Abstract
Purpose:
Iridocorneal endothelial (ICE) syndrome is a group of rare ocular conditions that result from abnormal corneal endothelial cells leading to secondary glaucoma, iris distortions, and corneal edema. The etiology of ICE is unknown although it has been associated with viral infections, such as herpes simplex virus (HSV). In this study, we sought to identify an infectious etiology for ICE using advanced molecular techniques.
Methods:
Metagenomic RNA sequencing (MDS) is a high-throughput sequencing approach that can identify all pathogens in any clinical sample, including RNA viruses. Descemet membrane and aqueous fluid from patients with ICE syndrome were subjected to MDS testing.
Results:
Samples from 3 ICE patients were analyzed. MDS was performed on the aqueous fluid of 3 patients and the Descemet membrane and endothelial cell tissue from 1 patient. Viral pathogens were not identified in any of the samples.
Conclusions:
We were unable to identify a viral etiology in the tissues of patients with the Chandler’s variant of ICE syndrome, although this study was limited by sample size.
INTRODUCTION
Iridocorneal endothelial (ICE) syndrome represents a spectrum of rare ocular conditions characterized by abnormal corneal endothelium leading to secondary glaucoma, iris changes, corneal edema, and peripheral anterior synechiae.1,2 It is non-hereditary and commonly presents unilaterally in middle-aged women. The etiology of ICE syndrome remains unclear although prior studies have suggested that the viral infections may be responsible for the clinical findings. The basis for the viral hypothesis was put forth by Alvarado and colleagues2–4 based on the following observations: (1) the alterations of the normal appearing corneal endothelium of ICE patients are suggestive of an acquired process, (2) the mild and chronic inflammation of the corneal endothelium and the unilaterality of the disease are suggestive of an infectious etiology, and (3) corneal specimens of ICE patients were found to be positive for HSV DNA on polymerase chain reaction (PCR). Subsequently, other studies have associated the detection of herpes simplex virus (HSV), varicella zoster virus (VZV), and Epstein-Barr virus (EBV) with ICE syndrome.4–7
In this study, we sought to identify an infectious etiology for ICE syndrome using pathogen directed-PCR and metagenomic deep sequencing (MDS). Unlike PCR, MDS is an unbiased sequencing approach that can identify any pathogen (RNA or DNA viruses, bacteria, fungi, parasites) in a clinical sample, which may identify additional viruses that could explain the pathophysiology of ICE syndrome.8
METHODS
This is a retrospective case study of patients with presumed intraocular infections who presented to the Francis I. Proctor Foundation at the University of San Francisco California (UCSF) for evaluation. This study adhered to the tenets of the Declaration of Helsinki. The Institutional Review Board of the UCSF approved the study. Informed consents were obtained from all patients. Clinical data, including eye examinations, pathology results, and laboratory testing, from patient files were extracted from August 2018 to June 2019. Samples from patients were subjected to HSV, VZV, and CMV PCRs (Viracor Laboratory, MN) and MDS. Viracor is a CLIA-certified laboratory. An internal control was added to each sample to ensure the extraction was performed correctly and that the PCR assay was not inhibited. The limit of detection for HSV-1, HSV-2, VZV, and CMV, were 2176 copies/mL, 748 copies/mL, 3160 copies/mL, and 1423 IU/mL, respectively.
MDS has been described elsewhere.8,9 Briefly, RNA was extracted from 50 uL of aqueous fluid or Descemet membrane and converted to cDNA prior to sequencing library preparation. The samples were sequenced on the Illumina HiSeq 4000 or NovaSeq 6000 using 150-nucleotide (nt) paired end sequencing. Sequencing reads were analyzed using an in-house pipeline that compared the reads to the NCBI nt database.10 Of the 4 samples sequenced in this study, the mean number of reads per sample was 15.38 x 106 ± 3.41 x 106 (SD). Of those, 8.5% ± 6.0% were non-host reads with 26.0% ± 28.1% of the reads were unmapped to any sequences in the NCBI reference database.
RESULTS
Case 1
A 47-year-old Caucasian woman complained of recurrent decreased vision and light sensitivity in the right eye and was referred for possible viral related anterior uveitis. On presentation, the patient’s best corrected visual acuity (BCVA) was 20/25 in the right eye and 20/20 in the left eye. Her intraocular pressure (IOP) was asymmetrical, 25 mm Hg OD and 15 mm Hg OS, despite being on timolol, brimonidine, tafluprost drops, and oral acetazolamide 500 mg twice a day. Slit lamp examination of the right eye was notable for the classic “beaten metal appearance” of the corneal endothelium nasally and temporally and a slight ovalized pupil. Gonioscopic exam showed broad peripheral anterior synechiae (PAS) inferiorly and temporally that extended to Schwalbe’s line. Anterior chamber cells were not seen, although the patient presented on prednisolone acetate 1% drops. Dilated fundus exam of the right eye was normal. Examination of the left eye was unremarkable. In vivo confocal microscopy of the right corneal endothelium revealed pleomorphic epithelioid-like cells with bright hyper-reflective nuclei and indistinct borders (Fig. 1A). The corneal endothelium of the left eye was normal (Fig. 1B). An anterior chamber paracentesis was performed, and aqueous fluid was sent to Viracor Laboratory for HSV, VZV, CMV, and Toxoplasma gondii PCRs.
FIGURE 1.
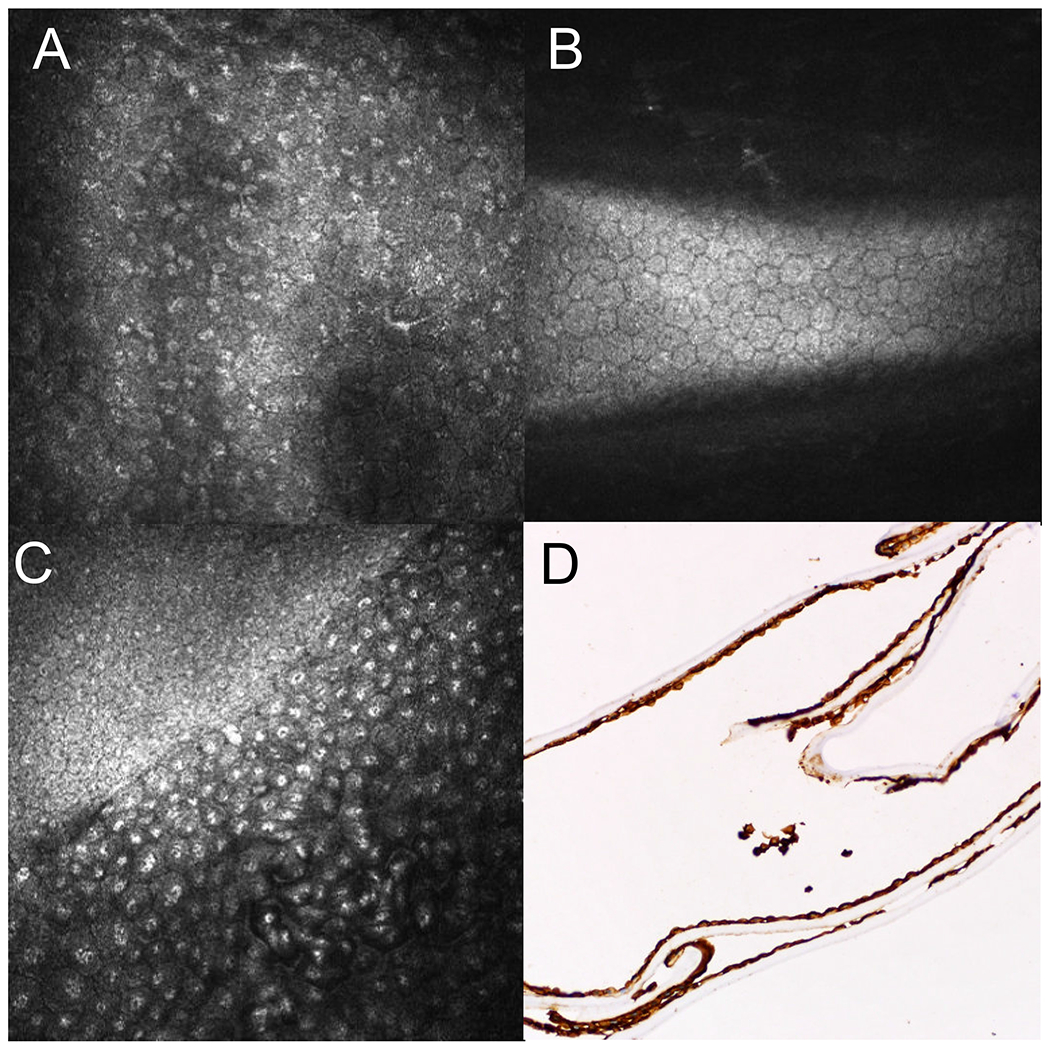
In vivo confocal microscopy images for Case 1 and 2. A, Confocal microscopy images of the affected right eye of Case 1 showing pleomorphic epithelioid-like cells with bright hyperreflective nuclei and indistinct borders of the endothelial layer. B, Confocal microscopy images of the unaffected left eye of Case 1 showing normal endothelial cells. C, Confocal microscopy images of the affected right eye of Case 2 showing loss of regularity in size and shape of the endothelial cells with irregular and indistinct of epithelial-like endothelial cells border containing prominent bright hyperreflective nuclei and epithelioid-like cells with a transition zone between normal endothelial and affected endothelial cells. D, Cytokeratin stain AE1/AE3 positive of the corneal endothelial membrane of Case 2 suggesting that cells are of epithelial origin.
MDS was performed on the residual fluid. No DNA or RNA viruses were detected in this patient. Based on the clinical and confocal findings, the patient was diagnosed with the Chandler’s variant of ICE syndrome.
Case 2
Patient was a 62-year-old Hispanic man who presented to the Cornea clinic for evaluation of chronic corneal edema in the right eye. BCVA was 20/50 OD and 20/25 OS. IOP was 8 mm Hg OD and 6 mm Hg OS. Examination of the left eye was unremarkable. Specular biomicroscopy of the affected eye showed a “beaten metal appearance” of the corneal endothelium. Central corneal thickness (CCT) was 702 μm OD and 572 μm OS. Confocal imaging demonstrated a loss of regularity of the endothelial cells with associated hyper-reflective nuclei and epithelioid-like cells with a distinct transition line to the normal endothelium (Fig. 1C). The patient underwent a combined cataract surgery and Descemet stripping automated endothelial keratoplasty (DSAEK) of the right eye. While histopathologic evaluation of the Descemet membrane demonstrated multilayered endothelial cells with epithelial-like features with thickened membranes, and positive keratin staining consistent with ICE syndrome (Figure 1D), MDS failed to identify either DNA or RNA viruses in the tissue. Furthermore, MDS also did not detect any viral pathogen in the patient’s aqueous fluid.
Case 3
A 47-year-old Hispanic woman, who complained of recurrent left eye blurred vision for 11 years, was referred to the Proctor Foundation on the suspicion of viral-related hypertensive anterior uveitis. BCVA was 20/20 OD and 20/40 in the affected left eye. IOP was 12 mm Hg OD and 22 mm Hg OS despite being on timolol and dorzolamide drops. Examination of the right eye was unremarkable. Slit lamp examination of the left eye showed diffused microcystic corneal edema (Fig. 2A). No obvious anterior chamber cells were seen given the degree of corneal edema. There was iris atrophy inferiorly and PAS noted on both gonioscopy and anterior segment OCT (AS-OCT) (Fig. 2B). The dilated fundus exam was normal in both eyes. In vivo confocal imaging was normal in the right eye (Fig. 2C), but showed irregular and indistinct endothelial cell borders with hyper-reflective nuclei (Fig. 2D) in the left eye. Both HSV, VZV, CMV-directed PCRs and MDS of the aqueous fluid were negative. Again, clinical features of unilaterality, elevated IOP, PAS, corneal edema, in the presence of abnormal endothelial cells were all consistent with the Chandler’s variant of ICE syndrome.
FIGURE 2.
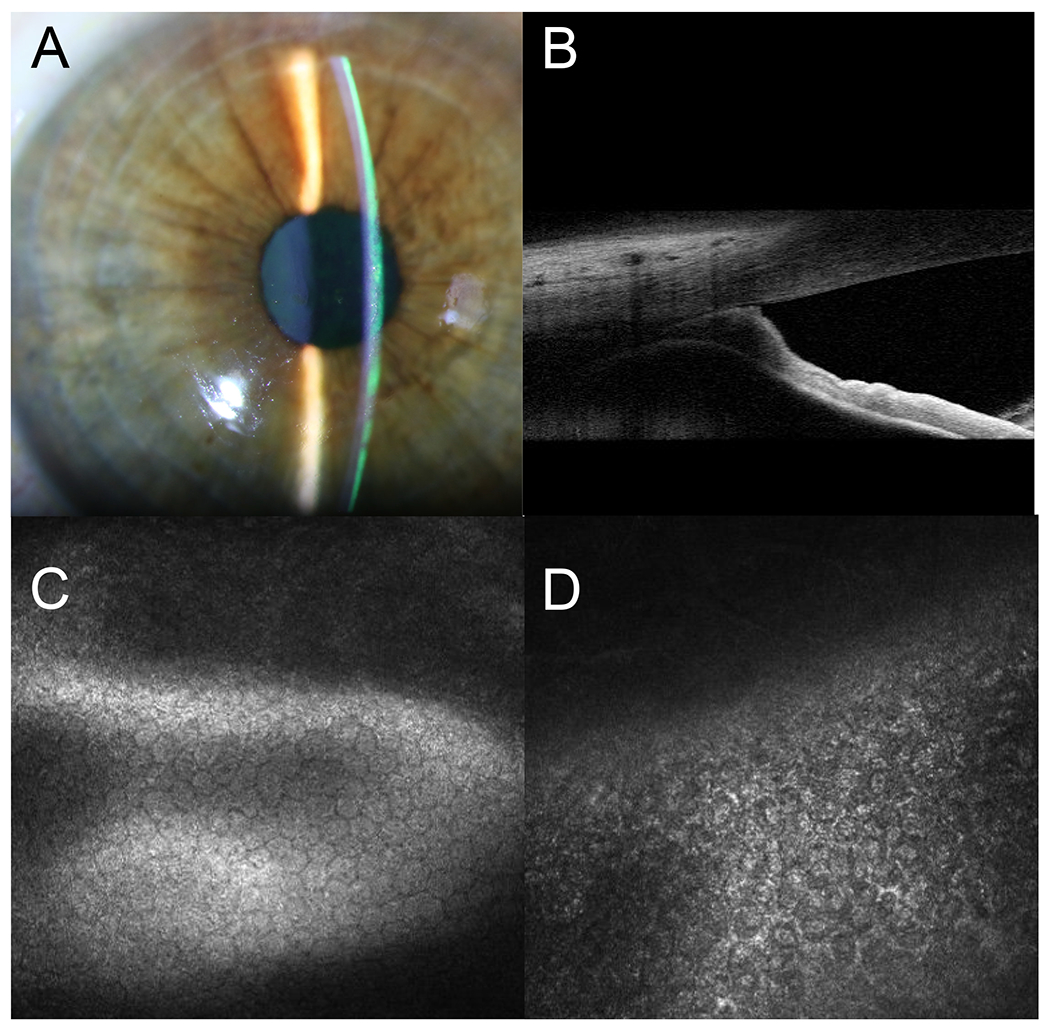
Diagnostic images for Case 3. A, Slit-lamp photograph of the left eye showing microcystic cornea edema. B, Anterior segment optical coherence tomography (AS-OCT) images of the affected eye showing high PAS. C, Normal endothelial cells of the unaffected right eye on confocal imaging. D, Pleomorphic epithelioid-like cells with bright hyperreflective nuclei and indistinct border of the affected left eye.
DISCUSSION
ICE syndrome is a rare and slowly progressive non-hereditary unilateral eye condition characterized by abnormal epithelialization and proliferation of endothelial cells with resultant obstruction of the iridocorneal angle and changes of the iris.1–3 There exist several hypotheses relating to the pathogenesis of this disease. The corneal endothelium originates embryonically from neural crest cells. Aberrant proliferation, membrane formation and contraction of these neural crest cells are thought to give rise to ICE syndrome.11 The initiation factor for the structural changes, however, remains unexplained.
Various theories suggested ICE is an acquired disorder that occurs in the postnasal period and takes decades to develop before diagnosis.2,3 Because the alterations of the endothelial cells in ICE patients observed with electron microscopy are reminiscent of those observed in viral infections, it is hypothesized that disease pathogenesis may be related to injury of the endothelial cells caused by viral infections.12,13 Scheie and Yanoff reported chronic inflammation in association with the onset of ICE syndrome.14 Notable are the findings described by Alvarado and colleagues3 in which they showed the first evidence of HSV genomic DNA being detected in the ocular specimens of patients with ICE syndrome using HSV-directed PCR. Subsequently, several studies either associated the presence of HSV and EBV IgG in the serum or HSV and VZV DNA in the aqueous fluid of patients with ICE syndrome.5–7 Despite the association of viral infections with ICE syndrome, ultrastructure methods have not been able to identify viral particles and viral cultures have failed to grow viruses from ICE specimens.4 In addition, viral infections in animal models have not been able to replicate the disease.6
It is possible that viruses other than HSV, VZV, and EBV are associated with ICE syndrome. Until recently, pathogen-directed molecular assays, such as ELISA or PCR, have been used to interrogate for suspected pathogens. These approaches are low throughput and pathogens can be missed if the specific pathogens are sufficiently rare and are not considered in the differential diagnosis. MDS, in contrast, is an unbiased sequencing approach that sequences all genomic material in any given sample. Therefore, if a pathogen’s genomic material is present in the tested material, then it will be detected, regardless of any a priori assumptions. Previously, we showed that MDS can detect both RNA and DNA viruses in intraocular samples with known and unknown infections.8,15 We applied the same approach to the aqueous fluid or Descemet membrane of 3 patients with clinical findings or histopathology consistent with ICE syndrome. No virus was detected in any of these samples.
This study is limited by the small sample size. While previous studies had patients with all 3 variants of ICE syndrome, the clinical findings of our patients were more consistent with the Chandler’s variant, limiting any generalization of this study’s findings to either the essential iris atrophy (EIA) or Cogan-Reese variant of ICE syndrome. It could also be argued that the samples obtained from the patients in this study had passed their active infectious stage and now only in the inflammation stage as the patients were all in their 40s and 60s. However, it should be noted that while prior studies had a few patients in their 20s and 30s, the majority of specimens were from patients in their 40s to 60s, as most of the corneal tissues were obtained from either enucleation or penetrating keratoplasty.2–5 Finally, it is possible that MDS may have failed to detect an entirely novel virus or a known virus but that the viral load was at a threshold below the limit of MDS detection. Whilst these are possibilities, we have consistently shown that MDS can detect missed pathogens compared to conventional diagnostics.8,9,15
CONCLUSION
We were unable to identify any DNA or RNA viruses in the intraocular fluid or tissues of patients with the Chandler’s variant of ICE syndrome. The exact pathogenesis remains unclear and may be multifactorial for the heterogenous group of ICE syndrome.
Acknowledgments
Funding:
Research reported in this manuscript was supported by the Research to Prevent Blindness Career Development Award (T.D.); the National Eye Institute of the National Institutes of Health under Award Number K08EY026986 (T.D.).
REFERENCES
- 1.Bahn CF, Falls HF, Varley GA, et al. Classification of corneal endothelial disorders based on neural crest origin. Ophthalmology. 1984;91(6):558–563. [DOI] [PubMed] [Google Scholar]
- 2.Alvarado JA, Murphy CG, Maglio M, et al. Pathogenesis of Chandler’s syndrome, essential iris atrophy and the Cogan-Reese syndrome. I. Alterations of the corneal endothelium. Investigative ophthalmology & visual science. 1986;27(6):853–872. [PubMed] [Google Scholar]
- 3.Alvarado JA, Murphy CG, Juster RP, et al. Pathogenesis of Chandler’s syndrome, essential iris atrophy and the Cogan-Reese syndrome. II. Estimated age at disease onset. Investigative ophthalmology & visual science. 1986;27(6):873–882. [PubMed] [Google Scholar]
- 4.Alvarado JA, Underwood JL, Green WR, et al. Detection of herpes simplex viral DNA in the iridocorneal endothelial syndrome. Archives of ophthalmology (Chicago, Ill : 1960). 1994;112(12):1601–1609. [DOI] [PubMed] [Google Scholar]
- 5.Tsai CS, Ritch R, Straus SE, et al. Antibodies to Epstein-Barr virus in iridocorneal endothelial syndrome. Archives of ophthalmology (Chicago, Ill : 1960). 1990;108(11):1572–1576. [DOI] [PubMed] [Google Scholar]
- 6.Li F, Liu Y, Sun Y, et al. Etiological mechanism of iridocorneal endothelial (ICE) syndrome may involve infection of herpes simplex virus (HSV) and integration of viral genes into human genome. Medical hypotheses. 2018;110:50–52. [DOI] [PubMed] [Google Scholar]
- 7.Groh MJ, Seitz B, Schumacher S, et al. Detection of herpes simplex virus in aqueous humor in iridocorneal endothelial (ICE) syndrome. Cornea. 1999;18(3):359–360. [PubMed] [Google Scholar]
- 8.Doan T, Wilson MR, Crawford ED, et al. Illuminating uveitis: metagenomic deep sequencing identifies common and rare pathogens. Genome medicine. 2016;8(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalitha P, Seitzman GD, Kotecha R, et al. Unbiased Pathogen Detection and Host Gene Profiling for Conjunctivitis. Ophthalmology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim D, Song L, Breitwieser FP, et al. Centrifuge: rapid and sensitive classification of metagenomic sequences. Genome research. 2016;26(12):1721–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell DG, Shields MB, Smith TR. The corneal endothelium and the spectrum of essential iris atrophy. American journal of ophthalmology. 1978;86(3):317–324. [DOI] [PubMed] [Google Scholar]
- 12.Buckley RJ. Pathogenesis of the ICE syndrome. The British journal of ophthalmology. 1994;78(8):595–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minkowski JS, Bartels SP, Delori FC, et al. Corneal endothelial function and structure following cryo-injury in the rabbit. Investigative ophthalmology & visual science. 1984;25(12):1416–1425. [PubMed] [Google Scholar]
- 14.Scheie HG, Yanoff M. Iris nevus (Cogan-Reese) syndrome. A cause of unilateral glaucoma. Archives of ophthalmology (Chicago, Ill : 1960). 1975;93(10):963–970. [DOI] [PubMed] [Google Scholar]
- 15.Gonzales JA, Hinterwirth A, Shantha J, et al. Association of Ocular Inflammation and Rubella Virus Persistence. JAMA ophthalmology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


