Abstract
Patients with sickle cell disease (SCD) can develop strokes and as a result, present neurologic and neurocognitive deficits. However, recent studies show that even without detectable cerebral parenchymal abnormalities on imaging studies, SCD patients can have significant cognitive and motor dysfunction, which can present as early as during infancy. As the cerebellum plays pivotal roles in motor and non-motor functions including sensorimotor processing and learning, we examined cerebellar behavior in humanized SCD mice using the Erasmus ladder. Homozygous (sickling) mice had significant locomotor malperformance characterized by miscoordination and impaired locomotor gait/stepping patterns adaptability. Conversely, Townes homozygous mice had no overall deficits in motor learning, as they were able to associate a conditioning stimulus (high-pitch warning tone) with the presentation of an obstacle and learned to decrease steptimes thereby increasing speed to avoid it. While these animals had no cerebellar strokes, these locomotor and adaptive gait/stepping patterns deficits were associated with oxidative stress, as well as cerebellar vascular endothelial and white matter abnormalities and blood brain barrier disruption, suggestive of ischemic injury. Taken together, these observations suggest that motor and adaptive locomotor deficits in SCD mice mirror some of those described in SCD patients and that ischemic changes in white matter and vascular endothelium and oxidative stress are biologic correlates of those deficits. These findings point to the cerebellum as a cerebral region that is vulnerable to vascular and white matter injury and support the use of SCD mice for studies of the underlying mechanisms of cerebellar dysfunction in SCD.
Keywords: sickle, anemia, cerebellum, behavior, Erasmus Ladder, balance
1. Introduction
In sickle cell disease (SCD), polymerization of sickle hemoglobin leads to sickling of red blood cells, hemolysis, vaso-occlusion, chronic anemia, inflammation, and vascular endothelial injury. As a result, SCD patients face lifetime complications including acute and chronic pain, multiorgan system damage, and decreased life expectancy. Complications involving the central nervous system, which include overt and silent cerebral infarcts, transient ischemic attacks, acute and chronic headaches, seizures, and neurocognitive deficits are rather prevalent in SCD (DeBaun and Kirkham, 2016; Farooq and Testai, 2019; Stotesbury et al., 2019; Vichinsky et al., 2010). Studies correlating abnormalities in imaging studies with neuropsychological testing demonstrate that in children and adults, silent and clinical strokes are associated with significant neurologic and neurocognitive deficits in domains of learning and memory, visual motor integration, visual processing, and processing speed (DeBaun et al., 1998; Mackin et al., 2014; Stotesbury et al., 2019). However, recent studies show that SCD patients even without detectable parenchymal abnormalities on brain imaging can have significantly reduced global cognitive function, working memory, processing speed and impaired executive function (Newby et al., 2018; Steen et al., 2003; Vichinsky et al., 2010; Wang et al., 2001). Conversely, others have shown that despite the absence of clinical symptoms and grey matter abnormalities, some SCD patients can have profound global white matter volume loss (6.8–8.1%) in the frontal, parietal and temporal lobes, corpus callosum, right brainstem and right cerebellum (Choi et al., 2017). Together, these data suggest that structural abnormalities identified by existing imaging modalities can be sufficient but are not necessary for the development of neurocognitive deficits in SCD patients (Choi et al., 2019; Glass et al., 2013; Kawadler et al., 2013; Stotesbury et al., 2019).
While locomotor deficits have not been characteristically described in SCD patients, locomotion and gait adaptability have not been systematically studied. However, there is growing evidence that the cerebellum and some cerebellar functions could be affected in SCD patients (Kawadler et al., 2013; Newby et al., 2018; Scantlebury et al., 2011). Studies using magnetic resonance imaging show significant reduction in cerebellar volume in some SCD patients independently of the presence of infarcts (Kawadler et al., 2013). Others showed that cerebellar white matter is altered in SCD patients and that these changes are associated with impaired processing speed in neurocognitive tests (Scantlebury et al., 2011). Still others have recently demonstrated that in SCD patients, the cerebellum has stronger connections to areas involved in pain inhibition such as the periaqueductal gray matter, and lesser connectivity to pain processing areas (Case et al., 2017) compared to healthy subjects. While core cerebellar functions have traditionally involved locomotor coordination, motor speed, and balance, it has become abundantly clear that the cerebellum is involved in non-motor functions including sensory processing, learning, and emotionality (Baillieux et al., 2008; Salman and Tsai, 2016). In fact, a number of human and animal studies demonstrate that cerebellar damage can be associated with adaptive motor learning alterations and cognitive and affective processes impairment (Baillieux et al., 2008). The cerebellum is also known to be involved in pain processing, which is highly relevant for SCD patients, as pain is a highly prevalent symptom (Apkarian et al., 2005). Therefore, increasing evidence suggests that cerebellar involvement may significantly contribute to neurocognitive deficits and pain processing in SCD.
Two well studied humanized mouse models of SCD, Townes and BERK strains, have been shown to display many of the phenotypes of human sickle cell trait (heterozygous) and SCD (homozygous) (Khaibullina et al., 2015; Khaibullina et al., 2018; Wang et al., 2016; Wang et al., 2018). These animals express normal and sickle human hemoglobin, exhibit hemolysis, severe anemia and leukocytosis, as well as severe organ dysfunction. We have previously shown that homozygous SCD mice have neurocognitive and memory deficits, decreased exercise capacity, and altered mood and emotionality (Wang et al., 2016; Wang et al., 2018). While these animals do not have strokes in early adulthood, the behavioral deficits in SCD mice are associated with significant morphologic alterations in the hippocampus and cerebellum, thus suggesting that the cerebellum and hippocampus are susceptible to injury in SCD mice (Wang et al., 2016). In this investigation, we sought to characterize cerebellar function in the SCD mouse model and to identify biologic correlates of cerebellar deficits.
2. Results
2. 1. Sickle cell mice have significant locomotor miscoordination
One hundred and thirty eight Townes (Wu et al., 2006) and BERK (Paszty et al., 1997) SCD mice were used in this study. As we included male and female mice in controls, heterozygote, and homozygous groups and examined the effect of sex on most variables, we show data by sex, genotype, and session. Using the Erasmus Ladder task, a fully automated instrument, we examined walking patterns, motivation, interlimb coordination, locomotion gait/stepping pattern adaptability, and adaptive cerebellar motor learning in SCD Townes mice, during unperturbed (daily sessions one to four, when no obstacles are presented) and perturbed sessions (sessions five to eight, when an obstacle was presented on the mouse path with or without a warning sound).
We first examined the percentage of missteps (steps in which the mouse made a faulty movement onto the lower misstep rung), a measure of interlimb coordination (Vinueza Veloz et al., 2015). In animals from all genotypes (controls, heterozygotes, and homozygotes), the percentage of missteps varied according to session. Regardless of sex and genotype, over the four unperturbed sessions (sessions one to four), mice displayed an overall decrease in percentage of missteps. Conversely, during perturbed sessions there were increases on percentage of missteps compared to session four, the last unperturbed session (all p<0.0001 Figure 1A–C, supplemental Figure 1A). However, overall, controlling for session and sex, homozygous Townes displayed significant interlimb miscoordination, as they committed more missteps than heterozygotes and controls (overall effect of genotype, p=0.031, Figure 1A–C, and supplemental Figure 1A). Controlling for genotype and sessions, overall, males and females mice displayed similar percentage of missteps (p=0.496, Figure 1A–C). However, ad-hoc analysis indicated that among homozygous Townes, male mice, compared to females, displayed higher percentage of missteps on sessions three (p=0.003), five (p=0.0012) and six (p=0.031), Figure 1C.
Figure 1. Sickle cell mice have locomotor miscoordination and decreased motivation.
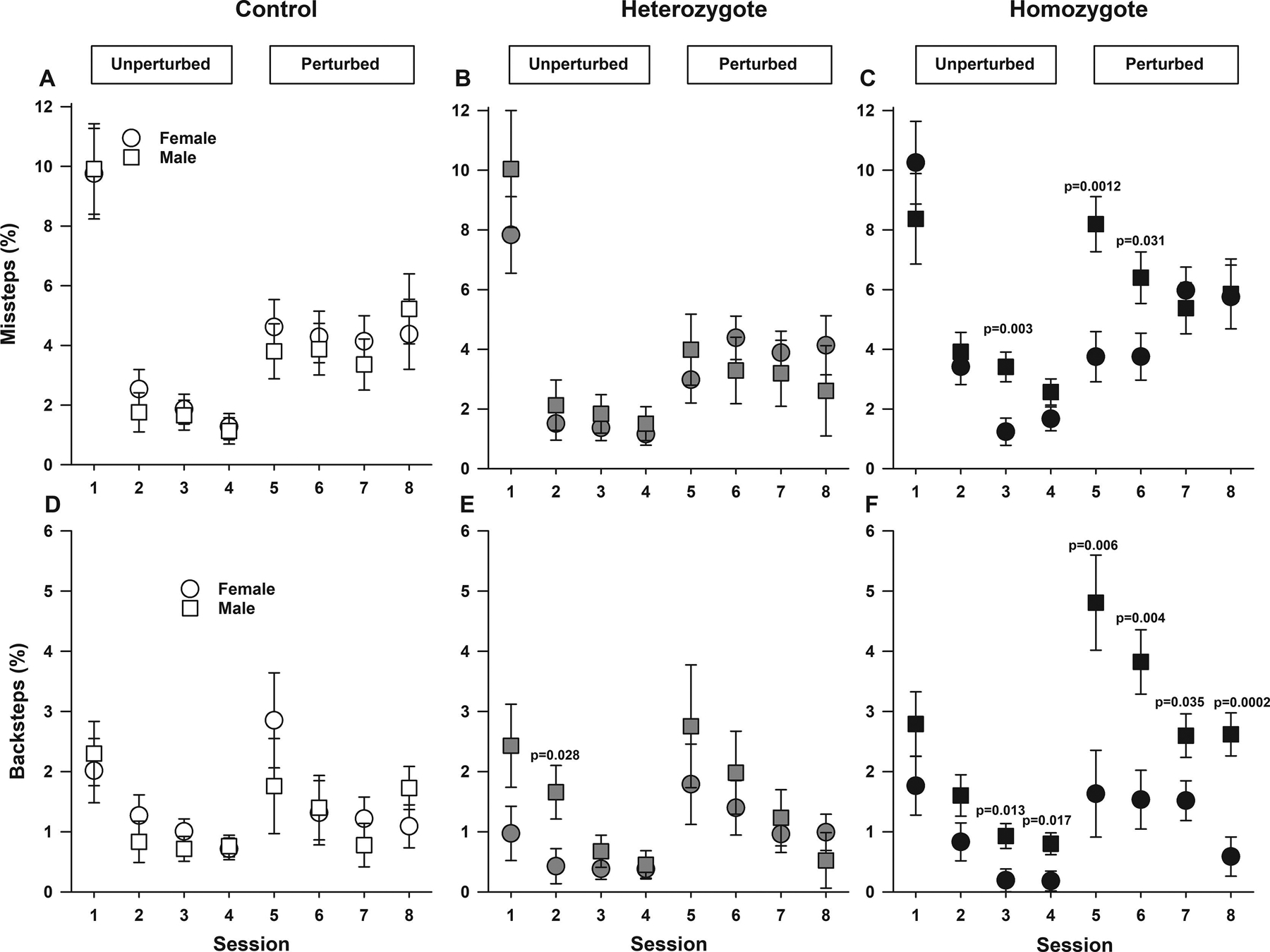
Data are shown as least-square means±standard error of the least-square means for male and female controls (A and D), heterozygotes (B and E), and homozygous (C and F). In animals from all genotypes (control, heterozygote, and homozygotes), the percentage of missteps displayed varied according to session (unperturbed or perturbed). P values reflect ad hoc male vs. female pairwise comparisons for respective genotypes and sessions. Controlling for sex and genotype, over the four unperturbed sessions (one-four), there was an overall decrease in percentage of missteps, which during perturbed (five-eight) sessions increased compared to the last unperturbed session (session 4, all p<0.0001). Controlling for session and sex, homozygous Townes committed a higher percentage of missteps compared with heterozygotes and control animals (overall effect of genotype, p=0.031, A, B, and C). Among homozygous Townes, male mice, compared to females displayed a higher percentage of missteps on sessions three (p=0.003), five (0.0012) and six (p=0.031), C. Regarding backsteps, the effect of genotype varied by session and by sex as there were genotype by session (p=0.012) and genotype by sex (p=0.016) interactions (D-F). During unperturbed sessions, control, heterozygous, and homozygous mice displayed similar percentage of backsteps. Regarding genotype by sex interaction, we found that among control mice male and female mice had similar percentage of backsteps in all sessions (all, p≥0.217, D). Among controls and heterozygotes, male and female mice had similar percentage of backsteps in all sessions (all, p>0.027, Figure 1D–E). In contrast, among homozygous Townes, males had a higher percentages of backsteps compared to females in unperturbed (three and four) and all perturbed sessions (five-eight), all p≤0.035, F. Controlling for session, females of all genotypes displayed similar percentage of backsteps (p≥0.149). In contrast, male homozygous mice showed a higher percentage of backsteps compared to controls (p=0.003) and heterozygous males (p=0.025), D-F. Thirty-one mice participated in the Erasmus Ladder test, N=10–11 per genotype including balanced numbers of age-matched male and females.
We then examined the percentage of backsteps [steps made in the direction opposite to that of the tail-wind (backwards) in an attempt to return to the originating shelter box], a variable thought to reflect decreased motivation to complete the trials (Sathyanesan et al., 2018a; Vinueza Veloz et al., 2015). The effect of genotype on backsteps percentage varied by session and by sex as there were genotype by session (p=0.012) and genotype by sex (p=0.016) interactions (Figure 1D–F). Overall, during unperturbed sessions, control, heterozygous, and homozygous mice displayed similar percentage of backsteps (supplemental Figure 1B). However, during perturbed sessions, compared to controls, homozygous Townes displayed a higher percentage of backsteps in sessions six (p=0.017) and seven (p=0.0054) and compared to heterozygotes in sessions seven (p=0.015) and eight (p=0.028), supplemental Figure 1B. We also found that among controls and heterozygotes, male and female mice displayed similar percentage of backsteps in all sessions (all, p>0.027, Figure 1D–E). In contrast, among homozygous Townes, males displayed higher percentages of backsteps compared to females during some unperturbed (sessions three and four) and perturbed sessions (five-eight), Figure 1F. Further, controlling for session, females of all genotypes had similar percentage of backsteps (p≥0.149). In contrast, male homozygous mice displayed a higher percentage of backsteps compared to male controls (p=0.003) and heterozygotes (p=0.025), Figure 1D–F.
2.2. Sickle cell mice have gait/stepping pattern adaptability deficits
We then conducted an overall assessment of multi-joint limb control, coordination capabilities and gait/stepping patterns adaptability by measuring the percentage of short and long steps as well as jump steps during unperturbed and perturbed sessions (Figures 2 and 3, supplemental Figure 2 and 3). Homozygous and heterozygous Townes displayed altered stepping patterns compared to controls in a way that varied according to session and sex.
Figure 2. Sickle cell mice have significant locomotor/gait adaptability deficits.
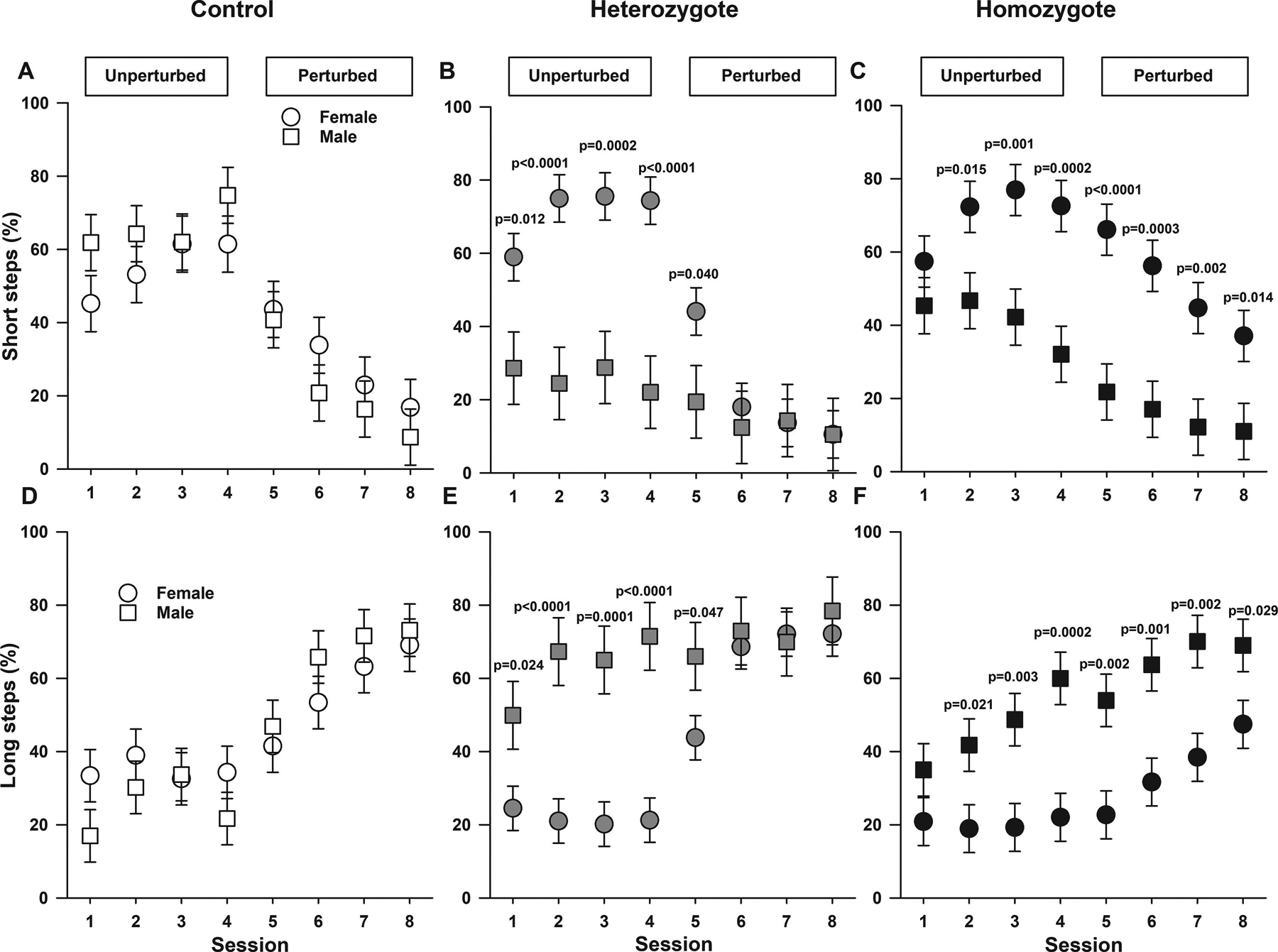
Data are shown as least-square means±standard error of the least-square means. The experimental paradigm in the Erasmus Ladder allows for an analysis of multi-joint limb control and coordination capabilities by measuring the percentage of short (A, B, and C) and long steps (D, E, and F) in male and female controls (A and D), heterozygotes (B and E), and homozygous (C and F). P values reflect ad hoc male vs. female pairwise comparisons for respective genotypes and sessions. Regarding short steps, there were genotype by session by sex interaction (p=0.0075, A-C) indicating that there were differences in percentage of short steps among genotypes that varied by sex and session. A. Among control mice, male and females showed similar percentage of short steps during unperturbed and perturbed sessions. B. In heterozygotes, female mice displayed higher percentage of short steps compared to males during unperturbed sessions (all p≤0.012). C. Among homozygotes, female mice displayed higher percentage of short steps compared to males in all sessions except during the first unperturbed session (all p<0.016). Regarding long steps, controlling for sex and genotype, during perturbed sessions animals displayed increases in percentage of long steps compared to the last unperturbed session (all p≤0.010, D-F). Additionally, there were also genotype by session by sex interactions (p=0.0024, D-F) indicating that there were differences in percentage of long steps among controls, heterozygotes, and homozygotes, which varied according to sex and session. D. Among control mice, male and females showed similar percentage of long steps during all sessions. E. Among heterozygotes, during unperturbed and the first perturbed sessions, male mice displayed higher percentage of long steps (all p<0.048) compared to females heterozygotes. F. In a different pattern compared to controls and heterozygotes (p=0.0024, for genotype by sex by session interaction), among homozygotes, male mice had a higher percentage of long steps compared to females in all sessions after the first unperturbed session (all p≤0.021). Overall, controlling for session, female homozygous displayed a lower percentage (p=0.016) whereas male homozygotes had similar (p=0.179) percentage of long steps compared to female and male controls respectively. Thirty-one mice participated in the Erasmus Ladder test, N=10–11 per genotype including balanced numbers of age-matched male and females.
Figure 3. Sickle cell mice have altered locomotor/gait adaptability.
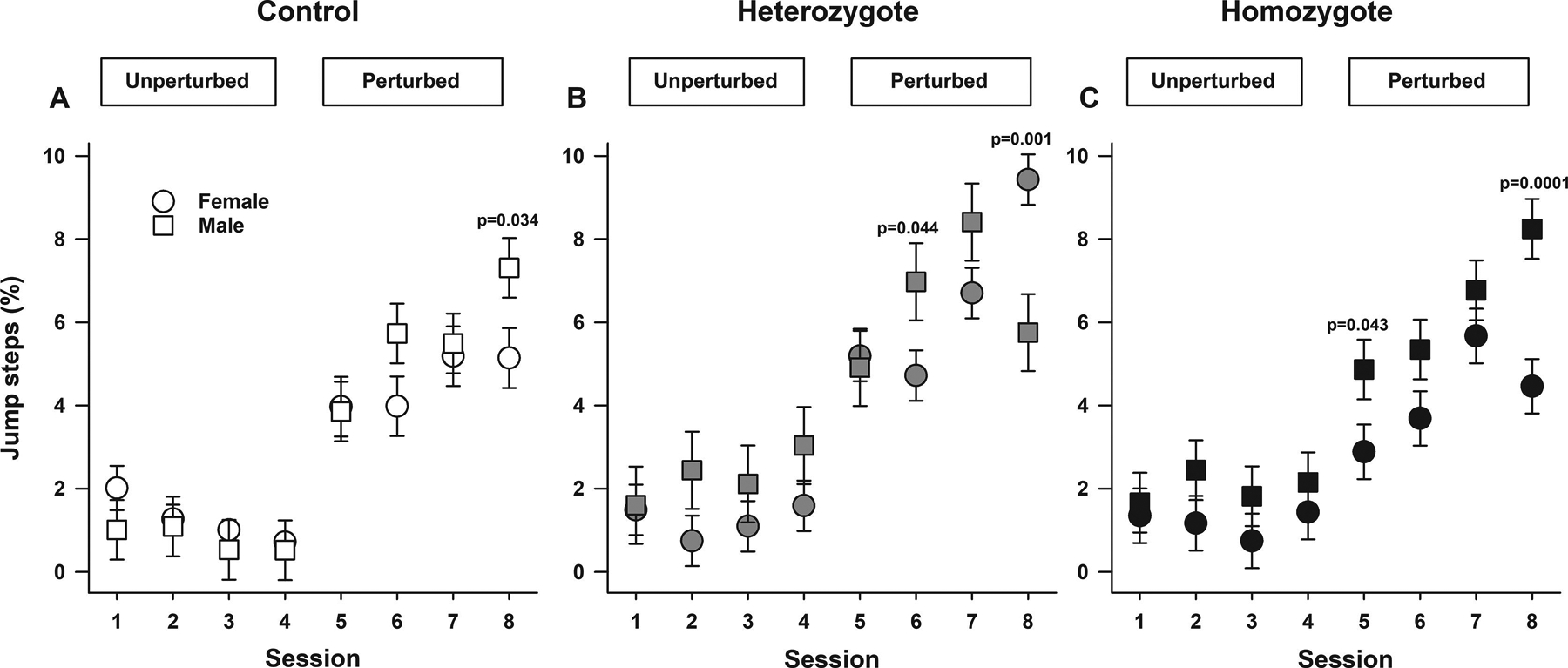
Data are shown as least-square means±standard error of the least-square means for male and female controls (A), heterozygotes (B), and homozygotes (C). P values reflect ad hoc male vs. female pairwise comparisons for respective genotypes and sessions. Overall, controlling for sex and genotypes, the percentage of jump steps during perturbed sessions were higher than the last unperturbed session (all p<0.0001, A, B, and C). Also, there were genotype by session by sex interactions (p<0.0001, A-C) indicating that there were differences in percentage of jump steps among controls, heterozygotes, and homozygotes, which varied according to sex and session. A. Among control mice, male and females showed similar percentage of jump steps during all sessions except for the last perturbed sessions when males had a higher percentage of jumps compared to females (p=0.034). B. Among heterozygotes, during all unperturbed and perturbed sessions five and seven male and females had similar percentage of jumps. However, on session six, males had higher (p<0.044) and in session eight lower (p=0.001) percentage of jumps compared to females. C. Homozygous males and females displayed similar percentage of jumps in all unperturbed session and in sessions six and seven (all p>0.089). In contrast, homozygote males had a higher percentage of jumps compared to females in perturbed sessions five (p=0.043) and eight (p=0.0001). Thirty-one mice participated in the Erasmus Ladder test, N=10–11 per genotype including balanced numbers of age-matched male and females.
Regarding short steps, controlling for sex and genotype, during perturbed sessions, animals displayed decreases in percentage of short steps compared to the last unperturbed session (all p≤0.0001, Figure 2A–C). Among controls, heterozygotes, and homozygotes there were differences in stepping patterns that varied by sex and session (p=0.0075 for genotype by session by sex interaction, Figure 2A–C). Among controls, male and females displayed similar percentage of short steps during unperturbed and perturbed sessions (Figure 2A). In contrast, among heterozygotes, female mice displayed higher percentage of short steps compared to males during unperturbed sessions (all p≤0.012). Lastly, among homozygotes, female mice displayed higher percentage of short steps compared to males in all sessions except during the first unperturbed session (all p<0.016, Figure 2C). Overall, controlling for session, female homozygous displayed higher, whereas male homozygotes lower percentage of short steps compared to female and male control mice respectively. Overall, controlling for sex, on the last unperturbed session, compared to controls, homozygous (p=0.038) and heterozygous (p=0.015) Townes had lower percentage of short steps (supplemental Figure 2A). Additionally, during perturbed session six, homozygotes had a higher percentage of short steps compared to heterozygotes (p=0.008, supplemental Figure 2A).
Regarding long steps, controlling for sex and genotype, during perturbed sessions (five-eight), coupled with decreases in short steps as indicated above, animals displayed increases in percentage of long steps compared to the last unperturbed session (all p≤0.0101, Figure 2D–F). Additionally, there was a modest effect of genotype (p=0.047, Figure 2 and supplemental Figure 2) and controlling for session and sex, homozygous Townes had a lower percentage of long steps compared to heterozygotes (p=0.015, supplemental Figure 2B). However, we found that there was a genotype by session by sex interaction (p=0.0024, Figure 2D–F) indicating that there were differences in percentage of long steps displayed by controls, heterozygotes, and homozygotes, which varied according to sex and session. Specifically, among control mice, male and females displayed similar percentage of long steps during all sessions (all p>0.108, Figure 2D). Among heterozygotes, during unperturbed sessions, male mice displayed a higher percentage of long steps (all p≤0.024) compared to female heterozygotes. In contrast, among homozygotes, male mice also displayed greater percentage of long steps compared to female homozygous in all sessions after the first unperturbed session (all p≤0.021, Figure 2F). Overall, controlling for session, female homozygotes displayed lower (p=0.016) whereas male homozygotes similar (p=0.179) percentage of long steps compared to female and male controls respectively Figure 2D and 2F.
Regarding jump steps, overall controlling for sex and genotype, during perturbed sessions, animals displayed increases in percentage of jump steps compared to the last unperturbed session (all, p<0.0001, Figure 3A–C and supplemental Figure 3). There was also a modest effect of genotype (p=0.046, Figure 3 and supplemental Figure 3). Controlling for session and sex, heterozygous Townes displayed similar percentage compared to homozygotes (p=0.103) but a greater percentage of jump steps compared to controls (p=0.014, supplemental Figure 3). However, there was a genotype by session by sex interaction (p<0.0001, Figure 3A–C) indicating that there were differences in percentage of jump steps among controls, heterozygotes, and homozygotes, which varied according to sex and session. Among control mice, male and females showed similar percentage of jump steps during all sessions except for the last perturbed sessions when male controls displayed a higher percentage of jumps compared to females (p=0.034, Figure 3A). In heterozygotes, during all unperturbed and perturbed sessions five and seven, male and females had similar percentage of jump steps. However, on session six, males had higher (p=0.044) and in session eight lower (p=0.001) percentage of jumps compared to female heterozygotes, Figure 3B. Homozygous males and females displayed similar percentage of jumps in all unperturbed sessions and in perturbed sessions six and seven (all p>0.089, Figure 3C). In contrast, homozygous males had higher percentage of jumps compared to females in perturbed sessions five (p=0.043) and eight (p=0.0001), Figure 3C.
2.3. Sickle cell mice have intact motor learning
Using the Erasmus Ladder, we also examined adaptive cerebellar motor learning by comparing rung activation steptimes in male and female control, heterozygous, and homozygous Townes (Figure 4 and supplemental Figure 4). During the first perturbed session, one typically observes an increase in post perturbation steptime. Over subsequent perturbed sessions, mice associate the conditioning stimulus (high pitch warning tone) with presentation of the obstacle and begin to adapt their gait/stepping pattern by decreasing steptimes thereby increasing speed to overcome the obstacle. During unperturbed sessions, when no obstacles were presented, overall, homozygous and heterozygous had similar steptimes (all p>0.191, supplemental Figure 4). However, during the fourth unperturbed sessions, homozygous (p=0.0005) and heterozygous (p=0.0085) had shorter steptimes compared to control animals (supplemental Figure 4). Over perturbed sessions, when obstacles were presented, overall, there were increases in post perturbation steptimes in sessions five (p<0.0001) and six (p=0.002) compared to session four (Figure 4, supplemental Figure 4). However, by sessions seven and eight, post perturbation steptimes decreased and were similar to those in session four (all p>0.065), thus indicating that controls, heterozygous, and homozygous Townes mice displayed similar adaptive cerebellar learning abilities (p=0.638 for genotype by session by perturbation interaction, Figure 4). Interestingly, during perturbed sessions, among control and homozygotes, males and females had similar pre and post perturbation steptimes (all p>0.17, Figure 4), whereas among heterozygotes, in sessions six and seven females had shorter pre and post perturbations steptimes compared to males (all p<0.034, Figure 4), thus suggesting that female heterozygotes had better motor learning compared to males. During perturbed sessions, females from all genotypes had similar post perturbation steptimes (all p>0.23, Figure 4). In contrast, during perturbed session seven, homozygous males had shorter post perturbation steptimes compared to control (p=0.027) and heterozygote (p=0.031) males, Figure 4.
Figure 4. Sickle cell mice have intact adaptive cerebellar motor learning.
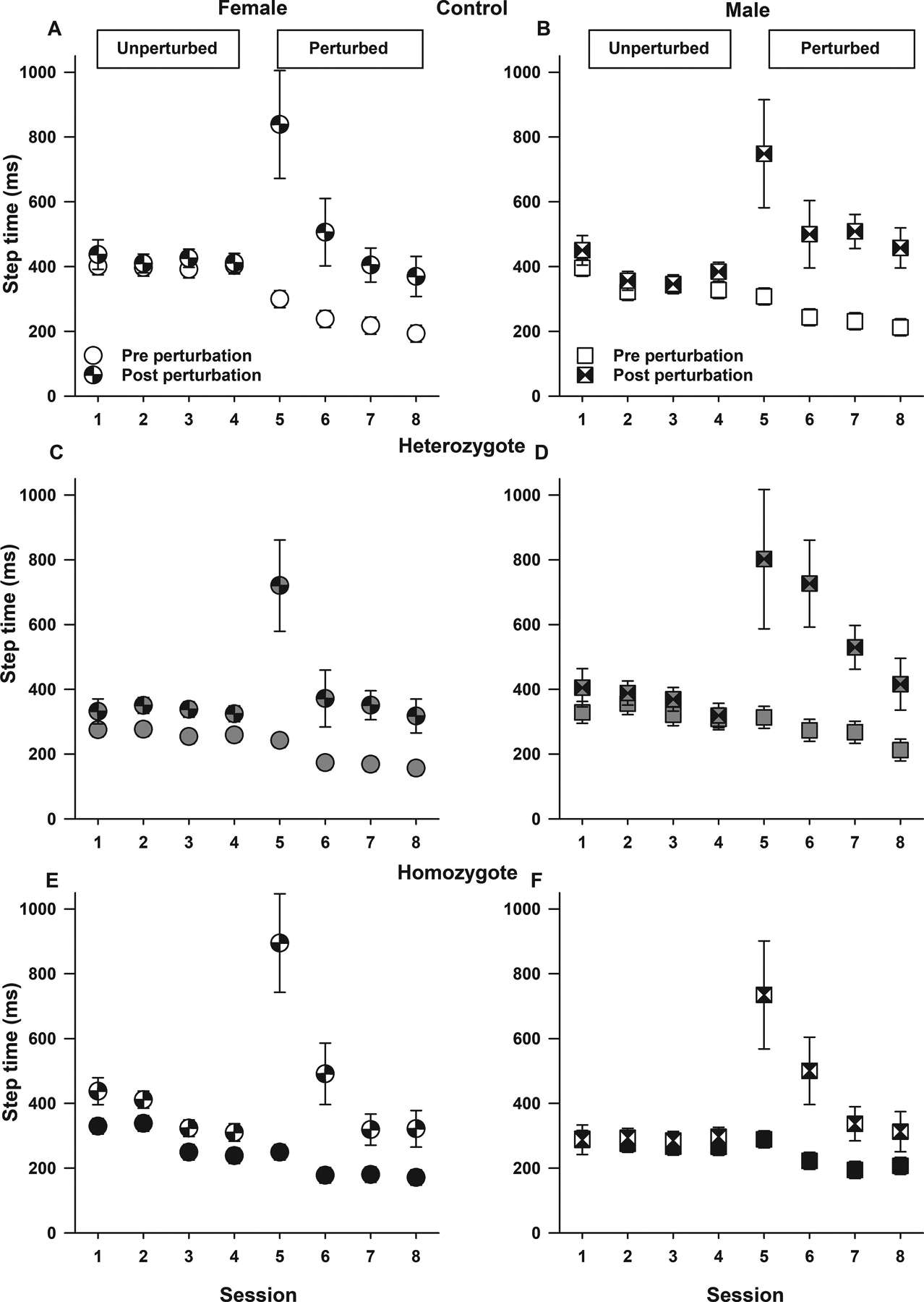
Data are shown as least-square means±standard error of the least-square means pre and post perturbation steptime by sex (females A, C, E and males B, D, F), genotype (controls, A and B; heterozygotes, C and D; and homozygotes E and F), and session. On the Erasmus Ladder, one can examine adaptive cerebellar motor learning. During unperturbed sessions, there are no conditioning stimuli or obstacle presentation. During the first perturbed session, one observes an increase in post perturbation steptime. At that time, animals begin to adapt their gait/stepping pattern as to avoid the upcoming obstacle. Over the subsequent perturbed sessions, mice associate the conditioning stimulus (high pitch warning tone) with presentation of the obstacle and learn to modify their gait/steps by decreasing post perturbation steptimes thereby increasing speed to overcome the obstacle. During perturbed sessions (five through eight), among control and homozygotes, males and females had similar pre and post perturbation step times (all p>0.17). Among heterozygotes, in sessions six and seven females had shorter pre and post perturbations steptimes compared to males (all p<0.034), thus suggesting that female heterozygotes had better motor learning compared to males. During perturbed sessions, females from all genotypes had similar post perturbation steptimes (all p>0.23). In contrast, during session seven, homozygous males had shorter post perturbation steptimes compared to control (p=0.027) and heterozygote (p=0.031) males. Thirty-one mice participated in the Erasmus Ladder test, N=10–11 per genotype including balanced numbers of age-matched male and females.
2.4. Sickle cell mice have impaired performance on the rotarod task.
While the Erasmus ladder allows for a comprehensive and automated evaluation of cerebellar behavior, it is not widely available. Therefore, we also used the rotarod task, a well-known tool used to evaluate motor coordination, balance, and motor learning, Figure 5. Overall, controlling for sex and trial, homozygotes had shorter latency to fall from the rotating rod compared to controls (p=0.030, for overall effect of genotype, Figure 5). In addition, controlling for genotype and sex, over the three trials, both controls and homozygotes displayed increases in latency to fall (p<0.0001 for overall effect of trial) indicating that homozygous have intact motor learning, a finding similar to that observed on the Erasmus ladder. However, there were sex by genotype by trial interaction (p=0.024) indicating that the trajectory of latency over the three trials varied according to genotype and sex. Specifically, over the three trials, among homozygotes, both males (p=0.014) and females (p=0.034) showed increases in latency to fall from trial one to trial three. In contrast among controls, female (p<0.0001), but not male mice (p=0.713), had increases in latency to fall from trial one to trial three (Figure 5).
Figure 5. Sickle cell mice have impaired performance on the rotarod task.
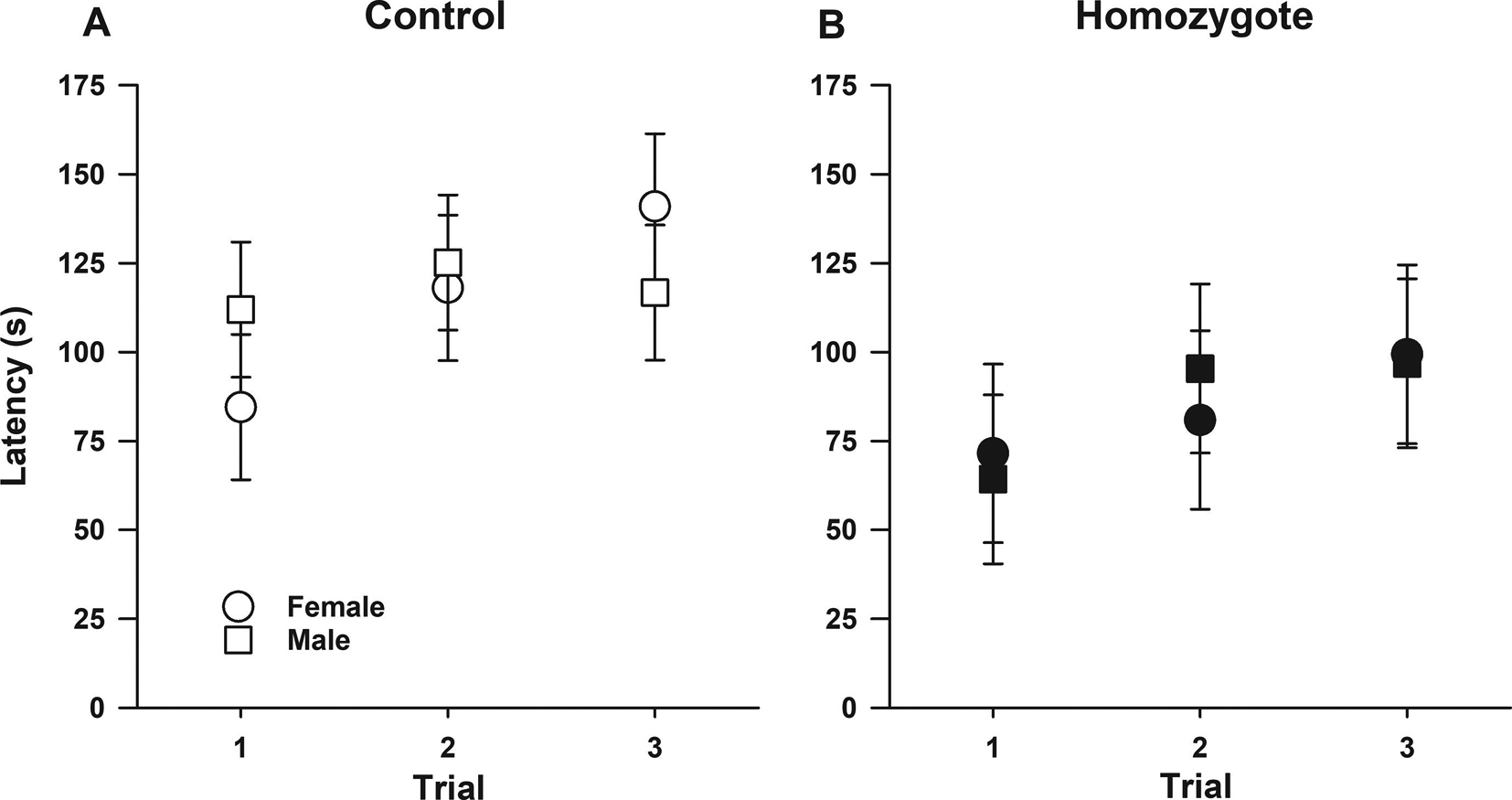
Data are shown as least-square means±standard error of the least-square means of latency to fall from the rod for male and female controls (A) and homozygotes (B). Overall, controlling for sex and trial, homozygotes had shorter latency to fall from the rotating rod compared to controls (p=0.030, for genotype effect). In addition, controlling for genotype and sex, overall, over the three trials, both controls and homozygotes displayed increases in latency to fall (p<0.0001 for overall effect of trial) indicating that homozygous have intact motor learning, a finding similar to that observed on the Erasmus ladder. However, there was a sex by genotype by trial interaction (p=0.024) indicating that comparing controls and homozygotes, the trajectory of latency to fall over the three trials varied according to trial and sex. Specifically, over the three trials, among homozygotes, both males (p=0.014) and females (p=0.034) showed increases in latency to fall from trial one to trial three. In contrast among controls, female (p<0.0001), but not male mice (p=0.713), had increases in latency to fall from trial one to trial three. Forty mice participated in the rotarod test N=20 per genotype including balanced numbers of age-matched male and females.
We then examined whether pain could play a role in the performance on the rotarod. We used dexmedetomidine at a dose (5μg/kg) shown to decrease visceral/inflammatory pain (Rangel et al., 2012) and to ameliorate mechanical hyperalgesia in a model of neuropathic pain with minimal sedation (Funai et al., 2014). At these doses, dexmedetomidine-treated mice were awake but slightly less active than vehicle-treated animals (Calhoun et al., 2015). Controlling for baseline latency, sex, and genotype, dexmedetomidine-treated controls and homozygous Townes had shorter latency to fall compared to respective vehicle-treated mice (p=0.0006, for treatment effect, supplemental Figure 5). Interestingly, controlling for baseline, genotype, sex, and treatment, overall from trial two to trial three, mice showed increases in latency to fall (p=0.0005 for overall effect of trial, supplemental Figure 5) suggesting that dexmedetomidine did not affect motor learning.
2.5. SCD mice have vascular and neural ultrastructural abnormalities in cerebellum
Others and we have previously shown that SCD mice have pyknotic Purkinje cells in the cerebellum (Manci et al., 2006; Wang et al., 2016). We sought to characterize the morphologic correlates of the cerebellar behavior deficits observed among homozygotes. Ultrastructural analysis of the cerebellum showed that, compared to controls, homozygotes had significant changes in vascular endothelial cells characterized by cytoplasmic vacuolization and extensive pseudopodia formation into the vascular lumen. Further, while control mice had normal foot processes of astrocytes, pericytes, and tight junctions/adherens junctions, homozygotes had significant white matter changes in the form of distended foot processes from astrocytes as well as attenuation/loss of tight junctions/adherens junctions (Hawkins and Davis, 2005; Salameh et al., 2016). These findings in homozygotes are strongly suggestive of damage to blood brain barrier integrity observed with hypoxic/ischemic injury (Figure 6).
Figure 6. Sickle cell mice have vascular and neural ultrastructural abnormalities in cerebellum.
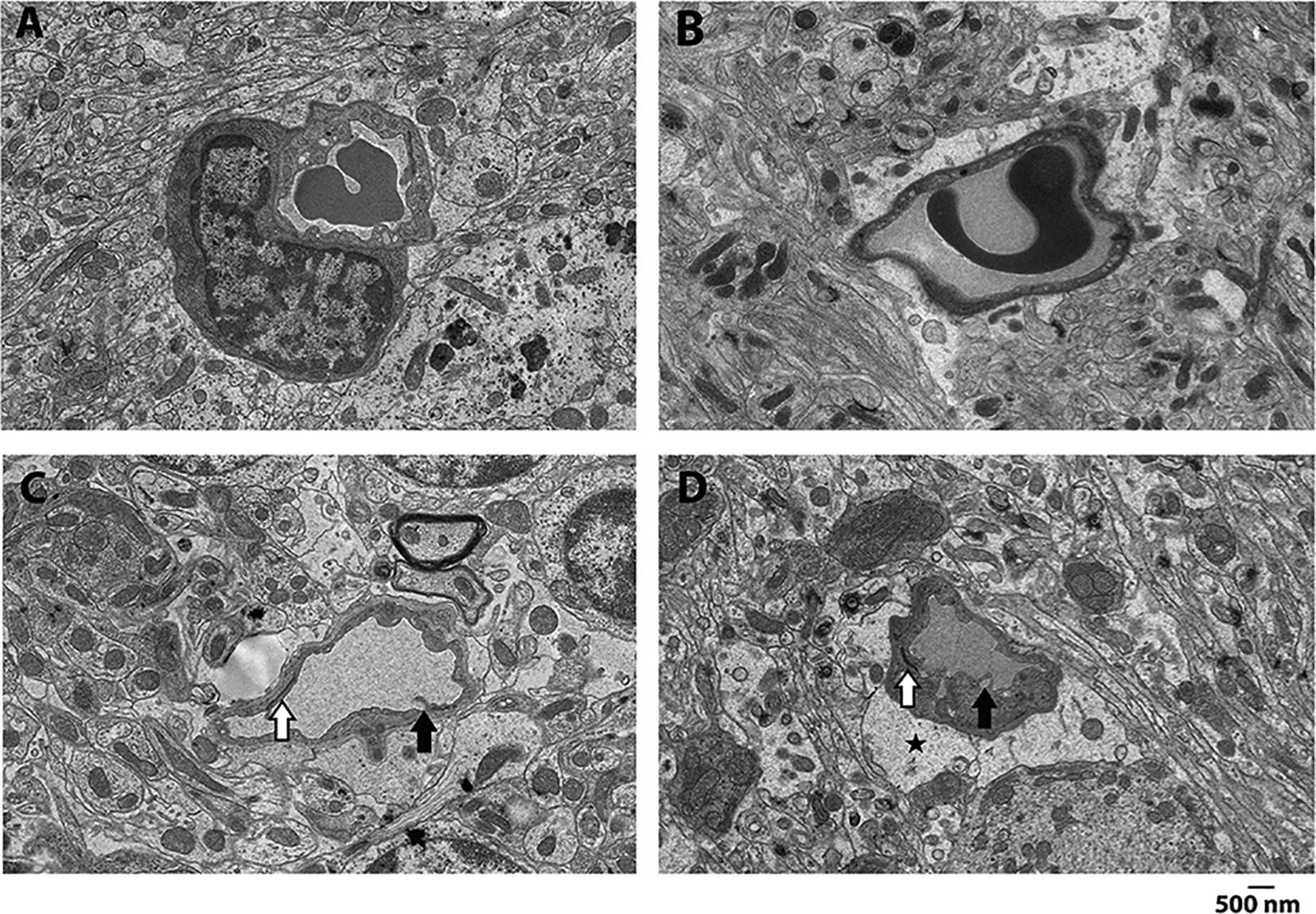
Representative images from control (A, B) and homozygotes (C, D) cerebellum. Compared to controls, homozygotes had significant changes in vascular endothelial cells characterized by cytoplasmic vacuolization and extensive pseudopodia formation into the vascular lumen (black arrows). Further, while control mice had normal foot processes of astrocytes, pericytes, and tight junctions/adherens junctions, homozygotes had significant white matter changes in the form of distended foot processes from astrocytes (black star) as well as attenuation/loss of tight junctions/adherens junctions (white arrows). These findings in homozygotes are strongly suggestive of damage to blood brain barrier integrity observed with hypoxic/ischemic injury.
2.6. Townes sickle cell mice have increased oxidative stress in cerebellum
There is growing evidence to suggest that repeated episodes of ischemia/reperfusion injury and oxidative stress contributes to the pathobiology of SCD. After we identified ultrastructural abnormalities suggestive of ischemic cerebellar injury, we tested the hypothesis that in cerebellum of SCD mice there would be increased oxidative stress. We first measured malondialdehyde formation, a surrogate measure of oxidative stress and lipid peroxidation. In cerebellar homogenates (Figure 7A), homozygous Townes had higher malondialdehyde formation compared to controls (p<0.001) and heterozygotes (p<0.001).
Figure 7. Townes sickle cell mice have increased oxidative stress in cerebellum.
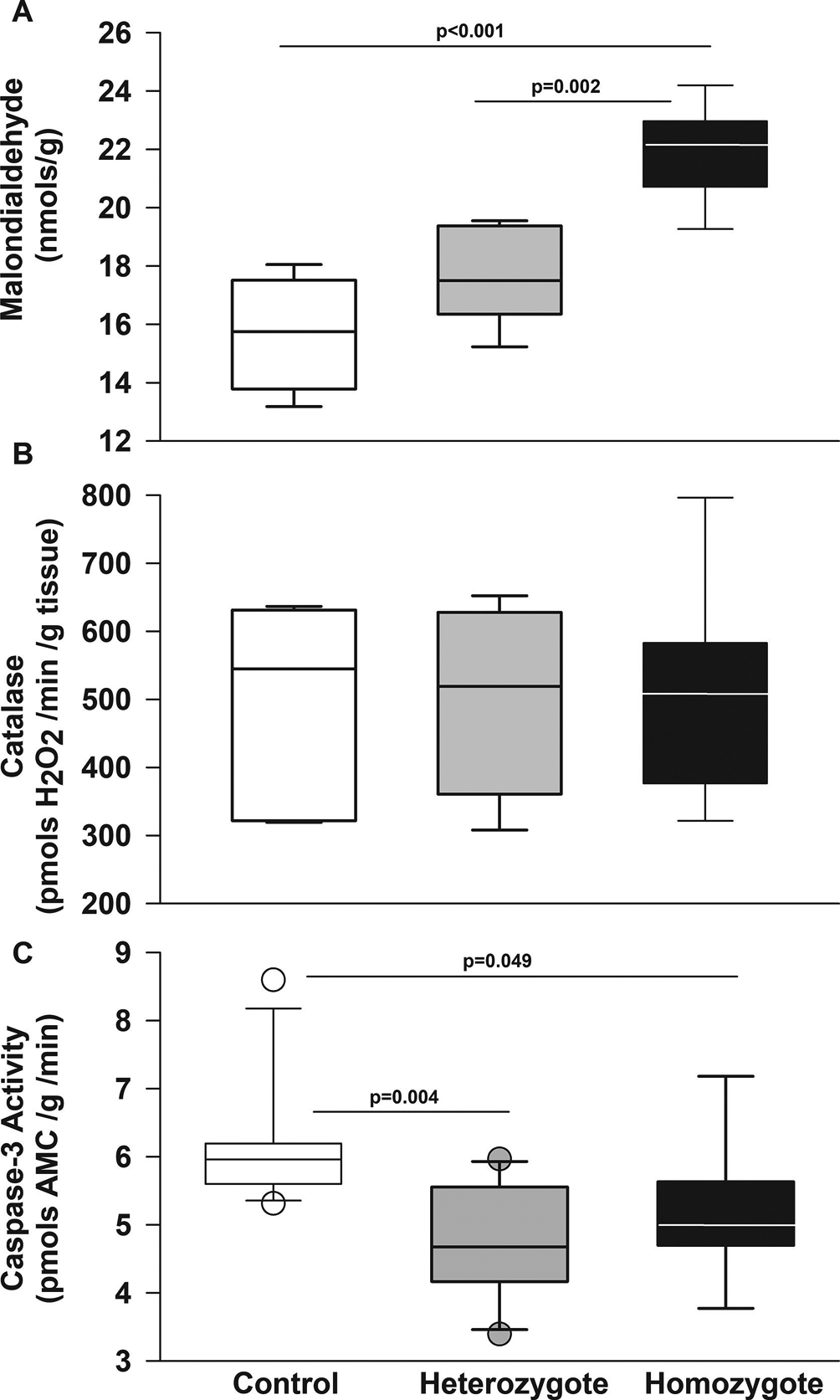
Box plots of respective variables show median and first and third quartiles and the whiskers 10th and 90th percentiles. A. In cerebellar homogenates, homozygous Townes had higher malondialdehyde, a surrogate measure of oxidative stress and lipid peroxidation formation compared to controls (p<0.001) and heterozygotes (p=0.002). B. Among Townes mice, levels of catalase activity were similar in controls, heterozygous, and homozygous Townes (p=0.983). C. In cerebellum, heterozygotes (p=0.004) and homozygotes (0.049) had lower levels of caspase-3 activity compared to control animals. N=4–11 per genotype and assay.
We also measured catalase activity, a component of antioxidant response, in cerebellum and found that among Townes mice, levels of catalase activity were similar in controls, heterozygous, and homozygous Townes (p=0.983, Figure 7B).
2.7. In the cerebellum of Townes SCD mice, oxidative stress is not associated with increased apoptosis
After we found evidence of oxidative stress in cerebellum of Townes mice, we examined whether there was evidence of increased caspase-3 activity and apoptosis. We first examined the effect of age and genotype on caspase-3 activity in cerebellum homogenates. We found that controlling for genotype, caspase-3 activity in the cerebellum was similar in young and old Townes mice (data not shown, p=0.292) and that in heterozygotes (p=0.004) and homozygotes (0.049) caspase-3 activity was lower than in control animals (Figure 7C). Additionally, TUNEL staining of cerebellum from control and homozygous Townes mice showed similarly scant number of apoptotic cells (data not shown).
2.8. The BERK strain of SCD mice also has oxidative stress in cerebellum
We then sought to determine whether these findings of increased oxidative stress and decreased caspase-3 activity were present on a second strain of SCD mice, the BERK strain (Figure 8). We found that in the cerebellum of BERK mice, akin to Townes animals, homozygotes had higher levels of malondialdehyde compared to hemizygotes (p=0.027, Figure 8A). Similar to the Townes mice, among BERKs homozygotes and hemizygotes had similar levels of catalase activity in the cerebellum (p=0.072), Figure 8B. Regarding caspase-3 activity, we found that hemizygous and homozygous BERKs had similar levels of caspase-3 activity in cerebellum homogenates (p=0.059, Figure 8C).
Figure 8. BERK sickle cell mice have increased oxidative stress in cerebellum.
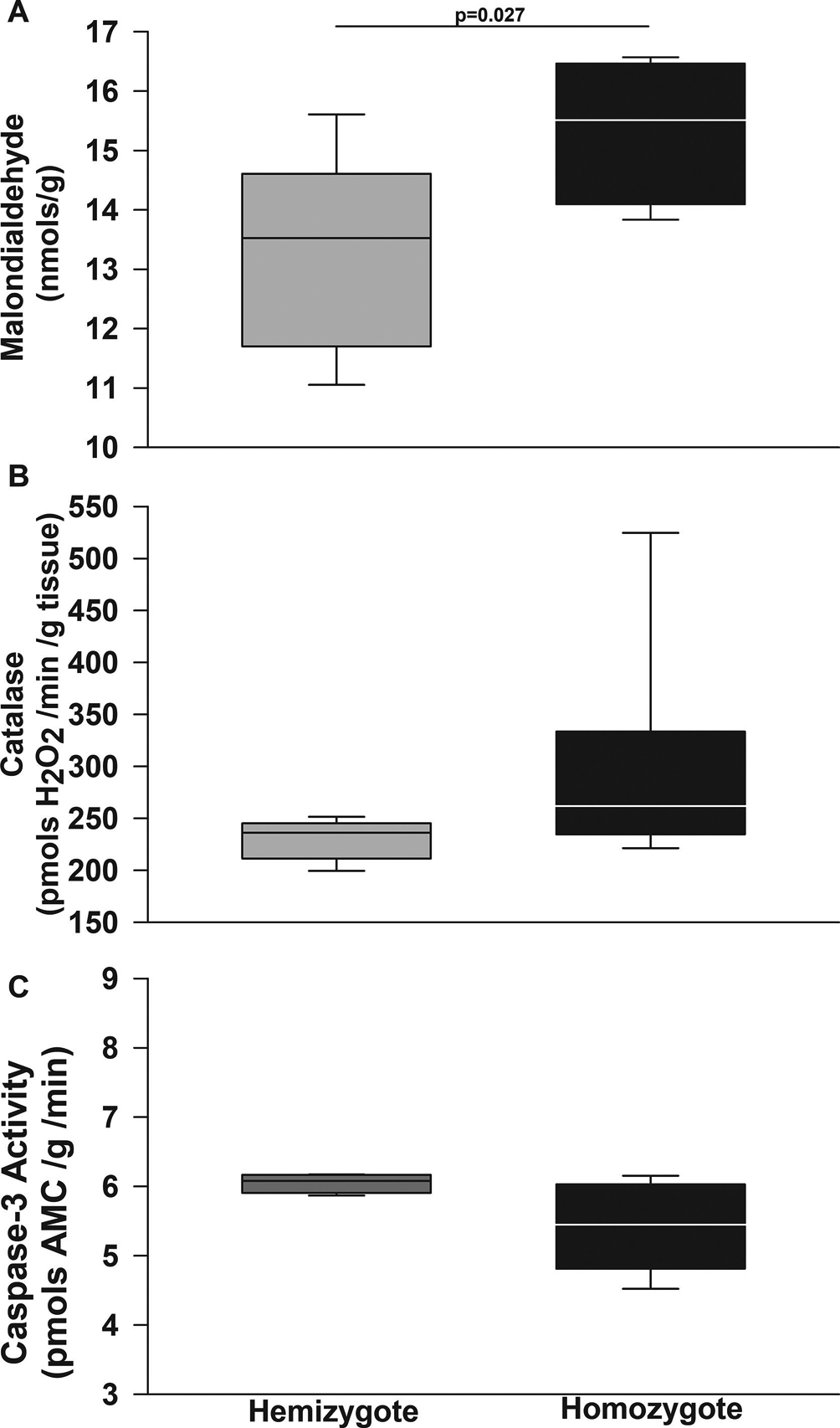
Box plots of respective variables show median and first and third quartiles and the whiskers 10th and 90th percentiles. In cerebellum homogenates, homozygous BERKs, akin to Townes animals, had higher levels of malondialdehyde, a surrogate measure of oxidative stress and lipid peroxidation formation compared to hemizygotes (p=0.027). B. Similar to the Townes mice, among BERKs, homozygotes and hemizygotes had similar levels of catalase activity in the cerebellum (p=0.072). C. Regarding caspase-3 activity, contrary to Townes mice, hemizygous and homozygous BERKs had similar levels of caspase-3 activity in cerebellum homogenates (p=0.059). N=4–8 per genotype and assay
3. Discussion
We showed that Townes mice, a clinically relevant model of SCD, have significant locomotor malperformance, motor miscoordination, and impaired gait/stepping adaptability. Interestingly, despite having locomotor malperformance, homozygous Townes had intact adaptive cerebellar motor learning, in that when warned with a conditioning stimulus (loud tone), animals were able to optimize steptimes in anticipation of and in an effort to avoid the upcoming obstacle. These findings of locomotor malperformance and miscoordination are suggestive of cerebellar injury in the Townes model. In support of this hypothesis are findings of disruption of the blood brain barrier, ultrastructural vascular endothelial abnormalities and cerebellar white matter changes suggestive of ischemic injury, oxidative stress, and altered caspase-3 activity levels in the cerebellum. Therefore, while these animals do not have cerebellar strokes, our findings suggest that SCD can be associated with locomotor malperformance, cerebellar endothelial vascular injury, abnormal blood brain barrier, and white matter abnormalities. These findings then support the use of Townes mice as suitable models for the study of mechanisms underlying cerebellar injury and white matter abnormalities in SCD.
Several tasks (balance beam, rotarod, open field, vestibulo-ocular reflex, delay eye blink conditioning) are used to evaluate locomotion and cerebellar learning. However, the Erasmus Ladder offers a robust approach to detect specific deficits in whole-body locomotion, gait adaptability, and adaptive cerebellar learning within a locomotor context. Using the Ladder, we were able to evaluate basic walking patterns, gait/stepping adaptability capacity during perturbed sessions, as well as cognitive parameters such as motivation, obstacle avoidance and adaptive cerebellar motor learning in SCD mice, which cannot be done with most commonly available tasks. We note that confounders inherent to the sickle cell model, such as pain, sensory fiber hypersensitivity (Kenyon et al., 2015), muscle hyperalgesia (Calhoun et al., 2015), and fear of pain could possibly have contributed to the phenotypes observed in Townes mice. Additionally, while it is possible that our testing paradigm could also detect extra cerebellar modulation of gait adaptability and the conditioned response, we posit that the experimental design used here, minimizes the likelihood of incorporating the contributions from regions other than cerebellum.
During unperturbed sessions, homozygous Townes mice had alterations of basic walking pattern shown by a higher percentage of missteps as they traversed the ladder. During perturbed sessions mice were forced to overcome physical obstacles presented on their path, which requires greater sensorimotor integration. The findings that homozygotes displayed significantly more missteps during perturbed sessions then suggest that SCD mice have sensorimotor integration impairment and resulting limb miscoordination. These observations were corroborated by the performance of the Townes mice on the rotarod and are in concert with findings in other sickle cell mouse strains, which were shown to have a lower latency to fall from a rotating rod (Fasipe et al., 2004). Taken together our findings and those of others (Fasipe et al., 2004) indicate that these animals have impaired motor coordination and balance, suggest the presence of sensorimotor integration deficits, and point to cerebellar involvement in SCD.
In humans, cerebellar damage due to injury or genetic abnormalities is often associated with deficits in multi-joint limb control and intra-limb coordination, thus leading to impairment in goal-directed leg movements and adaptive locomotion (Ilg et al., 2008). During perturbed sessions, control animals adapted gait patterns by increasing percentage of long steps while decreasing short steps in order to overcome the obstacles. Conversely, homozygous Townes displayed a deficit in gait/stepping adaptability in that, compared to controls, they had less increases in the percentage of long steps, a phenotype that was more pronounced in females. These findings suggest that female Townes homozygous adopted different strategies to cross the presented obstacles by preferentially using short rather than long steps. During obstacle crossing, the cerebellum has been shown to play a pivotal role in adaptation and optimization of stepping strategies (Kim et al., 2013). Patients with cerebellar damage are known to adopt adaptive stepping strategies aimed at minimizing execution demands and enhancing safe performance, i.e. avoid falls, during obstacle crossing (Kim et al., 2013). These deficits are similar to those observed in animals with mutations affecting Purkinje cell function (Vinueza Veloz et al., 2015). Further, the present findings parallels the reports in humans with SCD, who have been shown to have average fine motor dexterity and speed, but impaired visual motor integration, which is a more complex and challenging motor skill(Newby et al., 2018). Thus, taken together, the abnormal adaptive obstacle avoidance strategy observed here among homozygous mice and the evidence of impaired complex motor skills observed in SCD subjects (Newby et al., 2018) are indicative of behavioral consequences of and consistent with SCD-associated cerebellar damage.
On the Erasmus Ladder, percentage of backsteps can reflect motivation to finish the trials, such that an increased percentage of backsteps could suggest decreased motivation (Sathyanesan et al., 2018b; Vinueza Veloz et al., 2015). Homozygous males had significantly higher percentage of backsteps compared to female homozygotes and to male controls and heterozygous. This phenotype was even more prominent during perturbed session when male homozygotes had increased percentage of backsteps, which could be interpreted as decreased motivation when confronted with an aversive situation (the presentation of an obstacle). This decreased motivation in male homozygotes is in concert with our previous work demonstrating that male homozygotes run significantly shorter distances in the voluntary wheel compared to females (Wang et al., 2016). These findings are also in concert with reports that SCD humans have an increased incidence of depression and that SCD mice display depression-like behaviors (Wang et al., 2016). An alternative interpretation is that increases in backsteps could reflect avoidance behavior due to fear of pain. In fact, SCD mice are known to display muscle and sensory fiber hyperalgesia (Calhoun et al., 2015; Kenyon et al., 2015; Wang et al., 2018) and SCD patients to have kinesiophobia (fear of movement)(Pells et al., 2007). Nevertheless, we note that despite a decrease in motivation and or/avoidance to finish the trials, males were able to adapt stepping patterns better than females by displaying more long steps during perturbed sessions, which suggests that lack of motivation or kinesiophobia was not responsible for lack of gait adaptability.
We identified a number of behavioral phenotypes that differentially affected male and female Townes SCD mice. Such sex-related differences in SCD mice have been shown in some of our previous studies demonstrating differential nociception and behavior phenotypes among male and female SCD mice (Kenyon et al., 2015; Wang et al., 2016). Here we found significant genotype by sex interaction, in that male and female homozygous, but not controls, were differentially affected in their percentage of backsteps and on their overall stepping pattern adaptability (long and short steps percentages). In humans, some studies reveal significant sex-related differences in susceptibility to hemolysis, incidence of complications, and life expectancy among SCD patients (Ceglie et al., 2019; Gladwin et al., 2014; Kanias et al., 2016; Platt et al., 1994; Raslan et al., 2018). Additionally, researchers have shown that among SCD patients, white matter volume changes and silent infarcts yields different neurocognitive consequences in males compared to females with SCD (Choi et al., 2019). In that study, white matter abnormalities noted on brain imaging were associated with lower nonverbal intellectual functioning in males, but not in females (Choi et al., 2019). Therefore, our findings of different performance on the Erasmus Ladder comparing male and female Townes mice are in keeping with those human studies. While the causes of such sex-related differences remain unknown, these data add support to the notion that in SCD, complications affecting the central nervous system can differentially affect males and females and underscore the need to include males and females in human and murine studies.
When we sought to identify the biologic correlates of the behavior findings, which are suggestive of cerebellar dysfunction, we found that SCD homozygous mice displayed significant alterations in cerebellar vascular endothelium and white matter, which were suggestive of damage caused by hypoxia and or ischemia. As we and others have shown, SCD mice, at the age studied here, do not have strokes (Manci et al., 2006; Wang et al., 2016), these changes in white matter and vascular endothelium point to a mechanism unrelated to vascular stenosis and or strokes. In SCD, a number of factors could contribute to the anatomic and biochemical changes observed in the cerebellum. One possibility is that chronic anemia could contribute to the white matter changes (Wang et al., 2016). We note that while chronic anemia is present in SCD mice, it occurs both in male and female animals at similar levels (Wang et al., 2016), thus making it an unlikely explanation for sex-related differential behaviors observed. One could alternatively postulate that sex-related differences in circulating products of hemolysis (hemoglobin, heme, microparticles) and/or hematopoiesis capacity could possibly have contributed to the observed behavior deficits, anatomic changes, and oxidative stress observed in SCD mice (Gladwin et al., 2014; Raslan et al., 2018). Yet another possibility is that recurrent episodes of sickling could lead to microvascular occlusions, consequent multifocal ischemia, and increased oxidative stress leading to astrocyte damage. Therefore, our findings generate hypotheses to be tested in future studies as to improve our understanding of the underlying mechanisms of SCD-related cerebellar injury.
We acknowledge some limitations in this study. First, we conducted a cross-sectional, not longitudinal, analysis of locomotion and locomotion adaptability in young adult mice and therefore are unable to comment on the effect of time on cerebellar function and white matter damage in SCD. Given that SCD is coupled with severe anemia, as we did not correct it in the SCD mice, or studied anemia unrelated to SCD, we are unable to determine whether the cerebellar involvement in this model is related to chronic anemia or to SCD itself. However, we posit that the finding that some of the cerebellar function phenotypes affected males and females differently while both have similar degree of anemia (Wang et al., 2016), make this possibility less likely. Whereas the Erasmus Ladder enables the analysis of core cerebellar functions in the SCD mouse, it has limitations in other types of questions. The current approach cannot eliminate the possibility that control mice by expressing normal human hemoglobin could have an altered phenotype in the Erasmus Ladder. However, by including heterozygous mice, we could actually examine the effect of a lower expression of human sickle hemoglobin and did observe that heterozygous can present some impairment in locomotion performance. Pain is certainly an inherent confounder related to SCD mouse model and could possibly have contributed to some of the phenotypes observed here and hinder the interpretation of the results. In fact, other have shown that in neurologically intact rodents, prior pain episodes can negatively affect motor learning (Huot-Lavoie et al., 2019). When we used dexmedetomidine, an analgesic known to ameliorate muscle hyperalgesia is SCD mice (Calhoun et al., 2015), we observed a decrease in latency to fall from the rotarod in both controls and homozygotes but no interference with cerebellar learning. While, it remains unknown to what extent pain contributed to gait adaptability impairment in this model, the finding that dexmedetomidine did not impair motor learning might suggest that pain did not play a significant role on the performance of the rotarod task. Nevertheless, despite its limitations, this study points to the vulnerability of the cerebellum and suggests that the Townes SCD model is suitable for studies of the underlying mechanisms of cerebellar deficits in SCD.
This investigation showed that SCD mice have basal locomotor and gait/stepping pattern adaptability deficits, which are coupled with vascular endothelium and white matter abnormalities and increased oxidative stress in the cerebellum. This study builds on our previous work and on the work of others demonstrating that, SCD mice display neurocognitive deficits that are associated with morphologic changes in Purkinje cells in the cerebellum of Townes and BERK mice (Manci et al., 2006; Wang et al., 2016). As human studies have shown cerebellar involvement and associated behavioral deficits in visual motor integration, skills that are essential for academic development, the present findings call for improved neurodevelopmental interventions targeting cerebellar function for vulnerable SCD patients. Future studies using the Townes mice can provide novel insights on the mechanisms underlying cerebellar dysfunction in SCD and inform the design of therapies to improve the cerebellar deficits associated with the disease.
4. Experimental Procedures, Acknowledgements, References
4.1. Mice
The Townes and BERK SCD mouse strains were used in this study (Paszty et al., 1997; Wu et al., 2006). The NIH Clinical Center and Children’s Research Institute Animal Care and Use Committees approved animal protocols. In the Townes strain, mouse α and ß globin hemoglobin genes were replaced with corresponding human genes; the presence of a double or single dose of the ß sickle mutation marks these animals as homozygous or heterozygous, respectively. The absence of the ß sickle mutation marks them as controls, which express human hemoglobin A only (Wu et al., 2006). In the BERK strain, mouse hemoglobin α genes were removed and the human α and ß sickle genes were introduced in a transgene construct; the presence or absence of the wild type mouse ß gene marks these animals as hemizygotes or homozygotes, respectively (Paszty et al., 1997). Here we only used hemizygous or homozygous BERK mice. Detailed breeding and genotyping schemes have been published (Wang et al., 2016).
4.2. Erasmus Ladder task and experimental paradigm
We used the Erasmus Ladder, an automated system that allows for analysis of locomotion pattern, gait adaptability and cerebellar motor learning, to evaluate cerebellar function in the SCD mouse model (Rahmati et al., 2016; Van Der Giessen et al., 2008; Vinueza Veloz et al., 2015). Briefly, the system consists of two goal/shelter boxes located at the ends of a horizontal ladder (Noldus Information Technology, Leesburg, VA). The ladder is composed of 37 touch-sensitive rungs and each can be moved up vertically according to the experimental protocol. The experimental paradigm consisted of four unperturbed sessions (one to four) followed by four perturbed sessions (five to eight) and was controlled by the Erasmus Ladder software (v1.0 and 1.1). Each daily session consisted of 42 trials, when the mouse had to walk on the Ladder between shelter boxes. Each trial started when a mouse was positioned inside a starter shelter-box, where the animal had to remain randomly between 10 – 20 seconds. After that time, a light was turned on as to indicate that the mouse had to leave the box. If the mouse did not exit after 3 seconds, a tail wind was turned on to force the mouse to leave the starter shelter-box and walk on the ladder to begin the trial. During unperturbed sessions, we examined basal locomotion by allowing the mouse to traverse the ladder without challenges. In contrast, during perturbed sessions, we tested gait/stepping pattern adaptability by randomly presenting a12 mm-high obstacle along the mouse path during each trial. During perturbed sessions, there were three possible perturbation scenarios, which were randomly delivered. In one scenario, animals would face the obstacle without any warning (unconditioned stimulus only). In a second scenario, a high pitch warning tone was randomly delivered while the animal was traversing the Ladder (conditioning stimulus only). The third possible scenario was the presentation of the obstacle preceded by the warning tone with an inter-stimulus interval of 250 ms (paired conditioning and unconditioned stimuli).
Using the Ladder, we collected and examined the following outcomes for each perturbed and unperturbed sessions. 1) Average percentage of missteps, defined as steps in which the mouse made a faulty movement onto the lower misstep rung. 2) Average percentage of backsteps, defined as steps made in the direction opposite to that of the tail wind (backwards) in an attempt to return to the originating shelter box. 3) Average percentage of short steps, defined as steps made from one to the immediately next rung. 4) Average percentage of long steps, defined as steps made from one rung to the second next consecutive rung (skipping one rung). 5) Average percentage of jump steps, defined as steps made from one rung to the next third or more consecutive rungs (skipping two or more rungs). 6) Pre-perturbation step time, the time difference between subsequent rung activations immediately preceding the obstacle on the same side. 7) Post perturbation step time, the time difference between rung activation immediately preceding the obstacle and that immediately following the obstacle on the same side. During the first perturbed session, one will typically observe an increase in post perturbation step time, which signals the start of associative motor learning, when animals begin to adapt their gait/stepping pattern to overcome the obstacle. Over the following three perturbed sessions, intact mice associate the conditioning stimulus (high pitch warning tone) with the upcoming presentation of the obstacle and learn to modify their gait/steps by decreasing post perturbation step times thereby increasing speed to overcome the obstacle. While during unperturbed sessions there was no physical perturbation, post perturbation step times were measured based on the computer prediction of when and which a perturbation would have been presented but without physically presenting it. The post perturbation steptimes acquired during unperturbed sessions served as baseline measurements to compare with post-perturbation step times obtained across perturbed sessions.
4.3. Rotarod task
In another a different mouse, we used an accelerating Rotamex-5 System rotarod (Columbus Instruments, Columbus, OH) to evaluate balance and motor coordination in SCD mice (16–20 weeks of age) measuring latency to fall from the rotating spindle as described (Fasipe et al., 2004). All mice underwent training consisting of walking on the rotating rod for 30 seconds at two revolutions per minute (rpm). After the training phase, mice went through a series of three sequential trials 15 min apart. During each trial, the rod was set to accelerate at 0.1 rpm/second starting at 2 rpm. Latency to fall (in seconds) was recorded for each trial.
In yet a different cohort of Townes mice, we used dexmedetomidine at analgesic doses (Funai et al., 2014; Rangel et al., 2012) to investigate whether pain could affect performance on the rotarod. After baseline measurement (Trial one), control and homozygous Townes were subcutaneously injected with dexmedetomidine 5μg/kg or vehicle. Fifteen and 35 minutes after the injection, mice went through trials two and three on the rotarod and latency to fall was recorded.
4.4. Transmission electron microscopy
Brain sections approximately 1 mm3 were fixed for 48 hrs at 4°C in 2.5% glutaraldehyde and 1% paraformaldehyde in 0.1M cacodylate buffer (pH 7.4) and washed with cacodylate buffer three times. The tissues were fixed with 1% OsO4 for two hours, washed again with 0.1M cacodylate buffer three times, washed with water and placed in 1% uranyl acetate for one hour. The tissues were subsequently serially dehydrated in ethanol and propylene oxide and embedded in EMBed 812 resin (Electron Microscopy Sciences, Hatfield, PA, USA). Thin sections, approx. 80 nm, were obtained by utilizing the Leica ultracut-UCT ultramicrotome (Leica, Deerfield, IL, USA) and placed onto 300 mesh copper grids and stained with saturated uranyl acetate in 50% methanol and then with lead citrate. The grids were viewed with a JEM-1200EXII electron microscope (JEOL Ltd, Tokyo, Japan) at 80kV and images were recorded on the XR611M, mid mounted, 10.5M pixel, CCD camera (Advanced Microscopy Techniques Corp, Danvers, MA, USA).
4.5. Markers of oxidative stress
In cerebellar homogenates, we measured malondialdehyde formation, an indicator of free radical oxidation of polyunsaturated fatty acids, using the thiobarbituric acid reactive substance (TBARS) fluorometric assay (BioAssay Systems, Hayward, CA)(Yagi, 1998) with small modifications from the manufacturer’s instructions. Cerebellum samples were disrupted in 350μl of ice-cold PBS using rapid agitation with zirconia beads (2.3 mm) followed by precipitation of insoluble material with centrifugation (10,000g, 5 min, 4°C).
Catalase activity was measured fluorometrically (BioAssay Systems, Hayward, CA). Cerebellum homogenates were dissociated with 2.3mm zirconia beads in PBS with 0.1% digitonin and clarified by centrifugation (14,000g, 10 min, 4°C). Preliminary experiments were conducted to investigate if samples required further dilutions and only experiments in which all samples remained within the standard curve were accepted for analysis.
4.6. Apoptosis and caspase-3 activity.
After anesthesia and exsanguination, whole brain was dissected and kept in 10% buffered formalin for histological evaluation. Detection of apoptotic cells in the cerebellum was performed by terminal deoxynucleotidyl transferase (TdT) deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL) using Roche Anti-Digoxigenin (1:1000, Sigma-Aldrich cat# 11–093-274-910).
Caspase-3 activity was assayed fluorometrically with modifications from manufacturer’s instructions (Sigma-Aldrich cat# CASP3F). Caspase-3 activity was measured by caspase-3-induced hydrolysis of the substrate acetyl-Asp-Glu-Val-Asp-7-amido-4-methylcoumarin (Ac-DEVD-AMC) into 7-amino-4-methylcoumarin (AMC) which is fluorescent (Ex = 360nm and Em = 460nm). All samples included duplicates with the caspase-3 antagonist Ac-DEVD-CHO to document non-specific Ac-DEVD-AMC metabolism; only the fluorometric difference between samples without Ac-DEVD-CHO and those with it were accepted as specific caspase-3 activity. Preliminary experiments indicated that caspase-3 activity assay did not require protease inhibitors (data not shown). Cerebellar homogenates were prepared in 500μl lysis buffer by fast agitation with 2.3 mm zirconia beads and clarified by centrifugation (10,000g, 10min, 4°C). After reagent mixing, a 96 well plate was incubated at 37°C and fluorescence was acquired every 15 min (PerkinElmer EnSpire 2300) and was stable for at least 90 min; for standardization, caspase-3 activity was calculated after 60 min incubation.
4.7. Statistical Analysis
We used a repeated-measures model to analyze longitudinal outcomes. Each of the longitudinal outcome variables listed in the Experimental Procedures section was modeled separately as a response (dependent) variable. The following variables were used as explanatory (independent) variables: genotype, sex, and session main effects; and sex-by-genotype, genotype-by-session, sex-by-session, and sex-by-genotype-by-session interactions. For each model, the “best” variance-covariance matrix structure was determined based on the lowest Bayesian Information Criterion value. Model assumptions were checked based on model fit statistics, e.g. studentized residuals, normality of the errors. For the analysis to assess the effect of dexmedetomidine on latency to fall, the same type of model was used, except that its value at Trial 1 was used as a covariate in order to account for the mouse’s latency to fall prior to injection, which occurred between Trial 1 and Trial 2. For data recorded at one time point, a one-way analysis of variance or the Kruskal-Wallis test were used for analysis. These analyses were conducted using the SAS statistical software v. 9.4 (SAS Institute, Cary, NC, USA) and SigmaStat 13.0 (Systat Software, Inc., San Jose, CA). All reported p-values are two-sided and were not adjusted for multiple comparisons, given that we conducted an exploratory investigation with its findings being intended to generate testable hypothesis rather than being definitive (Rothman, 2014; Streiner, 2015). We encourage the reader to keep that in mind when interpreting p-values for pairwise comparisons, particularly those greater than 0.002. This p-value threshold was obtained by dividing 0.05 by 24, the number of pairwise comparisons, i.e. female vs. male for each of 8 sessions × 3 genotypes.
Supplementary Material
Highlights.
Sickle cell disease (SCD) patients can have abnormal cerebellum on imaging studies
SCD mice have evidence of cerebellar behavior deficits as described in SCD patients
Cerebellar behavior deficits in SCD mice are associated with white matter abnormalities
The deficits are associated with oxidative stress and vascular injury in cerebellum
SCD mice are valid models for studies of cerebellar injury in SCD
4.8. Acknowledgements
4.8.1. The authors are grateful to Paulette Price for technical support throughout the study and to Taylor Baugh, Lillian Hallmark, and Sherouk Hassan for editorial comments.
4.8.2. Funding
The Intramural Program from the National Institutes of Health Clinical Center, NIH (grant numbers 1ZIACL090052-01, 1ZIACL090053-01, and 1ZIACL090054-01) supported this work.
Abbreviations:
- SCD
Sickle cell disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. Declarations of competing interest
The authors have no conflict of interest to declare
4.9. References
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK, 2005. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 9, 463–84. [DOI] [PubMed] [Google Scholar]
- Baillieux H, De Smet HJ, Paquier PF, De Deyn PP, Marien P, 2008. Cerebellar neurocognition: insights into the bottom of the brain. Clin Neurol Neurosurg. 110, 763–73. [DOI] [PubMed] [Google Scholar]
- Calhoun G, Wang L, Almeida LE, Kenyon N, Afsar N, Nouraie M, Finkel JC, Quezado ZM, 2015. Dexmedetomidine ameliorates nocifensive behavior in humanized sickle cell mice. Eur J Pharmacol. 754, 125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case M, Zhang H, Mundahl J, Datta Y, Nelson S, Gupta K, He B, 2017. Characterization of functional brain activity and connectivity using EEG and fMRI in patients with sickle cell disease. Neuroimage Clin. 14, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceglie G, Di Mauro M, Tarissi De Jacobis I, de Gennaro F, Quaranta M, Baronci C, Villani A, Palumbo G, 2019. Gender-Related Differences in Sickle Cell Disease in a Pediatric Cohort: A Single-Center Retrospective Study. Frontiers in Molecular Biosciences. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Bush AM, Borzage MT, Joshi AA, Mack WJ, Coates TD, Leahy RM, Wood JC, 2017. Hemoglobin and mean platelet volume predicts diffuse T1-MRI white matter volume decrease in sickle cell disease patients. NeuroImage. Clinical 15, 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, O’Neil SH, Joshi AA, Li J, Bush AM, Coates TD, Leahy RM, Wood JC, 2019. Anemia predicts lower white matter volume and cognitive performance in sickle and non-sickle cell anemia syndrome. Am J Hematol. 94, 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun MR, Schatz J, Siegel MJ, Koby M, Craft S, Resar L, Chu JY, Launius G, Dadash-Zadeh M, Lee RB, Noetzel M, 1998. Cognitive screening examinations for silent cerebral infarcts in sickle cell disease. Neurology. 50, 1678–82. [DOI] [PubMed] [Google Scholar]
- DeBaun MR, Kirkham FJ, 2016. Central nervous system complications and management in sickle cell disease. Blood. 127, 829–38. [DOI] [PubMed] [Google Scholar]
- Farooq S, Testai FD, 2019. Neurologic Complications of Sickle Cell Disease. Curr Neurol Neurosci Rep. 19, 17. [DOI] [PubMed] [Google Scholar]
- Fasipe FR, Ubawike AE, Eva R, Fabry ME, 2004. Arginine supplementation improves rotorod performance in sickle transgenic mice. Hematology. 9, 301–5. [DOI] [PubMed] [Google Scholar]
- Funai Y, Pickering AE, Uta D, Nishikawa K, Mori T, Asada A, Imoto K, Furue H, 2014. Systemic dexmedetomidine augments inhibitory synaptic transmission in the superficial dorsal horn through activation of descending noradrenergic control: an in vivo patch-clamp analysis of analgesic mechanisms. Pain. 155, 617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin MT, Barst RJ, Gibbs JS, Hildesheim M, Sachdev V, Nouraie M, Hassell KL, Little JA, Schraufnagel DE, Krishnamurti L, Novelli E, Girgis RE, Morris CR, Berman Rosenzweig E, Badesch DB, Lanzkron S, Castro OL, Taylor J.G.t., Goldsmith JC, Kato GJ, Gordeuk VR, Machado RF, 2014. Risk factors for death in 632 patients with sickle cell disease in the United States and United Kingdom. PLoS One. 9, e99489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass P, Brennan T, Wang J, Luchtman-Jones L, Hsu L, Bass CM, Rana S, Martin B, Reed C, Cheng YI, Gordeuk V, 2013. Neurodevelopmental deficits among infants and toddlers with sickle cell disease. J Dev Behav Pediatr. 34, 399–405. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP, 2005. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 57, 173–85. [DOI] [PubMed] [Google Scholar]
- Huot-Lavoie M, Ting WK, Demers M, Mercier C, Ethier C, 2019. Impaired Motor Learning Following a Pain Episode in Intact Rats. Front Neurol. 10, 927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg W, Giese MA, Gizewski ER, Schoch B, Timmann D, 2008. The influence of focal cerebellar lesions on the control and adaptation of gait. Brain. 131, 2913–27. [DOI] [PubMed] [Google Scholar]
- Kanias T, Sinchar D, Osei-Hwedieh D, Baust JJ, Jordan A, Zimring JC, Waterman HR, de Wolski KS, Acker JP, Gladwin MT, 2016. Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease. Transfusion. 56, 2571–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawadler JM, Clayden JD, Kirkham FJ, Cox TC, Saunders DE, Clark CA, 2013. Subcortical and cerebellar volumetric deficits in paediatric sickle cell anaemia. Br J Haematol. 163, 373–6. [DOI] [PubMed] [Google Scholar]
- Kenyon N, Wang L, Spornick N, Khaibullina A, Almeida LE, Cheng Y, Wang J, Guptill V, Finkel JC, Quezado ZM, 2015. Sickle cell disease in mice is associated with sensitization of sensory nerve fibers. Exp Biol Med (Maywood). 240, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaibullina A, Almeida LE, Wang L, Kamimura S, Wong EC, Nouraie M, Maric I, Albani S, Finkel J, Quezado ZM, 2015. Rapamycin increases fetal hemoglobin and ameliorates the nociception phenotype in sickle cell mice. Blood Cells Mol Dis. 55, 363–72. [DOI] [PubMed] [Google Scholar]
- Khaibullina A, Adjei E, Afangbedji N, Ivanov A, Kumari N, Almeida L, Quezado Z, Nekhai S, Jerebtsova M, 2018. RON kinase inhibition reduces renal endothelial injury in sickle cell disease mice. Haematologica. 103, haematol.2017.180992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Song YG, Park IS, Rhyu IJ, Kim SB, Park JH, 2013. Effects of task constraints on obstacle avoidance strategies in patients with cerebellar disease. Gait Posture. 37, 521–5. [DOI] [PubMed] [Google Scholar]
- Mackin RS, Insel P, Truran D, Vichinsky EP, Neumayr LD, Armstrong FD, Gold JI, Kesler K, Brewer J, Weiner MW, 2014. Neuroimaging abnormalities in adults with sickle cell anemia: associations with cognition. Neurology. 82, 835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA, Coller BS, 2006. Pathology of Berkeley sickle cell mice: similarities and differences with human sickle cell disease. Blood. 107, 1651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby RF, Epping A, Geiger JA, Miller MS, Scott JP, 2018. Visual Motor Integration in Children With Sickle Cell Disease. J Pediatr Hematol Oncol. 40, 495–498. [DOI] [PubMed] [Google Scholar]
- Paszty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, Rubin EM, 1997. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 278, 876–8. [DOI] [PubMed] [Google Scholar]
- Pells J, Edwards CL, McDougald CS, Wood M, Barksdale C, Jonassaint J, Leach-Beale B, Byrd G, Mathis M, Harrison MO, Feliu M, Edwards LY, Whitfield KE, Rogers L, 2007. Fear of movement (kinesiophobia), pain, and psychopathology in patients with sickle cell disease. Clin J Pain. 23, 707–13. [DOI] [PubMed] [Google Scholar]
- Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP, 1994. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 330, 1639–44. [DOI] [PubMed] [Google Scholar]
- Rahmati N, Vinueza Veloz MF, Xu J, Barone S, Rodolfo Ben Hamida N, Schonewille M, Hoebeek FE, Soleimani M, De Zeeuw CI, 2016. SLC26A11 (KBAT) in Purkinje Cells Is Critical for Inhibitory Transmission and Contributes to Locomotor Coordination. eNeuro. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel R, Marinho B, Fernandes P, Moura R, Lessa M, 2012. Pharmacological mechanisms involved in the antinociceptive effects of dexmedetomidine in mice. Fundamental & clinical pharmacology. 28. [DOI] [PubMed] [Google Scholar]
- Raslan R, Shah BN, Zhang X, Kanias T, Han J, Machado RF, Gladwin MT, Gordeuk VR, Saraf SL, 2018. Hemolysis and hemolysis-related complications in females vs. males with sickle cell disease. Am J Hematol. 93, E376–e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, 2014. Six persistent research misconceptions. J Gen Intern Med. 29, 1060–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salameh TS, Shah GN, Price TO, Hayden MR, Banks WA, 2016. Blood-Brain Barrier Disruption and Neurovascular Unit Dysfunction in Diabetic Mice: Protection with the Mitochondrial Carbonic Anhydrase Inhibitor Topiramate. J Pharmacol Exp Ther. 359, 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman MS, Tsai P, 2016. The Role of the Pediatric Cerebellum in Motor Functions, Cognition, and Behavior: A Clinical Perspective. Neuroimaging Clin N Am. 26, 317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanesan A, Kundu S, Abbah J, Gallo V, 2018a. Neonatal brain injury causes cerebellar learning deficits and Purkinje cell dysfunction. Nature Communications. 9, 3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanesan A, Kundu S, Abbah J, Gallo V, 2018b. Neonatal brain injury causes cerebellar learning deficits and Purkinje cell dysfunction. Nat Commun. 9, 3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scantlebury N, Mabbott D, Janzen L, Rockel C, Widjaja E, Jones G, Kirby M, Odame I, 2011. White matter integrity and core cognitive function in children diagnosed with sickle cell disease. J Pediatr Hematol Oncol. 33, 163–71. [DOI] [PubMed] [Google Scholar]
- Steen RG, Miles MA, Helton KJ, Strawn S, Wang W, Xiong X, Mulhern RK, 2003. Cognitive impairment in children with hemoglobin SS sickle cell disease: relationship to MR imaging findings and hematocrit. AJNR Am J Neuroradiol. 24, 382–9. [PMC free article] [PubMed] [Google Scholar]
- Stotesbury H, Kawadler JM, Hales PW, Saunders DE, Clark CA, Kirkham FJ, 2019. Vascular Instability and Neurological Morbidity in Sickle Cell Disease: An Integrative Framework. Front Neurol. 10, 871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streiner DL, 2015. Best (but oft-forgotten) practices: the multiple problems of multiplicity-whether and how to correct for many statistical tests. Am J Clin Nutr. 102, 721–8. [DOI] [PubMed] [Google Scholar]
- Van Der Giessen RS, Koekkoek SK, van Dorp S, De Gruijl JR, Cupido A, Khosrovani S, Dortland B, Wellershaus K, Degen J, Deuchars J, Fuchs EC, Monyer H, Willecke K, De Jeu MT, De Zeeuw CI, 2008. Role of olivary electrical coupling in cerebellar motor learning. Neuron. 58, 599–612. [DOI] [PubMed] [Google Scholar]
- Vichinsky EP, Neumayr LD, Gold JI, Weiner MW, Rule RR, Truran D, Kasten J, Eggleston B, Kesler K, McMahon L, Orringer EP, Harrington T, Kalinyak K, De Castro LM, Kutlar A, Rutherford CJ, Johnson C, Bessman JD, Jordan LB, Armstrong FD, 2010. Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. JAMA. 303, 1823–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinueza Veloz MF, Zhou K, Bosman LW, Potters JW, Negrello M, Seepers RM, Strydis C, Koekkoek SK, De Zeeuw CI, 2015. Cerebellar control of gait and interlimb coordination. Brain Struct Funct. 220, 3513–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Almeida LEF, de Souza Batista CM, Khaibullina A, Xu N, Albani S, Guth KA, Seo JS, Quezado M, Quezado ZMN, 2016. Cognitive and behavior deficits in sickle cell mice are associated with profound neuropathologic changes in hippocampus and cerebellum. Neurobiol Dis. 85, 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Almeida LEF, Kamimura S, van der Meulen JH, Nagaraju K, Quezado M, Wakim P, Quezado ZMN, 2018. The role of nitrite in muscle function, susceptibility to contraction injury, and fatigability in sickle cell mice. Nitric Oxide. 80, 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Enos L, Gallagher D, Thompson R, Guarini L, Vichinsky E, Wright E, Zimmerman R, Armstrong FD, 2001. Neuropsychologic performance in school-aged children with sickle cell disease: a report from the Cooperative Study of Sickle Cell Disease. J Pediatr. 139, 391–7. [DOI] [PubMed] [Google Scholar]
- Wu L-C, Sun C-W, Ryan T, Pawlik K, Ren J, Townes T, 2006. Wu LC, Sun CW, Ryan TM, Pawlik KM, Ren J and Townes TM. Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood 108: 1183–1188. Blood. 108, 1183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi K, 1998. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol. 108, 101–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


