Abstract
HIV attacks the body’s immune cells, frequently compromises the integrity of the blood–brain barrier (BBB), and infects the CNS in the early stages of infection. Dysfunction of the BBB further potentiates viral replication within the CNS, which can lead to HIV-associated neuropathology. Antiretroviral therapy (ART) significantly improves HIV patient outcomes and reduces mortality rates. However, there has been limited progress in targeting latent viral reservoirs within the CNS, which may eventually lead to rebound viremia. While ART drugs are shown to be effective in attenuating HIV replication in the periphery, the protection of the brain by the BBB offers an isolated sanctuary to harbor HIV and maintains chronic and persistent replication within the CNS. In this review, we elucidate the pathology of the BBB, its ability to potentiate viral replication, as well as current therapies and insufficiencies in treating HIV-infected individuals.
The CNS as a Sanctuary for HIV
Infection with HIV is an epidemic affecting nearly 38 million people worldwide [1]. Antiretroviral therapy (ART) is the modern-day force for eradicating HIV in peripheral blood circulation while also maintaining quiescent HIV levels in the CNS. Most drug cocktails consist of a combination of antiretrovirals with the goal of intensifying virus elimination by suppressing viral replication and reducing HIV RNA to untraceable levels in the blood. However, ineffective penetrance of ART into the brain can lead to HIV recurrence and increase the risk for neurological, metabolic, cardiovascular, and cerebrovascular comorbidities [2,3]. Along with risk for comorbidities, these drug cocktails are accompanied by major drug delivery challenges, mainly in relation to crossing the blood–brain barrier (BBB) (see Glossary). Successful drug passage across the BBB remains an unsolved problem; however, it is necessary in order to effectively target viral reservoirs in the CNS that can accumulate HIV proviruses. Thus, one of the major challenges of ART drugs, beyond the goal of eradicating HIV, is to penetrate the BBB without altering its neurological barrier integrity and CNS function.
This presents the paradox that we seek to review: how do foreign viral components cross the BBB and transfer into the CNS, whereas crucial therapeutic drugs cannot (Figure 1, Key Figure)? In this review, we describe the neuropathology of HIV and the BBB, how BBB disruption enables and potentiates proviral replication, as well as current therapies and their shortcomings in treating HIV-infected individuals. Additionally, we draw attention to attractive therapeutic solutions for overcoming HIV-associated barriers, including use of nanoparticles for effective passage and delivery of ART to the brain. These novel approaches for effective drug delivery are critical for advancing CNS-targeting drug development efforts.
Figure 1. The Central Paradox of HIV Infection in the Brain.
HIV has the innate ability to bind to receptors that are expressed on circulating leukocytes in the blood. Once bound, recruitment of these leukocytes into the brain across the blood-brain barrier (BBB) is possible via different hypothesized mechanisms. The ease at which these infected host cells migrate into the brain surpasses the ability for therapeutic drugs to complete the same task. ART circulates in peripheral blood and has been shown to adequately suppress HIV replication levels; however, the inability for ART to penetrate the BBB leads to a safe haven for HIV to accumulate and lie dormant. This is a major challenge with treating HIV and preventing disease recurrence.
The Landscape of HIV Infection in the CNS
HIV is a form of lentivirus that mainly attacks CD4+ T cells of the human immune system, in turn, diminishing the immune system’s responses to fight off foreign infections (Box 1). In addition, HIV frequently compromises the integrity of the BBB and infects the CNS in the early stages of infection. To prevent complete immune system monopolization and progression of HIV to advanced stages of AIDS, HIV patients are prescribed antiretroviral drug cocktails. These drugs do not eliminate the virus, rather use various strategies to inhibit HIV entry into host cells and/or suppress viral replication within host cells to reduce viral load.
Box 1. Structural and Regulatory Components of HIV.
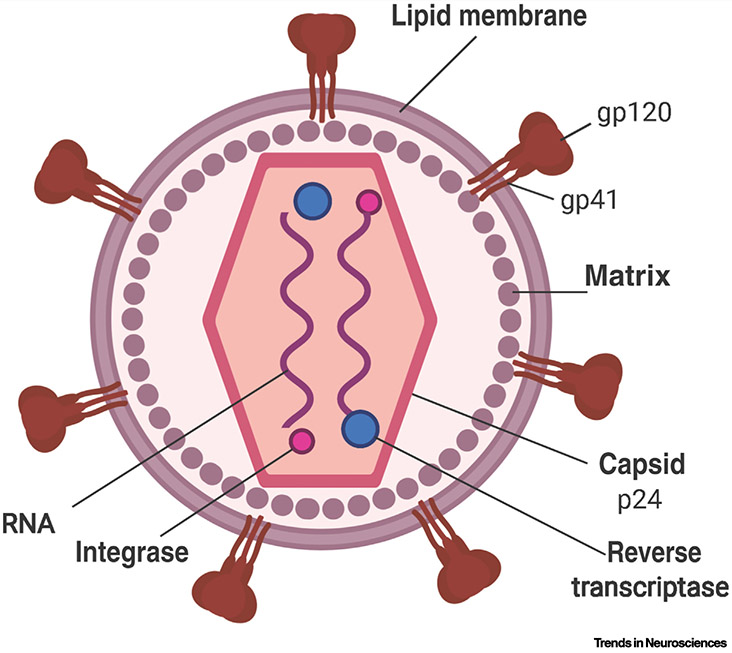
The HIV lentivirus structure is composed of two single strands of RNA enclosed within a capsid containing viral protein, p24, which is being utilized as an important marker for detecting HIV infection (Figure I). The RNA genome of HIV is highly organized into nine genes that can code for 15 different viral proteins. These include three structural genes: gag (group-specific antigen), pol (polymerase), and env (envelope); two essential regulatory genes: tat and rev; and four additional accessory genes nef, vpr, vpu, and vif [96]. These genes and their associated viral protein products are essential for viral replication and can be used as potential drug targets [97]. Anti-HIV peptides and small-molecule inhibitors have been used to target these specific genomic proteins, showing successful suppression of HIV RNA levels to below 50 copies/ml when measuring viral load [98]. The HIV RNA-enclosed capsid is surrounded by a lipoprotein-rich membrane. The surface glycoprotein (gp120) and transmembrane glycoprotein (gp41)are two crucial proteins embedded within this membrane that allow the virus to attack, bind, and transfect host cells. Gp120 binds to the cluster of differentiation 4 (CD4) receptors, which is expressed on the surfaces of multiple types of host cells that may traffic into the CNS, including T cell precursors (CD4+ T cells) in the bone marrow and thymus, monocytes, macrophages, and dendritic cells [99]. Infection with HIV is dependent upon an initial progression of protein–protein interactions. Upon the binding of surface gp120 to the CD4 receptor on a host cell, the viral lipoprotein membrane undergoes a structural change during which the gp120 protein domain is exposed, which allows for specific binding of chemokine receptors, or coreceptors of HIV, such as CCR5 and CXCR4. Furthermore, when gp120 binds to both CD4 glycoprotein and a chemokine coreceptor, the gp41 transmembrane protein contains a hydrophobic fusion domain that allows it to create a channel across the host cell’s plasma membrane. This results in the subsequent translocation of viral capsid into the host cell, activation of reverse transcriptase, and integration of the complementary DNA into the host cell’s nuclear DNA via integrase. This leads to continuous HIV replication and infection, which is the underlying mechanism of chronic HIV infection.
Figure I. The Structure of HIV.
The structure of HIV is depicted in this schematic. The viral RNA genome is enclosed within a protein capsid that is surrounded by a lipoprotein-rich membrane. The important structural proteins glycoprotein-120 (gp120) and glycoprotein-41 (gp41) are embedded within the lipid membrane and assist HIV in binding to receptors for viral transfection and fusion into the host cell. HIV contains an RNA genome consisting of two single strands at the core of the viral capsid. Reverse transcriptase is an enzyme responsible for catalyzing the transcription of viral RNA into complementary DNA (cDNA). HIV integrase is a crucial enzyme that can integrate HIV DNA into the host cell’s genome once it is transcribed. Once this viral DNA is inserted into a host cell, it is commonly referred to as proviral DNA.
The BBB as the Rate-Limiting Step in Drug Delivery
The BBB is a highly selective and impervious physiological barrier that separates the brain parenchyma from systemic blood circulation. Other barriers, such as the blood–cerebrospinal fluid (CSF) interface of the choroid plexus that is located in the ventricles of the brain, provide additional challenges for drug targeting and delivery into the brain. While these additional barriers exist, the BBB remains the largest drug delivery obstacle due to its expansive surface area and impermeable structure. Beyond this barrier, cells within the brain parenchyma are located 25 μm or less from brain capillaries. Therefore, once drugs pass the BBB, drug diffusion distances decrease and drug absorption into the brain is rapid [4]. The BBB is composed of a basement monolayer of microvascular endothelial cells that are linked via tight junction proteins that aim to preserve the microenvironment of the CNS by selective paracellular uptake of biomolecules into the brain [5].
The close proximity of endothelial cells in this structure limits the ability for molecules to paracellularly cross the BBB. Interestingly, some studies indicate that administration of ART can dysregulate the tight junction proteins, which is a major risk-factor induced by these drugs since it may contribute to HIV-associated neurocognitive disorder and neurological comorbidities [6]. To avoid this potential problem, alternative pathways need to be explored where the drug molecules are able to cross into the brain parenchyma via transcellular routes.
There are four molecular transcellular routes into the CNS: (i) lipid-mediated diffusion, (ii) carrier-mediated transport, (iii) receptor-mediated transport, or (iv) active efflux transport. Small, lipophilic therapeutic molecules less than 400 Da can successfully diffuse across the BBB via lipid-mediated diffusion [7]. However, these small molecules may be rapidly removed from the brain parenchyma via ATP-binding efflux pumps [i.e., p-glycoprotein, multidrug resistance proteins (MRP)] that are embedded within the endothelial monolayer [8,9]. These ATP-binding pumps are important for removal and circulation of biomolecules and drugs within the CNS. Consequentially, some ART drugs such as abacavir, efavirenz, and protease inhibitors, that are removed from the brain display a high brain efflux and therefore are unable to reach therapeutic concentrations in patients [10]. By targeting and providing antagonists of this pathway, one may be able to increase the bioavailability of therapeutics beyond the BBB and increase circulation time and efficacy. Overall, penetration of the BBB still remains the rate-limiting step in achieving efficient therapeutic interventions for most CNS diseases.
HIV Can Stealthily Breach the BBB
The most effective cellular route of HIV infection is by binding to a host cell via CD4 and chemokine receptors, CXCR4 and CCR5, followed by injecting its viral genome for replication [11]. These receptors are especially highly expressed on T lymphocytes and monocytes. HIV-infected T lymphocytes and monocytes circulate within the peripheral blood and can be recruited to cross the BBB [12,13]. It is hypothesized that these cells carry the HIV genome into the CNS in a Trojan horse-like mechanism, subsequently heightening chronic infection through the synergistic protection provided by the BBB [14]. Consequentially, once the virus has been replicated in macrophages and microglia within the CNS, it becomes difficult to eradicate. A more contested hypothesis suggests that viral entry of HIV across the BBB is due to the transcellular uptake of the virus by certain chemokine (i.e., APJ, CCR3, CXCR4, CCR5) receptors on the microvascular endothelial cells of the BBB (Figure 2) [15]. While CD4 receptors were described on brain microvascular endothelial cells [16], no active replication in brain micro vessels was demonstrated in HIV patients. In an in vitro model of the BBB, inhibition of chemokine receptors does not prevent HIV infection of microvascular endothelial cells [14]. The neuro-invasion mechanisms of HIV entering the CNS are enigmatic; however, the consequences of this process are crucial to understanding how to develop drugs that can target the brain.
Figure 2. Invasion of HIV into CNS via CD4+ T Cells and Monocytes.
Schematic of the hypothesized infiltration of HIV-infected immune cells. Receptors on CD4+ T cells (green) and monocytes (red) can bind to HIV, allowing for its replication and viral injection into a healthy host cell. The blood-brain barrier (BBB) expresses chemokines with chemokine-specific receptors for recruitment of immune cells, including T cell and monocytes depicted here. As a result, HIV can covertly cross the otherwise impermeable BBB, leading to invasion into the CNS.
Other routes of viral entry target the breakdown of the BBB structure. This may occur by altering the structure of tight junction proteins that tightly bind the microvascular endothelial cells (Figure 3). Several studies have shown that after an initial HIV infection, increased BBB permeability arises, contributing to significant neurological implications [17,18]. Part of this permeability change may be due to a reduction in pericyte coverage of the brain endothelium that may lead to monocyte neuro-infiltration, including trafficking of HIV-infected cells, and to elevated levels of proinflammatory cytokines [19]. Additionally, dysfunction of the BBB has been confirmed in the form of pericyte loss, contributing to the HIV-induced BBB dysregulation and associated comorbidities [20]. The importance of these events stems from the fact that endothelial cells are sensitive to inflammatory insult [21]. To quantify a neuro-invasion event, clinical studies have compared CSF albumin levels with serum albumin levels, represented as a quotient [QAlb] as the standard for determining BBB dysregulation in patients [22]. As a large macromolecule, albumin is unable to penetrate the BBB and should not be present at high concentrations within the CSF. Therefore, a low [QAlb] is indicative of a normal functioning BBB, while a high [QAlb] corresponds to a loss of tight junction protein integrity and subsequent dysregulation of the BBB. Clinical studies confirm that individuals with an acute HIV infection demonstrate early neuronal injury, viral infection, inflammatory onslaught of immune cells, and increased CSF to serum albumin quotients [23]. These clinical studies confirm the susceptibility of the BBB following early HIV infection, even though routes of entry are not entirely understood.
Figure 3. Comparison of Blood-Brain Barrier (BBB) Before and After Dysregulation and Subsequent HIV Infection.
In healthy individuals (left), the BBB functions as an impermeable structure to separate the CNS parenchyma from peripheral blood circulation and other pathogens. A main component of the BBB is endothelial cells, which are bridged by a tight junction protein complex. Illustrated are three critical tight junction proteins that make up the BBB: claudin (claudin-5 is the main claudin constituent of the BBB), zonula occludens (ZO-1, ZO-2, and ZO-3), and occludin. Right: HIV infection and long-term ART regimens can induce tight junction protein dysregulation, thereby potentiating further CNS HIV infection. Abbreviation: TJ, tight junction.
Several animal models of HIV infection have been developed to further assess whether the virus can cross into the brain parenchyma. In studies using simian immunodeficiency virus (SIV) in rhesus macaques, SIV-infected CD4+ T cells, macrophages, and dendritic cell markers were found in brain and bone marrow tissues. Additionally, SIV RNA was observed to be actively dividing, thus supporting the theory of clonal expansion of latent viral reservoirs in brain compartments [24,25]. Mice infected with a chimeric form of HIV, termed EcoHIV, have been shown to express the viral genome that was illuminated by the increased expression of C3, IL-1β, IL-6, CCL2, and STAT-1, which are factors that influence inflammatory responses to HIV in the brain [26]. EcoHIV induced antiviral responses and host cell infections while simultaneously downregulating tissue function and recovery that predispose the brain to cerebrovascular events [27,28].
The CNS Safeguards HIV Accumulation in Reservoirs Beyond the BBB
The phenomenon of HIV-associated reservoirs within the CNS is a more recently debated topic, yet it has already been observed in many animal models. Microglial cells make up the main reservoir of HIV within the CNS [29]. Additionally, in a T cell-only mouse model, mice were infected with HIV. Following ART initiation, CD4+ T cells were harvested from the virally suppressed mice. It was found that induction of HIV expression is possible ex vivo, suggesting latency was pre-established in these isolated CD4+ T cells in vivo [30]. Real-time PCR assays and genomic sequencing have also confirmed the presence of viral reservoirs within CD4+ T cells that can cause rebound viremia if ART drugs are terminated [25,28,31,32]. More recently, the novel development of an intact proviral DNA assay has enabled selective detection of the levels of proviral DNA within CD4+ T cells, thus discerning from any present defective proviruses [33]. However, there is still controversy as to how important T cells are in the HIV-infected brain in humans.
Other cell types have also been studied as potential hosts for latent HIV reservoirs within the CNS. Pericytes are still somewhat of an enigma in understanding how they interact with HIV. Recent studies have demonstrated their ability to shelter HIV as well as switch between latent and reactivated viral cycle stages. Latently infected pericytes were exposed to histone deacetylase inhibitors and tumor necrosis factor that resulted in increased p24 and HIV RNA levels, confirming a viral reactivation [34]. Pericytes express both CD4 and chemokine coreceptors, allowing them to be directly infected by HIV [20,35].
Beyond the neurovascular unit, perivascular spaces contain populations of cells capable of harboring HIV. In a macaque model, perivascular macrophages and microglia were shown to harbor SIV genomes, which could be reactivated, even after observed ART suppression, to actively reverse transcribe viral RNA to DNA upon QVOA analysis [36]. While there is still much debate with regards to the role that macrophages play in active viral reservoirs of HIV, findings in mice confirm the possible importance of this cell type. Indeed, studies indicated that HIV persists in humanized myeloid-only mice independent of other possible reservoir-capable cell types, such as T cells, supporting the role of macrophages in HIV replication and formation of viral reservoirs. This mouse model is generated by transplanting CD34+ hematopoietic stem cells into immunodeficient nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice, which are characterized by an absence of functional T and B cells [37,38]. Furthermore, there is mounting evidence that macrophages play an important role in their susceptibility to HIV even after ART initiation.
As evidenced by various animal models, the CNS can compartmentalize pockets of HIV in its latent form. CD4+ T cells can transition into a resting state that allows them to be the perfect host for a viral infection to enter a stage of dormancy [39-41]. The CD4+ T cells within viral reservoirs have been shown to have a half-life of 44 months even after ART administration; therefore, viral eradication by natural decay is highly unlikely [42]. For example, a latent viral reservoir consisting of just 1 million cells can potentially remain viable for 73 years [43]. It is also plausible to have multiple heterogeneous reservoirs consisting of millions of cells each, suggesting the difficulty in eradicating HIV due to reservoir buildup within the confines of the CNS. This environment of highly stable cells along with physical barriers offers protection for HIV strains to lie dormant. In humans, patients will typically display a CD4+ cell count below baseline, even after ART therapeutic regimens have begun, which signifies that HIV continues to suppress healthy cell cycle replication of CD4+ T cells, further indicating the inability of ART to adequately suppress viral replication beyond the BBB [44]. While the complete stability of a cell reservoir is still unclear, these models exemplify the longevity of HIV and the difficulty in eradicating it via ART or natural decay.
In addition to the BBB, viral entry can occur through the choroid plexus [45,46]. While both the BBB and choroid plexus offer protection to the CNS, the choroid plexus produces the CSF, which circulates molecules throughout the CNS. It is also well known that resident macrophages (i.e., the cells that frequently become infected with HIV in the CNS) can line the epithelium of the choroid plexus [47]. A feline model confirmed that feline immunodeficiency virus can cross into the brain through the choroid plexus via macrophages, T lymphocytes, and monocytes [48]. While these cells are dynamic in their movement across barriers, viral accumulation was observed to be significantly higher on the apical surface of this epithelial barrier. As a separate dynamic reservoir for HIV accumulation, the choroid plexus provides a possible path for neuro-invasion events and a conduit for future ART drug delivery. It should also be noted that the ease at which viruses can breach this epithelial barrier is coordinated by the high amount of MRP and P-glycoprotein expressed on the surface [49]. Paradoxically, the P-glycoprotein pump is oriented in a way that opposes the action of P-glycoprotein efflux transporter located in the BBB, whereby it prevents substrates and other molecules from escaping the CSF. This complex relationship further accentuates CNS and BBB homeostasis in trafficking therapeutics to the CNS.
HIV Therapeutics and Their Limitations in Targeting the Brain
A combination of three therapeutic antiretroviral drugs for HIV treatment was successfully introduced clinically in 1996; however, to date, there are no FDA approved ART agents that can diffuse across the BBB without altering its structural integrity [50]. The mechanism of BBB crossing of present FDA approved ART drugs involves transcellular uptake or rapid efflux across the BBB using transport proteins such as P-glycoprotein, MRP, and breast cancer resistance protein (BCRP) (Table 1). Ineffective ART delivery and suboptimal concentrations reaching the CNS are causes of HIV’s ability to manifest in the CNS and maintain a low level of replication [51]. This viral survival event can affect organs and tissues beyond the CNS, as is evident from its associated comorbidities. The ability for these drugs to penetrate the BBB depends on many coordinating factors, including molecular size and weight, protein-to-protein interactions, lipophilicity, molecular pump and uptake mechanisms, as well as physiochemical properties. Due to the recency of newer generations of ART drugs, lifetime longitudinal toxicity studies are somewhat limited. Neurovascular toxicity associated with taking ART as a chronic regimen has been documented as permitting mitochondrial dysfunction, disrupting or altering the BBB, neural progenitor cell senescence, and reducing electron transport chain function through Complex I [52-54]. While the clinical prescription of ART is the gold standard for suppressing actively replicating viral genomes in HIV-infected individuals, eliminating the virus is not foolproof and neurovascular toxicity should be further analyzed.
Table 1.
Uptake Mechanisms and Relative Concentrations of FDA Approved HIV Medications in the Braina
| Drug class | Drug name | Mechanism for BBB uptake | Plasma concentration [μmol/ml] |
CSF concentration [μmol/ml] |
Refs |
|---|---|---|---|---|---|
| CCR5 antagonists: block CCR5 HIV-coreceptors on the surface of immune cells. Inhibition of this pathway suppresses HIV entry into potential host cells. | Maraviroc | ND | 0.041–0.930 | 0.003–0.023 | [50,55,56] |
| Fusion inhibitors: block HIV from fusing with the host CD4 cellular receptor. | Enfuvirtide | ND | 3.69 | ND | [50,55,56] |
| Integrase inhibitors: block the enzyme HIV integrase, which covalently binds viral DNA and integrates it into the host DNA. | Dolutegravir | ND | ND | ND | [50] |
| Elvitegravir | ND | 0.005–0.026 | 0.0009–0.002 | [50,57] | |
| Raltegravir | ND | 0.083–11.6 | 0.004–0.283 | [50,55,56] | |
| Nucleoside reverse transcriptase inhibitors (NRTIs): NRTIs competitively inhibit reverse transcriptase, which is essential for HIV RNA to make DNA copies of itself. | Abacavir | P-glycoprotein, BCRP substrate | 5.2–10.9 | 0.5–1.8 | [50,55,56,58] |
| Emtricitabine | Inhibits MRP-1, MRP-2, MRP-3 | ND | 0.157–1.56 | [50,56,58,59] | |
| Lamivudine | Inhibits MRP-1, MRP-2, MRP-3 | 4.3–8.7 | 0.05–1.14 | [50,55,56,58] | |
| Tenofovir Disoproxil Fumarate | Inhibits MRP-1, MRP-2, MRP-3 | ND | 0.007–0.028 | [50,55,56,58,59] | |
| Zidovudine | P-glycoprotein, BCRP, MRP-4, and MRP-5 substrate | 4.5–6.7 | 0.21–0.41 | [50,55,56,58] | |
| Non-nucleoside reverse transcriptase inhibitors (NNRTIs): NNRTIs physically bind to reverse transcriptase, thereby preventing its binding to HIV RNA. | Doravirine | ND | ND | ND | [50] |
| Efavirenz | Induces P-glycoprotein, inhibits MRP-1, MRP-2, MRP-3 | 9.2–16.6 | 0.006–0.09 | [50,55,56,58-60] | |
| Etravirine | ND | 0.6 | 0.001 | [50,55,56] | |
| Nevirapine | Induces P-glycoprotein. Inhibits MRP-1, MRP-2, MRP-3 | 7.5–16.9 | 1.3–10.9 | [50,55,56,58,60] | |
| Rilpivirine | ND | ND | ND | [50] | |
| Pharmacokinetic enhancers: this medication is used along with other HIV medicines to enhance the effect of a particular medication within an ART regimen. | Cobicistat | ND | ND | ND | [50] |
| Post-attachment inhibitors: ibalizumab-uiyk is a humanized monoclonal antibody that acts as a CD4-directed post-attachment inhibitor. Recommended for the management of multidrug-resistant HIV infection. | Ibalizumab-uiyk | ND | ND | ND | [50] |
| Protease inhibitors (PIs): PIs inhibit the enzyme HIV protease, which is essential for HIV replication. | Atazanavir | P-glycoprotein substrate | 0.18–8.79 | 0.007–0.056 | [50,55,56] |
| Darunavir | P-glycoprotein substrate | 3.29–23.55 | 0.029–0.387 | [50,55,56] | |
| Fosamprenavir | P-glycoprotein substrate | ND | 0.017–0.210 | [50,55,56] | |
| Ritonavir | P-glycoprotein, MRP-1. MRP-2 substrate | 10.5–26.0 | ND–0.32 | [50,55,56,58] | |
| Saquinavir | P-glycoprotein, MRP-1. MRP-2 substrate | 1.84–3.23 | ND–0.008 | [50,55,56,58] | |
| Tipranavir | P-glycoprotein substrate | ND | ND | [50,56] |
Abbreviation: ND, not determined.
Antiretroviral Therapeutic Drugs Inability to Cross the BBB and Eradicate Viral Reservoirs
Currently, there is no cure for HIV and, consequently, ART drugs are the standard for HIV care. ART can successfully suppress HIV viral loads in peripheral blood circulation and also reduce the risk of transmission of HIV. An undetectable HIV RNA viral load equates to <50 copies/ml, while HIV suppression is associated with HIV RNA plasma levels below 200 copies/ml [61]. Conversely, rebound viremia is apparent once plasma viral loads are above 500 copies/ml after initial suppression using ART [62]. There is little doubt that ART drugs can reach the CNS, however their concentration is greatly diminished as compared with peripheral blood plasma levels. For instance, abacavir accumulates in the plasma at 5.2–10.9 μmol/ml while only reaching 0.5–1.8 μmol/ml in the CSF. Similarly, efavirenz accumulates in the plasma at 9.2–16.6 μmol/ml while only reaching 0.006–0.09 μmol/ml in the CSF [55]. As discussed earlier, while the BBB is most likely the rate-limiting step for ART to reach therapeutic concentrations in the CNS, an alternative route of entry may be through the blood–CSF barrier of the choroid plexus. The BBB demonstrates limited transport of many ART drugs into the brain; however, some studies suggest that cation/anion transporters in the choroid plexus can preferentially uptake drugs such as tenofovir disoproxil fumarate (PMPA) and lamivudine (3TC) [63,64].
Modern day ART therapeutics are typically administered in combination with each other based on their CNS penetration effectiveness (CPE) scores. A high CPE score correlates with a higher penetration across the BBB, whereas a low score indicates lower penetration; however, the risks associated with higher CPE scores and, therefore, higher concentrations of ART drugs is undetermined [65]. Even so, results from recent clinical trials did not observe any impact of CPE score on neurocognitive impairment [66]. HIV therapeutic regimens for antiretroviral-naïve patients are most often given as three combined medications from at least two distinct drug classes, typically consisting of two nucleoside reverse transcriptase inhibitors and one additional antiretroviral therapeutic [67]. These antiretroviral drugs are classified into eight categories that target the lifecycle of HIV.
The discovery of the aforementioned viral reservoirs within the CNS is indicative of ineffective ART drug penetration across the BBB. Therefore, adequate therapeutic concentrations within the CNS may not be reached in order to target latent viruses. These latent viruses have been shown to undergo clonal expansion events that are not targeted by current therapies. The HIV RNA genome contains a long terminal repeat (LTR) promoter region that has been shown to be important for driving cell-associated HIV expression [41]. Certain ART regimens do suppress ongoing viral replication; however, they do not attenuate the HIV LTR promoter region functionality. This allows the HIV proviruses to continue expressing RNA, produce viral loads, and eventual T cell activation [68]. This can lead to an event important for therapeutic targeting, namely, clonal expansion. It is estimated that over half of the cells in viral reservoirs are maintained via clonal expansion [69,70]. In that regard, for ART to be effective, these reservoirs must be targeted to prevent clonal expansion to surrounding tissues, while preserving normal functioning CNS cells.
Many studies have shown that HIV viral reservoirs in the CNS can continue to replicate due to poor ART drug accumulation. Subsequently, mutant-derived strains of HIV may arise due to antiretroviral multidrug resistance or discontinuation [71,72]. As a result, these strains of HIV may become distinct in their viral genome and difficult to target in comparison with parent HIV strains that can be more effectively targeted by existing drugs [73]. Eventually, rebound viremia may occur, causing de novo infection [41,74,75]. It should also be noted that if patients prematurely stop taking the prescribed ART treatments, there is a lack of viral reservoir targeting and the subsequent viremia and disease rebound is nearly inevitable. There is still some debate as to the extent of growth of latent reservoirs. For instance, studies indicate that there was no statistical significance in HIV-associated DNA, RNA, or infected cell levels before versus after treatment interruption; however, expanded clonal populations are undeniable [76,77]. Due to evidence of rebound viremia, even after undetectable viral plasma levels are established, attenuation of ART regimens is risky and generally not advised.
With a lack of fully effective ART, a bystander damaging effect may occur, where HIV-related secondary mechanisms promote apoptosis of uninfected cells that are in close proximity to infected cells. This event was comprehensively described in CD4 T cells; however, it may also affect neurons and glia. This process may also lead to downstream pyroptosis and inflammatory responses that lead to further comorbidities [78,79]. Therefore, reducing immune activation and preventing loss of immune cells are some of the main goals of maintaining ART regimens, in addition to their ability to prevent rebound viremia.
Nanomedicine as a Vehicle to Cross the BBB While Preserving its Neurological Integrity
Because ART drugs are administered orally, drug efficacy and bioavailability in the brain is reduced due to hepatic first-pass metabolism and slow absorption, in turn requiring a higher dosage and frequency of dosage to achieve desired effects [80]. In addition, the BBB remains one of the main obstacles for targeting a drug to the brain. Nanotechnology has recently been used in the design, formulation, and delivery of drugs that can overcome these challenges by improving BBB transmigration to increase drug delivery and minimize loss of ART drug load to the brain. A promising direction involves nanotechnology for optimization of molecule shape and size of the conventional ART drugs to achieve a nanoparticle to solve drug solubility and permeability issues [81-83].
In order to target ART drugs across the BBB, size considerations in the nanoscale should be utilized. To cross the BBB and ensure site-specific drug targeting, ART nanoparticles can be formulated to be even smaller than a typical HIV virus, which is around 100 nm in diameter, [84]. Typical nanoparticles for brain-specific drugs should be less than 120 nm and preferentially should be administered intranasally for direct delivery [85]. As a result, ART nanoparticles may effectively cross the BBB via transient pathways, without altering neurological integrity, and simultaneously preserve the innate therapeutic effects of the original drug. Other important factors limiting passage of nanoparticles into the brain include charge and surface moieties that can functionalize the nanoparticles to impart specificity to target desired receptors. One study used, for instance, PLGA-coated elvitegravir nanoparticles to increase transmigration across the BBB, decrease inflammation at the brain interface, and effectively suppress HIV replication [86]. In a separate in vitro model, surface-modified nanodiamonds were loaded with efavirenz to cross the BBB. In this case, nanodiamonds provided an inert, nontoxic carbon material for delivery of the therapeutic drug across the BBB, extended retention time of drug bioavailability in the CNS, and showed no deleterious effects on neuronal plasticity [87]. Functionalized surface moieties on nanoparticles may function to increase the binding surface area for desired drugs, thereby increasing drug bioavailability. By increasing bioavailability in restricted regions, nanomedicine may provide an avenue towards lower dosages of potentially neurotoxic ART drugs.
The use of nanoparticles and nanodrugs as carriers for therapeutic administration has the potential to improve the treatment of HIV in patients. In rodent models, nanomedicine-based approaches have demonstrated marked improvements in ART delivery, such as macrophage-mediated uptake of ART drugs [88-90]. An alternative approach that has been tested in mice for targeting proviral DNA in host genomes of latently infected cells is based on long-acting slow-effective release (LASER) ART and CRISPR-Cas9 injections to deliver hydrophobic lipophilic ART nanoparticles into the body, allowing for slow drug dissolution, macrophage uptake, and limited off-target toxicity [91]. Similar macrophage uptake has been observed in a long-acting dolutegravir prodrug encapsulated in a poloxamer nanoformulation [88]. To enhance transmigration across the BBB, nanoparticles utilizing a ferrous magnet-based liposome nanocarrier resulted in a 7.3-fold increase of transmigration with the synergistic support from transferrin receptors on the epithelium in vitro without affecting BBB integrity [92]. Similarly, the discovery of magnetic azidothymidine 5′-triphosphate (AZTTP) liposome demonstrated a threefold increase in migrating across the BBB compared with free AZTTP by utilizing the guidance of an external magnetic field to deliver the nanoparticle to a specific site of interest [93]. This approach, combined with the conjugation of ART, may potentially be used to target sequestered viral genomes and reach brain reservoirs in a controlled, sustained, and nontoxic manner. ART nanoparticles are envisioned to preserve the innate therapeutic and nontoxic properties of original drugs while increasing bioavailability in comparison with traditional pharmacokinetic properties [94]. Future directions suggest the use of nanomedicine as a vehicle for drug delivery that can be applied not only to targeting HIV viral reservoirs within the brain, but can be expanded to other targeted neurological therapeutics from treating diseases such as brain tumors, brain infections, brain degeneration, blood flow disruptions, and even autoimmune disorders. A novel drug delivery system of operationalizing ART nanoparticles may potentially overcome the shortcomings that conventional ART regimens in humans currently pose.
Concluding Remarks and Future Perspectives
HIV infection causes a rapid invasion of immune cells that inhibits the body’s ability to fight off diseases, leading to AIDS, if inadequately treated. Presently, ART is the gold standard for suppressing actively replicating viral genomes in HIV-infected individuals. While its effects have prolonged the life of patients infected with this disease, fully eradicating the virus has not been achieved. There are still many unresolved shortcomings of ART, including ineffective BBB penetration, failure to achieve necessary therapeutic concentrations within the CNS, and full elimination of CNS HIV reservoirs, which can lead to chronic HIV rebound viremia and reinfection. The presence of latent viral reservoirs within the brain that can potentiate HIV replication has focused efforts to develop drugs aimed at specific targeting. As one of the largest obstacles in the path of drug delivery, the necessity to overcome the BBB leaves many questions as to how to approach targeting reserves in the brain (see Outstanding Questions).
Outstanding Questions.
Through which pathways does infection of the brain with HIV potentiate BBB breakdown? To what extent does this breakdown further support entry of the virus into the CNS and/or viral escape back into the periphery?
There are many shortcomings to current antiretroviral drugs in relation to crossing the BBB. What entry method is best when formulating drugs to cross the BBB? Furthermore, if one could increase drug targeting across the BBB, would it be possible to also attenuate loss of barrier neurological integrity that leads to long-term neuroinflammation and comorbidities (e.g., HIV-associated neurocognitive disorders)?
To what extent can latent viruses in the brain have variable genomes that have selective advantages over a host immune response or are able to evade current ART drugs? If these HIV-mutants exist, therapeutics with high CPE scores might be ineffective towards genetic recombinants and new approaches to eradicating viral variants would be crucial.
What factors reactivate latent HIV reservoirs? Given canonical reactivation of latent viral cells in the brain, could latency reversal techniques be used as a strategy to target sequestered reservoirs that are not recognized by host immune cells? Would this strategy truly reduce viral reservoir size to prevent rebound viremia?
Can drug development strategies be improved to not only eradicate preexisting HIV infections, but also facilitate the development of novel vaccines to circumvent the disease altogether?
Nanomedicine has been proposed as a plausible approach to facilitate drugs crossing the BBB in a neuroprotective fashion, while preserving barrier integrity, limiting disruption of tight junction proteins, and delivering targeted therapeutic concentrations of drugs. Can nanotechnology approaches targeting HIV be designed to meet these goals?
As evident by studies in vitro, in animal models and clinical work, we are gaining a better understanding of critical factors that may prevent full systematic suppression of HIV replication within the body and, more specifically, the CNS. One of the future directions central to this field is the design of novel techniques to augment ART drug delivery across the BBB for increased targeted drug efficiency. Some of the treatment options to consider include increasing the penetration of FDA-approved ART agents across the BBB, while preserving physiological function of the brain, via the use of nanomedicine approaches. From a broader perspective, the use of nanomedicine has become paramount in efforts to develop HIV vaccines and microbicides, diagnostics, and therapeutics [95].
Highlights.
HIV can infect immune cells that are able to traffic into the brain by crossing the blood–brain barrier. Consequentially, HIV can disguise itself to bypass this biological barrier that restricts most other foreign molecules.
The vast majority of antiretroviral drugs are unable to effectively penetrate the BBB or are effectively removed from the brain parenchyma, resulting in ineffective elimination of HIV from the brain and the formation of reseivoirs.
There are heterogeneous cell reservoirs in the brain that are capable of harboring quiescent HIV. This buildup within the CNS can lead to viral recurrence and rebound infection.
Specific brain-targeting efficiency of clinically administered antiretroviral drugs is low. By using nano-formulations of these drugs, one could potentially increase bioavailability of effective drugs to targeted regions while preserving neurological integrity.
Acknowledgments
Supported by the National Institutes of Health (NIH), grants MH122235, MH072567, HL126559, DA044579, DA039576, DA040537, DA044579, and DA047157.
Glossary
- Blood–brain barrier (BBB)
an anatomo-physiological unit that interfaces between the blood and the brain. It is composed of brain vascular endothelial cells that are firmly bound by tight junction proteins and interact with surrounding astrocytes and pericytes. These cells interface with neurons to form a functional neurovascular unit that protects the brain from pathogens, maintains cerebral homeostasis, and regulates the exchange of molecules between blood and the CNS.
- Clonal expansion
HIV-infected cells that reside within the brain can maintain undergoing viral replication. These proliferating cell colonies are clones of original HIV-infected cells and can lie dormant within the CNS for an extensive period of time. This event is referred to as clonal expansion and is an important hallmark to the longevity of CNS viral reservoirs even during antiretroviral treatment.
- HIV-associated neurocognrtive disorder
more commonly referred to as HAND, this includes a broad range of comorbidities that arise due to immune activation as a result of infection with HIV.
- Latent viral reservoirs
HIV-infected cells incorporate HIV genome into their own DNA. The cells are able to enter a stage of dormancy by cessation of transcription machinery. Therefore, these cells become ‘invisible’ to the immune system and can evade the immune response. This makes developing drugs and eliminating HIV a difficult task.
- Proviruses
an inactive form of HIV that is incorporated into host cells as DNA. Once HIV infects a host, its RNA is reverse transcribed into DNA, which is termed the provirus. This viral DNA (provirus) is inserted into the host cell’s genome and, upon normal cell cycle replication, the virus is replicated into cell progeny.
- QVOA
an acronym for quantified viral outgrowth assay, which is important for measuring the level of viral reservoirs, particularly on CD4+ T cells. Typically, after ART administration, this assay can reverse latent cells to begin transcribing HIV. A high amount of HIV replication is indicative of possible viral reservoirs that were unaltered by ART. A downfall of QVOA is due to the heterogeneity of replication-competent cell reservoirs that have now been discovered. An accurate measurement of latent HIV that is present in the brain may be greatly underestimated by this assay.
- Rebound viremia
re-entering of HIV into the bloodstream via CSF HIV escape from CNS viral reservoirs. Rebound viremia can also occur due to patient noncompliance or premature discontinuation of ART regimens.
- Viral load
a common term used clinically to determine the amount of virus present in a specified volume of blood. In HIV-infected individuals, this quantity is usually expressed by measuring the amount of HIV RNA copies per milliliter of blood collected.
References
- 1.Centers for Disease Control and Prevention (2018) People with diagnosed HIV In Diagnoses of HIV Infection in the United States and Dependent Areas, 2018, CDC [Google Scholar]
- 2.Joseph J et al. (2016) HIV-1 induced CNS dysfunction: current overview and research priorities. Curr. HIV Res 14, 389–399 [DOI] [PubMed] [Google Scholar]
- 3.Gorantla S et al. (2012) Rodent models for HIV-associated neurocognitive disorders. Trends Neurosci. 35, 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbott NJ et al. (2010) Structure and function of the blood-brain barrier. Neurobiol. Dis 37, 13–25 [DOI] [PubMed] [Google Scholar]
- 5.Chow BW and Gu C (2015) The molecular constituents of the blood-brain barrier. Trends Neurosci. 38, 598–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahimy E et al. (2017) Blood-brain barrier disruption is initiated during primary HIV infection and not rapidly altered by antiretroviral therapy. J. Infect. Dis 215, 1132–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardridge WM (2012) Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab 32, 1959–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta P et al. (2015) Polymeric drug-delivery systems: role in P-gp efflux system inhibition. Crit Rev. Ther. Drug Carrier Syst 32, 247–275 [DOI] [PubMed] [Google Scholar]
- 9.Löscher W and Potschka H (2005) Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2, 86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harder BG et al. (2018) Developments in blood-brain barrier penetrance and drug repurposing for improved treatment of glioblastoma. Front. Oncol 8, 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alkhatib G and Berger EA (2007) HIV coreceptors: from discovery and designation to new paradigms and promise. Eur. J. Med. Res 12, 375–384 [PubMed] [Google Scholar]
- 12.Takeshita Y and Ransohoff RM (2012) Inflammatory cell trafficking across the blood-brain barrier: chemokine regulation and in vitro models. Immunol. Rev 248, 228–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larochelle C et al. (2011) How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 585, 3770–3780 [DOI] [PubMed] [Google Scholar]
- 14.Mukhtar M et al. (2002) Primary isolated human brain microvascular endothelial cells express diverse HIV/SIV-associated chemokine coreceptors and DC-SIGN and L-SIGN. Virology 297, 78–88 [DOI] [PubMed] [Google Scholar]
- 15.Argyris EG et al. (2003) Human immunodeficiency virus type 1 enters primary human brain microvascular endothelial cells by a mechanism involving cell surface proteoglycans independent of lipid rafts. J. Virol 77, 12140–12151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stins MF et al. (2004) CD4 and chemokine receptors on human brain microvascular endothelial cells, implications for human immunodeficiency virus type 1 pathogenesis. Endothelium 11, 275–284 [DOI] [PubMed] [Google Scholar]
- 17.Andersson LM et al. (2001) Increased blood-brain barrier permeability in neuro-asymptomatic HIV-1-infected individuals–correlation with cerebrospinal fluid HIV-1 RNA and neopterin levels. J. Neurovirol 7, 542–547 [DOI] [PubMed] [Google Scholar]
- 18.Leibrand CR et al. (2017) HIV-1 Tat disrupts blood-brain barrier integrity and increases phagocytic perivascular macrophages and microglia in the dorsal striatum of transgenic mice. Neurosci. Lett 640,136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piekna-Przybylska D et al. (2019) HIV-1 infection renders brain vascular pericytes susceptible to the extracellular glutamate. J. Neurovirol 25, 114–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohannon DG et al. (2019) Dysregulation of sonic hedgehog pathway and pericytes in the brain after lentiviral infection. J. Neuroinflammation 16, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YW et al. (2004) Estrogen-mediated protection against HIV Tat protein-induced inflammatory pathways in human vascular endothelial cells. Cardiovasc. Res 63, 139–148 [DOI] [PubMed] [Google Scholar]
- 22.Di Stefano A et al. (2019) Cerebrospinal fluid biomarkers in patients with central nervous system infections: a retrospective study. CNS Spectr. 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 23.Spudich S et al. (2011) Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J. Infect. Dis 204, 753–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pahar B et al. (2019) Quantification of viral RNA and DNA positive cells in tissues from simian immunodeficiency virus/simian human immunodeficiency virus infected controller and progressor rhesus macaques. Front. Microbiol 10, 2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long S et al. (2019) Evaluating the intactness of persistent viral genomes in simian immunodeficiency virus-infected rhesus macaques after initiating antiretroviral therapy within one year of infection. J. Virol 94, e01308–e01319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potash MJ et al. (2005) A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc. Natl. Acad. Sci. U. S. A 102, 3760–3765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He H et al. (2014) Enhanced human immunodeficiency virus Type 1 expression and neuropathogenesis in knockout mice lacking Type I interferon responses. J. Neuropathol. Exp. Neurol 73, 59–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertrand L et al. (2019) Targeting the HIV-infected brain to improve ischemic stroke outcome. Nat. Commun 10, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallet C et al. (2019) Microglial cells: the main HIV-1 reservoir in the brain. Front. Cell. Infect. Microbiol 9, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honeycutt JB et al. (2013) HIV-1 infection, response to treatment and establishment of viral latency in a novel humanized T cell-only mouse (TOM) model. Retrovirology 10, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abreu C et al. (2019) Brain macrophages harbor latent, infectious simian immunodeficiency virus. Aids 33, S181–S188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abreu CM et al. (2019) Myeloid and CD4 T cells comprise the latent reservoir in antiretroviral therapy-suppressed SIVmac251-infected macaques. mBio 10, e01659–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruner KM et al. (2019) A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 566, 120–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertrand L et al. (2019) Blood–brain barrier pericytes as a target for HIV-1 infection. Brain 142, 502–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagawa S et al. (2012) Infection of human pericytes by HIV-1 disrupts the integrity of the blood-brain barrier. J. Cell. Mol. Med 16, 2950–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nixon CC et al. (2020) Systemic HIV and SIV latency reversal via non-canonical NF-κB signalling in vivo. Nature 578, 160–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honeycutt JB et al. (2017) HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nat. Med 23, 638–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu CJ et al. (2018) EcoHIV infection of mice establishes latent viral reservoirs in T cells and active viral reservoirs in macrophages that are sufficient for induction of neurocognitive impairment. PLoS Pathog. 14, e1007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agosto LM et al. (2007) HIV-1 integrates into resting CD4+ T cells even at low inoculums as demonstrated with an improved assay for HIV-1 integration. Virology 368, 60–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horsburgh BA et al. (2020) High levels of genetically-intact HIV in HLA-DR+ memory T-cells indicates their value for reservoir studies. AIDS 34, 659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Scheerder MA et al. (2019) HIV rebound is predominantly fueled by genetically identical viral expansions from diverse reservoirs. Cell Host Microbe 26, 347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siliciano JD et al. (2003) Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med 9, 727–728 [DOI] [PubMed] [Google Scholar]
- 43.Crooks AM et al. (2015) Precise quantitation of the latent HIV-1 reservoir: implications for eradication strategies. J. Infect. Dis 212, 1361–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mzingwane ML and Tiemessen CT (2017) Mechanisms of HIV persistence in HIV resemoirs. Rev. Med. Virol 27, 28128885. [DOI] [PubMed] [Google Scholar]
- 45.Delery EC and MacLean AG (2019) Culture model for non-human primate choroid plexus. Front. Cell. Neurosci 13, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meeker RB et al. (2012) Cell trafffcking through the choroid plexus. Cell Adhes. Migr 6, 390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burkala EJ et al. (2005) Compartmentalization of HIV-1 in the central nervous system: role of the choroid plexus. AIDS 19, 675–684 [DOI] [PubMed] [Google Scholar]
- 48.Meeker RB et al. (2012) Transmigration of macrophages across the choroid plexus epithelium in response to the feline immunodeffciency virus. Cell Tissue Res. 347, 443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rao VV et al. (1999) Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood–cerebrospinal-fluid drug-permeability barrier. Proc. Natl. Acad. Sci. U. S. A 96, 3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Institutes of Health (2020) FDA-approved HIV medicines, NIH [Google Scholar]
- 51.Bertrand L et al. (2016) Solving the blood-brain barrier challenge for the effective treatment of HIV replication in the central nervous system. Curr. Pharm. Des 22, 5477–5486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertrand L et al. (2019) Cerebral vascular toxicity of antiretroviral therapy. J. NeuroImmune Pharmacol Published online June 17, 2019. 10.1007/s11481-019-09858-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Velichkovska M et al. (2019) Targeted mitochondrial COQ(10) delivery attenuates antiretroviral-drug-induced senescence of neural progenitor cells. Mol. Pharm 16, 724–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Apostolova N et al. (2015) Efavirenz and the CNS: what we already know and questions that need to be answered. J. Antimicrob. Chemother 70, 2693–2708 [DOI] [PubMed] [Google Scholar]
- 55.Ene L et al. (2011) How much do antiretroviral drugs penetrate into the central nervous system? J. Med. Life 4, 432–439 [PMC free article] [PubMed] [Google Scholar]
- 56.Yilmaz A et al. (2011) Antiretroviral drug treatment of CNS HIV-1 infection. J. Antimicrob. Chemother 67, 299–311 [DOI] [PubMed] [Google Scholar]
- 57.Calcagno A et al. (2016) Elvitegravir/cobicistat/tenofovir/emtricitabine penetration in the cerebrospinal fluid of three HIV-positive patients. AIDS Res. Hum. Retrovir 32, 409–411 [DOI] [PubMed] [Google Scholar]
- 58.Varatharajan L and Thomas SA (2009) The transport of anti-HIV drugs across blood–CNS interfaces: summary of current knowledge and recommendations for further research. Antivir. Res 82, A99–A109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiss J et al. (2007) Inhibition of MRP1/ABCC1, MRP2/ABCC2, and MRP3/ABCC3 by nucleoside, nucleotide, and non-nucleoside reverse transcriptase inhibitors. Drug Metab. Dispos 35, 340–344 [DOI] [PubMed] [Google Scholar]
- 60.Stormer E et al. (2002) Differential modulation of P-glycoprotein expression and activity by non-nucleoside HIV-1 reverse transcriptase inhibitors in cell culture. Pharm. Res 19, 1038–1045 [DOI] [PubMed] [Google Scholar]
- 61.Chun TW et al. (2010) Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: implications for eradication. AIDS 24, 2803–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greub G et al. (2002) Intermittent and sustained low-level HIV viral rebound in patients receiving potent antiretroviral therapy. AIDS 16, 1967–1969 [DOI] [PubMed] [Google Scholar]
- 63.Anthonypillai C et al. (2006) The distribution of the anti-HIV drug, tenofovir (PMPA), into the brain, CSF and choroid plexuses. Cerebrospinal Fluid Res. 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gibbs JE et al. (2003) Effect of transport inhibitors and additional anti-HIV drugs on the movement of lamivudine (3TC) across the guinea pig brain barriers. J. Pharmacol. Exp. Ther 306, 1035–1041 [DOI] [PubMed] [Google Scholar]
- 65.Letendre S et al. (2008) Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch. Neurol 65, 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santos GMA et al. (2019) Cross-sectional and cumulative longitudinal central nervous system penetration effectiveness scores are not associated with neurocognitive impairment in a well treated aging human immunodeffciency virus-positive population in Switzerland. Open Forum Infect. Dis 6, ofz277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pau AK and George JM (2014) Antiretroviral therapy: current drugs. Infect. Dis. Clin. N. Am 28, 371–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu R et al. (2020) The forces driving clonal expansion of the HIV-1 latent reservoir. Virol. J 17, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bui JK et al. (2017) Proviruses with identical sequences comprise a large fraction of the replication-competent HIV reservoir. PLoS Pathog. 13, e1006283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lorenzi JC et al. (2016) Paired quantitative and qualitative assessment of the replication-competent HIV-1 reservoir and comparison with integrated proviral DNA. Proc. Natl. Acad. Sci. U. S. A 113, E7908–E7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karade S et al. (2018) HIV drug resistance following a decade of the free antiretroviral therapy programme in India: a review. Int. J. Infect. Dis 66, 33–41 [DOI] [PubMed] [Google Scholar]
- 72.Bednar MM et al. (2016) Diversity and tropism of HIV-1 rebound virus populations in plasma level after treatment discontinuation. J. Infect. Dis 214, 403–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sunshine JE et al. (2015) Fitness-balanced escape determines resolution of dynamic founder virus escape processes in HIV-1 infection. J. Virol 89, 10303–10318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farhadian SF et al. (2019) Markers of CNS injury in adults living with HIV with CSF HIV not detected vs detected <20 copies/mL. Open Forum Infect. Dis 6, ofz528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Canestri A et al. (2010) Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin. Infect. Dis 50, 773–778 [DOI] [PubMed] [Google Scholar]
- 76.Salantes DB et al. (2018) HIV-1 latent reservoir size and diversity are stable following brief treatment interruption. J. Clin. Invest 128, 3102–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosas-Umbert M et al. (2017) Virological and immunological outcome of treatment interruption in HIV-1-infected subjects vaccinated with MVA-B. PLoS One 12, e0184929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meythaler M et al. (2009) Differential CD4+ T-lymphocyte apoptosis and bystander T-cell activation in rhesus macaques and sooty mangabeys during acute simian immunodeffciency virus infection. J. Virol 83, 572–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garg H et al. (2011) Single amino acid change in gp41 region of HIV-1 alters bystander apoptosis and CD4 decline in humanized mice. Virol. J 8, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tatham LM et al. (2015) Nanoformulation strategies for the enhanced oral bioavailability of antiretroviral therapeutics. Ther. Deliv 6, 469–490 [DOI] [PubMed] [Google Scholar]
- 81.Garrido C et al. (2015) Gold nanoparticles to improve HIV drug delivery. Future Med. Chem 7, 1097–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wan L et al. (2007) Peritoneal macrophage uptake, pharmacokinetics and biodistribution of macrophage-targeted PEG-fMLF (N-formyl-methionyl-leucyl-phenylalanine) nanocarriers for improving HIV drug delivery. Pharm. Res 24, 2110–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khandalavala K et al. (2017) Nanoparticle encapsulation for antiretroviral pre-exposure prophylaxis. J. Nanotechnol. Mater. Sci 4, 53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh R and Lillard JW Jr. (2009) Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol 86, 215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nair M et al. (2016) Getting into the brain: potential of nanotechnology in the management of NeuroAIDS. Adv. Drug Deliv. Rev 103, 202–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gong Y et al. (2020) Novel elvitegravir nanoformulation for drug delivery across the blood-brain barrier to achieve HIV-1 suppression in the CNS macrophages. Sci. Rep 10, 3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roy U et al. (2018) Characterization of nanodiamond-based anti-HIV drug delivery to the brain. Sci. Rep 8, 1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sillman B et al. (2018) Creation ofalong-acting nanoformulated dolutegravir. Nat. Commun 9, 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo D et al. (2017) Creation of a long-acting nanoformulated 2′,3′-dideoxy-3′-thiacytidine. JAIDS J. Acquir. Immune Defic. Syndr 74, e75–e83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Singh D et al. (2016) Development and characterization of a long-acting nanoformulated abacavir prodrug. Nanomedicine 11, 1913–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dash PK et al. (2019) Sequential LASER ART and CRISPR treatments eliminate HIV-1 in a subset of infected humanized mice. Nat. Commun 10, 2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thomsen LB et al. (2019)Evaluation of targeted delivery to the brain using magnetic immunoliposomes and magnetic force. Materials (Basel) 12, 3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nair M et al. (2013) Externally controlled on-demand release of anti-HIV drug using magneto-electric nanoparticles as carriers. Nat. Commun 4, 1707. [DOI] [PubMed] [Google Scholar]
- 94.Sneha R et al. (2018) Design of antiretroviral drug-polymeric nanoparticles laden buccal films for chronic HIV therapy in paediatrics. Colloid Interface Sci. Commun 27, 49–59 [Google Scholar]
- 95.Mamo T et al. (2010) Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine (London) 5, 269–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li G et al. (2015) An integrated map of HIV genome-wide variation from a population perspective. Retrovirology 12, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Engelman A and Cherepanov P (2012) The structural biology of HIV-1: mechanistic and therapeutic insights. Nat. Rev. Microbiol 10, 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perry CM (2014) Elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate single-tablet regimen (Stribild(R)): a review of its use in the management of HIV-1 infection in adults. Drugs 74, 75–97 [DOI] [PubMed] [Google Scholar]
- 99.Mundt S et al. (2019) The CNS immune landscape from the viewpoint of a T cell. Trends Neurosci. 42, 667–679 [DOI] [PubMed] [Google Scholar]