Abstract
Controlling norovirus transmission in units with immunocompromised patients is challenging. We present a cluster of norovirus cases that occurred on a stem cell transplant unit and the prevention efforts that were implemented to limit the outbreak. Protocols developed to control this cluster may provide a model for other facilities.
Keywords: norovirus, outbreak, bone marrow transplant, hematological malignancy, immunocompromised, environment of care, infection control
BACKGROUND
Norovirus infections are an increasingly recognized cause of diarrhea in hematopoietic stem cell transplantation (HSCT) recipients and are associated with prolonged periods of symptoms and viral shedding, high morbidity, and occasionally mortality.1–5 Although a well-recognized cause of hospital clusters of gastrointestinal illness, data on the containment of nosocomial norovirus clusters in HSCT populations are limited. Standard infection prevention strategies for preventing and controlling norovirus in non-immunocompromised settings include hand washing with soap and water, environmental cleaning with bleach, unit closure, staff education, and staff screening for symptoms with requirements to stay home if found to have gastrointestinal illness.6,7 However, the effectiveness of these strategies for controlling a cluster in the HSCT population ― where the duration of symptoms and shedding are much longer and the risk of transmission is higher — is not well characterized.8
During the period from January – May 2012, we identified a cluster of laboratory-confirmed norovirus infections among patients admitted to the HSCT unit. One patient with nosocomially-acquired norovirus died secondary to the infection, prompting a review of infection prevention programs and implementation of new processes to reduce future transmissions.
METHODS
Setting
Beth Israel Deaconess Medical Center (BIDMC) is an academic tertiary care center with a 28-bed inpatient HSCT unit during the study period (20/28 were single-room occupancy). The HSCT unit is a restricted-access unit in a building that is separate from most of the medical/surgical inpatient beds.
Population and Data Collection
All patients admitted to the HSCT unit during the study period from January 1 through May 31, 2012 were included. Demographic, administrative, pharmacy, and laboratory data were extracted electronically. Clinical variables, including gastrointestinal symptoms (GIS), were manually abstracted.
Description of Outbreak and Case-Cohort Study
An epidemic curve was generated; then a retrospective case-cohort study was conducted to identify risk factors for norovirus acquisition, including the role of facility factors, such as bed location. The primary outcome was laboratory-confirmed norovirus. Cases were matched to all patients admitted to the HSCT unit with GIS. Patients tested for norovirus and found to be positive were also compared to patients who were tested for norovirus and found to be negative.
Case Definitions
GIS were defined as any patient with loose stools at least three times per day and/or vomiting for at least 48 hours.3,8,9 Details of the norovirus case definition are included in the supplementary materials (Supplementary Table 1) and included fever, new GI complaints, and testing results. Norovirus testing was performed at the discretion of the treating clinician and thus not available for all GIS patients.
Infection Control Measures
Standard infection prevention protocols on the HSCT unit prior to January 2012 included hand hygiene with alcohol-based hand rub or soap and water; hand hygiene monitoring via product usage measurement10 validated with intermittent direct observation, cleaning of patient and common areas with quaternary ammonium-based disinfectants, and active screening and restriction of visitors with symptoms of viral infection. All patients with presumed norovirus are placed on contact precautions, and bleach cleaning daily and post-discharge was and remains standard for patients with norovirus. A hospital-wide no shared food policy was in effect during the study period. In addition, staff members with GIS are given early-access to sick leave time and thus are not required to use three vacation days before they can access sick leave pay. This policy is designed to encourage compliance with recommended absence from work to limit spread of infection.
Statistical Analysis:
Risk factors for norovirus acquisition were evaluated using Chi squared test and Fisher’s exact tests for categorical variables and ANOVA and Kruskal-Wallis test for continuous variables. All analyses were performed using STATA v.12.0 (College Station, TX).
RESULTS
During the period from 1/1/2012–5/31/2012, 148 patients were admitted to the HSCT unit (Figure 1). Of these, 99 (66.9%) patients had GIS, including 48 patients (48.5%) who were tested for norovirus. 14.6% (7/48) samples were EIA-positive (Supplementary Table 2). Eighty-four of the 99 patients with GIS (84.9%) were tested for C. difficile and 10/84 (11.9%) were positive; 0/10 had confirmed norovirus. Two GIS patients were admitted to the ICU and one died from norovirus infection. No other nosocomial clusters on other inpatient units were identified during the study period.
Figure 1.
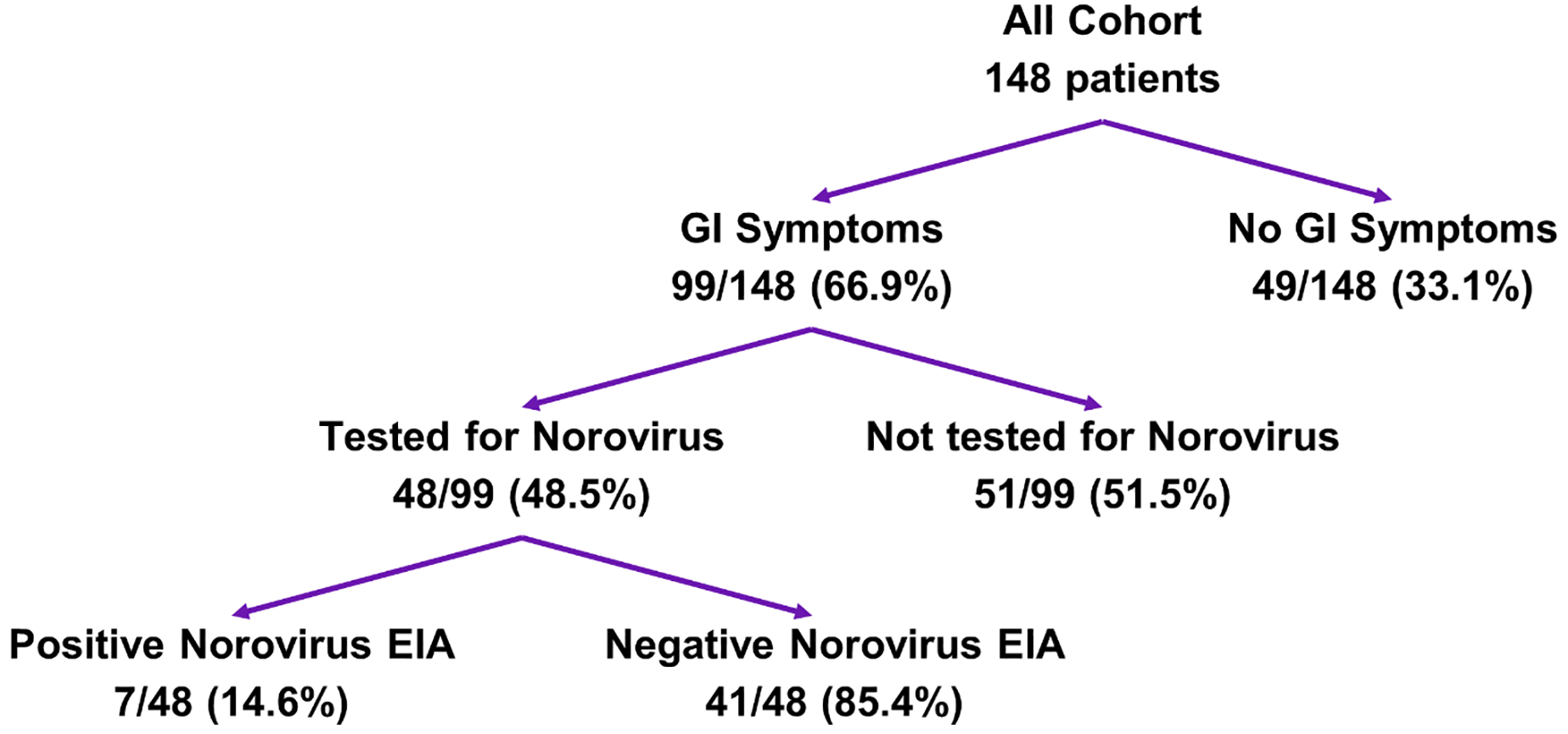
Description of Full Cohort and Case Selection
The epidemic curve and distribution of cases in the unit suggested point-source transmission with contamination of a limited environmental area (Figure 2). The only patient with GIS present on admission was the index case. Among the six subsequent cases, two occurred in the same room as the index patient and one occurred in an adjacent room after a median nine day inpatient stay; room location was the only significant predictor of infection acquisition (Table 1, Supplementary Figure 1).
Figure 2.
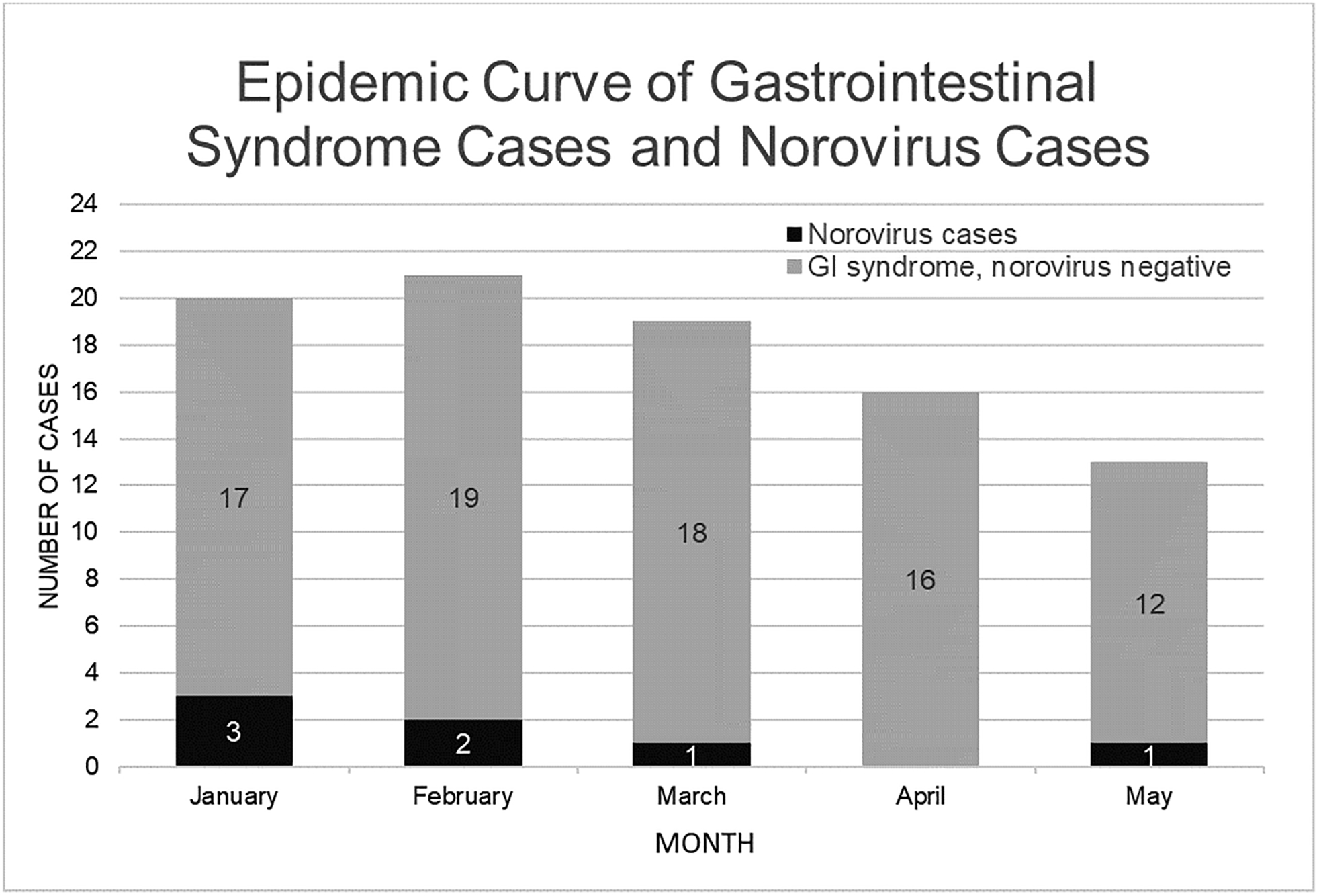
Norovirus Epidemic Curve
Table 1.
Differences between the patients with severe gastrointestinal symptoms who were tested and not tested by norovirus enzyme immunoassay, and between the patients who tested positive and negative for norovirus
| Not tested for Norovirus (N = 51) | Tested for Norovirus (N = 48) | p-value | Norovirus negative (N = 41) | Norovirus positive (N = 7) | p-value | |
|---|---|---|---|---|---|---|
| Male Sex | 29 (56.9%) | 28 (58.3%) | 0.88 | 24 (58.5%) | 4 (57.2%) | 1.00 |
| Race White | 37 (72.6%) | 33 (68.8%) | 0.75 | 27 (65.9%) | 6 (85.7%) | 0.46 |
| Age (Median) | 63.25 | 60.12 | 0.83 | 60.31 | 62.56 | 0.72 |
| Leukemia | 19 (37.3%) | 18 (37.5%) | 0.98 | 16 (39.0%) | 2 (28.6%) | 0.70 |
| Lymphoma | 13 (25.5%) | 14 (29.2%) | 0.68 | 11 (26.8%) | 3 (42.9%) | 0.40 |
| Myeloma | 7 (13.7%) | 11 (22.9%) | 0.24 | 10 (24.4%) | 1 (14.3%) | 1.00 |
| Myelodysplastic syndrome | 7 (13.7%) | 3 (6.3%) | 0.14 | 2 (4.9%) | 1 (14.3%) | 0.38 |
| Other | 5 (9.8%) | 2 (4.2%) | 0.22 | 2 (4.9%) | 0 (0.0%) | 1.00 |
| HSCTa during admission | 8 (15.7%) | 18 (37.5%) | 0.014 | 16 (39.0%) | 2 (28.6%) | 0.70 |
| Prior history of HSCT | 14 (27.5%) | 26 (54.2%) | 0.007 | 23 (56.1%) | 3 (42.9%) | 0.69 |
| Allogeneic | 7/14 (50.0%) | 9/26 (34.6%) | 8/23 (34.8%) | 1/3 (33.3%) | ||
| Autologous | 7/14 (50.0%) | 12/26 (46.2%) | 0.20 | 10/23 (43.5%) | 2/3 (67.3%) | 0.62 |
| Cord Blood transplant | 0/14 (0.0%) | 5/26 (19.2%) | 5/23 (21.7%) | 0/3 (0.0%) | ||
| Graft-versus-host disease | 4 (7.8%) | 6 (12.5%) | 0.32 | 5 (12.2%) | 1 (14.3%) | 0.32 |
| ANCb <500 at admission | 11 (21.6%) | 15 (31.3%) | 0.27 | 13 (31.7%) | 2 (28.6%) | 1.00 |
| IVIG | 5 (9.8%) | 11 (22.9%) | 0.076 | 10 (24.4%) | 1 (14.3%) | 1.00 |
| Medications | ||||||
| Steroids | 35 (68.6%) | 41 (85.4%) | 0.048 | 34 (82.9%) | 7 (100.0%) | 0.57 |
| Chemotherapy agents | 27 (52.9%) | 33 (68.8%) | 0.11 | 28 (68.3%) | 5 (71.4%) | 1.00 |
| Immunosuppressants | 7 (13.7%) | 15 (31.3%) | 0.036 | 14 (34.2%) | 1 (14.3%) | 0.41 |
| Biological Agents | 4 (7.8%) | 5 (10.4%) | 0.65 | 4 (9.8%) | 1 (14.3%) | 0.56 |
| Mean numbers of days between admission and symptom-onset | 9.76 | 8.56 | 0.53 | 8.46 | 9.14 | 0.42 |
| Index case room | 2 (3.9%) | 3 (6.3%) | 0.672 | 1 (2.4%) | 2 (28.6%) | 0.05* |
| Index case room or adjacent room | 13 (25.5%) | 17 (35.4%) | 0.283 | 7 (17.1%) | 3 (42.9%) | 0.15* |
Index case not included in the calculation
ANC = Absolute neutrophil count, in cells/mm3
HSCT = Hematopoietic stem cell transplant
Bundle & Outbreak Control
After the first nosocomial norovirus case was identified, an enhanced infection control bundle was instituted. The bundle included: hand washing with soap and water followed by alcohol-based hand rub; enhanced environmental cleaning with bleach, including in all common areas and bathrooms; direct observation of the post-discharge and daily cleaning process by Infection Control/Hospital Epidemiology (IC/HE) personnel; norovirus education for clinical staff, patients and visitors; symptom screening with work restriction for clinical staff and visit restriction for visitors; and enhanced surveillance for additional cases by IC/HE personnel. The screening process was triggered after identification of a nosocomial case and performed by unit staff on every shift. It includes a strict case definition with high sensitivity applied to patients and hospital staff and an algorithm for testing and identification of norovirus cases (Figure 3). Any patients with symptoms were isolated and tested; any staff were removed from work for 72 hours after their last symptom and given access to extended sick time pay immediately. Screening continued until transmission was no longer detected. Because HSCT patients shed virus for prolonged periods and are frequently readmitted, contact precautions were extended to a 30-day window following symptom onset, rather than until discharge. To assist with isolation on subsequent admissions, HSCT patients with norovirus are flagged in the electronic medical record. Enhanced post-discharge cleaning protocols were developed, including direct observation of the rooms prior to occupancy by the next patient, in addition to the standard bleach cleaning for all rooms with norovirus patients.11 Finally, in-house norovirus PCR replaced EIA to improve the speed and accuracy of detection of cases.
Figure 3. Inpatient Gastrointestinal Syndrome/Norovirus Evaluation and Precautions Algorithm.
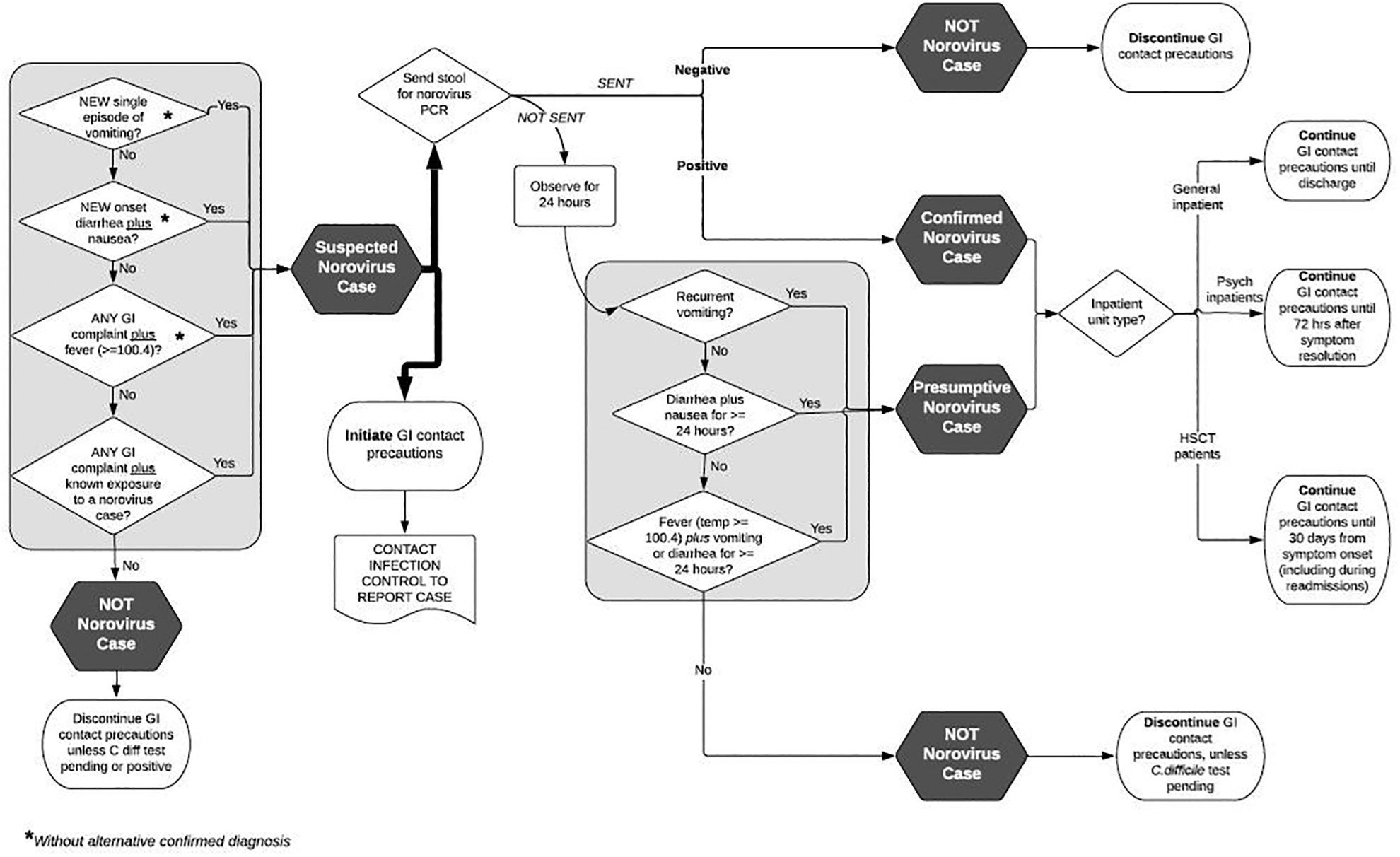
Contact precautions = gastrointestinal syndrome precautions. These included patient isolation, gown and glove for all room entry, and hand washing with soap and water followed by alcohol-based hand rub after room exit.
After implementation of enhanced infection prevention measures, including the newly developed protocol, no additional healthcare-associated cases have been identified on the HSCT unit.
DISCUSSION
In this norovirus cluster on the inpatient HSCT unit, the only significant factor associated with nosocomial norovirus acquisition was room location, congruent with other studies that established the importance of proximity and room contamination in healthcare-associated clusters.8,12 Nenonen et al demonstrated that, among hospital wards experiencing an outbreak, 47% of environmental swabs in the affected ward were positive for norovirus, compared to only 7% in the unaffected ward.13 Positive pressure ventilation present in HSCT units but not standard hospital floors may also play a role in environmental contamination in this population by distributing aerosols outside of a patient’s room.14 This study highlights the importance of early recognition and diagnosis of norovirus in the HSCT population, given the potential for rapid and sustained spread of norovirus long after the index patient has been discharged.
Based on our own findings and earlier studies on outbreaks in HSCT, we implemented a novel infection prevention protocol, which included a strict definition of norovirus infection that permitted earlier and broader implementation of control measures. This protocol successfully terminated the cluster and prevented outbreaks in subsequent norovirus seasons. The strict definition included in the triaging algorithm (Figure 3)—which was designed to be extremely sensitive but have low specificity—was an important element of the infection prevention strategy, because our data suggested that clinicians often did not consider norovirus as a potential cause of severe GIS in this population and early identification is critical for preventing clusters. Other causes, such as C. difficile infection, were more likely to be tested and evaluated. In addition to the highly sensitive case definition, strict screening of staff and patients was undertaken to ensure that cases were identified early in the disease course.
In the years following the implementation of this bundle, no additional healthcare-associated transmissions have been detected on the HSCT unit, and the protocol has been rolled out in modified form to other units with no additional sustained nosocomial clusters of infection in our facility.
Many of the interventions included in the infection prevention protocol are cost-saving measures, even at very low effectiveness (10%) for preventing additional norovirus transmissions.15 Healthcare worker presenteeism is an important factor in nosocomial outbreaks, including on transplant and immunocompromised patient wards; thus our screening protocol was applied to patients and staff.8 Healthcare worker screening and mandated time off if GIS develop is another intervention that may be both clinically effective and cost saving—even with paid time off and early access to paid sick leave-- depending upon the cause of the outbreak.15 Ward closure is another strategy that has been used to manage and prevent healthcare-associated transmissions; however, modeling studies suggest that this strategy is more expensive than other infection prevention interventions and only cost saving if prevention effectiveness exceeds 90%.15 Beyond cost effectiveness, due the specialized nature of the HSCT unit, moving these immunocompromised patients to other hospital wards is often not feasible due to facility constraints and thus ward closure may not be a practical option.
Another important finding was the limited testing for—and identification of—norovirus in the HSCT population.16 Due to the frequency of GIS in this population and the multitude of infectious and non-infectious cases, differentiating norovirus from other causes of GIS is challenging. Among the 99 patients with severe GIS, only 48 of them (48%) were tested for norovirus, but a majority were tested for other types of infections, including C. difficile and Enterobacteriaceae. This aligns with prior studies that suggesting that GIS are often misdiagnosed as other infections, including C. difficile.17, 18 Thus, education of staff is of critical importance—especially during norovirus season—to identify cases and to implement infection prevention strategies as soon as infections begin to occur. Electronic systems can be leveraged for early case ascertainment and intervention by helping to identify cases early in the course, to encourage testing and isolation before substantial environmental contamination and nosocomial transmissions have occurred. Another important diagnostic intervention is the use of PCR, rather than EIA, to confirm cases. The sensitivity and specificity of EIA are both poor, potentially leading to many missed cases before transmission is identified.
This study was limited by small sample size and retrospective design, the limited number of patients who were tested for norovirus, and the diagnostic test itself (EIA during the study period), which has poor sensitivity and specificity. Thus, it is feasible that the cluster may have been larger and that factors other than environmental contamination may have also played a role. However, limited testing and poor predictive value of the test are likely to bias findings toward the null, rather than toward finding an effect, thus it is unlikely that either of these factors caused a false-positive association between environmental exposure and norovirus acquisition. Another possible source of norovirus was staff presenteeism, particularly for the cases that occurred parts of the HSCT unit that were not in close proximity to the index case room (Supplementary Figure 1), however, we did not find any specific evidence of a healthcare worker source for this cluster. Because infection prevention interventions were bundled, we were not able to determine which, if any, individual interventions were the most important. However, given the strong association between location and acquisition, improved environmental cleaning coupled with the aggressive screening protocol is likely to have played a major role in ending the outbreak.
Conclusions
Norovirus presents similarly to other causes of GIS in the HSCT population, leading to delayed diagnosis and environmental contamination. Use of a standard algorithm for earlier identification of presumptive cases of GIS may help prevent future norovirus outbreaks.
Supplementary Material
Acknowledgements:
We would like to thank Dr. David S. Yassa, MD, MPH, Infection Control/Hospital Epidemiology staff members, and the clinical staff in the Bone Marrow Transplant Unit for their assistance in developing and implementing protocols that led to the successful control of this outbreak.
Funding:
WBE is supported by NIH NHLBI 1K12HL138049–01.
Footnotes
Conflicts:
All authors have no conflicts of interest to report.
REFERENCES
- 1.Bok K, Green KY. Norovirus gastroenteritis in immunocompromised patients. N Engl J Med 2012;367:2126–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green KY. Norovirus infection in immunocompromised hosts. Clin Microbiol Infect 2014;20:717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz S, Vergoulidou M, Schreier E, et al. Norovirus gastroenteritis causes severe and lethal complications after chemotherapy and hematopoietic stem cell transplantation. Blood 2011;117:5850–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avery RK, Lonze BE, Kraus ES, Marr KA, Montgomery RA. Severe chronic norovirus diarrheal disease in transplant recipients: Clinical features of an under-recognized syndrome. Transpl Infect Dis 2017;19. [DOI] [PubMed] [Google Scholar]
- 5.van Beek J, van der Eijk AA, Fraaij PL, et al. Chronic norovirus infection among solid organ recipients in a tertiary care hospital, the Netherlands, 2006–2014. Clin Microbiol Infect 2017;23:265 e269–265 e213. [DOI] [PubMed] [Google Scholar]
- 6.Barclay L, Park GW, Vega E, et al. Infection control for norovirus. Clin Microbiol Infect 2014;20:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC, Division of Viral Diseases, Respiratory Diseases. Updated norovirus outbreak management and disease prevention guidelines. MMWR Recomm Rep 2011;60:1–18. [PubMed] [Google Scholar]
- 8.Doshi M, Woodwell S, Kelleher K, Mangan K, Axelrod P. An outbreak of norovirus infection in a bone marrow transplant unit. Am J Infect Control 2013;41:820–823. [DOI] [PubMed] [Google Scholar]
- 9.Robles JD, Cheuk DK, Ha SY, Chiang AK, Chan GC. Norovirus infection in pediatric hematopoietic stem cell transplantation recipients: incidence, risk factors, and outcome. Biol Blood Marrow Transplant 2012;18:1883–1889. [DOI] [PubMed] [Google Scholar]
- 10.Branch-Elliman W, Snyder GM, King AD, et al. Correlation of Hand Hygiene Compliance Measured by Direct Observation with Estimates Obtained from Product Usage. Infect Control Hosp Epidemiol 2018;39:746–749. [DOI] [PubMed] [Google Scholar]
- 11.Greig JD, Lee MB. A review of nosocomial norovirus outbreaks: infection control interventions found effective. Epidemiol Infect 2012;140:1151–1160. [DOI] [PubMed] [Google Scholar]
- 12.Harris JP, Lopman BA, Cooper BS, O’Brien SJ. Does spatial proximity drive norovirus transmission during outbreaks in hospitals? BMJ Open 2013;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nenonen NP, Hannoun C, Svensson L, et al. Norovirus GII.4 detection in environmental samples from patient rooms during nosocomial outbreaks. J Clin Microbiol 2014;52:2352–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsved M, Fraenkel C-J, Bohgard M, et al. Sources of Airborne Norovirus in Hospital Outbreaks. Clin Infect Dis 2019. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee BY, Wettstein ZS, McGlone SM, et al. Economic value of norovirus outbreak control measures in healthcare settings. Clin Microbiol Infect 2011;17:640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roddie C, Paul JP, Benjamin R, et al. Allogeneic hematopoietic stem cell transplantation and norovirus gastroenteritis: a previously unrecognized cause of morbidity. Clin Infect Dis 2009;49:1061–1068. [DOI] [PubMed] [Google Scholar]
- 17.Koo HL, Ajami NJ, Jiang ZD, et al. A nosocomial outbreak of norovirus infection masquerading as clostridium difficile infection. Clin Infect Dis 2009;48:e75–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacAllister TJ, Stednick Z, Golob JL, Huang ML, Pergam SA. Underutilization of norovirus testing in hematopoietic cell transplant recipients at a large cancer center. Am J Infect Control 2018;46:100–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


