerase (RNAP) II on chromosome arms is released when cells enter mitosis, active forms of RNAP II were found to be retained at centromeres [63]. The centromeric RNAP II is under active transcription as it was shown to be able to produce RNAs [52,63]. We were wondering if active RNAP II at centromeres was responsible for Sgo1 relocation from the kinetochore-proximal region to the inner centromere. Treatment of α-amanitin, an RNAP II inhibitor that inhibits its elongation, induced significant centromeric cohesion defects in nocodazole-arrested mitotic cells [52]. Furthermore, inactivation of RNAP II activity using an auxin-induced protein degradation (AID) system also resulted in severe centromeric cohesion defects. These two lines of evidence strongly suggested that RNAP II-dependent transcription plays an important role in centromeric cohesion protection. The transcriptional inhibition in these experiments also prevented Sgo1 from being targeted to inner centromeres; as a result, Sgo1 was enriched to the kinetochore-proximal region, supporting a model that RNAP II transcription drives Sgo1 from the kinetochore-proximal region to the inner centromere [52] (Figure 1). The role of RNAP II on Sgo1 relocation might be direct as Sgo1 was found to physically interact with RNAP II [52]. Notably, a very recent study suggested that cohesin itself can also be a promoting factor for mitotic transcription [64]. Thus, combined with our findings, a positive feedback loop might be formed among cohesin, transcription and Sgo1 for centromeric cohesion maintenance.
Two recent studies suggested that mitotic transcription is not important for mitotic progression using triptolide, a chemical that specifically inhibits the activity of the transcriptional initiation factor TFIIH [64–66]. The observations are seemingly inconsistent with ours. The discrepancy could be due to the application of distinct transcriptional inhibitors. As a matter of fact, the efficacies of these inhibitors on centromeric transcription were not rigorously measured in these studies. Re-evaluating the effects of these inhibitors on centromeric transcription is needed to clarify this issue. It is worth mentioning that transcriptional inhibitors usually inhibit global transcription and do not distinguish the transcriptions on between centromeres and the other DNA sequences. In future, development of methods to specifically inactivate centromeric transcription without affecting global gene transcription is needed to re-evaluate the roles of centromeric transcription in chromosome segregation.
Inactivation of Sgo1 function
At metaphase-to-anaphase transition, centromeric cohesin is cleaved by Separase to trigger sister chromatid segregation. Then, a question was asked whether Separase-catalyzed cohesin cleavage requires the inactivation of Sgo1 function. Two previous studies suggested that cohesin could be protected from Separase cleavage when Sgo1 was overproduced in budding yeast cells or ectopically retained at anaphase centromeres in human cells [67,68], supporting that inactivation of Sgo1 function may be required for Separase cleavage of cohesin. Furthermore, we previously demonstrated that expression of Sgo1 T346D that can constitutively bind cohesin resulted in a significant increase in anaphase lagging chromosomes with cohesed sister centromeres. All these interesting findings implied that disruption of the Sgo1–cohesin binding could be a critical step toward inactivation of Sgo1 functions that is required for timely resolution of centromeric cohesion. In further support of it, various studies demonstrated that at both mitotic metaphase and meiotic metaphase II, Shugoshin is relocated from inner centromeres to kinetochore-proximal regions [50,68,69]. Therefore, removal of Sgo1 from centromeric cohesin might precede and be critical for Separase cleavage of centromeric cohesin albeit direct evidence is lacking. How is Sgo1 removed from centromeric cohesin? It has been shown that tension across sister chromatid kinetochores applied by spindle microtubule pulling force directly triggers it [50,68]. At early mitosis of less tension, Shugoshin mainly localizes to the inner centromere where it binds cohesin to protect centromeric cohesion; at metaphase, more tension by full kinetochore-microtubule attachments triggers Shugoshin translocation to the kinetochore-proximal region, which may initiate centromeric cohesion de-protection. Thus, Sgo1 function coordinates well with mitotic progression. Although Shugoshin was removed from the inner centromere in metaphase-arrested cells treated with MG132 for a short period of time, cohesin seemed to be retained at inner centromeres and centromeric cohesion was still intact [70], suggesting that centromeric cohesin is still under protection even though Sgo1 is absent. Of course, it is possible that under these conditions, a small portion of Sgo1 that may be invisible by immunostaining is retained and binds to centromeric cohesin, which might be sufficient for centromeric cohesion protection. Alternatively, in MG132-arrested metaphase cells, either the pathways resolving cohesion are not fully activated [71] or the other cohesion-protection pathways, such as Haspin/Pds5B, are still active [72,73]. Another possibility is that the cohesin concentration at centromeres is much higher than the threshold, under which sister chromatids will separate from each other [74]. In addition, degradation of Sgo1 by the APC/C at anaphase onset may also facilitate the inactivation of Sgo1 function [75,76].
As PP2A is essential for centromeric cohesion protection [44–46], it was therefore proposed that SET, a cellular PP2A inhibitor [77,78], could function at metaphase-to-anaphase transition to de-protect centromeric cohesion [79–82] (Figure 1), but compelling evidence to support it is still lacking. Interestingly, this very SET has also been found to physically interact with both Sgo1 and Sgo2 and the interaction is direct [45,70,83–85]. Further analyses on the Sgo1–SET interaction suggested that SET binding to Sgo1 can remove Sgo1 from centromeric regions and promote timely chromosome segregation, thus establishing SET as a key Sgo1 inhibitor [70,84]. Mechanistically, SET can inhibit Sgo1 in two ways (Figure 1): First, SET binding to Sgo1 discharges Sgo1 from chromatin, thus reducing Sgo1 concentration from centromeres [84]. This might be achieved through the inhibition of the binding between the Sgo1 C-terminus and nucleosomes. Second, a major SET-binding domain in Sgo1 is in very close proximity to the cohesin-binding domain [70]. SET binding to Sgo1 disrupts the Sgo1–cohesin binding that is essential for cohesion protection, thus resolving centromeric cohesion. These two compatible mechanisms well explain how Sgo1 is inactivated at metaphase-to-anaphase transition. Then, another important question is why SET mainly functional at metaphase-to-anaphase transition but not at early mitosis? Studying post-translational modifications or/and spatial regulation of SET could be key for addressing this question in future [86,87].
Extensive studies have shown that SET is highly expressed in various types of cancer cells and tissues [88]. Considering that impaired centromeric cohesion is a common phenomenon in cancer cells [89–91], it is possible that SET overexpression could be a main cause of the phenomenon. Actually, our recent preliminary results support that SET overexpression is a universal mechanism underlying impaired centromeric cohesion in cancer cells. In future, it would be intriguing to determine if the impaired Sgo1–SET pathway contributes to tumorigenesis in animal models.
Conclusion
A great deal of effort from many laboratories have established the essential role of Shugoshin in centromeric cohesion protection and uncovered the key factors and pathways that are important for the regulation of its functions. These findings have not only provided the critical mechanisms underlying sister-chromatid cohesion regulation, but also provoked further outstanding questions. First, how does centromeric transcription regulate chromosome segregation? The contradicting results about the roles of centromeric transcription during mitosis in chromosome segregation have been reported. As various types of transcriptional inhibitors were utilized and centromeric transcription was not rigorously evaluated in these studies, the seemingly contradictory results might reflect the outcomes of differential efficacies of various types of transcriptional inhibitors on centromeric transcription. Therefore, further experimentation is needed for clarification on this issue. More importantly, methods to specifically inactivate centromeric transcription are critically needed to explore its specific functions in chromosome segregation. Second, how does the Sgo1–SET pathway regulate cohesin dynamics? Cdk1-phosphorylated Sgo1 directly binds the cohesin sub-complex of Scc1/SA2 and Separase cleavage occurs on the cohesin subunit Scc1 [47,92,93]. Thus, in addition to enabling PP2A to suppress cohesin phosphorylation that promotes Separase cleavage [93–96], the Sgo1–cohesin binding might also be able to directly block Separase cleavage [68]. In future, it will be interesting to test this idea. In addition, if SET mainly deprotects centromeric cohesion at metaphase-to-anaphase transition, how is SET function suppressed at early mitosis or how is SET function activated at metaphase-to-anaphase transition? Determining SET regulation biochemically and cellularly could be key to address these questions. Third, does the impaired Sgo1–SET pathway contribute to tumorigenesis? Our preliminary results show that SET overexpression is a universal mechanism underlying impaired centromeric cohesion in cancer cells. The cohesion defects in cancer cells could be a legacy inherited from the process of tumorigenesis. These interesting observations raise a possibility that SET overexpression weakens centromeric cohesion, which leads to chromosomal instability and aneuploidy that contributes to tumorigenesis. In future, it would be intriguing to test if this is the case using transgenic mice that are overexpressing SET. Application of our isolated separation-of-function SET mutants would help dissect the molecular mechanisms [70]. Finally, in addition to its canonical functions in chromosome segregation, does Shugoshin have other non-canonical functions? Emerging evidence indicates that mutation or haploinsufficiency of Shugoshin may cause the CAID syndrome (Chronic Atrial and Intestinal Dysrhythmia) in human and Alzheimer-like disease in mice [97,98]. A recent study demonstrated that Sgo2 may function as a stress regulator by binding to and regulating RNAP II [99]. These interesting observations suggest that Shugoshin may have non-canonical functions other than canonical functions in chromosome segregation albeit further clarification is needed. In future, it would be important to determine whether and how non-canonical functions of Shugoshin are associated with human diseases.
Summary.
Cdk1-enabled Sgo1 binding to cohesin is required for centromeric cohesion protection and installs Sgo1 at inner centromeres. Bub1 phosphorylation facilitates Sgo1 enrichment at inner centromeres.
Transcription promotes Sgo1 installment to inner centromeres.
SET binding to Sgo1 promotes Sgo1 removal from inner centromeres at metaphase-to-anaphase transition, facilitating the inactivation of Sgo1 function of cohesion protection.
Funding
This work was supported by the Tulane startup and NIH funds [grant numbers NIH P20GM103629 and R01GM124018 (to H.L.)].
Abbreviations
- Cdk1
Cyclin-dependent kinase 1
- pH2A
phospho-histone H2A
- RNAP II
RNA polymerase II
- Sgo1
Shugoshin-1
- Sgo2
Shugoshin-2
Footnotes
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Watanabe Y (2005) Shugoshin: guardian spirit at the centromere. Curr. Opin. Cell Biol 17, 590–595, 10.1016/j.ceb.2005.10.003 [DOI] [PubMed] [Google Scholar]
- 2.Wang X and Dai W (2005) Shugoshin, a guardian for sister chromatid segregation. Exp. Cell Res 310, 1–9, 10.1016/j.yexcr.2005.07.018 [DOI] [PubMed] [Google Scholar]
- 3.Salic A, Waters JC and Mitchison TJ (2004) Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell 118, 567–578, 10.1016/j.cell.2004.08.016 [DOI] [PubMed] [Google Scholar]
- 4.McGuinness BE, Hirota T, Kudo NR, Peters JM and Nasmyth K (2005) Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol. 3, e86, 10.1371/journal.pbio.0030086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang Z, Sun Y, Harley SE, Zou H and Yu H (2004) Human Bub1 protects centromeric sister-chromatid cohesion through Shugoshin during mitosis. Proc. Natl. Acad. Sci. U.S.A 101, 18012–18017, 10.1073/pnas.0408600102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitajima TS, Hauf S, Ohsugi M, Yamamoto T and Watanabe Y (2005) Human Bub1 defines the persistent cohesion site along the mitotic chromosome by affecting Shugoshin localization. Curr. Biol 15, 353–359, 10.1016/j.cub.2004.12.044 [DOI] [PubMed] [Google Scholar]
- 7.Kitajima TS, Kawashima SA and Watanabe Y (2004) The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature 427, 510–517, 10.1038/nature02312 [DOI] [PubMed] [Google Scholar]
- 8.Hamant O, Golubovskaya I, Meeley R, Fiume E, Timofejeva L, Schleiffer A et al. (2005) A REC8-dependent plant Shugoshin is required for maintenance of centromeric cohesion during meiosis and has no mitotic functions. Curr. Biol 15, 948–954, 10.1016/j.cub.2005.04.049 [DOI] [PubMed] [Google Scholar]
- 9.Vaur S, Cubizolles F, Plane G, Genier S, Rabitsch PK, Gregan J et al. (2005) Control of Shugoshin function during fission-yeast meiosis. Curr. Biol 15, 2263–2270, 10.1016/j.cub.2005.11.034 [DOI] [PubMed] [Google Scholar]
- 10.Rabitsch KP, Gregan J, Schleiffer A, Javerzat JP, Eisenhaber F and Nasmyth K (2004) Two fission yeast homologs of Drosophila Mei-S332 are required for chromosome segregation during meiosis I and II. Curr. Biol 14, 287–301, 10.1016/j.cub.2004.01.051 [DOI] [PubMed] [Google Scholar]
- 11.Katis VL, Galova M, Rabitsch KP, Gregan J and Nasmyth K (2004) Maintenance of cohesin at centromeres after meiosis I in budding yeast requires a kinetochore-associated protein related to MEI-S332. Curr. Biol 14, 560–572, 10.1016/j.cub.2004.03.001 [DOI] [PubMed] [Google Scholar]
- 12.Marston AL, Tham WH, Shah H and Amon A (2004) A genome-wide screen identifies genes required for centromeric cohesion. Science 303, 1367–1370, 10.1126/science.1094220 [DOI] [PubMed] [Google Scholar]
- 13.Gregan J, Rabitsch PK, Sakem B, Csutak O, Latypov V, Lehmann E et al. (2005) Novel genes required for meiotic chromosome segregation are identified by a high-throughput knockout screen in fission yeast. Curr. Biol 15, 1663–1669, 10.1016/j.cub.2005.07.059 [DOI] [PubMed] [Google Scholar]
- 14.Kiburz BM, Reynolds DB, Megee PC, Marston AL, Lee BH, Lee TI et al. (2005) The core centromere and Sgo1 establish a 50-kb cohesin-protected domain around centromeres during meiosis I. Genes Dev. 19, 3017–3030, 10.1101/gad.1373005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Indjeian VB, Stern BM and Murray AW (2005) The centromeric protein Sgo1 is required to sense lack of tension on mitotic chromosomes. Science 307, 130–133, 10.1126/science.1101366 [DOI] [PubMed] [Google Scholar]
- 16.Indjeian VB and Murray AW (2007) Budding yeast mitotic chromosomes have an intrinsic bias to biorient on the spindle. Curr. Biol 17, 1837–1846, 10.1016/j.cub.2007.09.056 [DOI] [PubMed] [Google Scholar]
- 17.Vanoosthuyse V, Prykhozhij S and Hardwick KG (2007) Shugoshin 2 regulates localization of the chromosomal passenger proteins in fission yeast mitosis. Mol. Biol. Cell 18, 1657–1669, 10.1091/mbc.e06-10-0890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawashima SA, Tsukahara T, Langegger M, Hauf S, Kitajima TS and Watanabe Y (2007) Shugoshin enables tension-generating attachment of kinetochores by loading Aurora to centromeres. Genes Dev. 21, 420–435, 10.1101/gad.1497307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernius J and Hardwick KG (2007) Bub1 kinase targets Sgo1 to ensure efficient chromosome biorientation in budding yeast mitosis. PLos Genet. 3, e213, 10.1371/journal.pgen.0030213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiburz BM, Amon A and Marston AL (2008) Shugoshin promotes sister kinetochore biorientation in Saccharomyces cerevisiae. Mol. Biol. Cell 19, 1199–1209, 10.1091/mbc.e07-06-0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storchova Z, Becker JS, Talarek N, Kogelsberger S and Pellman D (2011) Bub1, Sgo1, and Mps1 mediate a distinct pathway for chromosome biorientation in budding yeast. Mol. Biol. Cell 22, 1473–1485, 10.1091/mbc.e10-08-0673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawashima S, Nakabayashi Y, Matsubara K, Sano N, Enomoto T, Tanaka K et al. (2011) Global analysis of core histones reveals nucleosomal surfaces required for chromosome bi-orientation. EMBO J. 30, 3353–3367, 10.1038/emboj.2011.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haase J, Stephens A, Verdaasdonk J, Yeh E and Bloom K (2012) Bub1 kinase and Sgo1 modulate pericentric chromatin in response to altered microtubule dynamics. Curr. Biol 22, 471–481, 10.1016/j.cub.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin F and Wang Y (2013) The signaling network that silences the spindle assembly checkpoint upon the establishment of chromosome bipolar attachment. Proc. Natl. Acad. Sci. U.S.A 110, 21036–21041, 10.1073/pnas.1307595111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peplowska K, Wallek AU and Storchova Z (2014) Sgo1 regulates both condensin and Ipl1/Aurora B to promote chromosome biorientation. PLos Genet. 10, e1004411, 10.1371/journal.pgen.1004411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eshleman HD and Morgan DO (2014) Sgo1 recruits PP2A to chromosomes to ensure sister chromatid bi-orientation during mitosis. J. Cell Sci 127, 4974–4983, 10.1242/jcs.161273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin F, Bokros M and Wang Y (2017) Premature Silencing of the Spindle Assembly Checkpoint Is Prevented by the Bub1-H2A-Sgo1-PP2A Axis in Saccharomyces cerevisiae. Genetics 205, 1169–1178, 10.1534/genetics.116.195727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meppelink A, Kabeche L, Vromans MJ, Compton DA and Lens SM (2015) Shugoshin-1 balances Aurora B kinase activity via PP2A to promote chromosome bi-orientation. Cell Rep. 11, 508–515, 10.1016/j.celrep.2015.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang C, Zhang Z, Chen Q, Yan H, Zhang M, Zhou L et al. (2020) Centromere-localized Aurora B kinase is required for the fidelity of chromosome segregation. J. Cell Biol 219, 10.1083/jcb.201907092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clift D and Marston AL (2011) The role of shugoshin in meiotic chromosome segregation. Cytogenet. Genome Res 133, 234–242, 10.1159/000323793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandler L, Romans P and Figenshow J (1974) An effect of centromere function on the behavior of ring-X chromosomes in Drosophila melanogaster. Genetics 77, 299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerrebrock AW, Miyazaki WY, Birnby D and Orr-Weaver TL (1992) The Drosophila mei-S332 gene promotes sister-chromatid cohesion in meiosis following kinetochore differentiation. Genetics 130, 827–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerrebrock AW, Moore DP, Wu JS and Orr-Weaver TL (1995) Mei-S332, a Drosophila protein required for sister-chromatid cohesion, can localize to meiotic centromere regions. Cell. 83, 247–256, 10.1016/0092-8674(95)90166-3 [DOI] [PubMed] [Google Scholar]
- 34.Yamada T, Tahara E, Kanke M, Kuwata K and Nishiyama T (2017) Drosophila Dalmatian combines sororin and shugoshin roles in establishment and protection of cohesion. EMBO J. 36, 1513–1527, 10.15252/embj.201695607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gandhi R, Gillespie PJ and Hirano T (2006) Human Wapl is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase. Curr. Biol 16, 2406–2417, 10.1016/j.cub.2006.10.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kueng S, Hegemann B, Peters BH, Lipp JJ, Schleiffer A, Mechtler K et al. (2006) Wapl controls the dynamic association of cohesin with chromatin. Cell 127, 955–967, 10.1016/j.cell.2006.09.040 [DOI] [PubMed] [Google Scholar]
- 37.Shintomi K and Hirano T (2009) Releasing cohesin from chromosome arms in early mitosis: opposing actions of Wapl-Pds5 and Sgo1. Genes Dev. 23, 2224–2236, 10.1101/gad.1844309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishiyama T, Ladurner R, Schmitz J, Kreidl E, Schleiffer A, Bhaskara V et al. (2010) Sororin mediates sister chromatid cohesion by antagonizing Wapl. Cell. 143, 737–749, 10.1016/j.cell.2010.10.031 [DOI] [PubMed] [Google Scholar]
- 39.Dreier MR, Bekier ME 2nd and Taylor WR (2011) Regulation of sororin by Cdk1-mediated phosphorylation. J. Cell Sci 124, 2976–2987, 10.1242/jcs.085431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Losada A, Hirano M and Hirano T (2002) Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis. Genes Dev. 16, 3004–3016, 10.1101/gad.249202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishiyama T, Sykora MM, Huis in ‘t Veld PJ, Mechtler K and Peters JM (2013) Aurora B and Cdk1 mediate Wapl activation and release of acetylated cohesin from chromosomes by phosphorylating Sororin. Proc. Natl. Acad. Sci. U.S.A 110, 13404–13409, 10.1073/pnas.1305020110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gimenez-Abian JF, Sumara I, Hirota T, Hauf S, Gerlich D, de la Torre C et al. (2004) Regulation of sister chromatid cohesion between chromosome arms. Curr. Biol 14, 1187–1193, 10.1016/j.cub.2004.06.052 [DOI] [PubMed] [Google Scholar]
- 43.Hauf S, Roitinger E, Koch B, Dittrich CM, Mechtler K and Peters JM (2005) Dissociation of cohesin from chromosome arms and loss of arm cohesion during early mitosis depends on phosphorylation of SA2. PLoS Biol. 3, e69, 10.1371/journal.pbio.0030069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riedel CG, Katis VL, Katou Y, Mori S, Itoh T, Helmhart W et al. (2006) Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature 441, 53–61, 10.1038/nature04664 [DOI] [PubMed] [Google Scholar]
- 45.Kitajima TS, Sakuno T, Ishiguro K, Iemura S, Natsume T, Kawashima SA et al. (2006) Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature 441, 46–52, 10.1038/nature04663 [DOI] [PubMed] [Google Scholar]
- 46.Tang Z, Shu H, Qi W, Mahmood NA, Mumby MC and Yu H (2006) PP2A is required for centromeric localization of Sgo1 and proper chromosome segregation. Dev. Cell 10, 575–585, 10.1016/j.devcel.2006.03.010 [DOI] [PubMed] [Google Scholar]
- 47.Liu H, Rankin S and Yu H (2013) Phosphorylation-enabled binding of SGO1-PP2A to cohesin protects sororin and centromeric cohesion during mitosis. Nat. Cell Biol 15, 40–49, 10.1038/ncb2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyarchuk Y, Salic A, Dasso M and Arnaoutov A (2007) Bub1 is essential for assembly of the functional inner centromere. J. Cell Biol 176, 919–928, 10.1083/jcb.200609044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawashima SA, Yamagishi Y, Honda T, Ishiguro K and Watanabe Y (2010) Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science 327, 172–177, 10.1126/science.1180189 [DOI] [PubMed] [Google Scholar]
- 50.Liu H, Jia L and Yu H (2013) Phospho-H2A and cohesin specify distinct tension-regulated Sgo1 pools at kinetochores and inner centromeres. Curr. Biol 23, 1927–1933, 10.1016/j.cub.2013.07.078 [DOI] [PubMed] [Google Scholar]
- 51.Hara K, Zheng G, Qu Q, Liu H, Ouyang Z, Chen Z et al. (2014) Structure of cohesin subcomplex pinpoints direct shugoshin-Wapl antagonism in centromeric cohesion. Nat. Struct. Mol. Biol 21, 864–870, 10.1038/nsmb.2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu H, Qu Q, Warrington R, Rice A, Cheng N and Yu H (2015) Mitotic Transcription Installs Sgo1 at Centromeres to Coordinate Chromosome Segregation. Mol. Cell 59, 426–436, 10.1016/j.molcel.2015.06.018 [DOI] [PubMed] [Google Scholar]
- 53.Liang C, Zhang Z, Chen Q, Yan H, Zhang M, Xiang X et al. (2019) A positive feedback mechanism ensures proper assembly of the functional inner centromere during mitosis in human cells. J. Biol. Chem 294, 1437–1450, 10.1074/jbc.RA118.006046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamagishi Y, Honda T, Tanno Y and Watanabe Y (2010) Two histone marks establish the inner centromere and chromosome bi-orientation. Science 330, 239–243, 10.1126/science.1194498 [DOI] [PubMed] [Google Scholar]
- 55.Resnick TD, Satinover DL, MacIsaac F, Stukenberg PT, Earnshaw WC, Orr-Weaver TL et al. (2006) INCENP and Aurora B promote meiotic sister chromatid cohesion through localization of the Shugoshin MEI-S332 in Drosophila. Dev. Cell 11, 57–68, 10.1016/j.devcel.2006.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dai J, Sullivan BA and Higgins JM (2006) Regulation of mitotic chromosome cohesion by Haspin and Aurora B. Dev. Cell 11, 741–750, 10.1016/j.devcel.2006.09.018 [DOI] [PubMed] [Google Scholar]
- 57.Wang F, Ulyanova NP, Daum JR, Patnaik D, Kateneva AV, Gorbsky GJ et al. (2012) Haspin inhibitors reveal centromeric functions of Aurora B in chromosome segregation. J. Cell Biol 199, 251–268, 10.1083/jcb.201205106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Waal MS, Saurin AT, Vromans MJ, Vleugel M, Wurzenberger C, Gerlich DW et al. (2012) Mps1 promotes rapid centromere accumulation of Aurora B. EMBO Rep. 13, 847–854, 10.1038/embor.2012.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Broad AJ, DeLuca KF and DeLuca JG (2020) Aurora B kinase is recruited to multiple discrete kinetochore and centromere regions in human cells. J. Cell Biol 219, 10.1083/jcb.201905144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hadders MA, Hindriksen S, Truong MA, Mhaskar AN, Wopken JP, Vromans MJM et al. (2020) Untangling the contribution of Haspin and Bub1 to Aurora B function during mitosis. J. Cell Biol 219, 10.1083/jcb.201907087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee NR, Kim HS, Kim YS, Kwon MH, Choi KS and Lee CW (2014) Regulation of the subcellular shuttling of Sgo1 between centromeres and chromosome arms by Aurora B-mediated phosphorylation. Biochem. Biophys. Res. Commun 454, 429–435, 10.1016/j.bbrc.2014.10.103 [DOI] [PubMed] [Google Scholar]
- 62.Eot-Houllier G, Magnaghi-Jaulin L, Fulcrand G, Moyroud FX, Monier S and Jaulin C (2018) Aurora A-dependent CENP-A phosphorylation at inner centromeres protects bioriented chromosomes against cohesion fatigue. Nat. Commun 9, 1888, 10.1038/s41467-018-04089-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chan FL, Marshall OJ, Saffery R, Kim BW, Earle E, Choo KH et al. (2012) Active transcription and essential role of RNA polymerase II at the centromere during mitosis. Proc. Natl. Acad. Sci. U.S.A 109, 1979–1984, 10.1073/pnas.1108705109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perea-Resa C, Bury L, Cheeseman IM and Blower MD (2020) Cohesin Removal Reprograms Gene Expression upon Mitotic Entry. Mol. Cell, 10.1016/j.molcel.2020.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Titov DV, Gilman B, He QL, Bhat S, Low WK, Dang Y et al. (2011) XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat. Chem. Biol 7, 182–188, 10.1038/nchembio.522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Novais-Cruz M, Alba Abad M, van IWF, Galjart N, Jeyaprakash AA, Maiato H et al. (2018) Mitotic progression, arrest, exit or death relies on centromere structural integrity, rather than de novo transcription. Elife 7, 10.7554/eLife.36898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clift D, Bizzari F and Marston AL (2009) Shugoshin prevents cohesin cleavage by PP2A(Cdc55)-dependent inhibition of separase. Genes Dev. 23, 766–780, 10.1101/gad.507509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee J, Kitajima TS, Tanno Y, Yoshida K, Morita T, Miyano T et al. (2008) Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat. Cell Biol 10, 42–52, 10.1038/ncb1667 [DOI] [PubMed] [Google Scholar]
- 69.Gomez R, Valdeolmillos A, Parra MT, Viera A, Carreiro C, Roncal F et al. (2007) Mammalian SGO2 appears at the inner centromere domain and redistributes depending on tension across centromeres during meiosis II and mitosis. EMBO Rep. 8, 173–180, 10.1038/sj.embor.7400877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qu Q, Zhang Q, Yang L, Chen Y and Liu H (2019) SET binding to Sgo1 inhibits Sgo1-cohesin interactions and promotes chromosome segregation. J. Cell Biol 218, 2514–2528, 10.1083/jcb.201810096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sapkota H, Wasiak E, Daum JR and Gorbsky GJ (2018) Multiple determinants and consequences of cohesion fatigue in mammalian cells. Mol. Biol. Cell 29, 1811–1824, 10.1091/mbc.E18-05-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou L, Liang C, Chen Q, Zhang Z, Zhang B, Yan H et al. (2017) The N-Terminal Non-Kinase-Domain-Mediated Binding of Haspin to Pds5B Protects Centromeric Cohesion in Mitosis. Curr. Biol 27, 992–1004, 10.1016/j.cub.2017.02.019 [DOI] [PubMed] [Google Scholar]
- 73.Liang C, Chen Q, Yi Q, Zhang M, Yan H, Zhang B et al. (2018) A kinase-dependent role for Haspin in antagonizing Wapl and protecting mitotic centromere cohesion. EMBO Rep. 19, 43–56, 10.15252/embr.201744737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carvalhal S, Tavares A, Santos MB, Mirkovic M and Oliveira RA (2018) A quantitative analysis of cohesin decay in mitotic fidelity. J. Cell Biol 217, 3343–3353, 10.1083/jcb.201801111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karamysheva Z, Diaz-Martinez LA, Crow SE, Li B and Yu H (2009) Multiple anaphase-promoting complex/cyclosome degrons mediate the degradation of human Sgo1. J. Biol. Chem 284, 1772–1780, 10.1074/jbc.M807083200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jonak K, Zagoriy I, Oz T, Graf P, Rojas J, Mengoli V et al. (2017) APC/C-Cdc20 mediates deprotection of centromeric cohesin at meiosis II in yeast. Cell Cycle 16, 1145–1152, 10.1080/15384101.2017.1320628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li M, Guo H and Damuni Z (1995) Purification and characterization of two potent heat-stable protein inhibitors of protein phosphatase 2A from bovine kidney. Biochemistry 34, 1988–1996, 10.1021/bi00006a020 [DOI] [PubMed] [Google Scholar]
- 78.Li M, Makkinje A and Damuni Z (1996) The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J. Biol. Chem 271, 11059–11062, 10.1074/jbc.271.19.11059 [DOI] [PubMed] [Google Scholar]
- 79.Chambon JP, Touati SA, Berneau S, Cladiere D, Hebras C, Groeme R et al. (2013) The PP2A inhibitor I2PP2A is essential for sister chromatid segregation in oocyte meiosis II. Curr. Biol 23, 485–490, 10.1016/j.cub.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 80.Qi ST, Wang ZB, Ouyang YC, Zhang QH, Hu MW, Huang X et al. (2013) Overexpression of SETbeta, a protein localizing to centromeres, causes precocious separation of chromatids during the first meiosis of mouse oocytes. J. Cell Sci 126, 1595–1603, 10.1242/jcs.116541 [DOI] [PubMed] [Google Scholar]
- 81.Moshkin YM, Doyen CM, Kan TW, Chalkley GE, Sap K, Bezstarosti K et al. (2013) Histone chaperone NAP1 mediates sister chromatid resolution by counteracting protein phosphatase 2A. PLos Genet. 9, e1003719, 10.1371/journal.pgen.1003719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Higgins JM and Herbert M (2013) Nucleosome assembly proteins get SET to defeat the guardian of chromosome cohesion. PLos Genet. 9, e1003829, 10.1371/journal.pgen.1003829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herzog F, Kahraman A, Boehringer D, Mak R, Bracher A, Walzthoeni T et al. (2012) Structural probing of a protein phosphatase 2A network by chemical cross-linking and mass spectrometry. Science 337, 1348–1352, 10.1126/science.1221483 [DOI] [PubMed] [Google Scholar]
- 84.Krishnan S, Smits AH, Vermeulen M and Reinberg D (2017) Phospho-H1 Decorates the Inter-chromatid Axis and Is Evicted along with Shugoshin by SET during Mitosis. Mol. Cell 67, 579e6–593e6, 10.1016/j.molcel.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Asai Y, Fukuchi K, Tanno Y, Koitabashi-Kiyozuka S, Kiyozuka T, Noda Y et al. (2019) Aurora B kinase activity is regulated by SET/TAF1 on Sgo2 at the inner centromere. J. Cell Biol 218, 3223–3236, 10.1083/jcb.201811060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yin L, Zeng Y, Xiao Y, Chen Y, Shen H and Dong J (2019) Cyclin-dependent kinase 1-mediated phosphorylation of SET at serine 7 is essential for its oncogenic activity. Cell Death. Dis 10, 385, 10.1038/s41419-019-1621-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seibert M, Kruger M, Watson NA, Sen O, Daum JR, Slotman JA et al. (2019) CDK1-mediated phosphorylation at H2B serine 6 is required for mitotic chromosome segregation. J. Cell Biol 218, 1164–1181, 10.1083/jcb.201806057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hung MH and Chen KF (2017) Reprogramming the oncogenic response: SET protein as a potential therapeutic target in cancer. Exp. Opin. Ther. Targets 21, 685–694, 10.1080/14728222.2017.1336226 [DOI] [PubMed] [Google Scholar]
- 89.Sajesh BV, Lichtensztejn Z and McManus KJ (2013) Sister chromatid cohesion defects are associated with chromosome instability in Hodgkin lymphoma cells. BMC Cancer 13, 391, 10.1186/1471-2407-13-391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barber TD, McManus K, Yuen KW, Reis M, Parmigiani G, Shen D et al. (2008) Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc. Natl. Acad. Sci. U.S.A 105, 3443–3448, 10.1073/pnas.0712384105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stoepker C, Ameziane N, van der Lelij P, Kooi IE, Oostra AB, Rooimans MA et al. (2015) Defects in the Fanconi Anemia Pathway and Chromatid Cohesion in Head and Neck Cancer. Cancer Res. 75, 3543–3553, 10.1158/0008-5472.CAN-15-0528 [DOI] [PubMed] [Google Scholar]
- 92.Uhlmann F, Lottspeich F and Nasmyth K (1999) Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400, 37–42, 10.1038/21831 [DOI] [PubMed] [Google Scholar]
- 93.Lin Z, Luo X and Yu H (2016) Structural basis of cohesin cleavage by separase. Nature 532, 131–134, 10.1038/nature17402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Katis VL, Lipp JJ, Imre R, Bogdanova A, Okaz E, Habermann B et al. (2010) Rec8 phosphorylation by casein kinase 1 and Cdc7-Dbf4 kinase regulates cohesin cleavage by separase during meiosis. Dev. Cell 18, 397–409, 10.1016/j.devcel.2010.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ishiguro T, Tanaka K, Sakuno T and Watanabe Y (2010) Shugoshin-PP2A counteracts casein-kinase-1-dependent cleavage of Rec8 by separase. Nat. Cell Biol 12, 500–506, 10.1038/ncb2052 [DOI] [PubMed] [Google Scholar]
- 96.Alexandru G, Uhlmann F, Mechtler K, Poupart MA and Nasmyth K (2001) Phosphorylation of the cohesin subunit Scc1 by Polo/Cdc5 kinase regulates sister chromatid separation in yeast. Cell 105, 459–472, 10.1016/S0092-8674(01)00362-2 [DOI] [PubMed] [Google Scholar]
- 97.Rao CV, Farooqui M, Zhang Y, Asch AS and Yamada HY (2018) Spontaneous development of Alzheimer’s disease-associated brain pathology in a Shugoshin-1 mouse cohesinopathy model. Aging Cell 17, e12797, 10.1111/acel.12797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chetaille P, Preuss C, Burkhard S, Cote JM, Houde C, Castilloux J et al. (2014) Mutations in SGOL1 cause a novel cohesinopathy affecting heart and gut rhythm. Nat. Genet 46, 1245–1249, 10.1038/ng.3113 [DOI] [PubMed] [Google Scholar]
- 99.Takii R, Fujimoto M, Matsumoto M, Srivastava P, Katiyar A, Nakayama KI et al. (2019) The pericentromeric protein shugoshin 2 cooperates with HSF1 in heat shock response and RNA Pol II recruitment. EMBO J. 38, e102566, 10.15252/embj.2019102566 [DOI] [PMC free article] [PubMed] [Google Scholar]


