Abstract
During infection, sickness behaviors, such as a hunched stance with piloerection, can facilitate host resistance by supporting the generation and maintenance of fever. Fever, in turn, is mediated by hypothalamic neuroimmune signaling. Sickness behaviors, however, can also be influenced by social stimuli. In the present study, guinea pig pups were injected with lipopolysaccharide to simulate a bacterial infection and then exposed to a novel, threatening environment while either with their mother or alone. We found the presence of the mother suppressed sickness behavior, but enhanced fever, and had no measureable effect on gene expression of hypothalamic mediators of fever. This 3-way dissociation induced by the mother’s presence is interpreted in terms of the differential adaptive consequences of behavioral and febrile responses for pups in this situation. The results contribute to a growing literature linking immunological and social processes.
Keywords: mother-infant, maternal influence, sickness behavior, fever, infection, maternal separation, stress
INTRODUCTION
“Sickness behaviors” designate a class of behavioral adjustments induced by neuroimmune signaling in response to infection (Dantzer & Kelley, 2007). Although specific patterns vary by species, a primary function of sickness behaviors is to support the energetically demanding task of generating and maintaining fever, which then slows pathogen reproduction and enhances immunological efficiency in combating infection (Hart, 1988; Sylvia & Demas, 2017). Some sickness behaviors (e.g., shivering, seeking warmth, hunched posture with piloerection) generate or conserve body heat, while others (e.g., lethargy, somnolence) conserve non-thermal energy. Diverse neuroimmune signaling pathways in brain stem, limbic, and cortical regions appear to mediate sickness behaviors (Konat, 2016; Konsman, Luheshi, Bluthé, & Dantzer, 2000; Maes et al., 2012) whereas proinflammatory cytokine and prostaglandin signaling affecting the hypothalamus is critical for generating the fever response (Conti, Tabarean, Andrei, & Bartfai, 2004; Saper, Romanovsky, & Scammell, 2012).
Despite their clear link to host resistance following infection, sickness behaviors are nonetheless sensitive to species-relevant social and other environmental stimuli. For instance, it has long been known that if male rats have an opportunity to mate, or lactating females detect a need to care for pups, sickness behaviors can be suppressed so that mating or care-taking of pups can occur (Aubert, Goodall, Dantzer, & Gheusi, 1997; Yirmiya, Avitsur, Donchin, & Cohen, 1995). Such observations led to the notion that sickness behaviors are motivated, in that an individual can suppress the expression of sickness behaviors when incompatible behaviors are required to meet a more-pressing need (Aubert, 1999). Viewed from an evolutionary perspective, sickness behaviors divert resources from other fitness-related activities, such as mating or territorial defense. An infected animal must, therefore, evaluate ecological and social conditions and opportunities as well as the threat imposed by infection to determine whether resources are best invested in sickness behaviors or other fitness activities at a particular point in time (Adelman & Martin, 2009; Lopes, 2014).
Not only can pathogen-induced sickness behaviors be suppressed or modulated by environmental events, these events—specifically many stressors—can induce sickness behaviors, fever, and underlying changes in neuroimmune signaling in the absence of pathogen exposure (Maier & Watkins, 1998). Indeed, administration of peptides with anti-inflammatory properties reverse stress-induced sickness behaviors (Arakawa, Blandino, & Deak, 2009) and fever (Milligan et al., 1998). These findings suggest the possibility that neuroimmune pathways subserving sickness behavior might be recruited to produce behavior that is adaptive for coping with environmental challenges unrelated to pathogen exposure or recuperation (Hennessy, Deak, & Schiml-Webb, 2001).
Infant guinea pigs isolated in a brightly lit novel environment exhibit a two-stage behavioral reaction: an initial active period of repetitive, high-pitched vocalizing, often with increased movement, followed an hour or so later by onset of a passive stage characterized by an immobile crouched stance, prolonged eye-closure, and extensive piloerection (Hennessy, Long, Nigh, Williams, & Nolan, 1995), responses recognized as classic sickness behaviors when they occur in infected individuals (Hart, 1988). The ability of COX inhibitors and other anti-inflammatories to suppress the passive behaviors of separated pups confirms that the responses are mediated by neuroimmune signaling, that is, that they represent true stress-induced sickness behaviors (Hennessy et al., 2007, Hennessy et al., 2015; Perkeybile, Schiml-Webb, O’Brien, Deak, & Hennesy, 2009; Schiml-Webb, Deak, Greenlee, Maken, & Hennessy, 2006). Further, although initially accompanied by fever, these sickness behaviors persist hours longer (Hennessy et al., 1995). This dissociation indicates that sickness behaviors in this context are not serving only to support generation and maintenance of fever. For a common prey species, such as the wild ancestor of the domestic guinea pig, isolation of a pup in a brightly lit, open environment is a particularly threatening situation. We have argued that emergence of sickness behavior when vocalizing has failed to re-establish contact with the mother is an adaptive response because it conserves energy and reduces detection by predators (Hennessy, Deak, & Schiml, 2014). The finding that the sickness behaviors of 3–4-week-old pups rapidly disappeared upon placement of the mother in the test cage (Hennessy et al., 2013) is consistent with this interpretation because suppression of sickness behavior in the natural environment would permit the pup to follow the mother to safer surroundings. Further, because the little active care guinea pig females provide pups has virtually ceased by this age (König, 1985; Schiml & Hennessy, 1990), it is the mere presence of the mother, rather than her treatment of the pup, that suppresses sickness behaviors.
The presence of the mother can also suppress pup sickness behaviors induced pharmacologically. Administration of lipopolysaccharide (LPS), which is derived from the cell wall of gram negative bacteria, simulates a bacterial infection resulting in increased neuroimmune signaling, fever, and sickness behaviors (Rummel, 2016). If guinea pig pups are injected with LPS and then isolated in novel surroundings, they begin exhibiting pronounced crouching, eye-closure, and piloerection during the first hour of isolation when vocalizing and other active behavior predominates in untreated pups (Hennessy et al., 2004). However, when the LPS-injected pups were placed in the novel environment together with the mother, sickness behaviors were suppressed, though this effect was clear only for males (Hennessy et al., 2013). This suppression raises the question of the extent of the mother’s impact. Is she reducing only sickness behavior or is she also suppressing underlying fever and neuroimmune signaling? A motivational perspective might suggest that the pups are only adjusting their behavior. That is, for an infected pup isolated in an open, threatening environment, motivation to exhibit sickness behaviors may be high both because these behaviors help prevent detection from predators, and because they support host defense. If, however, the mother is present, following her to safety would seem to be of greater immediate importance, and passive sickness behaviors might then be suppressed so that the pup can in engage in the incompatible behavior of following her. On the other hand, from an evolutionary perspective in which pups are more-generally altering their allocation of resources, one might expect resources devoted to fever, as well sickness behaviors, to be diverted to actively following the mother to safety. The present study examined these possibilities. Experiment 1 asked whether or not maternal suppression of LPS-induced sickness behavior in guinea pig pups was accompanied by suppression of fever. The relative influence of the mother on male versus female pups was also re-examined. Experiment 2 then assessed the mother’s impact on central neuroimmune signaling underlying the febrile response.
METHOD
Subjects
Albino guinea pigs (Cavia porcellus) of the Hartley strain were bred in our laboratory using breeders obtained from Hilltop Lab Animals Inc, Scottdale PA. Each mother and her litter were housed in an opaque plastic cage (73 × 54 × 24 cm) with wire front and sawdust bedding. Water and guinea pig chow were available ad libitum. Lights were maintained on a 12:12 light:dark cycle, with lights on at 0700 hr. Cages were changed twice per week. All procedures were approved by the Wright State University Institutional Animal Care and Use Committee.
Surgery to implant telemetry probes
In Experiment 1, telemetry probes (PD 4000 Emitter, Philips Respironics) were surgically implanted into the abdominal cavity under isoflurane anesthesia (2–4%) using aseptic procedures between 15 and 19 days of age. Pups were treated with 0.05 mg/kg atropine (i.p.) to reduce secretory activity. Buprenorphine (0.015 mg/0.05 ml) was given s.c. immediately following surgery and again 6–12 hr and 24 hr later. Behavioral testing did not begin for at least 3 days following surgery, at which time pups appeared fully recovered. Core temperature and movement were measured by the telemetry probe, which sent signals to a receiver plate (55 × 28 cm) placed under the cage during testing. The temperature and motor activity data were collected with computer software (Vital View), which aggregated data in 3-min bins.
Injection and test procedures
Ninety min prior to testing, pups were injected i.p. (1 ml/kg) with either 100 μg/kg LPS (E. coli, serotype 026:B6, Sigma-Aldrich, Lot #8274) or isotonic saline vehicle. This dose of LPS was chosen based on a preliminary study that indicated 100 μg/kg produced a clear elevation of core temperature in guinea pig pups. For testing, pups were carried in a transport cage (< 10 s) from the colony room to the testing room where they were placed into an empty, clear plastic cage (47 × 24 × 20 cm), either together with their mother or alone, on a table top under full room lighting. Behavior was observed through 1-way glass. The test cage was cleaned with detergent after each test. Pups were tested between 20 and 23 days of age, by which time they are nearly completely thermocompetent, including being able to mount a febrile response to LPS (Blatteis, 1975; Dawes & Mestyán, 1963; Fewell, Kang, & Eliason, 1997). Natural weaning occurs around Day 25 (König, 1985; Schiml & Hennessy, 1990) though young guinea pigs continue to show a strong attraction to the mother, as well as behavioral and physiological reactions to her presence and absence, for weeks thereafter (Hennessy, Maken, & Graves, 2002; Hennessy & Morris, 2005; Hennessy, Young, O’Leary, & Maken, 2003).
A mirror positioned ~ 0.75 m behind the cage helped determine the pup’s behavior if the mother blocked the direct view of the observer. Because passive behavior typically occurs over an extended period of time, these responses were scored with one-zero sampling as in previous studies (e.g., Hennessy et al., 2013). That is, a trained observer (85% or better inter-observer reliability) recorded the number of 1-min intervals in which pups exhibited the characteristic crouched posture in which the feet are tucked beneath the body, complete or near complete closure of one or both eyes (> 1 s), and extensive piloerection (over half the body). However, because the mother often occluded the observer’s view of the infant’s eyes, the measure of eye-closing was dropped from the study (as in Hennessy et al., 2013). Therefore, our measure of passive (i.e., sickness) behavior was the number of 1-min intervals in which crouch and piloerection both occurred. In addition, if the mother was positioned such that either crouching or piloerection could not be clearly determined, that 1-min interval was marked as unscored. If fewer than half of the 30, 1-min intervals were marked unscored for any scoring period, we calculated the percentage of scored 1-min intervals in which each behavior was seen and then assigned the pup the number corresponding to the same percentage of 30, 1-min intervals. If more than half of the pup’s observations were marked unscored in any 30-min scoring interval, the animal’s sickness behavior data were dropped from the study. This occurred for three males and one female in Experiment 1 and one male in Experiment 2. For each experiment, the time of initiation of testing did not vary by more than 3.5 hr.
Tissue collection and processing
In Experiment 2, brains were harvested after rapid decapitation, immediately flash frozen in 2-methylbutane, and stored at −80°C. Once testing was complete, the brains were partially thawed, and the hypothalamus was dissected and immediately refrozen. For processing, each tissue sample was placed in a 2.0 ml Eppendorf tube with 700 μl Trizol® RNA reagent and a 5 mm stainless steel bead. Tissue was then homogenized using a Qiagen TissueLyser II™ (Qiagen, Valencia, CA) for 2–4 min at 20 Hz to ensure thorough homogenization of samples. Total cellular RNA was extracted from tissue using Qiagen RNeasy Mini Kits according to the manufacturer’s instructions. RNA was separated from the supernatant through chloroform extraction performed at 12,000 g for 15 min at 4°C. Equal volume of 70% ethanol was added to the collected RNA and purified through RNeasy mini columns (Qiagen). Columns were washed and eluted with 30 μl of RNase-free water (warmed to 65°C). RNA yield and purity was determined using the NanoDrop 2000 spectrophotometer (NanoDrop, Wilmington, DE). RNA was stored at −80 °C prior to cDNA synthesis.
Real-time RT-PCR
All RT-PCR was conducted using procedures described in previous work (Hueston & Deak, 2014). Synthesis of cDNA was performed on 0.3–1.0 μg of normalized total RNA from each sample using QuantiTect Reverse Transcription kit (Qiagen), which included a DNase treatment step. All cDNA was stored at −20 °C until further processing. Probed cDNA amplification was performed in a 20 μl reaction consisting of 10 μl IQ SYBR Green supermix (BioRad Laboratories), 0.1 μl forward and reverse primer, 2 μl cDNA template, and 8.8 μl ribonuclease-free water. Samples were run in triplicate in a 384-well plate (BioRad Laboratories) using BioRad CFX 384 Real Time System C1000 Thermal Cycler (BioRad Laboratories). Relative gene expression was quantified using the 2 - ΔΔCT method (Livak & Schmittgen, 2001), with target gene expression normalized to the referenced gene, β-actin. Before analyses of target genes, reference gene expression was first separately analyzed in each tissue compartment to ensure stability. Primer sequences for all target genes were recently published in Hennessy et al (2019).
Experiment 1
To determine if the presence of the mother would reduce fever as well as sickness behavior following LPS injection and exposure to novelty, and whether there were sex differences in these effects, separate groups of 12 male and 12 female pups were placed into test cage either together with the mother or alone following LPS injection. Eleven or 12 separate litters contributed pups to each of these four groups. Pups were tested for 180 min, with sickness behavior observed during Min 0–30, 60–90, and 150–180 and temperature and movement data collected during the entire session.
Experiment 2
Here we assessed the effect of the mother’s presence on hypothalamic gene expression of neuroimmune signaling molecules. In addition to cyclooxygenase-2 (COX-2), which synthesizes the prostaglandin PGE-2, whose action in the hypothalamus appears essential for generating LPS-induced fever in guinea pigs (Sehic, Székely, Ungar, Oladehin, & Blatteis,1996), we also examined expression of three classic proinflammatory cytokines (IL-1β, TNF-α, IL-6), as well as of the chemokine MCP-1. We recently found hypothalamic expression of MCP-1, as well as COX-2, to be responsive to LPS injection and exposure to novelty in guinea pig pups (Hennessy et al., 2019). As in Experiment 1, LPS was injected 90 min prior to exposure to the test cage. We harvested brains to measure gene expression at two time points: 30 and 90 min following test cage exposure. The 30-min time point (i.e., 2-hr following LPS injection) was chosen because early pilot work found an increase in IL-1β immunoreactivity in hypothalamus 2 hr following LPS injection in guinea pig pups (Hennessy et al., 2004). The 90-min time point was included because it approximated the peak fever response in Experiment 1. Behavior was examined for 30 min prior to tissue sampling at each time point.
Because of the lack of sex differences in Experiment 1, groups in Experiment 2 included both sexes. Sample sizes for LPS-injected pups were as follows: 30 min with mother—n = 10 (4 males, 6 females); 90 min with mother—n = 10 (4 males, 6 females); 30 min alone—n = 9 (4 males, 5 females); 90 min alone—n = 10 (4 males, 6 females). For each group, pups were drawn from 9 or 10 separate litters. In addition, to allow determination of the magnitude of effect of the experimental manipulations, we also included control groups injected with saline vehicle and then returned to the home cage for either 120 min (n = 5, 2 males, 3 females) or 180 min (n = 5, 2 males, 3 females). For each control group, pups were drawn from 5 litters.
Statistical analysis
Due to non-normal distributions (many “zeros” and maximum responses), sickness behaviors were assessed with Mann-Whitney U tests and central tendency and variability are represented as medians and semi-interquartile ranges. In Experiment 1, core body temperature and motor activity data were grouped into 15-min time blocks as in previous studies (e.g., Schneider, Schiml, Deak, & Hennessy, 2012). These data were then analyzed in 2 (With Mother/Alone) x 2 (Sex) x 12 (Time Block) analyses of variance (ANOVA) with Time Block as a repeated measure. For both core temperature and motor activity, sphericity was problematic as indicated by the Mauchly test. Therefore, the Huynh-Feldt correction was used and corrected degrees of freedom are presented. In light of heterogeneity of variance as indicated by the Levene test, activity was subjected to a square root transformation prior to analysis. For ease of presentation, raw data are presented in the figure. In Experiment 2, preliminary t-tests showed that there was no difference between the saline control groups in expression of any of the five signaling molecules. These two control groups were then combined and values compared to those of the other four groups with Dunnett’s test to assess changes from baseline. For two of these comparisons (IL-1β, MCP-1), the Levene test indicated heterogeneity of variance. A square root transformation as used in Experiment 1 for activity was ineffective, so a logarithmic transformation was used (raw data are presented in figure). To examine the effect of the presence of the mother in the test cage on peptide expression, 2 (With Mother/Alone) x 2 (Time Point) x 2 (Sex) ANOVAs were conducted. For temperature, motor activity, and gene expression, central tendency and variability are represented by means and standard errors. A significance level of p < 0.05 (2-tailed) was accepted throughout. In Experiment 2, gene expression data from one animal tested with the mother for 30 min and behavioral data from one animal tested with the mother for 90 min were lost due to error.
RESULTS
Experiment 1
There were no differences in the level of sickness behavior shown by males versus females across the 3-hr test when either with the mother or alone. Because the sex difference in our earlier study (i.e., suppression of sickness behavior when with the mother in males not females; Hennessy et al., 2013) was apparent only during the first 30 min of testing, we performed additional analyses for each of the three separate 30-min observation periods. There was no indication of any male, female difference for either the With Mother or Alone conditions at any of the three periods (all p’s > .50). For both sexes the presence of the mother greatly reduced sickness behavior across the 3-hr test (p’s < .001; Table 1). Additional analyses of individual observation periods showed significant influence of the mother on both males and females only during the final 30 min (p’s < .001) when sickness behavior reached its highest level in animals tested alone.
Table 1.
Median levels (and semi-interquartile ranges) of number of minutes male and female pups injected with LPS and tested with the mother or alone in Experiment 1 exhibited sickness behavior during Min 0–30, 60–90, and 150–180 of exposure to the novel environment.
differs from With Mother, p < 0.001
ANOVA of temperature revealed a significant main effect of Time Block, F (3.8, 165.0) = 31.35, p <.001, and a With Mother/Alone x Time Block interaction, F (3.8, 165.0) = 4.79, p = .001. As is clear in Figure 1, this interaction was due to the pups tested with the mother unexpectedly showing a greater increase (rather than a decrease or no change) in core temperature during the middle portion of the 3-hr test than did pups tested alone.
Figure 1.
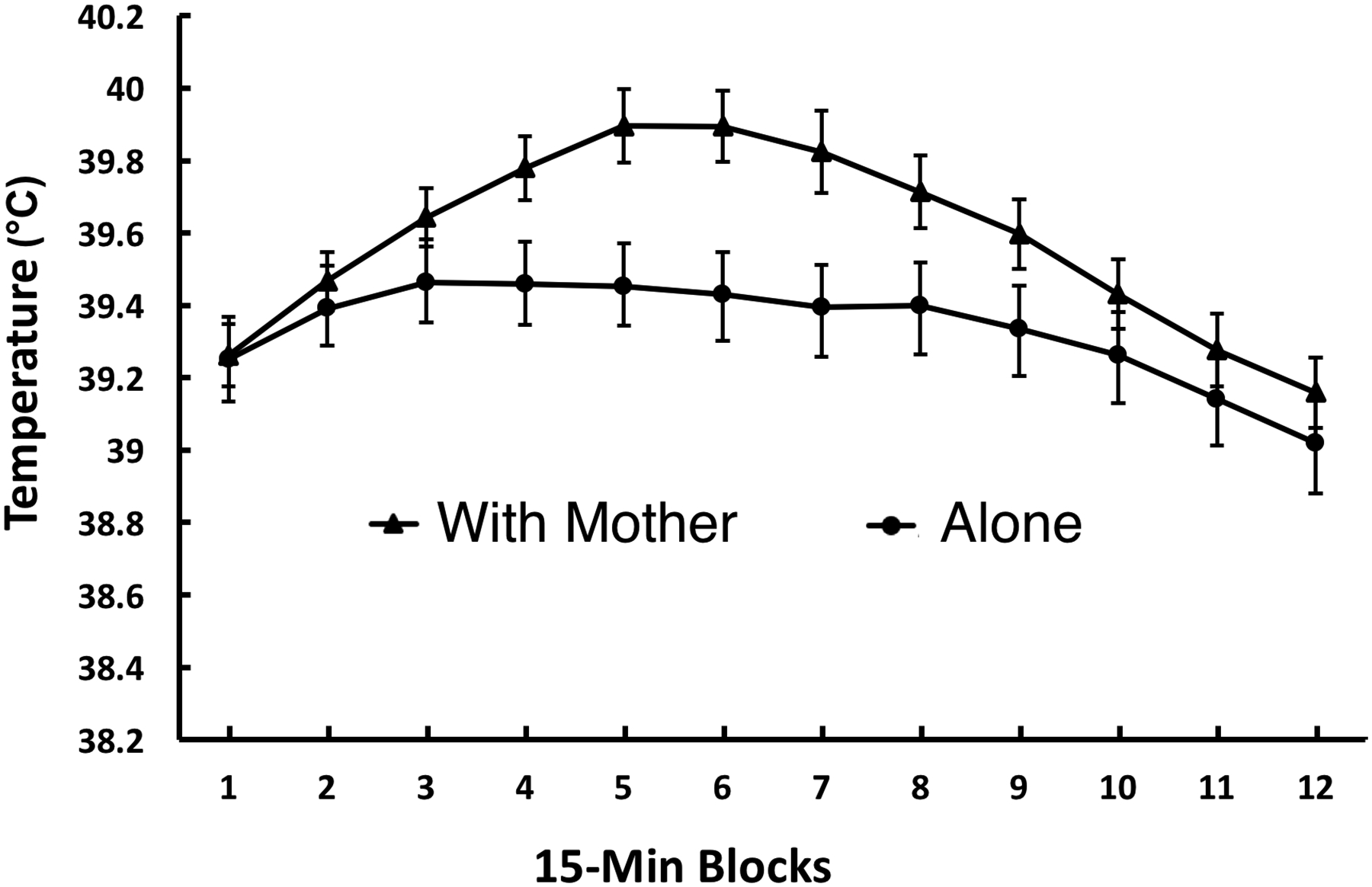
Mean core temperature of pups tested with the mother or alone in Experiment 1. Temperature is averaged across 15-min time blocks during a 3-hr exposure to a novel environment. Vertical lines indicate standard errors of the means. There was a significant With Mother/Alone x Time Block interaction, p = 0.001.
For the movement measure, there were significant main effects of With Mother/Aone, F (1, 44) = 9.10, p < .005, and Time Block, F (5.6, 244.9) = 3.63, p < .005, which were qualified by a significant interaction between these two variables, F (5.6, 244.9) = 4.76, p <.001. As seen in Figure 2, activity was greater in the pups tested alone during the early time blocks. That is, the greater core temperature in pups tested with the mother was not associated with heightened activity.
Figure 2.
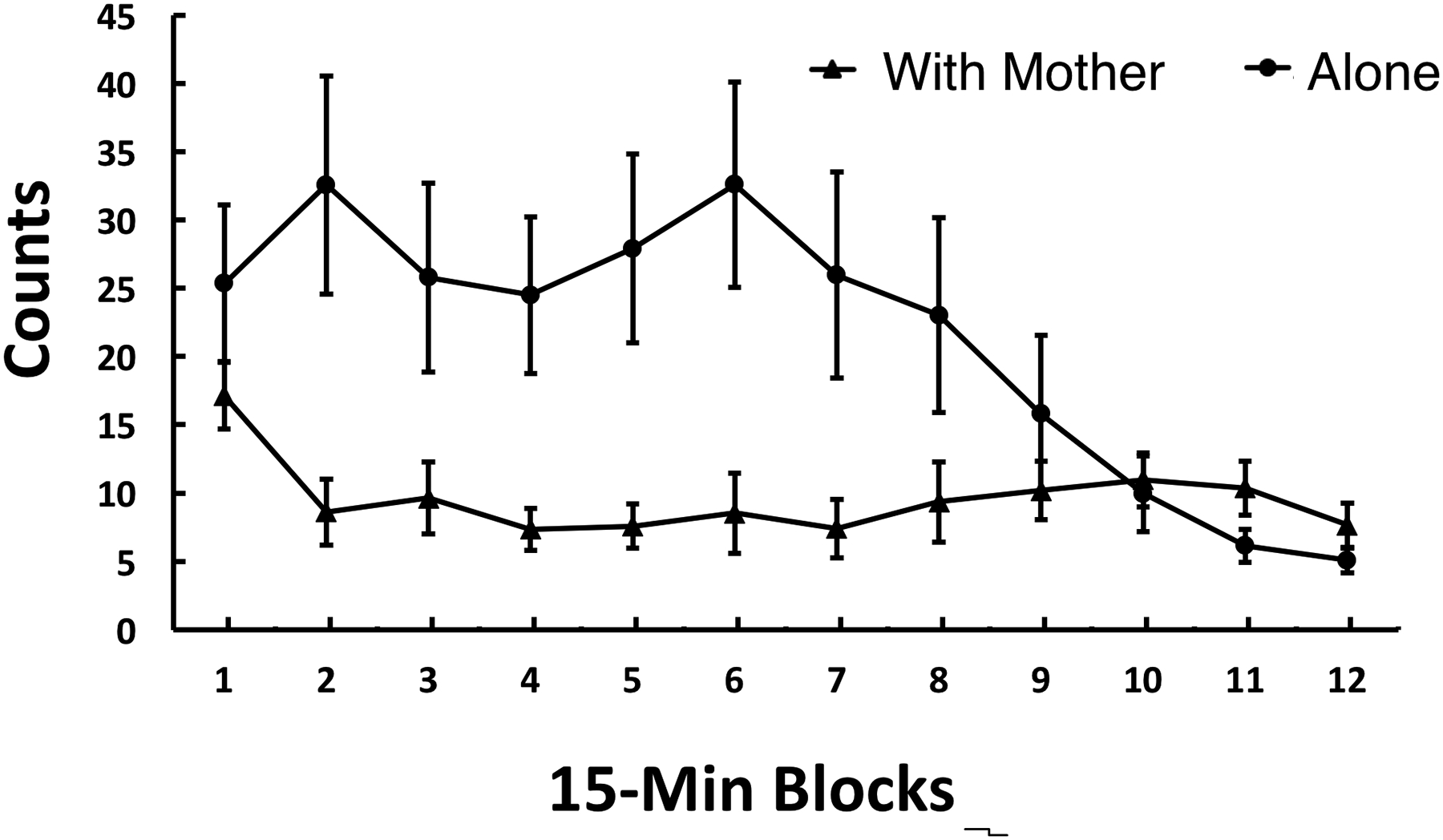
Mean movement counts of pups tested with the mother or alone in Experiment 1. Activity is averaged across 15-min time blocks during a 3-hr exposure to a novel environment. Vertical lines indicate standard errors of the means. There was a significant Mother/Alone x Time Block interaction, p < 0.001, however the pattern seen differed greatly from the pattern for core temperature.
Experiment 2
Pups tested alone showed more sickness behavior than did pups tested with their mother during both scoring intervals (0–30 min, p < .005; 60–90 min, p = .001; Table 2). Comparison of gene expression in pups injected with saline vs LPS using Dunnett’s procedure resulted in significant effects for all peptides other than IL-6. For COX-2 and MCP-1, LPS injection elevated expression relative to saline in pups tested either with the mother or alone at both the 30 and 90 min time points (p’s ≤ .001). For IL-1β and TNF-α, expression was increased by LPS in pups with the mother at the 90 min time point (IL-1β, p < .001; TNF-α, p < .05) and in pups tested alone at both 30 (IL-1β, p < .05; TNF-α, p < .005) and 90 min (IL-1β, p < .001; TNF-α, p < .05). ANOVAs of hypothalamic gene expression in pups injected with LPS yielded only a significant main effect of Time Point for IL-1β, F (1, 31) = 16.64, p < .001, with expression increasing from 30 to 90 min of testing. Importantly, there were no significant main or interaction effects involving the factor of With Mother/Alone (Fig. 3). Thus, despite the finding of the first experiment that being tested with the mother elevated core temperature, there was no evidence that the mother increased expression of hypothalamic neuroimmune signaling genes underlying fever in the pups.
Table 2.
Median levels (and semi-interquartile ranges) of number of minutes pups injected with LPS and tested with the mother or alone in Experiment 2 exhibited sickness behavior during Min 0–30 and 60–90 of exposure to the novel environment.
differs from With Mother, p < 0.005
Figure 3.
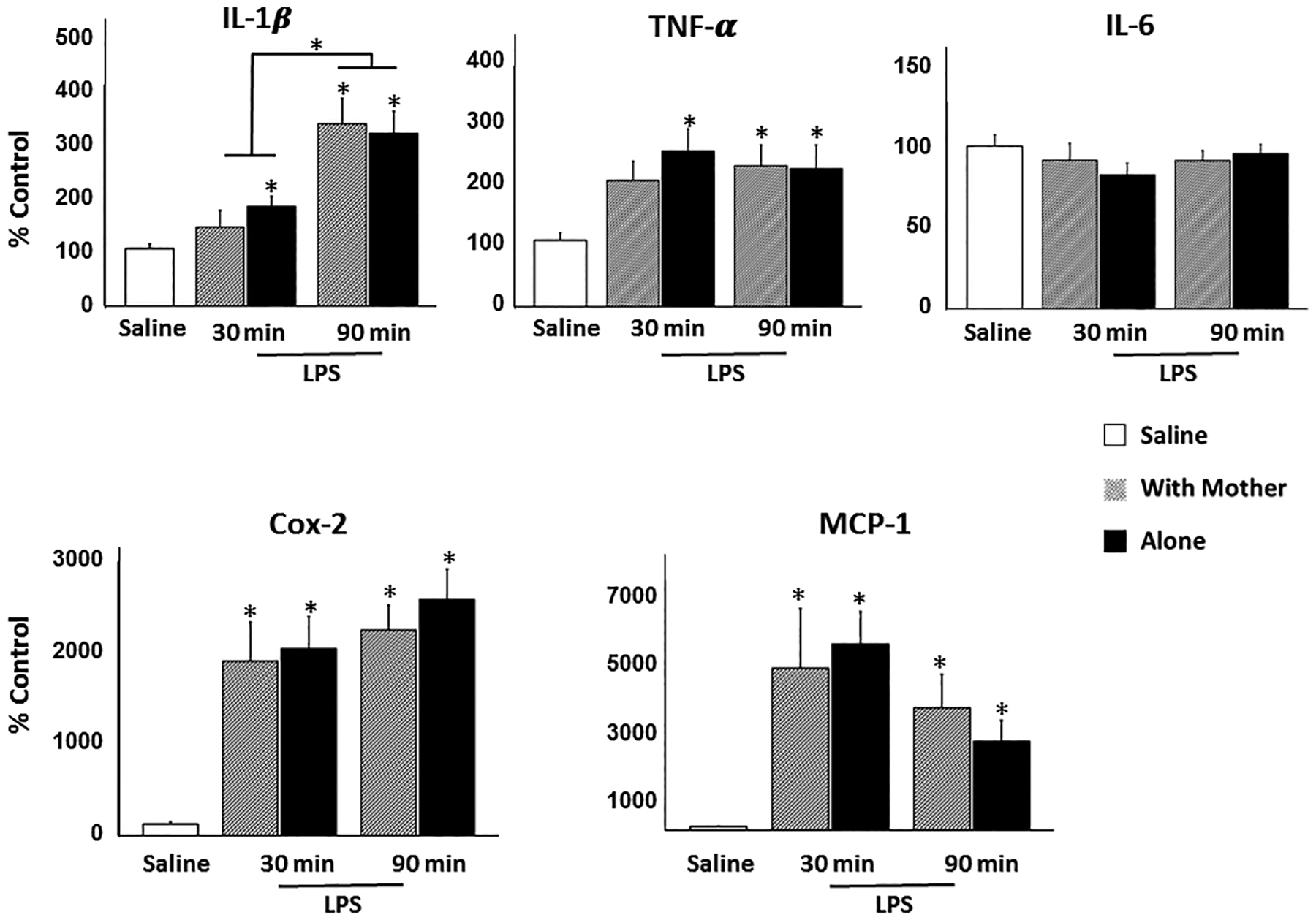
Mean expression relative to control of IL-1β, TNF-α, IL-6, COX-2, and MCP-1 in hypothalamus of pups injected with saline and returned to the home cage or injected with 100 μg/kg LPS and placed into a novel environment either with the mother or alone for 30 or 90 min prior to harvesting brain tissue. Vertical lines indicate standard errors of the means. * indicates a significant difference (p < 0.05) from saline or as indicated by overlying lines.
DISCUSSION
We conducted Experiment 1 to determine whether or not the mother’s suppression of sickness behavior of LPS-injected pups was accompanied by suppression of the pups’ febrile response. To our surprise, we found that the core temperature of pups increased when their mother was present. Although LPS clearly increases fever, a fair question is whether the greater increase in core temperature in pups tested with the mother as opposed to alone was due to some influence other than fever? It is clear that the increase cannot be accounted for by heat generated by increased activity because at the time of the largest temperature difference, pups tested with the mother were less active than those tested alone. Maternal contact would appear to be another possible influence. Given that the size of the test cage almost assured pups would be in frequent physical contact with the mother, one would expect maternal contact to reduce heat loss to the environment. However in an earlier study, untreated pups tested with the mother in a novel test cage spent 82% and 88% of two observation sessions in physical contact with her, yet a group of pups tested alone exhibited significantly higher core temperature during each session (Hennessy, Deak, Schiml-Webb, Carlisle, & O’Brien, 2010). Thus, bodily contact with the mother seems unable to account for the greater core temperature of pups in the With Mother condition of the present study. Finally, sympathetic activity has been shown to produce a short-lived increase in body temperature referred to as stress-induced hyperthermia (Shibata & Nagasaka, 1982). But this effect too appears unable to account for the current findings because pups placed into a brightly lit novel cage were found to show increased sympathetic activity when tested alone, but not when with the mother (Hennessy, Tamborski, Schiml, & Lucot, 1989). Overall, the greater increase in core temperature in pups tested together with the mother as opposed to alone appears to represent a true increase in fever. Although fever rose in the presence of the mother, the effect was transitory, with core body temperature in the With Mother and Alone conditions showing negligible difference by the end of the 3-hr test. Thus, while the presence of the mother initiated an enhanced febrile response, additional factors must be involved if the elevated fever is to be maintained.
In Experiment 2, we found that LPS increased gene expression of all hypothalamic signaling molecules other than IL-6, including COX-2, which synthesizes the prostaglandin PGE-2, considered the prime mediator of the fever response (Saper et al., 2012). Nonetheless, no further increase in expression of any of the molecules was seen in those young guinea pigs tested with the mother as opposed to those tested alone. That is, the presence of the mother in the novel environment resulted in a three-way dissociation among the hypothalamic neuroimmune genes, the fever generated by these molecules, and the sickness behaviors thought to have evolved primarily to support fever. It is possible that effects of the mother’s presence on gene expression in the preoptic area, the chief site of action of fever-inducing PGE-2 (Saper et al., 2012), was obscured by unrelated changes in peptide expression in other regions of the hypothalamic samples. It also is possible that the lack of effects were due to the time points selected, though the 90 min time point matched the peak fever response in Experiment 1, and the time points chosen were within the range at which the presence or absence of the mother in an unfamiliar cage recently was found to affect expression of some of the same signaling molecules in the hypothalamus of preweaning mice (Zajdel, Zagr, Blomqvist, Engblom, & Shionoya, 2019). Finally, though gene expression was not altered, it may be that processes downstream of gene expression such as translational or post-translational effects on peptide expression or enzymatic activity of prostaglandin-synthesizing enzymes might have occurred. In addition, altered expression of the EP3 receptor, which PGE-2 binds and activates to mediate fever (Saper et al., 2012) could have been enhanced in the presence of the mother.
Regardless of the neural mechanisms, the relation between fever and sickness behavior is of interest from a functional viewpoint. For those pups tested with the mother, the near complete suppression of sickness behavior as fever was reaching its peak provides additional evidence that supporting fever is not the primary function of the sickness behaviors shown by infant guinea pigs in a brightly lit, open environment. Rather, the results are consistent with the hypothesis (Hennessy et al., 2014) that in a threatening, open environment sickness behaviors serve as a means of making pups less conspicuous to predators and conserving energy if an initial period of active behavior (vocalizing, movement) fails to reunite the pup with its mother. The present results suggest this applies to infected as well as untreated pups. If the mother is present, however, the most adaptive response for the pup, whether infected or not, is almost certainly to follow her, an action inconsistent with sickness behavior.
But what of the function of the increased fever in the mother’s presence? A hint may be afforded by study of the evolution of the placebo effect, in which the placebo is seen as involving a trade-off between the costs and benefits of allocating resources to a particular problem (Trimmer, Marshall, Fromhage, McNamara, & Houston, 2013). Although a robust immunological response might best counter a particular disease state, physiological resources are limited so that mounting the immunological response might be at the expense of other critical activities (e.g., migration or warding off starvation). Thus under stressful circumstances, generating a full immunological response may be postponed until environmental cues suggest that it is safe and advantageous to do so. In the case of the human patient, these cues may be the placebo itself, a medical setting, or other signs that increase positive expectancy. For the infant guinea pig, the environmental cue may be the presence of the mother. That is, just as an animal might choose to divert limited resources from sickness behavior when external cues (e.g., presence of a receptive female for males, or pups requiring care for lactating females) indicate greater fitness benefit from other activities, resources for mustering a higher fever might have been withheld in the threatening novel environment of the present study when the mother was absent. Her presence, on the other hand, may have been the signal indicating that conditions were favorable for investing resources in augmented fever to combat the apparent infection.
In addition, however, one needs to consider substantial evidence that mechanisms of inflammation, including the overall manifestation of core body temperature changes evoked by LPS exposure, do not follow a linear dose-response function. Under severe environmental or immunological conditions (e.g., starvation, particularly high dose of LPS) a regulated hypothermic response, i.e., anapyrexia, may be triggered rather than fever (Romanovsky & Székely, 1998). The mechanisms underlying fever and anapyrexia are distinct (Steiner & Romanovsky, 2019) and although fever and anapyrexia are typically viewed as alternative strategies, we cannot rule out the possibility that the core temperature of pups tested alone were influenced by some relative activation of both anapyrexic and febrile pathways, while only febrile pathways were activated in pups tested with the mother.
In our earlier study (Hennessy et al., 2013), the presence of the mother reduced sickness behavior of male, but not female, pups during the first 30 min of exposure to the novel test environment. The lack of sex difference in the present study may be due to the different doses of LPS used in the two studies. Pups were administered 250 μg/kg LPS in the earlier study, while in the present study, we chose a 100 μg/kg dose based on its ability to produce a clear elevation of core temperature in pilot work. If females are more sensitive to LPS than are males, the mother’s presence may have been sufficient to reduce sickness behavior in both sexes when administered the lower dose, but the impact of the higher dose on females may have been too great for the mother to moderate.
In all, the present results contribute to a growing literature linking immunological processes and social behavior (e.g., Hennessy et al., 2014; Lopes, 2014; Moieni & Eisenberger, 2018). It is now well documented that social stimuli (potential mates, competitors, or offspring in need) can suppress sickness behaviors in infected animals in order to serve more immediate fitness purposes (Aubert et al., 1997; Lopes, Adelman, Wingfield, & Bentley et al., 2012; Yirmiya et al., 1995). Evidence indicates, however, that the cost of suppressing sickness behaviors is reduced host resistance with potentially increased mortality (Lopes, Springthorpe, & Bentley, 2014; Murray & Murray, 1979). The present results document a situation in which a social stimulus—the presence of the mother in a threatening environment—can both suppress sickness behavior, apparently to enable the pup to follow the mother to safety, and at the same time trigger an enhanced febrile response. Although we do not know if or how the increased fever can be maintained, the findings suggest that the cost of sickness behavior suppression may in some way be compensated in this situation by an enhanced febrile response.
Acknowledgments
The authors thank Kendra Carter, Kate Berberich, Jessica Martin, Tiphani Moss, and Jadyn Barga for assistance in various aspects of the project. We also acknowledge Federica Sgorbissa for bringing the literature on the evolution of the placebo effect to our attention. The work was supported by grant MH068228 from the National Institute of Mental Health. The authors have no conflicts of interest to report.
Footnotes
DATA AVAILABILITY STATEMENT
The original data the support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Adelmman JS, & Martin LB (2009). Vertebrate sickness behaviors: adaptive and integrated neuroendocrine immune responses. Integrative and Comparative Biology, 49, 201–214. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Blandino P, & Deak T (2009). Central infusion of Interleukin-1 receptor antagonist blocks the reduction in social behavior produced by prior stressor exposure. Physiology and Behavior, 98, 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert A (1999). Sickness and behaviour in animals: a motivational perspective. Neuroscience and Biobehavioral Reviews, 23, 1029–1036. [DOI] [PubMed] [Google Scholar]
- Aubert A, Goodall G, Danzer R, & Gheusi G (1997). Differential effects of lipopolysacchride on pup retrieving and nest building in lactating mice. Brain Behavior and Immunity. 11, 107–118. [DOI] [PubMed] [Google Scholar]
- Blatteis CM (1975). Postnatal development of pyrogenic sensitivity in guinea pigs. Journal of Applied Physiology, 39, 251–257. [DOI] [PubMed] [Google Scholar]
- Conti B, Tabarean I, Andrei C, & Bartfai T (2004). Cytokines and fever. Frontiers in Bioscience, 9, 1433–1449. [DOI] [PubMed] [Google Scholar]
- Dantzer R, & Kelley KW (2007). Twenty years of research on cytokine-induced sickness behavior. Brain, Behavior and Immunity, 21, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes GS, & Mestyán G (1963). Changes in the oxygen consumption of new-born guinea-pigs and rabbits on exposure to cold. Journal of Physiology, 168, 22–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell JE, Kang M, & Eliason HL (1997). Autonomic and behavioral thermoregulation in guinea pigs during postnatal maturation. Journal of Applied Physiology, 83, 830–836. [DOI] [PubMed] [Google Scholar]
- Hart BL (1988). Biological basis of the behavior of sick animals. Neuroscience and Biobehavioral Reviews, 12, 123–137. [DOI] [PubMed] [Google Scholar]
- Hennessy MB & Morris A (2005). Passive responses of young guinea pigs during exposure to a novel environment: influences of social partners and age. Developmental Psychobiology, 46, 86–96. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, & Schiml PA (2014). Sociality and sickness: Have cytokines evolved to serve social functions beyond times of pathogen exposure? Brain, Behavior, and Immunity, 37, 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, & Schiml-Webb PA (2001). Stress-induced sickness behavior: an alternative hypothesis for responses during maternal separation. Developmental Psychobiology, 39, 76–83. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, Schiml-Webb PA, Carlisle CW, O’Brien E (2010). Maternal separation produces, and a second separation enhances, core temperature and passive behavioral responses in guinea pig pups. Physiology and Behavior, 100, 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, Schiml-Webb PA, Wilson SE, Greenlee TM, & McCall E (2004). Responses of guinea pig pups during isolation in a novel environment may represent stress-induced sickness behaviors. Physiology and Behavior, 81, 5–13. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, Sensenbaugh JD, Gallimore DM, Garybush AM, Mondello JE, & Schiml PA (2019). Central neuroimmune activity and depressive-like behavior in response to repeated maternal separation and injection of LPS. Physiology and Behavior, 199, 366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Jacobs S, Schiml PA, Hawk K, Stafford N, & Deak T (2013). Maternal inhibition of infant behavioral response following isolation in novel surroundings and inflammatory challenge. Developmental Psychobiology, 55, 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Long SJ, Nigh CK, Williams MT, & Nolan D (1995). Effects of peripherally administered corticotropin-releasing factor (CRF) and a CRF antagonist: Does peripheral CRF activity mediate behavior of guinea pig pups during isolation? Behavioral Neuroscience, 109, 1137–1145. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Maken DS, & Graves FC (2002). Presence of mother and unfamiliar female alters levels of testosterone, progesterone, cortisol, ACTH and behavior in maturing guinea pigs. Hormones and Behavior, 42, 42–52. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Schiml-Webb PA, Miller EE, Maken DS, Bullinger KL, & Deak T (2007). Anti-inflammatory agents attenuate the passive responses of guinea pig pups: evidence for stress-induced sickness behavior during maternal separation. Psychoneuroendocrinology, 32, 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Stafford NP, Yusko-Osborne B, Schiml PA, Xanthos ED, & Deak T (2015). Naproxen Attenuates Sensitization of Depressive-Like Behavior and Fever during Maternal Separation. Physiology and Behavior, 139, 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Tamborski A, Schiml P, & Lucot J (1989). The influence of maternal separation on plasma concentrations of ACTH, epinephrine, and norepinephrine in guinea pig pups. Physiology and Behavior, 45, 1147–1152. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Young TL, O’Leary SK, & Maken DS (2003). Social preferences of developing guinea pigs (Cavia porcellus) from the preweaning to the periadolescent period. Journal of Comparative Psychology, 117, 406–413. [DOI] [PubMed] [Google Scholar]
- Hueston CA, & Deak T (2014). The inflamed axis: The interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic–pituitary–adrenal axis. Physiology and Behavior, 124, 77–91. [DOI] [PubMed] [Google Scholar]
- Konat G (2016). Cerebral response to peripheral challenge with a viral mimetic. Neurochemical Research, 41, 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König B (1985). Maternal activity budget during lactation in two species of Caviidae (Cavia porcellus and Galea musteloides). Zeitschrift für Tierpsychologie, 68, 215–230. [Google Scholar]
- Konsman JP, Luheshi GN, Bluthé R-M, & Dantzer R (2000). The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. European Journal of Neuroscience, 12, 4434–4446. [DOI] [PubMed] [Google Scholar]
- Livak KJ, & Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lopes PC (2014). When is it socially acceptable to feel sick? Proceedings of the Royal Society B, 281: 2014.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes PC, Adelman J, Wingfield JC, & Bentley GE (2012). Social context modulates sickness behavior. Behavior, Ecology and Sociobiology 66, 1421–1428. [Google Scholar]
- Lopes PC, Springthorpe D, & Bentley GE (2014). Increased activity correlates with reduced ability to mount immune defenses to endotoxin in zebra finches. Journal of Experimental Zoology, 321A, 422–431. [DOI] [PubMed] [Google Scholar]
- Maes M, Berk M, Goehler L, Song C, Anderson G, Galecki P, & Leonard B (2012). Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Medicine, 10:66. doi: 10.1186/1741-7015-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, & Watkins LR (1998). Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological Review 105, 83–107. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Nguyen KT, Deak T, Hinde JL, Fleshner M, Watkins LR, & Maier SF (1998). The long term acute phase-like responses that follow acute stressor exposure are blocked by alpha-melanocyte stimulating hormone. Brain Research, 810, 48–58. [DOI] [PubMed] [Google Scholar]
- Monieni M, & Eisenberger NI (2018). Effects of inflammation on social processes and implications for health. Annals of the New York Academy of Sciences, 1428, 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MJ, & Murray AB (1979). Anorexia of infection as a mechanism of host defense. American Journal of Clinical Nutrition, 32, 593–596. [DOI] [PubMed] [Google Scholar]
- Perkeybile AM, Schiml-Webb PA, O’Brien E, Deak T, & Hennessy MB (2009). Anti-inflammatory influences on behavioral, but not cortisol, responses during maternal separation. Psychoneuroendocrinology, 34, 1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovsky AA, & Székely M (1998). Fever and hypothermia: two adaptive thermoregulatory responses to systemic inflammation. Medical Hypotheses, 50, 219–226. [DOI] [PubMed] [Google Scholar]
- Rummel C (2016). Inflammatory transcription factors as activation markers and functional readouts in immune-to-brain communication. Brain, Behavior and Immunity, 54, 1–14. [DOI] [PubMed] [Google Scholar]
- Saper CB, Romanovsky AA, & Scammell T (2012). Neural circuitry engaged by prostaglandins during the sickness syndrome. Nature Neuroscience, 15, 1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiml PA & Hennessy MB (1990). Light-dark variation and changes across the lactational period in the behaviors of undisturbed mother and infant guinea pigs (Cavia porcellus). Journal of Comparative Psychology, 104, 283–288. [DOI] [PubMed] [Google Scholar]
- Schiml-Webb PA, Deak T, Greenlee T, Maken DS, & Hennessy MB (2006). Alpha melanocyte stimulating hormone reduces putative stress-induced sickness behaviors in isolated guinea pig pups. Behavioural Brain Research, 168, 326–330. [DOI] [PubMed] [Google Scholar]
- Schneider RL, Schiml PA, Deak T, & Hennessy MB (2012). Persistent sensitization of depressive-like behavior and thermogenic response during maternal separation in pre- and post-weaning guinea pigs. Developmental Psychobiology, 54, 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehic E, Székely M, Ungar AL, Oladehin A, & Blatteis CM (1996). Hypothalamic prostaglandin E2 during lipopolysaccharide-induced fever in guinea pigs. Brain Research Bulletin, 39, 391–399. [DOI] [PubMed] [Google Scholar]
- Shibata H, & Nagasaka T (1982). Contribution of nonshivering thermogenesis to stress-induced hyperthermia in rats. Japanese Journal of Physiology, 32, 991–995. [DOI] [PubMed] [Google Scholar]
- Steiner AA, & Romanovsky AA (2019). Energy trade-offs in host defense: immunology meets physiology. Trends in Endocrinology & Metabolism, 30, 875–878. [DOI] [PubMed] [Google Scholar]
- Sylvia K,E, & Demas GE (2017). A return to wisdom: Using sickness behaviors to integrate ecological and translational research. Integrative and Comparative Biology, 57, 1204–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer PC, Marshall JAR, Fromhage L, McNamara JM, & Houston AI (2013). Understanding the placebo effect from an evolutionary perspective. Evolution and Human Behavior, 34, 8–15. [Google Scholar]
- Yirmiya R, Avitsur R, Donchin O, Cohen E (1995). Interleukin-1 inhibits sexual behavior in female but not male mice. Brain, Behavior and Immunity, 9, 220–233. [DOI] [PubMed] [Google Scholar]
- Zajdel J, Zager A, Blomqvist A, Engblom D, Shionoya K (2019). Acute maternal separation potentiates the gene expression and corticosterone response induced by inflammation. Brain, Behavior and Immunity, 77,141–149. [DOI] [PubMed] [Google Scholar]


