Abstract
Frequently the results generated when testing novel anti-tumor immunotherapies in vitro do not correlate with data collected in in vivo models and/or in clinical settings. It is our hypothesis that this discrepancy is caused by the use of in vitro conditions, such as normoxia, a two-dimensional surface, optimal growth media and lack of cell complexity and heterogeneity. These conditions do not accurately reflect the tumor microenvironment (TME) that the tested immunotherapeutic strategies experience in vivo.
Whilst there are many variables which can have an impact upon the anti-tumor efficacy of an immunotherapy, the immunosuppressive TME is one in which several of the conditions commonly found in vivo can be mimicked in vitro. These conditions, which include hypoxia, low pH, low glucose, presence of adenosine, cell complexity and heterogeneity as well as the three-dimensional structure of TME, can all affect immune cell-tumor cell interactions. Here we discuss the impact that these conditions, either individually or in combination, can have on these interactions. Furthermore, we propose that performing in vitro assays under TME-like conditions improves the clinical relevance of the yielded results. This in turn contributes to accelerate the speed, reduce the cost and increase efficiency of screening novel immunotherapies and eventually the development of prospective clinical trials.
Keywords: Immunotherapy, Combination immunotherapy, Cellular immunotherapy, Tumor Microenvironment, Immune cells and tumor microenvironment, Tumor/immune editing
1. Introduction
Our increased understanding at the molecular level of the complex interactions between a host’s immune system and tumor cells has informed the generation of a large number of novel immunotherapeutic strategies to treat many types of cancer. Screening these immunotherapies to identify the most effective reagents or combinatorial strategies is a huge challenge, especially because of the need to accomplish it in a fast, economic and efficient manner. At present, preclinical efficacy testing of novel immunotherapies against tumor cell lines or primary samples, both under ‘normal’ in vitro conditions and in animal models, is the conventional way of assessing their potential therapeutic activity. Conventional or classical approaches to test the anti-tumor activity of immunotherapies in vitro commonly involve co-culture experiments measuring target cell viability, effector cell activation or cytokine release through methods such as colorimetric assays (1,2) and FACS-based analysis (3,4) (Table 1). However, often there are discrepancies between the results generated when testing immunotherapies in in vitro assays and those derived from testing them in in vivo experiments (5–7). A lack of in vivo eradication of tumor cells following immunotherapeutic interventions, despite their complete in vitro elimination, likely reflects the impact several factors can have upon the anti-tumor efficacy of an immunotherapy in vivo (Table 2) (8,9). Of these the negative impact of tumor microenvironment (TME) on effector cell-target tumor cell interactions is of particular interest. It raises the question as to how well results acquired from anti-tumor activity assays under ‘normal in vitro conditions’ truly reflect the interactions that tumor cells and effector cells, selected for their immunotherapeutic capacity, experience in vivo, and whether they truly have any in vivo relevance. TME is characterized by cell types, morphology and environmental conditions significantly different from those associated with normal tissues (10,11). These conditions can affect tumor cell antigen expression, proliferation and survival as well as alter effector lymphocyte differentiation, trafficking and function. The resulting changes can ultimately lead to tumor cell evasion from a host’s immune surveillance and suppression of immune effector cell function. However, the characteristics of TME, which can dramatically affect tumor cell and immune cell function, are not considered in most, if not all, the commonly used in vitro assays. These conditions can be mimicked in vitro and we believe that their use would increase the clinical relevance of the results generated by experiments evaluating the potential in vivo efficacy of immunotherapeutic strategies. These methodological changes will improve the currently slow development of effective treatment options and their translation from the bench to the bedside. We discuss the potential effects that conditions which mimic TME, hence referred to as ‘TME-like conditions’ can have on both tumor cells and immune cells, highlighting the significant impact that TME plays in tumor cell-immune cell interactions.
Table 1:
Examples of conventional in vitro effector-target co-culture assays
| Assay Type | Examples | Summary |
|---|---|---|
| Cell Viability | Chromium release | Radioactively labelled target cells release chromium when lysed |
| MTT, XTT, ATP assays | Metabolically activated target cells produce substrate detected using a spectrophotometer | |
| Live/dead | Fluorescently labelled or transfected target cells measured by FACS or microscopy analysis | |
| Cytokines | IFN-gamma expression and release | Cytokine release by effector cells measured by ELISPOT/ELISA |
| Apoptosis | Annexin V and 7-AAD/PI | Binding to phosphatidylserine and DNA respectively to detect early and late apoptosis by FACS |
| TUNEL assay | Labelling of fragmented DNA produced during apoptosis | |
| Degranulation | CD107a assay | Detection of cytotoxic granule marker at the extracellular membrane of effector cells |
Table 2:
Summary of abnormalities influencing in vivo efficacy of immunotherapeutic strategies
| Factor | Summary | Reason |
|---|---|---|
| Trafficking | Insufficient immune effector cell trafficking or drug payload delivery to the site of tumor cells |
|
| Recognition | Defective recognition of the targeted tumor antigen by cognate immune cells |
|
| Survival | Low proliferation and limited persistence of effector immune cells in TME |
|
| Specificity | Side effects caused by the targeting of normal cells |
|
| Control | Side effects such as those caused by cytokine release syndrome |
|
| TME | Multiple escape mechanisms utilized by tumor cells |
|
2. TME Characteristics
2.1. Hypoxia, Low pH and Presence of Adenosine
Often, the combination of excessive proliferation of malignant cells and inadequate angiogenesis in tumors results in a lack of blood supply, and therefore insufficient levels of oxygen, creating areas of hypoxia within TME (11). Under normal conditions hypoxia-inducible factors (HIFs) are constitutively expressed in the cytoplasm of cells, where they are subject to von Hippel-Lindau (VHL) complex dependent degradation. However, during hypoxia the lack of oxygen necessary to promote their degradation allows HIFs to relocate to the nucleus and drive transcriptional changes in cancer and stromal cells as well as in effector lymphocytes. Whether tumor cells are under normoxia or hypoxia, either in vitro or in vivo, they will metabolize via glycolysis, a process termed the Warburg effect (12). The increased glucose uptake by tumor cells supports tumor progression and results in production of lactate and a reversed intra-extracellular pH gradient, producing a hostile acidic extracellular environment. Commonly the extracellular pH level within TME can reach values between 6–6.5. Acidic conditions such as these have been shown to negatively impact immune cell function and proliferation (13). An additional consequence of the glycolytic metabolism of tumor cells following ATP degradation is adenosine accumulation in TME; this contributes to the immunosuppressive properties of TME (14). In vitro assays performed under normoxia with continually replenished growth media therefore do not replicate these TME conditions.
2.1.1. Effects of Hypoxia, Low pH and Presence of Adenosine on Tumor Cell Phenotype and Functional Properties
As a result of the above mentioned transcriptional changes, the expression of immunologically relevant molecules which appear to play a role in tumor progression, as well as of those molecules having a detrimental effect on immune cells, can be modulated. One of the most well-known examples of these changes is the induction and/or upregulation of the immune checkpoint protein PD-L1 on tumor cells under hypoxic conditions (15). These phenotypic changes can promote tumor cell mediated inhibition of cytotoxic effector T cell activity (16) and help drive tumor growth, invasion, migration and neo-angiogenesis (17,18). Similarly, from our own experience analyzing tumor cell lines, hypoxia can upregulate the expression of tumor antigens such as chondroitin sulfate proteoglycan 4 (CSPG4) and B7-H3, both of which are associated with tumor progression (19,20).
Hypoxia induced secretion of chemokines and cytokines by cancer cells recruits immunosuppressive T regulatory (Treg) cells and myeloid derived suppressor cells (MDSCs) into TME, further dampening cytolytic T cell immune function (21,22). Cytokine expression can also transform fibroblasts into cancer associated fibroblasts (CAFs), which along with secretion of enzymes by tumor cells can remodel the extracellular matrix (ECM), promoting cancer cell invasion and metastasis (23). Furthermore, hypoxia has been linked to promotion of the cancer stem cell phenotype which can result in rapid growth of tumor cells and increase in their metastatic potential (24). Hypoxia can also result in a decrease of pro-apoptotic molecules (Bid and Bax) (25) and an increase in anti-apoptotic molecules (Survivin and Bcl-2), generating pro-survival signals for tumor cells (26,27).
The effect of an acidic extracellular but alkaline intracellular TME on tumor cells favors cancer progression. Cell proliferation, differentiation, migration, clastogenesis, suppression of apoptosis and malignant transformation have all been linked to alkaline intracellular pH (28). The acidic extracellular pH is associated with activation of some lysosomal enzymes, stimulation of signaling pathways, disruption of adherence junctions and promotion of metastasis (29,30). Additionally, binding of adenosine to tumor cells can promote their proliferation, increase their adhesion and migration as well as reduce their susceptibility to apoptosis (14).
Differential effects on HLA class-I and tumor antigen expression have been observed in our preliminary studies when culturing various types of cancer cell lines under TME-like conditions of hypoxia, low pH and presence of adenosine. Some illustrative examples are shown in Figure 1. These phenotypic changes may provide tumor cells with an escape mechanism from cognate cytotoxic T cells or tumor antigen targeted immunotherapies.
Figure 1: Differential modulation of B7-H3 and CSPG4 tumor antigen expression on cancer cells cultured under TME-like conditions.
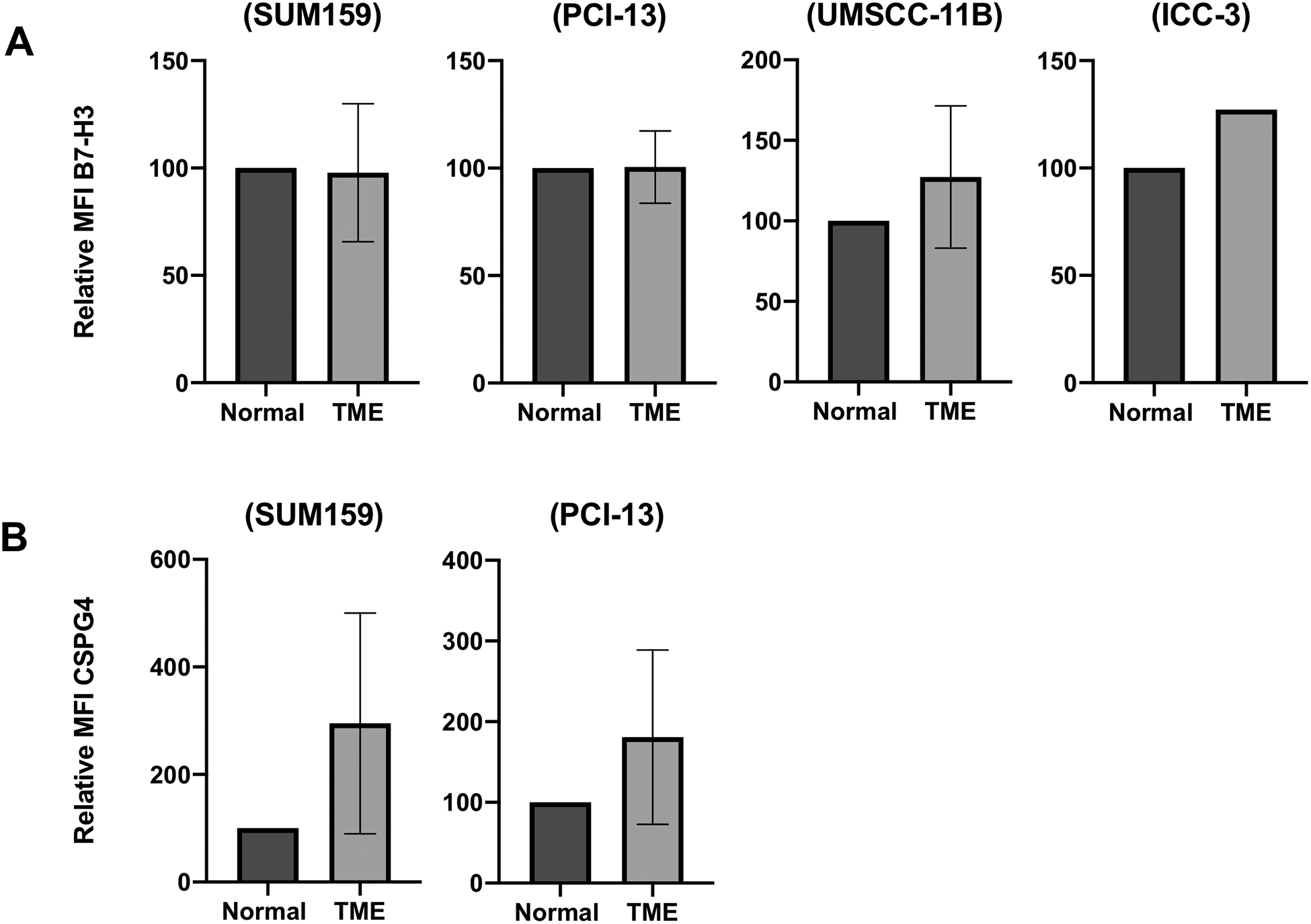
Cancer cell lines (Triple negative breast cancer, SUM159; Squamous cell carcinoma of the head and neck, PCI-13 and UMSCC-11B; Cholangiocarcinoma, ICC-3) were cultured under TME-like conditions (1% oxygen, pH6.5, adenosine IC5) for six days. Cells cultured under normoxia in unmodified media (‘normal’ in vitro conditions) were used as controls. After a six day culture at 37°C cells were harvested and stained using the B7-H3 mAb 376.96 (A) and the CSPG4 mAb 225.28 (B). Stained cells were then analysed by flow cytometry for antigen expression level. MFI comparison between the normal and TME-like conditions is shown. Error bars represent standard deviation.
2.1.2. Effects of Hypoxia, Low pH and Presence of Adenosine on Immune Cell Phenotype and Functional Properties
Hypoxia can have a detrimental effect on the anti-tumor activity of immune effector cells. HIF1a can promote differentiation of CD4+ T cells into immunosuppressive Treg cells through FOXP3 induction (31). Additionally checkpoint protein CTLA4, LAG3 and Tim-3 expression is upregulated in VHL-deficient CD8+ T cells (32).
NK cell cytotoxic ability is diminished in an acidic in vitro environment (pH<7.2), along with their susceptibility to cytokine stimulation (33). Tumor antigen specific CD8+ T cells in an acidic environment are characterized by a decrease in T cell receptor and IL-2 receptor alpha-chain expression. The latter changes, coupled with defective STAT5 and ERK pathway signaling, result in reduced cytotoxic activity and cytokine secretion (34,35). However the precise mechanism of the reduced functionality of cytotoxic T cells seems to vary based upon the specific cancer type tested (36).
Activated T cells rely upon glycolytic metabolism to rapidly proliferate and provide a dynamic effector function. However, in TME increased glucose uptake by tumor cells results in metabolic competition with T cells, thus limiting their glycolytic capacity, mTOR activity and IFN-y production (37). Post-translational downregulation of the glycolytic pathway enzyme enolase 1 is thought to be the mechanism behind this effect, which can be restored through provision of pyruvate (38). Similarly, NK cells metabolize through aerobic glycolysis and therefore a lack of glucose and other nutrients within TME is thought to limit NK cell activity through disruption of this process (39).
Binding of adenosine to immune cells including T cells, NK cells, NKT cells, macrophages, dendritic cells and neutrophils leads to suppression of their functional properties (14). Stimulation of the adenosine receptor A2A on T cells can induce FOXP3, stimulating development of CD4+FOXP3+ immunosuppressive Treg cells. Additionally, adenosine can induce expansion of immunosuppressive MDSCs.
2.2. Morphology and Composition of TME
TME is a unique and heterogenous structure composed of multiple cell types alongside the malignant cancer cells (10) (Table 3). Excessive tumor cell proliferation and insufficient vascularization produce areas which vary in oxygen and nutrient levels, therefore generating a significant level of heterogeneity in the above described characteristics. These features also vary broadly among types of solid cancer, stage of the malignancy, the host’s genetic makeup, as well as between primary and metastatic tumors within the same patient (41,42). Another variable to consider is represented by the complex and multifaceted changes which occur in vivo over the time course of a malignancy and its treatment. An in vitro assay may give a reflection of circumstances occurring during a snapshot of conditions in vivo but cannot recapitulate the plasticity occurring within an animal system which is constantly in flux. Given the current interest in the use of immune checkpoint blockade treatment for various types of cancer (43), it is noteworthy that the mentioned cellular heterogeneity in TME may impact upon the evaluation of immunotherapeutic strategies. In vitro assays do not provide the complexity and dynamic changes to identify the potentially multiple mechanisms of actions through which the immunotherapy may function.
Table 3:
Cellular components of TME
| TME components | Cell type |
|---|---|
| Tumor cells | Differentiated malignant cells |
| Cancer initiating cells | |
| Endothelial cells | Vascular endothelial cells |
| Lymphatic endothelial cells | |
| Lymphocytes | T lymphocytes |
| NK lymphocytes | |
| B lymphocytes | |
| Myeloid Cells | Myeloid derived suppressor cells |
| Tumor associated macrophages | |
| Dendritic cells | |
| Stromal cells | Mesenchymal stem cells |
| Cancer associated fibroblasts | |
| Adipocytes | |
| Pericytes |
In vitro experiments with tumor cells are predominantly performed on a two-dimensional (2D) surface within the well of a plastic culture plate. This environment is clearly dissimilar from the morphology within TME. Culture of cells on a 2D surface affects their proliferation, differentiation and survival as well as interactions among cells cultured together (44,45). For example, hypoxia mediated MHC class I antigen downregulation in mouse cancer cell lines does not occur in a 2D monolayer, but can be induced when cells are grown in three-dimensional (3D) spheroids, generating heterogeneity in MHC class I antigen expression among cells (46). The development of novel 3D microfluidics devices however now allows a closer approximation of TME structure in vitro. Tumor cells are grown on a low cell attachment plate forcing the formation of tumor spheroids. These spheroids can then be added to a microfluidics device where they bind to an internal collagen channel presenting themselves as a 3D structure into which drugs or effector cells can be introduced in a more relevant spatiotemporal environment (47).
3. Expert Opinion
Ultimately the hallmarks of TME described above are inextricably linked, contributing to the pro-tumor effects of one another and providing tumor cells with beneficial immune evasion mechanisms (Figure 2). These factors likely contribute to the large disparity between exciting in vitro results of immunotherapeutic strategies and their limited efficacy in vivo. It is noteworthy that the same limitation may apply to results obtained from clinical trials based on information generated using animal models. An absence of toxicity when treating mice bearing human tumors with antibody-based therapies, due to the lack of cross reactivity of the human tumor antigen specific antibodies tested with its respective mouse counterpart, may suggest the misleading conclusion that the tested antibody will not cause toxicity when injected into patients. In spite of these limitations the results obtained from pre-clinical studies may provide useful information concerning the safety and feasibility of immunotherapies if interpreted in a suitable way.
Figure 2:
Conditions of TME alter tumor cell and immune cell phenotype and function creating a disparity between data collected in vitro under ‘normal conditions’ compared to those produced in vivo.
We hypothesize that the clinical significance of in vitro results could be easily improved in a cost and time efficient manner by conducting assays under characteristics which can be mimicked through relatively simplistic means, namely, hypoxia, low pH and presence of adenosine. More complex methodology such as the use of 3D microfluidics devices or the co-culture of multiple cell types is also possible, however requires more in-depth user experience and resources. Furthermore to mimic TME conditions in more complex and potentially more relevant ways it is necessary to realize that different types of solid cancer have distinct TME compositions and therefore the assay conditions should be adjusted to the malignancy under investigation. For example, a hallmark of pancreatic ductal adenocarcinoma (PDAC) TME is the presence of desmoplasia, which is not associated with other types of solid cancer (48). Similarly, the extent of immune cell infiltration between ‘hot’ and ‘cold’ tumors differs substantially (49). These differences should be taken into account when designing immune effector cell-based immunotherapeutic strategies. When testing immunotherapies involving effector lymphocytes such as T cells transduced with T cell receptors or with chimeric antigen receptors (CARs) targeting tumor antigens, their expression, as well as that of molecules/pathways regulating their expression, should be monitored, since it may be markedly modulated by hypoxia, low pH and/or glycolytic state. Similarly, the regulatory effect of acidic extracellular pH on drug efficacy should be considered, especially when designing combination strategies with chemotherapeutic agents or the delivery of antibody drug conjugates with a pH sensitive linker.
It is important to note that these hallmarks of TME can be targeted with treatments to alleviate their negative effects on immune function. Use of CTLA4, PD-1 and Tim-3 blocking antibodies can enhance the presence of CD8+ T cells in murine models of melanoma with restored enolase activity (38). Blocking of the A2A adenosine receptor through small molecule inhibitors can lower adenosine levels (40) counteracting immunosuppressive mechanisms mediated by adenosine signaling pathways. Several clinical studies are currently assessing ways to inhibit the adenosine pathway as an anti-tumor mechanism for cancer immunotherapy. Similarly, HIF prolyl hydroxylase enzyme inhibitors are able to stabilize the HIF complex (50), which allows for inhibition of tumor growth through TGFβ signaling. However the applicability of this strategy to all types of solid cancer is questionable.
The information resulting from in vitro assays incorporating TME-like conditions will help identify major changes caused by in vivo TME which may affect the efficacy of the tested immunotherapeutic strategies. These results, in turn, will enable optimization of suitable combination therapies with much lower experimental requirements and at a much lower cost.
Statement of translational relevance.
Results of in vitro assays evaluating the efficacy of immunotherapeutic strategies for the treatment of malignant diseases do not often correlate with those generated by in vivo assays in animal models and/or by clinical investigations. This discrepancy is caused by many variables, among which conditions within the tumor microenvironment (TME) such as hypoxia, low pH, presence of adenosine as well as cell heterogeneity and complexity appear to play a major role, since they affect immune cell-tumor cell interactions. Here, we discuss whether conducting in vitro assays under ‘TME-like conditions’ will improve the clinical relevance of the generated results and therefore facilitate the translation of pre-clinical data into a clinical trial.
Acknowledgements:
Funding: This work was supported by NIH grants R01DE028172, R03CA239193, R03CA231766, R03CA216114 and R03CA223886 and by DOD grant W81XWH-16-1-0500.
Omar Ahmed Barakat contributed to a grammatical review of the manuscript. Dr. Filippos Kontos and Dr. Ali Emre Dal provided portions of the data used to prepare Figure 1.
Footnotes
Conflict of interest statement: The authors declare no potential conflicts of interest.
References
- 1.Malyguine AM, Strobl S, Dunham K, Shurin MR, Sayers TJ. ELISPOT Assay for Monitoring Cytotoxic T Lymphocytes (CTL) Activity in Cancer Vaccine Clinical Trials. Cells. 2012. May 10;1(2):111–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karimi MA, Lee E, Bachmann MH, Salicioni AM, Behrens EM, Kambayashi T, et al. Measuring cytotoxicity by bioluminescence imaging outperforms the standard chromium-51 release assay. PLoS One. 2014;9(2):e89357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jedema I, van der Werff NM, Barge RMY, Willemze R, Falkenburg JHF. New CFSE-based assay to determine susceptibility to lysis by cytotoxic T cells of leukemic precursor cells within a heterogeneous target cell population. Blood. 2004. April 1;103(7):2677–82. [DOI] [PubMed] [Google Scholar]
- 4.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004. November;294(1–2):15–22. [DOI] [PubMed] [Google Scholar]
- 5.Martinez M, Moon EK. CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment. Front Immunol. 2019. February 5;10:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filley AC, Henriquez M, Dey M. CART Immunotherapy: Development, Success, and Translation to Malignant Gliomas and Other Solid Tumors. Front Oncol. 2018. October 17;8:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nayyar G, Chu Y, Cairo MS. Overcoming Resistance to Natural Killer Cell Based Immunotherapies for Solid Tumors. Front Oncol. 2019. February 11;9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim WA, June CH. The Principles of Engineering Immune Cells to Treat Cancer. Cell. 2017. February 9;168(4):724–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scarfò I, Maus MV. Current approaches to increase CAR T cell potency in solid tumors: targeting the tumor microenvironment. J Immunother cancer. 2017;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010. December 15;123(Pt 24):4195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006. May 24;441(7092):437–43. [DOI] [PubMed] [Google Scholar]
- 12.Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016. March;41(3):211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol. 2001. April;69(4):522–30. [PubMed] [Google Scholar]
- 14.Ohta A. A Metabolic Immune Checkpoint: Adenosine in Tumor Microenvironment. Front Immunol. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 2016. March;27(3):409–16. [DOI] [PubMed] [Google Scholar]
- 16.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A Mechanism of Hypoxia-Mediated Escape from Adaptive Immunity in Cancer Cells. Cancer Res. 2014. February 1;74(3):665–74. [DOI] [PubMed] [Google Scholar]
- 17.Murfee WL, Rehorn MR, Peirce SM, Skalak TC. Perivascular Cells Along Venules Upregulate NG2 Expression During Microvascular Remodeling. Microcirculation. 2006. January;13(3):261–73. [DOI] [PubMed] [Google Scholar]
- 18.Pucciarelli D, Lengger N, Takacova M, Csaderova L, Bartosova M, Breiteneder H, et al. Anti-chondroitin sulfate proteoglycan 4-specific antibodies modify the effects of vemurafenib on melanoma cells differentially in normoxia and hypoxia. Int J Oncol. 2015. July;47(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campoli M, Ferrone S, Wang X. Functional and Clinical Relevance of Chondroitin Sulfate Proteoglycan 4. In: Advances in cancer research. 2010. p. 73–121. [DOI] [PubMed] [Google Scholar]
- 20.Fauci JM, Straughn JM, Ferrone S, Buchsbaum DJ. A review of B7-H3 and B7-H4 immune molecules and their role in ovarian cancer. Gynecol Oncol. 2012. November;127(2):420–5. [DOI] [PubMed] [Google Scholar]
- 21.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn J-I, Cheng P, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010. October 25;207(11):2439–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang L-P, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature. 2011. July 13;475(7355):226–30. [DOI] [PubMed] [Google Scholar]
- 23.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016. September 1;16(9):582–98. [DOI] [PubMed] [Google Scholar]
- 24.Schwab LP, Peacock DL, Majumdar D, Ingels JF, Jensen LC, Smith KD, et al. Hypoxia-inducible factor 1α promotes primary tumor growth and tumor-initiating cell activity in breast cancer. Breast Cancer Res. 2012. February 7;14(1):R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erler JT, Cawthorne CJ, Williams KJ, Koritzinsky M, Wouters BG, Wilson C, et al. Hypoxia-mediated down-regulation of Bid and Bax in tumors occurs via hypoxia-inducible factor 1-dependent and -independent mechanisms and contributes to drug resistance. Mol Cell Biol. 2004. April;24(7):2875–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng X-H, Karna P, Cao Z, Jiang B-H, Zhou M, Yang L. Cross-talk between Epidermal Growth Factor Receptor and Hypoxia-inducible Factor-1α Signal Pathways Increases Resistance to Apoptosis by Up-regulating Survivin Gene Expression. J Biol Chem. 2006. September 8;281(36):25903–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan Y, He Q, Yue K, Si H, Wang J, Zhou X, et al. Hypoxia induced Bcl-2/Twist1 complex promotes tumor cell invasion in oral squamous cell carcinoma. Oncotarget. 2017. January 31;8(5):7729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swietach P, Vaughan-Jones RD, Harris AL, Hulikova A. The chemistry, physiology and pathology of pH in cancer. Philos Trans R Soc B Biol Sci. 2014. February 3;369(1638):20130099–20130099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rofstad EK, Mathiesen B, Kindem K, Galappathi K. Acidic Extracellular pH Promotes Experimental Metastasis of Human Melanoma Cells in Athymic Nude Mice. Cancer Res. 2006. July 1;66(13):6699–707. [DOI] [PubMed] [Google Scholar]
- 30.Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T, et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013. September 3;13(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J. Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1α. Eur J Immunol. 2008. September;38(9):2412–8. [DOI] [PubMed] [Google Scholar]
- 32.Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, et al. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol. 2013. November;14(11):1173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller B, Fischer B, Kreutz W. An acidic microenvironment impairs the generation of non-major histocompatibility complex-restricted killer cells. Immunology. 2000. March;99(3):375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calcinotto A, Filipazzi P, Grioni M, Iero M, De Milito A, Ricupito A, et al. Modulation of Microenvironment Acidity Reverses Anergy in Human and Murine Tumor-Infiltrating T Lymphocytes. Cancer Res. 2012. June 1;72(11):2746–56. [DOI] [PubMed] [Google Scholar]
- 35.Bellone M, Calcinotto A, Filipazzi P, De Milito A, Fais S, Rivoltini L. The acidity of the tumor microenvironment is a mechanism of immune escape that can be overcome by proton pump inhibitors. Oncoimmunology. 2013. January 27;2(1):e22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huber V, Camisaschi C, Berzi A, Ferro S, Lugini L, Triulzi T, et al. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin Cancer Biol. 2017. April 1;43:74–89. [DOI] [PubMed] [Google Scholar]
- 37.Chang C-H, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015. September 10;162(6):1229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gemta LF, Siska PJ, Nelson ME, Gao X, Liu X, Locasale JW, et al. Impaired enolase 1 glycolytic activity restrains effector functions of tumor-infiltrating CD8 + T cells. Sci Immunol. 2019. January 25;4(31):eaap9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Brien KL, Finlay DK. Immunometabolism and natural killer cell responses. Nat Rev Immunol. 2019. May 26;19(5):282–90. [DOI] [PubMed] [Google Scholar]
- 40.Leone RD, Emens LA. Targeting adenosine for cancer immunotherapy. J Immunother Cancer. 2018. December 18;6(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Meara T, Safonov A, Casadevall D, Qing T, Silber A, Killelea B, et al. Immune microenvironment of triple-negative breast cancer in African-American and Caucasian women. Breast Cancer Res Treat. 2019. May 6;175(1):247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szekely B, Bossuyt V, Li X, Wali VB, Patwardhan GA, Frederick C, et al. Immunological differences between primary and metastatic breast cancer. Ann Oncol. 2018. November 1;29(11):2232–9. [DOI] [PubMed] [Google Scholar]
- 43.Hoos A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov. 2016;15(4):235–47. [DOI] [PubMed] [Google Scholar]
- 44.Duval K, Grover H, Han L-H, Mou Y, Pegoraro AF, Fredberg J, et al. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology (Bethesda). 2017;32(4):266–77. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 45.Kapałczyńska M, Kolenda T, Przybyła W, Zajączkowska M, Teresiak A, Filas V, et al. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch Med Sci. 2018. June;14(4):910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sethumadhavan S, Silva M, Philbrook P, Nguyen T, Hatfield SM, Ohta A, et al. Hypoxia and hypoxia-inducible factor (HIF) downregulate antigen-presenting MHC class I molecules limiting tumor cell recognition by T cells. Simos G, editor. PLoS One. 2017. November 20;12(11):e0187314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jenkins RW, Aref AR, Lizotte PH, Ivanova E, Stinson S, Zhou CW, et al. Ex Vivo Profiling of PD-1 Blockade Using Organotypic Tumor Spheroids. Cancer Discov. 2018. February;8(2):196–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang H, Hegde S, DeNardo DG. Tumor-associated fibrosis as a regulator of tumor immunity and response to immunotherapy. Cancer Immunol Immunother. 2017. August 27;66(8):1037–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019. March 4;18(3):197–218. [DOI] [PubMed] [Google Scholar]
- 50.Singh L, Aldosary S, Saeedan AS, Ansari MN, Kaithwas G. Prolyl hydroxylase 2: a promising target to inhibit hypoxia-induced cellular metabolism in cancer cells. Drug Discov Today. 2018. November;23(11):1873–82. [DOI] [PubMed] [Google Scholar]


