Abstract
Background:
Consumption of soy foods has been associated with protection against cardiometabolic disease, but the mechanisms are incompletely understood.
Objective:
We hypothesized that habitual soy food consumption associates with gut microbiome composition, metabolite production, and the interaction between diet, microbiota and metabolites.
Design:
We analyzed dietary soy intake, plasma and stool metabolites, and gut microbiome data from two independent cross-sectional samples of healthy US individuals (N = 75 lean or overweight, and N = 29 obese).
Results:
Habitual soy intake associated with several circulating metabolites. There was a significant interaction between soy intake and gut microbiome composition, as defined by gut enterotype, on metabolites in plasma and stool. Soy consumption associated with reduced systolic blood pressure, but only in a subset of individuals defined by their gut microbiome enterotype, suggesting that responsiveness to soy may be dependent on microbiome composition. Soy intake was associated with differences in specific microbial taxa, including two taxa mapping to genus Dialister and Prevotella which appeared to be suppressed by high soy intake We identified context-dependent effects of these taxa, where presence of Prevotella was associated with higher blood pressure and a worse cardiometabolic profile, but only in the absence of Dialister.
Conclusions:
The gut microbiome is an important intermediate in the interplay between dietary soy intake and systemic metabolism. Consumption of soy foods may shape the microbiome by suppressing specific taxa, and may protect against hypertension only in individuals with soy-responsive microbiota.
Clinical trials registry:
Keywords: Metabolome, Microbiome, Soy, Nutrition, Blood pressure, Cardiometabolic health
Introduction
Recent studies have firmly established the relevance of commensal microbiota to cardiometabolic health [1,2]. Beyond traditional determinants of health and disease, the human gut microbiome is a complex intermediate that interacts bi-directionally with the human host as well as the environment to impact metabolic and immune function [3]. In this new and expanding field, many questions remain to be answered to further our understanding of how the microbiome shapes cardiometabolic health. Diet is known to affect both cardiometabolic health, and microbiome composition [4], and may be mediated by effects on metabolism [5]. Soy food consumption has been associated with reduced blood pressure [6–9], athero-protection [10] and mediation of inflammatory cytokine expression [11,12]. Soy foods contain multiple nutrients that may act through independent mechanisms, including protein, fiber, and phytochemicals [13] including isoflavones (genistein, daidzein and glycitein) [14].
There are at least two potential mechanisms whereby interaction between soy food consumption and the microbiome may lead to metabolic variation. First, the composition of the gut microbiota confers differences in the affinity of the microbiome to metabolize dietary precursors. This has been established in individuals with gut microbiome-dependent differences in their ability to metabolize the isoflavone daidzein to equol [15,16]. Second, the consumption of soy foods exert selective pressure on the microbiota, leading to increases or decreases in the abundance of specific microbial taxa, some of which may have additional roles independent of their ability to metabolize components of soy foods. In support of this mechanism, gut microbiome composition was shown to be altered after a soy-diet intervention [17]. Soy-derived compounds interact with enzymes and pathways that are involved in the metabolism of a wide range of nutrients and drugs [18], suggesting that effects of soy food intake on cardiometabolic health may be mediated directly and indirectly through multiple pathways.
We previously reported that dietary soy intake associates with inflammation and glycemic control in evoked settings [19]. Notably, we observed these effects in US and European-based populations with relatively low soy food intake, equivalent to one portion a week, suggesting that inclusion of even small amounts of soy foods in the diet may have biologically meaningful effects on metabolism. We hypothesized that soy food composition associates with differences in metabolite signaling, in a gut microbiota-dependent manner. We further hypothesized that soy food intake shapes microbiome composition in soy consumers, further modulating systemic metabolism. We probed the relationship between soy food intake, the metabolome and the microbiome in healthy lean, overweight and obese individuals.
Materials and methods
Study population
ABO study
The ABO Glycoproteomics in Platelets and Endothelial Cells (ABO) Study recruited healthy volunteers (men and non-pregnant/lactating women age 18–50) to a single study visit at the University of Pennsylvania from 2012 to 2014, as described previously [20,21]. Briefly, a blood sample was obtained from participants on the morning of their study visit following a 12-h overnight fast, and a subset of individuals provided a stool sample for metagenomic analysis as part of a diet and microbiome-focused sub-study. Data from this cross-sectional microbiome sub-study are presented here (N = 132).
Estimates of dietary soy consumption were extracted from dietary records for analysis in this study. Participants completed 3-day food records prior to the study visit [20], including on the day directly before the visit, and a weekend day. We consider these data to represent short-term intake of soy foods. Nutrient composition was analyzed using Food Processor 8.1 (ESHA Research, Salem, OR). Participants also completed food frequency questionnaires (FFQ) to assess habitual dietary intake, including serving size, of 134 food items over the previous year (the National Cancer Institute’s Diet History Questionnaire, DHQ II) [22]. This included specific questions relating to intake of soy and soy-derived foods (soy beans, soy milk, tofu, soy meat-substitutes, soy supplements). We consider these data to represent long-term soy food intake. Completed responses were analyzed using Diet*Calc version 1.5.1, to convert food data to nutrient intake values. Because the nutrient estimates for the soy isoflavones: genistein, daidzein and glycitein are almost perfectly related to soy food consumption, we used the sum of soy isoflavones as the variable best representing overall soy food intake. We excluded individuals whose responses (for food records or DHQII) were incomplete or implausible (e.g. extremely low overall food intake implying high missingness). Characteristics of the 132 participants with complete dietary records and metabolite and/or stool analyses are shown in Table 1. All individuals provided written informed consent. The study was approved by the Institutional Review Boards of the University of Pennsylvania and Vanderbilt University.
Table 1.
Characteristics of the study participants across the two independent studies.
| ABO Study (N = 132) |
Fair Study (N = 29) |
|
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age (Years) | 29.8 (7.9) | 36.0 (8.7) |
| Sex (M/F) | 50/82 | 9/20 |
| Race (EA/AA/OA) | 75/32/25 | 18/8/3 |
| BMI (kg/m2) | 25.4 (5.4) | 36.1 (5.5) |
| Blood Pressure (mmHg) | 115/69 (11.1/8.2) | 119/71 (9.5/6.8) |
| Short-term diet | ||
| Daidzein (mg/day) | 2.3 (5.8) | – |
| Genistein (mg/day) | 3.1 (7.8) | – |
| Glycitein (mg/day) | 0.58 (1.6) | – |
| Long-term diet | ||
| Daidzein (mg/day) | 1.1 (1.6) | 0.64 (0.5) |
| Genistein (mg/day) | 1.4 (2.3) | 0.68 (0.7) |
| Glycitein (mg/day) | 0.18 (0.3) | 0.08 (0.1) |
EA = European Ancestry, AA = African Ancestry, OA = Other ancestry (Asian, Hispanic, multi-racial).
FAIR study
Diet and metabolite associations identified in the ABO Study were validated in a comparison sample, which had measured habitual diet and global metabolomics using identical methods, in individuals recruited from the same geographical area. The Fish oils and Adipose Inflammation Reduction (FAIR) Study recruited obese individuals (N = 29; men and non-pregnant/lactating women age 25–50, BMI≥30) from the Philadelphia area to a randomized controlled fish oil intervention trial at the University of Pennsylvania from 2014 to 2017 (NCT02010359). Baseline pre-intervention samples and data were analyzed for the current study. Participants provided a fasting blood sample (following a 12-h overnight fast) and completed the National Cancer Institute’s Diet History Questionnaire (DHQ II) [22]. Completed diet responses were analyzed using Diet*Calc version 1.5.1, and estimates for soy isoflavones, genistein, daidzein and glycitein used to represent soy food intake for further analysis. Participant characteristics are shown in Table 1. All participants provided written informed consent. The study was approved by the Institutional Review Board of the University of Pennsylvania.
Biomarker and metabolomics profiling
Plasma C-reactive protein (CRP) in the ABO Study was measured by ELISA (Quantikine CRP Immunoassay, R&D Systems, Minneapolis, MN), in accordance with the manufacturer’s protocol. Metabolomics profiling of plasma and stool samples (N = 75 plasma and stool in the ABO Study, N = 29 plasma from the FAIR Study) was carried out at Metabolon (Metabolon Inc, Morrisville, NC; global metabolomics platform). Briefly, samples were prepared at Metabolon using the automated MicroLab STAR® system (Hamilton Company). Proteins were precipitated with methanol under vigorous shaking for 2 min (Glen Mills GenoGrinder 2000) followed by centrifugation, removal of organic solvent (TurboVap®, Zymark), and sample extracts stored overnight under nitrogen prior to analysis To obtain a global metabolomics profile, samples were analyzed in four aliquots; two separate reverse phase (RP)/UPLC-MS/MS methods with positive ion mode electrospray ionization (ESI), one RP/UPLC-MS/MS with negative ion mode ESI, and one HILIC/UPLC-MS/MS with negative ion mode ESI. Raw data were extracted, peak-identified and QC processed using Metabolon’s hardware and software. Samples for the ABO and FAIR Studies were run in separate batches. Because of variation in platform detection limits for reporting across batches and tissues, not all metabolites were equally detected across both studies. In plasma, 812 metabolites were reported for ABO, and 787 for FAIR. In stool, 770 metabolites were reported. These included several soy metabolites, including daidzein, genistein, and their sulfate derivative. For each metabolite passing thresholds, the raw peak intensity was rescaled to set the median across all samples equal to 1, and values below the limit of detection were imputed with the lowest observed value in the dataset. Metabolite pathway enrichment analysis was conducted using MetaboAnalyst [23].
Sample processing, DNA extraction and sequencing
As previously described [20,21], ABO Study participants collected a stool sample in the 24 h prior to their study visit, using a stool collection kit (Commode Specimen Collection System, Fisher Scientific, Pittsburgh PA), and stored their samples at 4 °C prior to the study visit. Of the 132 individuals studied here, 131 provided stool samples. Aliquots of stool were made within 36 h of sample collection and stored at −80 °C prior to nucleic acid extraction (PSP Spin Stool DNA Plus Kit, Stratec, Germany). The 16S rRNA gene region was amplified using barcoded primers [24] (Eurofins Genomics, Louisville, KY) and DNA libraries were cleaned (MinElute PCR Purification kit, Qiagen, Germantown, MD) prior to quantification and pooling. Pooled DNA libraries were sequenced on the MiSeq platform, 300bp paired-end reads, at an average depth of 158,000 reads/sample (Illumina Inc., San Diego, CA). Samples were sequenced in two batches, at the University of Pennsylvania Next-Generation Sequencing Center (UPenn NGSC, n = 104) and the Vanderbilt University Technologies for Advanced Genomics (VANTAGE, n = 27) Core. As we previously reported [20], there was evidence of a small global difference in microbiome composition by sequencing batch, but this did not change any conclusions of the analysis. There was no significant difference in enterotype distribution by batch (P > 0.6, Chi2 test), or in the abundance of the specific taxa analyzed here (P > 0.1, T-test), and thus data are presented for both batches combined. DNA sequences in Fastq files were de-multiplexed, assembled, clustered, and phylogenetically classified using the Mothur pipeline [25]. Phylogenetic classification was performed against the Silva V123 16S database. Mothur was run using standard cutoffs, creating operational taxonomic unit (OTU) clusters at 97% identity. Enterotypes have been used in microbiome studies as a way to identify major sub-groups of individuals with broadly similar microbiota [4]. To identify microbial enterotypes in our dataset, we conducted distance-based clustering using the Partitioning Around Medoids (PAM) method [26] with various distances including Euclidean, Bray–Curtis and Jaccard, and identified two enterotypes for gut microbiota, Enterotype 1 (n = 53) and Enterotype 2 (n = 78).
Statistical analysis
Descriptive and anthropomorphic measures are reported as mean ± SD. Relationships between soy intake and metabolites (as continuous variables) were assessed by Spearman correlation. Given known inter-relationships between metabolites, we chose to use a nominal significance value of p < 0.05 as suggestive, and used validation approaches where possible to identify biologically relevant associations, rather than applying multiple testing correction. To test the hypothesis that overall gut microbiome composition alters how soy foods are metabolized, we identified relationships between soy and metabolites that differed by gut enterotype. We tested diet–enterotype interaction through linear regression models for each pair of soy isoflavone-metabolite variables, using enterotype as the interaction term, and each metabolite as the outcome. For interactions reaching statistical significance that validated across both independent dietary measures, we generated scatterplots to visually assess individual soy–metabolite associations by enterotype. To test the hypothesis that consumption of soy foods may select for presence or absence of specific bacterial taxa, we identified relationships between soy (as a continuous variable) and bacterial taxa through Spearman correlation analysis. We then dichotomized the sample into low and moderate consumers (based on a cut-point of 3 mg/day total isoflavone, which represents about one serving of soy per week). Differences in microbiome composition by these dichotomized groups were analyzed by Linear discriminant analysis Effect Size (LEfSe) using MicrobiomeAnalyst. Differences in other variables between groups were assessed by independent sample t test, Mann–Whitney test, or by ANOVA, as appropriate. To reduce the likelihood of false positives, we required the diet-gut-metabolite associations to independently reach statistical significance using both the long and short-term estimates of soy isoflavones. This served as a quasi-replication in the absence of a true independent replication sample for microbiome data. Global microbiome data processing and statistical analysis was previously performed in R, as described [20]. Additional analyses and visualizations for this study were conducted using R, MicrobiomeAnalyst [27], IBM SPSS Statistics 25, and GraphPad Prism 7.
Results
Levels of daidzein in plasma and stool reflect recent soy food intake but not long-term habitual intake
Characteristics for ABO and FAIR Study participants are shown in Table 1. Self-reported isoflavone intake (sum of daidzein, genistein and glycitein) across all subjects ranged from 0.00 to 110.3 mg/day for short-term estimates, and 0.04–31.1 mg/day for long-term estimates, and did not differ by sex. Soy food intake from short- and long-term estimates correlated (Spearman correlation, daidzein r = 0.42, genistein r = 0.36, glycitein r = 0.32, all p < 0.0001) in the ABO Study, suggesting that people who consumed soy foods in the days before their study visit were also regular soy food consumers. Plasma daidzein sulfate correlated with short-term intake of daidzein (r = 0.361, p = 0.002), but not with long-term intake (r = 0.152, p = 0.2) in ABO. Similarly, daidzein levels in stool correlated significantly with short term (r = 0.319, p = 0.006) but not long-term (r = 0.222, p = 0.057) daidzein intake. Levels of daidzein in plasma and stool correlated with each other (r = 0.349, p = 0.002). These data suggest that circulating and stool daidzein reflect recent soy food intake, but may not reflect long-term habitual soy consumption.
Dietary soy intake is associated with metabolites in plasma, with significant enrichment for caffeine metabolites
We identified 147 metabolites in the ABO Study (N = 75) with nominal associations with habitual soy intake (p < 0.05 for correlation with at least one of the 3 isoflavones measured). We replicated associations in an independent sample (FAIR Study, N = 29), and identified 8 metabolites that replicated across both studies (Spearman correlation p < 0.05 in ABO and FAIR for at least one isoflavone, association in same direction, Supplement Table 1). These included a negative correlation with the glycemic marker 1,5-Anhydroglucitol, and positive correlations with amino acid metabolites (isobutyrylcarnitine C4, xanthurenate and 2-hydroxyphenylacetate), and several xenobiotics (acesulfame, 1,7-dimethylurate, 5-acetylamino-6-amino-3-methyluracil, 5-acetylamino-6-formylamino-3-methyluracil). These xenobiotics include known caffeine-related metabolites. Enrichment analysis for the soy-linked metabolites confirmed significant enrichment for caffeine metabolism (p = 0.0005, FDR p = 0.05). However, there was a positive correlation between soy food intake and caffeine intake in our sample, suggesting that the enrichment for caffeine metabolites could be confounded by caffeine intake. We assessed the correlations between caffeine intake and metabolites in the ABO and FAIR studies, and identified 8 metabolites that validated across both studies. All were caffeine metabolites, but only one (1,7-dimethylurate) overlapped with those identified as associated with soy. However, given the complex inter-relationships between metabolites in a given pathway, our cross-sectional data cannot disentangle the relative contributions of soy and caffeine. Further studies are required to address the specific contributions of soy and caffeine in xenobiotic metabolism.
Gut microbiome enterotype is a significant modulator of the relationship between dietary soy and plasma and stool metabolites, and with systolic blood pressure
We hypothesized that the gut microbiome modulates the association between dietary soy and metabolites in stool and plasma. We identified two distinct gut enterotypes within participants of the ABO Study, Enterotype 1 (n = 53) and Enterotype 2 (n = 78). We then used enterotype as a composite measure of inter-individual variability in gut microbiome composition. There was no difference in soy intake between the two gut enterotypes, using either short- or long-term estimates, consistent with the hypothesis that consumption of soy foods does not cause broad alterations in microbiota. There were significant interactions by gut enterotype on the association between dietary isoflavones and several plasma and stool metabolites. Using an internal validation approach, which required a significant association using the two independently-surveyed measures of soy food intake (short- and long-term), we identified 15 plasma metabolites and 4 stool metabolites whose association with soy isoflavone intake differed by gut enterotype (interaction p < 0.05 for both long and short-term estimates) (Table 2). There was no significant enrichment in any particular metabolic pathway. However, several metabolites have known relevance to hypertension and diabetes, including 2-hydroxybutyrate, glycine, and liquiritigenin (Fig. 1, A–C). Further, there was evidence for an effect of gut enterotype on the relationship between soy food and blood pressure; individuals in Enterotype 1 had a negative association between increasing soy consumption and systolic blood pressure, but this was not observed in individuals with Enterotype 2 (Fig. 1D).
Table 2.
Metabolites with significant gut-enterotype mediated association with soy intake.
| Metabolites | Short-term diet (p value) | Long-term diet (p Value) | Relationship with increasing soy intake |
|---|---|---|---|
| Plasma | |||
| 2-hydroxybutyrate/2-hydroxyisobutyrate | 0.004 | 0.008 | ↓ Enterotype 1 No association Enterotype 2 |
| 3-methoxycatechol sulfate | 7.7 × 10−5 | 1.0 × 10−5 | ↑ Enterotype 1 No association Enterotype 2 |
| 3-methoxytyrosine | 0.04 | 0.03 | ↑ Enterotype 1 No association Enterotype 2 |
| 3-methylglutaconate | 0.02 | 0.006 | ↑ Enterotype 1 No association Enterotype 2 |
| Gamma-glutamylglycine | 0.01 | 0.001 | ↑ Enterotype 1 No association Enterotype 2 |
| Gamma-glutamylleucine | 0.048 | a0.05 | ↓ Enterotype 1 No association Enterotype 2 |
| Glycine | 0.003 | 0.0005 | ↑ Enterotype 1 ↓ Enterotype 2 |
| Isovalerylcarnitine (C5) | 0.005 | 0.003 | ↓ Enterotype 1 No association Enterotype 2 |
| Leucine | 0.02 | 0.02 | ↓ Enterotype 1 No association Enterotype 2 |
| Myo-inositol | a0.06 | 0.04 | ↓ Enterotype 1 ↑ Enterotype 2 |
| O-acetylhomoserine | 0.004 | 0.004 | ↑ Enterotype 1 No association Enterotype 2 |
| Oxalate (ethanedioate) | 0.02 | 0.008 | ↑ Enterotype 1 No association Enterotype 2 |
| Tartarate | 0.002 | 0.004 | ↑ Enterotype 1 No association Enterotype 2 |
| Tartronate (hydroxymalonate) | 0.007 | 0.03 | ↑ Enterotype 1 No association Enterotype 2 |
| Threonate | 0.002 | 0.001 | ↑ Enterotype 1 No association Enterotype 2 |
| Stool | |||
| Guanine | 0.01 | 0.03 | ↑ Enterotype 1 No association Enterotype 2 |
| Indolepropionate | 0.01 | 0.03 | ↑ Enterotype 1 No association Enterotype 2 |
| Isocaproate | 3.3 × 10−5 | 0.02 | ↑ Enterotype 1 No association Enterotype 2 |
| Liquiritigenin | 4.5 × 10−14 | 0.02 | ↑ Enterotype 1 No association Enterotype 2 |
P values reported are interaction p values from the association with genistein. Where indicated, a metabolite was included even if the p value for genistein was >0.05, if the p value for daidzein and/or glycitein was <0.05.
Figure 1. Significant gut-enterotype interactions on the association between dietary soy intake and metabolites in plasma (A, B), Stool (C), and Systolic Blood Pressure (D).
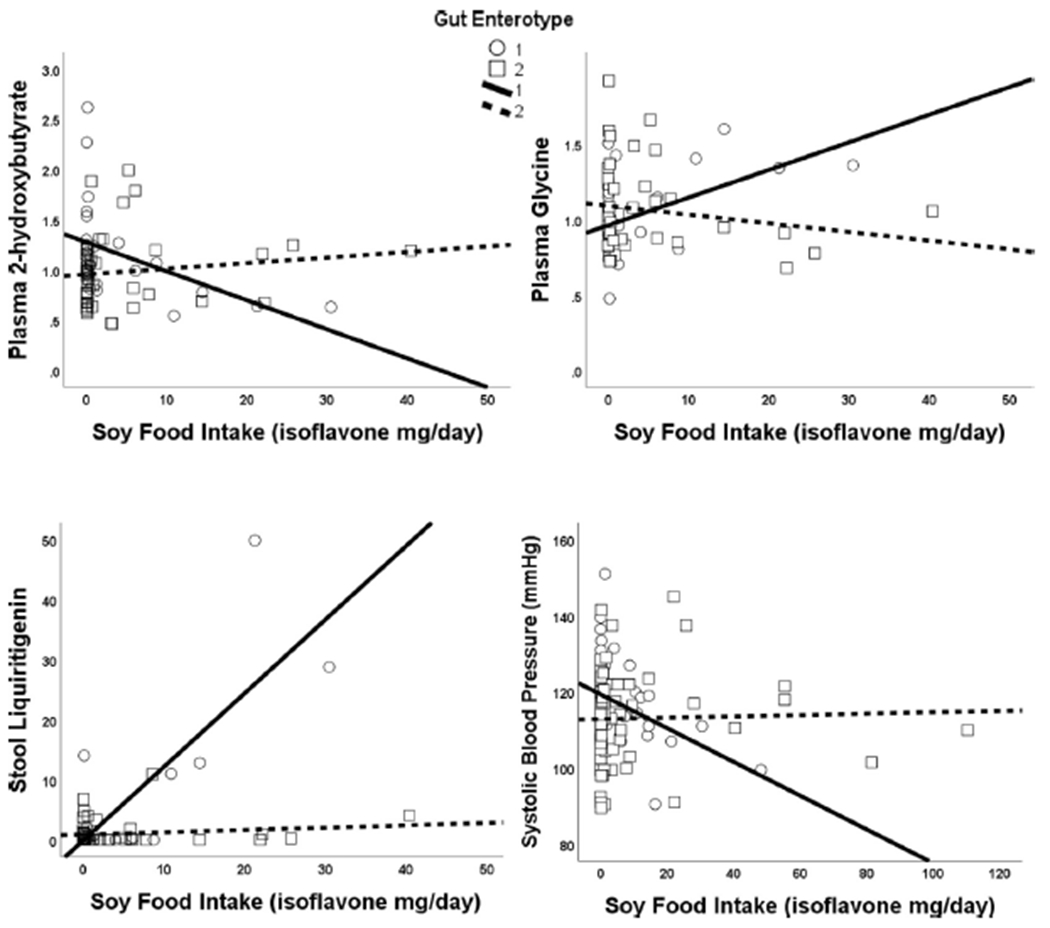
Data shown for short-term soy intake. Results for long-term soy intake were almost identical. All interactions p < 0.05. While there were no associations between soy and outcomes within enterotype 2, individuals had lower plasma 2-hydroxybutyrate, higher plasma glycine, higher stool liquiritigenin, and lower systolic blood pressure with increasing soy intake.
Dietary soy intake is associated with differences in the abundance of OTUs mapping to genus Dialister and Prevotella
We next assessed whether soy intake was associated with differences in abundance of any specific microbial taxa. We again required associations to be present using both the short- and long-term diet estimates, as a quasi-internal replication to reduce the likelihood of false positives. There were significant associations between soy intake and two independent OTUs, one mapping to the genus Dialister, and one mapping to the genus Prevotella. To probe this further, we dichotomized our sample based on a cut-point of 3 mg/day total isoflavones. This was selected because it represents one average portion of soy (e.g. 3.5oz tofu, or 8oz soy milk) per week, and thus separates no-low consumers from moderate-high consumers in our dataset. Using soy intake as either a continuous or dichotomized variable, individuals with higher soy intake had significantly lower abundance of both OTUs, suggesting that soy consumption may associate with suppression of these specific taxa (Fig. 2). There was no difference in the abundance of either taxon by gut enterotype, consistent with independent mechanisms linking soy food and microbiota to downstream metabolism.
Figure 2. Significant association between dietary soy isoflavone intake and abundance of individual OTUs mapping to Prevotella (A) and Dialister (B).
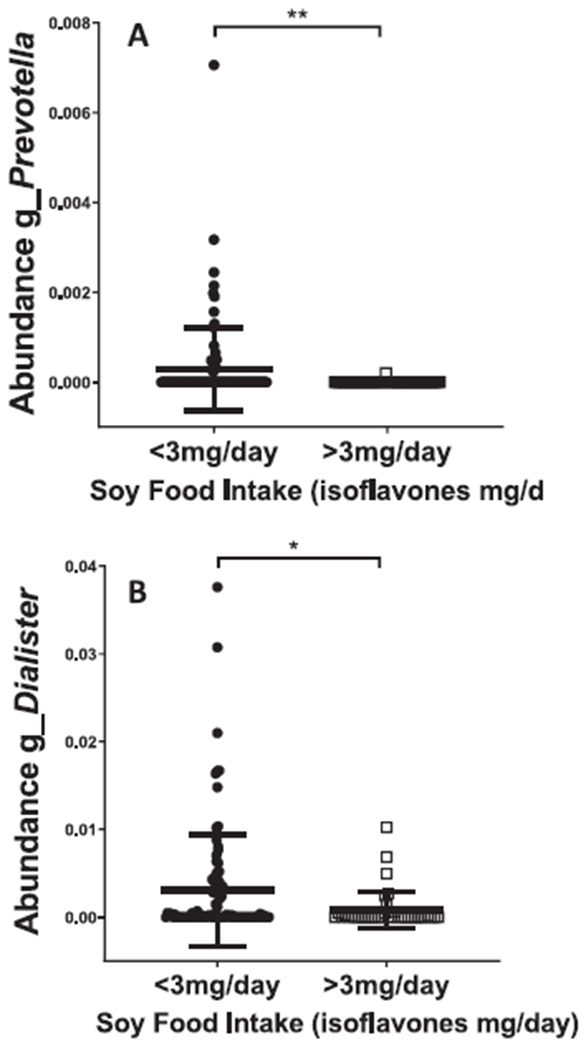
Data shown for short-term soy intake. Results for long-term soy intake were almost identical. Using soy isoflavone intake dichotomized by a cut-point of 3 mg/day (selected to separate low consumers from moderate-high consumers), individuals with higher soy intake had significantly lower abundance of both taxa (p < 0.05), suggesting that soy consumption may associate with suppression of these specific taxa.
Abundance of Prevotella OTU associates with blood pressure, but is mitigated by the presence of Dialister
We hypothesized that soy mediates effects on cardiometabolic risk factors through modulation of gut microbial taxa. We therefore investigated whether the soy-linked OTUs associated with markers of cardiometabolic health. There was a significant association between the OTUs (Dialister and Prevotella) and systolic blood pressure (p < 0.05). Surprisingly, the association was in opposite directions, with blood pressure increasing with Prevotella, and decreasing with Dialister, despite soy being associated with lower abundance in both cases. We divided the sample into 4 groups based on the presence or absence of either taxon, and found significantly higher blood pressure, adiposity markers (waist circumference, weight, BMI) and C-reactive protein in individuals carrying Prevotella alone (all p < 0.05), but not when carrying Prevotella and Dialister. Individuals carrying Dialister alone had the same risk profiles as people carrying either both or neither taxon (Fig. 3). These associations did not differ by sex. Overall, our data suggest that the Prevotella taxon, common in individuals with low soy food intake, may exert deleterious effects on health, which are mitigated when Dialister is also present.
Figure 3. Prevotella associates with increased blood pressure and cardiometabolic risk markers, but not when Dialister is also present.
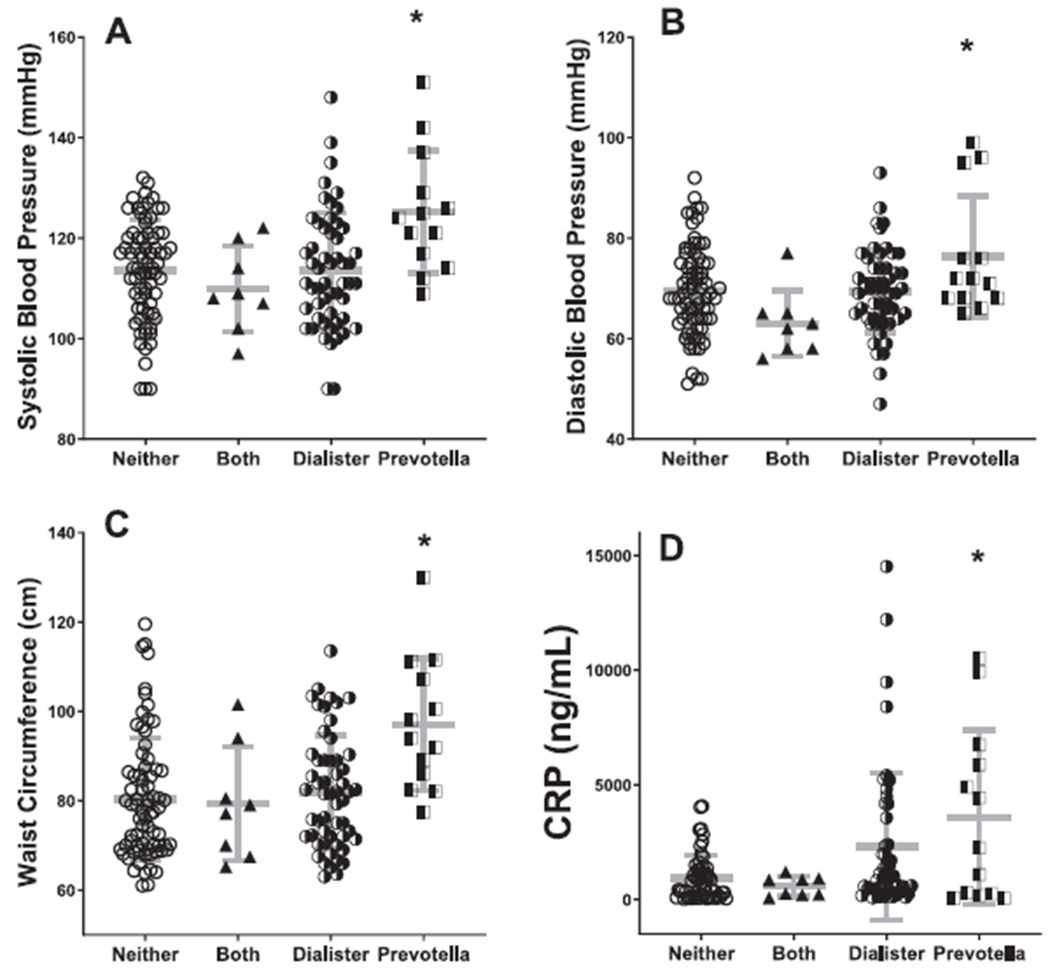
After splitting the sample into 4 groups based on the presence or absence of either taxon, we found significantly higher blood pressure, waist circumference, and c-reactive protein in individuals carrying Prevotella alone, but not when carrying Prevotella and Dialister (overall ANOVA p < 0.0001, all post-hoc comparisons p < 0.05). Individuals carrying Dialister alone had the same risk profiles as people carrying either both or neither taxon.
Presence of Prevotella without Dialister is associated with differences in circulating and stool metabolites
We hypothesized that individuals carrying Prevotella, without the protective co-occurrence of Dialister, would have differences in their metabolite profiles. Indeed, 53 metabolites in plasma, and 65 metabolites in stool, differed significantly by group (comparison of 4 groups by ANOVA), with the “Prevotella only” group different from everyone else (significant post-hoc test, and significant when comparing Prevotella-only to all other groups). In particular, plasma indoleacetyl glutamine, a tryptophan metabolite known to be produced by gut bacteria, was significantly higher in Prevotella-only compared with other groups. Enrichment analysis revealed significant enrichment in sphingolipid (P = 2.9 × 10−4, FDR P = 0.01) and nitrogen (P = 3.2 × 10−4, FDR P = 0.01) metabolism, and in Aminoacyl-tRNA biosynthesis (P = 0.002, FDR P = 0.05).
Soy- and microbiome-mediated effects on blood pressure are independent of dietary sodium
We have recently shown in the ABO Study that dietary sodium is associated with increased blood pressure, which is mediated through the microbiome [21 ]. We assessed the relationship between dietary sodium and dietary isoflavones in our sample to exclude the possibility of confounding by sodium. Interestingly, there was a significant positive correlation between long-term soy isoflavone and sodium intake (r = 0.4, p < 0.0001), and no relationship with short-term dietary estimates. Because higher sodium intake was associated with increased blood pressure, while higher isoflavone intake was associated with lower blood pressure, and because the specific taxa identified as related to sodium-mediated hypertension differ from those related to soy-mediated blood-pressure reduction in the current study, we conclude that the microbiome-mediated effects of soy food are not related to sodium.
Discussion
In this study, we aimed to characterize the relationship between soy food intake, the gut microbiome, and the metabolome, and to probe the role of the microbiome as a mediator of soy effects on cardiometabolic health. Metabolites in stool and plasma were associated with soy intake. For several metabolites, this association differed by gut microbiome enterotype. Higher soy consumption was associated with suppression of two bacterial taxa, mapping to Prevotella and Dialister. However, these taxa had distinct relationships with downstream markers; Prevotella, in the absence of Dialister, positively correlated with increased markers of cardiometabolic risk and differences in plasma and stool metabolites. However, when Dialister and Prevotella were both present, these effects were mitigated, suggesting complex interaction between different bacterial taxa. Our findings highlight a relationship between soy foods and metabolic parameters in a gut-microbiome dependent manner.
Levels of the soy isoflavone daidzein in blood and stool correlated with short-term but not long-term soy intake, suggesting its utility as a biomarker for recent, but not habitual soy consumption. This novel finding suggests that studies that rely on circulating isoflavones as a proxy for soy food intake could mis-classify habitual soy food consumers as non-consumers if they did not consume soy foods in the days preceding the blood draw [28].
Several plasma metabolites with known cardiometabolic associations were correlated to long-term soy intake and were validated in both ABO and FAIR studies. 1,5-Anhydroglucitol, which negatively correlated with daidzein levels is a biomarker for hyperglycemic excursions [29]. The amino acid metabolite isobutyrylcarnitine C4, has been associated with risk of gestational diabetes [30]. Xanthurenate, a member of the kynurenine pathway, has been associated with glucose metabolism and diabetes [31,32]. We previously found that soy intake associated with protection against inflammation-induced decline in insulin sensitivity, lower fasting insulin, and more effective insulin response to oral glucose challenge [19]. These metabolites may directly or indirectly mediate the protective effects of soy intake on insulin and glucose homeostasis, however the mechanisms are unclear and warrant further study.
We found several xenobiotics that positively correlated with soy intake, including enrichment for those related to caffeine metabolism (1,7-dimethylurate, 5-acetylamino-6-amino-3-methyluracil, 5-acetylamino-6-formylamino-3-methyluracil). Because caffeine consumption was correlated with soy consumption in our sample, we cannot exclude the possibility that this association is attributable to dietary caffeine. However, the specific metabolites associated with soy mostly differed from those associated with dietary caffeine, suggesting that soy food may interact with, or potentiate caffeine metabolism. Indeed, a link between isoflavone and caffeine metabolism has been reported previously, with genistein supplementation linked to alteration of caffeine metabolism through cytochrome p450 metabolism [18]. While intriguing, this question remains understudied. Well-controlled mechanistic or human interventional studies are required to characterize potential interaction between intake of soy- and caffeine-containing foods, and downstream metabolism.
Microbiota are known to differentially confer soy-metabolizing ability, most notably in the ability to metabolize daidzein to equol, and this ability is lower in non-Asian populations [33]. Further, it appears that equol itself may not be responsible for the beneficial effects of soy foods in metabolizers, but that equol-generating capacity serves as a biomarker for beneficial microbiota [34]. We used gut enterotype profiling as a tool to broadly separate our sample by gut microbial phenotype. We observed gut-enterotype interactions between soy intake and metabolites, as well as with systolic blood pressure. Notably, soy consumption associated with metabolite variation, and with decreased systolic blood pressure primarily in individuals with gut enterotype 1. In contrast, individuals with enterotype 2 appeared relatively unresponsive to high soy intake. Several of the metabolites have known cardiometabolic implications. In plasma, 2-hydroxybutyrate levels have been associated with hypertension [35], gestational diabetes [36] and reduced by bariatric surgery [37]. 3-methoxytyrosine is associated with increased incidence of type 2 diabetes [38].Isovalerylcarnitine has been negatively correlated with development of gestational diabetes [39]. The amino acids glycine and leucine have extensive literature supporting their role in type 2 diabetes and obesity [40,41 ] in addition to hypertension [42,43]. Notably, myo-inositol can activate insulin secretion and is being studied therapeutically for treatment of polycystic ovarian syndrome [44] and reduction of gestational diabetes [45]. Comparison of published directions of association with the direction associated with soy intake in our sample revealed a lack of consistency in the directionality of the associations. However, even in large epidemiological studies, it is difficult to determine whether a change in metabolite levels in disease states is causal or compensatory. Our cross-sectional data do not resolve whether soy-related changes in metabolites are protective or deleterious for glycemic control, however in general, soy intake is considered to be protective against diabetes [46]. In stool, the significant soy-microbiome mediated metabolites included indole-3-propionic acid, a known product of specific gut microbiota which has been associated with protection against diabetes [47 ] and mediates vasodilation [48] and liquiritigenin (4′,7-Dihydroxyflavanone), a flavanone shown to inhibit NLRP3 inflammasome, protect against hypertension and lower glucose in cell and animal models [49–51]. Both were positively associated with higher soy consumption in enterotype 1, consistent with potential protective effects of soy in individuals with compatible microbiota. The long-term health implications of alterations in circulating metabolites resulting from differential gut microbial metabolism of dietary soy isoflavones is unknown, but our data suggest that gut microbiome enterotype may be a factor in determining levels of metabolites of particular relevance to hypertension and diabetes.
Soy intake was negatively correlated with systolic blood pressure in a gut-microbiota-dependent manner. Effects of soy intake or supplementation on blood pressure have been widely studied in clinical trials, and while results are not always consistent, most studies show an anti-hypertensive effect [8,9,52]. Of note, our sample was normotensive, and younger than the subjects in most of the reported literature, but replicates the effects of soy intake on blood pressure, while additionally highlighting a gut-dependent mechanism. Inconsistencies in previous reports may be due to confounding by differences in gut microbiome composition, and in the proportion of individuals with soy-responsive microbiota. Mechanisms by which soy components lower blood pressure are not definitively known but may involve effects on flow-mediated dilation, via increased nitric oxide synthesis in endothelial cells; similar to the effects of estrogen [7]. Animal studies have also suggested angiotensin-converting enzyme inhibitory activity in fermented soy proteins [53]. Our study highlights metabolites that vary by soy and enterotype, and may be considered as novel candidate mediators for anti-hypertensive effects. Future mechanistic studies are needed to probe these further.
While determining association of the gut microbiome to cardiometabolic profiles, we found a complex interaction between two taxa: Prevotella and Dialister. While both were suppressed by soy consumption, Prevotella in the absence of Dialister was associated with a distinct metabolomic profile as well as cardiometabolic risk factors including elevated blood pressure, weight, waist circumference, and CRP. Prevotella has previously been reported to be altered by soy intake in rat and human models [54,55]. Dialister is a member of the firmicute phylum and has also been associated with insulin secretion [56] and found to be more prevalent in lean compared to obese human subjects. Our data highlight the microbiome as a potential mediator of the protective effects of soy on blood pressure and overall cardiometabolic risk, and may explain the heterogeneity in findings from previous studies, given variability of gut microbiome composition across and within populations.
In contrast to many published reports, we studied individuals with relatively low soy intake, as part of a typical US western diet. In previous work, we similarly demonstrated that low intake of soy foods, equivalent to a portion a week, were sufficient to detect metabolic effects [19]. From a public health perspective, it would be a major challenge to increase levels of soy food consumption in the US and Europe up to the high levels of consumption in some parts of Asia. Our cross-sectional data in healthy individuals suggest that high soy food intake may not be required to provide cardiometabolic benefit. However, carefully-controlled interventional trials in diverse samples are needed to address this important question.
Our study is uniquely strengthened by the concomitant collection of cardiometabolic phenotyping, dietary intake data, stool and plasma metabolomics and gut microbiome which allowed for analysis of the diet-metabolome-microbiome interactions and associations with metabolic health. By sampling from a healthy population, our results are not affected by chronic inflammation, medication usage, or other sequelae of chronic illness that are known to alter the microbiome and metabolome. We were also able to validate our plasma metabolite associations in an independent sample. Our study also has some limitations, including the lack of external validation of the microbiome associations in an independent sample. To partially address this limitation, we conducted internal validation using the two independent measures of soy intake. While this may result in exclusion of metabolites that are rapidly metabolized in the short term, or those that only reflect long-term consumption, requiring associations with two independent measures of soy isoflavones gives greater confidence that the reported metabolites have consistent biological relevance. Conversion of dietary daidzein to equol is a known gut-microbiota dependent process, that varies between individuals. Unfortunately equol was not detectable in our sample using the global metabolomics profiling platform, limiting our ability to characterize individuals by equol production capacity. Soy contains several components that may promote health, including isoflavones and fiber which are likely to interact with gut microbiota, as well as being a source of protein and other nutrients. Our data do not resolve whether the observed effects are attributable to whole soy foods or individual components. While our findings identify key metabolites and taxa that may link soy foods to cardioprotection, these are of an associative nature, and mechanistic studies are needed to determine causality.
Overall our findings demonstrate that moderate-low soy intake has significant effects on the metabolome that are mediated by the gut microbiome. Soy intake was associated with modest cardiometabolic benefits, which was evident even in our healthy sample. Soy food consumption may shape microbiome composition, suppressing certain taxa, which may provide health benefits. Targeted dietary manipulation of the microbiome is an extremely attractive therapeutic option to improve health outcomes. Future interventional studies are needed to fully characterize the effects of soy foods on the microbiome, metabolome, and cardiometabolic risk.
Supplementary Material
Acknowledgements
The authors have no conflicts of interest to disclose.
Sources of support
The project was supported by an AHA Scientist Development Grant (15SDG24890015, PI: Ferguson) and a P&F Award to Dr. Ferguson from the Vanderbilt University Medical Center’s Digestive Disease Research Center supported by NIH grant P30DK058404. The project was also supported by the Data Science Initiative Award (PI: Tang) provided by the University of Wisconsin-Madison Office of the Chancellor and the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation. The project was also supported by NIH Grant DK095913 (PI: Shah). Dr. Ferguson is also supported by R01DK117144 and R01HL142856.
Abbreviations:
- BMI
body mass index
- C
Celsius
- DHQ
diet history questionnaire
- DNA
deoxyribonucleic acid
- FFQ
food frequency questionnaire
- PCR
polymerase chain reaction
- OTU
operational taxonomic unit
Footnotes
Data availability statement
The 16S microbiome data generated for this study are available in the Sequence Read Archive SRA accession number SRP153041.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.numecd.2020.05.001.
References
- [1].Mohammadkhah AI, Simpson EB, Patterson SG, Ferguson JF. Development of the gut microbiome in children, and lifetime implications for obesity and cardiometabolic disease. Children 2018;5 10.3390/children5120160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ferguson JF, Allayee H, Gerszten RE, Ideraabdullah F, Kris-Etherton PM, Ordovas JM, et al. Nutrigenomics, the microbiome, and gene-environment interactions: new directions in cardiovascular disease research, prevention, and treatment: a scientific statement from the American heart association. Circ Cardiovasc Genet 2016;9:291–313. 10.1161/HCG.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature 2011;474:327–36. 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105–8. 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Guertin KA, Moore SC, Sampson JN, Huang W-Y, Xiao Q, Stolzenberg-Solomon RZ, et al. Metabolomics in nutritional epidemiology: identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. Am J Clin Nutr 2014;100:208–17, 10.3945/ajcn.113.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mahn K, Borras C, Knock GA, Taylor P, Khan IY, Sugden D, et al. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J 2005;19:1755–7. 10.1096/fj.05-4008fje. [DOI] [PubMed] [Google Scholar]
- [7].Rivas M, Garay RP, Escanero JF, Cia P, Cia P, Alda JO. Soy milk lowers blood pressure in men and women with mild to moderate essential hypertension. J Nutr 2002;132:1900–2. 10.1093/jn/132.7.1900. [DOI] [PubMed] [Google Scholar]
- [8].Liu XX, Li SH, Chen JZ, Sun K, Wang XJ, Wang XG, et al. Effect of soy isoflavones on blood pressure: a meta-analysis of randomized controlled trials. Nutr Metabol Cardiovasc Dis 2012;22:463–70. 10.1016/j.numecd.2010.09.006. [DOI] [PubMed] [Google Scholar]
- [9].Taku K, Lin N, Cai D, Hu J, Zhao X, Zhang Y, et al. Effects of soy isoflavone extract supplements on blood pressure in adult humans: systematic review and meta-analysis of randomized placebo-controlled trials. J Hypertens 2010;28:1971–82. 10.1097/HJH.0b013e32833c6edb. [DOI] [PubMed] [Google Scholar]
- [10].Kokubo Y, Iso H, Ishihara J, Okada K, Inoue M, Tsugane S, et al. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: the Japan Public Health Center-based (JPHC) study cohort I. Circulation 2007;116:2553–62. 10.1161/CIRCULATIONAHA.106.683755. [DOI] [PubMed] [Google Scholar]
- [11].Cheong SH, Furuhashi K, Ito K, Nagaoka M, Yonezawa T, Miura Y, et al. Daidzein promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and improves glucose homeostasis in Type 2 diabetic model mice. J Nutr Biochem 2014;25:136–43. 10.1016/j.jnutbio.2013.09.012. [DOI] [PubMed] [Google Scholar]
- [12].Kim EK, Kwon KB, Song MY, Seo SW, Park SJ, Ka SO, et al. Genistein protects pancreatic beta cells against cytokine-mediated toxicity. Mol Cell Endocrinol 2007;278:18–28. 10.1016/j.mce.2007.08.003. [DOI] [PubMed] [Google Scholar]
- [13].Ramdath DD, Padhi EMT, Sarfaraz S, Renwick S, Duncan AM. Beyond the cholesterol-lowering effect of soy protein: a review of the effects of dietary soy and its constituents on risk factors for cardiovascular disease. Nutrients 2017;9 10.3390/nu9040324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].USDA Food Composition Databases n.d htts://ndb.nal.usda.gov/ndb/(accessed September 24, 2019).
- [15].Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Canc 2000;36:27–32. 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- [16].Bowey E, Adlercreutz H, Rowland I. Metabolism of isoflavones and lignans by the gut microflora: a study in germ-free and human flora associated rats. Food Chem Toxicol: An International Journal Published for the British Industrial Biological Research Association 2003;41:631–6. [DOI] [PubMed] [Google Scholar]
- [17].Nakatsu CH, Armstrong A, Clavijo AP, Martin BR, Barnes S, Weaver CM. Fecal bacterial community changes associated with isoflavone metabolites in postmenopausal women after soy bar consumption. PloS One 2014;9:e108924 10.1371/journal.pone.0108924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ronis Mjj. Effects of soy containing diet and isoflavones on cytochrome P450 enzyme expression and activity. Drug Metab Rev 2016;48:331–41. 10.1080/03602532.2016.1206562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ferguson JF, Ryan MF, Gibney ER, Brennan L, Roche HM, Reilly MP. Dietary isoflavone intake is associated with evoked responses to inflammatory cardiometabolic stimuli and improved glucose homeostasis in healthy volunteers. Nutr Metabol Cardiovasc Dis 2014;24:996–1003. 10.1016/j.numecd.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tang Z-Z, Chen G, Hong Q, Huang S, Smith HM, Shah RD, et al. Multi-omic analysis of the microbiome and metabolome in healthy subjects reveals microbiome-dependent relationships between diet and metabolites. Front Genet 2019;10 10.3389/fgene.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ferguson JF, Aden LA, Barbaro NR, Van Beusecum JP, Xiao L, Simmons AJ, et al. High dietary salt-induced dendritic cell activation underlies microbial dysbiosis-associated hypertension. JCI Insight 2019;5 10.1172/jci.insight.126241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the block, Willett, and National cancer Institute food frequency questionnaires : the eating at America’s table study. Am J Epidemiol 2001;154: 1089–99. [DOI] [PubMed] [Google Scholar]
- [23].Xia J, Wishart DS. Metabolomic data processing, analysis, and interpretation using MetaboAnalyst. Curr Protoc Bioinformatics 2011. 10.1002/0471250953.bi1410s34 [Chapter 14]: Unit 14 10. [DOI] [PubMed] [Google Scholar]
- [24].Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 2012; 6:1621–4. 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 2009; 75:7537–41. 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kaufman L, Rousseeuw PJ. Clustering by means of medoids In: Dodge Y, editor. Statistical data analysis based on the L1 norm and related methods. Amsterdam: Elsevier; 1987. p. 405–16. [Google Scholar]
- [27].Dhariwal A, Chong J, Habib S, King IL, Agellon LB, Xia J. MicrobiomeAnalyst: a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res 2017;45:W180–8. 10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Miles FL, Lloren JIC, Haddad E, Jaceldo-Siegl K, Knutsen S, Sabate J, et al. Plasma, urine, and Adipose tissue biomarkers of dietary intake differ between vegetarian and non-vegetarian diet groups in the adventist health study-2. J Nutr 2019;149:667–75. 10.1093/jn/nxy292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sun J, Dou J-T, Wang X-L, Yang G-Q, Lu Z-H, Zheng H, et al. Correlation between 1,5-anhydroglucitol and glycemic excursions in type 2 diabetic patients. Chin Med J 2011;124:3641–5. [PubMed] [Google Scholar]
- [30].Roy C, Tremblay P-Y, Anassour-Laouan-Sidi E, Lucas M, Forest J-C, Giguere Y, et al. Risk of gestational diabetes mellitus in relation to plasma concentrations of amino acids and acylcarnitines: a nested case-control study. Diabetes Res Clin Pract 2018;140:183–90. 10.1016/j.diabres.2018.03.058. [DOI] [PubMed] [Google Scholar]
- [31].Oxenkrug GF. Increased plasma levels of xanthurenic and kynurenic acids in type 2 diabetes. Mol Neurobiol 2015;52:805–10. 10.1007/s12035-015-9232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Favennec M, Hennart B, Verbanck M, Pigeyre M, Caiazzo R, Raverdy V, et al. Post-bariatric surgery changes in quinolinic and xanthurenic acid concentrations are associated with glucose homeostasis. PloS One 2016;11:e0158051 10.1371/journal.pone.0158051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Setchell KDR, Clerici C. Equol: pharmacokinetics and biological actions. J Nutr 2010;140:1363S–8S. 10.3945/jn.109.119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hazim S, Curtis PJ, Schar MY, Ostertag LM, Kay CD, Minihane A-M, et al. Acute benefits of the microbial-derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: a double-blind randomized controlled trial. Am J Clin Nutr 2016;103:694–702. 10.3945/ajcn.115.125690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chakraborty S, Galla S, Cheng X, Yeo J-Y, Mell B, Singh V, et al. Salt-responsive metabolite, β-hydroxybutyrate, attenuates hypertension. Cell Rep 2018;25:677–89. 10.1016/jxel-rep.2018.09.058 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dudzik D, Zorawski M, Skotnicki M, Zarzycki W, Garcia A, Angulo S, et al. GC-MS based Gestational Diabetes Mellitus longitudinal study: identification of 2-and 3-hydroxybutyrate as potential prognostic biomarkers. J Pharmaceut Biomed Anal 2017; 144:90–8. 10.1016/j.jpba.2017.02.056. [DOI] [PubMed] [Google Scholar]
- [37].Wijayatunga NN, Sams VG, Dawson JA, Mancini ML, Mancini GJ, Moustaid-Moussa N. Roux-en-Y gastric bypass surgery alters serum metabolites and fatty acids in patients with morbid obesity. Diabetes Metab Res Rev 2018;34:e3045 10.1002/dmrr.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yu D, Moore SC, Matthews CE, Xiang Y-B, Zhang X, Gao Y-T, et al. Plasma metabolomic profiles in association with type 2 diabetes risk and prevalence in Chinese adults. Metabolomics 2016;12 10.1007/s11306-015-0890-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nevalainen J, Sairanen M, Appelblom H, Gissler M, Timonen S, Ryynanen M. First-trimester maternal serum amino acids and acylcarnitines are significant predictors of gestational diabetes. Rev Diabet Stud 2016;13:236–45. 10.1900/RDS.2016.13.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Alves A, Bassot A, Bulteau A-L, Pirola L, Morio B. Glycine metabolism and its alterations in obesity and metabolic diseases. Nutrients 2019;11 10.3390/nu11061356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Di Camillo B, Eduati F, Nair SK, Avogaro A, Toffolo GM. Leucine modulates dynamic phosphorylation events in insulin signaling pathway and enhances insulin-dependent glycogen synthesis in human skeletal muscle cells. BMC Cell Biol 2014;15:9 10.1186/1471-2121-15-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yu Q, Tai Y-Y, Tang Y, Zhao J, Negi V, Culley MK, et al. BOLA (BolA family member 3) deficiency controls endothelial metabolism and Glycine homeostasis in pulmonary hypertension. Circulation 2019;139:2238–55. 10.1161/CIRCULATIONAHA.118.035889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang H, Wang X, Qi D, Sun M, Hou Q, Li Y, et al. Establishment of the circadian metabolic phenotype strategy in spontaneously hypertensive rats: a dynamic metabolomics study. J Transl Med 2020;18:38 10.1186/s12967-020-02222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pundir J, Charles D, Sabatini L, Hiam D, Jitpiriyaroj S, Teede H, et al. Overview of systematic reviews of non-pharmacological interventions in women with polycystic ovary syndrome. Hum Reprod Update 2019;25:243–56. 10.1093/humupd/dmy045. [DOI] [PubMed] [Google Scholar]
- [45].Crawford TJ, Crowther CA, Alsweiler J, Brown J. Antenatal dietary supplementation with myo-inositol in women during pregnancy for preventing gestational diabetes. Cochrane Database Syst Rev 2015:CD011507 10.1002/14651858.CD011507.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Li W, Ruan W, Peng Y, Wang D. Soy and the risk of type 2 diabetes mellitus: a systematic review and meta-analysis of observational studies. Diabetes Res Clin Pract 2018;137:190–9. 10.1016/j.diabres.2018.01.010. [DOI] [PubMed] [Google Scholar]
- [47].Abildgaard A, Elfving B, Hokland M, Wegener G, Lund S. The microbial metabolite indole-3-propionic acid improves glucose metabolism in rats, but does not affect behaviour. Arch Physiol Biochem 2018;124:306–12. 10.1080/13813455.2017.1398262. [DOI] [PubMed] [Google Scholar]
- [48].Pulakazhi Venu VK, Saifeddine M, Mihara K, Tsai Y-C, Nieves K, Alston L, et al. The pregnane X receptor and its microbiota-derived ligand indole 3-propionic acid regulate endothelium-dependent vasodilation. Am J Physiol Endocrinol Metab 2019;317:E350–61. 10.1152/ajpendo.00572.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhu X, Shi J, Li H. Liquiritigenin attenuates high glucose-induced mesangial matrix accumulation, oxidative stress, and inflammation by suppression of the NF-kB and NLRP3 inflammasome pathways. Biomed Pharmacother 2018;106:976–82. 10.1016/j.biopha.2018.07.045. [DOI] [PubMed] [Google Scholar]
- [50].Carnovali M, Luzi L, Terruzzi I, Banfi G, Mariotti M. Liquiritigenin reduces blood glucose level and bone adverse effects in hyperglycemic adult zebrafish. Nutrients 2019;11 10.3390/nu11051042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kim KU, Lee S-J, Lee I. Development of an improved menopausal symptom-alleviating licorice (Glycyrrhiza uralensis) by biotransformation using monascus albidulus. J Microbiol Biotechnol 2020; 30:178–86. 10.4014/jmb.1909.09037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kou T, Wang Q, Cai J, Song J, Du B, Zhao K, et al. Effect of soybean protein on blood pressure in postmenopausal women: a meta-analysis of randomized controlled trials. Food Funct 2017;8: 2663–71. 10.1039/c6fo01845a. [DOI] [PubMed] [Google Scholar]
- [53].Wu J, Ding X. Hypotensive and physiological effect of angiotensin converting enzyme inhibitory peptides derived from soy protein on spontaneously hypertensive rats. J Agric Food Chem 2001;49: 501–6. 10.1021/jf000695n. [DOI] [PubMed] [Google Scholar]
- [54].Cross T-WL, Zidon TM, Welly RJ, Park Y-M, Britton SL, Koch LG, et al. Soy improves cardiometabolic health and cecal microbiota in female low-fit rats. Sci Rep 2017;7:9261 10.1038/s41598-017-08965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fernandez-Raudales D, Hoeflinger JL, Bringe NA, Cox SB, Dowd SE, Miller MJ, et al. Consumption of different soymilk formulations differentially affects the gut microbiomes of overweight and obese men. Gut Microb 2012;3:490–500. 10.4161/gmic.21578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Naderpoor N, Mousa A, Gomez-Arango LF, Barrett HL, Dekker Nitert M, de Courten B. Faecal microbiota are related to insulin sensitivity and secretion in overweight or obese adults. J Clin Med 2019;8 10.3390/jcm8040452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


