Abstract
Background
Shortened sleep and affective disturbances are both prevalent in adolescents with attention-deficit/hyperactivity disorder (ADHD), yet the causal link between these domains has not been examined. This study investigated whether shortened sleep duration is causally linked to affective functioning in adolescents with ADHD.
Methods
Participants were 48 adolescents (75% male) ages 14–17 years with ADHD who successfully completed a three-week sleep protocol using an experimental crossover design. The protocol included a phase stabilization week, followed, in randomized counterbalanced order, by one week of sleep restriction (6.5 hours in bed) and one week of sleep extension (9.5 hours in bed). Sleep was monitored with objective actigraphy, and all participants included in this study obtained ≥1 hour actigraphy-measured sleep duration during extension compared to restriction. Parents and adolescents provided daily ratings of positive and negative affect during the extension and restriction conditions. Ratings of affect, internalizing symptoms, and emotion regulation were collected at laboratory visits conducted at the end of each week.
Results
Both parents and adolescents reported greater depressive symptoms and lower positive affect during restriction compared to extension. Parents also reported greater negative affect and emotion dysregulation among adolescents during sleep restriction than extension. No effects were found for parent- or adolescent-reported anxiety symptoms or for adolescent-reported emotion regulation or negative affect.
Conclusions
Findings from this study provide the first evidence that shortened sleep duration is a causal contributor to the affect and mood disturbances frequently experienced by adolescents with ADHD, particularly as observed by parents. Targeting sleep may be important to reduce affective disturbances in adolescents with ADHD.
Keywords: ADHD, adolescence, affect, anxiety, attention-deficit/hyperactivity disorder, comorbidity, depression, emotion regulation, functional impairment, sleep deprivation
Introduction
Adolescents with attention-deficit/hyperactivity disorder (ADHD) experience more sleep problems, including insufficient sleep duration, delayed sleep onset latency, more variable sleep/wake patterns, and increased daytime sleepiness, compared to adolescents without ADHD (for reviews, see Becker, 2019; Lunsford-Avery, Krystal, & Kollins, 2016; Mulraney, Sciberras, & Becker, 2020). For example, adolescents with ADHD are 6.2 times more likely than their peers without ADHD to have a parent-reported sleep disturbance (Becker, Langberg, Eadeh, Isaacson, & Bourchtein, 2019). Further, correlational studies have found sleep problems to be associated with poorer daytime functioning in adolescents with ADHD (Becker, Langberg, & Evans, 2015; Hysing, Lundervold, Posserud, & Sivertsen, 2016; Stein et al., 2002). To examine whether shortened sleep duration is causally linked to daytime functioning in adolescents with ADHD, we recently conducted a crossover sleep restriction/extension study and found shortened sleep to cause greater inattention, oppositionality, and sleepiness (Becker, Epstein, et al., 2019). The current paper extends these findings by examining the causal impact of shortened sleep on affective functioning in adolescents with ADHD.
Observational studies find poor or insufficient sleep to be associated with poorer affective functioning in adolescents (Becker, Langberg, & Byars, 2015; Brand et al., 2016; Shochat, Cohen-Zion, & Tzischinsky, 2014). Studies with typically developing adolescents also have found sleep deprivation (Short & Louca, 2015) or varying degrees of sleep restriction (Baum et al., 2014; McMakin et al., 2016; Talbot, McGlinchey, Kaplan, Dahl, & Harvey, 2010) to causally impact affective functioning. Using a similar protocol as the present study, Baum and colleagues (2014) found that adolescents had worsened mood (e.g., increased anxiety and anger, lower vigor) following five days of sleep restriction (6.5 hours in bed) compared to five days of sleep extension (10 hours in bed), though no effect was found for depressive symptoms specifically. Both adolescents and parents also reported poorer emotion regulation and greater irritability following sleep restriction (Baum et al., 2014). Other studies have also found sleep restriction (ranging from 2 to 5 hours in bed) to cause increases in negative affect and/or decreases in positive affect (Lo, Ong, Leong, Gooley, & Chee, 2016; McMakin et al., 2016; Talbot et al., 2010). McMakin et al. (McMakin et al., 2016) extended these findings to the social context and found evidence for observed negative affective behavior during a peer conflict task after sleep restriction.
In building from previous work conducted in typically developing adolescents, it is especially important to examine the impact of sleep restriction on the affective functioning of adolescents with ADHD. Adolescents with ADHD are at increased risk for experiencing co-occurring internalizing psychopathology, including anxiety and depression (Becker & Fogleman, 2020; Smalley et al., 2007). In addition, deficits in emotion regulation are increasingly recognized as a core feature of ADHD (Faraone et al., 2019). Furthermore, emotion regulation deficits in children with ADHD are associated with a more severe ADHD phenotype (Sobanski et al., 2010) and prospectively predict greater functional impairment in adulthood (Barkley & Fischer, 2010). Given these findings, there has been recent interest in developing and testing interventions targeting emotion and mood for children and adolescents with ADHD (Meinzer, Hartley, Hoogesteyn, & Pettit, 2018; Rosen et al., 2018). Sleep is not currently included in these interventions. If shortened sleep contributes to poorer affective functioning in adolescents with ADHD, then sleep may be an important, yet currently unaddressed, treatment target for this population.
The present study used an experimental sleep restriction/extension protocol to examine shortened sleep duration as a causal contributor to poorer affective functioning in adolescents with ADHD. Based on previous research with typically developing adolescents (Baum et al., 2014; Lo et al., 2016; McMakin et al., 2016; Talbot et al., 2010), we hypothesized that adolescents with ADHD would experience lower positive affect, higher negative affect, increased internalizing symptoms, and greater emotion dysregulation during a sleep restriction condition compared to a sleep extension condition.
Methods
Participants
Participants were 48 adolescents (75% male) ages 14–17 years (M = 15.21, SD = 1.15) diagnosed with ADHD who successfully completed the three-week sleep protocol described below. All participants had an IQ ≥ 70 (Range = 79–132) based on the Kaufman Brief Intelligence Test, Second Edition (Kaufman & Kaufman, 2004). Sample characteristics, including comorbid diagnoses based on the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS) (Kaufman et al., 1997) interview conducted separately with the adolescent and parent, are provided in Table 1.
Table 1.
Sample Characteristics (N = 48)
| M ± SD | |
|---|---|
| Age | 15.21 ± 1.15 |
| IQ | 102.90 ± 11.64 |
|
N (%) |
|
| Sex | |
| Male | 36 (75.0%) |
| Female | 12 (25.0%) |
| Race/Ethnicity | |
| White | 37 (77.1%) |
| Black | 5 (10.4%) |
| Hispanic | 1 (2.1%) |
| Multiracial | 5 (10.4%) |
| Baseline Medication Statusa | |
| Stimulant | 35 (72.9%) |
| Nonstimulant | 4 (8.3%) |
| Melatonin | 1 (2.1%) |
| Any medication | 37 (77.1%) |
| Family Incomeb | |
| Up to $40,000 | 4 (8.3%) |
| $40,001 - $60,000 | 8 (17.0%) |
| $60,001 - $80,000 | 4 (8.5%) |
| Over $80,000 | 31 (66.0%) |
| ADHD Presentationc | |
| Combined | 11 (22.9%) |
| Inattentive | 37 (77.1%) |
| Comorbid Diagnosesc | |
| Depression/Dysthymia | 1 (2.1%) |
| GAD | 4 (8.3%) |
| PTSD | 0 (0%) |
| Mania | 0 (0%) |
| ODD | 2 (4.2%) |
| CD | 0 (0%) |
| Any Comorbidity | 6 (12.5%) |
Note: ADHD = attention-deficit/hyperactivity disorder. CD = conduct disorder. GAD = generalized anxiety disorder. ODD = oppositional defiant disorder. PTSD = posttraumatic stress disorder.
All participants were taken off any medication prior to starting the three-week sleep protocol.
One parent declined to answer the family income question.
ADHD and comorbid diagnoses established using the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS) conducted separately with the parent and adolescent (using an “or” rule), with ADHD diagnosis and presentation based on interview with the adolescent’s parent.
As in our previous study (Becker, Epstein, et al., 2019), although 72 adolescents entered the sleep protocol, only the 48 who were adherent to the sleep protocol (defined a priori as ≥1 hour longer nightly sleep during sleep extension compared to sleep restriction) were included in current analyses given the aim to test the impact of sleep restriction and extension. There were no significant differences between adherent and non-adherent participants in demographic characteristics, ADHD symptom severity, ADHD presentation, sleep/sleepiness, or psychiatric comorbidity (see Becker et al., 2019 for details). In addition, there were no significant differences between adherent and non-adherent participants in parent- or adolescent-reported internalizing symptoms or emotion regulation assessed at the inclusion visit (all ps > .20).
Procedures
All study procedures were approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board (IRB). Signed informed consent and assent were obtained. Recruitment materials were distributed via local schools, in the community, and at Cincinnati Children’s Hospital Medical Center where the study was conducted during the summers of 2016 and 2017. Materials described a study examining sleep in adolescents with ADHD but did not specifically mention (nor target) adolescents with sleep problems.
Full Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) (American Psychiatric Association, 2013) criteria for ADHD Predominantly Inattentive or Combined Presentation on the K-SADS parent interview was required for eligibility. Exclusion criteria included autism, bipolar disorder, obsessive-compulsive disorder, or psychosis; meeting screening criteria for the possible presence of sleep-disordered breathing or restless leg syndrome using the Pediatric Sleep Questionnaire (Chervin & Hedger, 2001; Chervin et al., 2007); history of epilepsy or head trauma resulting in loss of consciousness; IQ < 70; regular high caffeine use (>1 coffee/energy drink/day or 3 caffeinated soft drinks/day); highly atypical sleep duration (routinely obtaining <6 hours of >9.5 hours on school nights); or obligations that required a bedtime later than 10:00PM or waking prior to 6:00AM. Participants taking stimulant medication were allowed if the family was willing to discontinue the medication for the three-week sleep protocol during the summer. In the first year, all participants taking melatonin or a non-stimulant psychiatric medication were excluded; in the second year, these were not exclusionary but only allowable if discontinued during the summer.
Sleep protocol
As in previous studies of typically developing adolescents (Baum et al., 2014; Beebe et al., 2008), participants were involved in a three-week sleep manipulation protocol administered during the summer break from school to avoid impacting scholastic performance. All sleep occurred in the home environment and was monitored via sleep diaries and actigraphy. After a stabilization week whereby participants were asked to wake at a time that would allow them to arrive at the research location by 8:00AM, participants were asked to systematically change their bedtimes to accommodate sleep extension and sleep restriction conditions. A within-subjects, crossover design was used, such that all adolescents participated in both the sleep restriction and sleep extension conditions, with the order of conditions randomly counterbalanced across participants. During the sleep extension condition, adolescents adjusted their bedtime to allow 9.5 hours in bed. This 9.5-hour “in bed” window was selected because, allowing for 30 minutes to fall asleep, (a) 9 hours is how long adolescents sleep during controlled trials of sleep satiation (Carskadon et al., 1980) and naturally on non-school nights (National Sleep Foundation, 2006), (b) this results in a well-rested state in adolescents (Beebe et al., 2008), and (c) this conforms to clinical recommendations for adolescents (Hirshkowitz et al., 2015; Paruthi et al., 2016). During the sleep restriction condition, adolescents adjusted their bedtime to allow 6.5 hours in bed, which in previous studies using this protocol resulted in an average of 6.1–6.3 hours of nightly sleep (Beebe et al., 2008). This sleep restriction condition reflects a realistic dose of sleep restriction (similar to school-night sleep of 15–20% of healthy adolescents; Wolfson & Carskadon, 1998) that is feasible and induces daytime sleepiness, inattention, and oppositionality in typically developing adolescents (Baum et al., 2014; Beebe et al., 2008).
Primary data collection occurred throughout the week (via actigraphy and daily diaries) and at a laboratory visit each Friday at the end of each condition. To monitor sleep and adherence to the protocol, participants wore a wrist-mounted actigraph (Micro Motionlogger©, Ambulatory Monitoring, Inc.) throughout the sleep protocol to gather an objective measure of sleep. At each Friday assessment, actigraph data were downloaded and both the actigraphy data and sleep diaries were reviewed with the adolescent and their parent. In tandem with visually inspecting the sleep diaries, a validated algorithm (Sadeh, Sharkey, & Carskadon, 1994) was used to obtain objective estimates of sleep duration. The 48 adolescents included in current analyses averaged 1.6 hours longer actigraphy-measured sleep per night during the sleep extension condition than during sleep restriction (p < .001; additional details available in Becker, Epstein, et al., 2019).
Measures
Daily affect
Adolescents and parents completed the 10-item Positive and Negative Affect Scale, Short Version (PANAS) (Ebesutani, Okamura, Higa-McMillan, & Chorpita, 2011) at the laboratory visits at the conclusion of the sleep extension and sleep restriction weeks. The 10-item PANAS includes a positive affect scale (joyful, cheerful, happy, lively, proud) and a negative affect scale (miserable, mad, afraid, scared, sad), with each item rated on a five-point scale (1 = very slightly or not at all, 5 = extremely). Total mean scale scores were calculated (adolescent positive affect αs = .90 and .91, for sleep extension and sleep restriction, respectively; adolescent negative affect αs = .90 and .75; parent positive affect αs = .93 and .90; parent negative affect αs = .70 and .78). In addition, both adolescents and parents completed a daily diary which included the 10-item PANAS.
Emotion regulation
The Emotion Regulation Checklist (ERC) (Shields & Cicchetti, 1997) and Difficulties in Emotion Regulation Scale (DERS) (Gratz & Roemer, 2004) were used at each laboratory visit to assess parent- and adolescent-reported emotion regulation, respectively. The ERC consists of 24 items rated on a four-point scale (1 = never; 4 = always). The DERS consists of 36 items rated on a five-point scale (1 = almost never; 5 = almost always), with higher scores indicating more difficulties with emotion regulation. Total mean scale scores were calculated (ERC αs = .77 and .76 for sleep extension and sleep restriction, respectively; DERS αs = .88 and .90).
Internalizing symptoms
At each laboratory visit, adolescents and parents completed the Revised Child Anxiety and Depression Scales (RCADS) (Chorpita, Moffitt, & Gray, 2005; Chorpita, Yim, Moffitt, Umemoto, & Francis, 2000; Ebesutani et al., 2010; Ebesutani, Chorpita, et al., 2011), a 47-item measure that assesses DSM-based anxiety and depression disorder symptoms on a four-point scale (1 = never, 4 = always). To ensure that any differences across sleep conditions were not attributable to the protocol’s expected impact on sleep, two separation anxiety items (“feel scared to sleep on my own”, “I worry when I go to bed at night”) and one depression item (“I have trouble sleeping”) related to sleep were removed before computing scale scores. Total mean scale scores were calculated for the remaining 35 anxiety symptoms (adolescent αs = .95 and .95 for sleep extension and sleep restriction, respectively; parent αs = .93 and .89) and the remaining nine depressive symptoms (adolescent αs = .83 and .80 for sleep extension and sleep restriction, respectively; parent αs = .79 and .71).
In addition, at each laboratory visit adolescents completed the depressed mood subscale of the Sleep Habits Survey (SHS) (Wolfson & Carskadon, 1998). The depressed mood scale includes six items (e.g., “Feeling unhappy, sad or depressed”) rated on a three-point scale (1 = not at all; 2 = somewhat; 3 = much). For the present study, two items of sleepiness (“feeling too tired to do things”) and sleep problems (“having trouble going to sleep or staying asleep”) were removed before calculating total sum scale scores (αs = .82 and .75 for sleep extension and sleep restriction, respectively).
Analyses
Our primary analyses examined the extent to which sleep restriction was associated with internalizing symptoms, affect, and emotion regulation. A paired samples t-test for each outcome variable was conducted comparing the sleep restriction and sleep extension conditions, with an alpha threshold of 0.051. Cohen’s d, corrected for dependence in within-subjects data, was computed as a measure of effect size, with 0.2, 0.5, and 0.8 as benchmarks for small, medium, and large effects, respectively (Cohen, 1988). Secondary analyses were conducted examining daily-level positive and negative affect across the sleep restriction and sleep extension weeks. Specifically, repeated-measures general linear models were conducted with both sleep condition (sleep restriction and sleep extension) and day (days 1 through 5) as within-subjects factors, with separate models conducted for adolescent and parent ratings and for positive and negative affect. Our effect of interest was whether there was a main effect of sleep condition, though we also examined whether there was a main effect of day of the week as well as the sleep condition × day interaction. Partial eta-squared (ηp2) was computed as a measure of effect size, with 0.01, 0.06, and 0.14 as benchmarks for small, medium, and large effects, respectively (Cohen, 1988).
Results
Impact on Weekly Ratings of Affect, Emotion Regulation, and Internalizing Symptoms
Table 2 summarizes findings for the outcome variables assessed at the laboratory visits following sleep restriction and sleep extension. Parents reported significantly lower positive affect, higher negative affect, and poorer emotion regulation during sleep restriction compared to sleep extension, with medium to large effects for positive affect and emotion regulation (ds = 0.82 and 0.56, respectively) and a small to medium effect for negative affect (d = 0.44). In contrast to parent ratings, significant differences were not found for adolescent-reported negative affect or emotion regulation. Although statistically significant effects were not found for adolescent-reported positive and negative affect, small effects were found in the expected direction for these domains (both ds = 0.20).
Table 2.
Differences in Affective Functioning During Sleep Restriction and Sleep Extension in Adolescents with ADHD
| Sleep Restriction |
Sleep Extension |
Paired samples t-tests comparing sleep restriction and extension |
|||
|---|---|---|---|---|---|
| M ± SD | M ± SD | t | p | d | |
| Positive affect | |||||
| PANAS (parent) | 2.66 ± 0.80 | 3.13 ± 0.83 | 4.96 | <.001 | 0.72 |
| PANAS (adolescent) | 2.88 ± 0.93 | 3.09 ± 0.95 | 2.01 | 0.051 | 0.20 |
| Negative affect | |||||
| PANAS (parent) | 1.33 ± 0.47 | 1.15 ± 0.31 | −3.05 | 0.004 | 0.44 |
| PANAS (adolescent) | 1.25 ± 0.42 | 1.18 ± 0.29 | −1.34 | 0.182 | 0.20 |
| Emotion regulation | |||||
| ERC (parent) | 1.88 ± 0.28 | 1.76 ± 0.28 | −3.83 | <.001 | 0.56 |
| DERS (adolescent) | 2.04 ± 0.45 | 2.02 ± 0.42 | −0.69 | 0.496 | 0.10 |
| Anxiety | |||||
| RCADS (parent) | 0.24 ± 0.21 | 0.21 ± 0.23 | −1.72 | 0.093 | 0.25 |
| RCADS (adolescent) | 0.36 ± 0.35 | 0.34 ± 0.37 | −0.68 | 0.501 | 0.10 |
| Depression | |||||
| RCADS (parent) | 0.45 ± 0.32 | 0.26 ± 0.30 | −5.80 | <.001 | 0.84 |
| RCADS (adolescent) | 0.41 ± 0.38 | 0.32 ± 0.38 | −1.81 | 0.077 | 0.26 |
| SHS (adolescent) | 4.83 ± 1.42 | 4.56 ± 1.30 | −2.16 | 0.036 | 0.31 |
Note: All measures were completed at a laboratory visit following sleep restriction and sleep extension. ADHD = attention-deficit/hyperactivity disorder. DERS = Difficulties in Emotion Regulation Scale. ERC = Emotion Regulation Checklist. PANAS = 10-item Positive and Negative Affect Scale. RCADS = Revised Child Anxiety and Depression Scales. SD = standard deviation. SHS = Sleep Habits Survey.
In considering internalizing symptoms, parents reported significantly greater depressive symptoms during sleep restriction compared to sleep extension, with a large effect (d = 0.84). Adolescents reported significantly greater depression on the SHS, but not on the RCADS, during sleep restriction compared to sleep extension, with small effects for both measures (ds = 0.31 and 0.26, respectively). Significant differences were not found for either parent- or adolescent-reported anxiety2.
Impact on Daily Ratings of Positive and Negative Affect
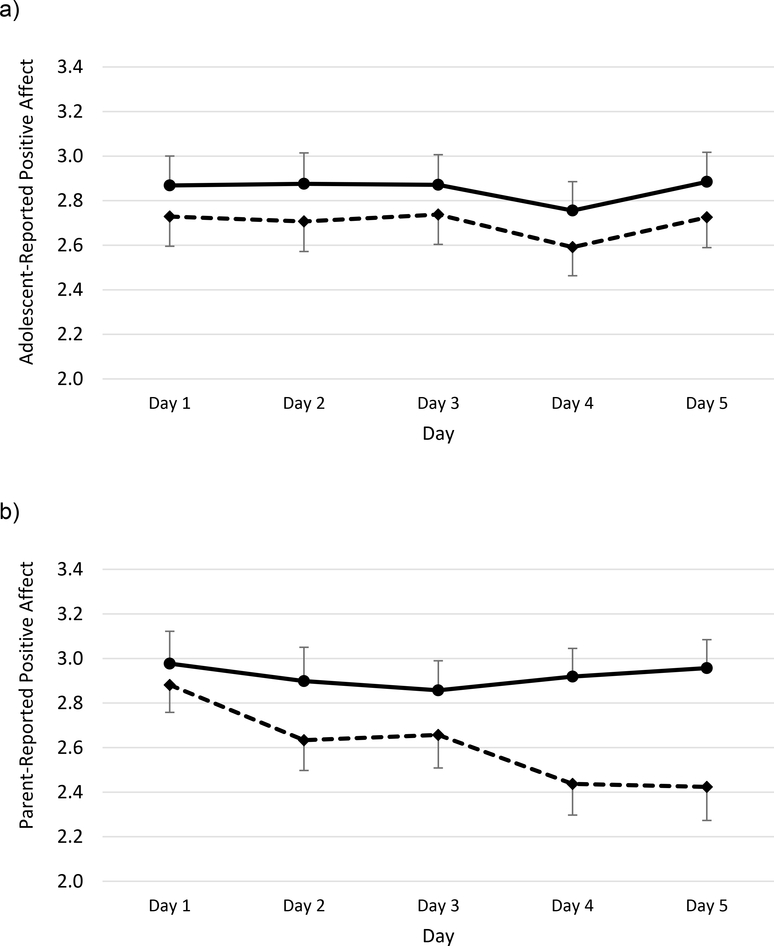
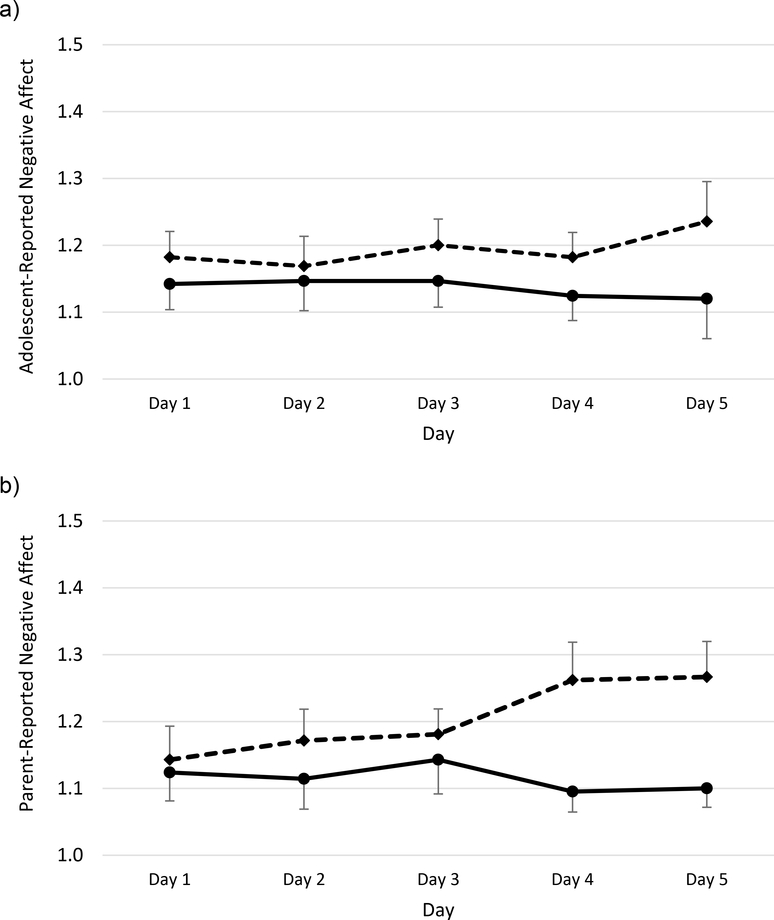
Repeated-measures general linear models indicated a significant effect of sleep condition on both parent-reported positive affect, F(1,41) = 10.02, p = .003, ηp2 = .196, and adolescent-reported positive affect, F(1,44) = 4.20, p = .046, ηp2 = .087. As shown in Figure 1, parent- and adolescent-reported mean scores for positive affect were lower across all five days of sleep restriction than sleep extension. In addition, there was a significant effect of day for both parent- and adolescent-reported positive affect (ps = .026 and .008, respectively), with positive affect trending lower later in the week. As shown in Figure 2, daily negative affect ratings were generally higher during sleep restriction than extension, but this reached statistical significance only on parent-report, F(1,41) = 6.07, p = .018, ηp2 = .129, not adolescent-report, F(1,44) = 2.45, p = .125, ηp2 = .053. Although visual inspection of both figures suggests that sleep restriction resulted in progressively lower parent-reported positive affect and higher parent-reported negative affect over time, there was not a significant effect of day or a significant week × day interaction for either parent- or adolescent-reported negative affect (ps > .05).
Figure 1.
Daily positive affect during sleep extension (solid lines) and sleep restriction (dashed lines). Higher scores indicate greater positive affect. Adolescent-reported positive affect is dispalyed in panel a, parent-report of adolescent positive affect is displayed in panel b. Bars indicate standard errors.
Figure 2.
Daily negative affect during sleep extension (solid lines) and sleep restriction (dashed lines). Higher scores indicate greater negative affect. Adolescent-reported positive affect is displayed in panel a, parent-report of adolescent positive affect is displayed in panel b. Bars indicate standard errors.
Discussion
The present study provides evidence that shortened sleep duration causally contributes to poorer affective functioning in adolescents diagnosed with ADHD. Insufficient sleep duration worsens mood and emotion regulation in adolescents with ADHD, particularly as observed by parents.
Across both daily diary and laboratory visit measures, parents reported less positive affect and more negative affect during sleep restriction compared to extension, with medium to large effect sizes. Although effects of the sleep manipulation on affect ratings were in the same direction on adolescent self-reports, effect sizes were much smaller effects, generally falling just short of statistical significance. Previous research with typically developing adolescents has found sleep restriction to impact a) both positive and negative affect (Study 1 in McMakin et al., 2016), b) negative but not positive affect (Study 2 in McMakin et al., 2016), or c) positive but not negative affect (Dagys et al., 2012; Lo et al., 2016; Talbot et al., 2010). Of note, each of these previous studies used variations of the self-report PANAS and did not collect parent ratings of adolescents’ affect. We are unaware of any study that has used the parent-report PANAS to assess affect across sleep restriction and extension in adolescents. However, in considering parent-report of adolescents’ affect, our findings are consistent with Baum et al. (2014) who found sleep restriction to impact both positive mood (e.g., vigor/activity) and negative mood (e.g., anger/hostility).
Sleep restriction increased emotion regulation difficulties according to parent-report but not adolescent-report. This contrasts with a previous study that used a similar sleep protocol and found sleep restriction to negatively impact emotion regulation across both parent and adolescent informants (Baum et al., 2014). However, our findings are consistent with an experimental sleep study conducted with school-aged children (ages 8–12 years) which found restricted sleep to worsen parent-reported, but not child-reported, emotion regulation (Vriend et al., 2013). In addition, emotion dysregulation is closely linked to irritability in youth with ADHD (Faraone et al., 2019), and previous work with typically developing adolescents has found restricted sleep to increase parent-reported irritability but not adolescent-reported irritability (Beebe et al., 2008). It may be that youth themselves are less able to reflect on their emotional reactivity, or to observe changes over the relatively short periods used in the sleep protocol. Also, as parents and adolescents completed different measures of emotion regulation in the present study, it is unclear if the discrepant pattern of findings across raters is due to informant differences or measurement differences. It would be beneficial for future research to further examine how restricted sleep impacts emotion in adolescents with ADHD, including attending to informant differences and distinguishing between emotion generation and emotion regulation (Palmer & Alfano, 2017).
A previous observational study found sleep problems to longitudinally predict increases in depressive symptoms one year later in adolescents with ADHD, whereas sleep problems were not concurrently or prospectively associated with anxiety symptoms (Becker, Langberg, & Evans, 2015). Our findings provide experimental support for differentiating the impact of sleep on depression and anxiety in adolescents with ADHD. Specifically, sleep restriction resulted in significantly increased depressive symptoms, but not anxiety symptoms, with findings largely consistent across parent and adolescent informants. This pattern fits with our findings that sleep restriction impacted positive affect more so than negative affect, as low positive affect is a defining feature that distinguishes depression from anxiety (Clark & Watson, 1991). Our findings suggest that insufficient sleep should be incorporated into models that aim to identify mechanisms linking ADHD and depression (Meinzer & Chronis-Tuscano, 2017). This is especially important given the increased impairment and suicide behaviors among individuals with ADHD who have co-occurring depression (Balazs & Kereszteny, 2017; Daviss, 2008).
Given the prevalence and impact of affective disturbances in ADHD, interventions have recently been developed to target emotion and mood problems specific to this population (Meinzer et al., 2018; Rosen et al., 2018). Our findings suggest that targeting sleep may be an important factor to include for optimizing treatment effects. Taking a different approach, directly treating sleep may lead to improvements in mood and emotion regulation. There is some evidence that improving sleep also improves emotional functioning in school-aged children with ADHD (Hiscock et al., 2015; Keshavarzi et al., 2014; Sciberras et al., 2019), though improvements in emotional functioning were not found when a brief sleep intervention was delivered by community-based clinicians (Hiscock et al., 2019). Studies have yet to evaluate evidence-based cognitive-behavioral sleep interventions in adolescents with ADHD, which will be important given the developmental changes and contexts that occur in adolescence that have implications for intervention delivery. Our findings showing shortened sleep to cause poorer daytime functioning point to this as an important area for clinical attention (Becker, 2019).
Several limitations are important to acknowledge. First, our measures were limited to parent and adolescent rating scales, which may lack objectivity and could not be masked to sleep condition. It is reassuring that we did not find universal effects across all domains examined in this study, bolstering confidence that findings were not attributable to response bias. Nevertheless, it would be beneficial for future research to incorporate other performance-based measures (e.g., emotional Stroop task), and administering the sleep protocol during the school year would allow for collecting ratings from teachers and peers who could be masked to the sleep conditions. Second, our study was limited to adolescents with ADHD and did not include a comparison sample of adolescents without ADHD. We were thus unable to examine whether the magnitude of effects differs for adolescents with and without ADHD or whether certain effects (e.g., depressive symptoms) are especially relevant to youth with ADHD. Third, the sample was of a modest size, which limited statistical power to detect small effects (e.g., on adolescent self-report). Finally, the sample had relatively low rates of psychiatric comorbidity. The extent to which findings generalize to more clinically severe samples of adolescents with ADHD is unknown.
In conclusion, the current study provides the first evidence that shortened sleep is causally contributes to poorer affective functioning in adolescents with ADHD. These findings are especially important given the prevalence and impact of emotion dysregulation and mood disturbances in this population. Findings suggest that targeting sleep may be important to reduce these problems in adolescents with ADHD.
Key Points.
Shortened sleep and affective disturbances are both prevalent in adolescents with attention-deficit/hyperactivity disorder (ADHD).
This experimental sleep restriction/extension study provides evidence that shortened sleep duration causally contributes to poorer affective functioning in adolescents diagnosed with ADHD, particularly as observed by parents.
Findings align with correlational research in demonstrating sleep restriction to result in increased depressive symptoms, but not anxiety symptoms, in adolescents with ADHD.
There has been recent interest in developing and testing interventions targeting emotion and mood disturbances in children and adolescents with ADHD. Findings from this study suggest that targeting sleep may be an important factor to include for optimizing treatment effects.
Acknowledgements
This research was supported by grant R03MH109787 from the National Institute of Mental Health (NIMH). S.B. is supported by award number K23MH108603 from the NIMH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. National Institutes of Health (NIH). The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
The pattern of findings was unchanged when nonparametric Wilcoxon signed-rank tests were used.
Sleep manipulation order did not interact with any of the affective functioning variables to moderate effects of the sleep manipulation (all ps > .10), with one exception: there was a significant interaction between manipulation order and adolescent self-reported anxiety symptoms (p = .03). However, follow-up tests indicated that the sleep protocol did not significantly impact anxiety regardless of manipulation order (ps > .05).
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: Fifth Edition (5th ed.). Washington, D.C.: American Psychiatric Association. [Google Scholar]
- Balazs J, & Kereszteny A (2017). Attention-deficit/hyperactivity disorder and suicide: A systematic review. World J Psychiatry, 7, 44–59. doi: 10.5498/wjp.v7.i1.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, & Fischer M (2010). The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. J Am Acad Child Adolesc Psychiatry, 49, 503–513. [DOI] [PubMed] [Google Scholar]
- Baum KT, Desai A, Field J, Miller LE, Rausch J, & Beebe DW (2014). Sleep restriction worsens mood and emotion regulation in adolescents. J Child Psychol Psychiatry, 55, 180–190. doi: 10.1111/jcpp.12125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker SP (2019). The triple threat of sleep, adolescence, and ADHD In Hiscock H & Sciberras E (Eds.), Sleep and ADHD: An evidence-based guide to assessment and treatment (pp. 257–294). San Diego, CA: Elsevier/Academic Press. [Google Scholar]
- Becker SP, Epstein JN, Tamm L, Tilford AA, Tischner CM, Isaacson PA, … Beebe DW (2019). Shortened sleep duration causes sleepiness, inattention, and oppositionality in adolescents with attention-deficit/hyperactivity disorder: Findings from a crossover sleep restriction/extension study. J Am Acad Child Adolesc Psychiatry, 58, 433–442. doi: 10.1016/j.jaac.2018.09.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker SP, & Fogleman ND (2020). Psychiatric co-occurrence (comorbidity) in adolescents with ADHD In Becker SP (Ed.), ADHD in Adolescents: Development, Assessment, and Treatment (pp. 170–203). New York: Guilford. [Google Scholar]
- Becker SP, Langberg JM, & Byars KC (2015). Advancing a biopsychosocial and contextual model of sleep in adolescence: a review and introduction to the special issue. J Youth Adolesc, 44, 239–270. doi: 10.1007/s10964-014-0248-y [DOI] [PubMed] [Google Scholar]
- Becker SP, Langberg JM, Eadeh HM, Isaacson PA, & Bourchtein E (2019). Sleep and daytime sleepiness in adolescents with and without ADHD: Differences across ratings, daily diary, and actigraphy. J Child Psychol Psychiatry, 60, 1021–1031. doi: 10.1111/jcpp.13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker SP, Langberg JM, & Evans SW (2015). Sleep problems predict comorbid externalizing behaviors and depression in young adolescents with attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry, 24, 897–907. doi: 10.1007/s00787-014-0636-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DW, Fallone G, Godiwala N, Flanigan M, Martin D, Schaffner L, & Amin R (2008). Feasibility and behavioral effects of an at-home multi-night sleep restriction protocol for adolescents. J Child Psychol Psychiatry, 49, 915–923. doi: 10.1111/j.1469-7610.2008.01885.x [DOI] [PubMed] [Google Scholar]
- Brand S, Kirov R, Kalak N, Gerber M, Schmidt NB, Lemola S, … Holsboer-Trachsler E (2016). Poor sleep is related to lower emotional competence among adolescents. Behav Sleep Med, 14, 602–614. doi: 10.1080/15402002.2015.1048450 [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, & Dement WC (1980). Pubertal changes in daytime sleepiness. Sleep, 2, 453–460. [DOI] [PubMed] [Google Scholar]
- Chervin RD, & Hedger KM (2001). Clinical prediction of periodic leg movements during sleep in children. Sleep Med, 2, 501–510. [DOI] [PubMed] [Google Scholar]
- Chervin RD, Weatherly RA, Garetz SL, Ruzicka DL, Giordani BJ, Hodges EK, … Guire KE (2007). Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg, 133, 216–222. doi: 10.1001/archotol.133.3.216 [DOI] [PubMed] [Google Scholar]
- Chorpita BF, Moffitt CE, & Gray J (2005). Psychometric properties of the Revised Child Anxiety and Depression Scale in a clinical sample. Behav Res Ther, 43, 309–322. doi: 10.1016/j.brat.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Chorpita BF, Yim L, Moffitt C, Umemoto LA, & Francis SE (2000). Assessment of symptoms of DSM-IV anxiety and depression in children: a revised child anxiety and depression scale. Behav Res Ther, 38, 835–855. [DOI] [PubMed] [Google Scholar]
- Clark LA, & Watson D (1991). Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol, 100, 316–336. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Dagys N, McGlinchey EL, Talbot LS, Kaplan KA, Dahl RE, & Harvey AG (2012). Double trouble? The effects of sleep deprivation and chronotype on adolescent affect. J Child Psychol Psychiatry, 53, 660–667. doi: 10.1111/j.1469-7610.2011.02502.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviss WB (2008). A Review of Co-Morbid Depression in Pediatric ADHD: Etiologies, Phenomenology, and Treatment. J Child Adolesc Psychopharmacol, 18, 565–571. doi: 10.1089/cap.2008.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebesutani C, Bernstein A, Nakamura BJ, Chorpita BF, Weisz JR, & Research Network on Youth Mental, H. (2010). A psychometric analysis of the revised child anxiety and depression scale--parent version in a clinical sample. J Abnorm Child Psychol, 38, 249–260. doi: 10.1007/s10802-009-9363-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebesutani C, Chorpita BF, Higa-McMillan CK, Nakamura BJ, Regan J, & Lynch RE (2011). A psychometric analysis of the Revised Child Anxiety and Depression Scales--parent version in a school sample. J Abnorm Child Psychol, 39, 173–185. doi: 10.1007/s10802-010-9460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebesutani C, Okamura K, Higa-McMillan C, & Chorpita BF (2011). A psychometric analysis of the Positive and Negative Affect Schedule for Children-Parent Version in a school sample. Psychol Assess, 23, 406–416. doi: 10.1037/a0022057 [DOI] [PubMed] [Google Scholar]
- Faraone SV, Rostain AL, Blader J, Busch B, Childress AC, Connor DF, & Newcorn JH (2019). Practitioner Review: Emotional dysregulation in attention-deficit/hyperactivity disorder - implications for clinical recognition and intervention. J Child Psychol Psychiatry, 60, 133–150. doi: 10.1111/jcpp.12899 [DOI] [PubMed] [Google Scholar]
- Gratz KL, & Roemer L (2004). Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. J Psychopathol Behav Assess, 26, 41–54. doi:Doi 10.1023/B:Joba.0000007455.08539.94 [DOI] [Google Scholar]
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, … Kheirandish-Gozal L (2015). National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health, 1, 40–43. doi: 10.1016/j.sleh.2014.12.010 [DOI] [PubMed] [Google Scholar]
- Hiscock H, Mulraney M, Heussler H, Rinehart N, Schuster T, Grobler AC, … Sciberras E (2019). Impact of a behavioral intervention, delivered by pediatricians or psychologists, on sleep problems in children with ADHD: a cluster-randomized, translational trial. J Child Psychol Psychiatry, 60, 1230–1241. doi: 10.1111/jcpp.13083 [DOI] [PubMed] [Google Scholar]
- Hiscock H, Sciberras E, Mensah F, Gerner B, Efron D, Khano S, & Oberklaid F (2015). Impact of a behavioural sleep intervention on symptoms and sleep in children with attention deficit hyperactivity disorder, and parental mental health: randomised controlled trial. BMJ, 350, h68. doi: 10.1136/bmj.h68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysing M, Lundervold AJ, Posserud MB, & Sivertsen B (2016). Association between sleep problems and symptoms of attention deficit hyperactivity disorder in adolescence: Results from a large population-based study. Behav Sleep Med, 14, 550–564. doi: 10.1080/15402002.2015.1048448 [DOI] [PubMed] [Google Scholar]
- Kaufman AS, & Kaufman NL (2004). Kaufman Brief Intelligence Test, Second Edition (KBIT-2): Pearson. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry, 36, 980–988. doi: 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Keshavarzi Z, Bajoghli H, Mohamadi MR, Salmanian M, Kirov R, Gerber M, … Brand S (2014). In a randomized case-control trial with 10-years olds suffering from attention deficit/hyperactivity disorder (ADHD) sleep and psychological functioning improved during a 12-week sleep-training program. World Journal of Biological Psychiatry, 15, 609–619. doi: 10.3109/15622975.2014.922698 [DOI] [PubMed] [Google Scholar]
- Lo JC, Ong JL, Leong RL, Gooley JJ, & Chee MW (2016). Cognitive performance, sleepiness, and mood in partially sleep deprived adolescents: The need for sleep study. Sleep, 39, 687–698. doi: 10.5665/sleep.5552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunsford-Avery JR, Krystal AD, & Kollins SH (2016). Sleep disturbances in adolescents with ADHD: A systematic review and framework for future research. Clin Psychol Rev, 50, 159–174. doi: 10.1016/j.cpr.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMakin DL, Dahl RE, Buysse DJ, Cousins JC, Forbes EE, Silk JS, … Franzen PL (2016). The impact of experimental sleep restriction on affective functioning in social and nonsocial contexts among adolescents. J Child Psychol Psychiatry, 57, 1027–1037. doi: 10.1111/jcpp.12568 [DOI] [PubMed] [Google Scholar]
- Meinzer MC, & Chronis-Tuscano A (2017). ADHD and the development of depression: Commentary on the prevalence, proposed mechanisms, and promising interventions. Current Developmental Disorders Reports, 4, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer MC, Hartley CM, Hoogesteyn K, & Pettit JW (2018). Development and open trial of a depression preventive intervention for adolescents with attention-deficit/hyperactivity disorder. Cognitive and Behavioral Practice, 25, 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulraney M, Sciberras E, & Becker SP (2020). Sleep functioning in adolescents with ADHD ADHD in Adolescents: Development, Assessment, and Treatment (pp. 204–227). New York: Guilford. [Google Scholar]
- National Sleep Foundation. (2006). Summary of Findings: 2006 Sleep In America Poll. Retrieved from Washington, DC: [Google Scholar]
- Palmer CA, & Alfano CA (2017). Sleep and emotion regulation: An organizing, integrative review. Sleep Med Rev, 31, 6–16. doi: 10.1016/j.smrv.2015.12.006 [DOI] [PubMed] [Google Scholar]
- Paruthi S, Brooks LJ, D’Ambrosio C, Hall WA, Kotagal S, Lloyd RM, … Wise MS (2016). Consensus statement of the American Academy of Sleep Medicine on the recommended amount of sleep for healthy children: Methodology and discussion. J Clin Sleep Med, 12, 1549–1561. doi: 10.5664/jcsm.6288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen PJ, Leaberry KD, Slaughter K, Fogleman ND, Walerius DM, Loren REA, & Epstein JN (2018). Managing Frustration for Children (MFC) group intervention for ADHD: An open trial of a novel group intervention for deficient emotion regulation. Cognitive and Behavioral Practice. [Google Scholar]
- Sadeh A, Sharkey KM, & Carskadon MA (1994). Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep, 17, 201–207. [DOI] [PubMed] [Google Scholar]
- Sciberras E, Mulraney M, Mensah F, Oberklaid F, Efron D, & Hiscock H (2019). Sustained impact of a sleep intervention and moderators of treatment outcome for children with ADHD: a randomised controlled trial. Psychol Med, 1–10. doi: 10.1017/S0033291718004063 [DOI] [PubMed] [Google Scholar]
- Shields A, & Cicchetti D (1997). Emotion regulation among school-age children: the development and validation of a new criterion Q-sort scale. Dev Psychol, 33, 906–916. [DOI] [PubMed] [Google Scholar]
- Shochat T, Cohen-Zion M, & Tzischinsky O (2014). Functional consequences of inadequate sleep in adolescents: a systematic review. Sleep Med Rev, 18, 75–87. doi: 10.1016/j.smrv.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Short MA, & Louca M (2015). Sleep deprivation leads to mood deficits in healthy adolescents. Sleep Med, 16, 987–993. doi: 10.1016/j.sleep.2015.03.007 [DOI] [PubMed] [Google Scholar]
- Smalley SL, McGough JJ, Moilanen IK, Loo SK, Taanila A, Ebeling H, … Jarvelin MR (2007). Prevalence and psychiatric comorbidity of attention-deficit/hyperactivity disorder in an adolescent Finnish population. J Am Acad Child Adolesc Psychiatry, 46, 1575–1583. doi: 10.1097/chi.0b013e3181573137 [DOI] [PubMed] [Google Scholar]
- Sobanski E, Banaschewski T, Asherson P, Buitelaar J, Chen W, Franke B, … Faraone SV (2010). Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): clinical correlates and familial prevalence. J Child Psychol Psychiatry, 51, 915–923. doi: 10.1111/j.1469-7610.2010.02217.x [DOI] [PubMed] [Google Scholar]
- Stein D, Pat-Horenczyk R, Blank S, Dagan Y, Barak Y, & Gumpel TP (2002). Sleep disturbances in adolescents with symptoms of attention-deficit/hyperactivity disorder. J Learn Disabil, 35, 268–275. [DOI] [PubMed] [Google Scholar]
- Talbot LS, McGlinchey EL, Kaplan KA, Dahl RE, & Harvey AG (2010). Sleep deprivation in adolescents and adults: changes in affect. Emotion, 10, 831–841. doi: 10.1037/a0020138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriend JL, Davidson FD, Corkum PV, Rusak B, Chambers CT, & McLaughlin EN (2013). Manipulating sleep duration alters emotional functioning and cognitive performance in children. J Pediatr Psychol, 38, 1058–1069. doi: 10.1093/jpepsy/jst033 [DOI] [PubMed] [Google Scholar]
- Wolfson AR, & Carskadon MA (1998). Sleep schedules and daytime functioning in adolescents. Child Dev, 69, 875–887. [PubMed] [Google Scholar]