Abstract
Background:
Posttraumatic stress disorder (PTSD) is characterized by hyperarousal, avoidance, and intrusive/re-experiencing symptoms. The periaqueductal gray (PAG), which generates behavioral responses to physical and psychological stressors, is also implicated in threat processing. Distinct regions of the PAG elicit opposing responses to threatening or stressful stimuli: the ventrolateral PAG (vlPAG) evokes passive coping strategies (e.g., analgesia), whereas the dorsolateral PAG (dlPAG) promotes active responses (e.g., fight or flight). We investigated whether altered PAG resting state functional connectivity (RSFC) prospectively predicted PTSD symptoms.
Method:
Forty-eight trauma-exposed individuals underwent a RSFC scan two-weeks post-traumatic injury. Self-report measures, including the Visual Analogue Scale of Pain and Impact of Event Scale, were collected at two-weeks and six-months post-trauma. We analyzed whether acute bilateral PAG RSFC was a marker of risk for total six-month symptom severity and specific symptom clusters. In an exploratory analysis, we investigated whether dlPAG RSFC predicted PTSD symptoms.
Results:
After adjusting for physical pain ratings, greater acute post-trauma PAG-frontal pole and PAG-posterior cingulate cortex connectivity was positively associated with six-month total PTSD symptoms. Weaker dlPAG-superior/inferior parietal lobule connectivity predicted both higher hyperarousal and intrusive symptoms, while weaker dlPAG-supramarginal gyrus RSFC was only associated with hyperarousal symptoms.
Conclusions:
Altered connectivity of the PAG two-weeks post-trauma prospectively predicted PTSD symptoms. These findings suggest aberrant PAG function may serve as a marker of risk for chronic PTSD symptoms, possibly by driving specific symptom clusters and more broadly, that connectivity of specific brain regions may underlie specific symptom profiles.
Keywords: periaqueductal gray, posttraumatic stress disorder, resting-state functional connectivity, fMRI, PTSD, trauma
1. Introduction
Up to 90% of American adults will experience a traumatic event (1, 2). A minority of trauma-exposed individuals (8–10%) will develop posttraumatic stress disorder (PTSD; 2, 3). PTSD is characterized by a constellation of symptoms, including hyperarousal, avoidance of trauma-related stimuli, negative alterations in mood and cognition, and intrusive thoughts (4). Functional magnetic resonance imaging (fMRI) studies suggest distinct patterns of brain activity differentiate individuals with PTSD from trauma-exposed controls. PTSD is linked with aberrations in neural substrates mediating threat processing and fear learning, including the amygdala (5, 6, 7), medial prefrontal cortex (8, 9, 10), and hippocampus (11, 12, 13, 14, 15).
Establishing early neural markers of PTSD is critical as it would allow for preventive interventions to minimize the risk of PTSD development (16, 17). However, identifying sensitive and specific markers of risk is challenging (18, 19). Although factors including education, marital status, and gender, as well as peritraumatic psychological processes (e.g. peritraumatic dissociation) confer vulnerability to PTSD, these are estimated to independently predict only 30% of cases (20, 21). Thus, research exploring potential early biomarkers may provide more specific process-based markers of risk. Acute post-trauma studies indicate structural, resting-state, and task-based functional imaging may be useful in identifying biomarkers of PTSD (22, 23, 24).
Consistent with the overarching neurobiological model of PTSD, which suggests fear-learning circuitry is disrupted (25, 26, 27, 28), greater amygdala reactivity is predictive of future PTSD (22, 23) and treatment response (29). Amygdala resting-state functional connectivity (RSFC) is disrupted acutely post-trauma (30). In general, greater RSFC between regions in the salience network, including the amygdala (30) and insula (24, 31), is associated with current, and predictive of future, PTSD symptoms. Importantly, widespread disruption of RSFC in parietal, occipital, and prefrontal regions predicts PTSD symptoms (32). Thus, there is evidence that regions not traditionally defined in neurobiological frameworks of PTSD (e.g. medial prefrontal cortex, amygdala), may be involved.
The periaqueductal gray (PAG), a small structure in the midbrain with a critical role in generating behavioral responses to threat, has emerged in theoretical models of PTSD (33, 34). Essential for pain modulation (35), the PAG is comprised of four columns: dorsolateral (dlPAG), dorsomedial, ventrolateral (vlPAG), and lateral (36, 37). Stimulation of the dlPAG evokes active behavioral responses (e.g. fight/flight; 38), whereas the activation of the vlPAG elicits passive behavioral strategies (e.g. analgesia; 39). The human PAG is functionally connected to numerous brain regions, including the thalamus, hypothalamus, prefrontal cortex, amygdala, and insular cortex (40). Previously identified as part of the salience network (41), the PAG appears to have a role in rapidly generating a response to threat (42).
In preclinical studies, the PAG is implicated in threat detection (43), estimating threat probability (44, 45), and initiating defensive behaviors (46). As threats transition from distal to more imminent, brain activity appears to shift from top-down processing to greater bottom-up control. As threat becomes closer, activity transitions from the ventromedial prefrontal cortex to the PAG (47). Although both the amygdala and PAG appear to be sensitive to threat, the PAG is especially responsive to approaching threatening stimuli (i.e. “looming threat”; 48). After a threatening encounter, fear learning circuitry, including the hippocampus, amygdala, and subgenual anterior cingulate cortex, are recruited (49). In the context of PTSD neurobiology, the PAG is well-positioned to drive symptoms from the bottom-up.
Individuals with PTSD demonstrate greater connectivity between the dlPAG and motor regions, potentially driving symptoms by perpetually preparing for a defensive behavioral response (50). Effective connectivity studies have suggested PTSD is characterized by bottom-up connections between the PAG and vmPFC (51). A neural circuit comprising the amygdala, PAG, frontal cortex, and pons, may indeed underlie behavior strategies in response to threat and stress (52). The PAG relays information to the amygdala to initiate fear responding (53), whereas activation of prefrontal projecting neurons decreases sensitivity to pain, potentially reducing defensive behavior (54).
As a heterogeneous disorder, unique neural correlates may characterize specific features of PTSD (55, 56, 57, 58). A small number of studies suggest that, while there are common neural correlates of PTSD, distinct neural patterns underly specific symptom profiles (56, 57). The PAG has been particularly insightful in distinguishing PTSD from its dissociative subtype, which is characterized by depersonalization or derealization and conceptualized as an “overmodulation of affect” (30; 33). Beyond dissociation, however, few studies have proposed the PAG as a potential driver of specific symptoms. The PAG is particularly well-positioned to underlie both avoidance and hyperarousal (50, 51). Hyperarousal can be conceptualized as hypervigilance to potential threats and may facilitate the behavioral responses (e.g. startle response) often presented in individuals with PTSD.
Using a prospective, longitudinal design, this study examined the role of the PAG in PTSD. We investigated whether PAG RSFC in the early aftermath of a traumatic injury (two-weeks post-trauma) would uniquely predict PTSD symptom severity six-months post-trauma. Based on previous studies, we hypothesized greater acute PAG-prefrontal cortex connectivity would predict six-month post-trauma PTSD symptoms (47). We also expected increased PAG-cingulate cortex connectivity would be predictive of symptom severity (50). Considering the PAG is widely recognized as responsible for descending pain modulation and has been extensively implicated in fMRI studies on pain (59, 60, 61), we also analyzed whether PAG RSFC was associated with physical pain. PTSD is highly co-morbid with chronic pain (62) and patient-perceived injury severity is a significant predictor of PTSD development (63). To our knowledge, no previous studies have considered physical pain when investigating the PAG in the context of PTSD (50, 51).
Finally, using a method similar to Harrricharan and colleagues (2016), we conducted an exploratory analysis investigating the relationship between the dlPAG and specific symptom clusters. Based on Harrricharan and colleagues (2016) and considering the dlPAG’s crucial role in coordinating active defensive strategies (38), we hypothesized altered dlPAG connectivity with the dorsal anterior cingulate cortex would prospectively predict both hyperarousal and avoidance symptoms. Hypervigilance may increase the brain’s preparedness for the “fight or flight” response by specifically priming the dlPAG. Broadly, we anticipated increased dlPAG RSFC with brain regions responsible for “fight or flight” responses, including the premotor areas.
2. Methods and Materials
2.1. Participants
Participants were recruited from a Level 1 Trauma center emergency department (ED) in southeastern Wisconsin. Prospective participants were identified via the ED’s discharge database and telephonically screened. Eligible participants were between the ages of 18–65, right-handed, able to lay flat on their back for two hours, less than 300 pounds, and could schedule an appointment within two weeks of the traumatic event. Individuals were excluded if they scored below 13 on the Glasgow Coma Scale upon ED arrival, experienced head injury with loss of consciousness, or had contraindications to MRI (e.g., pregnancy, irremovable metal in body). Importantly, the recruitment of individuals who were deemed “MRI-safe” may have excluded those with more serious injuries (e.g. gun-shot wounds, injuries that required surgical implants). Moderate to severe cognitive impairment, an intentional self-inflicted injury, antipsychotics prescription(s), and a history of seizures were also exclusionary criteria. Two-weeks post-trauma, participants underwent a resting state fMRI scan and completed self-report measures of PTSD symptom severity and pain. In addition, participants reported current medication use (pain and psychotropic). Six-months post-trauma, individuals completed the same self-report measures. Sample characteristics are reported in Table 1.
Table 1.
Sample Characteristics.
| Characteristics | Mean (SD) or % |
|---|---|
| Age (years) | 33.40 (11.99) |
| Sex | |
| Females | 71.83% |
| Education | |
| Did not complete high school | 4.17% |
| High school/GED | 33.33% |
| Some post-secondary education/college | 35.42% |
| Completed secondary education or vocational degree | 25.00% |
| No information | 2.08% |
| Race and Ethnicity | |
| African American/Black | 47.92% |
| White | 43.75% |
| Hispanic/Latino | 2.08% |
| Biracial | 4.17% |
| No information | 2.08% |
| Mechanism of Injury | |
| Motor vehicle crash | 70.83% |
| Physical assault | 18.75% |
| Other | 10.42% |
| Prescription Medication Use | 43.75% |
| Pain medication (e.g. opioids) | 31.25% |
| Psychotropics (e.g. SSRI) | 22.92% |
| Past Psychiatric Diagnosis | 22.92% |
| Depression | 10.42% |
| Other | 12.50% |
| Two-week assessment | |
| VAS Pain Rating | 3.27 (2.39) |
| IES Total | 33.09 (19.02) |
| Avoidance | 1.44 (0.95) |
| Hyperarousal | 1.55 (0.87) |
| Intrusive/Re-experiencing | 1.53 (1.02) |
| Six-month assessment | |
| VAS Pain Rating | 2.29 (2.68) |
| IES Total | 20.53 (21.89) |
| Avoidance | 0.97 (1.05) |
| Hyperarousal | 0.94 (1.20) |
| Intrusive/Re-experiencing | 0.81 (1.00) |
Abbreviations: SSRI: Selective Serotonin Reuptake Inhibitor; VAS: Visual Analogue of Pain Scale; IES: Impact of Event Scale-Revised
All participants provided written informed consent to partake in the study and were compensated with cash payment for their participation. This study was approved by the Medical College of Wisconsin Institutional Review Board. Four individuals were removed from analysis due to poor neuroimaging data quality (i.e., excessive motion), and 11 participants were lost to follow-up. Forty-eight participants were included in the final analyses.
2.2. Self-Report Measures
At approximately two-weeks and six-months post-trauma, participants completed the Visual Analogue Scale for Pain (VAS; 64) and the Impact of Events Scale-Revised (IES-R; 65) inventories to assess posttraumatic stress symptoms and physical pain severity. The VAS is widely used to evaluate one’s subjective experience of pain (64). Participants rated their physical pain using a numbered line with labels ranging from 0 (no pain) to 10 (worst possible pain). The IES-R consists of 22 questions rated on a scale from 0 (not at all) to 4 (extremely; 65). The items covered three distinct symptom clusters: hyperarousal, avoidance, and intrusive symptoms. Total symptom severity was calculated by taking the sum of all 22 items, while separate scores for each symptom cluster were calculated by averaging the responses to sub-scale specific questions. Although the IES-R is not widely used to diagnosis PTSD, a total score of 24 or higher reflects clinical concern (66).
2.3. Imaging Acquisition
Within two-weeks post-trauma, individuals completed a structural and resting state fMRI scan. During image acquisition, participants were instructed to keep their eyes open and a view a blue screen. All functional images were acquired using a T2* weighted gradient-echo, echoplanar pulse sequence. fMRI data was collected using an interleaved slice acquisition order in a sagittal orientation.
Twenty-one participants completed a six-minute resting state scan on a 3.0 Tesla (3T) long bore Signa Excite MRI system. Thirty-eight slices were acquired with the following parameters: Time Repetition (TR)/Echo Time (TE)= 2000 ms/25 ms, Field of View (FOV) = 24 mm, Matrix = 64 × 64, Slice Thickness = 3.7 mm, Flip Angle (FA) = 77°, voxel size = 3.45 × 3.75 × 3.7. For registration of functional data, high-resolution T1-weighted anatomical images were also obtained (TR/TE = 8.2ms/3.2ms, FOV = 240mm, Matrix = 256×224, FA = 12°, voxel size = 0.9375 × 0.9375 × 1 mm3).
Twenty-seven participants completed a five-minute resting state scan on a 3T short bore Signa Excite MRI system. Forty-one slices were acquired with the following parameters: TR/TE = 2000 ms/25 ms, FOV = 24 mm, Matrix = 64 × 64, Slice Thickness = 3.5 mm, FA = 77°, and voxel size = 3.75 × 3.75 × 3.5. High-resolution T1-weighted anatomical images were obtained with the parameters described above.
2.4. Data Analysis
2.4.1. Image Preprocessing
Images were preprocessed using the CONN toolbox (67; http://www.nitrc.org/projects/conn). The first three TRs were discarded to allow for magnetic field stabilization. Preprocessing steps included motion correction using a six-parameter linear transformation and normalization to Montreal Neurological Institute (MNI 152). Images were spatially smoothed using a three-dimensional Gaussian kernel of 4-mm full-width at half-maximum (FWHM; 40, 50). ROIs were created with unsmoothed images. To reduce the signal-to-noise ratio a temporal band-pass filter was applied (0.01 to 0.1 Hz).
To address any confounding effects of motion, volumes with frame-wise displacement over 0.3 mm were excluded from analysis (i.e. “scrubbed”). Nuisance covariates including head motion parameters (and their first-order derivatives), white matter signal, and cerebrospinal fluid signal were regressed out during first-level analysis. Participants were excluded from analysis if more than 20% of the volumes were scrubbed.
2.4.2. Statistical Analysis
Seed regions-of-interest (ROIs) were created in MNI space. Based on average coordinates reported in a meta-analysis on neuroimaging of the PAG (40), two ROIs were defined as 5-mm radius spheres around the left (−4, 29, −12) and right (4, 29, −12) PAG. For analyses, the primary ROI was created by combining these ROIs (Figure 1). For the exploratory analyses, a box-shaped ROI was created for the dlPAG (0, −32, −8.5 plus 6 × 2 × 1.5 mm extensions; 50, 51).
Figure 1.
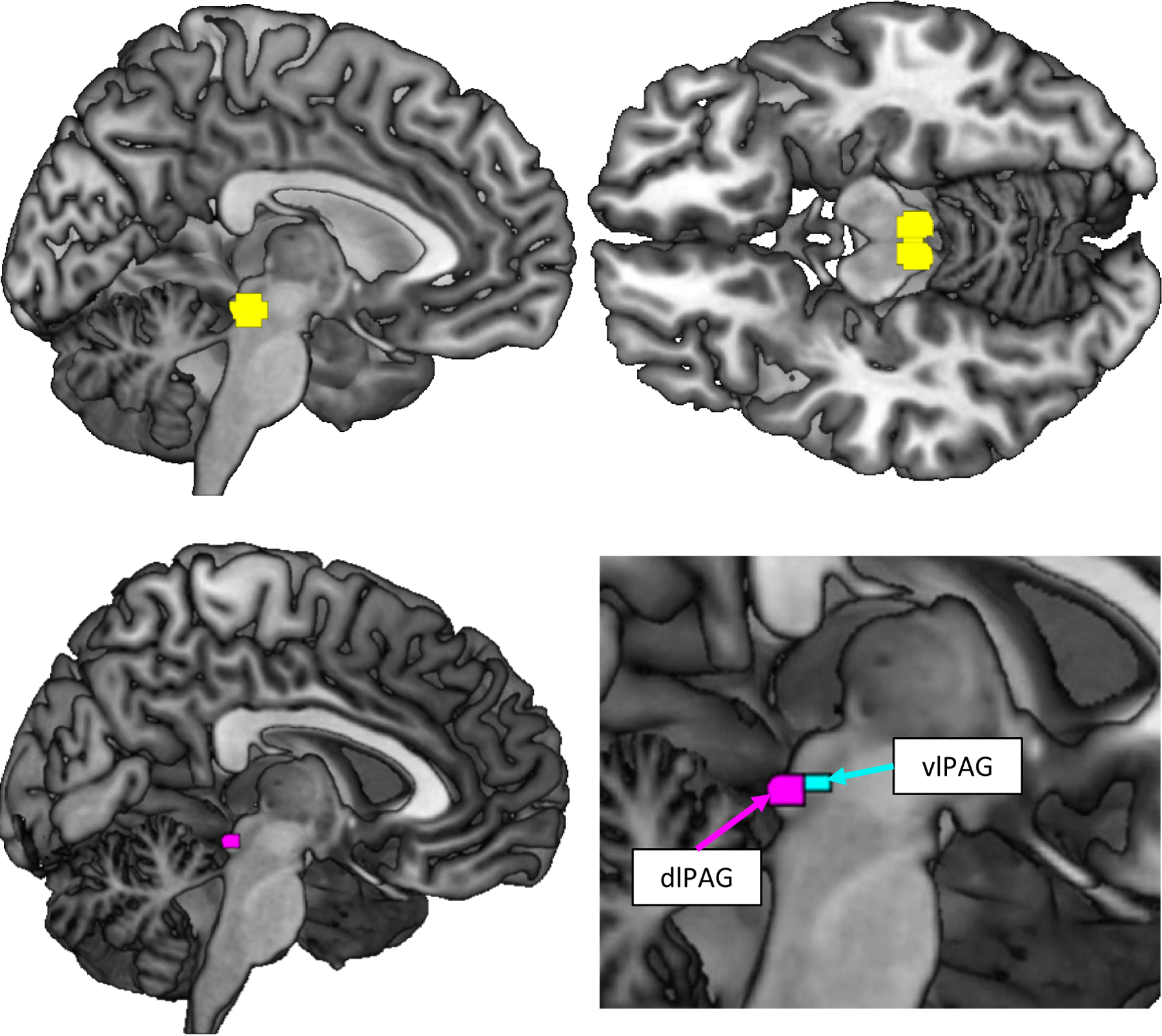
Regions of interest. A bilateral PAG mask (yellow; left: −4, 29, −12; right: 4, 29 −12) and one subregion mask (dlPAG; red; x: 0; y: −32; z: −8.5 plus 6 × 2 × 1.5 mm extensions; 50) were created based on coordinates reported in a meta-analysis (40) and an atlas (50, 68), respectively. For reference, the vlPAG is also pictured (green; 50, 68).
We investigated whether two-week post-trauma PAG RSFC predicted six-month self-reported symptoms of PTSD. In an exploratory analysis, we examined whether acute dlPAG RSFC also predicted six-month self-reported symptoms of PTSD. Mean BOLD time series were extracted from each seed region and correlated with the time series of every other voxel in the brain to produce a three-dimensional correlation (r) map for each subject. For group analyses, correlations were normalized using a Fisher transformation. The statistical threshold for all analyses was set to p < .05. The height threshold was set at p < .001 uncorrected and the cluster-size threshold was set to an adjusted p < .05 false discovery rate (FDR) corrected. To correct for multiple regressions for each seed, we applied the Benjamini–Hochberg procedure (69) using a p < .05 threshold. This correction did not alter our results, therefore only the cluster corrected p-value is presented.
First, a multiple regression analysis examined whether PAG RSFC was associated with two-week or six-month post-trauma VAS scores. We then conducted a multiple regression analysis investigating whether PAG RSFC predicted IES total score, after adjusting for two-week physical pain. Additional analyses (reported in Supplemental Material) examined whether PAG RSFC predicted hyperarousal, avoidance, and intrusion symptoms. Finally, we explored whether dlPAG RSFC predicted specific PTSD symptoms. As data were collected using two MR scanners, scanner was included as a covariate in all analyses.
3. Results
3.1. Self-report Measures
Correlations were computed amongst VAS pain ratings, IES total scores, and IES symptom subscales (Table 2). There was a significant positive correlation between six-month post-trauma physical pain ratings and total six-month PTSD symptoms, r(46) = .54, p < .001; however, two-week VAS scores were not predictive of six-month PTSD symptoms, r(46) = .14, p = .353. Although none of the six-month IES subscales were associated with two-week VAS scores (hyperarousal: r(46) = .16, p = .237; avoidance: r(46) = .15, p = .323; intrusive: r(46) = .19, p = .200), they were all positively associated with six-month pain ratings (hyperarousal: r(46) = .41, p = .003; avoidance: r(46) = .49, p < .001; intrusive: r(46) = .42, p = .003).
Table 2.
Pearson correlation matrix for self-report measures.
| Measure | VAS (2 week) | VAS (6 month) | IES Total (6 month) | Avoidance | Hyperarousal | Intrusive |
|---|---|---|---|---|---|---|
| VAS (2 week) | -- | |||||
| VAS (6 month) | .40b | -- | ||||
| IES Total (6 month) | .14 | .54a | -- | |||
| Avoidance | .15 | .49 a | .88 a | -- | ||
| Hyperarousal | .16 | .41b | .88 a | .61a | -- | |
| Intrusive | .19 | .42 b | .90 a | .67a | .91a | -- |
p < .001;
p < .01
As expected, symptom clusters were highly intercorrelated. At six-months, avoidance symptoms were significantly associated with intrusive symptoms (r(46) = .67, p < .001) and hyperarousal symptoms (r(46) = .61, p < .001), and intrusive symptoms were significantly correlated with hyperarousal symptoms, r(46) = .91, p < .001. Furthermore, the six-months subscales were highly correlated with six-month total IES score (hyperarousal: r(46) = .88, p < .001; avoidance: r(46) = .88, p < .001; intrusive: r(46) = .90, p < .001).
3.2. Resting-State Functional Connectivity
RSFC analyses can be sensitive to confounding factors, particularly head motion (70; 71). We confirmed that average head motion was not correlated with two-week pain symptoms (r(46) = .16, p = .265) or six-month PTSD symptom severity (r(46) = .13, p = .398). Medication use had no effect on RSFC.
Altered PAG connectivity associated with physical pain ratings.
There was a significant association between PAG RSFC and two-week physical pain ratings. PAG RSFC with the precentral gyrus (42, −18, 65; cluster size k = 100; t(45) = 5.07, pFDR = .031) was associated with increased pain ratings (Figure 2). PAG RSFC did not predict six-month physical pain. Results of multiple regression analyses examining PAG and dlPAG RSFC are reported in Tables 3 and 4, respectively.
Figure 2.
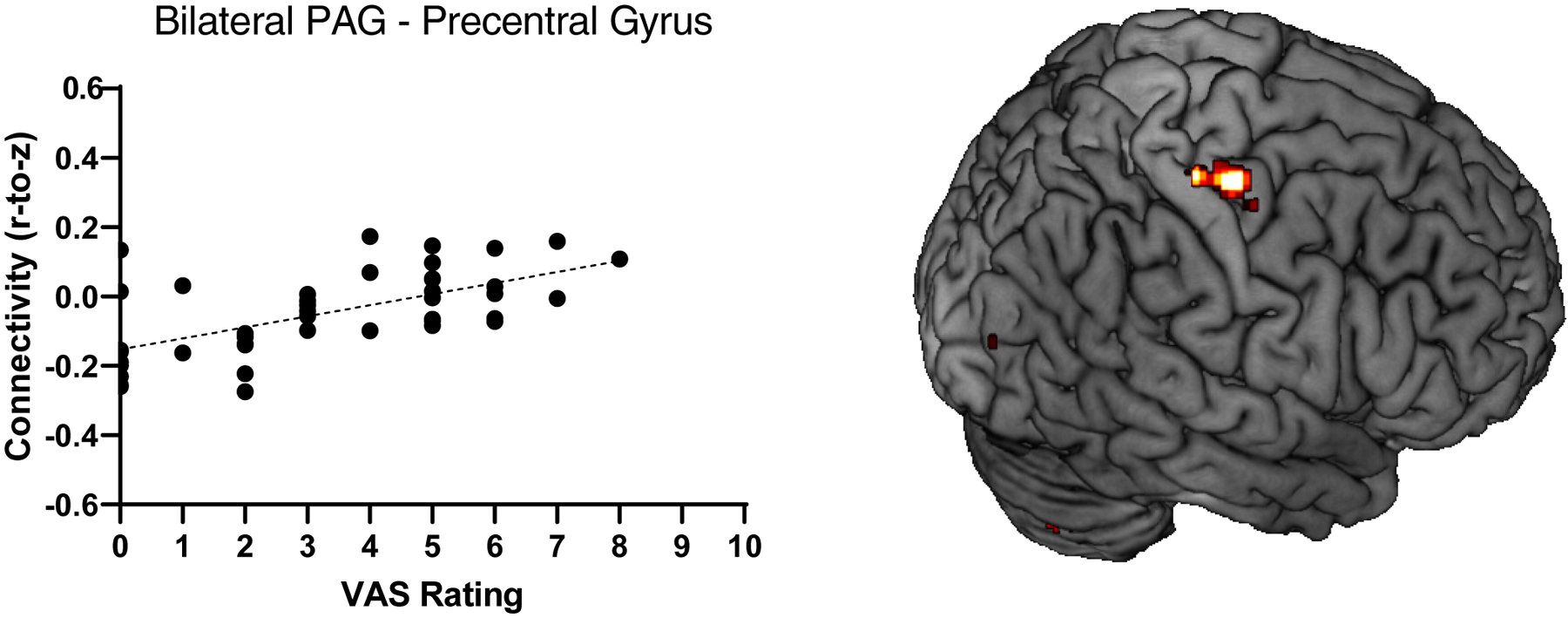
Higher physical pain ratings were associated with greater PAG RSFC (Fisher’s Z) with the precentral gyrus (42, −18, 65; t(45) = 5.07, pFDR = .031).
Table 3.
Altered PAG functional connectivity associated with pain ratings and posttraumatic symptoms.
| Contrast | Symptom(s) | Brain Region | No. of voxels | t(45) | pFDR-corrected | Peak Coordinates (MNI) | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Positive | Pain | Precentral gyrus | 100 | 5.07 | .031 | 42 | −18 | 65 |
| Total PTSD | PCC | 243 | 4.81 | <.001 | −8 | −58 | 36 | |
| Frontal Pole | 117 | 5.57 | .004 | 0 | 68 | 0 | ||
Note. PCC, posterior cingulate cortex.
Table 4.
Altered dlPAG functional connectivity associated with posttraumatic symptoms.
| Contrast | Symptom(s) | Brain Region | No. of voxels | t(45) | pFDR-corrected | Peak Coordinates (MNI) | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Negative | Hyperarousal | SPL/IPL | 131 | 5.39 | .003 | −24 | −36 | 54 |
| SMG | 118 | 4.22 | .003 | −50 | −36 | 34 | ||
| Intrusive | SPL/IPL | 147 | 4.68 | .002 | −24 | −36 | 54 | |
SPL, superior parietal lobule; IPL, inferior parietal lobule; SMG, supramarginal gyrus.
Altered PAG connectivity predicted six-month total PTSD symptoms.
After adjusting for two-week pain ratings, greater PAG RSFC with the frontal pole (0, 68, 0; cluster size k = 117; t(45) = 5.57, pFDR = .004; Figure 3A) and the posterior cingulate cortex (PCC; −8, −58, 36; cluster size k = 243; t(45) = 4.81, pFDR < .001; Figure 3B) prospectively predicted six-month PTSD symptoms. Altered PAG connectivity was also predictive of specific symptom clusters (results reported in Supplemental Material).
Figure 3.
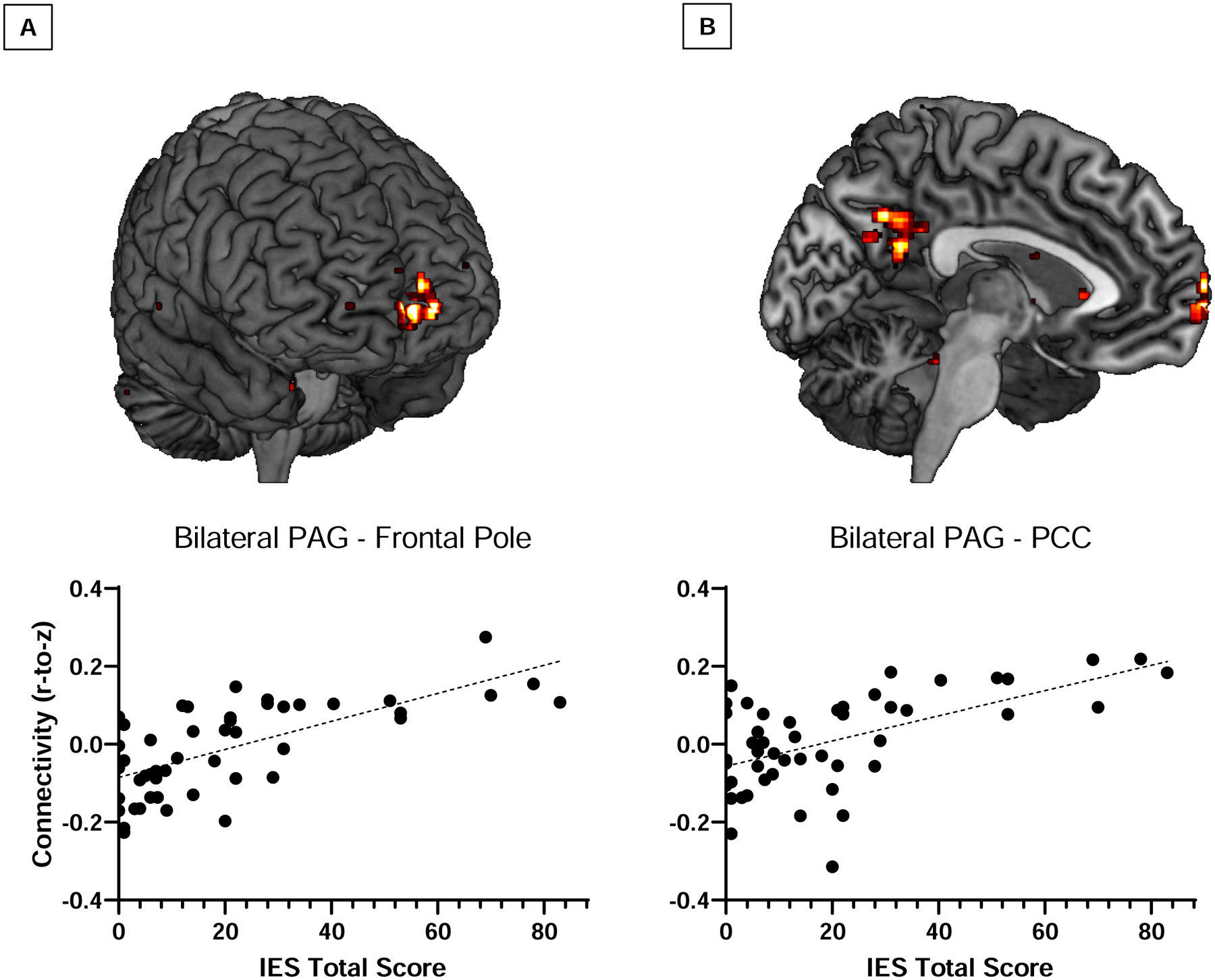
Increased functional connectivity of the PAG with the (A) frontal pole (0, 68, 0; t(45) = 5.57, pFDR = .004) and (B) the posterior cingulate cortex (−8, −58, 36; t(45) = 4.81, pFDR < .001) predicted total posttraumatic stress symptom severity at six-months post-trauma.
Altered connectivity of the dlPAG predicted symptom clusters.
Results of the exploratory analysis revealed weaker dlPAG RSFC with the superior/inferior parietal lobule (−24, −36, 54; cluster size k = 131; t(45) = 5.39, pFDR = .003) and the supramarginal gyrus (−50, −36, 34; cluster size k = 118; t(45) = 4.22, pFDR = .003) predicted hyperarousal symptoms (Figure 4A). Weaker dlPAG RSFC with the superior/inferior parietal lobule also predicted intrusive symptoms (−24, −36, 54; cluster size k = 147; t(45) = 4.68, pFDR = .002; Figure 4B). dlPAG RSFC did not predict Total PTSD symptoms nor avoidance symptoms.
Figure 4.
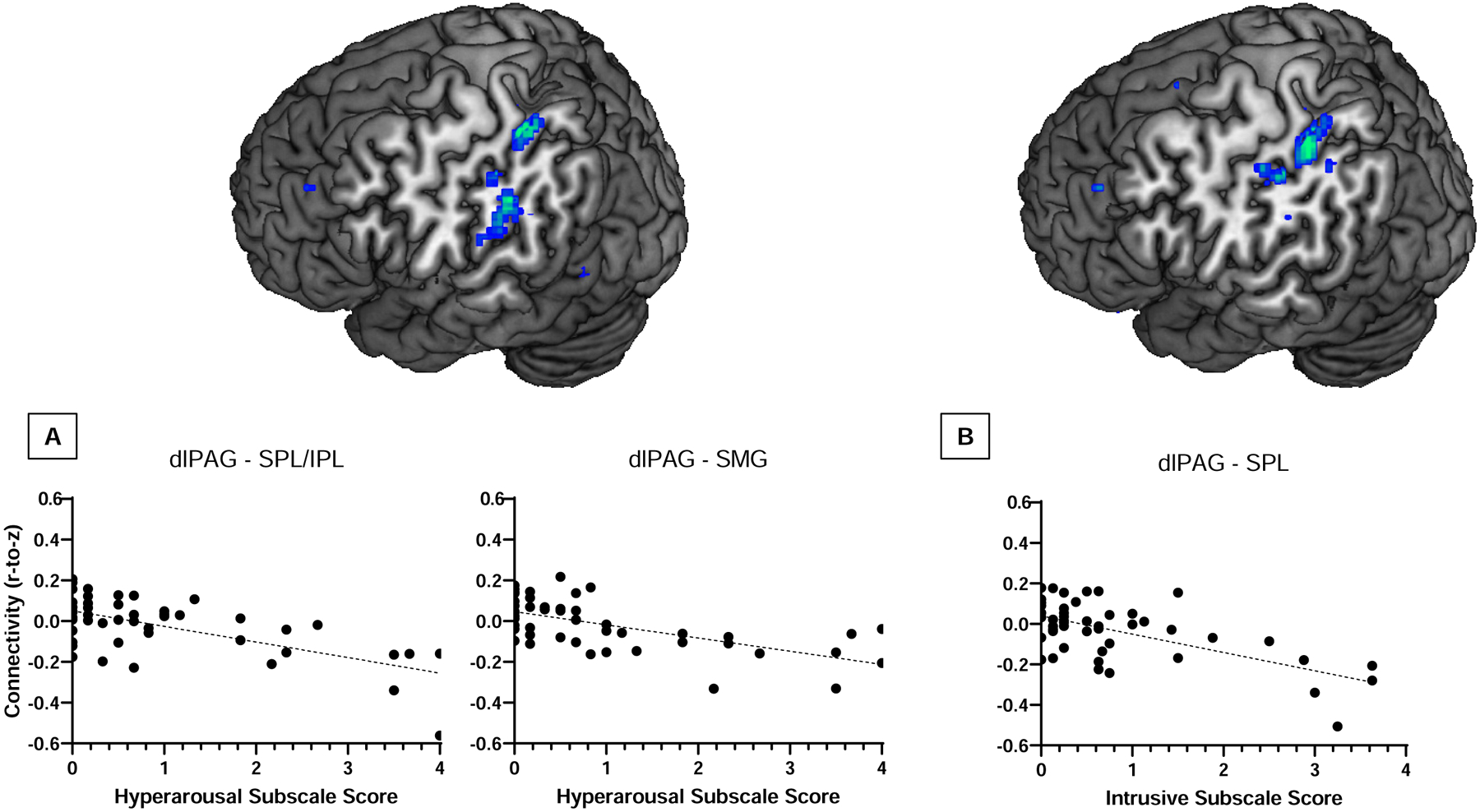
(A) Weaker functional connectivity of the dlPAG with the superior/inferior parietal lobule (−24, −36, 54; t(45) = 5.39, pFDR = .003) and supramarginal gyrus (−50, −36, 34; t(45) = 4.22, pFDR = .003) predicted hyperarousal scores, whereas (B) weaker dlPAG RSFC with the superior parietal lobule (−24, −36, 54; t(45) = 4.68, pFDR = .002) predicted intrusive symptoms.
DISCUSSION
We examined whether PAG RSFC acutely post-trauma predicted PTSD symptoms six months later. Greater PAG-frontal pole connectivity predicted total PTSD symptoms, adding to the growing body of literature indicating the PAG and frontal lobe are sensitive to threats (47,49). For individuals with PTSD, threat-related attentional bias may result in stimuli feeling increasingly threatening and imminent, even when stimuli are distal or non-threatening (72). Increased PAG-frontal pole connectivity acutely post-trauma is likely capturing the transition from top-down to bottom-up control. Greater connectivity between the PAG and the PCC, a region that directs attention to information and drives states of arousal (73), also predicted symptoms. The PCC is responsible for getting “caught-up” in one’s own experiences and feelings (74). Increased PAG-PCC connectivity may reflect increased arousal to both internal negative affect and external trauma-related cues.
Regarding the exploratory dlPAG analysis, weaker dlPAG connectivity with the parietal lobules (superior and inferior) and supramarginal gyrus (also an area of the parietal lobe) predicted hyperarousal symptoms, whereas intrusive symptoms were only predicted by weaker dlPAG-parietal lobules. These regions are involved with cognitive and attentional control (75, 76) and may be disrupted in PTSD (77, 78, 79). Individuals with PTSD demonstrate decreased recruitment of the parietal cortex (77, 80). The frontal-parietal network assists with controlling “emotional influence” on working memory and attention (80, 81). Although the PAG-parietal relationship has been understudied, PAG-parietal connections have been reported (82, 83).
Initiating active avoidance is a key function of the PAG (84); however, dlPAG RSFC did not predict avoidance symptoms. This may have been a limitation of the self-report measure selected. While the IES avoidance subscale includes some assessment of behavioral avoidance (e.g., “I stayed away from reminders about it.”), it more thoroughly assesses cognitive avoidance (e.g., “I tried not to think about it,” “I tried to remove it from my memory”) (65). Therefore, this cognitive avoidance may be weighed more heavily on the IES and less sufficiently capture behavioral avoidance, such as changing driving route to avoid crash site. Future work would benefit from utilizing the Clinician-Administered PTSD Scale for DSM-5 (85), which includes more comprehensive assessment of behavioral avoidance, and assessing the role of the PAG during behavioral tasks of avoidance in both trauma-unexposed and trauma-exposed individuals.
Our results vary from previous research demonstrating extensive altered PAG connectivity in individuals with PTSD compared to healthy controls (50, 51). Harricharan and colleagues (2016) found widespread PAG connectivity with prefrontal and cingulate regions was associated with PTSD. In the present study, PAG RSFC with only the frontal pole and PCC significantly predicted PTSD symptoms. This likely reflects the temporality of the study design; participants completed scanning acutely post-trauma. We demonstrated altered PAG RSFC is not only present in individuals diagnosed with PTSD (50) but is also predictive of symptom development. Although studies have demonstrated a PAG-hippocampus-amygdala circuit, which is theorized to underlie the re-experiencing of traumatic memories (86, 87), we did not observe altered PAG-amygdala or PAG-hippocampus connectivity. Despite strong theoretical evidence and support from preclinical models, altered activation of the amygdala is surprisingly inconsistent in human studies on PTSD (88). We postulate investigations into the relationship between midbrain and cortical structures may offer explanation into these inconsistencies.
Despite pain modulation and PAG activity being nearly synonymous (84, 89) and high comorbidity between chronic pain and PTSD (90), most of the research on the PAG’s role in PTSD does not consider physical pain. We discovered a relationship between PTSD symptoms and pain ratings. Importantly, there was also a significant association between PAG-precentral gyrus connectivity and two-week pain. In healthy humans, the lateral PAG and vlPAG are functionally connected to the precentral gyrus, which is responsible for voluntary movements (68). In certain populations pain may be less relevant to PTSD development; however, pain has been demonstrated as a significant predictor of PTSD in motor vehicle crash survivors (70% of our sample; 91). Our findings demonstrate pain is an important consideration when examining the PAG in the context of PTSD.
Several limitations are noteworthy. First, the scan length of both acquisitions was relatively short. In general, reliability of resting-state scans can be improved by increasing scan duration (92); however, the ideal scan length is disputed, with recommendations ranging from five minutes (93) to over an hour (94). Notwithstanding, if a proposed neural biomarker is unreliable and/or unstable across time, then it cannot be classified as a predictive risk indicator. Additionally, after one scanner was phased out for research, data were collected on two scanners. We cannot entirely rule out any effects of scanner on the current findings; however, consistent with previous work (95, 96), scanner was controlled for in all analyses. Moreover, other multisite studies with pooled neuroimaging data suggest minimal effects of scanner differences in RSFC (97). Our sample is relatively homogenous in nature. Seventy percent of participants were female and involved in a motor vehicle crash. In general, individuals in this sample had lower/sub-threshold symptoms: 14 individuals had a total score greater than 24 on the IES, with scores ranging overall from 0–83. This reduces the generalizability of our findings and future work should replicate this study with a larger, more heterogenous sample. Participants were excluded for antipsychotic medication use, but not prescription pain medication. Even after controlling for medication use, PAG RSFC predicted PTSD symptoms, suggesting our results do not reflect medication exposure. Still, future research should assess the effect of medication on PAG connectivity in PTSD. Finally, our analysis of dlPAG was exploratory. We did not have the optimal spatial resolution to explore the PAG subregions, therefore these specific results should be interpreted with caution. Nevertheless, even with these considerations in mind, our findings highlight the importance of examining regions outside the traditional neurobiological framework of PTSD.
Our results align with previous work demonstrating PAG RSFC is disrupted in PTSD. Importantly, we demonstrated this connectivity prospectively predicts PTSD symptoms, suggesting it may be a useful biomarker of PTSD risk. Future work should continue disentangling the heterogeneity of PTSD, as knowledge of the distinct neural patterns underlying specific symptom profiles may aid in the development of more precise theoretical models and targeted therapeutic interventions.
Supplementary Material
Acknowledgments
The authors extend a special thanks to the research assistants involved in this study. This research was supported by a National Institute of Mental Health grant (R01 MH106574; PI: Larson), a Medical College of Wisconsin CTSI grant (PI: Larson), and a Medical College of Wisconsin Injury Research Center grant (PI: deRoon-Cassini).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Benjet C, Bromet E, Karam EG, Kessler RC, McLaughlin KA, Ruscio AM, et al. (2016). The epidemiology of traumatic event exposure worldwide: results from the World Mental Health Survey Consortium. Psychological medicine, 46(2), 327–343. doi: 10.1017/S0033291715001981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, & Friedman MJ (2013). National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. Journal of traumatic stress, 26(5), 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breslau N (2009). The Epidemiology of Trauma, PTSD, and Other Posttrauma Disorders. Trauma, Violence, & Abuse, 10(3), 198–210. 10.1177/1524838009334448 [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. (2013): Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing. [Google Scholar]
- 5.Patel R, Girard TA, Pukay-Martin N, & Monson C (2016). Preferential recruitment of the basolateral amygdala during memory encoding of negative scenes in posttraumatic stress disorder. Neurobiology of learning and memory, 130, 170–176. [DOI] [PubMed] [Google Scholar]
- 6.Rabellino D, Densmore M, Frewen PA, Théberge J, McKinnon MC, & Lanius RA (2016). Aberrant functional connectivity of the amygdala complexes in PTSD during conscious and subconscious processing of trauma-related stimuli. PloS one, 11(9), e0163097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, et al. (2000). Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological psychiatry, 47(9), 769–776. [DOI] [PubMed] [Google Scholar]
- 8.Clausen AN, Francisco AJ, Thelen J, Bruce J, Martin LE, McDowd J, et al. (2017). PTSD and cognitive symptoms relate to inhibition-related prefrontal activation and functional connectivity. Depression and anxiety, 34(5), 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahlgren MK, Laifer LM, VanElzakker MB, Offringa R, Hughes KC, Staples-Bradley LK, et al. (2018). Diminished medial prefrontal cortex activation during the recollection of stressful events is an acquired characteristic of PTSD. Psychological medicine, 48(7), 1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. (2005). A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of general psychiatry, 62(3), 273–281. [DOI] [PubMed] [Google Scholar]
- 11.Chalavi S, Vissia EM, Giesen ME, Nijenhuis ER, Draijer N, Cole JH, et al. (2015). Abnormal hippocampal morphology in dissociative identity disorder and post-traumatic stress disorder correlates with childhood trauma and dissociative symptoms. Human brain mapping, 36(5), 1692–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, & Pitman RK (2002). Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature neuroscience, 5(11), 1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalavi S, Vissia EM, Giesen ME, Nijenhuis ER, Draijer N, Cole JH, et al. (2017). Compromised hippocampus-striatum pathway as a potential imaging biomarker of mild-traumatic brain injury and posttraumatic stress disorder. Human brain mapping, 38(6), 2843–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Rooij SJ, Stevens JS, Ely TD, Hinrichs R, Michopoulos V, Winters SJ, et al. (2018). The role of the hippocampus in predicting future posttraumatic stress disorder symptoms in recently traumatized civilians. Biological psychiatry, 84(2), 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garfinkel SN, & Liberzon I (2009). Neurobiology of PTSD: A review of neuroimaging findings. Psychiatric Annals, 39(6). [Google Scholar]
- 16.Colvonen PJ, Glassman LH, Crocker LD, Buttner MM, Orff H, Schiehser DM, et al. (2017). Pretreatment biomarkers predicting PTSD psychotherapy outcomes: a systematic review. Neuroscience & Biobehavioral Reviews, 75, 140–156. [DOI] [PubMed] [Google Scholar]
- 17.van Zuiden M, Kavelaars A, Geuze E, Olff M, & Heijnen CJ (2013). Predicting PTSD: pre-existing vulnerabilities in glucocorticoid-signaling and implications for preventive interventions. Brain, Behavior, and Immunity, 30, 12–21. [DOI] [PubMed] [Google Scholar]
- 18.Zannas AS, Provençal N, & Binder EB (2015). Epigenetics of posttraumatic stress disorder: current evidence, challenges, and future directions. Biological psychiatry, 78(5), 327–335. [DOI] [PubMed] [Google Scholar]
- 19.Savitz JB, Rauch SL, & Drevets WC (2013). Clinical application of brain imaging for the diagnosis of mood disorders: the current state of play. Molecular psychiatry, 18(5), 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozer EJ, Best SR, Lipsey TL, & Weiss DS (2003). Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull,129, 52–73. [DOI] [PubMed] [Google Scholar]
- 21.Shalev AY, Gevonden M, Ratanatharathorn A, Laska E, Van Der Mei WF, Qi W, et al. (2019). Estimating the risk of PTSD in recent trauma survivors: results of the International Consortium to Predict PTSD (ICPP). World Psychiatry, 18(1), 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, & Sheridan MA (2014). Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depression and anxiety, 31(10), 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, et al. (2017). Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. Biological psychiatry, 81(12), 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harricharan S, Nicholson AA, Thome J, Densmore M, McKinnon MC, Théberge J,et al. (2020). PTSD and its dissociative subtype through the lens of the insula: Anterior and posterior insula resting-state functional connectivity and its predictive validity using machine learning. Psychophysiology, 57(1), e13472. [DOI] [PubMed] [Google Scholar]
- 25.Bryant RA (2019). Post-traumatic stress disorder: a state-of-the-art review of evidence and challenges. World Psychiatry, 18(3), 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fullana MA, Dunsmoor JE, Schruers KRJ, Savage HS, Bach DR, & Harrison BJ (2020). Human fear conditioning: From neuroscience to the clinic. Behaviour research and therapy, 124, 103528. [DOI] [PubMed] [Google Scholar]
- 27.Janak PH, & Tye KM (2015). From circuits to behaviour in the amygdala. Nature, 517(7534), 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolassa IT, Illek S, Wilker S, Karabatsiakis A, & Elbert T (2015). Neurobiological findings in post-traumatic stress disorder In Evidence based treatments for trauma-related psychological disorders (pp. 63–86). Springer, Cham. [Google Scholar]
- 29.Cisler JM, Sigel BA, Kramer TL, Smitherman S, Vanderzee K, Pemberton J, et al. (2015). Amygdala response predicts trajectory of symptom reduction during trauma-focused cognitive-behavioral therapy among adolescent girls with PTSD. Journal of psychiatric research, 71, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, et al. (2010). Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. American Journal of Psychiatry, 167(6), 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, Scherr M, Schwerthöffer D, et al. (2014). Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Frontiers in human neuroscience, 7, 930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong Q, Li L, Du M, Pettersson-Yeo W, Crossley N, Yang X, et al. (2014). Quantitative Prediction of Individual Psychopathology in Trauma Survivors Using Resting-State fMRI. Neuropsychopharmacol, 39, 681–687 doi: 10.1038/npp.2013.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terpou BA, Harricharan S, McKinnon MC, Frewen P, Jetly R, & Lanius RA (2019). The effects of trauma on brain and body: A unifying role for the midbrain periaqueductal gray. Journal of neuroscience research, 97(9), 1110–1140. [DOI] [PubMed] [Google Scholar]
- 34.Brandão ML, & Lovick TA (2019). Role of the dorsal periaqueductal gray in posttraumatic stress disorder: mediation by dopamine and neurokinin. Translational psychiatry, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benarroch EE (2001). Pain-autonomic interactions: a selective review. Clinical Autonomic Research, 11(6), 343–349. [DOI] [PubMed] [Google Scholar]
- 36.Bandler R, & Shipley M (1994). Columnar organization in the midbrain periaqueductal gray modules for emotional expression. Trends in Neuroscience, 17(11), 445–445. [DOI] [PubMed] [Google Scholar]
- 37.Satpute AB, Wager TD, Cohen-Adad J, Bianciardi M, Choi JK, Buhle JT, et al. (2013). Identification of discrete functional subregions of the human periaqueductal gray. PNAS, 110(42), 17101–17106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fanselow MS (1994). Neural organization of the defensive behavior system responsible for fear. Psychonomic bulletin & review, 1(4), 429–438. [DOI] [PubMed] [Google Scholar]
- 39.Bellgowan PS, & Helmstetter FJ (1996). Neural systems for the expression of hypoalgesia during nonassociative fear. Behavioral neuroscience, 110(4), 727. [DOI] [PubMed] [Google Scholar]
- 40.Linnman C, Moulton EA, Barmettler G, Becerra L, & Borsook D (2012). Neuroimaging of the periaqueductal gray: state of the field. Neuroimage, 60(1), 505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lanius RA, Rabellino D, Boyd JE, Harricharan S, Frewen PA, & McKinnon MC (2017). The innate alarm system in PTSD: conscious and subconscious processing of threat. Current Opinion in Psychology, 14, 109–115. [DOI] [PubMed] [Google Scholar]
- 43.Silva BA, Gross CT, & Gräff J (2016). The neural circuits of innate fear: detection, integration, action, and memorization. Learning & memory, 23(10), 544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright KM, & McDannald MA (2019). Ventrolateral periaqueductal gray neurons prioritize threat probability over fear output. eLife, 8, e45013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker RA, Wright KM, Jhou TC, & McDannald MA (2019). The ventrolateral periaqueductal grey updates fear via positive prediction error. European Journal of Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng H, Xiao X, & Wang Z (2016). Periaqueductal gray neuronal activities underlie different aspects of defensive behaviors. Journal of Neuroscience, 36(29), 7580–7588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mobbs D et al. (2007). When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science, 317(5841), 1079–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coker-Appiah DS, White SF, Clanton R, Yang J, Martin A, & Blair RJ (2013). Looming animate and inanimate threats: the response of the amygdala and periaqueductal gray. Social neuroscience, 8(6), 621–630. doi: 10.1080/17470919.2013.839480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, et al. (2009). From threat to fear: the neural organization of defensive fear systems in humans. Journal of Neuroscience, 29(39), 12236–12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harricharan S, Rabellino D, Frewen PA, Densmore M, Théberge J, McKinnon MC, et al. (2016). fMRI functional connectivity of the periaqueductal gray in PTSD and its dissociative subtype. Brain and behavior, 6(12), e00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicholson AA, Friston KJ, Zeidman P, Harricharan S, McKinnon MC, Densmore M, et al. (2017). Dynamic causal modeling in PTSD and its dissociative subtype: Bottom–up versus top–down processing within fear and emotion regulation circuitry. Human brain mapping, 38(11), 5551–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.George DT, Ameli R, & Koob GF (2019). Periaqueductal gray sheds light on dark areas of psychopathology. Trends in neurosciences. [DOI] [PubMed] [Google Scholar]
- 53.Johansen JP, Tarpley JW, LeDoux JE, & Blair HT (2010). Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nature neuroscience, 13(8), 979–986. doi: 10.1038/nn.2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hardy SG, & Haigler HJ (1985) Prefrontal influences upon the midbrain: a possible route for pain modulation. Brain Res, 665, 285–293. [DOI] [PubMed] [Google Scholar]
- 55.Bonanno GA, & Mancini AD (2012). Beyond resilience and PTSD: Mapping the heterogeneity of responses to potential trauma. Psychological trauma: Theory, research, practice, and policy, 4(1), 74. [Google Scholar]
- 56.Carrion VG, Haas BW, Garrett A, Song S, & Reiss AL (2009). Reduced hippocampal activity in youth with posttraumatic stress symptoms: an FMRI study. Journal of pediatric psychology, 35(5), 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tursich M, Ros T, Frewen PA, Kluetsch RC, Calhoun VD, & Lanius RA (2015). Distinct intrinsic network connectivity patterns of post-traumatic stress disorder symptom clusters. Acta Psychiatrica Scandinavica, 132(1), 29–38. [DOI] [PubMed] [Google Scholar]
- 58.Young G (2014). PTSD, endophenotypes, the RDoC, and the DSM-5. Psychological Injury and Law, 7(1), 75–91. 10.1007/s12207-014-9187-x [DOI] [Google Scholar]
- 59.Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, et al. (2004). Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain—an fMRI analysis. Pain, 109(3), 399–408. [DOI] [PubMed] [Google Scholar]
- 60.Tracey I Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, et al. (2002). Imaging attentional modulation of pain in the periaqueductal gray in humans. Journal of Neuroscience, 22(7), 2748–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knudsen L, Petersen GL, Nørskov KN, Vase L, Finnerup N, Jensen TS, et al. (2011). Review of neuroimaging studies related to pain modulation. Scandinavian Journal of Pain, 2(3), 108–120. [DOI] [PubMed] [Google Scholar]
- 62.Otis JD, Keane TM, & Kerns RD (2003). An examination of the relationship between chronic pain and post-traumatic stress disorder. Journal of rehabilitation research and development, 40(5), 397–406. [DOI] [PubMed] [Google Scholar]
- 63.Brasel KJ, deRoon-Cassini T, & Bradley CT (2010). Injury severity and quality of life: whose perspective is important? Journal of Trauma and Acute Care Surgery, 68(2), 263–268. [DOI] [PubMed] [Google Scholar]
- 64.Holdgate A, Asha S, Craig J, & Thompson J (2003). Comparison of a verbal numeric rating scale with the visual analogue scale for the measurement of acute pain. Emergency Medicine, 15(5–6), 441–446. [DOI] [PubMed] [Google Scholar]
- 65.Weiss DS (2007). The impact of event scale: revised In Cross-cultural assessment of psychological trauma and PTSD (pp. 219–238). Springer, Boston, MA. [Google Scholar]
- 66.Tiemensma J, Depaoli S, Winter SD, Felt JM, Rus HM, & Arroyo AC (2018). The performance of the IES-R for Latinos and non-Latinos: Assessing measurement invariance. PloS one, 13(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connectivity, 2(3), 125–141. [DOI] [PubMed] [Google Scholar]
- 68.Ezra M, Faull OK, Jbabdi S, & Pattinson KT (2015). Connectivity-based segmentation of the periaqueductal gray matter in human with brainstem optimized diffusion MRI. Human brain mapping, 36(9), 3459–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological), 57(1), 289–300. [Google Scholar]
- 70.Van Dijk KR, Sabuncu MR, & Buckner RL (2012). The influence of head motion on intrinsic functional connectivity MRI. Neuroimage, 59(1), 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buckner RL, Krienen FM, & Yeo BT (2013). Opportunities and limitations of intrinsic functional connectivity MRI. Nature neuroscience, 16(7), 832. [DOI] [PubMed] [Google Scholar]
- 72.Weber DL (2008). Information processing bias in post-traumatic stress disorder. The open neuroimaging journal, 2, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leech R, & Sharp DJ (2013). The role of the posterior cingulate cortex in cognition and disease. Brain, 137(1), 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brewer J, Garrison K, & Whitfield-Gabrieli S (2013). What about the “self” is processed in the posterior cingulate cortex? Frontiers in human neuroscience, 7, 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Behrmann M, Geng JJ, & Shomstein S (2004). Parietal cortex and attention. Current opinion in neurobiology, 14(2), 212–217. [DOI] [PubMed] [Google Scholar]
- 76.Falconer E et al. (2008). The neural networks of inhibitory control in posttraumatic stress disorder. Journal of psychiatry & neuroscience: JPN, 33(5), 413. [PMC free article] [PubMed] [Google Scholar]
- 77.Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, & Charney DS (2004). Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biological psychiatry, 55(6), 612–620. [DOI] [PubMed] [Google Scholar]
- 78.Morey RA et al. (2015). Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Translational psychiatry, 5(12), e700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun D, Phillips RD, Mulready HL, Zablonski ST, Turner JA, Turner MD, et al. (2019). Resting-state brain fluctuation and functional connectivity dissociate moral injury from posttraumatic stress disorder. Depression and Anxiety, 36(5), 442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blair KS, Vythilingam M, Crowe SL, McCaffrey DE, Ng P, Wu CC, et al. (2013). Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychological medicine, 43(1), 85–95. doi: 10.1017/S0033291712000840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang JN, Xiong KL, Qiu MG, Zhang Y, Xie B, Wang J, et al. (2013). Negative emotional distraction on neural circuits for working memory in patients with posttraumatic stress disorder. Brain Research, 1531, 94–101. [DOI] [PubMed] [Google Scholar]
- 82.Kong J, Tu PC, Zyloney C, & Su TP (2010). Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behavioural brain research, 211(2), 215–219. doi: 10.1016/j.bbr.2010.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mainero C, Boshyan J, & Hadjikhani N (2011). Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Annals of neurology, 70(5), 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keay KA, & Bandler R (2015). Periaqueductal gray In The rat nervous system (pp. 207–221). Academic Press. [Google Scholar]
- 85.Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, et al. (2018). The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military Veterans. Psychological Assessment, 30, 383–395. doi: 10.1037/pas0000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim JJ, Rison RA, & Fanselow MS (1993). Effects of amygdala, hippocampus, and periaqueductal gray lesions on short-and long-term contextual fear. Behavioral neuroscience, 107(6), 1093. [DOI] [PubMed] [Google Scholar]
- 87.Nicholson AA, Ros T, Frewen PA, Densmore M, Théberge J, Kluetsch RC, et al. (2016). Alpha oscillation neurofeedback modulates amygdala complex connectivity and arousal in posttraumatic stress disorder. NeuroImage: Clinical, 12, 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forster GL, Simons RM, & Baugh LA (2017). Revisiting the role of the amygdala in posttraumatic stress disorder. The amygdala-where emotions shape perception, learning and memories, 113–136. [Google Scholar]
- 89.Behbehani MM (1995). Functional characteristics of the midbrain periaqueductal gray. Progress in neurobiology, 46(6), 575–605. [DOI] [PubMed] [Google Scholar]
- 90.Palyo SA, & Beck JG (2005). Post-traumatic stress disorder symptoms, pain, and perceived life control: associations with psychosocial and physical [DOI] [PMC free article] [PubMed]
- 91.Beck JG, Gudmundsdottir B, & Shipherd JC (2003). PTSD and emotional distress symptoms measured after a motor vehicle accident: Relationships with pain coping profiles. Journal of Psychopathology and Behavioral Assessment, 25(4), 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Birn RM, Molloy EK, Patriat R, Parker T, Meier TB, Kirk GR, et al. (2013). The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage, 83, 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liao X-H, Xia M-R, Xu T, Dai Z-J, Cao X-Y, Niu H-J, et al. (2013). Functional brain hubs and their test–retest reliability: a multiband resting-state functional mri study. Neuroimage, 83, 969–982 [DOI] [PubMed] [Google Scholar]
- 94.Laumann TO, Gordon EM, Adeyemo B, Snyder AZ, Joo SJ, Chen M-Y, Gilmore AW, et al. (2015). Functional system and areal organization of a highly sampled individual human brain. Neuron, 87(3), 657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Casanova R, Srikanth R, Baer A, Laurienti PJ, Burdette JH, Hayasaka S, et al. (2007). Biological parametric mapping: a statistical toolbox for multimodality brain image analysis. Neuroimage, 34(1), 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roy AK, Benson BE, Degnan KA, Perez-Edgar K, Pine DS, Fox NA, et al. (2014). Alterations in amygdala functional connectivity reflect early temperament. Biological psychology, 103, 248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Noble S, Scheinost D, Finn ES, Shen X, Papademetris X, McEwen SC, et al. (2017). Multisite reliability of MR-based functional connectivity. Neuroimage, 146, 959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


