Abstract
Objective
To estimate whether the frequency of adverse maternal and neonatal outcomes differs between low-risk nulliparous and multiparous women at 39 to 41 weeks.
Methods
This is a secondary analysis of an observational obstetrics cohort of maternal-neonatal dyads at 25 hospitals. Low-risk women with non-anomalous singletons who delivered between 390 and 416 weeks were included. The composite neonatal adverse outcome included Apgar score < 5 at 5 min, ventilator support or cardiopulmonary resuscitation, seizure, hypoxic ischemic encephalopathy, sepsis, bronchopulmonary dysplasia, persistent pulmonary hypertension, necrotizing enterocolitis, birth injury or perinatal death. The composite maternal adverse outcome included infection, 3rd or 4th degree perineal laceration, thromboembolism, transfusion of blood products, or maternal death. Small-gestational-age (SGA), large-gestational-age (LGA), and shoulder dystocia requiring maneuvers were also evaluated. Multivariable regression was used to estimate adjusted relative risks (aRR) and odds ratios (aOR) with 95% confidence intervals (CI).
Results
Of the 115,502 women in the overall cohort, 39,870 (34.5%) met eligibility criteria for this analysis; 18,245 (45.8%) were nulliparous. The risk of the composite neonatal adverse outcome (1.5% vs 1.0%, aRR 1.80, 95% CI 1.48–2.19), composite maternal adverse outcome (15.1% vs. 3.3%, aRR 5.04, 95% CI 4.62–5.49) and SGA (8.9% vs. 5.8%, aOR 1.45, 95% CI 1.33–1.57) was significantly higher in nulliparous than multiparous patients. The risk of LGA (aOR 0.65, 95% CI 0.60–0.71) and shoulder dystocia with maneuvers (aRR 0.68, 95% CI 0.60–0.77) was significantly lower in nulliparous rather than multiparous patients.
Conclusions
The risk of composite adverse outcomes and SGA among low-risk nulliparous women at 39–41 weeks is significantly higher than among multiparous counterparts. However, nulliparous woomen had a lower risk of shoulder dystocia with maneuvers and LGA.
Précis
Nulliparity was associated with a significantly higher likelihood of composite adverse neonatal and maternal outcomes than multiparity at 39 to 41 weeks of gestation in low-risk pregnancies.
Introduction
In 2018, upwards of 2.47 million children were born at 39 weeks of gestation or later, with most being delivered to low-risk women (1–3). Randomized trials (4), population-based reports (2–7) and meta-analyses (8,9) all indicate that adverse outcomes for the maternal-newborn dyad incrementally increase with advancing gestational age after 39 weeks. The higher likelihood of maternal complications include hypertensive disorder of pregnancy, cesarean delivery, 3rd or 4th degree lacerations, or transfusion; the increasing rate of neonatal complications includes low Apgar score at 5 min, shoulder dystocia, admission to neonatal intensive care unit, seizure, or perinatal mortality (2–11). Yet, it remains uncertain whether and to what degree there is a differential rate of complications for low-risk nulliparous versus multiparous women at 39 weeks of gestation and beyond.
The primary objective of our analysis was to compare a composite of adverse neonatal outcomes among low-risk nulliparous versus multiparous women at 39–41 weeks who underwent labor with the intent to deliver vaginally. The secondary purpose was to compare the composite of adverse maternal outcomes among these two groups. Small- and large-for gestational age (SGA, LGA) and shoulder dystocia were also evaluated. We hypothesized that adverse outcomes among low-risk women are significantly more likely for nulliparous than multiparous patients.
Methods
We undertook a secondary analysis of an observational obstetric cohort, Assessment of Perinatal Excellence (APEX), of maternal-newborn dyads born in 25 geographically-dispersed medical centers of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Maternal characteristics, peripartum outcomes, and neonatal morbidities were culled by trained research staff for all women who had a live fetus on admission and delivered a newborn of at least 23 weeks of gestation on randomly selected days. The data collected represented one third of deliveries over a 3-year period. Neonatal data were collected until discharge, death, or 120 days of age, whichever came first. Under the waiver of informed consent, the institutional review board at each participating center approved the study. Further details of this study have been published previously (12).
Women were included in this secondary analysis if they delivered non-anomalous singletons between 390 and 416 weeks of gestation with a live fetus at the start of the intrapartum period, and had a pregnancy that had been dated by last menstrual period concordant with first or second trimester ultrasound, first or second trimester ultrasound alone, or assisted reproductive technology. A woman was considered to be high-risk, and excluded, if any of the following were present: diabetes (pregestational or gestational), chronic hypertension, history of deep venous thrombus or pulmonary embolism, thrombophilia, anticoagulant use, placenta previa, or – at admission for delivery – deep venous thrombosis, asthma exacerbation, hypertensive disorder of pregnancy (HDP; gestational hypertension, preeclampsia, or eclampsia), or abruption. In the absence of these conditions, women were considered to have low-risk pregnancies. This analysis was also restricted to women who labored with a fetus in the vertex position and excluded women with a scheduled cesarean or those missing data on parity.
The composite of adverse neonatal outcomes consisted of any of the following: Apgar score < 5 at 5 min, ventilator support within 24 hrs of birth (mechanical ventilation; not continuous positive airway pressure), cardiopulmonary resuscitation, hypoxic ischemic encephalopathy, seizure, sepsis, bronchopulmonary dysplasia, persistent pulmonary hypertension, necrotizing enterocolitis class 2 or 3, intraventricular hemorrhage grade III / IV, fracture, neonatal brachial plexus palsy, facial nerve palsy, or perinatal death. The composite adverse maternal outcomes included any of the following: endometritis, antibiotics for chorioamnionitis, 3rd or 4th degree laceration, deep venous thrombosis / pulmonary embolism, transfusion of blood products, wound infection or maternal death. Using the nomogram by Alexander et al (13 and personal communication), which corrected for sex, maternal race/ethnicity, newborns were categorized as: small for gestational age (SGA; birth weight < 10th percentile gestational age); appropriate for gestational age (AGA; birthweight of 10th to 90th percentile for gestational age) and large for gestational age (LGA; birthweight greater than 90th for gestational age). An additional outcome included shoulder dystocia requiring maneuvers (i.e. McRoberts, suprapubic pressure, rotational maneuvers, or extraction of the posterior arm).
Comparative analyses between nulliparous and multiparous women were performed with the chi-square test or Fisher’s exact test for categorical variables and the Wilcoxon rank sum test for continuous variables. For descriptive purposes we evaluated the frequency of outcomes by gestational age using the Cochran-Armitage test for trend. Adjusted relative risks (aRR) with 95% confidence intervals (CI), accounting for potential confounding factors (maternal age, race/ethnicity, body mass index at delivery, and insurance), were estimated with modified Poisson models for the binomial outcomes. Adjusted odds ratios (aOR) with 95%CIs were estimated with multinomial logistic regression for the multinomial outcome of size for gestational age. Because cesarean delivery and time from labor and delivery admission to delivery or cesarean decision were considered potentially in the causal pathway for the composite outcomes,they were not adjusted for in the main multivariable models, but were in sensitivity analyses for the composite outcomes. An additional sensitivity analysis included birthwight in the model evaluating the association between parity and shoulder dystocia with maneuvers. We also evaluated whether associations varied by gestational week and included interaction terms between parity and gestational age at delivery in multivariable models. SAS software version 9.4 was used for the analyses. All tests were two-tailed, and p<.05 was used to define statistical significance. No imputation for missing data was performed.
Results
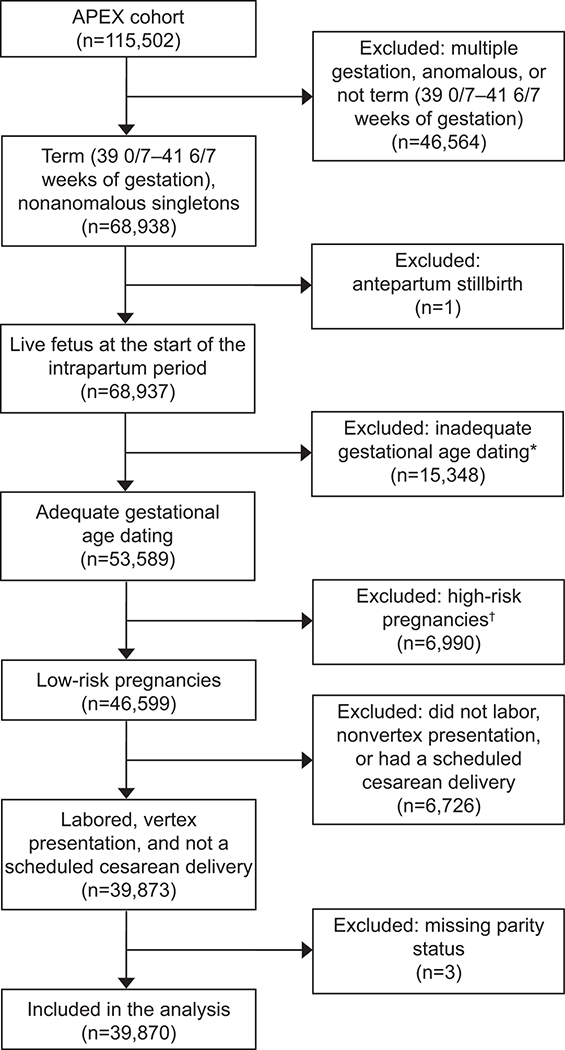
Of the 115, 502 women in the APEX study, 39,870 (34.5%) were eligible for this analysis (Fig 1). Among eligible women, 45.8% (N=18,245) were nulliparous and 54.2% (N=21,625) multiparous.
Figure 1:
Flowchart of eligibility determination for inclusion in this secondary analysis. APEX, Assessment of Perinatal Excellence (for details see reference 12). *Confirmed by first or second trimester ultrasonogram or assisted conception. †Pregnancy complicated by chronic hypertension, diabetes, history of thromboembolism, thrombophilia, anticoagulation use, abruption, previa, delivery admission for evaluation of a maternal medical condition (eg, asthma exacerbation, seizures), or pregnancy-associated hypertension before admission.
Several maternal characteristics differed significantly between low-risk nulliparous and multiparous women, including maternal age, race /ethnicity, insurance status, alcohol consumption, and body mass index at delivery (Table 1). The intrapartum course differed between the two groups by whether membranes were ruptured artificially, type of labor, gestational age at delivery, time from labor and delivery admission to delivery or cesarean decision, regional anesthesia, mode of delivery, birthweight, and the likelihood of newborn weighing at least 4,000 g (Table 2). Among those who had a cesarean delivery, the frequency of nonreassuring fetal status as the indication for cesarean was 32% in the nulliparas and 34% in the multiparas. Significant trends across gestational age were observed for each of the outcomes (Table 3). The frequencies of the neonatal composite, the maternal composite, shoulder dystocial requiring maneuvers, and LGA increased with increasing gestational age at delivery, whereas the frequency of small for gestational age decreased. None of the associations between parity and outcomes varied by gestational week (all tests for interaction p>0.05).
Table 1.
Baseline maternal characteristics by parity
| Nulliparous n=18,245 | Multiparous n=21,625 | P* | |
|---|---|---|---|
| Age, yr | <0.001 | ||
| <20 | 3,280 (18.0) | 633 (2.9) | |
| 20–24.9 | 5,061 (27.7) | 4,190 (19.4) | |
| 25–29.9 | 4,743 (26.0) | 6,586 (30.5) | |
| 30–34.9 | 3,689 (20.2) | 6,423 (29.7) | |
| ≥35 | 1,472 (8.1) | 3,793 (17.5) | |
| Race and ethnicity | <0.001 | ||
| White | 9,270 (50.8) | 9,550 (44.2) | |
| Black | 3,446 (18.9) | 3,824 (17.7) | |
| Asian | 1,151 (6.3) | 820 (3.8) | |
| Hispanic | 3,381 (18.5) | 6,478 (30.0) | |
| Other | 997 (5.5) | 953 (4.4) | |
| Insurance† | <0.001 | ||
| Private | 10,303 (56.9) | 10,009 (46.6) | |
| Government assisted | 6,384 (35.2) | 8,541 (39.8) | |
| Uninsured | 1,431 (7.9) | 2,934 (13.7) | |
| Smoked cigarettes† | 1,504 (8.3) | 1,833 (8.5) | 0.40 |
| Drank alcohol† | 704 (3.9) | 573 (2.7) | <0.001 |
| BMI at delivery, kg/m2† | 29 (27–33) | 30 (27–34) | <0.001 |
| BMI at delivery, kg/m2† | <0.001 | ||
| <25 | 2,382 (13.2) | 2,454 (11.6) | |
| 25–29 | 7,562 (42.0) | 8,096 (38.4) | |
| 30–34 | 5,020 (27.9) | 6,497 (30.8) | |
| 35–39 | 1,953 (10.9) | 2,658 (12.6) | |
| ≥40 | 1,076 (6.0) | 1,406 (6.7) |
Data are n (%) or median (IQR) unless otherwise specified
BMI, body mass index
Based on χ2 or Wilcoxon
Missing in 766 (BMI); 22 (smoking); 43 (drinking); 268 (insurance)
Table 2.
Labor and delivery characteristics by parity
| Nulliparous n=18,245 |
Multiparous n=21,625 |
P* | |
|---|---|---|---|
| Artificial rupture of membrane | 10,780 (59.4) | 14,091 (65.7) | <0.001 |
| Type of labor | <0.001 | ||
| Spontaneous | 4,731 (25.9) | 9,388 (43.4) | |
| Spontaneous, augmented | 7,265 (39.8) | 5,257 (24.3) | |
| Induced | 6,249 (34.3) | 6,980 (32.3) | |
| Induction indication† | <0.001 | ||
| Not induced | 11,996 (65.8) | 14,645 (67.7) | |
| Intrauterine growth restriction | 122 (0.7) | 89 (0.4) | |
| Oligohydramnios | 710 (3.9) | 399 (1.9) | |
| Nonreassuring fetal status | 322 (1.8) | 267 (1.2) | |
| Prelabor rupture of membranes | 834 (4.6) | 333 (1.5) | |
| Macrosomia | 76 (0.4) | 137 (0.6) | |
| Post term | 2,907 (15.9) | 1,874 (8.7) | |
| Elective | 1,029 (5.6) | 3,518 (16.3) | |
| Other or missing indication | 249 (1.4) | 363 (1.7) | |
| Gestational age at delivery, wk | <0.001 | ||
| 39.0 to 39.6 wk | 6,882 (37.7) | 11,491 (53.1) | |
| 40.0 to 40.6 wk | 7,620 (41.8) | 7,771 (35.9) | |
| 41.0 to 41.6 wk | 3,743 (20.5) | 2,363 (10.9) | |
| Regional anesthesia (epidural or spinal) | 16,408 (89.9) | 15,788 (73.0) | <0.001 |
| Time from L&D admission to delivery or cesarean decision, hrs | 11.1 (7.3–16.3) | 6.0 (3.2–9.2) | <0.001 |
| Mode of delivery† | <0.001 | ||
| Non-operative vaginal | 12,012 (65.8) | 19,155 (88.6) | |
| Operative vaginal (forceps or vacuum) | 1,916 (10.5) | 815 (3.8) | |
| Cesarean | 4,316 (23.7) | 1,655 (7.7) | |
| Cesarean indication† | <0.001 | ||
| Not delivered by cesarean | 13,928 (76.3) | 19,970 (92.4) | |
| Nonreassuring fetal status | 1,383 (7.6) | 570 (2.6) | |
| Labor dystocia or failed induction | 2,774 (15.2) | 567 (2.6) | |
| Prior cesarean | 0 (0.0) | 412 (1.9) | |
| Other or missing indication | 160 (0.9) | 106 (0.5) | |
| Male fetal sex | 9,102 (49.9) | 10,851 (50.2) | 0.56 |
| Birthweight, g | 3410 (3148–3700) | 3490 (3215–3780) | <0.001 |
| Birthweight ≥ 4,000 g | 1609 (8.8) | 2658 (12.3) | <0.001 |
L&D, labor and delivery
Data are n (%) or median (IQR) unless otherwise specified
Based on χ2 or Wilcoxon
Missing in 241 (type of membrane rupture); 13 (induction indication); 1 (regional anesthesia and mode of delivery); 14 (cesarean indication)
Table 3.
Outcomes by gestational age at delivery
| 39 weeks n=18,373 | 40 weeks n=15,391 | 41 weeks n=6,106 | Cochran-Armitage P for trend | |
|---|---|---|---|---|
| Composite of neonatal adverse outcomes† | 192 (1.0) | 198 (1.3) | 99 (1.6) | <0.001 |
| Composite of maternal adverse outcomes | 1,222 (6.7) | 1,474 (9.6) | 778 (12.7) | <0.001 |
| Shoulder dystocia requiring maneuvers | 430 (2.3) | 472 (3.1) | 192 (3.1) | <0.001 |
| Size for gestational age | ||||
| Small | 1,522 (8.3) | 1,023 (6.6) | 324 (5.3) | <0.001 |
| Large | 1,413 (7.7) | 1,316 (8.6) | 637 (10.4) | <0.001 |
Data are n (%) unless otherwise noted.
Missing in 53
Though the neonatal composite occurred infrequently, it was more likely among nulliparous (1.5%) then multiparous women (1.0%; P < 0.001). For both groups, the three most common morbidities were ventilator support or cardiopulmonary support, Apgar score < 5 at 5 min, and fracture. After adjusting for confounding factors, the significant differences in the neonatal composite remained, with the adjusted relative risk being 80% higher for nulliparous patients (aRR 1.80; 95% CI 1.48–2.19) (Table 4). In sensitivity analysis, the association between parity and adverse neonatal outcome was attenuated but remained significant after adjustment either for cesarean delivery (aRR 1.68; 95% CI 1.37–2.06) or for time from labor and delivery admission to delivery or cesarean decision (aRR 1.54; 95% CI 1.26–1.90).
Table 4.
Outcomes by parity
| Nulliparous n=18,245 | Multiparous n=21,625 | aRR (95%CI)* | |
|---|---|---|---|
| Composite of neonatal adverse outcomes† | 274 (1.5) | 215 (1.0) | 1.80 (1.48–2.19) |
| Perinatal death | 4 (0.0) | 4 (0.0) | |
| Ventilator support or cardiopulmonary resuscitation | 101 (0.6) | 39 (0.2) | |
| Apgar score <5 at 5 min | 60 (0.3) | 23 (0.1) | |
| Hypoxic-ischemic encephalopathy | 39 (0.2) | 17 (0.1) | |
| Seizure | 26 (0.1) | 8 (0.0) | |
| Sepsis—confirmed | 21 (0.1) | 12 (0.1) | |
| Bronchopulmonary dysplasia | 2 (0.0) | 1 (0.0) | |
| Persistent pulmonary hypertension of the newborn | 24 (0.1) | 14 (0.1) | |
| Necrotizing enterocolitis, stage 2 or 3 | 1 (0.0) | 0 (0.0) | |
| Intraventricular hemorrhage, grade III or IV | 1 (0.0) | 0 (0.0) | |
| Intracranial hemorrhage other than intraventricular hemorrhage | 29 (0.2) | 21 (0.1) | |
| Fracture—any | 47 (0.3) | 110 (0.5) | |
| Brachial plexus palsy | 21 (0.1) | 14 (0.1) | |
| Facial nerve palsy | 4 (0.0) | 1 (0.0) | |
| Composite of maternal adverse outcomes | 2,756 (15.1) | 718 (3.3) | 5.04 (4.62–5.49) |
| Endometritis | 155 (0.9) | 60 (0.3) | |
| Antibiotics for chorioamnionitis | 1,496 (8.2) | 287 (1.3) | |
| Wound infection | 29 (0.2) | 13 (0.1) | |
| 3rd or 4th degree perineal laceration | 1,090 (6.0) | 247 (1.1) | |
| Blood transfusion | 191 (1.0) | 136 (0.6) | |
| Thromboembolism | 0 (0.0) | 1 (0.0) | |
| Intensive care unit admission | 20 (0.1) | 31 (0.1) | |
| Death | 0 (0.0) | 0 (0.0) | |
| Shoulder dystocia requiring maneuvers | 402 (2.2) | 692 (3.2) | 0.68 (0.60–0.77) |
| Size for gestational age | aOR (95%CI)* | ||
| Small | 1,617 (8.9) | 1,252 (5.8) | 1.45 (1.33–1.57) |
| Large | 1,105 (6.1) | 2,261 (10.5) | 0.65 (0.60–0.71) |
Data are n (%) unless otherwise noted.
aRR, adjusted relative risk from modified Poisson regression; CI, confidence interval; aOR, adjusted odds ratio from multinomial logistic regression.
Adjusted for age, race/ethnicity, body mass index at delivery, and insurance. All associations were p<0.001.
Missing in 53
The maternal composite also was higher among nulliparous (15.1%) then among multiparous women (3.3%; P < 0.001). The most common maternal complications in both groups were antibiotics for chorioamnionitis and 3rd or 4th degree perineal lacerations. After adjusting for confounders, nulliparous women were at a significantly higher risk of the maternal composite than were multiparous women (aRR 5.04; 95% CI 4.62–5.49) (Table 4). Once again, adjustment for additional covariates in sensitivity analysis did not materially change the results: The association between parity and maternal adverse outcome remained significant after adjustment for cesarean delivery (aRR 4.66; 95% CI 4.26–5.10), and after adjustment for time from labor and delivery admission to delivery or cesarean decision (aRR 3.89; 95% CI 3.55–4.26).
The rate of SGA was substantially higher among nulliparous (8.9%) than multiparous women (5.8%; P < 0.001). After adjustment for confounders, the odds of SGA was 45% higher (aOR 1.45; 95% CI 1.33–1.57) among nulliparous than parous women (Table 4). The rate of LGA, however, was significantly lower among nulliparous (6.1%) than multiparous (10.5%; P < 0.001). The significant difference in accelerated growth remained after adjustment, with the adjusted odds ratio being 35% lower among nulliparous (aOR 0.65; 95% CI 0.60–0.71). Shoulder dystocia with maneuvers occurred less often in nulliparous (2.2%) than multiparous women (3.2%; P<0.001), which remained significant after adjustment for confounders (aRR 0.68; 95%CI 0.60–0.77; Table 4). In sensitivity analysis, the association between parity and shoulder dystocia remained significant after adjustment for birthweight (aRR 0.75; 95% CI 0.66–0.85).
Discussion
Our analysis demonstrates that low-risk nulliparous women have a significantly higher rate of composite adverse neonatal and maternal outcomes than low-risk multiparous women. Although the absolute rate of the neonatal composite was low (less than 2%) in both groups, after adjustment it was 80% higher among nulliparous patients. Even though neonatal morbidity is uncommon, it is noteworthy that annually there are over 2.4 million low-risk deliveries in the US (1,2), meaning that even though an event may be infrequent, its total burden in terms of absolute numbers may be high. The maternal composite, more common than the neonatal composite, also occurred more frequently among nulliparous than multiparous women. Additionally, SGA occurred more frequently among nulliparous women. LGA and shoulder dystocia requiring maneuvers, however, were more common among multiparous women.
This report differs from prior studies of low-risk pregnant women in several ways. First, we focused on deliveries at 39 weeks or later, while other studies included term delivery at 37 or 38 weeks (6,11,14,15). By focusing on women at 39 to 41 weeks, clinicians can provide counseling for the 2.4 million women who deliver during this gestational age range (1–3). Second, previous reports either reported on nulliparous (3,4,7,16) or multiparous (2) patients, without directly comparing the outcomes based on parity. Third, after adjustment for time interval from admission to delivery, and whether cesarean delivery was done, the differential morbidity between nulliparous versus parous women persisted. Fourth, although birthweight is a known risk factor for shoulder dystocia, (17–18), the increased likelihood of shoulder dystocia among parous women persisted even after adjusting for birthweight.
The limitations of the analysis should be acknowledged. Our sample size of over 39,000 women was insufficient to assess whether there might be relatively small differences in uncommon outcomes like uterine rupture or mortality. Even though we excluded several conditions that are regarded as high-risk during pregnancy, some complications (e.g. thrombophilia) may not have been fully ascertained. However, our exclusion criteria are akin to prior publications on the topic (19–21). It is also uncertain how fetal surveillance and intrapartum management may have influenced the frequency of outcomes (22–25). Since the parent data set did not collect information on stillbirths occurring before admission, we cannot capture the frequency of antepartum stillbirth during this gestational age range.
Notwithstanding the limitations, the strengths of the analysis include ascertainment of data directly from the charts by trained research personnel from 25 geographically and demographically diverse populations (10). The data are from 2008 to 2011, which reflects contemporary practice. The gestational age determination was reliable since we required either reproductive assisted technology or sonographic examination by the second trimester to be present. Additionally, uniform definitions of neonatal outcomes were pre-specified and included outcomes that are associated with long-term sequelae.
These findings have potential implications for the management of low-risk women approaching 39 weeks. Our data provide insights—for women meeting the eligibility criteria, and for clinicians planning trials—into the rate and type of adverse outcomes for those who do attain 39 weeks. Our findings could also be helpful in cost-effective analysis and health policy for low-risk pregnant women.
Supplementary Material
Acknowledgements
The authors thank Cynthia Milluzzi, R.N., and Joan Moss, R.N.C., M.S.N. for protocol development and coordination between clinical research centers; Vinay Bhandaru, M.S. for statistical analysis; and Elizabeth Thom, Ph.D., Brian M. Mercer, M.D. and Catherine Y. Spong, M.D. for protocol development and oversight.
The project described was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [HD21410, HD27869, HD27915, HD27917, HD34116, HD34208, HD36801, HD40500, HD40512, HD40544, HD40545, HD40560, HD40485, HD53097, HD53118] and the National Center for Research Resources [UL1 RR024989; 5UL1 RR025764]. Comments and views of the authors do not necessarily represent views of the NIH.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
See Appendix 1 for a list of other members of the NICHD MFMU Network.
Each author has confirmed compliance with the journal’s requirements for authorship.
Contributor Information
Suneet P. Chauhan, Department of Obstetrics and Gynecology of the University of Texas Health Science Center at Houston, McGovern Medical School-Children’s Memorial Hermann Hospital, Houston, TX
Madeline Murguia Rice, George Washington University Biostatistics Center, Washington, DC
William A. Grobman, Northwestern University, Chicago, IL
Jennifer Bailit, MetroHealth Medical Center-Case Western Reserve University, Cleveland, OH
Uma M. Reddy, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD
Ronald J. Wapner, Columbia University, New York, NY
Michael W. Varner, University of Utah Health Sciences Center, Salt Lake City, UT
John M. Thorp, Jr., University of North Carolina at Chapel Hill, Chapel Hill, NC
Steve N. Caritis, University of Pittsburgh, Pittsburgh, PA
Mona Prasad, The Ohio State University, Columbus, OH
Alan T. N. Tita, University of Alabama at Birmingham, Birmingham, AL
George R. Saade, University of Texas Medical Branch, Galveston, TX
Yoram Sorokin, Wayne State University, Detroit, MI
Dwight J. Rouse, Brown University, Providence, RI
Jorge E. Tolosa, Oregon Health & Science University, Portland, OR
References
- 1.Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2018. NCHS Data Brief, no 346. Hyattsville, MD: National Center for Health Statistics; 2019. [PubMed] [Google Scholar]
- 2.Chen HY, Grobman WA, Blackwell SC, Chauhan SP. Neonatal and Maternal Adverse Outcomes Among Low-Risk Parous Women at 39–41 Weeks of Gestation. Obstet Gynecol. 2019;134:288–294. [DOI] [PubMed] [Google Scholar]
- 3:Chen HY, Grobman WA, Blackwell SC, Chauhan SP. Neonatal and Maternal Morbidity Among Low-Risk Nulliparous Women at 39–41 Weeks of Gestation. Obstet Gynecol. 2019;133:729–737. [DOI] [PubMed] [Google Scholar]
- 4.Grobman WA, Rice MM, Reddy UM, Tita ATN, Silver RM, Mallett G, Hill K, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Labor Induction versus Expectant Management in Low-Risk Nulliparous Women. N Engl J Med. 2018;379:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caughey AB, Washington AE, Laros RK Jr. Neonatal complications of term pregnancy: rates by gestational age increase in a continuous, not threshold, fashion. Am J Obstet Gynecol. 2005;192:185–90. [DOI] [PubMed] [Google Scholar]
- 6.Cheng YW, Nicholson JM, Nakagawa S, Bruckner TA, Washington AE, Caughey AB. Perinatal outcomes in low-risk term pregnancies: do they differ by week of gestation? Am J Obstet Gynecol 2008;199:370.e1–7. [DOI] [PubMed] [Google Scholar]
- 7.Tita AT, Lai Y, Bloom SL, Spong CY, Varner MW, Ramin SM, Caritis SN, Grobman WA, Sorokin Y, Sciscione A, Carpenter MW, Mercer BM, Thorp JM Jr, Malone FD, Harper M, Iams JD. Timing of delivery and pregnancy outcomes among laboring nulliparous women. Am J Obstet Gynecol. 2012;206:239.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saccone G, Berghella V. Induction of labor at full term in uncomplicated singleton gestations: a systematic review and metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2015;213:629–36. [DOI] [PubMed] [Google Scholar]
- 9.Grobman WA, Caughey AB. Elective induction of labor at 39 weeks compared with expectant management: a meta-analysis of cohort studies. Am J Obstet Gynecol. 2019. February 25. [DOI] [PubMed] [Google Scholar]
- 10.Gibbs Pickens CM, Kramer MR, Howards PP, Badell ML, Caughey AB, Hogue CJ. Term Elective Induction of Labor and Pregnancy Outcomes Among Obese Women and Their Offspring. Obstet Gynecol. 2018;131:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibson KS, Waters TP, Bailit JL. Maternal and neonatal outcomes in electively induced low-risk term pregnancies. Am J Obstet Gynecol. 2014;211:249.e1–249.e16. [DOI] [PubMed] [Google Scholar]
- 12.Bailit JL, Grobman WA, Rice MM, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Risk-adjusted models for adverse obstetric outcomes and variation in risk-adjusted outcomes across hospitals. Am J Obstet Gynecol. 2013;209:446.e1–446.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander GR, Kogan MD, Himes JH. 1994–1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J. 1999;3:225–31. [DOI] [PubMed] [Google Scholar]
- 14.Darney BG, Snowden JM, Cheng YW, Jacob L, Nicholson JM, Kaimal A, Dublin S, Getahun D, Caughey AB. Elective induction of labor at term compared with expectant management: maternal and neonatal outcomes. Obstet Gynecol. 2013;122:761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page JM, Snowden JM, Cheng YW, Doss AE, Rosenstein MG, Caughey AB. The risk of stillbirth and infant death by each additional week of expectant management stratified by maternal age. Am J Obstet Gynecol. 2013;209:375.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hersh AR, Skeith AE, Sargent JA, Caughey AB. Induction of labor at 39 weeks of gestation versus expectant management for low-risk nulliparous women: a cost-effectiveness analysis. Am J Obstet Gynecol. 2019;220:590.e1–590.e10. [DOI] [PubMed] [Google Scholar]
- 17.Chauhan SP, Laye MR, Lutgendorf M, et al. A multicenter assessment of 1,177 cases of shoulder dystocia: lessons learned. Am J Perinatol. 2014;31:401–406. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman MK, Bailit JL, Branch DW, et al. A comparison of obstetric maneuvers for the acute management of shoulder dystocia. Obstet Gynecol. 2011;117:1272–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parchem JG, Gupta M, Chen HY, Wagner S, Mendez-Figueroa H, Chauhan SP. Adverse Infant and Maternal Outcomes Among Low-Risk Term Pregnancies Stratified by Race and Ethnicity. Obstet Gynecol. 2020;135:925–934. [DOI] [PubMed] [Google Scholar]
- 20.Wagner SM, Chen HY, Gupta M, Chauhan SP. Association of Time of Delivery With Composite Adverse Outcomes in Low-Risk Pregnancies. Obstet Gynecol. 2020;135:527–534. [DOI] [PubMed] [Google Scholar]
- 21.Chauhan SP, Rice MM, Grobman WA, et al. , for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Neonatal Morbidity of Small- and Large-for-Gestational-Age Neonates Born at Term in Uncomplicated Pregnancies. Obstet Gynecol. 2017;130:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chauhan SP, Beydoun H, Chang E, Sandlin AT, Dahlke JD, Igwe E, et al. Prenatal detection of fetal growth restriction in newborns classified as small for gestational age: correlates and risk of neonatal morbidity. Am J Perinatol 2014;31:187–94. [DOI] [PubMed] [Google Scholar]
- 23.Lindqvist PG, Molin J. Does antenatal identification of small for-gestational age fetuses significantly improve their outcome? Ultrasound Obstet Gynecol 2005;25:258–64. [DOI] [PubMed] [Google Scholar]
- 24.Heywood RE, Magann EF, Rich DL, Chauhan SP. The detection of macrosomia at a teaching hospital. Am J Perinatol 2009;26:165–8. [DOI] [PubMed] [Google Scholar]
- 25.Boulvain M, Senat MV, Perrotin F, Winer N, Beucher G, Subtil D, et al. Induction of labour versus expectant management for large-for-date fetuses: a randomised controlled trial. Lancet 2015;385:2600–5. [DOI] [PubMed] [Google Scholar]
- 26.Society of Maternal-Fetal (SMFM) Publications Committee. Electronic address: pubs@smfm.org SMFM Statement on Elective Induction of Labor in Low-Risk Nulliparous Women at Term: the ARRIVE Trial. Am J Obstet Gynecol. 2019;221(1):B2–B4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.