Abstract
Phytochemical investigation on the leaves of Tarenna obtusifolia Merr. (Rubiaceae) led to the isolation and identification of vomifoliol (1), p-coumaric acid (2), and stigmasterol (3) based on spectroscopic analyses and comparison with the literature data. Compound 1 moderately inhibited the aggregation of amyloid-beta (Aβ1–42) using the ThT assay (55.71% at 50 μM) and exhibited neuroprotective effects against amyloid-beta (Aβ1–42)-induced cytotoxicity in neuroblastoma SH-SY5Y cells at 20 μM concentration. This is the first phytochemical study on T. obtusifolia and the first report on the Aβ aggregation activity and neuroprotective potential of vomifoliol (1).
Keywords: Amyloid-beta, Neuroprotective effects, Rubiaceae, Tarenna, Vomifoliol
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disorder among the elderlies, which is characterized by memory loss, dementia, and steady deterioration of cognition (Bagyinzky et al. 2017). An estimate of 47 million elderlies worldwide are suffering from AD, and the figure may balloon to 131 million in 2050 (Emmerzaal et al. 2015). The pathogenesis of AD involves a complicated and an interconnected network of genetic and biochemical factors which have not been fully elucidated yet. The abnormal amyloid-beta deposition, tau protein aggregation, low levels of acetylcholine, oxidative stress, and neuroinflammation are some of the pathological characteristics associated with AD (Xia et al. 2019). There is no cure for AD yet, but strategic treatments in its early stage of detection prove to be beneficial.
Extensive studies on natural products are leading the way of extensive studies aimed at disclosing potential anti-AD drugs. These small molecules incorporates high structural chemical diversity. A good number of research focusing on natural products as therapeutic agents for AD have demonstrated that these small molecules favors neuroprotection against Aβ cytotoxicity and aggregation (Espargaro et al. 2017). Hence, the identification of plants as a possible source of pharmacologically relevant compounds against AD is warranted.
Members of the genus Tarenna Gaertn. (Rubiaceae) are shrubs or trees occurring from low to high elevation and distributed in tropical and subtropical regions, including Africa, Asia, and the Pacific islands (Naiki et al. 2017). There are about 200 species of Tarenna worldwide (Bridson and Robbrecht 1985; De Block et al. 2001). Among these, 19 out of 24 existing species in the Philippines are endemic (Pelser et al. 2011). Among the Tarenna species, T. asiatica is an economically important plant used in traditional folk medicine for a variety of conditions, including boils, external ulcers, sores, and wounds (Ramabharathi et al. 2014). Several studies on various Tarenna species have described their medicinal properties, such as antimicrobial (Jayasinghe et al. 2002; Karthikkumaran et al. 2014; Ramabharathi et al. 2014), antioxidant (Ramabharathi et al. 2014; Yang et al. 2007, 2009), anti-inflammatory (Amutha et al. 2012), and toxicity (Oloro et al. 2016) effects. Phytochemical investigation on the Tarenna species revealed the presence of triterpenoids and triterpenoid glycosides (Harinantenaina et al. 2019; Zhao et al. 2008, 2011, 2013), alkaloid (Takayama et al. 1992), iridoids and iridoid glycosides (Takeda et al. 1976; Yang et al. 2006), lignans (Yang et al. 2007, 2009), and sesquiterpenes (Salmoun et al. 2007). In our continuing search for bioactive compounds from the Rubiaceae plants, we herein report the first phytochemical study and biological activity on the Tarenna obtusifolia Merr. Because our research interests likewise include potential AD prevention natural products, we also report the neuronal protective SH-SY5Y cells and anti-amyloidogenic activities of vomifoliol (1).
Materials and methods
General experimental procedures
NMR spectra were recorded on a JEOL ECZR 600 spectrometer. Silica gel 7734 (Merck, Germany) or silica gel 9385 (Merck, Germany) was used for column chromatography (CC). Thin-layer chromatography (TLC) was performed on aluminum-backed plates coated with Si gel F254; plates were visualized by spraying with vanillin sulfuric acid and warming. Distilled technical grade CH2Cl2 was used for extraction of the plant material, while analytical grade solvents were used for column chromatography.
Plant material
Fresh leaves of Tarenna obtusifolia were collected in the seashore of Virgin Island, Cebu, Philippines (11° 21′ 66.00″ N, 123° 79′ 0.23″ E), in April 2017. This species is a shrub characterized by having glabrous styles and noticeable small flowers. It was identified and authenticated by Dr. Grecebio Jonathan Alejandro, a Philippine Rubiaceae specialist. A voucher specimen (USTH-17-007) was deposited at the University of Santo Tomas (UST) Herbarium.
Extraction and isolation of constituents
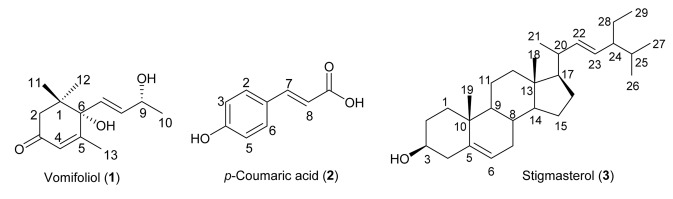
The air-dried, ground leaves (1.1 kg) were extracted exhaustively with CH2Cl2 (7.5 L) for three consecutive days and filtered. The combined filtrates were concentrated under reduced pressure to obtain the CH2Cl2 crude extract (83 g). The crude extract was subjected to silica gel CC using gradient hexane-CH2Cl2 and gradient CH2Cl2-MeOH to obtain four fractions DA–DD. Fraction DC was chromatographed on silica gel using gradient CHCl3-MeOH to afford subfractions DC1–DC5. Subfraction DC3 was passed through silica gel (gradient hexane–EtOAc/EtOAc–MeOH) to obtain DC3A–DC3D. Subfraction DC3A was chromatographed using gradient CHCl3-EtOAc to afford vomifoliol (1, 2.8 mg, white solid). Subfraction DC3B afforded stigmasterol (3, 28 mg, white solid) after silica gel chromatography (7:3 CHCl3-EtOAc). Subfraction DC3C was purified on silica gel (gradient hexane–EtOAc) to afford p-coumaric acid (2, 0.9 mg, yellow solid). The isolated compounds (Fig. 1) were subjected to spectral analysis.
Fig. 1.
Structures of compounds isolated from Tarenna obtusifolia
Vomifoliol (1): 1H NMR (600 MHz, CDCl3): δ 2.25 (1H, d, J = 16.8 Hz, H-2a); 2.45 (1H, d, J = 16.8 Hz, H-2b); 5.91 (1H, br s, H-4); 5.78 (1H, d, J = 15.7 Hz, H-7); 5.87 (1H, dd, J = 15.7 Hz, 5.1 Hz, H-8); 4.42 (1H, m, H-9); 1.31 (3H, d, J = 6.3 Hz, H3-10); 1.01 (3H, s, H3-11); 1.09 (3H, s, H3-12); 1.90 (3H, d, 1.5 Hz, H3-13). 13C NMR (150 MHz, CDCl3): δ 41.1 (C-1); 49.7 (C-2); 197.9 (C-3); 127.0 (C-4); 162.6 (C-5); 79.0 (C-6); 135.7 (C-7); 129.0 (C-8); 68.1 (C-9); 23.8 (C-10); 22.9 (C-11); 24.0 (C-12); 18.9 (C-13).
p-Coumaric acid (2): 1H NMR (600 MHz, CD3OD): δ 7.56 (1H, d, J = 15.9 Hz, H-7), 7.44 (2H, d, J = 8.6 Hz, H-2, H-6), 6.79 (2H, d, J = 8.6 Hz, H-3, H-5), 6.28 (1H, d, J = 15.9 Hz, H-8).
Stigmasterol (3): 1H NMR (600 MHz, CDCl3): δ 1.08 (1H, m, H-1a); 1.83 (1H, m, H-1b); 1.49 (1H, m, H-2a); 1.82 (1H, m, H-2b); 3.53 (1H, m, H-3); 2.26 (2H, m, H2-4); 5.35 (1H, d, J = 4.7 Hz, H-6); 1.52 (1H, m, H-7a); 1.98 (1H, m, H-7b); 1.46 (1H, m, H-8); 0.94 (1H, m, H-9); 1.47 (2H, m, H2-11); 1.15 (1H, m, H-12a); 1.97 (1H, m, H-12b); 1.00 (1H, m, H-14); 1.06 (1H, m, H-15a); 1.55 (1H, m, H-15b); 1.27 (1H, m, H-16a); 1.71 (1H, m, H-16b); 1.13 (1H, m, H-17); 0.70 (3H, s, H3-18); 1.01 (3H, s, H3-19); 2.04 (1H, m, H-20); 1.02 (3H, d, J = 6.8 Hz, H3-21); 5.15 (1H, dd, J = 15.1 Hz, 8.4 Hz, H-22); 5.02 (1H, dd, J = 15.1 Hz, 8.4 Hz, H-23); 1.53 (1H, m, H-24); 1.44 (1H, m, H-25); 0.84 (3H, d, J = 6.4 Hz, H3-26); 0.83 (3H, d, J = 6.3 Hz, H3-27); 1.15 (1H, m, H-28); 0.80 (3H, t, J = 6.0 Hz, H3-29).
Cytotoxicity assay
Neuroblastoma cells (SH-SY5Y), purchased from the American Type Culture Collection, were maintained in DMEM supplemented with 10% FBS, 1% kanamycin, and 1% penicillin. Cell cultures were maintained at 37 °C in 5% CO2 and passaged once per week. The SH-SY5Y cells were subcultured into a 96-well plate at 1 × 104 cells/well and incubated for 24 h. After incubation, the cells were treated with compound 1 and incubated for another 72 h. The media were removed, and the wells were washed with PBS. Fresh media (100 μL) were added and incubated for another 30 min. After incubation, CellTiter-Glo® luminescent reagent (100 μL; Promega, Madison, WI, USA) was added, and the luminescence was measured using a PerkinElmer Victor-3® multi-plate reader (PerkinElmer, Waltham, MA, USA). The values representing cell viability are expressed as means ± standard deviation (SD) of three trial experiments.
Thioflavin T (Tht) fluorescense assay
Amyloid-beta1-42 (10 μM) in PBS (pH 7.4) was incubated with or without the compound 1 at 37 °C for 24 h. Twenty microliters of ThT solution (50 μM) in glycine–NaOH buffer (pH 9) was then added. The fluorescence signal was measured (excitation wavelength, 450 nm; emission wavelength, 510 nm) using a PerkinElmer Victor-3® multi-plate reader. The percentage of aggregation inhibition was calculated using the following equation: [(1 − IFi/IFc) × 100%], where IFi and IFc are the fluorescence absorbance with and without the inhibitors, respectively, after subtracting the background fluorescence of the ThT solution.
Neuroprotective activity
Neuroblastoma SH-SY5Y cells (2 × 104 cells/well) were cultured in 96-well plate and incubated for 24 h. After incubation, the cells were pre-treated with compound 1 (20 μM, 10 μM, 1 μM) for 2 h. After 2 h, the cells were combined with 1 μM Aβ1–42 and incubated for another 24 h at 37 °C and 5.0% CO2. A negative control (DMEM + 10% FBS) was used to normalize the % cell viability. The toxicity of SH-SY5Y cells treated with the extracts only and the SH-SY5Y cells treated with Aβ1-42 only was also performed. After incubation, the percentage cell viability was determined using the CellTiter-Glo® luminescent reagent.
Statistical analysis
The results are expressed as the mean ± SD of at least three trial experiments. The statistical significance was analyzed by one-way ANOVA or Student’s t test and p < 0.05 was considered statistically significant.
Results and discussion
In our screening of biologically active Rubiaceae plant extracts using the neuroblastoma SH-SY5Y cell cytotoxicity (Table 1) and Thioflavin-T assay (Table 2), the CH2Cl2 extracts of T. obtusifolia showed inhibition of amyloid-beta aggregation and toxicity to the neuroblastoma cells. At the highest concentration (50 μg/mL), the extract exhibited a 75% cell growth inhibition (~ 35% cell viability). A significant difference in the cell viability (Table 1) was shown in the percentage cell viability of the extract at 5, 10, and 50 μg/mL concentrations when compared to the negative control (p < 0.05). The extract exhibited a moderate (63.42%) inhibition of amyloid-beta aggregation at 50 μg/mL (Table 2). This result is also comparable to the phenol red as positive control (Necula et al. 2007; Wu et al. 2006) at p < 0.05.
Table 1.
SH-SY5Y Cytotoxicity of Tarenna obtusifolia CH2Cl2 Extract
| Concentration, μg/mL | Cell viability (%)a |
|---|---|
| 50 | 34.91 ± 3.21* |
| 10 | 53.77 ± 2.46* |
| 5 | 73.59 ± 1.57* |
| 1 | 84.05 ± 0.56 |
| 0 | 100 ± 3.44 |
*Significant difference with the control cells (0 μg/mL) at p < 0.05
aThe values are expressed as mean ± SD of three trial experiments
Table 2.
Aβ1-42 aggregation using Thioflavin T assay
| Sample | % Inhibitiona |
|---|---|
| Tarenna obtusifolia CH2Cl2 extract (50 μg/mL) | 63.42 ± 3.42* |
| Tarenna obtusifolia CH2Cl2 extract (5 μg/mL) | 14.10 ± 1.67 |
| 1 (50 μM) | 55.71 ± 0.97 |
| 1 (5 μM) | 11.08 ± 1.23 |
| Phenol Redb (50 μM) | 69.85 ± 0.29 |
*Statistically comparable to the postive control (p < 0.05)
aThe values are expressed as mean ± SD of three trial experiments
bThe positive control
Fractionation of the crude CH2Cl2 extract of T. obtusifolia using various chromatographic techniques led to the isolation of three compounds 1‒3. Structures of the isolated compounds were established using spectroscopic analyses as well as comparison with the literature data. The compounds were identified as vomifoliol (1) (Mogana et al. 2014), p-coumaric acid (2) (Rho and Yoon 2017), and stigmasterol (3) (Pateh et al. 2008) (Fig. 1). Compound 2 was previously isolated from T. madagascariensis (Djoudi et al. 2007). To the best of our knowledge, this is the first isolation of compounds 1 and 3 from the genus Tarenna.
As part of our research interest of searching for potential anti-neurodegenerative agents from nature, we screened the isolated compounds in a thioflavin T assay. Results of the ThT assay of 1 are presented in Table 2. The ThT assay describes the capacity of a compound or plant extract to inhibit the aggregation of β-amyloid (Aβ), one of the pathological characteristics identified with AD. Compound 1 showed a moderate activity in the prevention of the aggregation of Aβ at 50 μM. Both p-coumaric acid (2) (10.51% at 50 μM) and stigmasterol (3) (13.74% at 50 μM) did not exhibit any potent activity. Interestingly, vomifoliol isolated from Canarium patentinervium of family Burseraceae also showed anticholinesterase activity with an IC50 96.64 ± 0.09 μg/mL (Mogana et al. 2014). In another study, the anti-neuroinflammatory activity of three isomers of vomifoliol were determined by measuring the NO levels produced in LPS-activated microglial cell line BV-2 with IC50 values ranging from 39 to 76 μM (Kim et al. 2015).
Compound 1 also exhibited an IC50 of 39.6 μM against the neuroblastoma SH-SY5Y cells utilizing the ATP assay after 72 h of cell incubation. Previous studies have also reported the cytotoxicity of 1 on various human cancer cell lines, such as HL-60 (IC50 55.6 μM ± 0.5), Hep G2 (IC50 45.5 μM ± 2.0), and COLO 205 (IC50 6.8 μM ± 0.5) (Bai et al. 2011).
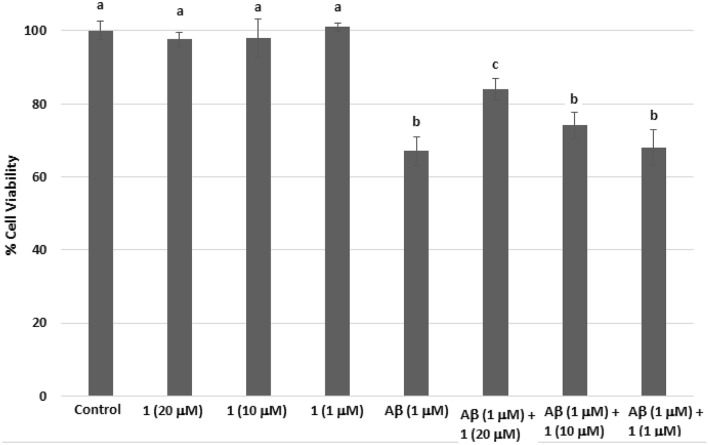
The neuroprotective effects of 1 in the SH-SY5Y cells were evaluated using Aβ1–42-induced cells (Fig. 2). A 20 μM, 10 μM, and 1 μM concentrations of 1 were used based on the initial SH-SY5Y toxicity screening. At these concentrations, 1 did not exhibit significant cytotoxicity in the SH-SY5Y cells when compared to the control (p < 0.05) (Fig. 2). To assess the protective effects of 1 on SH-SY5Y damaged cells, the neuroblastoma cells were pre-treated with compound 1 for 2 h and incubated for 24 h with 1 μM Aβ1-42 (Meng et al. 2018). Pre-treatment of the cells with 1 (20 μM) significantly protected (p < 0.05) the SH-SY5Y cells from the Aβ1–42 when compared to the cells exposed only with the Aβ1–42. In contrast, pre-treatment with 10 μM and 1 μM of 1 did not exhibit any protective effects to the cells when compared to the Aβ1-42 only-treated cells (p < 0.05).
Fig. 2.
Neuroprotective effects of vomifoliol (1) on amyloid-beta1–42-induced neuroblastoma SH-SY5Y cells after 24 h of treatment. The results represent % cell viability vs control (no treatment) and indicate means ± SD of three trials. Significant difference at p < 0.05 is indicated by different lowercase letters. Only the 20 μM of 1 showed a significant neuroprotective effects on the amyloid-beta-damaged SH-SY5Y cells
AD is often characterized by progressive loss of memory, mental ability, and language aptitude. Several key factors to combat the development and progression of AD is being undertaken, including the inhibition of Aβ deposition, oxidative stress reduction, and inhibition of tau protein aggregation. Plant extracts and their natural products have been given attention to disclose their capacity as anti-AD agents (Silva et al. 2014), focusing more into their ability to inhibit the Aβ aggregation (Espargaro et al. 2017). In this study, we have described the potential of vomifoliol (1), a norsesquiterpenoid, to inhibit the aggregation of Aβ. These type of small molecules, including iridoids and monoterpenoids, show diverse pharmacological activities which make them potential candidates as neuroprotective agents against AD (Habtemariam 2018).
Conclusion
This is the first phytochemical study on the aerial parts of T. obtusifolia. This is also the first report on the capability of vomifoliol (1) to inhibit the aggregation of Aβ1–42 using the ThT assay and its neuroprotective potential in Aβ1–42-treated SH-SY5Y cells at 20 μM concentration. Collective results on vomifoliol may suggest its promising potential as a possible candidate for neurodegenerative diseases warranting more chemical and pharmacological investigations including its mechanism of action.
Acknowledgements
The De La Salle University (Laguna Campus, Philippines) is gratefully acknowledged for the NMR measurements and the National Research Foundation of Korea (NRF) Grants awarded by the Korean government (MEST, No. 2017R1A2B4012636). Dr. Felicidad Christina Ramirez is also gratefully acknowledged for the help on the statistical analysis.
Author contributions
MAT and SSAN conceptualized the study. MAT and SJBG performed the experiments. GJDA collected and identified the plant. MAT wrote the manuscript. GJDA and SSAN revised the manuscript. All authors read and approved the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethics approval and consent to participate
The article does not contain any studies involving human participants or animals.
References
- Amutha D, Shanthi S, Mariappan V. Anti-inflammatory effect of Tarenna asiatica (L) in carrageenan induced lung inflammation. Int J Pharm Pharm Sci. 2012;4:344–347. [Google Scholar]
- Bagyinszky E, Giau VV, Shim K, Suk K, An SSA, Kim SY. Role of inflammatory molecules in the Alzheimer’s disease progression. J Neurol Sci. 2017;376:242–254. doi: 10.1016/j.jns.2017.03.031. [DOI] [PubMed] [Google Scholar]
- Bai N, He K, Roller M, Lai CS, Shao X, Pan MH, Bily A, Ho CT. Flavonoid glycosides from Microtea debilis and their cytotoxic and anti-inflammatory effects. Fitoterapia. 2011;82:168–172. doi: 10.1016/j.fitote.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Bridson DM, Robbrecht E. Further notes on the tribe Pavetteae (Rubiaceae) Bull Jard Bot Nat Belg. 1985;55:83–115. doi: 10.2307/3668012. [DOI] [Google Scholar]
- De Block P, Degreef J, Robbrecht E. Reinstatement of the Afro-Madagascan genus Coptosperma (Rubiaceae) Syst Geogr Plants. 2001;71:455–492. doi: 10.2307/3668694. [DOI] [Google Scholar]
- Djoudi R, Bertrand C, Fiasson K, Fiasson J, Comte G, Fenet B, Rabesa Z. Polyphenolics and iridoid glycosides from Tarenna madagascariensis. Biochem Syst Ecol. 2007;35:314–316. doi: 10.1016/j.bse.2006.10.012. [DOI] [Google Scholar]
- Emmerzaal TL, Kiliaan AJ, Gustafson DR. 2003–2013: a decade of body mass index, Alzheimer’s disease, and dementia. J Alzheimers Dis. 2015;43:739–755. doi: 10.3233/JAD-141086. [DOI] [PubMed] [Google Scholar]
- Espargaro A, Ginex T, del Mar VM, Busquets MA, Estelrich J, Munoz-Torrero D, Luque FJ, Sabate R. Combined in vitro cell-based/in silico screening of naturally occurring flavonoids and phenolic compounds as potential anti-Alzheimer drugs. J Nat Prod. 2017;80:278–289. doi: 10.1021/acs.jnatprod.6b00643. [DOI] [PubMed] [Google Scholar]
- Habtemariam S. Iridoids and Other Monoterpenes in the Alzheimer’s brain: recent development and future prospects. Molecules. 2018;23:117. doi: 10.3390/molecules23010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harinantenaina L, Brodie P, Callmander M, Razafitsalama L, Rasamison V, Rakotobe E, Kingston DG. Two antiproliferative saponins of Tarenna grevei from Madagascar dry forest. Nat Prod Commun. 2019;7:705–708. [PMC free article] [PubMed] [Google Scholar]
- Jayasinghe U, Jayasooriya C, Bandara B, Ekanayake S, Merlini L, Assante G. Antimicrobial activity of some Sri Lankan Rubiaceae and Meliaceae. Fitoterapia. 2002;73:424–427. doi: 10.1016/S0367-326X(02)00122-3. [DOI] [PubMed] [Google Scholar]
- Karthikkumaran S, Sajeesh T, Parimelazhagan T, Vinodhkumar V, Kamalanathan D, Natarajan T. Evaluation of the antioxidant and antimicrobial activities of Tarenna asiatica (L.) O. Ktze. Ex K. Schum Asian J Pharm Clin Res. 2014;7:102–110. [Google Scholar]
- Kim K, Moon E, Lee S, Park K, Kim S, Choi S, Lee K. Chemical constituents of the seeds of Raphanus sativus and their biological activity. J Braz Chem Soc. 2015;26:2307–2312. [Google Scholar]
- Meng L, Xin G, Li B, Li D, Sun X, Yan T, Li L, Shi L, Cao S, Meng X. Anthocyanins extracted from Aronia melanocarpa protect SH-SY5Y cells against amyloid-beta (1–42)-induced apoptosis by regulating Ca2+ homeostasis and inhibiting mitochondrial dysfunction. J Agric Food Chem. 2018;66:12967–12977. doi: 10.1021/acs.jafc.8b05404. [DOI] [PubMed] [Google Scholar]
- Mogana R, Adhikari A, Debnath S, Hazra S, Hazra B, Teng-Jin K, Wiart C. The antiacetylcholinesterase and antileishmanial activities of Canarium patentinervium Miq. BioMed Res Int. 2014;2014:903529. doi: 10.1155/2014/903529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiki N, Tagane S, Chang P, Dang V, Toyama H, Nagamasu H, Yahara T. Two new taxa and one new report of Tarenna (Rubiaceae) for the flora of Cambodia and Vietnam. Acta Phytotax Geobot. 2017;68:93–100. [Google Scholar]
- Necula M, Kayed R, Milton S, Glabe CG. Small molecule inhibitors of aggregation indicate that amyloid β oligomerization and fibrillization pathways are independent and distinct. J Biol Chem. 2007;282:10311–10324. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- Oloro J, Tanayen J, Barbra K, Lawrence I, Paul W, Francis B, Amon A. Toxicity of four herbs used in erectile dysfunction; Mondia whiteii, Cola acuminata, Urtica massaica, and Tarenna graveolens in male rats. Afr J Pharm Pharmacol. 2016;9:756–763. doi: 10.5897/AJPP2015.4299. [DOI] [Google Scholar]
- Pateh U, Haruna A, Garba M, Iliya I, Sule I, Abubakar M, Ambi A. Isolation of stigmasterol, β-sitosterol and 2-hydroxyhexadecanoic acid methyl ester from the rhizomes of Stylochiton lancifolius Pyer and Kotchy (Araceae) Niger J Pharm Sci. 2008;8:19–25. [Google Scholar]
- Pelser PB, Barcelona JF, Nickrent DL (2011 onwards) Co's Digital Flora of the Philippines. Retrieved from www.philippineplants.org on 26 September 2019.
- Ramabharathi V, Appa Rao A, Rajitha G. Phytochemical investigation and evaluation of antibacterial and antioxidant activities of leaf-bud exudate of Tarenna asiatica (L.) Kuntze ex K. Schum Indian J Nat Prod Res. 2014;5:48–51. [Google Scholar]
- Rho T, Yoon KD. Chemical constituents of Nelumbo nucifera seeds. Nat Prod Sci. 2017;23:253–257. doi: 10.20307/nps.2017.23.4.253. [DOI] [Google Scholar]
- Salmoun M, Braekman J, Ranarivelo Y, Rasamoelisendra R, Ralambomanana D, Dewelle J, Darro F, Kiss R. New calamine sesquiterpenes from Tarenna madagascariensis. Nat Prod Res. 2007;21:111–120. doi: 10.1080/14786410600899084. [DOI] [PubMed] [Google Scholar]
- Silva T, Reis J, Teixeira J, Borges F. Alzheimer's disease, enzyme targets and drug discovery struggles: from natural products to drug prototypes. Ageing Res Rev. 2014;15:116–145. doi: 10.1016/j.arr.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Takayama H, Katsura M, Seki N, Kitajima M, Aimi N, Sakai S, Santiarworn D, Liawruangrath B. Elaeocarpidine, a naturally occurring racemate, from Tarenna vanprukii. Planta Med. 1992;58:289–291. doi: 10.1055/s-2006-961462. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Nishimura H, Inouye H. Studies on monoterpene glucosides and related natural products. XXXII. Iridoid glucosides of Tarenna kotensis var. gyokushinka. Chem Pharm Bull. 1976;24:1216–1218. doi: 10.1248/cpb.24.1216. [DOI] [Google Scholar]
- Wu C, Lei H, Wang Z, Zhang W, Duan Y. Phenol red interacts with the protofibril-like oligomers of an amyloidogenic hexapeptide NFGAIL through both hydrophobic and aromatic contacts. Biophys J. 2006;91:3664–3672. doi: 10.1529/biophysj.106.081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C-L, Tang G-H, Guo Y-Q, Xu Y-K, Huang Z-S, Yin S. Mulberry Diels-Alder-type adducts from Morus alba as multi-targeted agents for Alzheimer’s disease. Phytochem. 2019;157:82–91. doi: 10.1016/j.phytochem.2018.10.028. [DOI] [PubMed] [Google Scholar]
- Yang X-W, He H-P, Du Z-Z, Liu H-Y, Di Y-T, Ma Y, Ling L, Hao X. Iridoid constituents of Tarenna attenuata. J Nat Prod. 2006;69:971–974. doi: 10.1021/np0600301. [DOI] [PubMed] [Google Scholar]
- Yang X-W, Zhao P, Ma Y-L, Xiao H-T, Zuo Y-P, He H-P, Li L, Hao X-L. Mixed lignan-neolignans from Tarenna attenuata. J Nat Prod. 2007;70:521–525. doi: 10.1021/np0603931. [DOI] [PubMed] [Google Scholar]
- Yang X-W, He H-P, Du Z-Z, Liu H-Y, Di Y-T, Ma Y-L, Wang F, Lin H, Zuo Y-Q, Li L. Tarennanosides A–H, eight new lignin glucosides from Tarenna attenuata and their protective effect on H2O2-induced impairment in PC12 cells. Chem Biodivers. 2009;6:540–550. doi: 10.1002/cbdv.200800022. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Matsunami K, Otsuka H, Shinzato T, Takeda Y. Tareciliosides A–G: Cycloartane glycosides from leaves of Tarenna gracilipes (Hay.) Ohwi. Chem Pharm Bull. 2008;56:1153–1158. doi: 10.1248/cpb.56.1153. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Matsunami K, Otsuka H, Shinzato T, Takeda Y. Tareceliosides H–M: further cycloartane glycosides from leaves of Tarenna gracilipes. Chem Pharm Bull. 2011;59:902–905. doi: 10.1248/cpb.59.902. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Matsunami K, Otsuka H, Shinzato T, Takeda Y. Tareciliosides N–S. Further cycloartanesaponins from the leaves of Tarenna gracilipes, and cytotoxicity of saponins and triterpenes. J Nat Med. 2013;67:503–511. doi: 10.1007/s11418-012-0707-2. [DOI] [PubMed] [Google Scholar]