Abstract
The lamina cribrosa is the initial site of glaucomatous injury. Pathological changes to the lamina cribrosa include posterior displacement of the lamina cribrosa, loss of trophic support, and remodeling of the extracellular matrix. Optic nerve head (ONH) astrocytes and lamina cribrosa cells synthesize extracellular matrix proteins to support and maintain the lamina cribrosa under physiological conditions. During glaucoma, these cells respond to mechanical strain and other stimuli, which leads to pathological remodeling of the ONH. Although ONH astrocytes and lamina cribrosa cells have been previously cultured, there is no well-accepted, straightforward technique to isolate both cell types from a single dissected human ONH. To better understand the pathophysiology of glaucoma, we obtained and cultured lamina cribrosa explants from human donor eyes. Initially, cells that grew out from the explant were ONH astrocytes and lamina cribrosa cells. Using a specialized medium, we isolated pure populations of lamina cribrosa cells and ONH astrocytes. ONH astrocytes expressed glial fibrillary acidic protein (GFAP). Lamina cribrosa cells expressed alpha-smooth muscle actin (α-SMA), but were negative for GFAP. This method of ONH cell isolation and cell-culture will provide a technique to better understand the molecular and cell-specific changes in glaucomatous damage to the ONH.
Keywords: Human ONH, astrocytes, lamina cribrosa cells, explant
1. Introduction
The optic nerve head (ONH) is an opening in the posterior sclera, where more than a million unmyelinated retinal ganglion cell axons converge at the optic disc and exit the eye to form the optic nerve. Histological analysis of the ONH shows distinct regional differences recognized as the surface nerve fiber layer, pre-laminar, lamina cribrosa, and post-laminar myelinated optic nerve. For all types of glaucoma, the primary site of injury is the lamina cribrosa region of the ONH (Quigley and Addicks, 1981; Quigley et al., 1983). Cells in the lamina cribrosa actively respond to physiological and pathophysiological conditions; therefore, isolating and culturing these cells may help understand normal lamina cribrosa function and glaucoma pathophysiology.
Cells of the human ONH include five cell types: astrocytes, lamina cribrosa cells, microglia, vascular endothelial cells, and vascular pericytes (Hernandez, 2000; Hernandez et al., 1988). The lamina cribrosa explant yields two primary cell types, ONH astrocytes and lamina cribrosa cells (Hernandez et al., 1988). ONH astrocytes are glial fibrillary acidic protein (GFAP) positive and the major glial cell type found throughout the ONH, which mechanically supports and provides neurotrophic support to retinal ganglion cell axons (Hernandez et al., 2008; Lambert et al., 2001; Tovar-Vidales et al., 2016; Vecino et al., 2016; Yang and Hernandez, 2003). Lamina cribrosa cells are broad, polygonal-shaped cells, localized within and between connective tissue plates in the lamina cribrosa (Hernandez et al., 1988; Tovar-Vidales et al., 2016). Lamina cribrosa cells express alpha-smooth muscle actin (α-SMA), and similar to ONH astrocytes, these cells synthesize extracellular matrix proteins that form the cribriform network (Hernandez et al., 1988; Lambert et al., 2001; Zode et al., 2011). Glaucomatous ONH astrocytes and lamina cribrosa cells show increased expression of growth factors and fibrotic genes; these differences are likely responsible for the fibrotic remodeling of the glaucomatous lamina cribrosa (Hernandez, 2000; Hernandez et al., 2002; Kirwan et al., 2009; Pena et al., 1999; Schneider and Fuchshofer, 2016; Wallace and O’Brien, 2016; Zode et al., 2011).
Our lab aims to gain a better understanding of the role of ONH cells and remodeling of the extracellular matrix. Previous methods have been described to isolate astrocytes and lamina cribrosa cells from human ONH tissue (Hernandez et al., 1988; Lambert et al., 2001; Rogers et al., 2012a; Rogers et al., 2012b; Yang and Hernandez, 2003). Here, we describe a modified method where both lamina cribrosa cells and ONH astrocytes can be isolated from a single ONH explant from human donor eyes. We characterize lamina cribrosa cells and ONH astrocytes by cell morphology, intracellular, and extracellular matrix markers.
2. Materials and Supplies
2.1. Human donor eyes
Human donor eyes without a history of ocular or neurodegenerative diseases were obtained within 24 hours of death from the Lions Eye Institute for Transplant and Research (Tampa, FL).
2.2. Equipment for dissection of the eye
NUNC cell culture petri dishes (100×21mm; Thermo Fisher Scientific, USA; Cat # 172931)
NUNC cell culture treated flasks with filter caps (T-25; Thermo Fisher Scientific, USA; Cat #150628)
NUNC treated 12 well cell culture multi-dish (Thermo Fisher Scientific, USA; Cat #136196)
NUNC 15mL conical tube (Thermo Fisher Scientific, USA; Cat # 339651)
Eppendorf Centrifuge 5810R 15 amp (Eppendorf, NY; Cat # 022625501)
Olympus 1.7mL microtubes (Genesee Scientific, USA; Cat # 24–282)
1000uL reach barrier tips (Genesee Scientific, USA; Cat # 24–430)
10mL serological pipets (Genesee Scientific, USA; Cat # 12–104)
Surgical scissors (Fine science tools, USA; Cat # 14002–16)
Fine scissors (Fine science tools, USA; Cat # 14106–09)
Graefe curve forceps (Fine science tools, USA; Cat # 11051–10)
Graefe straight forceps (Fine science tools, USA; Cat # 11050–10)
Wescott spring scissors (Fine science tools, USA; Cat # 15015–11)
Vannas spring scissors (Fine science tools, USA; Cat # 15000–10)
Surgical knife (Surgical Specialties Corporation; Cat # 72–2201)
AirClean Systems ductless microscope enclosure (AC648TMIC)
SMZ-800 Zoom Stereo Microscope System (Nikon Instruments, Inc, USA; Cat# MNA41000) that includes the following components: (Binocular tube (MNB42100); Widefield Eyepiece (MMK30102); Plan Achromat Objective (MNH43100), Plain stand (MMD31000), Beam splitter (MNB45901); LV-TV Tube for Ti3 (MBB63430); Cover type 104-lab/OptE400/E600/SMZ (MXA22061); C-NI-150 Fiber optic light source with heat filament 115V (83365); C-dual gooseneck light pipes (83371); C-focusing lens for gooseneck light pipes (83373); DS-L2 Camera control unit (MQA21010); DSVi1 color digital camera head (MQA120–10), DS camera I/F cable (MQF11000); AC adapter for 55iLED microscope (MQF52055); power cord (79035); C-0.7x DXM relay lens (MQD42070); DS-L2 Deluxe support kit (97049); (Nikon Instruments, Inc, USA)
Gauze sponges (Fisher Scientific, USA; Cat # 22–037-902)
2.3. Chemicals and reagents for cell culture
Betadine (Thermo Fisher Scientific, USA; Cat # 19–027136)
Nutrient mixture Ham’s F-10 (Sigma-Aldrich, St Louis, MO; Cat # N6013)
Astrocyte basal medium complete kit (ScienCell Research Laboratories Cat #1801, Carlsbad, CA)
Fetal bovine serum (Atlas Biologicals, USA; Cat # FP-0500-A)
L-glutamine (Thermo Fisher Scientific, USA; Cat # SH3003402)
Penicillin streptomycin (Sigma-Aldrich, St Louis, MO; Cat #P4333)
Dulbecco’s phosphate-buffered saline (PBS; Sigma-Aldrich, St Louis, MO; Cat # D8537)
TrypLE Express (Thermo Fisher Scientific, USA; Cat # 12605028)
Transforming Growth Factor Beta 2 (R&D Systems, Minneapolis, MN; Cat # 302-B2–010)
2.4. Antibodies for immunocytochemistry and western blot (see Table 1)
Table 1.
List of antibodies
| Antibody | Dilution and application | Source | Cat # |
|---|---|---|---|
| Mouse monoclonal α-SMA-FITC conjugated | 1:200 ICC | Sigma | F3777 |
| Rabbit α-SMA | 1:500 WB 1:100 IHC |
Abcam | Ab5694 |
| Mouse monoclonal GFAP | 1:500 WB 1:100 ICC |
Thermo Fisher |
MA5-12023 |
| Mouse monoclonal NCAM | 1:100 ICC | Sigma | C9672 |
| Rabbit s100β | 1:100 ICC | Abcam | Ab52642 |
| Rabbit Laminin | 1:100 ICC | Sigma | L9393 |
| Anti-Mouse HRP | 1:1000 WB | Cell Signalling |
7076S |
| Anti-Rabbit HRP | 1:1000 WB | Cell Signalling |
7074S |
| Donkey Anti Mouse 594 | 1:200 ICC | Invitrogen | A-21207 |
| Donkey Anti- Rabbit 488 | 1:200 ICC | Invitrogen | A-21206 |
| Mouse monoclonal GFAP | 1:100 IHC | NeoMarkers | Ab-6 |
ICC=immunocytochemistry, IHC=immunohistochemistry, WB=western blot
3. Detailed Methods
3.1. Human eye donor source, time of enucleation and age
Human donor eyes were obtained from the Lions Eye Institute for Transplant and Research (Tampa, Florida) within 24 hours of death. All donor eyes obtained were negative for the human immunodeficiency virus 1 and 2, hepatitis B virus, and the hepatitis C virus. The eyes were obtained and managed in compliance with the Declaration of Helsinki. The human eyes used for each experiment ranged from 56 to 99 years old.
3.2. Dissection of the lamina cribrosa from human tissue
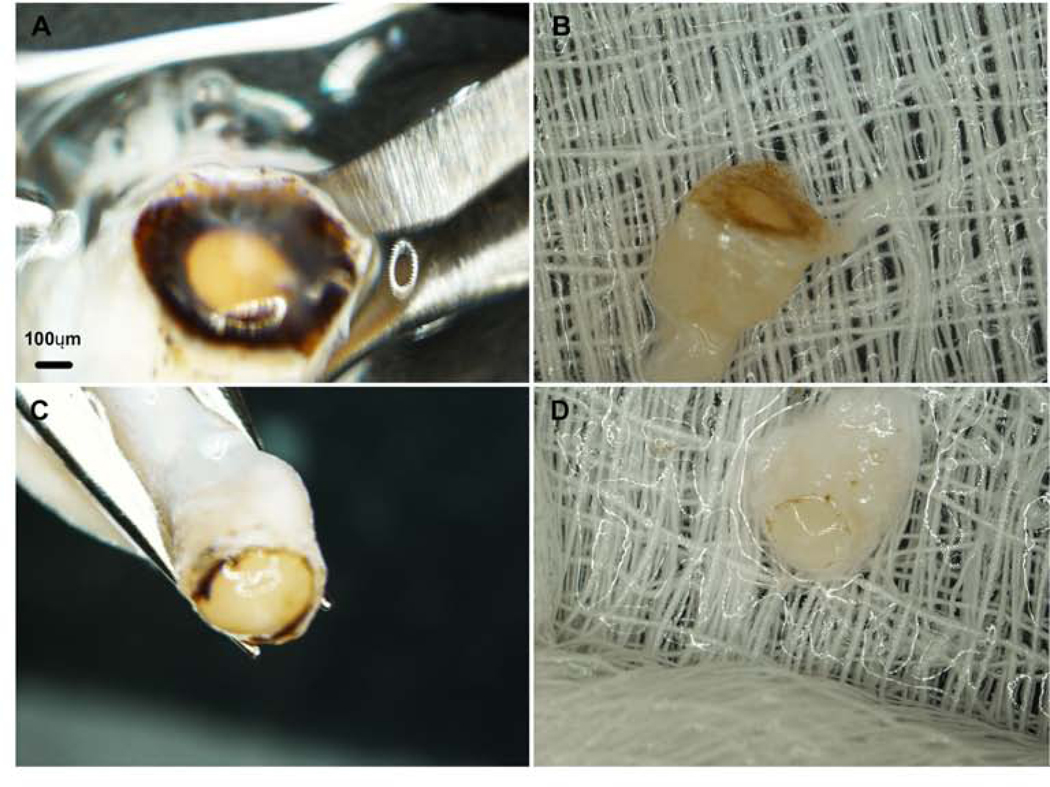
Eye globes were sterilized in a specimen container with Betadine (Thermo Fisher Scientific, USA) for 1 minute and then submerged in PBS into a new sterile container. Next, the straight Graefe forcep was used to transfer the eye from the PBS specimen container into a 10cm petri dish lined with a PBS-moist mesh gauze (Figure 2 A). Extraneous tissue from the eye globe was removed by using both the Graefe forceps and surgical scissors. A surgical knife was used to make a small incision into the sclera at the equator and surgical scissors were used to bisect the eye (Figure 2 B). The posterior eye segment was secured with straight Graefe forceps with one hand, and with the other hand, the curved Graefe forceps were used to remove the vitreous, retinal pigment epithelium and choroid from the posterior segment of the eye (Figure 2 C–D). With the posterior segment facing down (Figure 2B), the sheath surrounding the optic nerve should be gently grasped and removed using the surgical scissors and dissected up to the scleral canal surrounding the ONH. As the posterior segment faces up, grasp the stalk of the optic nerve with the Graefe forceps, and use the surgical scissors to trim up to the peripapillary sclera. Under a dissecting microscope, the peripapillary sclera should be removed using the Wescott spring scissors and the prelaminar region of the ONH delicately trimmed using the Vannas spring scissors (Figure 1 and Figure 3 B and D). At this point, use the fine or Westcott spring scissors to carefully dissect the lamina cribrosa region of the ONH, approximately 1mm down and section into 2 or 3 segments for explant cell culture. If desired, section the optic nerve, and culture the explant in ABM with supplements to culture optic nerve astrocytes. We did not separately culture the prelaminar region, which should contain both type 1A and type 1B astrocytes. We choose to specifically look at the lamina cribrosa since this tissue is remodeled during glaucomatous optic neuropathy.
Figure 2. Dissection of the human ONH.
Dissection images of the human ONH. (A) A whole human eye globe, (B) the posterior segment with the lens and vitreous humour removed, (C) lastly, the choroid and RPE are removed from the posterior segment of the human eye, leaving the scleral shell, optic nerve head, and the optic nerve (D).
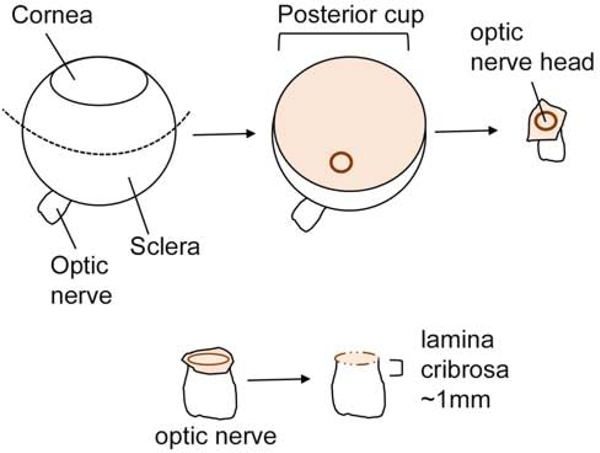
Figure 1. Schematic of eye dissection.
A schematic diagram illustrating the different steps involved in ONH dissections from a human eye. An incision at the equator is made to remove the posterior segment of the eye, the scleral of the ONH up to the scleral canal is removed, removal of the peripapillary sclera and scleral canal. Dotted line: the equator.
Figure 3. Light microscopy images of the ONH with the optic nerve.
Progressive removal of the RPE and peripapillary sclera from the ONH. (A-B) Peripapillary sclera with pigment surrounding the ONH, (C-D) removal of the peripapillary sclera with the pigment is trimmed. Scale bar: 100μm. Note: Pigment remains are visible for identification of the ONH, but was removed prior to dissection.
3.3. Tissue culture of the lamina cribrosa explant
The dissected explants should be placed into a 12-well plate without medium for approximately 1–2 minutes to allow adhesion to the well. Ham’s F-10 (500μL-1000μL) growth media, containing10% FBS L-glutamine (0.292 mg/ml), and penicillin (100units/ml)/ streptomycin (0.1mg/ml) was used to incubate the explants in a humidified chamber at 37°C in 5% CO2. ONH cell outgrowth and migration from the explant can take up to 3–4 weeks. The medium was replaced once per week until cells migrated out of the explant; after that, the medium was changed every 2–3 days. We recommend leaving approximately 10–20% of the conditioned medium in the wells when changing medium to ensure cells have constant contact with growth factors that were secreted into the culture medium.
3.4. Culturing ONH astrocytes and lamina cribrosa cells
To obtain both ONH astrocytes and lamina cribrosa cells from the same explant, we cultured the explant in Ham’s F-10 medium supplemented with 10% FBS L-glutamine (0.292 mg/ml), penicillin (100units/ml)/streptomycin (0.1mg/ml) in a 12-well culture plate. Cell confluency takes about 8–12 weeks in a single well. Initially, cells were passaged with TrypLE Express reagent and diluted 1:2 ratio (passage 1) into a new 12-well culture plate. One well in the 12-well plate was used to maintain lamina cribrosa cells in Ham’s F-10 medium with 10% FBS and supplements. The other well was used to obtain ONH astrocytes. For astrocyte isolation, the trypsinized cells were resuspended and cultured in astrocyte basal medium (ABM) without FBS for 72 hours, and then switched to ABM with 5% FBS and supplements. Note, this step is essential to isolate astrocytes since lamina cribrosa cells fail to attach in serum-free conditions (Lambert et al., 2001; Yu et al., 2008). Cells were grown to confluency and the passage protocol repeated in their respective mediums until cells can be expanded into a T25 or T75 flask. We then used Cytodex 3 microcarrier beads instead of trypsin for subsequent cell passage. We have observed that Cytodex 3 microcarrier beads allow these cells to be carried to about 8 passages. We recommend continuing the passaging of cells until these cells begin to become senescent or unhealthy. Cells serially passaged with trypsin become senescent more quickly at lower passages. We generally perform experiments on cells from passages 3–6. Be sure to cryopreserve early passage numbers of each cell strain.
3.5. Characterization of ONH cells using western blot
Whole cell lysates were collected from cultured ONH astrocytes and lamina cribrosa cells using MPER lysis buffer with Halt protease inhibitor cocktail (Pierce Biotech, Rockford, IL). The protein concentration was determined using the BioRad Dc Protein Assay kit (Bio-Rad Laboratories; Hercules, CA) according to manufacturers’ instructions. After protein estimation, 15–30μg total protein from each sample was loaded and separated on an SDS-PAGE denaturing 10% acrylamide gel, and then electrophoretically transferred onto a polyvinylidene difluoride (PVDF) membrane. PVDF membranes were subsequently blocked with 5% non-fat dry milk in Tris-Buffered Saline, 0.1% Tween-20 (TBST) for 1 hour at room temperature, and then incubated with specific primary antibodies (Table 1) at 4°C on a rotating shaker overnight. The membranes were washed three times using TBST and then incubated with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 hour on a rotating shaker at room temperature. The signal was detected using a chemiluminescence substrate and blots were exposed to an imager (Bio-Rad, USA).
3.6. Characterisation of ONH cells using immunocytochemistry
To document the presence of GFAP, α-SMA, NCAM, s100β, and laminin in human ONH cells, ONH astrocytes and lamina cribrosa cells were cultured on round glass coverslips in 24 well plates until confluent. ONH cells were fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) and permeabilized with 0.2% Triton X-100 (Fisher Scientific, Pittsburgh, PA, USA) in PBS at room temperature. Donkey serum in superblock PBS was used for 1 hour to prevent non-specific binding of the antibody. The cells were incubated with or without the primary antibody (negative control) overnight at 4°C. After the incubation period, the cells were washed three times with PBS and then incubated with the appropriate fluorescent conjugated secondary antibody for 1 hour in dark conditions at room temperature. The cells were washed three times with PBS, followed by two quick H2O rinses. Coverslips were mounted to glass slides with mounting medium containing 4’6’diamino-2-phenylindole (DAPI; Prolong with DAPI; Invitrogen Molecular Probes) to stain nuclei. Slides were kept in dark conditions and dried overnight at room temperature before imaging using the Nikon Eclipse TieU Microscope (Melville, NY) containing the Nuance FX imaging system (CRI Burlington, MA).
3.7. Localization of ONH cells using immunohistochemistry
Immunocytochemistry details for ONH tissues have been published previously (Tovar-Vidales et al., 2016). Human ONH tissues were fixed and immunostained with α-SMA and GFAP, followed by the appropriate secondary antibodies. Immunofluorescence images were taken using a Zeiss 410 confocal imaging system (Carl Zeiss, Thornwood, NY).
4. Results
4.1. Primary cell culture from the ONH
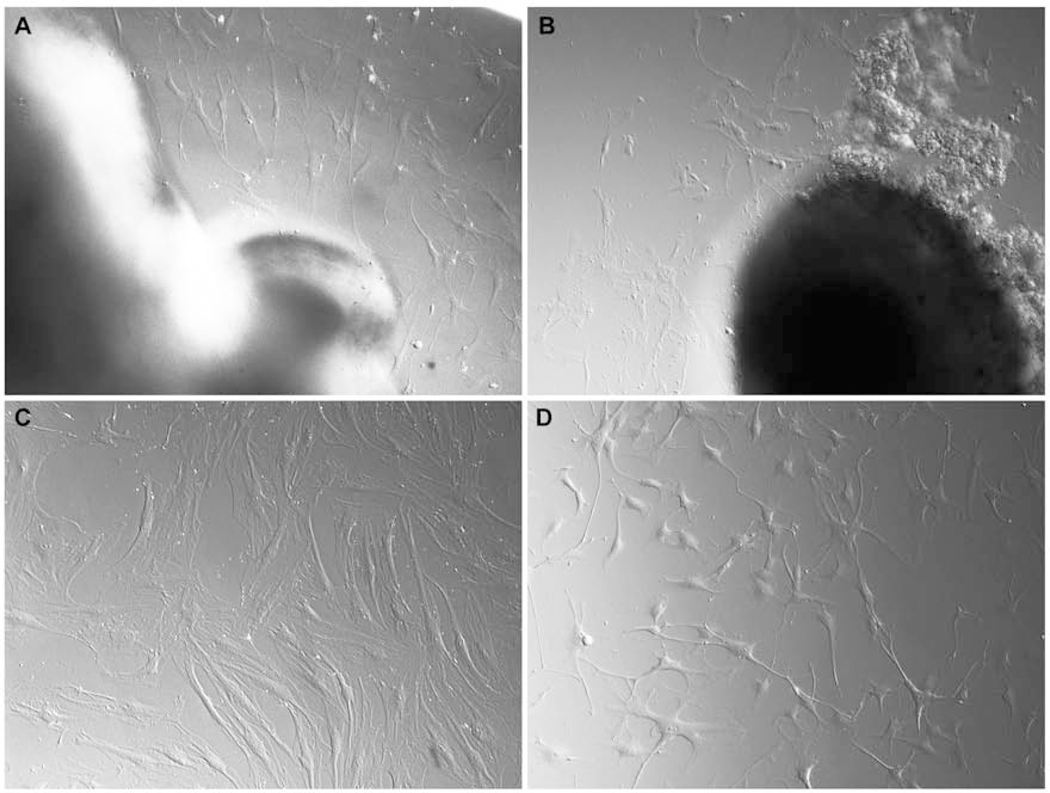
Initial cell migration from the lamina cribrosa explant appeared after approximately three weeks in culture. Cells grew as a monolayer surrounding the lamina cribrosa explant (Figures 4 A–B). Cells were a mixed population of ONH astrocytes and lamina cribrosa cells with few to multiple processes. After passage 1, we cultured the cells in either Ham’s F-10 medium for optimal growth of lamina cribrosa cells or ABM for astrocytes, as described in the detailed methods. Lamina cribrosa cells appeared flat, polygonal-shaped with few processes, and grew as a confluent monolayer (Figure 4C). ONH astrocytes have several long thin processes with star-shaped morphology and do not grow as a confluent monolayer; however, as soon as cell processes contact neighboring processes, then cell proliferation ceases (Figure 4D). It is important to note that these cells cultured were not microglia cells. Microglia cells have a different morphology, do not express GFAP, and are difficult to isolate and grow under the conditions we used (Hernandez, 2000).
Figure 4. Morphology of human lamina cribrosa explant and migrating cells.
(A-B) ONH cells migrating from lamina cribrosa explant using Hoffman modulus optics (Nikon, Inc.), (C) lamina cribrosa cells appeared as flat, broad shaped cells, (D) ONH astrocytes had several processes with star shape morphology.
4.2. Characterization of lamina cribrosa cells and ONH astrocytes
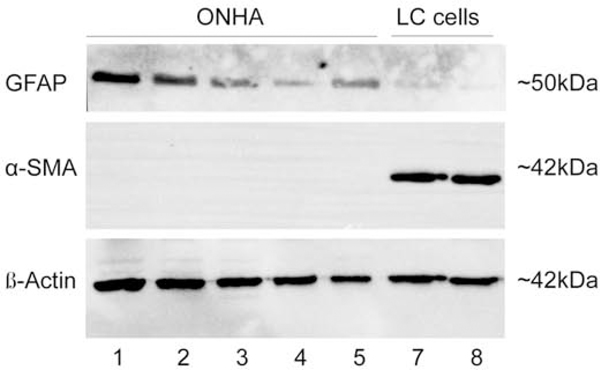
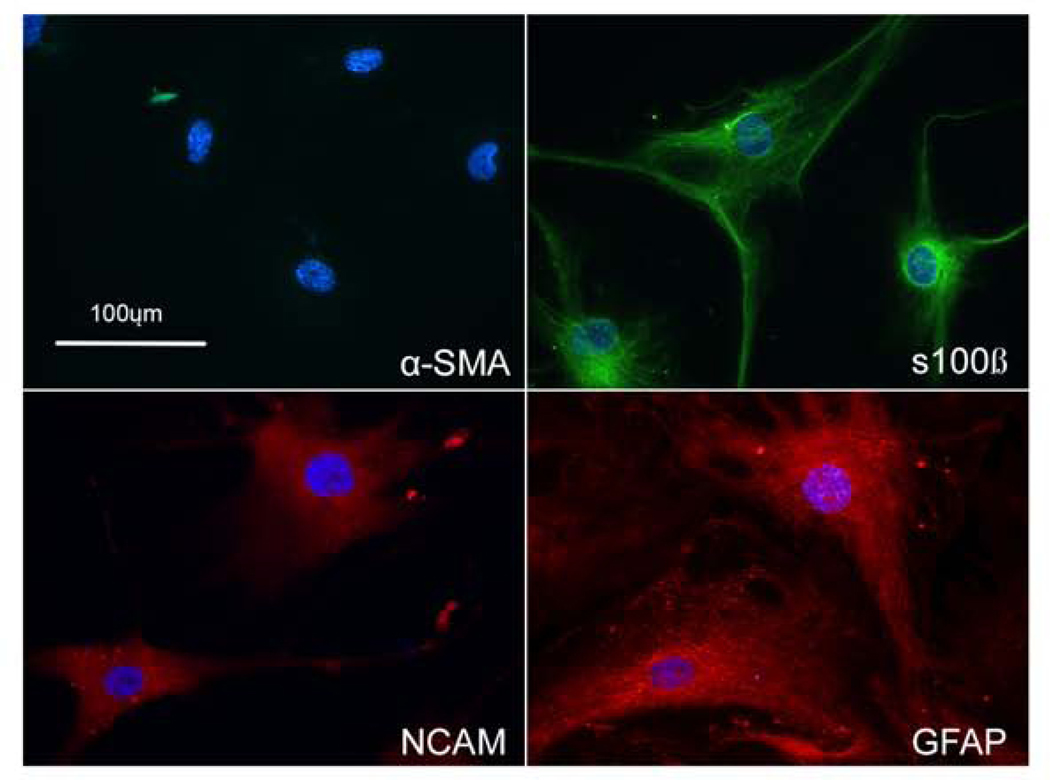
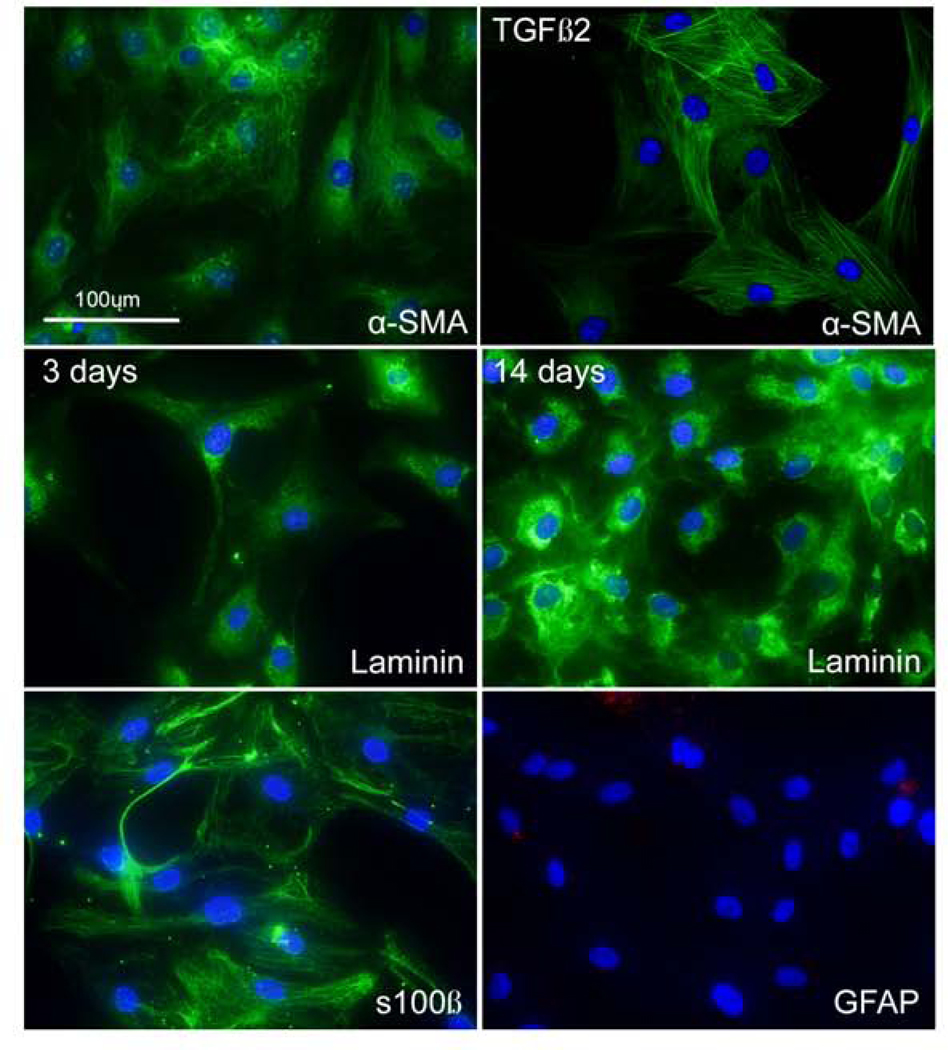
Western blot analysis (Figure 5) and immunofluorescent staining (Figure 6 –7) using cellular markers were used to characterize the ONH cells. ONH astrocytes stained for the intermediate filament marker GFAP, NCAM, s100β (Figure 6), but were negative for α-SMA. In contrast, lamina cribrosa cells were positive for α-SMA, NCAM (data not shown), s100β, but negative for GFAP (Figure 7). Treatment with TGF-β2 resulted in more pronounced α-SMA fibers compared to control treated lamina cribrosa cells (Figure 7). The vast majority of ONH astrocytes stained GFAP positive and the vast majority of lamina cribrosa cells were α-SMA positive (i.e. nearly all of the DAPI stained lamina cribrosa cells were α-SMA positive and DAPI stained ONH astrocytes were GFAP positive). Lamina cribrosa cells were positive for laminin, confirming they were not scleral fibroblasts. Scleral fibroblasts do not express the basement membrane proteins laminin or collagen type IV, have a long spindle-shaped morphology, and generally grow in multiple layers (Clark et al., 1995; Hernandez et al., 1987; Hernandez et al., 1991). Lamina cribrosa cells grow as a monolayer and are considered as a unique cell type within the lamina cribrosa (Hernandez, 2000).
Figure 5. Western blot image of GFAP and α-SMA in ONH cells.
(Lanes 1–5) ONH astrocytes were positive for GFAP and negative for α-SMA. In contrast, lamina cribrosa cells (Lanes 7–8) were negative for GFAP and positive for α-SMA. β-actin was used as a loading control.
Figure 6. Characterization of ONH astrocytes.
Immunostaining of in ONH astrocytes for GFAP, s100β, and NCAM, and α-SMA. ONH astrocytes expressed GFAP, s100β, and NCAM; however, were negative for α-SMA. Nuclei (blue) were stained with DAPI. Negative control consisted of PBS-BSA without primary antibody (data not shown) (Scale bar: 100μm)
Figure 7. Characterization of lamina cribrosa cells.
Immunostaining of lamina cribrosa cells for α-SMA, s100β, laminin, and GFAP. Lamina cribrosa cells treated with or without TGF-β2 expressed α-SMA. Also, lamina cribrosa cells expressed s100β, laminin; however, they were negative for GFAP. Nuclei (blue) were stained with DAPI. The Negative control consisted of PBS-BSA without the primary antibody (data not shown). (Scale bar: 100μm)
4.3. Localization of lamina cribrosa cells and ONH astrocytes in human ONH tissue
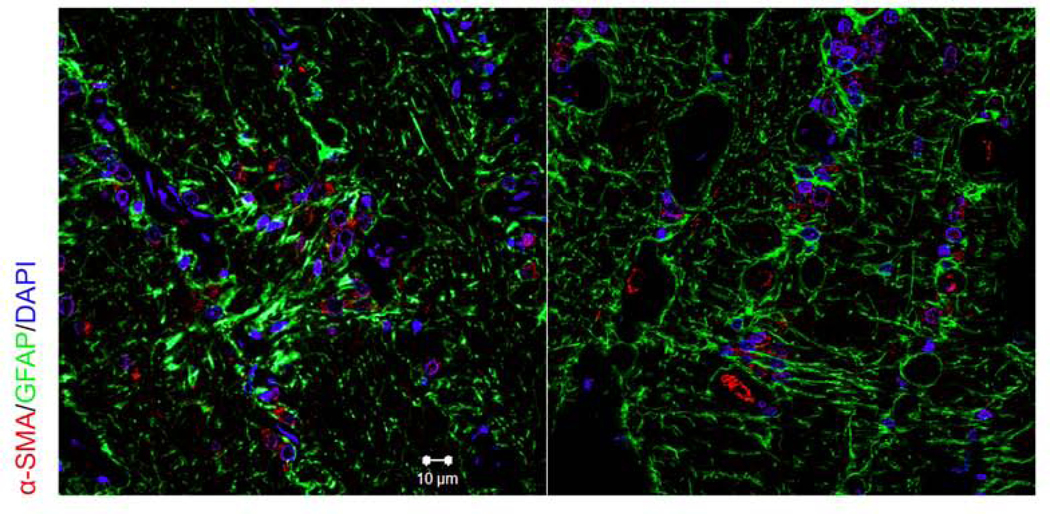
We used immunofluorescent staining of human donor eyes to identify ONH astrocytes and lamina cribrosa cells within the lamina cribrosa (Figure 8). We used antibodies against α-SMA (red) and GFAP (green) to show expression and localization of GFAP positive ONH astrocytes and α-SMA positive lamina cribrosa cells. As previously described, lamina cribrosa cells were localized within lamina cribrosa plates, while ONH astrocytes were present within the lamina cribrosa and their processes seem to surround RGC axon bundles (Hernandez, 2000; Tovar-Vidales et al., 2016). Nuclei of ONH cells were visualized by DAPI.
Figure 8. Immunohistochemical localization of GFAP and α-SMA in the human ONH.
ONH tissues were fixed, sectioned and stained with antibodies for GFAP and α-SMA. Immunohistochemical analysis shows the presence of α-SMA positive lamina cribrosa cells and GFAP positive astrocytes within the lamina cribrosa region. Nuclei (blue) was stained with DAPI. The negative controls consisted of PBS-BSA without primary antibody, IgG, and mouse ascites (data not shown). (Scale bar: 10μm)
4.4. Summary of ONH cell characterization (See Table 2)
Table 2.
ONH astrocytes isolated from lamina cribrosa expressed GFAP, NCAM, and s100β, but were negative for α-SMA. Lamina cribrosa cells expressed α-SMA, laminin, and s100β, but were negative for GFAP.
| Intracellular/extracellular marker | ONH astrocytes | Lamina Cribrosa cell |
|---|---|---|
| α-SMA | − | + |
| GFAP | + | − |
| NCAM | + | + |
| s100β | + | + |
| Laminin | + (Clark et al., 1995; Lambert et al., 2001) |
+ |
Previously, our laboratory has shown that both ONH astrocytes and lamina cribrosa cells express laminin (Clark et al., 1995; Lambert et al., 2001). We have summarized the markers used to characterize these two ONH cell types in Table 2.
5. Potential Pitfalls and Trouble Shooting
5.1. Presence of scleral fibroblasts
Fibroblast contamination is avoided by careful dissection of the lamina cribrosa explant. This procedure involves the removal of the scleral tissue, pigment, and nerve sheath surrounding the ONH. Incomplete removal of scleral tissue may result in scleral fibroblast proliferation from the explant. We recommend distinguishing between lamina cribrosa cells and scleral fibroblasts by using cell morphology (i.e. scleral fibroblasts grow in multiple layers) and immunocytochemical analysis of collagen type IV or laminin (Clark et al., 1995; Hernandez et al., 1987; Hernandez et al., 1991).
5.2. Location of the lamina cribrosa
Determining the location of the lamina cribrosa may be challenging. Nerve fibers at the posterior margins (post laminar region) of the lamina cribrosa are myelinated and increase in thickness; therefore, we recommend using the unmyelinated and myelinated regions of the optic nerve as a landmark for dissection. We recommend making the posterior cut approximately 1mm down from the anterior surface of the ONH before the increased thickness of the myelinated region (Figure 1).
5.3. Passage number and isolation of ONH astrocytes
Choosing the correct passage to culture ONH astrocytes can influence the growth and proliferation of astrocytes. At passages 2–3, there is a co-culture of lamina cribrosa cells and ONH astrocytes; at later passages of 4 or more, the ability to isolate ONH astrocytes declines.
5.4. Adherence of the lamina cribrosa explant
Try to be certain that the lamina cribrosa explant pieces adhere to the plate before the addition of medium so that the explants do not float in the medium. We recommend not to add more than 1mL of medium to the 12-well culture plate and avoid any disturbances while moving the plate. The explant must adhere to the culture dish to increase the likelihood of cell migration from the explant. The explant may need to be cultured for up to 4 weeks in vitro to generate ONH cell outgrowth. It may help to cut the explant into 2–3 segments before culturing or use a collagenase enzyme to digest the collagen extracellular matrix to enhance cell migration from the explant. We have not used collagenase to digest the ONH explant; however, other researchers were successful in using collagenase to dissociate cells from the trabecular meshwork explants (Keller et al., 2018; Stamer et al., 1995).
5.5. Type 1B astrocytes
Astrocytes can be categorized into subclasses within the ONH: type 1 (A and B) and type 2. Type 1 astrocytes are located in the pre-laminar region and lamina cribrosa, whereas type 2 astrocytes are located in the myelinated optic nerve. Type 1 B is the major astrocyte subtype present in the lamina cribrosa and are NCAM positive, while type 1 A astrocytes are NCAM negative.
5.6. Age of donor
Age of human donor eyes may have implications in cell isolation. The infant lamina cribrosa is composed mostly of collagen type III, in contrast to the adult lamina, which is mostly collagen type I, suggesting that the lamina cribrosa is not fully developed and organized (Hernandez et al., 1991; Morrison et al., 1989). The margins may not be evident in infant lamina cribrosa, and there could be contamination from other cell types. We recommend obtaining eyes from donors > 1 year.
5.7. Time of enucleation
The time of death to culture can influence the growth rate and yield of cells from the explant. The use of eyes enucleated and received within 24 hours of death is recommended for increased recovery of viable cells. However, we have successfully cultured cells from lamina cribrosa explants up to 72 hours post enucleation.
6. Discussion
The lamina cribrosa is a region of dense fibroelastic connective tissue, forming the border between the intraocular and retrobulbar tissues. Retinal ganglion cell axons traverse this region from a relatively high pressure to a low-pressure environment. The lamina cribrosa is the initial site of glaucomatous damage- the earliest detectable change is compression of connective tissue plates (Quigley et al., 1983). Isolating cells that populate this region will increase our understanding of cellular and extracellular matrix changes that occur in glaucoma.
Previous methods have been described isolating human, rat, and porcine ONH astrocytes using immunopanning or astrocyte selective medium (Lukas and Wang, 2012; Murphy et al., 2011; Obazawa et al., 2004; Rogers et al., 2012b; Yang and Hernandez, 2003; Yu et al., 2008; Yu et al., 2007). Techniques to isolate human ONH astrocytes have been published from other research laboratories. Previously, investigators have isolated ONH astrocytes from lamina cribrosa cells by using serum-free astrocyte growth medium (Rogers et al., 2012b; Yu et al., 2007). Yang and Hernandez have previously cultured ONH explants for up to four weeks and selected ONH astrocytes by immunopanning using specific antibodies to remove non-astrocytes (Yang and Hernandez, 2003).
Our lab has developed a modified technique using a selective medium to isolate both lamina cribrosa cells and ONH astrocytes from a single lamina cribrosa explant from human donor eyes. Initially, cells that grew from the explant were a co-culture of ONH astrocytes and lamina cribrosa cells. For ONH astrocytes, we serum-deprive co-cultures to isolate astrocytes from non-adhered lamina cribrosa cells. Researchers have shown that ONH astrocytes can be isolated from lamina cribrosa cells after trypsinization and placed in astrocyte serum-free medium for 24 hours from a co-culture consisting of ONH astrocytes and lamina cribrosa cells (Yu et al., 2008). To ensure complete removal of lamina cribrosa cells, we have used serum-deprivation for up to 72 hours and then ONH astrocytes were placed in astrocyte medium with 5% FBS. The 72-hour time point was determined empirically as providing the best astrocyte yield without lamina cribrosa cell contamination. The ability to isolate ONH astrocytes decreases as the co-culture cell passage increases. We recommend isolating ONH astrocytes no later than passage 3 from ONH co-cultures. Lambert and co-workers used high serum (10%) Ham’s F-10 Nutrient Mixture to isolate lamina cribrosa cells from ONH astrocytes (Lambert et al., 2001). Passaging cells a few times may be necessary to remove all ONH astrocytes to obtain a pure lamina cribrosa cell population.
We used both western blot and immunocytochemistry to characterize ONH cells using markers previously described (Hernandez et al., 1988; Lambert et al., 2001; Rogers et al., 2012a; Rogers et al., 2012b; Yang and Hernandez, 2003). Astrocytes isolated from the lamina cribrosa expressed GFAP, but were negative for α-SMA. In contrast, lamina cribrosa cells expressed α-SMA, but were negative for GFAP (Table 2). ONH astrocytes also expressed NCAM, suggesting these cells are type 1B astrocytes (Kobayashi et al., 1997; Ricard et al., 1999).
With this cell culture isolation technique, we can study cell-specific changes that occur during disease pathophysiology, such as glaucoma. Primary open-angle glaucoma is associated with elevated intraocular pressure. Ocular hypertension may lead to mechanical deformation of the ONH, including compression and stretching. Increased pressure within the eye may directly affect pressure-sensitive cells in the ONH, including ONH astrocytes and lamina cribrosa cells. Using this technique, we can study gene expression changes and activated pathways in response to glaucomatous conditions such as mechanical stretching or activated growth factors.
ONH astrocytes are the focus of many studies because they are the main glial cell type in the ONH. ONH astrocytes provide structural and trophic support to RGC axons, as well as communicate with the surrounding extracellular matrix, tissues, cells, and vasculature. Although ONH astrocytes have many normal homeostatic functions, they also are responsible for many pathological changes in the glaucomatous ONH (Hernandez, 2000; Hernandez et al., 2002; Hernandez et al., 2008; Schneider and Fuchshofer, 2016). Several investigators have utilized biomechanical strain to mimic the effects of pressure induced strain on ONH astrocytes in order to identify early cellular events/changes in glaucoma (Rogers et al., 2012b). Lamina cribrosa cells also respond to stretch and investigators have used the Flexercell system to induce cyclical strain and evaluate changes in gene expression (Kirwan et al., 2005; Rogers et al., 2012a). Key upregulated pathways in response to strain are transforming growth factor-beta (TGF-β) and extracellular matrix synthesis (Kirwan et al., 2005; Quill et al., 2011; Rogers et al., 2012a; Rogers et al., 2012b). The pro-fibrotic cytokines TGF-β2 and gremlin are elevated in the glaucomatous ONH and promote extracellular matrix synthesis (Pena et al., 1999; Zode et al., 2009; Zode et al., 2011). Our lab has previously used recombinant TGF-β2 protein to replicate endogenous conditions for glaucoma in cultured ONH cells to determine the effects on extracellular matrix proteins (Zode et al., 2011). These techniques and experimental approaches will allow more scientists to isolate both ONH astrocytes and lamina cribrosa cells to improve our understanding of cell-specific responses to glaucomatous conditions and potential treatments. For example, we have used ONH cells isolated from the human lamina cribrosa to identify the expression and secretion of neurotrophins and trk receptors, suggesting they may provide neurotrophic support for RGC neurons (Lambert et al., 2001). Also, we have shown using isolated ONH astrocytes and lamina cribrosa cells that TGF-β2 and the bone morphogenetic protein antagonist gremlin is implicated in the pathology of the glaucoma ONH (Zode et al., 2009; Zode et al., 2011).
7. Conclusions
The lamina cribrosa is progressively remodeled in glaucoma. Cells within the lamina cribrosa may be responsible for this pathological remodeling. Two major cell types have been identified in the lamina cribrosa: GFAP positive ONH astrocytes and α-SMA positive lamina cribrosa cells. There may be cell specific changes during glaucoma pathology; therefore, isolating and culturing these cell types may help understand the cellular and molecular changes that occur in glaucoma.
Highlights.
Dissection of human optic nerve head (ONH)
A technique to isolate human ONH astrocytes and lamina cribrosa cells from a single ONH explant
Characterization of ONH cells
Acknowledgments
The authors would like to thank the Lions Eye Institute for Transplant and Research. This research was supported by the NIH training grant T32 AG 020494 (NL).
Footnotes
Navita N. Lopez designed experiments, analyzed data, and wrote the paper. Tara Tovar-Vidales and Abbot F. Clark helped design experiments and assisted in the manuscript preparation. All authors discussed the results and implications, and commented on the manuscript at all stages.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Clark A, Browder SL, Steely HT, Wilson K, Cantu-Crouch D, McCartney MD, 1995. Cell biology of the human lamina cribosa. Optic Nerve in Glaucoma, 79–105. [Google Scholar]
- Hernandez MR, 2000. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res 19, 297–321. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Agapova OA, Yang P, Salvador-Silva M, Ricard CS, Aoi S, 2002. Differential gene expression in astrocytes from human normal and glaucomatous optic nerve head analyzed by cDNA microarray. Glia 38, 45–64. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Igoe F, Neufeld AH, 1988. Cell culture of the human lamina cribrosa. Invest Ophthalmol Vis Sci 29, 78–89. [PubMed] [Google Scholar]
- Hernandez MR, Luo XX, Igoe F, Neufeld AH, 1987. Extracellular matrix of the human lamina cribrosa. Am J Ophthalmol 104, 567–576. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Miao H, Lukas T, 2008. Astrocytes in glaucomatous optic neuropathy, in: Nucci C, Cerulli L, Osborne NN, Bagetta G (Eds.), Progress in Brain Research. Elsevier, pp. 353–373. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Wang N, Hanley NM, Neufeld AH, 1991. Localization of collagen types I and IV mRNAs in human optic nerve head by in situ hybridization. Invest Ophthalmol Vis Sci 32, 2169–2177. [PubMed] [Google Scholar]
- Keller KE, Bhattacharya SK, Borrás T, Brunner TM, Chansangpetch S, Clark AF, Dismuke WM, Du Y, Elliott MH, Ethier CR, Faralli JA, Freddo TF, Fuchshofer R, Giovingo M, Gong H, Gonzalez P, Huang A, Johnstone MA, Kaufman PL, Kelley MJ, Knepper PA, Kopczynski CC, Kuchtey JG, Kuchtey RW, Kuehn MH, Lieberman RL, Lin SC, Liton P, Liu Y, Lütjen-Drecoll E, Mao W, Masis-Solano M, McDonnell F, McDowell CM, Overby DR, Pattabiraman PP, Raghunathan VK, Rao PV, Rhee DJ, Chowdhury UR, Russell P, Samples JR, Schwartz D, Stubbs EB, Tamm ER, Tan JC, Toris CB, Torrejon KY, Vranka JA, Wirtz MK, Yorio T, Zhang J, Zode GS, Fautsch MP, Peters DM, Acott TS, Stamer WD, 2018. Consensus recommendations for trabecular meshwork cell isolation, characterization and culture. Experimental eye research 171, 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan RP, Fenerty CH, Crean J, Wordinger RJ, Clark AF, O’Brien CJ, 2005. Influence of cyclical mechanical strain on extracellular matrix gene expression in human lamina cribrosa cells in vitro. Mol Vis 11, 798–810. [PubMed] [Google Scholar]
- Kirwan RP, Wordinger RJ, Clark AF, O’Brien CJ, 2009. Differential global and extra-cellular matrix focused gene expression patterns between normal and glaucomatous human lamina cribrosa cells. Molecular Vision 15, 76–88. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Vidal I, Pena JD, Hernandez MR, 1997. Expression of neural cell adhesion molecule (NCAM) characterizes a subpopulation of type 1 astrocytes in human optic nerve head. Glia 20, 262–273. [DOI] [PubMed] [Google Scholar]
- Lambert W, Agarwal R, Howe W, Clark AF, Wordinger RJ, 2001. Neurotrophin and neurotrophin receptor expression by cells of the human lamina cribrosa. Invest Ophthalmol Vis Sci 42, 2315–2323. [PubMed] [Google Scholar]
- Lukas TJ, Wang AL, 2012. Isolation and culture of astrocytes from the retina and optic nerve. Methods Mol Biol 814, 105–115. [DOI] [PubMed] [Google Scholar]
- Morrison JC, Jerdan JA, Dorman ME, Quigley HA, 1989. Structural Proteins of the Neonatal and Adult Lamina Cribrosa. JAMA Ophthalmology 107, 1220–1224. [DOI] [PubMed] [Google Scholar]
- Murphy JA, Archibald ML, Baldridge WH, Chauhan BC, 2011. Endothelin-1induced proliferation is reduced and Ca(2)(+) signaling is enhanced in endothelin Bdeficient optic nerve head astrocytes. Invest Ophthalmol Vis Sci 52, 7771–7777. [DOI] [PubMed] [Google Scholar]
- Obazawa M, Mashima Y, Sanuki N, Noda S, Kudoh J, Shimizu N, Oguchi Y, Tanaka Y, Iwata T, 2004. Analysis of Porcine Optineurin and Myocilin Expression in Trabecular Meshwork Cells and Astrocytes from Optic Nerve Head. Investigative Ophthalmology & Visual Science 45, 2652–2659. [DOI] [PubMed] [Google Scholar]
- Pena JD, Taylor AW, Ricard CS, Vidal I, Hernandez MR, 1999. Transforming growth factor beta isoforms in human optic nerve heads. Br J Ophthalmol 83, 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, Addicks EM, 1981. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Archives of Ophthalmology 99, 137–143. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Hohman RM, Addicks EM, Massof RW, Green WR, 1983. Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. Am J Ophthalmol 95, 673–691. [DOI] [PubMed] [Google Scholar]
- Quill B, Docherty NG, Clark AF, O’Brien CJ, 2011. The effect of graded cyclic stretching on extracellular matrix-related gene expression profiles in cultured primary human lamina cribrosa cells. Invest Ophthalmol Vis Sci 52, 1908–1915. [DOI] [PubMed] [Google Scholar]
- Ricard CS, Pena JD, Hernandez MR, 1999. Differential expression of neural cell adhesion molecule isoforms in normal and glaucomatous human optic nerve heads. Brain Res Mol Brain Res 74, 69–82. [DOI] [PubMed] [Google Scholar]
- Rogers R, Dharsee M, Ackloo S, Flanagan JG, 2012a. Proteomics analyses of activated human optic nerve head lamina cribrosa cells following biomechanical strain. Invest Ophthalmol Vis Sci 53, 3806–3816. [DOI] [PubMed] [Google Scholar]
- Rogers RS, Dharsee M, Ackloo S, Sivak JM, Flanagan JG, 2012b. Proteomics analyses of human optic nerve head astrocytes following biomechanical strain. Mol Cell Proteomics 11, M111.012302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Fuchshofer R, 2016. The role of astrocytes in optic nerve head fibrosis in glaucoma. Experimental eye research 142, 49–55. [DOI] [PubMed] [Google Scholar]
- Stamer WD, Seftor RE, Williams SK, Samaha HA, Snyder RW, 1995. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Current eye research 14, 611–617. [DOI] [PubMed] [Google Scholar]
- Tovar-Vidales T, Wordinger RJ, Clark AF, 2016. Identification and localization of lamina cribrosa cells in the human optic nerve head. Experimental eye research 147, 94–97. [DOI] [PubMed] [Google Scholar]
- Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC, 2016. Glia–neuron interactions in the mammalian retina. Progress in Retinal and Eye Research 51, 1–40. [DOI] [PubMed] [Google Scholar]
- Wallace DM, O’Brien CJ, 2016. The role of lamina cribrosa cells in optic nerve head fibrosis in glaucoma. Experimental eye research 142, 102–109. [DOI] [PubMed] [Google Scholar]
- Yang P, Hernandez MR, 2003. Purification of astrocytes from adult human optic nerve heads by immunopanning. Brain Res Brain Res Protoc 12, 67–76. [DOI] [PubMed] [Google Scholar]
- Yu AL, Fuchshofer R, Birke M, Kampik A, Bloemendal H, Welge-Lüssen U, 2008. Oxidative Stress and TGF-β2 Increase Heat Shock Protein 27 Expression in Human Optic Nerve Head Astrocytes. Investigative Ophthalmology & Visual Science 49, 5403–5411. [DOI] [PubMed] [Google Scholar]
- Yu AL, Fuchshofer R, Birke M, Priglinger SG, Eibl KH, Kampik A, Bloemendal H, Welge-Lussen U, 2007. Hypoxia/reoxygenation and TGF-β increase αB-crystallin expression in human optic nerve head astrocytes. Experimental eye research 84, 694706. [DOI] [PubMed] [Google Scholar]
- Zode GS, Clark AF, Wordinger RJ, 2009. Bone morphogenetic protein 4 inhibits TGF-beta2 stimulation of extracellular matrix proteins in optic nerve head cells: role of gremlin in ECM modulation. Glia 57, 755–766. [DOI] [PubMed] [Google Scholar]
- Zode GS, Sethi A, Brun-Zinkernagel AM, Chang IF, Clark AF, Wordinger RJ, 2011. Transforming growth factor-beta2 increases extracellular matrix proteins in optic nerve head cells via activation of the Smad signaling pathway. Mol Vis 17, 1745–1758. [PMC free article] [PubMed] [Google Scholar]