Abstract
Objectives:
Tremendous progress has been made in the treatment of multiple myeloma; however, the majority of this success has been demonstrated in younger patients. With 36% of patients >80 years-old at diagnosis, it is important to understand if older patients are receiving similar benefits.
Materials and methods:
We identified 2,155 patients diagnosed with myeloma at age 80 or older in the Surveillance, Epidemiology, and End Results Program (SEER)-Medicare database from 2007–2013. A cohort of 2,933 similar patients diagnosed with myeloma at age 70–79 was used for comparison using a difference-in-differences design.
Results:
Only 51% of patients >80 years-old at diagnosis received systemic anti-myeloma treatment. Treatment was associated with a 26% decrease in hazard for death, independent of age, race, gender, poverty, comorbidities, and proxy measures of performance status. In the 70–79 cohort, treatment was associated with a 22% decrease in hazard for death. Based on the difference-in-differences design, there is no statistically significant difference in treatment benefit based on age cohort (p = 0.610).
Conclusions:
Anti-myeloma treatment produces a similar survival benefit amongst the oldest patients. The population over 80, when myeloma incidence peaks, is projected to triple over the next few decades. It is imperative that we continue to advance our understanding of the needs of this vulnerable subgroup of patients with myeloma.
Keywords: Older patients, multiple myeloma, novel agents
INTRODUCTION
There has been tremendous progress made in the treatment of myeloma over the past two decades; however, the majority of this success has been demonstrated in clinical trials of younger patients.1 But myeloma predominantly impacts older adults; the median age at diagnosis of 69 years-old and 36% of patients are 80 years-old or older.2 It is important to understand if similar treatment benefits are observed even among the oldest patients.
Among participants in clinical trials, older patients have been shown to have similar progression-free survival as younger patients.3–5 However, population-based studies have shown little improvement in overall survival of older patients following the approval of newer treatments.6–11 Further, in our prior work we have shown that real-world populations tend to have poorer outcomes than clinical trial populations and that increasing age is associated with a lower likelihood of receiving treatment.12,13 Therefore, the relative benefit of treatment in people with myeloma over age 80 is still unclear. In this study, we sought to better understand the characteristics, treatment, and outcomes of the oldest-old patients with myeloma, those over age 80, and determine if these patients receive a similar benefit from anti-myeloma treatment as younger patients, those aged 65 to 79.
MATERIALS AND METHODS
Data Sources
We used the Surveillance, Epidemiology, and End Results (SEER) database to obtain information regarding incidence of myeloma in the United States from 2007–2013. Details on how incidence is calculated can be found in the SEER data description.14 SEER collects demographics, tumor characteristics, and survival data from 18 population-based cancer registries throughout the United States covering approximately 35% of the population.15 Collectively, the demographics of the registries approximate that of the entire U.S. population.
We then performed a retrospective analysis of myeloma cases diagnosed from 2007–2013 in the SEER-Medicare linked dataset. The SEER-Medicare database has been described in detail elsewhere.16 In the database, the SEER registry data is linked to Medicare enrollment and claims data. Of all people 65 years of age or older in the SEER registry, 95% have been matched to their corresponding Medicare data.17 This linked database contains information regarding patient demographics, tumor characteristics, and survival for those with a cancer diagnosis who reside in the coverage area and broadly represents the health care of older patients in the United States diagnosed with cancer who are insured through traditional fee-for-service Medicare plans. At the time this study was conducted, the SEER-Medicare linkage included all Medicare-eligible persons appearing in the SEER data through 2013 and their Medicare claims and survival data through 2014. The study was performed with approval from SEER-Medicare and under the supervision of the local institutional review board.
Participants
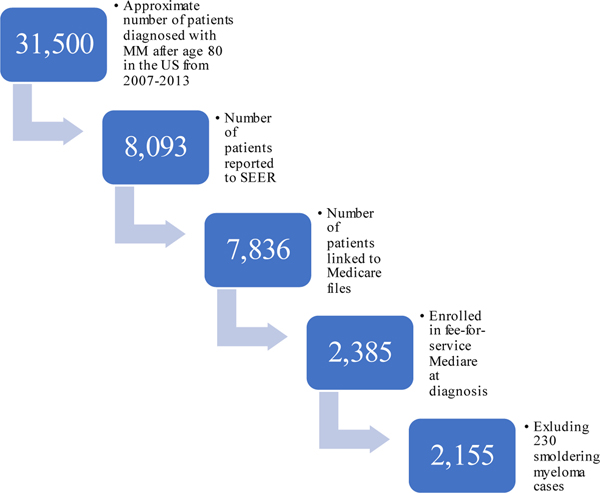
We identified all patients diagnosed with myeloma at age 65 or older in the SEER database to determine incidence (see SEER Cancer Statistics Review for methods used to estimate incidence)2 and survival outcomes. Using the SEER-Medicare linked database, we identified adults age 80 or older with newly-diagnosed multiple myeloma (International Classification of Disease for Oncology [third edition] code 9732). Of the 8,093 cases of myeloma reported to SEER during the study period, 7,836 were present in the SEER-Medicare linked dataset. Patients who were not enrolled in fee-for-service Medicare Parts A, B, and D were excluded, as treatments could not be determined, leaving 2,385 patients. In order to distinguish between patients with active myeloma and those with smoldering myeloma, we used an established algorithm described previously.18 In short, patients considered to have active myeloma required both the diagnosis code for myeloma and for one or more of the classically defined CRAB features (hypercalcemia, renal impairment, anemia, bone lesions) or receipt of anti-myeloma treatment within six months of diagnosis. Of these, 230 were identified as smoldering myeloma using a previously established algorithm and were excluded leaving 2,155 for the analyses. Patient selection is summarized in Figure 1. A similar group of patients (n=2,933) aged 70–79 years-old at diagnosis of myeloma were used for comparison.
Figure 1:
Cohort Selection
Of the estimated 31,500 cases of myeloma diagnosed in patients older than 80 from 2007–2013 in the United States, 2,155 (approximately 7%) were included in the analysis.
Statistical Analysis
Demographic and clinical characteristics collected included: age, gender, race (White/Caucasian, Black/African-American, or other as self-reported to Medicare), poor performance status indicators, Charlson Comorbidity Index, Medicaid enrollment, anti-myeloma treatment and survival. Charlson comorbidity index and poor performance status was assessed by commonly used algorithms for claims data.19,20 All statistical analyses were performed using SAS Enterprise Guide 5.1. A p value of < 0.05 was considered statistically significant. Cohort characteristics were compared with bivariate tests, t-tests or chi-square, as appropriate. The impact of myeloma treatments on survival was compared between the two cohorts using Cox Proportional Hazards in a difference-in-difference design, in which the respective improvement in hazard for those receiving treatment was compared for those diagnosed at age 70–79 and those after age 80. Three models were created, one for each age group and one to analyze the interaction between age group and anti-myeloma treatment, which included all patients.
RESULTS
The incidence of myeloma increases over lifetime, peaking after age 80. The annual incidence for those aged 65–69, 70–74, 75–79, 80–84 and 85+ was 24.4, 32.7, 39.5, 42.8 and 36.4 per 100,000, respectively. Approximately 31,500 new cases of myeloma were diagnosed 2007–2013, 4,500 annually, in patients age 80 or older. In that period, 8,093 cases, approximately 1,150 per year, were reported to SEER. The estimated median overall survival of these patients was 14.0 months (95% CI 13.2–14.8).
The median age at diagnosis for the group of interest was 84 years-old (range 80–100). The characteristics of the two cohorts are compared in Table 1. Compared to the 70–79 cohort, the 80+ cohort had a higher proportion of female and white patients (both p < 0.001). Patients in the 80+ group had a greater mean CCI score and a higher portion had indicators of poor performance status (both p < 0.001). There were significant differences in the presence of myeloma-related anemia and renal impairment. Anemia was more often seen in the 70–79 group, while renal impairment occurred more frequently in the older cohort (both p < 0.001). There was no significant difference in the proportion of patients with hypercalcemia or bone involvement between the two groups, nor was there a difference in year of diagnosis.
Table 1:
Patient Characteristics
| 80+ (n = 2,155) | 70–79 (n = 2,933) | P | |
|---|---|---|---|
| Gender | |||
| Male | 45% | 53% | < .0001 |
| Female | 55% | 47% | |
| Race | |||
| White/Caucasian | 81% | 77% | .0013 |
| Black/African-American | 13% | 16% | |
| Other | 7% | 7% | |
| Charlson Comorbidity Index Score (mean±standard deviation) | 2.27±2.02 | 1.95±2.01 | < .0001 |
| Poor Performance Status Indicator | 32% | 19% | < .0001 |
| Medicaid Enrollment | 28% | 31% | .0570 |
| Myeloma“CRAB” Criteria | |||
| Hypercalcemia | 22% | 22% | .7051 |
| Renal Impairment | 61% | 55% | < .0001 |
| Anemia | 59% | 63% | .0019 |
| Bone Involvement | 34% | 35% | .8843 |
| Received anti-myeloma treatment | 51% | 71% | < .0001 |
| Year of Diagnosis | |||
| 2007 | 12% | 13% | .6628 |
| 2008 | 13% | 13% | |
| 2009 | 14% | 13% | |
| 2010 | 13% | 14% | |
| 2011 | 15% | 15% | |
| 2012 | 16% | 17% | |
| 2013 | 16% | 16% | |
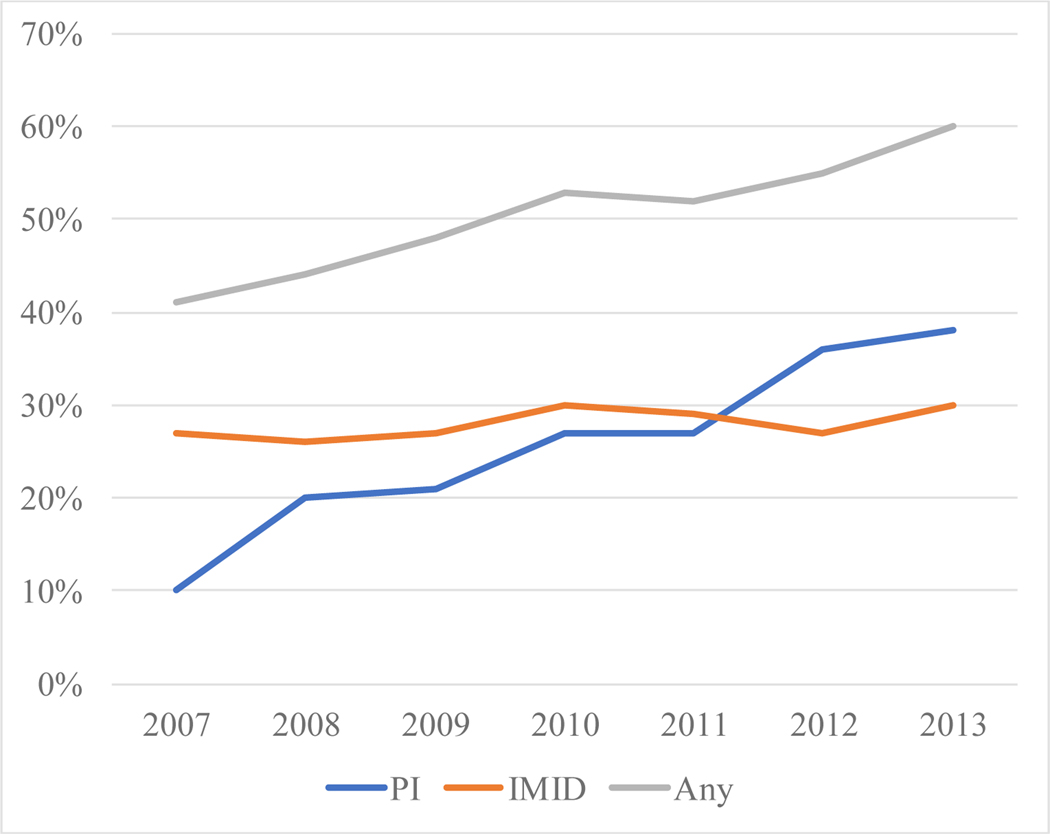
The number of patients receiving treatment for myeloma within 6 months of diagnosis was significantly lower in the 80+ group compared with the 70–79 group. Only 51% of patients in the 80+ cohort received systemic treatment within 6 months following diagnosis, whereas 71% of patients in the 70–79 cohort received systemic treatment in the same timeframe (p < 0.001). However, treatment rates among patients in the 80+ cohort have increased in more recent years. In 2007, only 41% of patients received treatment compared to 61% in 2013. Nearly all patients treated systemically received novel agents. Interestingly, the use of proteasome inhibitors (PIs) increased incrementally from 10% of patients diagnosed in 2007 to 38% in 2013, while immunomodulatory drug (IMiD) utilization remained relatively stable at around 30% (Figure 2).
Figure 2:
First-Line Treatment Patterns by Drug Class and Year
The rate of myeloma treatment in the over 80 year old population increased from 41% to 60% from 2007 to 2013. While immunomodulatory drug (IMID) use was relatively stable over that period, proteasome inhibitor use increased nearly three-fold.
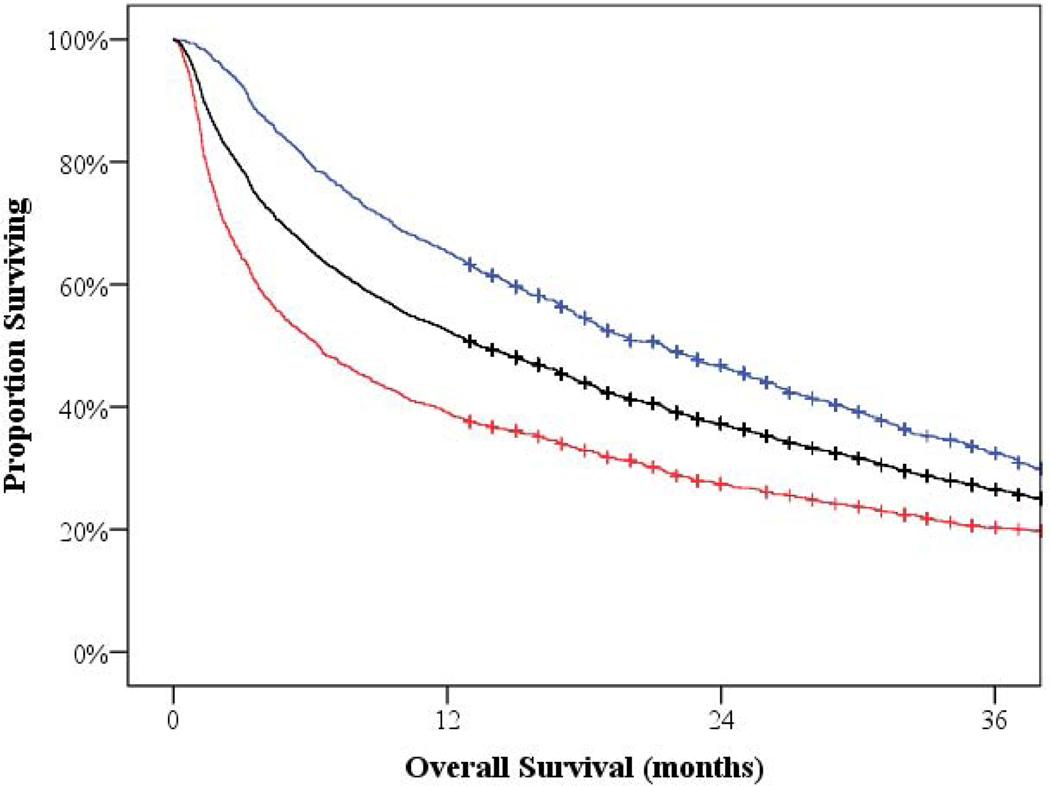
At time of data cutoff, 69% of patients had expired. The median overall survival for patients diagnosed at age 80 or older was 13.4 months (95% CI 12.2–15.2). However, those patients who received systemic treatment had a median overall survival of 21.4 months (95% CI 18.7–23.4), compared to a mere 6.4 months (95% CI 5.3–7.3) (p < 0.001) for those not receiving treatment (Figure 3). For reference, patients diagnosed with myeloma between age 70 and 79 had a median overall survival of 30.1 months (95% CI 28.4–32.4).
Figure 3:
Overall Survival
The median overall survival for patients diagnosed at age 80 or older was 13.4 months (shown in black). However, those patients who received systemic treatment had a median overall survival of 21.4 months (shown in blue), compared to 6.4 months for those not receiving treatment (shown in red).
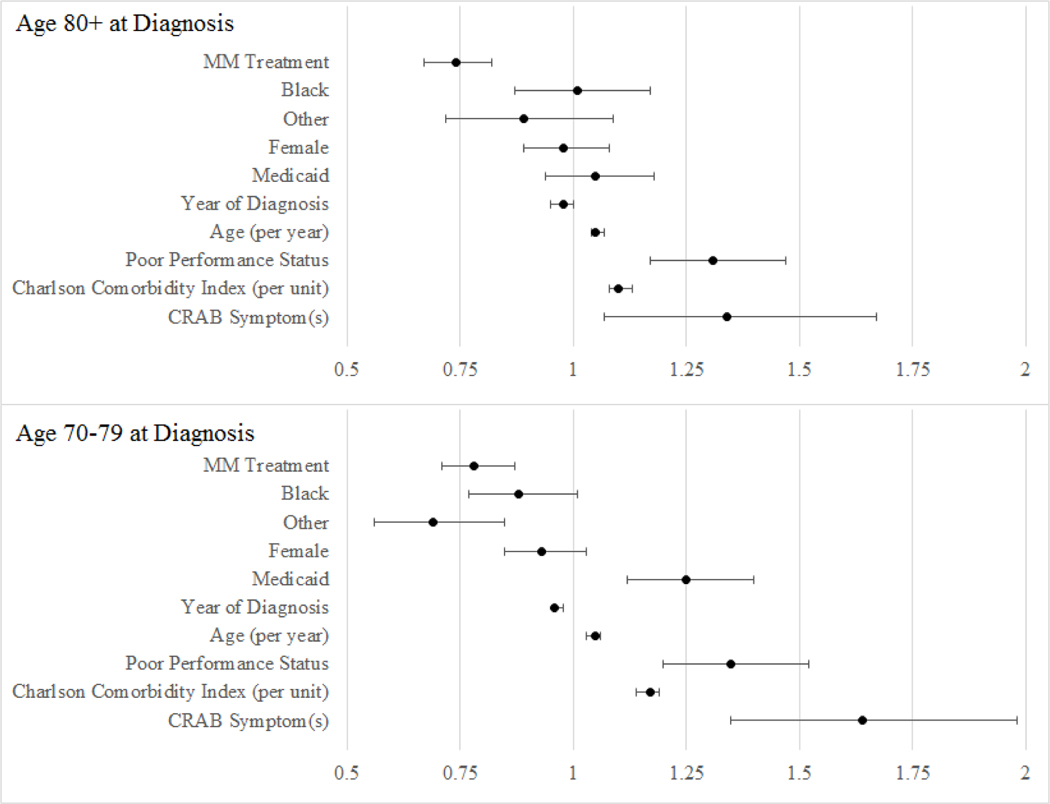
In the 80 and older cohort, myeloma treatment was associated with a 26% decrease in hazard for death (adjusted hazard ratio [aHR] 0.74; 95% CI 0.67–0.82; p < 0.0001) independent of age, race, gender, poverty, comorbidities, and proxy measures of performance status. Survival improved for patients in more recent years; the hazard for death decreased by 3% (HR 0.97; 95% CI 0.94–0.99; p = 0.010) each year 2007–2013. After controlling for myeloma treatment, the year of diagnosis was no longer a significant predictor of survival.
In the 70–79 cohort, myeloma treatment was associated with a 22% decrease in hazard for death (aHR 0.78; 95% CI 0.70–0.86; p < 0.0001) independent of age, race, gender, poverty, comorbidities, and proxy measures of performance status. Based on the difference-in-difference design, there is no statically significant difference in treatment benefit based on age cohort (p = 0.610). More specifically, patients over 80 at myeloma diagnosis who receive systemic treatment obtain proportional benefit to those age 70–79, in respect to their peers who do not receive treatment. Multivariate results are summarized in Table 2 and Figure 4.
Table 2:
Multivariate Survival Analyses
| Model 1 80+ (n = 2,155) | Model 2 70–79 (n = 2,933) | |||||
|---|---|---|---|---|---|---|
| aHR | 95% CI | P | aHR | 95% CI | P | |
| Myeloma Treatment | 0.74 | 0.67–0.82 | <.0001 | 0.78 | 0.71–0.87 | <.0001 |
| White | REF | REF | ||||
| Black | 1.01 | 0.87–1.17 | 0.9349 | 0.88 | 0.77–1.01 | 0.0615 |
| Other Race | 0.89 | 0.72–1.09 | 0.2479 | 0.69 | 0.56–0.85 | 0.0006 |
| Male | REF | REF | ||||
| Female | 0.98 | 0.89–1.08 | 0.6330 | 0.93 | 0.85–1.03 | 0.1577 |
| Medicaid Enrollment | 1.05 | 0.94–1.18 | 0.4099 | 1.25 | 1.12–1.40 | <.0001 |
| Year of Diagnosis (per year) | 0.98 | 0.95–1.00 | 0.0813 | 0.96 | 0.93–0.98 | 0.0006 |
| Age (per year) | 1.05 | 1.04–1.07 | <.0001 | 1.05 | 1.03–1.06 | <.0001 |
| Poor Performance Status | 1.31 | 1.17–1.47 | <.0001 | 1.35 | 1.20–1.52 | <.0001 |
| Charlson Comorbidity Index (per unit) | 1.10 | 1.08–1.13 | <.0001 | 1.17 | 1.14–1.19 | <.0001 |
| CRAB Criteria | 1.34 | 1.07–1.67 | 0.0110 | 1.64 | 1.35–1.98 | <.0001 |
Figure 4:
Forest Plot of Hazard Ratios
The adjusted hazard ratios with 95% confidence intervals are displayed for each cohort. There were no statistically significant (p < .05) differences observed suggesting that all factors influence survival similarly for the older and younger patients.
DISCUSSION
The results of our study indicate that novel agents produce a similar survival benefit among oldest patients. However, use of any systemic therapy in older patients with myeloma is limited by tolerability. The goal of clinicians is to avoid undertreating the fit older patient while also avoiding overtreating the frail older patient. While undertreatment can lead to greater disease burden, overtreatment gives way to toxicities limiting further therapy – either of which scenario having the potential to compromise quality and length of life. Possibly due to this consideration, a large, although decreasing, proportion of patients older than 80 years are not receiving systemic treatment for myeloma. Consequently the survival benefit of novel agents in these patients may not be apparent at the population level.
Previous studies have found minimal, if any, survival benefit for the oldest subgroups of patients with newly diagnosed myeloma.6–9 A post-hoc sub-analysis of octogenarians in pivotal randomized controlled trials demonstrated equivalent median progression-free survival for patients 80 as compared to younger patients;21 however, median overall survival was significantly inferior for patients 80 years-old. Comparing absolute overall survival between younger and older patients is flawed as the expected survival of these groups differ in the general population. We would suggest that the more relevant comparison is the relative benefit compared to those of the same age group. Data from our study using this approach suggest that survival improvements, while not equal, are proportional.
A smaller fraction of those over 80 receive treatment which mutes the benefit at the population level. However, treatment rates have been increasing in more recent years. This could be due to the greater availability of novel agents and/or increasing clinical experience in how to adapt therapy in order to minimize toxicity and willingness to recommend treatment to older patients. It is interesting to note that while treatment rates increased during the 6-year time period under study, IMiD use was relatively stable, but the use of PIs increased nearly three-fold. This may have been the result of increased utilization of the subcutaneous formulation of bortezomib, which has a lower incidence of peripheral neuropathy while continuing to have equal efficacy as compared with the intravenous preparation.22–25 The subcutaneous administration may have made treatment with bortezomib more feasible in the older population. Unfortunately, administrative codes do not allow us to ascertain the exact method of parenteral administration.
This study also suggests that comorbidities and poor performance indicators have a similar effect on overall survival in the 70–79 and 80+ cohorts. While these factors are more prevalent in the 80+ cohort, they do not seem to produce a disproportionate effect on survival. In the year preceding myeloma diagnosis all patients have a greater likelihood than the general population to have comorbid disease such as renal impairment, chronic pulmonary disease, congestive heart failure, and diabetes mellitus.26 Regardless of age, the presence of these comorbid conditions warrant a specialized approach to myeloma treatment, but do not seem to merit additional concerns in patients above 80-years-old.
A major limitation of our study is the use of claims to identify symptoms and treatments. Although the sensitivity and specificity of Medicare claims for anti-myeloma treatment have not been reported, previous work indicates a sensitivity of 93% (95% CI, 88%−96%) for administration of parenteral chemotherapy.27 As many patients with myeloma receive oral regimens, it is not clear if the sensitivity is similar. Another limitation is the lack of important prognostic information, including laboratory data and cytogenetic information, which form the basis of staging for multiple myeloma. Prior studies have found that older patients with myeloma have worse prognostic features at diagnosis, notably a higher ISS stage, when compared with younger patients.28,29 The generalizability of the results may also be limited, as the study was limited to patients reported to SEER and enrolled in Medicare Parts A, B, and D at time of diagnosis, approximately 7% of all cases, and may not represent the entire population. Despite the limitations inherent to studies such as this, the study provides valuable data on understudied segment of the myeloma population.
In conclusion, anti-myeloma treatment in the era of novel therapies seems to have a similar improvement on survival for the oldest-old, those beyond 80 years, as other patients. With growing knowledge of and experience with novel agents in older patients with myeloma, treatment rates have increased, which have in turn improved survival. The population over 80, when myeloma incidence peaks, is projected to triple over the next few decades. It is imperative that we continue to advance our understanding of the needs of this vulnerable subgroup of patients with myeloma.
Acknowledgements
This research was made possible by Grant Number K12CA167540 through the National Cancer Institute (NCI) at the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. The Center for Administrative Data Research is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH), and Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality (AHRQ). This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare & Medicaid Services; Information Management Services Inc; and the SEER program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Conflicts of Interest: None
Disclosures and Conflict of Interest Statements
T. Wildes has received research funding from Jannsen. The other authors have no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kanapuru B, Singh H, Myers A, Beaver JA, Kwitkowski VE, Farrell AT, Pazdur R. Enrollment of Older Adults in Clinical Trials Evaluating Patients with Hematologic Malignancies – the Food and Drug Administration (FDA). ASH; 2017. [Abstract 861]. [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. (eds). SEER Cancer Statistics Review, 1975–2016, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. [Google Scholar]
- 3.Facon T, Dimopoulos MA, Dispenzieri A, Catalano JV, Belch A, Cavo M, et al. Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma.Blood. 2018. January 18;131(3):301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N Engl J Med. 2018. February 8;378(6):518–528. [DOI] [PubMed] [Google Scholar]
- 5.Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N Engl J Med. 2019. May 30;380(22):2104–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa LJ, Brill IK, Omel J, et al. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States. Blood Advances. 2017;1(4):282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristinsson SY, Landgren O, Dickman PW, Derolf ÅR, Björkholm M. Patterns of Survival in Multiple Myeloma: A Population-Based Study of Patients Diagnosed in Sweden From 1973 to 2003. JCO. 2007;25(15):1993–1999. [DOI] [PubMed] [Google Scholar]
- 8.Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111(5):2521–2526. [DOI] [PubMed] [Google Scholar]
- 9.Kristinsson SY, Anderson WF, Landgren O. Improved long-term survival in multiple myeloma up to the age of 80 years. Leukemia. 2014;28(6):1346–1348. [DOI] [PubMed] [Google Scholar]
- 10.Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014. May;28(5):1122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nandakumar B, Binder M, Dispenzieri A, Kapoor P, Buadi F, Gertz MA, et al. Continued improvement in survival in multiple myeloma (MM) including high-risk patients [Abstarct 8039]. ASCO Annual Meeting 2019. Chicago, IL. [Google Scholar]
- 12.Fakhri B, Fiala MA, Tuchman SA, Wildes TM. Undertreatment of Older Patients With Newly Diagnosed Multiple Myeloma in the Era of Novel Therapies. Clin Lymphoma Myeloma Leuk. 2018. March;18(3):219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malecek MK, Fiala M, Schroeder M, Dukeman J, Ghobadi A, Stockerl-Goldstein K, et al. Multiple Myeloma Patients Ineligible for Randomized Controlled Trials Have Poorer Outcomes Irrespective of Treatment. Clin Lymphoma Myeloma Leuk. 2018. September;18(9):e363–e364. [DOI] [PubMed] [Google Scholar]
- 14.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov). Documentation for SEER Data. SEER. https://seer.cancer.gov/data-software/documentation/. Accessed January 2, 2020. [Google Scholar]
- 15.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov). About the SEER Program. SEER. https://seer.cancer.gov/about/overview.html. Accessed April 29, 2019. [Google Scholar]
- 16.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–18. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute. How the SEER & Medicare Data are Linked. https://healthcaredelivery.cancer.gov/seermedicare/overview/linked.html. Accessed April 29, 2019.
- 18.Fiala MA, Dukeman J, Tuchman SA, et al. Development of an Algorithm to Distinguish Smoldering Versus Symptomatic Multiple Myeloma in Claims-Based Data Sets. JCO Clinical Cancer Informatics. 2017;(1):1–8. 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klabunde C, Potosky AL, Legler JM, Warren J. Development of a comorbidity index using physician claims data. JCE, 2000; 53(12): 1258–1267. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths R, Mikhael J, Gleeson M, Danese M, Dreyling M. Addition of rituximab to chemotherapy alone as first-line therapy improves overall survival in elderly patients with mantle cell lymphoma. Blood, 2011; 118(18), 4808–4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zweegman S Octogenarians with Multiple Myeloma. ASCO Annual Meeting 2019. Chicago, IL. [Google Scholar]
- 22.Palumbo A, Bringhen S, Rossi D, et al. Bortezomib-Melphalan-Prednisone-Thalidomide Followed by Maintenance With Bortezomib-Thalidomide Compared With Bortezomib- Melphalan-Prednisone for Initial Treatment of Multiple Myeloma: A Randomized Controlled Trial. JCO. 2010;28(34):5101–5109. [DOI] [PubMed] [Google Scholar]
- 23.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12(5):431–440. [DOI] [PubMed] [Google Scholar]
- 24.Mateos M-V, San Miguel JF. Safety and efficacy of subcutaneous formulation of bortezomib versus the conventional intravenous formulation in multiple myeloma. Therapeutic Advances in Hematology. 2012;3(2):117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Liu H, Li L, et al. Clinical features and treatment outcome of elderly multiple myeloma patients with impaired renal function. J. Clin. Lab. Anal. 2019;e22888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregersen H, Vangsted AJ, Abildgaard N, et al. The impact of comorbidity on mortality in multiple myeloma: a Danish nationwide population-based study. Cancer Medicine. 2017;6(7):1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamont EB, Herndon JE 2nd, Weeks JC, et al. Criterion validity of Medicare chemotherapy claims in Cancer and Leukemia Group B breast and lung cancer trial participants. Journal of the National Cancer Institute 2005;97:1080–3. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig H, Durie BGM, Bolejack V, et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: an analysis of 10 549 patients from the International Myeloma Working Group. Blood. 2008;111(8):4039–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimopoulos MA, Kastritis E, Delimpasi S, et al. Multiple myeloma in octogenarians: Clinical features and outcome in the novel agent era. European Journal of Haematology. 2012;89(1):10–15. [DOI] [PubMed] [Google Scholar]