Abstract
The ideal modality for generating sensation in sensorimotor brain computer interfaces (BCI) has not been determined. Here we report the feasibility of using a high-density “mini”-electrocorticography (mECoG) grid in a somatosensory BCI system.
Thirteen subjects with intractable epilepsy underwent standard clinical implantation of subdural electrodes for the purpose of seizure localization. An additional high-density mECoG grid was placed (Adtech, 8 by 8, 1.2-mm exposed, 3-mm center-to-center spacing) over the hand area of primary somatosensory cortex. Following implantation, cortical mapping was performed with stimulation parameters of frequency: 50 Hz, pulse-width: 250 μs, pulse duration: 4 s, polarity: alternating, and current that ranged from 0.5 mA to 12 mA at the discretion of the epileptologist. Location of the evoked sensory percepts was recorded along with a description of the sensation. The hand was partitioned into 48 distinct boxes. A box was included if sensation was felt anywhere within the box.
The percentage of the hand covered was 63.9% (± 34.4%) (mean ± s.d.). Mean redundancy, measured as electrode pairs stimulating the same box, was 1.9 (± 2.2) electrodes per box; and mean resolution, measured as boxes included per electrode pair stimulation, was 11.4 (± 13.7) boxes with 8.1 (± 10.7) boxes in the digits and 3.4 (± 6.0) boxes in the palm. Functional utility of the system was assessed by quantifying usable percepts. Under the strictest classification, “dermatomally exclusive” percepts, the mean was 2.8 usable percepts per grid. Allowing “perceptually unique” percepts at the same anatomical location, the mean was 5.5 usable percepts per grid.
Compared to the small area of coverage and redundancy of a microelectrode system, or the poor resolution of a standard ECoG grid, a mECoG is likely the best modality for a somatosensory BCI system with good coverage of the hand and minimal redundancy.
Keywords: Somatosensory, Brain Computer Interface (BCI), Brain Machine Interface (BMI), Electrocorticography, Cortical Stimulation
Introduction
Loss of limb function is a common and debilitating outcome of spinal cord injuries, strokes, neuropathies, and limb amputation. Brain-computer interfaces (BCI) provide a mechanism for restoring function to these individuals. To date, BCI has focused on motor functions through recordings in the motor or parietal cortex1-7. However, an equally important component of functional restoration is somatosensation, which has implications for injury prevention as well as motor execution8-10. Work in non-human primates (NHPs) has shown that intracortical microsimulation of primary somatosensory cortex (S1) can serve as a replacement for tactile feedback and improve performance in closed loop BCI systems2,11,12. However, without verbal descriptions of the quality or location of the sensation, NHP work alone cannot provide information crucial to the design of clinically useful BCI systems. The human experience is crucial to the design of clinically useful sensory BCI. The quality and anatomic boundaries of the somatosensory percepts are important to designing a successful system and absent in NHP studies. To maximize the functionality of a somatosensory BCI system, the “degrees of freedom”, the topography, coverage area, and spread of activation from electrical stimulation, should all be considered.
Early work in human somatosensory BCI has established feasibility. Johnson et al. demonstrated reliably discriminable percepts using variations in stimulation frequency and amplitude with standard electrocorticography (ECoG) grids13. Hiremath et al. completed a 28-day study of upper extremity sensory restoration of in a patient with chronic sensory impairment due to brachial plexus injury14. Flesher et al. used microelectrode grids to deliver intracortical stimulation, finding that perceived intensity scaled with increased amplitude. Over the course of five months, the somatotopy of evoked sensations remained consistent15. Lee et al. used “mini”-electrocorticography (mECoG) grids to evaluate evoked somatosensation in the hand. They demonstrated changes in perceived intensity by varying pulse width, frequency, and current strength, and integrated these percepts into target acquisition tasks16. Overall, safe and consistent stimulation has been observed using various stimulation modalities, opening the door to studies of best practices for functional restoration of somatosensation.
Motor BCI reads out signals from the brain to decode behavior17, requiring primarily intracortical implants. Since micro-electrode grids require insertion into the cortex, the trade-off to protect the cortex (and avoid traversing vessels) is a small profile, with standard human-implanted micro-electrode arrays (the Utah array from BlackRock Systems) covering a cross-sectional area of just 0.144-cm2. While this small area has been adequate for motor signal extraction, somatosensory BCI relies on delivering signal to existing architecture and requires greater coverage18, more in line with prostheses focused on vision restoration. Like S1, the visual field is mapped in an orderly and precise retinotopic map19-21. High spatial resolution provided by penetrating microelectrodes is needed for foveal representation, while epicortical stimulation using macroelectrodes, or minielectrodes may be better suited for peripheral vision20. Somatosensory cortex is also organized topographically, but without an analogy to the fovea. Devices designed to restore sensation to the hand will need to consider underlying functional architecture. Microelectrode grids have provided high-resolution coverage over limited spatial areas of the hand region, even with multiple grids15,22. Standard ECoG grids may cover the entire hand region, but at very low resolution due to electrodes that are large relative to underlying cortical physiology13. Additionally, motor BCI relies on reading single-units, but the ability to stimulate single-units is not readily achievable with current electrical stimulation technology. A compact, multi-electrode penetrating array may not be as well suited for somatosensory stimulation as a high-density, small spaced ECoG array.
As somatosensory BCI gains momentum, establishing the best equipment practices is essential. This study continues prior work describing our experience with high-density mECoG grids to produce somatosensory percepts with cortical stimulation in S1. Electrical stimulation was delivered though bipolar mECoG electrodes covering the hand region in 13 patients being monitored for epilepsy. The dermatomal distribution of sensory percepts and a description of the sensation was recorded for each stimulation site. Measures of surface area, redundancy, and resolution were evaluated for each grid to determine the utility of such a modality going forward.
Methods
Subjects and Implantation
Patients with medically intractable epilepsy planned for surgical implantation of subdural electrodes for the purpose of seizure localization were included in this study. If the S1 hand area was clinically accessible, and coverage would not interfere with clinical testing, written informed consent for implantation of a mECoG grid over this area was obtained prior to implantation. This study was carried out with approval from the University of Southern California Health Sciences Campus Institutional Review Board.
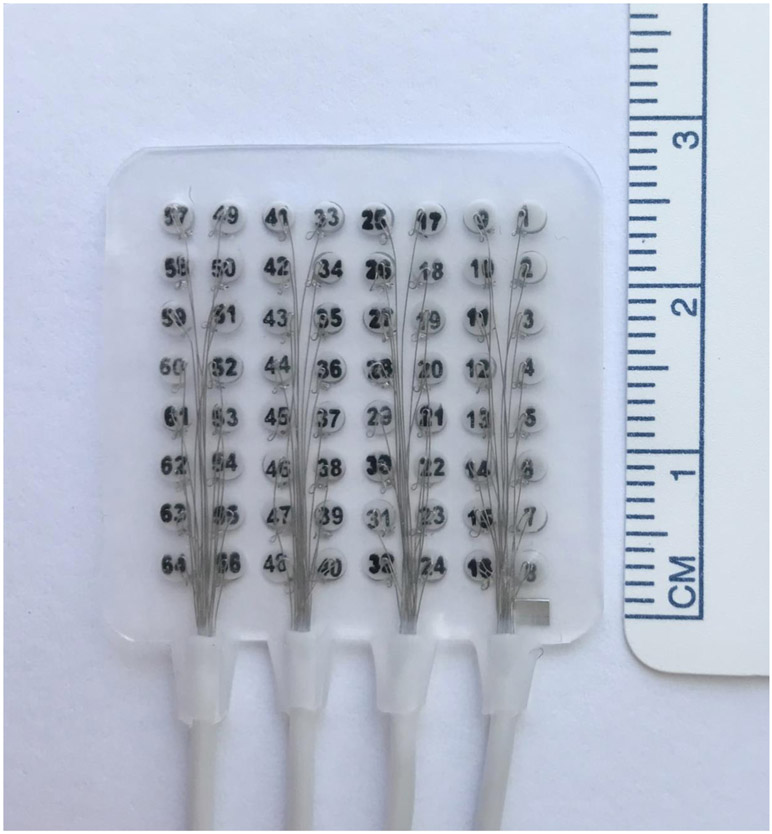
Subjects underwent a standard frontotemporoparietal region craniotomy for electrode placement based on recommendations for seizure localization. An additional 64-channel high density mECoG grid (8x8 array of 2-mm contacts, 1.2-mm exposed area, 3-mm center-to-center spacing; FG64C-MP03, Ad-Tech Medical Instrumentation Corporation, Oak Creek, WI, USA; see Figure 1) was placed over the hand region of S1. Though the hand area was accessible by the craniotomy it was not always directly visualized and therefore placement was guided by neuronavigation. Grids were anchored to the dura to prevent movement. The dura, bone, and scalp were closed in a standard water-tight fashion.
Figure 1.
The “mini-electrocorticography” (mECoG) grid. It is comprised of 64 2.0mm platinum contacts, 1.2mm of exposed contact, with 3mm center to center spacing.
After surgery, patients were then transferred to the epilepsy monitoring unit and tapered off antiepileptic medications. Once adequate seizure activity had been recorded, patients resumed anti-epileptic medications. At this time, clinical mapping of the grids was performed, followed by testing for this study. Both clinical testing and mapping for this study were done under the supervision of an epileptologist. The stimulation parameters were overseen by the patient’s epileptologist, with monitoring for epileptiform discharges, after-stimulation dischargers, or other concerning features, as well as clinical judgement of safe and appropriate parameter limits.
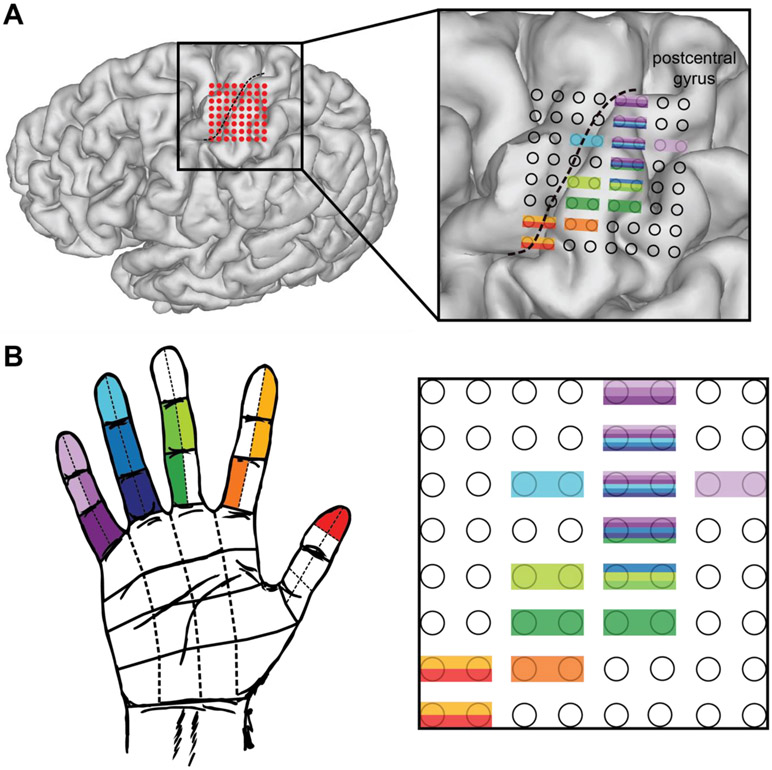
Thirteen right-handed subjects were included for this study. The grid was placed on the left for 8 subjects. See Table 1 for additional demographic information. Figure 2A shows an example grid placement for subject S06 on a three-dimensional reconstruction of the subject’s brain.
Table 1.
Subject demographics and details of epilepsy history.
| Subject | Age | Sex | Grid Laterality |
Handedness | Hand Tested |
Epileptic Foci | Years with epilepsy |
|---|---|---|---|---|---|---|---|
| 1 | 24 | M | L | R | R | L middle and inferior temporal gyri | 3 |
| 2 | 33 | F | R | R | L | R premotor cortex and R primary somatosensory cortex | 24 |
| 3 | 35 | F | L | R | R | L inferior frontal gyrus | 25 |
| 4 | 37 | F | R | R | L | R temporal lobe | 1 |
| 5 | 27 | F | R | R | L | R parietal focal cortical dysplasia | 21 |
| 6 | 55 | M | L | R | R | bitemporal | 36 |
| 7 | 48 | F | L | R | R | L hippocampus and temporal pole | 43 |
| 8 | 21 | F | L | R | R | L amygdala and hippocampus | 3 |
| 9 | 62 | F | R | R | L | R inferior temporal gyrus including calcified lesion | 48 |
| 10 | 50 | F | L | R | R | L mesial temporal | 45 |
| 11 | 43 | M | L | R | R | L amygdala | 24 |
| 12 | 19 | M | L | R | R | L hippocampus | 18 |
| 13 | 43 | F | R | R | L | R amygdala and hippocampus | 9 |
Figure 2.
Somatotopy of percepts for a representative subject. S06 anatomic distribution of percepts (self-reported) after stimulation testing. A. Grid placement (red dots) with the central sulcus illustrated (black dotted line) to highlight the location of primary somatosensory and motor cortices. Although some electrodes appear to cross into primary motor cortex, the percepts were somatosensory only, with no motor involvement. The 3D rendering of the grid locations does not perfectly capture the actual locations due to software estimates and possible grid migration between imaging (post-operative day 1) and testing (post-operative day 6 or 7). B. Hand regions where sensation was reported for bipolar stimulation of a given electrode pair matched to the grid layout by color. All percepts were purely sensory.
Experimental Design
Grid mapping was performed by an epileptologist using a Grass Technologies 212X Cortical Stimulator (Natus Neurology Incorporated, Warwick, RI, USA). Bipolar stimulation was delivered using charge-balanced biphasic waveforms, with alternating anodic- or cathodic-first polarity, to electrode pairs, with constant frequency (50Hz), pulse-width (250μs), and pulse duration (4s). Stimulation amplitude (0.5-12mA) was varied at the discretion of the epileptologist. Stimulation began at low amplitudes and continued with incrementally increasing amplitudes until either sensation and/or muscle activity was noted, or the epileptologist felt that a higher stimulation was not safe given electrocorticographic changes or clinical judgement. At locations deemed to have no activity, amplitude was raised to 2mA above the highest amplitude that produced activity at the other locations that produced somatosensory percepts (e.g. 4mA triggered sensory percepts at one location, all locations without activity were stimulated to 6mA). For the purposes of this study, once a safe and effective amplitude was established for the patient, it remained the same throughout testing.
Analysis
Percepts at each stimulation location were classified as sensory, motor, mixed or none. Subjects provided a description of the sensation and reported the dermatomal location of the sensation according to a hand map with 48 anatomically distinct boxes (herein referred to as simply, ”box” or “boxes”), described in Lee et al.16 (see Figure 2). If somatosensation appeared in any of the 48 boxes, the entire box was included. In areas of somatosensation, the pairs were repeated to assess stability and only included as S1 stimulation if the percept was consistent on three repeat stimulations. When assessing electrode pairs, not all combinations were included due to limited time in the ICU, however the entire grid was included, with all electrodes being included in at least one pair.
To assess utility for a BCI system, total surface area was calculated, as well as two concepts to capture the spatial dynamics of the evoked percepts. “Redundancy” was calculated as the number of electrodes that stimulated each dermatomal box and thereby not allowing for a functional differentiation between the two electrode pairs. “Resolution” was calculated based on the number of boxes stimulated by each electrode, to indicate whether each electrode pair was capturing a small or large portion of the surface area of the hand. Bipolar pairs of electrodes were used for stimulation, each pair is referred to as one “electrode”. Chi squared test was used for comparison, using MATLAB (The Mathworks, Natick, MA, USA).
Results:
Somatosensory Cortex Localization
Grid location in relation to the somatosensory cortex was determined by co-registration of a post-operative computed tomography scan and a pre-operative magnetic resonance imaging scan. 3D reconstructions of grid placement were created using Freesurfer and Statistical Parametric Mapping software SPM12 using the img_pipe package described in Hamilton et al.23 with MATLAB software.
The anatomical locations of the elicited percepts were found to be consistent with Penfield’s homunculus24. For grids where discrete digit representation was found, D5 was represented in the most superior aspect of the grid with D4, D3, D2, and D1 percepts seen in electrode pairs moving inferiorly down the grid, as illustrated in Figure 2b. 8 of the 13 subjects had more than one discrete digit represented, and this relationship held true for those 8 subjects.
Grid Coverage
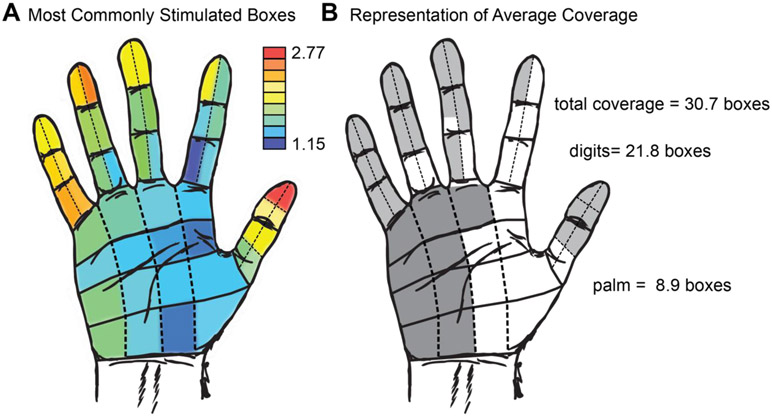
On average, grids covered 63.9% of the surface area of the hand, characterized as 30.7 boxes per grid (see Figure 3). Of these, 21.8 boxes were in the digits, representing 68.1% of possible digit coverage, and 8.9 boxes were in the palm, representing 55.3% of possible palm coverage. Broken down by specific dermatomal area, the most commonly stimulated area was the tip of the thumb (2.77 electrodes per grid), and the palm near the thumb was the least commonly stimulated area (1.15 electrodes per grid). Finger tips were more frequently stimulated than any other part of the hand (see Figure 3b for a complete breakdown by region). Surface area coverage across all subjects ranged from 2 to 48 boxes. The patient with the lowest coverage was S05, with just two boxes included. The highest coverage was all 48 boxes, seen in S07, S09, S10 and S13.
Figure 3.
Surface area coverage of the grids across all subjects. A. Heatmap illustrating the number of electrodes that illicit a percept in a given box averaged over all subjects. The tip of the thumb had the most representation per grid, while the palm beneath the thumb had the least. B. Surface area coverage per subject, on average. The mean number of boxes for digits and palm are shaded with the most commonly represented sites. Digits were more commonly stimulated than the palm. Lighter grey represents digits and darker grey represents the palm.
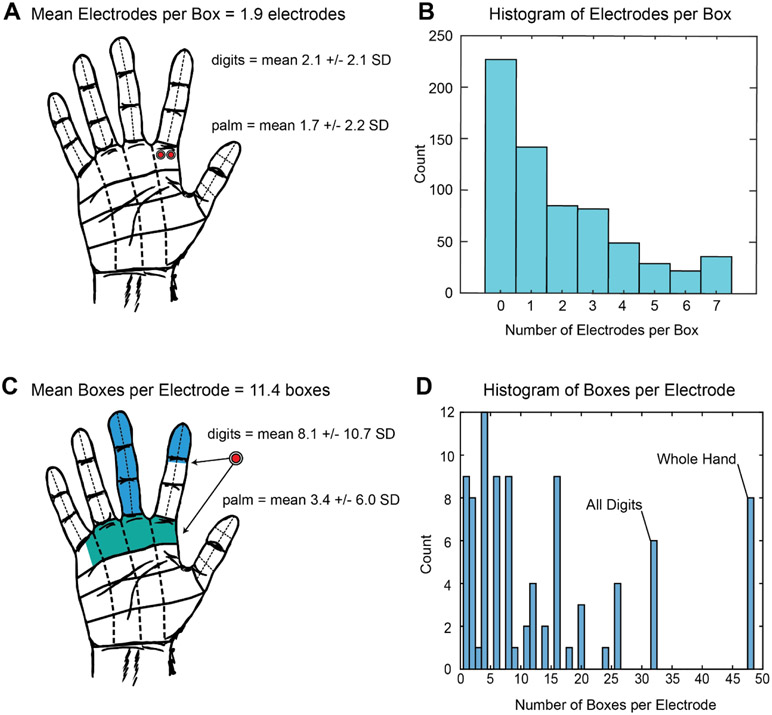
“Redundancy” was evaluated as the number of electrodes per grid that stimulate the same box (Figure 4a). The mean number of electrodes that stimulated a given box for the whole hand was 1.9 ± 2.2 (mean ± s.d.) electrodes. In the digits there was an average of 2.1 ± 2.1 electrodes per box, and in the palm 1.7 ± 2.2 electrodes per box. There was no statistical difference in redundancy between the digits than the palm (p = 0.052, χ2 test). Most electrodes on the grids did not stimulate any dermatomal areas of the hand. Among electrodes on which stimulation evoked percepts, most stimulated just one box. The highest number of electrodes stimulating a single box was seven.
Figure 4. Measures of “redundancy” and “resolution”.
A. Representation of mean number of electrodes that stimulate a given dermatomal box (redundancy). Mean number of electrodes stimulating each box was 1.9 (+/− 2.2 SD) total, 2.1 for digits boxes and 1.7 for palm boxes. B. A histogram shows that most electrodes did not stimulate any boxes, but of those that did, the mode was one electrode per box. C. Representation of mean boxes that a given electrode provided sensation to, the “resolution”. The mean number of boxes stimulated by an electrode was 11.4 (+/−13.7 SD), divided into 8.1 digit boxes and 3.4 palm boxes. D. A histogram demonstrates the mode being four boxes per electrode. Electrodes that caused sensation across all digits or the whole hand covered 32 and 48 boxes, respectively, and were relatively frequent.
“Resolution” was calculated as the number of boxes with somatosensory percepts elicited by each electrode (Figure 4c). The mean number of boxes per electrode was 11.4 ± 13.7, with 8.1 ± 10.7 boxes in the digits, and 3.4 ± 6.0 boxes in the palm. Electrodes were more likely to stimulate the digits than the palm (p < 0.05, χ2 test). Results were partially skewed by electrodes that stimulated the whole hand (48 boxes); or electrodes that stimulated all of the digits (32 boxes) as well. The mode was four boxes per electrode (Figure 4d).
Functionality for BCI System
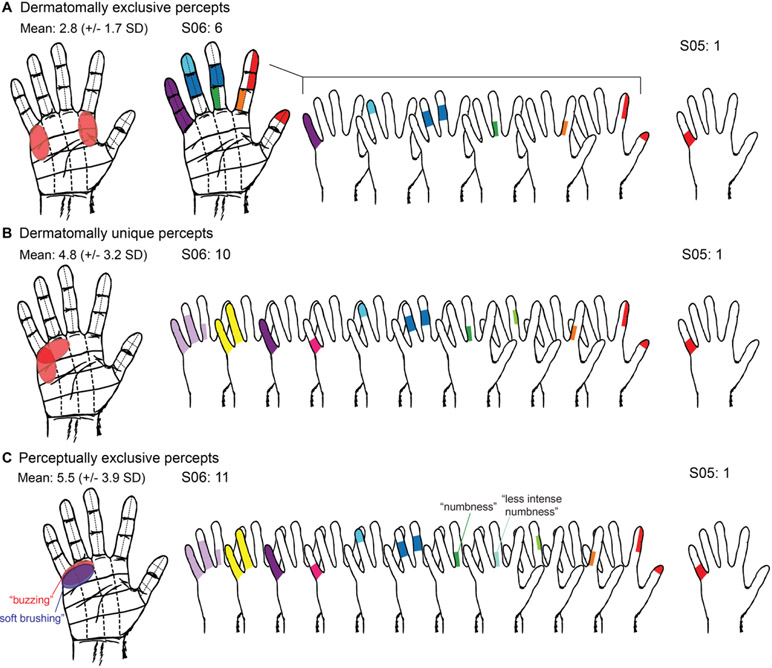
To assess the usefulness of the evoked percepts for a BCI system, receptive fields were evaluated under three different classification paradigms. Each unique receptive field would equate to an extra “degree of freedom” for coverage of the hand. For instance, if two unique areas of the hand can be differentiated through stimulation, then two degrees of freedom are available. The most conservative paradigm was “dermatomally exclusive” receptive fields (Figure 5a). Dermatomally exclusive receptive fields were defined as evoked sensations covering a unique set of boxes, without any dermatomal overlap with another set of evoked sensations. The subjects with the highest number of dermatomally exclusive receptive fields were S06 and S13, each with six dermatomally exclusive receptive fields. S05 had the fewest dermatomally exclusive receptive fields with one. The mean number of dermatomally exclusive receptive fields across all 13 grids was 2.8 ± 1.7 fields.
Figure 5.
Usable percepts, or “degrees of freedom” based on dermatomally exclusive somatosensory percepts. A. Representation of dermatomally exclusive receptive fields, in which percepts were not allowed to include a portion of any other percept. On average, 2.8 boxes were noted per grid. A subject with a high number of dermatomally exclusive receptive fields was S06, shown for illustration. This subject had 5 dermatomally exclusive receptive fields, each represented by one color. The subject with the least number of dermatomally exclusive receptive fields was S05, with only one dermatomally exclusive receptive field. B. Representation of dermatomally unique receptive fields, which may have some overlap of boxes, but not complete overlap. A mean of 4.8 per grid was noted. A representative subject with a high number of dermatomally unique receptive fields is S06, with 10 dermatomally unique receptive fields, each represented by one color. The subject with the least number of dermatomally unique receptive fields was S05, with only one dermatomally unique receptive field. C. Representation of perceptually exclusive percepts, where completely overlapping boxes that had distinguishable sensations were included. Overall, a mean of 5.5 were noted per grid. A subject with a high number of perceptually exclusive receptive fields is S06, with 11 perceptually exclusive percepts fields, each represented by one color. Digit 3 at the base is represented twice with two different percepts that were stable and distinguishable on repeat stimulations. The subject with the least number of perceptually exclusive percepts was S05, with only one perceptually exclusive percept.
The second most conservative classification of receptive fields was “dermatomally unique” receptive fields, shown in Figure 5b. Dermatomally unique receptive fields were allowed overlap in the dermatomal coverage so long as there were some unique boxes (and therefore would be discernable to the subject). The subject with the highest number of dermatomally unique receptive fields was S13, with 12 fields. The subject with the fewest dermatomally unique receptive fields was S05, with one field. The mean number of dermatomally unique receptive fields across all 13 grids was 4.8 ± 3.2 fields.
The final category, and least conservative, was “perceptually exclusive” (Figure 5c). Here, any percepts identified as unique over repeated testing, regardless of the dermatomal overlap, were included. For example, “buzzing” versus “soft brushing” in the same set of boxes would be two perceptually exclusive percepts. The subject with the highest number of perceptually exclusive percepts was S13, with 15 fields. The subject with the fewest perceptually exclusive percepts was S05, with one field. The mean number of perceptually exclusive percepts across all 13 grids was 5.5 ± 3.9 fields (Supplementary Table 1).
Discussion:
Here we studied the utility of 64-contact mECoG grids for the delivery of stimulation to S1 to elicit somatosensory percepts in 13 human subjects. Our results here and previously16 contribute to the growing body of evidence in support of safe and effective cortical stimulation to evoke perceptual sensations to distinct dermatomes. On average across subjects, mECoG grids covered 63.9% of the hand, with a range of 4% to 100%. Redundancy was low with most dermatomal “boxes” being stimulated by one electrode; resolution was high with most electrodes stimulating 4 boxes (the equivalent of 2/3rds of a finger or an edge of the palm; Figure 4). Three classifications of usable percepts were used to estimate degrees of freedom available for a sensory BCI system. We estimate the average degrees of freedom ranges from 2.8 - 5.5, depending on how distinct the percepts need to be.
Our results followed the basic homuncular topographic organization in S1 described by Penfield24. A more recent study from Roux et al., detailed the somatosensory homunculus in 50 operative patients undergoing awake electrical stimulation. The medial-to-lateral, little-finger-to-thumb, and rostral-to-caudal distribution of somatosensation in the hand was consistently observed across patients25. For the eight patients where multiple discrete digits were found, the little-finger-to-thumb, medial-to-lateral layout of somatosensation across S1 was observed. Each grid covered the majority of the surface area of the hand, with more coverage of the digits than the palm. The dermatomal boxes over the thumb tip were the most commonly stimulated site, and the tips of the digits were more commonly represented (Figure 3a), which is likely more useful for closed-loop BCI. Good representation of the palm was also observed. Ten out of 13 subjects had four out of five digits represented. Nine out of 13 subjects had some part of the palm represented. Even without feedback about the location or quality of the sensations, O’Doherty et al. found that NHPs operating a closed-loop BCI with S1 stimulation exhibited improved motor performance2. With specific information from digits and the palm, one would expect performance to improve considerably.
We evaluated percepts based on measures of “redundancy” and “resolution” to assess the efficiency of stimulation through mECoG. Redundancy was estimated by describing the number of electrodes that stimulated the same box. The mean was fairly low at 1.9 electrodes per box (Figure 4). Most electrodes stimulated 0 boxes. For the electrodes that did evoke somatosensory percepts, the mode was 1 electrode per box. This result suggests that the spacing of the electrodes is well matched to the underlying spatial architecture of hand dermatomes in S1. A prior analysis of microelectrode arrays implanted in human showed this same measure as 11.2 electrodes per box, showing a high degree of redundancy15,18. Mean resolution, described by the number of boxes stimulated per electrode, was high at 11.4 (out of 48 possible), but the mode was just 4, suggesting this result is skewed by electrodes that evoked sensation in the whole hand, or in all of the digits. Discounting these few electrodes, stimulation through mECoG grids yields a reasonably functional resolution. In contrast, a standard ECoG grid (1 cm center-to-center spacing) showed an average of 19.1 boxes stimulated by each electrode pair18. Comparing ECoG and microelectrode array results to the present study highlights the balance in resolution and redundancy achieved with the mECoG array. We have previously used these metrics to suggest that the mECoG is superior in both resolution and redundancy to either standard ECoG spacing, or microelectrode arrays. Here, in a larger series, we report low redundancy and high resolution when compared to a microelectrode array or a standard ECoG grid.
Electrodes that elicit whole hand or whole digit percepts are an interesting phenomenon that we have described previously16, but have not been explicitly observed or described in further ECoG studies13,16, during intraoperative cortical stimulation25, or with micro-electrode grids15,22. This may be due to the location, as an electrode pair that spans a sulcus might spread to more cortex as it travels down the sulcus. Alternatively, some areas of cortex may have more broad connections than others, resulting in a spread that activates multiple digits at the same time. S1 is organized into functionally distinct layers (area 3a, 3b, 1, and 2)26, and electrodes may activate these areas with different anatomic spreads. Regardless, whole hand and whole digit responses from an electrode pair would have a certain utility in somatosensory BCI; it would be easier to stimulate a single electrode pair to enlist all digits than to stimulate simultaneously through multiple pairs.
Degrees of freedom of stimulation was evaluated based on three different classification methods: dermatomally exclusive (no overlap), dermatomally unique (some overlap), and perceptually exclusive (complete overlap, different percept). The lowest number of unique percepts was 1, and the highest was 15. Overall, these findings suggest a BCI system with mECoG stimulation could achieve a high level of spatial differentiation, albeit individually there was a considerable degree of variability as evidenced by the large standard deviations. Furthermore, the number of unique percepts would likely expand rapidly with the addition of other changes to the stimulation parameters. This study did not explore varying combinations of frequency, amplitude, pulse-width, and duration of stimulation—all of which could potentially add to the available degrees of freedom. A somatosensory BCI system could combine variations across several parameters and offer reasonable degrees of freedom. Similarly, we did not address the range of percepts felt by participants in this study. We have previously reported that the percepts were generally an “electric” or “buzzing” feeling, and varied primarily in intensity with changes in amplitude, frequency, and pulse-width16. In this study, our most generous interpretation of available degrees of freedom included these variations in perception that occurred exclusively from different electrodes, but by altering percepts using different stimulation parameters, greater variety can be achieved. Similarly, with a better understanding of how different percepts are encoded in the cortex27, and therefore able to be reproduced or approximated, it will allow for a wider range of available parameters. Of course, the mECoG itself is only one option, and systems designed specifically for this purpose may aim for more electrodes, with closer spacing, and alternative shapes28-30.
Limitations
Several limitations to this study exist. The grid was placed without direct visualization of S1, which potentially led to suboptimal placement and could explain why some subjects had more hand coverage and overall more useful receptive fields. A somatosensory BCI implant would presumably be done with direct visualization of S1. Additionally, the ability to confirm optimal placement with awake intraoperative stimulation would be a useful step to optimize grid placement. Stimulation was performed at the discretion of an epileptologist to minimize the risk of adverse events. Therefore, while stimulation parameters were consistent within a single patient, they were not consistent between subjects. In addition, not all combinations of electrodes were tested in each grid, owing to time constraints in the ICU setting and the discretion of the epileptologist. These testing conditions may have limited the number of percepts that were observed for each patient. The constraints on stimulation parameters, including discrete options (e.g. frequency of 60 Hz and 100 Hz, but not 70 Hz), and square wave pulses may have reduced the possible percepts or dermatomal regions reachable with the grid. Future work will be necessary to test the effects of more complex stimulation parameters. Testing only occurred on a single day and do not speak to the stability between sessions, let alone over time. The dermatomal grid spacing used was in keeping with prior literature15,16, but may over or underestimate the true dermatomal perceptions.
Conclusion:
We used a high-density, mECoG device implanted over S1 to evaluate stimulation evoked sensation in the hand. We observed percepts following consistent somatotopy over a useful surface area, with good representation of the digits. Low redundancy and good resolution were observed. The degrees of freedom available vary widely, but suggest a reasonable topographic representation of the hand, albeit incomplete. Going forward, high-density ECoG is a viable modality for generating artificial sensation in a somatosensory BCI system.
Supplementary Material
Acknowledgments
We wish to acknowledge the generous support of Cal-BRAIN: A Neurotechnology Program for California, National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health (KL2TR001854), National Institutes of Health (R25 NS099008-01), The Neurosurgery Research and Education Foundation (NREF), the Tianqiao and Chrissy Chen Brain-machine Interface Center at Caltech, the Boswell Foundation and the Della Martin Foundation.
Footnotes
Publisher's Disclaimer: Accepted Manuscript is “the version of the article accepted for publication including all changes made as a result of the peer review process, and which may also include the addition to the article by IOP Publishing of a header, an article ID, a cover sheet and/or an ‘Accepted Manuscript’ watermark, but excluding any other editing, typesetting or other changes made by IOP Publishing and/or its licensors”
References
- 1.Lee B, Liu CY & Apuzzo MLJ A primer on brain-machine interfaces, concepts, and technology: A key element in the future of functional neurorestoration. World Neurosurg 79, 457–471 (2013). [DOI] [PubMed] [Google Scholar]
- 2.O’Doherty JE et al. Active tactile exploration using a brain-machine-brain interface. Nature 479, 228–231 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SP et al. Point-and-click cursor control with an intracortical neural interface system by humans with tetraplegia. IEEE Trans. Neural Syst. Rehabil. Eng 19, 193–203 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen RA & Cui H Intention, Action Planning, and Decision Making in Parietal-Frontal Circuits. Neuron 63, 568–583 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Hochberg LR et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442, 164 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Andersen RA, Musallam S & Pesaran B Selecting the signals for a brain-machine interface. Curr. Opin. Neurobiol 14, 720–726 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Andersen RA, Burdick JW, Musallam S, Pesaran B & Cham JG Cognitive neural prosthetics. Trends Cogn. Sci 8, 486–493 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Cramer SC, Lastra L, Lacourse MG & Cohen MJ Brain motor system function after chronic, complete spinal cord injury. Brain 128, 2941–2950 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Kikkert S et al. Revealing the neural fingerprints of a missing hand. Elife 5, 1–19 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sainburg RL, Poizner H & Ghez C Loss of proprioception produces deficits in interjoint coordination. J. Neurophysiol 70, 2136–2147 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klaes C et al. A cognitive neuroprosthetic that uses cortical stimulation for somatosensory feedback. J. Neural Eng 11, 056024 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabot GA et al. Restoring the sense of touch with a prosthetic hand through a brain interface. Proc. Natl. Acad. Sci. U. S. A 110, 18279–84 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson LA et al. Direct electrical stimulation of the somatosensory cortex in humans using electrocorticography electrodes: A qualitative and quantitative report. J. Neural Eng 10, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiremath SV et al. Human perception of electrical stimulation on the surface of somatosensory cortex. PLoS One 12, e0176020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flesher SN et al. Intracortical microstimulation of human somatosensory cortex Intracortical microstimulation of human somatosensory cortex. Sci. Transl. Med 1–11 (2016). doi: 10.1126/scitranslmed.aaf8083 [DOI] [PubMed] [Google Scholar]
- 16.Lee B et al. Engineering Artificial Somatosensation Through Cortical Stimulation in Humans. 12, 1–11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen RA, Kellis S, Klaes C & Aflalo T Toward more versatile and intuitive cortical brain-machine interfaces. Curr. Biol 24, R885–R897 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer DR et al. Technical considerations for generating somatosensation via cortical stimulation in a closed-loop sensory/motor brain-computer interface system in humans. J. Clin. Neurosci (2019). doi: 10.1016/j.jocn.2019.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosking WH, Beauchamp MS & Yoshor D Electrical Stimulation of Visual Cortex: Relevance for the Development of Visual Cortical Prosthetics. Annu. Rev. Vis. Sci 3, annurev-vision-111815-114525 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christie BP, Ashmont KR, House PA & Greger B Approaches to a cortical vision prosthesis: Implications of electrode size and placement. J. Neural Eng 13, (2016). [DOI] [PubMed] [Google Scholar]
- 21.Normann RA et al. Toward the development of a cortically based visual neuroprosthesis. J. Neural Eng 6, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armenta Salas M et al. Proprioceptive and cutaneous sensations in humans elicited by intracortical microstimulation. Elife 7, 1–11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton LS, Chang DL, Lee MB & Chang EF Semi-automated Anatomical Labeling and Inter-subject Warping of High-Density Intracranial Recording Electrodes in Electrocorticography. Front. Neuroinform. 11, 62 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penfield W & Boldrey E Somatic Motor and Sensory Representation in. Brain A J. Neurol 60, 389–443 (1937). [Google Scholar]
- 25.Roux F, Durand J, Taylor J & Carson R Functional architecture of the somatosensory homunculus detected by electrostimulation. 5, 941–956 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaas JH The functional organization of somatosensory cortex in primates. Ann. Anat 175, 509–18 (1993). [DOI] [PubMed] [Google Scholar]
- 27.Kramer DR et al. Electrocorticographic changes in field potentials following natural somatosensory percepts in humans. Exp. brain Res 237, 1155–1167 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson LJ et al. A novel high electrode count spike recording array using an 81,920 pixel transimpedance amplifier-based imaging chip. J. Neurosci. Methods 205, 223–32 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Kellis S et al. Multi-scale analysis of neural activity in humans: Implications for microscale electrocorticography. Clin. Neurophysiol 127, 591–601 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Escabí MA et al. A high-density, high-channel count, multiplexed μECoG array for auditory-cortex recordings. J. Neurophysiol 112, 1566–83 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.