Abstract
Objective:
To characterize national trends in oncologic imaging (OI) utilization.
Methods:
This retrospective cross-sectional study used 2004 and 2016 CMS 5% Carrier Claims Research Identifiable Files. Radiologist-performed, primary non-invasive diagnostic imaging exams were identified from billed CPT codes; CT, MRI and PET/CT examinations were categorized as “advanced” imaging. OI examinations were identified from imaging claims’ primary ICD-9 and ICD-10 codes. Imaging services were stratified by academic practice status and place of service. State-level correlations of oncologic advanced imaging utilization (examinations per 1000 beneficiaries) with cancer prevalence and radiologist supply were assessed by Spearman correlation coefficient.
Results:
The national Medicare sample included 5,030,955 diagnostic imaging exams (1,218,144 of them “advanced”) in 2004 and 5,017,287 diagnostic imaging exams (1,503,490 of them “advanced”) in 2016. In 2004 and 2016, OI represented 3.9% and 4.3%, respectively, of all imaging vs. 10.8% and 9.5%, respectively, of advanced imaging. The percentage of advanced OI done in academic practices rose from 18.8% in 2004 to 34.1% in 2016, leaving 65.9% outside academia. In 2016, 58.0% of advanced OI was performed in the hospital outpatient setting and 23.9% in the physician office setting. In 2016, state-level oncologic advanced imaging utilization correlated with state-level radiologist supply (r=+0.489, p<0.001) but not with state-level cancer prevalence (r=−0.139, p=0.329).
Discussion:
Oncologic imaging usage varied between practice settings. While the percentage of advanced OI done in academic settings nearly doubled from 2004 to 2016, the majority remained in non-academic practices. State-level oncologic advanced imaging utilization correlated with radiologist supply but not cancer prevalence.
Summary Statement
Oncologic imaging amounted to just 3.9% of all diagnostic imaging and 9.5% of advanced diagnostic imaging tests nationally, and radiologists’ exposure to oncologic imaging varied significantly across practice settings.
Introduction
Although the incidence of cancer in the United States is rising, the overall, age-adjusted mortality rate from cancer has been declining steadily: The cancer death rate for men and women combined fell 27% between 1991 and 2016 (1). Unfortunately, however, not all populations are benefitting equally. While the racial gap in cancer deaths is slowly diminishing, socioeconomic inequalities are growing (2), and notable disparities in outcomes have been found depending on the care setting as well as patients’ insurance status and race/ethnicity (3, 4). These disparities point to a need to promote greater consistency in the practice of cancer care, from diagnosis through treatment and follow-up.
Imaging today plays essential roles in the management of almost all non-cutaneous cancers, influencing diagnosis, assessment of prognosis, treatment selection, and therapeutic monitoring (5). Achieving consistently high-quality oncologic imaging interpretations poses an increasing challenge in light of the growing complexity of such imaging and of oncologic care in general (6). In recent years, there have been rapid advances in cancer diagnosis and treatment including the introduction of new technologies, changes in standard treatment protocols, and a shift toward the implementation of precision medicine based on a growing understanding of tumor biology (7, 8). Numerous studies have demonstrated added value when oncologic imaging examinations undergo secondary interpretations at expert centers (9–12); a metaanalysis found that such secondary interpretations resulted in discrepancies from the primary interpretations in 32.7% of cases and modifications of patient management in 22.1% (13). Moreover, an analysis of the radiologist workforce in the United States found that only about one fifth of counties have a radiologist with subspecialty expertise in any area of imaging and that, even if these radiologists could be redistributed geographically, there would be not be enough subspecialists to cover all areas of the country (14). These findings suggest that efforts to ensure consistent access to high-quality cancer care must includes strategies to more broadly disseminate cancer imaging expertise, particularly to settings where radiologists’ exposure to such imaging may be low (15). It should also be noted that high-quality care depends on evidence-based practice, which should include adherence to evidence-based cancer imaging guidelines (15, 16).
The idea for the present study arose from the 2018 National Cancer Policy Forum workshop, “Improving Cancer Diagnosis and Care: Patient Access to Oncologic Imaging and Pathology Expertise and Technologies,”(15) where it became clear that to address variations in the accessibility and quality of oncologic imaging, there was a need for benchmark data on the prevalence of oncologic imaging and variations in its use between different care settings and geographic locations. Thus, we conducted this study to characterize radiologists’ oncologic imaging practice patterns across the United States.
Methods
This retrospective, observational cross-sectional study was HIPAA-compliant and was approved by the institutional review board of the American College of Radiology with a waiver of the requirement for written informed consent.
The data sources for this analysis were the 5% Carrier Claims Research Identifiable Files obtained from the Centers for Medicare & Medicaid Services. These files contain all Medicare Part B claims for a 5% random sample of Medicare fee-for-service beneficiaries. We selected the files from 2004 and 2016, representing the oldest and most recent files, respectively, to which we had access at the time of completing the investigation.
We selected from the files all services performed by radiologists, defined as providers registered with CMS as diagnostic radiologists, interventional radiologists, or nuclear medicine physicians, representing the only classes of radiologists formally recognized by CMS provider codes. We then further selected all primary non-invasive diagnostic imaging services performed by radiologists, defined using the Neiman Imaging Types of Service (NITOS) (17) as radiography/fluoroscopy, ultrasonography, CT, MRI, and nuclear medicine; image-guided invasive procedures and secondary imaging services, such as 3D rendering and computer-aided reconstructions, were not included. Among included imaging services, CT, MRI, and PET/CT were further classified as advanced diagnostic imaging.
Imaging examinations were classified as oncologic based on primary ICD-9 (2004) and ICD-10 (2016) codes associated with the imaging claims. Imaging services were further classified using the claims information in terms of academic practice status (academic vs. nonacademic), place of service (emergency department, inpatient, physician office, hospital outpatient, or other) and U.S. state in which the service occurred. The determination of academic practice status was made using a classification system that considers the presence of an affiliated medical school or radiology residency program (18).
Statistical Analysis
For 2004 and 2016, the distributions of all oncologic imaging and all oncologic advanced imaging were determined across all places of service mentioned above (emergency department, inpatient, etc.) and also across all places of service stratified by academic vs. nonacademic status (i.e., academic emergency departments and non-academic emergency departments, academic inpatient settings and non-academic inpatient settings, etc.). Then, the percentages of radiologists’ diagnostic imaging services and advanced diagnostic imaging services constituted by oncologic imaging were determined overall, for each place of service, and for each place of service stratified by academic vs. non-academic status. The percentages of advanced diagnostic imaging services that were oncologic at the state level were also calculated and depicted graphically. State-level population-normalized cancer prevalence was determined using data from the National Cancer Institute and U.S. Census Bureau and assessed for correlation with state-level oncologic fraction of advanced imaging using the Spearman correlation coefficient. State-level radiologist supply was determined using the Medicare Physician Compare National Downloadable File (19) and also assessed for correlation with state-level oncologic fraction of advanced imaging using the Spearman correlation coefficient. Correlations were also computed of both state-level radiologist supply and population-normalized cancer prevalence with state-level oncologic advanced diagnostic imaging utilization expressed as service counts per 1,000 Medicare fee-for-service beneficiaries. Data were compared between 2004 and 2016 using the compound annual growth rate (CAGR). P<0.05 was considered significant for all comparisons.
Given the possibility of the 5% Medicare sample not being generalizable, the percentage of advanced imaging (CT, MRI, and PET/CT) that was oncologic in nature, based on ICD-10 codes associated with the examinations, was determined for a large tertiary care academic medical center. This percentage was computed for all such examinations performed in adult patients within the health system from January through December 2018. For this portion of the investigation, institutional IRB approval was obtained, with a waiver of written informed consent.
Analysis was performed using SAS 9.4 (SAS Institute; Cary, North Carolina), Excel for Windows (Microsoft Corporation; Redmond, Washington), and MedCalc (MedCalc Software; Ostend, Begium).
Results
The national Medicare sample included 5,030,955 diagnostic imaging and 1,218,144 advanced diagnostic imaging examinations in 2004, and 5,017,287 diagnostic imaging and 1,503,490 advanced diagnostic imaging examinations in 2016. Table 1 shows the distributions of oncologic imaging by academic practice status and place of service in 2004 and 2016. The distributions were very similar for all oncologic imaging and all oncologic advanced imaging. In 2004 and 2016, the distributions of oncologic advanced diagnostic imaging were as follows: 58.1% and 58.0%, respectively, in hospital outpatient settings; 25.9% and 23.9%, respectively, in physician office settings; 14.4% and 7.7%, respectively, in inpatient settings; 1.2% and 2.9%, respectively, in ED settings; and 0.4% and 7.6%, respectively, in all other settings (Table 1). Between 2004 and 2016, the percentage of oncologic advanced imaging performed in academic practices increased from 18.8% to 34.1% (CAGR 5.1%). Stratification by both academic status and place of service showed that oncologic advanced imaging was most commonly performed in non-academic hospital outpatient facilities (37.4%) and academic hospital outpatient facilities (20.6%).
Table 1-.
Distribution of radiologist-performed non-invasive oncologic diagnostic imaging (ODI) by academic practice status and place of service. CAGR = compound annual growth rate.
| 2004 | 2016 | CAGR | |||||
|---|---|---|---|---|---|---|---|
| Academic Status | Place of Service | All ODI (218,294) | Advanced ODI (131,394) | All ODI (195,182) | Advanced ODI (142,459) | All ODI | Advanced ODI |
| All | ED | 1.3% (2,863) | 1.2% (1584) | 3.0% (5886) | 2.9% (4090) | 7.2% | 7.5% |
| All | Inpatient | 18.8% (41,075) | 14.4% (18,932) | 8.9% (17,344) | 7.7% (10,994) | −6.1% | −5.1% |
| All | Physician Office | 23.0% (50,161) | 25.9% (34,035) | 22.9% (44,715) | 23.9% (33,984) | 0.0% | −0.7% |
| All | Hospital Outpatient | 56.6% (123,461) | 58.1% (76,329) | 58.2% (113,546) | 58.0% (82,615) | 0.2% | 0.0% |
| All | Other | 0.3% (724) | 0.4% (514) | 7.0% (13,691) | 7.6% (10,776) | 29.0% | 28.0% |
| Academic | All | 17.6% (38,326) | 18.8% (24,752) | 33.2% (64,734) | 34.1% (48,549) | 5.4% | 5.1% |
| Academic | ED | 0.2% (533) | 0.2% (318) | 0.9% (1,709) | 0.9% (1,220) | 11.2% | 11.1% |
| Academic | Inpatient | 3.3% (7,129) | 2.8% (3,650) | 3.0% (5,909) | 2.6% (3,678) | −0.6% | −0.6% |
| Academic | Physician Office | 4.5% (9,763) | 5.2% (6,897) | 5.6% (10,990) | 5.8% (8,296) | 1.9% | 0.9% |
| Academic | Hospital Outpatient | 9.5% (20,751) | 10.5% (13,771) | 19.8% (38,646) | 20.6% (29,323) | 6.3% | 5.8% |
| Academic | Other | 0.1% (150) | 0.1% (116) | 3.8% (7,480) | 4.2% (6,032) | 39.8% | 38.1% |
| Non-academic | All | 82.4% (179,958) | 81.2% (106,642) | 66.8% (130,448) | 65.9% (93,910) | −1.7% | −1.7% |
| Non-academic | ED | 1.1% (2,330) | 1.0% (1,266) | 2.1% (4,177) | 2.0% (2,870) | 6.0% | 6.3% |
| Non-academic | Inpatient | 15.6% (33,946) | 11.6% (15,282) | 5.9% (11,435) | 5.1% (7,316) | −7.8% | −6.6% |
| Non-academic | Physician Office | 18.5% (40,398) | 20.7% (27,138) | 17.3% (33,725) | 18.0% (25,688) | −0.6% | −1.1% |
| Non-academic | Hospital Outpatient | 47.1% (102,710) | 47.6% (62,558) | 38.4% (74,900) | 37.4% (53,292) | −1.7% | −2.0% |
| Non-academic | Other | 0.3% (574) | 0.3% (398) | 3.2% (6,211) | 3.3% (4,744) | 23.1% | 22.1% |
In 2016, oncologic imaging represented 3.9% of all radiologist-performed diagnostic imaging and 9.5% of all radiologist-performed advanced diagnostic imaging (Table 2, supplemental Table 3 online). The oncologic share (or “fraction”) of advanced imaging was higher in the academic (12.1%) than in the non-academic (8.5%) practice setting. By place of service, the oncologic fraction of all advanced diagnostic imaging was higher in the hospital outpatient (18.1%) and physician office (15.3%) settings than in the inpatient (3.2%) or ED (0.9%) settings. It was highest (22.7%) in the academic hospital outpatient setting. Variations in the oncologic fraction of advanced imaging were statistically significant (p<0.001) for all subsets defined by academic practice status and place of service.
Table 2-.
Percentage of radiologist-performed non-invasive diagnostic imaging (DI) that is oncologic imaging. CAGR = compound annual growth rate.
| 2004 | 2016 | CAGR | |||||
|---|---|---|---|---|---|---|---|
| Academic Status | Place of Service | All DI | Advanced DI | All DI | Advanced DI | All DI | Advanced DI |
| All | All | 4.3% | 10.8% | 3.9% | 9.5% | −0.9% | −1.1% |
| All | ED | 0.4% | 1.0% | 0.5% | 0.9% | 1.6% | −0.6% |
| All | Inpatient | 2.1% | 4.7% | 1.3% | 3.2% | −3.9% | −3.1% |
| All | Physician Office | 6.7% | 16.0% | 5.7% | 15.3% | −1.3% | −0.4% |
| All | Hospital Outpatient | 7.3% | 17.2% | 7.1% | 18.1% | −0.3% | 0.4% |
| All | Other | 2.9% | 13.7% | 7.2% | 23.3% | 7.8% | 4.6% |
| Academic | All | 5.1% | 12.3% | 5.1% | 12.1% | 0.0% | −0.1% |
| Academic | ED | 0.6% | 1.3% | 0.7% | 1.2% | 1.2% | −0.9% |
| Academic | Inpatient | 2.5% | 5.5% | 1.7% | 4.0% | −3.0% | −2.5% |
| Academic | Physician Office | 7.5% | 17.0% | 5.6% | 14.3% | −2.3% | −1.4% |
| Academic | Hospital Outpatient | 8.6% | 19.7% | 9.7% | 22.7% | 1.0% | 1.2% |
| Academic | Other | 3.6% | 17.4% | 10.0% | 29.7% | 9.0% | 4.5% |
| Non-academic | All | 4.2% | 10.5% | 3.5% | 8.5% | −1.5% | −1.7% |
| Non-academic | ED | 0.4% | 1.0% | 0.5% | 0.9% | 1.3% | −0.9% |
| Non-academic | Inpatient | 2.1% | 4.5% | 1.2% | 2.9% | −4.6% | −3.7% |
| Non-academic | Physician Office | 6.5% | 15.8% | 5.7% | 15.7% | −1.0% | 0.0% |
| Non-academic | Hospital Outpatient | 7.1% | 16.8% | 6.3% | 16.2% | −1.1% | −0.3% |
| Non-academic | Other | 2.8% | 12.9% | 5.4% | 18.3% | 5.6% | 3.0% |
From 2004 to 2016, the oncologic fraction of advanced diagnostic imaging decreased slightly overall (CAGR −1.1%) and in the non-academic (CAGR −1.7%) setting and remained essentially stable in the academic (CAGR −0.1%) setting (Table 2). Aside from a slight increase (CAGR +0.4%) in the hospital outpatient setting, this fraction decreased in all practice settings analyzed, with the largest decrease being a CAGR of −3.1% in the inpatient setting. By combination of academic status and defined place of service, the only increase was for the hospital outpatient setting in academic practices (CAGR +1.2%) (Table 2).
Figure 1 presents state-level oncologic fractions of advanced diagnostic imaging in 2016. There was an approximately 3:1 ratio between the highest and lowest state-level fractions. The highest fractions were in Arkansas (15.3%), Kansas (13.0%), and South Dakota (12.8%), and the lowest were in Wyoming (4.9%), Nevada (5.6%), and Rhode Island (6.0%). The oncologic fraction of advanced imaging did not correlate significantly with state-level cancer prevalence (r=−0.118, p=0.410) or state-level radiologist supply (r=0.126, p=0.375).
Figure 1.
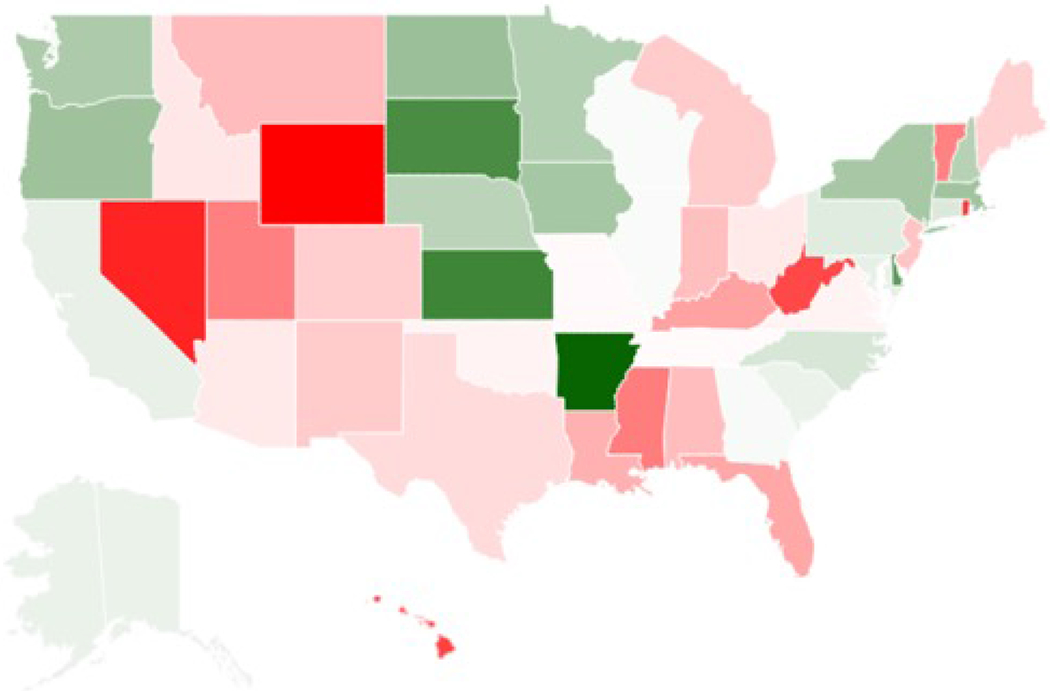
Chloropleth map depicting the state-level variation in the percent of radiologist-performed non-invasive advanced diagnostic imaging in 2016 that was oncologic in nature. White corresponds with the overall national rate (9.5%), deeper shades of green correspond with increasingly higher rates above the national above, and deeper shades of red correspond with decreasing rates below the national average.
There was an approximately 6:1 ratio between the highest and lowest state-level oncologic advanced diagnostic imaging utilization rates. The highest utilization was in Washington DC (162 examinations per 1,000 beneficiaries), Arkansas (118), Delaware (101), Kansas (94), and Massachusetts (91). The lowest utilization was in Wyoming (25), Vermont (28), New Mexico (41), Nevada (44), and Rhode Island (46). Oncologic advanced diagnostic imaging utilization showed a statistically significant moderate positive correlation with radiologist supply (r=+0.489; p<0.001), but no significant association with state-level cancer prevalence (r=−0.139; 0=0.329).
In the large tertiary care academic medical center, 9.1% (41,366/456,384) of all adult advanced imaging was oncologic in nature.
Discussion
To our knowledge, the present study was the first to use a national claims database to quantify oncologic diagnostic imaging exams and assess their distribution among different practice settings in the United States. We found that oncologic diagnostic imaging tests amounted to just 3.9% of all diagnostic imaging and 9.5% of advanced diagnostic imaging (i.e., CT, MRI and PET/CT) tests—percentages considerably lower than those often quoted anecdotally in the oncology community. We also found that radiologists’ exposure to oncologic imaging varied significantly across practice settings, lending further weight to existing concerns about the need to broaden access to cancer imaging expertise (6).
A few studies have attempted similar analyses, but none is directly comparable. For example, a study from Canada reported that oncologic indications accounted for approximately 23% of CT examinations, almost all PET/CT exams and a considerable share of MRI exams (20). However, that study was based largely on a survey of imaging facilities that called for subjective assessments of the distribution of indications rather than analysis of objective and reproducible exam-level claims data. Such subjective assessments are prone to biases and inaccuracies; furthermore, the majority of the surveyed sites failed to provide a response to the question at issue here, leading to potential sampling bias. A study from England noted that approximately 95% of CT units were used for cancer staging purposes (21), but such information fails to provide insight into the oncologic share of imaging at the examination level. The limitations of the existing literature in addressing this issue indicate the challenges of identifying the true oncologic share of advanced imaging and the value of our present investigation using an objective and reproducible methodology and a national claims dataset. Like our study, a recent study in Israel showed the oncologic share of imaging to be highly variable across settings, but it focused solely on the pediatric population (22).
We found that in 2016, approximately two-thirds of oncologic imaging was performed in non-academic practices, which aligns with NCI data indicating that the majority of cancer care takes place in community settings (3); however, the share of imaging that was oncologic was higher in academic than in nonacademic practices as well as in physician offices and hospital outpatient offices as compared to inpatient or ED settings. In addition, the share of advanced imaging that was oncologic varied considerably by state and failed to correlate with geographic variations in cancer prevalence or radiologist supply. Although the lack of such correlations could be due to multiple factors, it suggests a lack of adherence to oncologic imaging guidelines—such as those of the National Comprehensive Cancer Network—and highlights the importance of the clinical decision support initiative mandated by the Protecting Access to Medicare Act (PAMA). While state-level utilization of oncologic advanced imaging was likewise not correlated with cancer prevalence, it was significantly correlated with radiologist supply. The apparent role of the number of physicians—rather than actual cancer prevalence—in driving oncologic imaging utilization in a region further supports the need for more evidence-based decision-making regarding oncologic imaging.
Of note, we found that the share of oncologic imaging studies performed at academic practices had approximately doubled over the 12-year window leading into 2016. Drawing an analogy from cardiovascular (23) and surgical literature (24–26), such progressive concentration of oncologic imaging at academic centers signals a potential worsening of disparities in radiologists’ exposure to, and familiarity with, such exams, and consequently growing challenges in maintaining high-quality oncologic imaging services throughout the full breadth of radiology practices.
Action is urgently needed to disseminate the current expertise at cancer centers and major academic institutions to community and smaller radiology practices that may have very low oncologic imaging exposure. This could entail various changes to current organizational frameworks, similar to the kinds of initiatives that have been described for improving the quality of oncologic imaging globally (6, 27). Second-opinion networks and cancer imaging consortia could be established to create the infrastructure for rapid secondary interpretations by oncologic imaging experts through teleradiology (15). Telementoring, coaching, peer learning, and other forms of collaboration with feedback could also enhance the local expertise embedded within community settings (15, 28). For such approaches to be successful, formalized and longitudinal assessments and standards could be instituted to ensure ongoing accountability. Furthermore, drawing on the models provided by the Mammography Quality and Standards Act and American College of Radiology accreditation process for breast imaging, some form of accreditation or certification to recognize special competency in oncologic imaging (or in subspecialty areas of oncologic imaging other than breast imaging) could be established (15, 29). Also, in addition to more mini-fellowships and fellowships, maintenance-of-certification pathways in oncologic imaging could be developed (6, 27). Finally, more granular insight into the kinds of oncologic imaging performed in particular practice settings (e.g., distribution of initial diagnostic exams vs. subsequent scans for monitoring response to therapy, based on academic status, site of service, or geography) could also help determine the particular imaging knowledge and expertise that is required and help guide some of the outlined solutions.
Our estimate of the percentage of all Medicare advanced imaging that was oncologic in nature in 2016 (9.5%) was nearly identical to the percentage of adult advanced imaging we identified as oncologic in a single large academic medical center (9.1%). We expect these percentages to represent underestimates because we identified oncologic imaging based on the primary ICD codes associated with the examinations themselves. Thus, large volumes of imaging examinations performed in oncology patients but for other signs and symptoms, such as abdominal pain or suspected pulmonary embolus, were likely not categorized as oncologic, even though such examinations may be considerably more complex and difficult to interpret than those conducted in non-oncologic patients, involving greater physician time and benefitting from oncologic imaging expertise. For example, the radiologist would need to be familiar with imaging manifestations of radiation therapy, chemotherapy, immunotherapy, and other treatment regimens that might be contributing to the new clinical symptoms. In addition, cancer survivors remain at long-term risk for recurrent or secondary neoplastic disease as well as for complications of earlier therapies, such that follow-up imaging examinations in this cohort also present higher complexity, even if not done for an immediate oncologic indication. For these reasons, our methodology was likely highly specific in identifying oncologic imaging examinations and providing minimum estimates but still needs to be complemented with other approaches to estimate all oncologic imaging in cancer patients.
Limitations
In addition to the reliance on primary ICD codes to identify oncologic imaging exams, this study has a number of other limitations. First, given our underlying data source, we only assessed imaging performed for Medicare fee-for-service beneficiaries. Patients with private insurance, Medicaid, or Medicare Advantage plans were not evaluated. Because the older population enrolling in Medicare is anticipated to have a higher prevalence of cancer, it is possible that the oncologic fraction of imaging would in fact be even lower in the overall U.S. adult population. In addition, we only evaluated radiologist-performed diagnostic imaging. We did not assess imaging by non-radiologists (e.g., self-referral of oncologic imaging) or radiologist-performed interventional radiology services. Also, while we determined the oncologic fractions of radiologist-performed imaging and consider such fractions a marker of oncologic expertise, we are not able to assess the quality of the included imaging examinations or to evaluate the impact of the fraction on patient outcomes using our present approach. Finally, since we relied solely on ICD codes to identify oncologic imaging exams, we could not determine the reasons they were characterized as oncologic (e.g., whether they were performed for assessment of suspected cancer, evaluation of established cancer, treatment follow-up, or perhaps were done for other indications but yielded incidental findings of cancer).
Supplementary Material
Statement of Data Access and Integrity:
The authors declare that they had full access to all of the data in this study and the authors take complete responsibility for the integrity of the data and the accuracy of the data analysis.
Take-home Messages.
This study was the first to use a national claims database to gather information on the share of radiologist-performed diagnostic imaging done for oncologic indications in the US and on the distribution of such imaging among different care settings.
The oncologic share of advanced diagnostic imaging was found to be just 9.5%.
While from 2004 to 2016, the proportion of oncologic advanced imaging done in academic settings nearly doubled (rising from 18.8% to 34.1%), roughly two-thirds of oncologic advanced imaging continued to be done in non-academic practices in 2016.
At the state level, utilization of oncologic advanced imaging correlated with radiologist supply but not with cancer prevalence.
These findings indicate that strategies are warranted to disseminate oncologic imaging expertise throughout the radiology community. They also highlight a need for stricter application of evidence-based cancer imaging guidelines and clinical decision support nationwide.
Acknowledgments:
The authors thank Ada Muellner, MS for assistance in editing the manuscript.
Funding: The work of HH is supported by a P30 Cancer Center Support Grant (P30 CA008748) to Memorial Sloan Kettering Cancer Center (MSKCC), a National Cancer Institute-designated comprehensive cancer center. AR is supported by a research grant from the Harvey L. Neiman Health Policy Institute. Role of funding sources: Apart from the co-authors DH and LC (who are affiliated with the Harvey L. Neiman Health Policy Institute), no one else affiliated with the aforementioned funding sources played any role in the data analysis or the writing, editing or final approval of the manuscript.
Potential Conflicts of Interest (as disclosed on ICMJE form): Dr. Hricak reports that she receives compensation for her role as a member of the Board of Directors of Ion Beam Applications, a publicly traded company. Dr. Hughes reports grant funding from Harvey L. Neiman Health Policy Institute, outside the submitted work. Dr. Nass reports grants (sponsoring the National Cancer Policy Forum) from Bristol Myers Squibb, Helsinn Healthcare, Merck, Novartis, Pfizer, Flatiron Health, and the CEO Roundtable on Cancer, outside the submitted work. Dr. Rosenkrantz reports grant funding from Harvey L. Neiman Health Policy Institute, during the conduct of the study. The other authors report no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Employment Status: All authors are in the “non-partner/non-partnership track/employee” category.
References
- 1.Simon S American Cancer Society Facts & Figures 2019: US Cancer Death Rate has Dropped 27% in 25 Years. https://www.cancer.org/latest-news/facts-and-figures-2019.html. Published on January 8, 2019. Accessed on February 17, 2020.
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 3.Pfister DG, Rubin DM, Elkin EB, Neill US, Duck E, Radzyner M, Bach PB. Risk Adjusting Survival Outcomes in Hospitals That Treat Patients With Cancer Without Information on Cancer Stage. JAMA Oncol 2015;1(9):1303–1310. doi: 10.1001/jamaoncol.2015.3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagley AF, Anscher MS, Choi S, Frank SJ, Hoffman KE, Kuban DA, McGuire SE, Nguyen QN, Chapin B, Aparicio A, Pezzi TA, Smith GL, Smith BD, Hess K, Tang C. Association of Sociodemographic and Health-Related Factors With Receipt of Nondefinitive Therapy Among Younger Men With High-Risk Prostate Cancer. JAMA Netw Open 2020;3(3):e201255. doi: 10.1001/jamanetworkopen.2020.1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connor JP, Aboagye EO, Adams JE, Aerts HJ, Barrington SF, Beer AJ, Boellaard R, Bohndiek SE, Brady M, Brown G, Buckley DL, Chenevert TL, Clarke LP, Collette S, Cook GJ, deSouza NM, Dickson JC, Dive C, Evelhoch JL, Faivre-Finn C, Gallagher FA, Gilbert FJ, Gillies RJ, Goh V, Griffiths JR, Groves AM, Halligan S, Harris AL, Hawkes DJ, Hoekstra OS, Huang EP, Hutton BF, Jackson EF, Jayson GC, Jones A, Koh DM, Lacombe D, Lambin P, Lassau N, Leach MO, Lee TY, Leen EL, Lewis JS, Liu Y, Lythgoe MF, Manoharan P, Maxwell RJ, Miles KA, Morgan B, Morris S, Ng T, Padhani AR, Parker GJ, Partridge M, Pathak AP, Peet AC, Punwani S, Reynolds AR, Robinson SP, Shankar LK, Sharma RA, Soloviev D, Stroobants S, Sullivan DC, Taylor SA, Tofts PS, Tozer GM, van Herk M, Walker-Samuel S, Wason J, Williams KJ, Workman P, Yankeelov TE, Brindle KM, McShane LM, Jackson A, Waterton JC. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol 2017;14(3):169–186. doi: 10.1038/nrclinonc.2016.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nass SJ, Cogle CR, Brink JA, Langlotz CP, Balogh EP, Muellner A, Siegal D, Schilsky RL, Hricak H. Improving Cancer Diagnosis and Care: Patient Access to Oncologic Imaging Expertise. J Clin Oncol 2019;37(20):1690–1694. doi: 10.1200/JCO.18.01970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herold CJ, Lewin JS, Wibmer AG, Thrall JH, Krestin GP, Dixon AK, Schoenberg SO, Geckle RJ, Muellner A, Hricak H. Imaging in the Age of Precision Medicine: Summary of the Proceedings of the 10th Biannual Symposium of the International Society for Strategic Studies in Radiology. Radiology 2016;279(1):226–238. doi: 10.1148/radiol.2015150709 [DOI] [PubMed] [Google Scholar]
- 8.Thrall JH. Moreton Lecture: Imaging in the Age of Precision Medicine. J Am Coll Radiol 2015;12(10):1106–1111. doi: 10.1016/j.jacr.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 9.Coffey K, D’Alessio D, Keating DM, Morris EA. Second-Opinion Review of Breast Imaging at a Cancer Center: Is It Worthwhile? AJR Am J Roentgenol 2017;208(6):1386–1391. doi: 10.2214/AJR.16.16871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gollub MJ, Panicek DM, Bach AM, Penalver A, Castellino RA. Clinical importance of reinterpretation of body CT scans obtained elsewhere in patients referred for care at a tertiary cancer center. Radiology 1999;210(1):109–112. doi: 10.1148/radiology.210.1.r99ja47109 [DOI] [PubMed] [Google Scholar]
- 11.Lakhman Y, D’Anastasi M, Micco M, Scelzo C, Vargas HA, Nougaret S, Sosa RE, Chi DS, Abu-Rustum NR, Hricak H, Sala E. Second-Opinion Interpretations of Gynecologic Oncologic MRI Examinations by Sub-Specialized Radiologists Influence Patient Care. Eur Radiol 2016;26(7):2089–2098. doi: 10.1007/s00330-015-4040-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wibmer A, Vargas HA, Donahue TF, Zheng J, Moskowitz C, Eastham J, Sala E, Hricak H. Diagnosis of Extracapsular Extension of Prostate Cancer on Prostate MRI: Impact of Second-Opinion Readings by Subspecialized Genitourinary Oncologic Radiologists. AJR Am J Roentgenol 2015;205(1):W73–78. doi: 10.2214/AJR.14.13600 [DOI] [PubMed] [Google Scholar]
- 13.Rosenkrantz AB, Duszak R Jr., Babb JS, Glover M, Kang SK. Discrepancy Rates and Clinical Impact of Imaging Secondary Interpretations: A Systematic Review and Meta-Analysis. J Am Coll Radiol 2018;15(9):1222–1231. doi: 10.1016/j.jacr.2018.05.037 [DOI] [PubMed] [Google Scholar]
- 14.Rosenkrantz AB, Wang W, Hughes DR, Duszak R Jr. A County-Level Analysis of the US Radiologist Workforce: Physician Supply and Subspecialty Characteristics. J Am Coll Radiol 2018;15(4):601–606. doi: 10.1016/j.jacr.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 15.National Academies of Sciences, Engineering, and Medicine. 2018. Improving cancer diagnosis and care: Patient access to oncologic imaging and pathology expertise and technologies: Proceedings of a workshop Washington, DC: The National Academies Press; 10.17226/25163. [DOI] [PubMed] [Google Scholar]
- 16.IOM (Institute of Medicine). 2001. Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academy Press. [PubMed] [Google Scholar]
- 17.Harvey L Neiman Health Policy Institute. Neiman Imaging Types of Service. http://www.neimanhpi.org/neiman-imaging-types-of-service-nitos/.
- 18.Harvey L Neiman Health Policy Institute. Identification of Academic Radiology Practices. https://www.neimanhpi.org/academic-radiology-practices/. Accessed on April 24, 2020.
- 19.Centers for Medicare & Medicaid Services. Physician Compare. https://data.medicare.gov/data/physician-compare. Accessed on January 31, 2020.
- 20.Canadian Agency for Drugs and Technologies in Health. The Canadian Medical Imaging Inventory, 2017. https://cadth.ca/canadian-medical-imaging-inventory-2017. Accessed on February 2, 2020.
- 21.Clinical Imaging Board. CT Equipment, Operations, Capacity and Planning in the NHS, June 2015. https://www.rcr.ac.uk/sites/default/files/ct_equipment_in_the_nhs_report_cib_may_2015_v2_final240615.pdf. Accessed on February 2nd, 2020.
- 22.Chodick G, Levin M, Kleinerman RA, Shwarz M, Shalev V, Ashkenazi S, Horev G. Differences in characteristics of pediatric patients undergoing computed tomography between hospitals and primary care settings: implications for assessing cancer follow-up studies. Isr J Health Policy Res 2015;4:33. doi: 10.1186/s13584-015-0031-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao J, Redberg RF, Carroll JD, Marinac-Dabic D, Laschinger J, Thourani V, Mack M, Sedrakyan A. Association Between Hospital Surgical Aortic Valve Replacement Volume and Transcatheter Aortic Valve Replacement Outcomes. JAMA Cardiol 2018;3(11):1070–1078. doi: 10.1001/jamacardio.2018.3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abouassaly R, Finelli A, Tomlinson GA, Urbach DR, Alibhai SM. Volume-outcome relationships in the treatment of renal tumors. J Urol 2012;187(6):1984–1988. doi: 10.1016/j.juro.2012.01.076 [DOI] [PubMed] [Google Scholar]
- 25.Kulkarni GS, Urbach DR, Austin PC, Fleshner NE, Laupacis A. Higher surgeon and hospital volume improves long-term survival after radical cystectomy. Cancer 2013;119(19):3546–3554. doi: 10.1002/cncr.28235 [DOI] [PubMed] [Google Scholar]
- 26.Kutlu OC, Lee JE, Katz MH, Tzeng CD, Wolff RA, Varadhachary GR, Vauthey JN, Fleming JB, Conrad C. Open Pancreaticoduodenectomy Case Volume Predicts Outcome of Laparoscopic Approach: A Population-based Analysis. Ann Surg 2018;267(3):552–560. doi: 10.1097/SLA.0000000000002111 [DOI] [PubMed] [Google Scholar]
- 27.Schlemmer HP, Bittencourt LK, D’Anastasi M, Domingues R, Khong PL, Lockhat Z, Muellner A, Reiser MF, Schilsky RL, Hricak H. Global Challenges for Cancer Imaging. J Glob Oncol 2018;4:1–10. doi: 10.1200/JGO.17.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curci NE, Gartland P, Shankar PR, Montgomery JS, Miller DC, George AK, Davenport MS. Long-distance longitudinal prostate MRI quality assurance: from startup to 12 months. Abdom Radiol (NY) 2018;43(9):2505–2512. doi: 10.1007/s00261-018-1481-8 [DOI] [PubMed] [Google Scholar]
- 29.U.S. Food & Drug Administration. Mammogrqphy Quality Standards Act and Program. https://www.fda.gov/radiation-emittingproducts/mammographyqualitystandardsactandprogram/default.htm. Updated on December 3, 2018 Accessed on December 23, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


