Abstract
Background:
Non-invasive screening tools of cardiac function can play a significant role in the initial triage of patients with suspected acute coronary syndrome. Numerous ECG features have been previously linked with cardiac contractility in the general population. We sought to identify ECG features that are most predictive for real-time screening of reduced left ventricular ejection fraction (LVEF) in the acute care setting.
Methods:
We performed a secondary analysis of a prospective, observational cohort study of patients evaluated for suspected acute coronary syndrome. We included consecutive patients in whom an echocardiogram was performed during indexed encounter. We evaluated 554 automated 12-lead ECG features in multivariate linear regression for predicting LVEF. We then used regression trees to identify the most important predictive ECG features.
Results:
Our final sample included 297 patients (aged 63 ± 15, 45% females). The mean LVEF was 57% ± 13 (IQR 50%–65%). In multivariate analysis, depolarization dispersion in the horizontal plane; global repolarization dispersion; and abnormal temporal indices in inferolateral leads were all independent predictors of LVEF (R2 = 0.452, F = 6.679, p < 0.001). Horizontal QRS axis deviation and prolonged ventricular activation time in left ventricular apex were the most important determinants of reduced LVEF, while global QRS duration was of less importance.
Conclusions:
Poor R wave progression in precordial leads with dominant QS pattern in V3 is the most predictive feature of reduced LVEF in suspected ACS. This feature constitutes a simple visual marker to aid clinicians in identifying those with impaired cardiac function.
Keywords: left ventricular ejection fraction, 12-lead ECG, acute coronary syndrome, QRS axis, ventricular activation time
INTRODUCTION
There is a strong relationship between acute coronary syndrome (ACS) and cardiac contractile dysfunction. About 10% of patients presenting with ACS either develop a new onset heart failure or show acute decompensation of existing heart failure.1–3 Early recognition of impaired cardiac contractility and prompt initiation of adequate therapy in suspected ACS has been shown to improve outcomes and significantly reduce the risk of mortality and serious adverse events.4–6 Thus, the availability of non-invasive screening tools of cardiac function can play a critical role in the initial triage of this high risk population.
The 12-lead ECG has been widely used for predicting new onset heart failure in different clinical settings. Numerous ECG metrics seem to strongly correlate with subsequent development of heart failure, including temporal ECG intervals (e.g., QRS, QTc, etc.), ECG vectors (QRS axis, QRS-T angle, etc.), and abnormal ECG morphologies (e.g., LBBB, LVH, ST/T changes, etc.).7 The predictive value of such features for real-time detection of underlying poor left ventricular ejection fraction (LVEF), however, remains unclear. Moreover, a recent study successfully predicted cardiac dysfunction (LVEF ≤ 0.35) using raw ECG signal from a large general population.8 Despite the high accuracy (AUC = 0.93) achieved using deep learning in this study, it is difficult to speculate on the exact electrophysiological mechanisms of such association, which constitutes an important missed opportunity. Accordingly, we sought to evaluate the value of 554 automated 12-lead ECG features for real-time prediction of LVEF in patients evaluated at the emergency department for suspected ACS. We also sought to deploy supervised machine learning algorithms to rank the most important ECG features for this clinical task.
METHODS
Design, Sample, and Setting
This was a secondary analysis of data collected from the EMPIRE study (ECG Methods for the Prompt Identification of Coronary Events). Study design and methods were previously described in detail.9 Briefly, EMPIRE was an observational cohort study that enrolled consecutive patients 18 years of age or older who had chief complaint of non-traumatic chest pain and were transported via ambulance by Pittsburgh Emergency Medical Services (EMS) to one of three participating UPMC-affiliated tertiary care centers. For this analysis, we included consecutive patients from the first study cohort (n=750) who were in supraventricular rhythm and in whom an echocardiogram was performed during the indexed admission. The parent study was approved by the Institutional Review Board of the University of Pittsburgh.
Data Collection
Clinical data were obtained for each patient from the in-hospital electronic health record by independent reviewers per an a priori defined data coding scheme. Clinical data elements included demographics, past medical history, clinical presentation, laboratory tests, diagnostic studies, treatments, and adverse cardiac events. The presence of ACS was defined as elevation of cardiac troponin (i.e., > 99th percentile) and/or the presence of focal myocardial ischemia on cardiac imaging (i.e., echocardiogram, nuclear imaging, or angiographic evidence).10 Cardiac function was defined as per LVEF (%) as documented on the closest echocardiogram to the ECG.
ECG Methods
For this analysis, we used the prehospital 12-lead ECG obtained during first medical contact. Raw ECG data were acquired using HeartStart MRX monitor-defibrillator at 500 samples/second (Philips Healthcare). Standard ECG signal pre-processing was completed at Philips Healthcare Advanced Algorithm Research Center (Andover, MA). ECG signals were first decompressed and ECG leads were extracted. Noise, artifact, and ectopic beats were removed, and the representative average beat for each ECG lead was computed to eliminate residual baseline noise and artifacts, yielding a high signal-to-noise ratio and stable average waveform signal for each of the 12 leads.
ECG features extraction was performed on these representative beats. The details of ECG features extraction from this dataset were previously described in details.11 In short, the amplitude, duration, area, inflection, and slope measures of the P wave, Q wave, R wave, S wave, qR wave, qS wave, rS wave, QRS, R peak, ST segment, J+80, JT interval, T wave, T peak-end, JT peak, STT wave, QT interval, PP interval, RP interval, and SP interval were computed from each of the 12-leads. We then calculated principal component analysis (PCA) beats and computed the ratios between eigenvalues of QRS, STT, J, and T subintervals. Finally, we computed the mean, initial (first 40 milliseconds) and terminal (last 40 milliseconds) QRS and T axes from in the frontal, horizontal, and spatial planes and the corresponding angles and gradients between these vectors. Overall, there was a total of 554 ECG features available for analysis.
Statistical Analysis
Continuous variables were described with mean and standard deviation. Variables were evaluated for normality of distribution and log transform was used if a variable was considered non-normally distributed. Outliers were replaced by the trimmed 95% confidence limit boundary. Less than 3% of ECG features were missing and were also replaced with the mean. Association between each ECG feature and LVEF was evaluated using linear regression. Features significant at 0.05 in univariate analysis were entered into a stepwise multivariate linear regression model to identify the regression coefficients and 95% CI of independent ECG predictors. R square was used to evaluate the final model goodness of fit. Next, we used regression tree with deviance method to rank the most important ECG features for the clinical task of estimating LVEF (max number of nodes = 10). We then built a regression tree for estimating LVEF using the initial decision nodes based on the most important ECG features. The significance level was set at 0.05 for two-sided hypothesis testing. SPSS® version 25 (IBM, Armonk, NY) and R software package were used for all the analyses.
RESULTS
Of 750 patients enrolled in the parent study, an echocardiogram was performed in 313 patients (42%). After excluding those with pacing or ventricular rhythms (n=16), our final sample included 297 patients (mean age 63±15, 45% women, and 35% black). Baseline clinical characteristics are summarized in Table 1. Overall, coronary artery disease risk factors were prevalent in this population, including 16% with known history of heart failure. Most (87%) were in normal sinus rhythm. Approximately one third had a confirmed ACS. The main culprit lesion was in the left anterior descending (LAD) coronary artery in nearly half of those ACS events.
Table 1.
Baseline Demographic and Clinical Characteristics of Study Sample
| All Patients (n= 297) | |
|---|---|
| Demographics | |
| Age (years) | 63 ± 15 |
| Female Sex | 134 (45%) |
| Black Race | 104 (35%) |
| Past Medical History | |
| Obesity | 119 (40%) |
| Hypertension | 208 (70%) |
| Diabetes Mellitus | 80 (27%) |
| Dyslipidemia | 110 (37%) |
| Known CAD | 101 (34%) |
| Known CHF | 47 (16%) |
| Presenting Chief Complaints | |
| Chest Pain | 264 (89%) |
| Shortness of Breathing | 98 (33%) |
| Gastrointestinal Upset | 62 (21%) |
| Syncope or Dizziness | 53 (18%) |
| Palpitation | 41 (14%) |
| Presenting Vital Signs | |
| Heart Rate | 82 [70–100] |
| Respiratory Rate | 18.3 ± 3.3 |
| Systolic Blood Pressure | 149 ± 34 |
| Oxygen Saturation | 98 [97–99 |
| Presenting 12-Lead ECG | |
| Normal Sinus Rhythm | 259 (87%) |
| Atrial Fibrillation | 38 (13%) |
| Bundle Branch Block | 23 (7.7%) |
| ECG LVH (Sokolow-Lyon Criteria) | 14 (4.7%) |
| Clinical Outcomes | |
| Confirmed ACS | 85 (29%) |
| LAD lesion | 42 (14.1%) |
| LCX lesion | 26 (8.8%) |
| RCA lesion | 28 (9.4%) |
Values are mean ± SD, median [IQR], or count (%).
The mean LVEF of the sample was 57% ± 13 (range 10%–93%, IQR 50%–65%). On univariate analysis, 66 ECG features were associated with LVEF. In multivariate analysis, controlling for age, sex, patient history, vital signs, electrolytes, and medications, 14 ECG features remained in the final multivariate model (Table 2). Our model explained 45% of variability in the data. Among these independent predictors were global temporal measures (i.e., JTc and T peak–Tend intervals); horizontal plane QRS axis; repolarization dispersion indices (i.e., PCA ratio at J point and non-dipolar component of ST segment and T wave); and various temporal indices from individual ECG leads, primarily from inferolateral myocardial walls.
Table 2:
Multivariate Linear regression for Predicting LVEF
| Coefficient | SE | t value | P value | |
|---|---|---|---|---|
| Clinical Data | ||||
| Sex | −1.971 | 1.286 | −1.533 | 0.126 |
| Systolic Blood Pressure | 0.027 | 0.019 | 1.425 | 0.155 |
| Hypertension | 2.326 | 1.478 | 1.574 | 0.117 |
| Diabetes Mellitus | −2.628 | 1.524 | −1.724 | 0.086 |
| Known CAD | 3.150 | 1.628 | 1.934 | 0.054 |
| Old Myocardial Infarction | −4.900 | 1.692 | −2.897 | 0.004 |
| Known Heart failure | −5.779 | 1.828 | −3.162 | 0.002 |
| Global ECG Metrics | ||||
| JTc interval | 0.048 | 0.016 | 3.018 | 0.003 |
| T peak-T end | −0.058 | 0.027 | −2.177 | 0.030 |
| Initial hp-QRS axis | 0.077 | 0.015 | 5.212 | 0.000 |
| Terminal hp-QRS axis | −0.030 | 0.012 | −2.561 | 0.011 |
| PCA ratio at J point | −6.131 | 2.483 | −2.469 | 0.014 |
| NDV of ST-segment | 0.628 | 0.192 | 3.265 | 0.001 |
| NDV of T wave | −0.321 | 0.163 | −1.973 | 0.049 |
| QRS Complex | ||||
| Q duration Lead V5 | −0.069 | 0.040 | −1.736 | 0.084 |
| R amplitude Lead II | 0.005 | 0.002 | 2.883 | 0.004 |
| R amplitude Lead III | −0.006 | 0.002 | −3.271 | 0.001 |
| R duration Lead V4 | −0.058 | 0.034 | −1.689 | 0.092 |
| R peak Lead aVR | −0.043 | 0.021 | −2.096 | 0.037 |
| S amplitude Lead I | −0.013 | 0.005 | −2.775 | 0.006 |
| S duration Lead V4 | −0.047 | 0.030 | −1.543 | 0.124 |
| QS peak Lead V3 | 0.098 | 0.041 | 2.376 | 0.018 |
| S` amplitude Lead V3 | −0.071 | 0.040 | −1.808 | 0.072 |
| ST/T Wave | ||||
| ST amplitude Lead III | 0.016 | 0.006 | 2.840 | 0.005 |
| T duration lead aVR | −0.025 | 0.011 | −2.264 | 0.024 |
Model summary: R square = 0.452, F = 6.679, p < 0.001
CAD: coronary artery disease; hp: horizontal plane; PCA: principal component analysis; NDV: non-dipolar voltage;
Next, we used regression tree to evaluate and rank the most important ECG features for the clinical task of estimating LVEF. Starting from all available ECG features (k=554), our algorithm identified 11 features as the most important. These features are listed in descending order in Figure 1A. Notably, the horizontal plane QRS axis from the initial 40 milliseconds of depolarization remained as the most important feature for real-time estimation of LVEF, followed by the ventricular activation time (VAT) in lead aVR. These two features also determined the leading decision splits in the regression tree (Figure 1B), which indicates these two features are conditional for subsequent ECG findings. A horizontal plane QRS axis <–47 degrees suggests an electrical shift in QRS axis from left lateral myocardial wall to left posterior myocardial wall (Figure 2A, red arrow on horizontal plane), while VAT > 106 in aVR suggests delayed depolarization in left ventricular (LV) apex (Figure 2A, red arrow at –aVR). In post hoc analyses, there was no significant correlation between these two ECG features and the presence or location of culprit lesions, or any other commonly interpreted conduction disturbances like bundle branch block or left ventricular hypertrophy.
Figure 1: Most Important 12-Lead ECG features for EF Prediction.
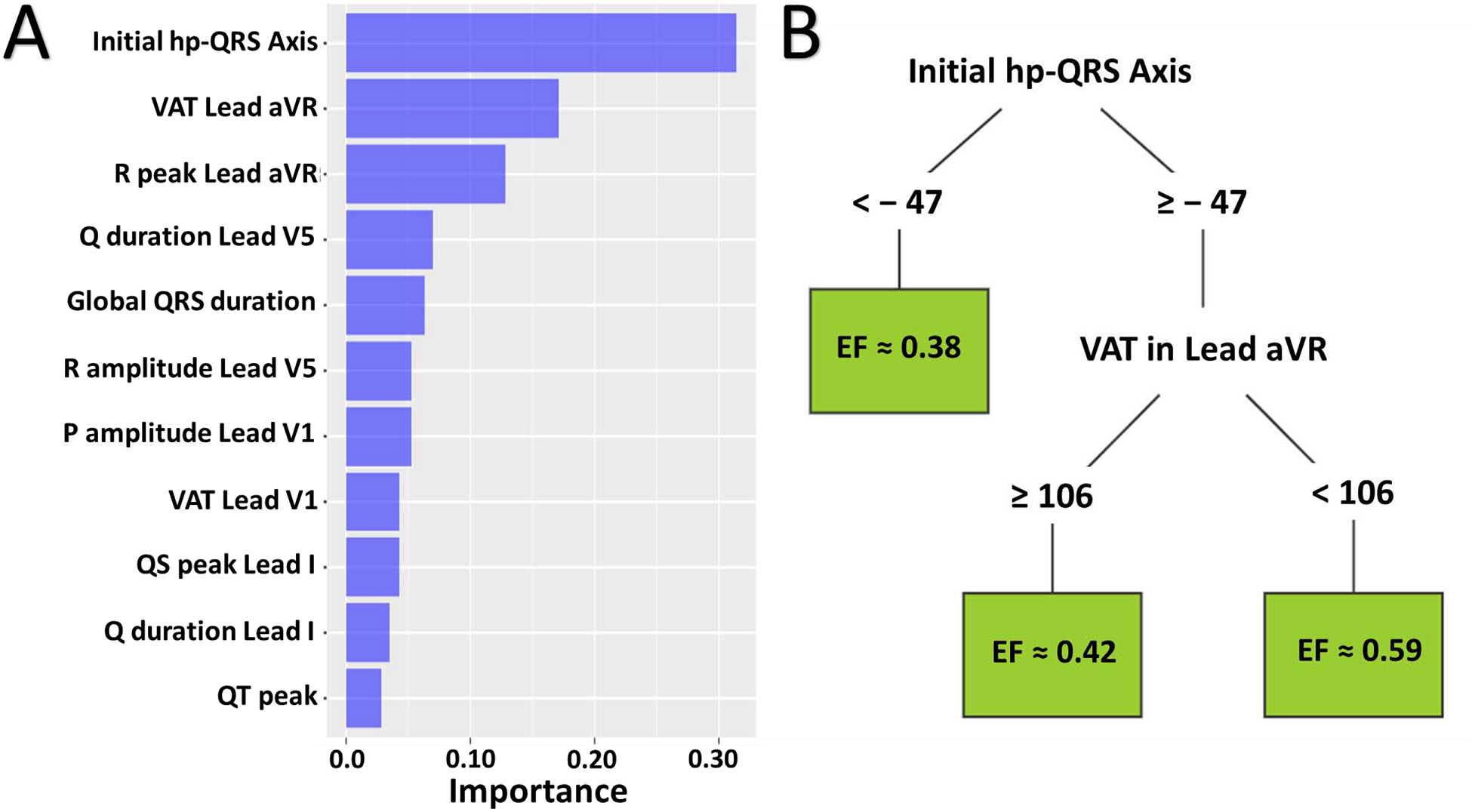
(A) Out of 554 features evaluated, this histogram shows the 11 most important 12-lead ECG features predictive of ejection fraction in descending order. (B) This regression tree shows the leading decision nodes in the estimation of ejection fraction using the 11 most important ECG features. VAT = ventricular activation time.
Figure 2: Spatial View of the Most Important 12-Lead ECG Features for EF Prediction.

(A) The spatial view of the 12 surface leads in relation to myocardial walls. Leads I–III and aVR–aVF constitute the frontal plane and leads V1–V6 constitute the horizontal plane. (B) The computation of initial horizontal QRS axis and ventricular activation time (VAT) from two selected patients: a 33-year-old female diagnosed with pericarditis (Patient 1) and a 49-year-old male diagnosed with acute myocardial infarction (Patient 2). Note the dominant QS pattern in V3, compared to a biphasic QRS, in the second patient.
DISCUSSION
In this study, we evaluated the value of 554 automated 12-lead ECG features for real-time prediction of LVEF in patients evaluated at the emergency department for suspected ACS. Using only 14 ECG features, our final multivariate linear regression model explained 45% of the variability in the data. Depolarization dispersion in the horizontal plane; global repolarization dispersion; and abnormal temporal indices in inferolateral leads were all independent predictors of LVEF in this parsimonious model. In terms of importance, depolarization dispersion in the horizontal plane and slow VAT in LV apex were the most important determinants of reduced LVEF, while duration of global depolarization was of less importance. These findings simply imply that Poor R wave progression in precordial leads with dominant QS pattern in V3, a visual marker that can be easily spot on the 12-lead ECG, is the most predictive feature of reduced LVEF in suspected ACS. To our knowledge, this is the first study to identify the single most important ECG feature for predicting LVEF in acute care settings.
Role of Horizontal QRS Axis Deviation
Our study demonstrates that left QRS axis deviation in the horizontal plane is the most important determinant of reduced LVEF, independent of culprit lesion and other ECG findings. This is consistent with prior literature that links QRS axis deviation to poor cardiac contractile function. Han and colleagues12 have previously shown that in elderly patients with known heart failure, the horizontal shift of QRS axis from left lateral to left posterior is associated with greater LV cavity size, lower LV systolic function and worse NYHA functional class, independent from LV wall thickness. Such left posterior deviation of horizontal QRS axis is associated with a relatively increased electrical force in the posterior myocardial wall, which could be attributed to myocardial loss in the intraventricular septum, asymmetric hypertrophy of LV posterior wall, or QRS broadening.12 Increased electrical heterogeneity, possibly from coronary ischemic disease, has been also shown to play a role in the distortion of spatial QRS axis.13 In our data, the initial 40 millisecond of horizontal QRS axis, rather than mean or terminal portions, was most important in determining LVEF. The initial axis segment could be attributed to the electrical dispersion between anterior and posterior-lateral myocardial walls, whereas the mean or terminal portions of the axis could be attributed to the electromechanical forces between these walls. Thus, ventricular heterogeneity could provide a plausible explanation for our findings in this population, especially that other measures of ventricular electrical dispersion, such as T peak – T end, were also significant independent predictors of LVEF in our study.
Role of VAT of the LV Apex
Our study demonstrates that slow ventricular activation in lead aVR is among the most important determinants of reduced LVEF, independent from global QRS duration. Whereas the total ventricular activity of depolarization is assessed through global QRS duration from the 12-lead ECG, localized regional depolarization can be assessed using individual leads facing that myocardial region. Thus, VAT measured from lead aVR constitutes altered depolarization in the LV apex (Figure 2A, red arrow at –aVR). The mechanism of longer VAT could be related to impaired rapid conduction through part of the His Purkinje system (e.g. LAFB, etc.) or may be related to poor cellular coupling in the myocardium itself due to ischemia or other metabolic disturbances leading to cell-to-cell uncoupling secondary to lateralization of gap junctions (mainly Connexin 43 in the ventricles).
Our findings are consistent with some prior studies that show slow ventricular activation is associated with lower LVEF in patients with ACS.14,15 Larger ischemic myocardium, higher syntax score, and more extensive coronary disease have been suggested as potential mechanistic links between altered depolarization and reduced LVEF. Another study suggested that slow VAT could be attributed to impaired coronary microcirculation and endothelial dysfunction.16 These mechanisms simply support the notion that the slow depolarization observed in our population of suspected ACS could be simply attributed to regional perfusion abnormalities in LV apex.
Role of Other ECG Features in LVEF
Although being of less importance, many ECG features assessed in this study were independent predictors of reduced LVEF. Most notable were indices of repolarization dispersion. A prolonged T peak – T end interval, a measure of transmural dispersion, was associated with lower LVEF. A larger 2nd to 1st PCA ratio at J wave, a measure of the presence of ischemic injury vector, was also associated with lower LVEF. Lower non-dipolar component of ST segment, a measure of localized ST changes, and larger non-dipolar component of T wave, a measure of diffuse T wave changes, were also associated with lower LVEF. Interestingly, a prior study has specifically linked abnormal repolarization in lead aVR with reduced LVEF in patients with ischemic cardiomyopathy.17 These findings support the notion that the strong association we observed between regional depolarization distortions at LV apex with LVEF could be attributed to underlying myocardial ischemia in this patient population. With ischemia, there is more anisotropic conduction. The ratio of conduction velocity along versus across myocardial cells decreases significantly due to redistribution of gap junctions from the cardiomyocyte ends to the lateral sides, leading to slower overall activation, more dispersion of depolarization and repolarization and higher risk of reentrant arrhythmias.
Clinical Implications:
Assessment of LVEF in patients with suspected ACS yields prognostic implications and has been shown to be associated with hospital quality of care and use of appropriate evidence-based prophylactic therapeutics.18 Echocardiography requires skilled manpower which may not be available immediately in acute care settings and therefore the LVEF reading may be delayed. Echocardiography availability in remote areas is also limited. Thus, given the immediate availability of 12-lead ECG data in this patient population, ECG predictors of reduced LVEF could provide an important noninvasive triage tool to identify those with impaired cardiac function and need subsequent evaluation or management. The features identified in our study can be automatically computed and can be visually seen on the 12-lead ECG. Figure 2B shows the automated calculations of horizontal plane QRS axis and VAT from the prehospital 12-lead ECG of two patients presenting with suspected ACS. The figure also show the corresponding visual morphology of lead V3 and lead aVR as simple markers during clinical evaluation. Patient 2 had greater left posterior horizontal plane axis deviation and slower ventricular activation time in LV apex, both of which correlated with lower LVEF compared to Patient 1. With such easy tools, ECG screening of impaired LVEF can be extended to the majority of patients evaluated at acute care setting, which constitutes an important opportunity to improve patient triage, especially in those in whom an echocardiogram was not available. For instance, in our parent study, the echocardiogram was performed on only 313 out of 750 patients (42%). Among those in whom no echocardiogram was done (i.e., 437 out of 750, 58%), 32% had known CAD, 17% had known heart failure, and 6% had confirmed ACS, suggesting that LVEF readings could have presented useful prognostic information.
Limitations
This study has some limitations, most notably is the relatively small sample size. Although we had adequate power to examine the main effect of predictors of interest using multivariate models, we were unable to perform sensitivity analyses to evaluate our models in various clinical subgroups (e.g., ACS vs. non-ACS, LAD vs. LCX vs RCA, etc.). Future work should focus on evaluating our results on an independent testing set to verify the accuracy of the prediction of the proposed diagnostic algorithm. In addition, by design, this study only included those with both ECG and echocardiogram performed during the indexed encounter, which would have enriched our population with sicker patients. Thus, the generalizability of our findings to the general chest pain population should be made with caution.
CONCLUSIONS
In patients with suspected ACS, the 12-lead ECG constitutes an important noninvasive tool to screen for reduced LVEF. Indices of depolarization dispersion in the horizontal plane and global repolarization dispersion are independent predictors of LVEF. Left QRS axis deviation in the horizontal plane and slow ventricular activation in LV apex constitute the most important determinants of reduced LVEF. Poor R wave progression in precordial leads with dominant QS pattern in V3 can be a simple visual marker to aid clinicians in identifying those with impaired cardiac function. In this clinical population of suspected ACS, electrical dispersion induced by the underlying myocardial ischemia and regional perfusion abnormalities could provide a plausible explanation of these findings.
Funding:
National Institute of Health R01-HL-137761
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
REFERENCES
- 1.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. European journal of heart failure. 2012;14(8):803–869. [DOI] [PubMed] [Google Scholar]
- 2.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Archives of internal medicine. 2001;161(7):996–1002. [DOI] [PubMed] [Google Scholar]
- 3.Jeger RV, Pfister O, Radovanovic D, et al. Heart failure in patients admitted for acute coronary syndromes: A report from a large national registry. Clinical cardiology. 2017;40(10):907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox KA, Steg PG, Eagle KA, et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. Jama. 2007;297(17):1892–1900. [DOI] [PubMed] [Google Scholar]
- 5.AlFaleh H, Elasfar AA, Ullah A, et al. Acute heart failure with and without acute coronary syndrome: clinical correlates and prognostic impact (From the HEARTS registry). BMC cardiovascular disorders. 2016;16(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahit MC, Kochar A, Granger CB. Post-myocardial infarction heart failure. JACC: Heart Failure. 2018;6(3):179–186. [DOI] [PubMed] [Google Scholar]
- 7.O’Neal WT, Mazur M, Bertoni AG, et al. Electrocardiographic predictors of heart failure with reduced versus preserved ejection fraction: the multi-ethnic study of atherosclerosis. Journal of the American Heart Association. 2017;6(6):e006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attia ZI, Kapa S, Lopez-Jimenez F, et al. Screening for cardiac contractile dysfunction using an artificial intelligence–enabled electrocardiogram. Nature medicine. 2019;25(1):70–74. [DOI] [PubMed] [Google Scholar]
- 9.Al-Zaiti SS, Martin-Gill C, Sejdic E, Alrawashdeh M, Callaway C. Rationale, development, and implementation of the Electrocardiographic Methods for the Prehospital Identification of Non-ST Elevation Myocardial Infarction Events (EMPIRE). J Electrocardiol. 2015;48(6):921–926. [DOI] [PubMed] [Google Scholar]
- 10.Rivero D, Alhamaydeh M, Faramand Z, et al. Nonspecific electrocardiographic abnormalities are associated with increased length of stay and adverse cardiac outcomes in prehospital chest pain. Heart Lung. 2019;48(2):121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Zaiti S, Besomi L, Bouzid Z, et al. Machine Learning-Based Prediction of Acute Coronary Syndrome Using Only the Pre-Hospital 12-Lead Electrocardiogram. Nature Communications. 2020;Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao HB, McCan S, Kaufman B. The prognostic significance of horizontal plane QRS axis in elderly heart failure. International journal of cardiology. 2006;106(2):196–200. [DOI] [PubMed] [Google Scholar]
- 13.Chumarnaia T, Solov’eva O, Sukhareva S, Vargina T, Markhasin V. Spatio-temporal heterogeneity of human left ventricle contractions in norm and under ischemic heart disease. Rossiiskii fiziologicheskii zhurnal imeni IM Sechenova. 2008;94(11):1217–1239. [PubMed] [Google Scholar]
- 14.Bendary A, El-Husseiny M, Azm TA, Moneim AA. The predictive value of R-wave peak time on no-reflow in patients with ST-elevation myocardial infarction treated with a primary percutaneous coronary intervention. The Egyptian Heart Journal. 2018;70(4):415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rencüzoğulları İ, Çağdaş M, Karakoyun S, et al. The association between electrocardiographic R wave peak time and coronary artery disease severity in patients with non-ST segment elevation myocardial infarction and unstable angina pectoris. Journal of electrocardiology. 2018;51(2):230–235. [DOI] [PubMed] [Google Scholar]
- 16.Kalçık M, Bayam E, Güner A, et al. Increased Ventricular Activation Time in Patients with the Diagnosis of Cardiac Syndrome X. Koşuyolu Heart Journal. 2019;22(3):145–151. [Google Scholar]
- 17.Al-Zaiti SS, Fallavollita JA, Canty JM, Carey MG. The prognostic value of discordant T waves in lead aVR: a simple risk marker of sudden cardiac arrest in ischemic cardiomyopathy. Journal of electrocardiology. 2015;48(5):887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller AL, Dib C, Li L, et al. Left ventricular ejection fraction assessment among patients with acute myocardial infarction and its association with hospital quality of care and evidence-based therapy use. Circulation: Cardiovascular Quality and Outcomes. 2012;5(5):662–671. [DOI] [PubMed] [Google Scholar]


