Abstract
Objective :
To conduct interviews with a multiyear sample of parents of infants found to have heterozygous status for sickle cell hemoglobinopathy (SCH) or cystic fibrosis (CF) during newborn blood screening (NBS).
Study design:
Interviewers with clinical backgrounds telephoned parents, and followed a structured script that blended follow-up and research purposes. Recruiting followed several steps to minimize recruiting bias as much as possible for a NBS study.
Results:
Follow-up calls were conducted with parents of 426 SCH carrier infants, and 288 parents of CF carriers (34.8% and 49.6% of those eligible). Among these, 27.5% and 7.8% had no recollection of being informed of NBS results. Of those who recalled a provider explanation, 8.6% and 13% appraised the explanation negatively. Overall, 7.4% and 13.2% were dissatisfied with the experience of learning about the NSB result. Mean anxiety levels were low but higher in the SCH group (P < .001). Misconceptions that the infant might get the disease were present in 27.5% and 7.8% of parents (despite zero actual risk for disease). Several of these data were significantly predicted by NBS result, health literacy, parental age, and race/ethnicity factors.
Conclusions:
Patient-centered public health follow-up can be effective after NBS identifies carrier status. Psychosocial complications were uncommon, but harms were substantial enough to justify mitigation.
Keywords: communication, newborn screening, genetic screening, sickle cell hemoglobinopathy, cystic fibrosis, incidental findings
Newborn blood screening (NBS) saves lives and reduces morbidity, but its population-wide benefits are accompanied by risk of adverse psychosocial consequences for families of infants with false positive results and/or heterozygote “carrier” detection. 1–28 Receipt of abnormal NBS results has been associated with parental stress,5–12 depressive symptoms,24–26 and anxiety/worry about the infant’s well-being. 3–6 Parents may have misconceptions about results and implications.20 Some parents have lingering concerns about their child’s vulnerability,15–17 over-utilize health services,12–14 and have difficulty with future reproductive plans.1, 2, 5 Concerns about the infant’s paternity may arise. 17, 21, 23 Parents also may struggle with decisions about when, how, and what to communicate about genetic information to their child or other family members.25, 29
Psychosocial complications have been cited in arguments to modify, delay, or even cease some NBS or genetics programs.27,28,30 Given NBS’ benefits, however, we developed a mechanism for follow-up and Communication Quality Assurance: the Wisconsin Project on Improvement of Communication Process and Outcomes after Newborn Screening (referred to hereafter as “the Project”). 31–40 The Project’s efforts focused on carrier results for sickle cell hemoglobinopathy (SCH) and cystic fibrosis (CF) because false-positive and carrier results are common with the laboratory methods used for NBS.28, 41, 42
METHODS
This Project report presents analyses of data from a statewide cohort of parents whose newborns had been identified as carriers for either SCH or CF. Parents were contacted by trained nurses or a genetic counselor following a standardized script. The Project team functioned as a contracted agent of the Wisconsin State Laboratory of Hygiene (a public health entity responsible for Wisconsin’s NBS program), and Institutional Review Board (IRB) approval was also obtained.
Participants
Participants were parents of infants found to have genetic carrier status for SCH or CF. The NBS methods were standard at the time we enrolled parents during 2008–2012. Specifically, hemoglobin molecular assessment was done by cellulose acetate electrophoresis. SCH carrier infants had an NBS result showing fetal, adult, and sickle hemoglobin (the “FAS” result). CF NBS employed the 2-tier evaluation of immunoreactive trypsinogen (IRT) with a 96th percentile cutoff and, if above this level, a panel of 23 mutations was used to identify CF-causing variants in the CFTR gene. CF carrier infants had an NBS result showing elevated IRT and a single mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, followed by a normal result on the infant’s sweat chloride testing. The term “likely CF carrier” was used in the Project for infants with an elevated IRT and a single mutation on NBS, but who had not yet had a sweat test.
Standard practice in Wisconsin is for parent notification to be done by primary care providers (PCPs), who have access to guidance and support materials provided by the clinicians and education subcommittee of Wisconsin’s NBS program.
Infants were excluded if:more than one abnormality was found on NBS, the NBS specimen was a repeat test, the gestational age was < 35 weeks, the calendar age at the time of specimen collection was > 180 days, or if a PCP could not be identified from NBS records or by calling the birthing facility or home birth provider. In the case of CF, several infants without a known PCP became our clinical responsibility and our IRBs required us to retrospectively censor them from the dataset. During a call to the PCP, infants were excluded if (6) the infant had spent more than 5 days in the hospital, (7) the infant was re-hospitalized after discharge from the nursery, (8) the infant was undergoing evaluation for another serious medical condition, or (9) the parent(s) were reported to need a language interpreter for the interview (because all the interviews were conducted in English due to limited resources). Finally, (10) infants with CF were excluded if they were found on a periodic review of sweat test results logged by the NBS laboratory.
A multistage process for informed consent was developed to prevent psychosocial harm, mitigate recruiting bias, and respect autonomy. For the Project’s first access to results within the NBS program, a waiver was granted by our IRBs. Second, we enabled PCPs to decline participation on behalf of their parents for any perceived contraindication.31, 33 Third, parents were mailed an introductory letter that did not describe the result, and only described the Project as an attempt to learn about parents’ experiences after NBS. This letter included a “decline of contact” card, which (unlike an opt-out card) enabled parents to decline participation without becoming fully informed about the purpose of the Project. During the telephone call, informed consent was sought at two points: an initial consent for audio-recording and a detailed consent once the parent knew about the NBS result and the study’s purpose. We clarified that parents could participate in the clinical portion of the call, but decline from the research portion (some did this).
Data Collection and Analysis
The NBS result documents provided infants’ gestational ages, birth weights, and birthdays. Birthdays were used to calculate infants’ ages on the interview days before identifying information was scrubbed from the database.
A standardized call script was developed with input and feedback from convenience discussions with parents, clinicians, and NBS experts. The script’s first draft was aimed at clinical follow-up in order to verify receipt of the NBS result, screen for misunderstanding, and provide counseling for mitigation of psychosocial complications. The script’s wording was then re-structured in places to facilitate collection of research data, often as fixed, ordinal-scale questions followed by an opportunity for an open-ended comment. Finally, some research-only questions were embedded in the script in such a way that they would not interfere with the call’s clinical purpose. The counseling/support portions were excerpted into subscripts that could be implemented whenever the interviewer felt that the parent was becoming alarmed or confused. The resulting script (Table 1; available at www.jpeds.com) was 9 pages long for a 20–30 minute interview (actual duration averaged 25.3 minutes, SD 8.5).
Table 1.
Questions and timing of segments in the interview script*
| Segment | Description |
|---|---|
| Information giving | Introduction, initial consent for recording a call about NBS** (newborn blood screening) |
| Data collection | Assessment of recall |
| “What do you remember from when the NBS test took place?” | |
| If parent does not mention the result… | |
| “Do you follow up with [source] for [baby]?” | |
| “Do you remember having a conversation with [source] about the screening results?” | |
| If not result not known or recalled, inform result without details | |
| “Screening showed that [baby] is probably something called a carrier of the gene for a disease called [disease]. [If CF carrier, ask about sweat test.] Is that information you had heard before?” | |
| Information giving | Detailed informed consent for interview about the specific NBS result |
| Data collection | Marteau instrument for anxiety at the time of the interview |
| Health literacy questions adapted from Chew39 | |
| “When you were in the hospital for [baby]’s delivery, how confident or comfortable did you feel with reading the brochures and handouts that the hospital gave you? ‘Extremely,’ ‘Quite a bit,’ ‘Somewhat,’ ‘A little bit,’ or ‘Not at all?’ “ | |
| “When you were in the hospital for [baby]’s delivery, how much (if any) help did you need to fill out the medical forms related to the birth? ‘Quite a bit,’ ‘Some,’ or ‘None?’ “ | |
| “Before [baby] was born, how often did you have a hard time learning about medical problems because of difficulty understanding things that are written down? ‘Always,’ ‘Often,’ ‘Sometimes,’ ‘Occasionally,’ or ‘Never?’ “ | |
| Reaction to communication services (if parent had heard about the result) | |
| “You mentioned earlier that you heard about [baby’s] result before … Can you rate on a scale of 1 to 5 how satisfied or happy you were with the way you heard about the result, with ‘5’ being ‘very satisfied’ and ‘1’ being ‘very dissatisfied?’“ | |
| “How well did the [source] explain the screening result to you, on a scale of 1 to 5, ‘5’ being ‘explained very well’ and ‘1’ being ‘no explanation at all?’ “ | |
| “How worried did you feel when you first heard about the screening result, on a scale of 1 to 5, ‘5’ being ‘very anxious,’ ‘3’ being ‘a little concerned,’ and ‘1’ being ‘not worried at all?’“ | |
| Age, race/ethnicity questions | |
| “Did you feel like your interactions, meetings, or conversations with the [source] were affected by differences between you and the [source], like maybe differences in age or differences in race or ethnicity? … How old are you? … How would you describe your race or ethnicity? … How would you describe your baby’s race or ethnicity?” | |
| Misconception about risk for developing the disease | |
| “Based on what you know now, how likely is it that [baby] is going to have [disease], the disease, on a scale of 1 to 5, with ‘5’ being ‘definitely going to have it,’ ‘3’ being ‘unsure’ and ‘1’ being ‘definitely NOT going to have it?’ “ | |
| Information giving | Detailed education, counseling, and support |
| Data collection | Plans for the future |
| “So, do you mind if I asked about your plans now?” | |
| If necessary… | |
| “Are you planning to have another baby in the future?” | |
| “Do you think that you will get yourself and [other parent] tested to see if you are carriers, too?” | |
| Information giving | Debriefing and closure |
Not shown: subscripts for ad hoc counseling and support
Abbreviation: NBS, Newborn Blood Screening
A set of questions about vulnerable baby syndrome were included, but these data have been moved to another manuscript because of the complexity of the results.16 Results of the debriefing questions were also reported separately.35
The telephone call was scheduled when each infant was between 3 and 5 months old, to allow for at least one well-baby visit. Contact information for the mother was sought via publicly accessible databases such as telephone directories and the search website Intellius (Bellevue, Washington). The located mothers were mailed the introductory letter, the decline-of-contact card, and an offer of a $20 gift certificate.
When the call began, the interviewer identified him/herself and asked to speak to “the mother or whoever takes the infant to doctor visits.”
Telephone calls were digitally audio-recorded, and transcribed without names or other identifying information. Interviewers kept written notes during the call, and both notes and transcripts were abstracted for fixed answers and other fields in the Project database.
Five outcome variables for communication were derived from the questions shown in Table I: (#1) whether the parent recalled being told about the NBS result, (#2) whether the parent recalled the provider giving an explanation, (#3) parent’s appraisal of the explanation, (#4) parental satisfaction with the entire experience, and (#5) misconception about risk for carrier status developing into the actual disease. The fifth outcome was operationalized as adverse if the parent chose any ordinal response other than “definitely not going to have the disease.”
Anxiety was assessed using 2 approaches (Table 1; online). First, anxiety at the time of the interview was measured using the Marteau version of the Spielberger State subscale.18, 43, 44 The Marteau questions were asked immediately after the second informed consent section, so the parent had just been reminded about the NBS result. In the second approach to anxiety, we asked parents to think back to the time they first learned about the NBS result, and rate their “original anxiety” on an ordinal scale. Original anxiety responses were excluded from analysis if the parent had just learned about the NBS result from our call.
We also inquired about plans for subsequent pregnancy and genetic testing. However, it is worth clarifying that in our view reproductive and testing plans should be not considered “outcomes of communication,” because counseling is supposed to be nondirective.
Race/ethnicity data were obtained using open-ended questions (Table 1; online), and abstracted responses into one or more binary fields for each of the standard NIH categories.45 For example, if a parent described his/her ethnicity as “mixed Latino and White” then the database fields for Hispanic and White were flagged.
Health literacy was evaluated with a three-item screening tool adapted from Chew et al.46 Analyses were done as applicable for the nature of each variable (Chi-square test, t-test, correlation, the Wilcoxon rank-sum test, or logistic and linear modeling) using JMP software (SAS Institute, Cary, NC).
RESULTS
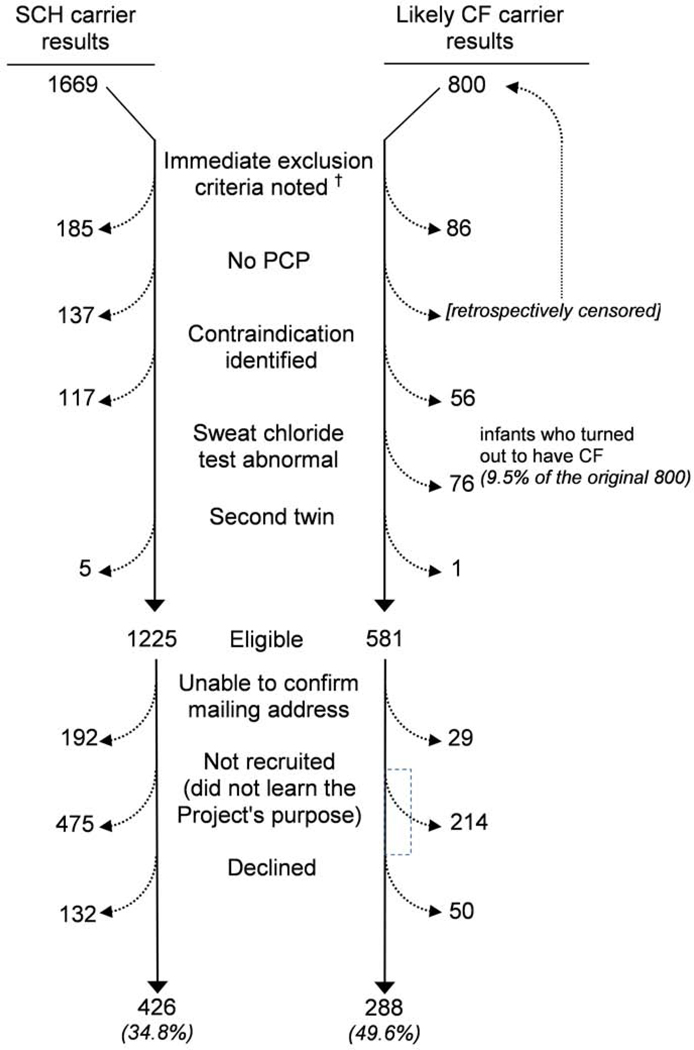
We logged NBS results until we identified 1669 infants with SCH carrier status and 800 infants with likely CF carrier status and a known PCP (Figure 1; available at www.jpeds.com). After exclusion criteria were applied and parents agreed to participate in the research portion of the Project, the final sample consisted of 426 in the SCH group and 288 in the CF group (participation rates 34.8% and 49.6% of those eligible).
Figure 1.
(online). Exclusions and losses during recruitment.
Table 2 lists participant characteristics. For non-participants there were no significant differences in the available data (age gestational age, birth weight, or birthing facility).
Table 2.
Participant Characteristics
| SCH carrier group | CF carrier group | |||
|---|---|---|---|---|
| Numeric data | mean | SD | mean | SD |
| Gest age at birth, weeks | 38.9 | 1.3 | 39.1* | 1.2 |
| Birth weight, grams | 3288 | 484 | 3413** | 496 |
| Baby’s age at interview (days) | 107.1 | 23.9 | 110.8 | 25.8 |
| Infant is female | 214 | (50.2) | 169 | (58.7) |
| Parent’s age (years) | 25.8 | (5.9) | 28.7 | (5.6) |
| Categorical data | n | (%) | n | (%) |
| Interviewee is infant’s mother | 418 | (98.1) | 278 | (96.5) |
| Parent knows another genetic carrier † | 325 | (76.3) | 137 | (47.6) |
| Screen positive for health literacy problem | 150 | (37.7) | 104 | (36.5) |
| Parent race data§ | ||||
| Race-included Black-included | 280 | (65.7) | 20 | (6.9) |
| White-included | 87 | (20.4) | 250 | (86.8) |
| Hispanic-included | 29 | (6.8) | 8 | (2.8) |
| Other-included | 14 | (3.3) | 7 | (2.4) |
| Race-only Black-only | 265 | (62.2) | 16 | (5.6) |
| White-only | 72 | (16.9) | 246 | (85.4) |
| Hispanic-only | 19 | (4.5) | 5 | (1.7) |
| Other-only | 7 | (16.4) | 4 | (1.4) |
| Multiracial unspecified | 5 | (1.2) | 1 | (0.4) |
| Not asked or answered | 36 | (8.4) | 9 | (3.1) |
| Infant race data§ | ||||
| Race-included Black-included | 330 | (77.5) | 24 | (8.3) |
| White-included | 82 | (19.3) | 255 | (88.5) |
| Hispanic-included | 38 | (8.9) | 14 | (4.9) |
| Other-included | 23 | (5.4) | 11 | (3.8) |
| Race-only Black-only | 249 | (58.5) | 14 | (4.9) |
| White-only | 13 | (3.1) | 235 | (81.6) |
| Hispanic-only | 15 | (3.5) | 4 | (1.4) |
| Other-only | 2 | (0.5) | 4 | (1.4) |
| Multiracial unspecified | 52 | (12.0) | 7 | (2.4) |
| Not asked or answered | 14 | (3.2) | 6 | (2.1) |
p < 0.04.
p < 0.001.
p < 0.0001
Columns for race data do not sum to 100% because data are not mutually exclusive
Also shown in Table 2 are the race/ethnicity data abstracted from answers to our open-ended questions into “included” and “only” categories for each of the NIH categories. Both CF and SCH groups demonstrated diversity in the race/ethnicity data. For example, responses in the SCH group led to the “Black-only” variable in only 62.2% of parents and 58.5% of infants.
Screening for limited health literacy was positive in similar proportions of the SCH and CF groups. Limited health literacy was more common for older parents (p < 0.0001 on regression). We also found two race/ethnicity differences: health literacy limitations were rarest for parents with White-only race/ethnicity (32.8% versus 50.1%, p < 0.03 on χ2), and more common for parents with Hispanic-included race (56.8% versus 36.1%, p = 0.01 onχ2).
Communication Outcomes
The five outcome variables are presented in Table 3.. For the misconception question, 7.8% of parents in the CF group indicated some lingering question that their infant might still develop the disease. The SCH group had more misconceptions (27.5%, p < 0.0001 on χ2). A favorable combination of outcomes in the SCH group was present for 73% of parents who were informed, recalled an explanation, and appraised the explanation positively. In the CF group, 84% of those informed recalled an explanation and appraised it positively.
Table 3.
Communication outcomes after newborn screening identifies genetic carrier status
| Adverse outcomes | Proportion of eligible cases | |||||
|---|---|---|---|---|---|---|
| SCH carrier group | CF carrier group | |||||
| n | eligibles | (%) | n | eligibles | (%) | |
| 1. Parent not informed about the NBS result | 60 | 426 | (14.1%) | n/a | ||
| 2. An explanation was not given about the NBS result | 26 | 366 | (7.1%) | 7 | 288 | (2.4%)* |
| 3. Negative appraisal of provider’s explanation about the NBS result* | 30 | 351 | (8.6%) | 36 | 278 | (13.0%)** |
| 4. Dissatisfaction with the experience of learning about the NBS result | 26 | 352 | (7.4%) | 37 | 281 | (13.2%)** |
| 5. Misconception about carrier status | 102 | 371 | (27.5%) | 22 | 281 | (7.8%)** |
p < 0.01 versus SCH group
p < 0.0001 versus SCH group
There were several characteristics associated (using stepwise regression) with the five adverse outcomes. In the SCH group, failure to recall being informed about the result was independently associated with parental White-included race/ethnicity (O.R. 9.1, p < 0.0001) and infant Hispanic-included race/ethnicity (O.R. 6.7, p < 0.03). In the CF group, bi/multiracial status was independently associated with failure to recall an explanation (O.R. 12.5, p < 0.01), and negative appraisal of explanation if it was recalled (O.R. 2.5, p < 0.03). Negative appraisal of SCH carrier explanations was independently associated with positive health literacy screen (O.R. 1.5, p < 0.01).
Dissatisfaction with the entire experience in both groups was independently associated with failure to recall an explanation (SCH group O.R. 4.0, CF group O.R. 3.8, both p < 0.0001). Dissatisfaction was also correlated with negative appraisal of the explanation (SCH group r = 0.71, CF group r = 0.67, both p < 0.0001). In the SCH group, dissatisfaction was independently associated with infant White-only race/ethnicity (O.R. 1.8, p < 0.03) and parental bi/multiracial status (O.R. 0.54, p < 0.05).
The misconception outcome (#5) was independently associated in the SCH group with younger parental age (O.R. 1.13 per year, p < 0.0001) and in the CF group with bi/multiracial status (O.R. 7.7, p < 0.03).
Anxiety
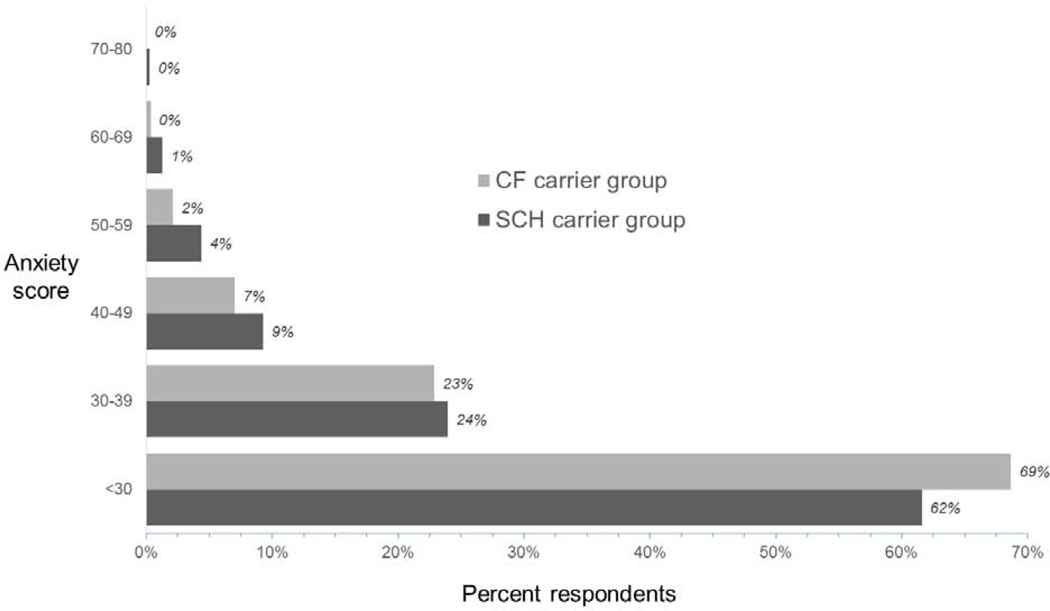
Figure 2 depicts anxiety immediately after being reminded of the NBS result, with the Marteau data prorated to the 20–80 range used by Spielberger.18, 43, 44 Median anxiety scores were 26.6 and 23.3 respectively for the SCH group and CF group (p < 0.002 on Wilcoxon). On average these results indicate low anxiety levels, but about 7% of parents had scores higher than 50. In the SCH group higher anxiety was associated with lower birth weight and limited health literacy.
Figure 2.
State anxiety assessed at the time of the interview.
Higher Marteau responses were correlated with more negative appraisal of the explanation for both groups (CF group, r = −0.15, p < 0.02; SCH group, r = −0.12, p < 0.02). In the SCH group, Marteau data correlated with dissatisfaction (r = −0.14, p < 0.01) and was associated with a misconception that the baby might develop sickle cell disease (O.R. 1.05, p < 0.0001).
When parents were asked about anxiety from when they originally learned about the NBS result, their ordinal ratings were in the three most worried responses (of five options) in 35.6% of the SCH group and 77.2% of the CF group. Comparing all ordinal responses, the CF group recalled more anxiety than the SCH group (medians 5 and 3, p < 0.0001 on Wilcoxon).
In the SCH group, rating of original anxiety was independently associated with older parental age (O.R. 0.94 per year), infant race/ethnicity other-included (O.R. 1.6), and for parents who knew another carrier (O.R. 0.47).
Satisfaction with the NBS experience was associated with rating of original anxiety (CF group O.R. 0.70, p < 0.04, SCH group O.R. 0.68, p < 0.04). In the SCH group only, rating of original anxiety was associated with a misconception that the infant might develop sickle cell disease (O.R. 1.6, p < 0.0001).
In the SCH group, there was a modest correlation between anxiety at the time of the interview and rating of original anxiety (r = +0.18, p = 0.0007). In the CF group, there was no correlation between the two anxiety assessments.
Parent’s reproductive plans for the future
Table 4 shows the proportions of parents who planned a future pregnancy or testing for carrier status.
Table 4.
Parents’ future plans for pregnancy and/or testing
| Adverse outcomes | Proportion of eligible cases | |||||
|---|---|---|---|---|---|---|
| SCH carrier group | CF carrier group | |||||
| n | eligibles | (%) | n | eligibles | (%) | |
| Parent plans another pregnancy | 99 | 231 | (42.9%) | 97 | 173 | (56.1%)* |
| Parent plans to have himself/herself tested for carrier status | 251 | 289 | (86.9%) | 189 | 225 | (84%) |
| Parent plans to have partner tested for carrier status | 211 | 250 | (84.4%) | 156 | 210 | (74.3%)* |
p < 0.01 versus SCH group
Likelihood of planning another pregnancy was lower in both groups for older parents (O.R.s 0.9 per year, each p < 0.0001), and in the SCH group for parents with Black-only race/ethnicity (O.R. 0.58, p < 0.05). In the CF group, parents with a possible health literacy problem were more likely to plan another pregnancy (O.R. 4.3, p < 0.001).
Likelihood of planned testing for self was higher for parents who reported knowing another carrier (for SCH group O.R. 3.1; for CF group O.R. 3.4, both p < 0.01), and for CF group parents with a possible health literacy problem (O.R. 3.0, p < 0.03). For race/ethnicity, plans for testing were more likely with the Black-included and Black-only states (O.R.s 5.7 and 7.4, p < 0.0001 in the SCF group, and approaching certainty in the CF group, p < 0.05). There was an independent effect for the White-included and White-only states (0.13 and 0.09, p < 0.0001), and for bi/multiracial status in the SCH group (O.R. 0.24, p < 0.0001).
Similar race/ethnicity factors were associated with likelihood of planning testing for a partner. Another pregnancy was planned by more parents in the CF group than the SCH group (O.R. 1.7, p < 0.009 on χ2).
All predictors of pregnancy planning were independent of each other. When a parent planned to have him/herself tested, then s/he was very likely to plan having the infant’s other parent tested (O.R. 36.5 for CF group, O.R. 24.9 for SCH group, p < 0.0001 for both). However, significantly fewer of the CF group planned testing of partners than parents in the SCH group (O.R. 0.53, p < 0.008).
Parents were more likely to plan testing if they had higher ratings of original anxiety at the time of learning about the NBS result (O.R. 2.3, p < 0.03).
DISCUSSION
Genetic screening programs for disease risk have been debated for decades, especially when test methods incidentally identify carriers. NBS is even more subject to debate because formal consent is not sought before testing. We sought to improve NBS “safety,” ie, mitigate harms that are incidental to NBS’s benefits.31–40 We describe our systematic experience with a NBS follow-up program for Communication Quality Assurance that was both public health and patient-centered and provide updated epidemiological insights about NBS after incidental findings.
Our experience was that a public health follow-up program for Communication Quality Assurance is clearly feasible, and we believe affordable (less than 2 full-time equivalents of personnel for our entire state’s average 68,000 births/year). As part of a public health program, we theoretically could have been exempt from some IRB regulations, but we requested oversight because of the limited literature on follow-up programs. As a result we lost a few hundred parents from the research portion of the Project, but only a small percentage of the lost parents could have known that we were studying communication and psychosocial complications related to carrier status (10.8% loss for SCH, 8.6% for CF). The final sample was large enough to detect modest effect sizes and adjust for covariance.
Adverse outcomes had a modest-sized incidence in our sample, but individually were still troubling. Our most concerning result may be the number of parents with the misconception that their infant might develop the disease. In the SCH group some misconceptions could be attributed to confusion about carrier adults’ small risk of events. 47–51 Even so, both groups’ misconceptions are surprising because all such parents in Wisconsin are offered genetic counseling.
We identified risk factors that might help to improve follow-up by NBS programs or PCPs. Health literacy covaried with several factors, but health literacy’s only independent association was for appraisal of explanations in the SCH group.
SCH results were independently less likely to be communicated by PCPs if the parent was White-included or the infant was Hispanic-included. CF results were less likely to be explained for bi/multiracial families. We can only speculate that these effects were influenced by PCP confusion about genetic epidemiology of CF and SCH. Perhaps parents were also confused by stereotypes between genetics and race/ethnicity. Regardless, our Project clearly documented the error of assuming that all SCH carriers are Black, and all CF carriers are White. We believe that because NBS will continue to include CF and SCH (i.e. infants with disease continue to need early identification), then NBS reports should be accompanied by better information for PCPs about carrier status and race/ethnicity.
Our findings about parents’ reproductive and testing plans were informative. However, these parent decisions should not be considered outcomes of communication after NBS. We agree with the predominant view that NBS exists to identify infants with diseases (who therefore will benefit from treatment or surveillance), rather than for carrier identification or reproductive decision-making.
Some limitations are worth considering. Most participants were mothers because the NBS card identified the mother. We sought to minimize recruiting bias, but regret that limited resources did not allow us to include parents with language barriers. Health literacy was assessed with a widely-used screening tool, but we recognize that some may be concerned about the tools’ applicability to NBS studies. Our analyses are of association rather than causation, but such results are still important for projecting risk of adverse communication outcomes. Most of our regression modeling assumed linear relationships, but we could have missed more complex effects because linearity could not be guaranteed for the entire distribution. Many of the results depend on the parents’ summative recall or heterogeneous experiences; a PCP could have explained the NBS result but the parent may not have recalled it vividly enough to report in the interview. On the other hand, this limitation could be seen as a strength, because an explanation about an important issue is presumably ineffective if it is not memorable 2–3 months later.
Notwithstanding these limitations, our epidemiologic findings and experience seem to emphasize the need for Communication Quality Assurance after NBS. We previously reported how our intervention seemed to have been effective and well-received by parents.35
Our experience and epidemiologic insights may also be informative for broader efforts with genetics in public health screening. Future study will be needed to test communication gaps, risk factors like race/ethnicity or health literacy, and the use of Communication Quality Assurance.
Finally, this Project has led us to reconsider some basic aspects of scholarship on the ethical, legal, and social implications (ELSIs) of genetics. Much of ELSI scholarship has considered whether genetics should (or should not) expand into new diseases or methods. We have seen NBS expansions prompted mostly by advocacy, politics, and the attractiveness of new laboratory techniques. We suspect that genome sequencing on blood spots will be routine within the coming generation, regardless of ELSI concerns. We suggest that geneticists and ELSI scholars adopt a safety perspective, and invest more effort to mitigate harm after molecular technologies.52 We believe that the time has come to shift from the question, “Should we screen or not?” to ask, “How can we make DNA-based screening tests safe for all infants and families?”
Supplementary Material
ACKNOWLEDGEMENTS
We thank Jill Paradowski, Jenelle Collins, Hollie Beaudry, Faith O’Tool, and Stephanie Christopher for << >>. We also thank our colleagues at the Wisconsin State Laboratory of Hygiene, especially the late Dr Ronald Laessig.
Supported by the National Institutes of Health (R01 HL086691 and HL086691–02S1). The authors declare no conflicts of interest.
Abbreviations:
- SCH
sickle cell hemoglobinopathy
- CF
cystic fibrosis
- NBS
newborn screening
Footnotes
Data Sharing Statement: Deidentified data are nonetheless sensitive because of age, genetic state, and origin, and so cannot be made available.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mischler EH, Wilfond BS, Fost N, Laxova A, Reiser C, Sauer CM, et al. Cystic fibrosis newborn screening: impact on reproductive behavior and implications for genetic counseling. Pediatrics. 1998;102:44–52. [DOI] [PubMed] [Google Scholar]
- 2.Gallo AM, Wilkie DJ, Yao Y, Molokie RE, Stahl C, Hershberger PE, et al. Reproductive Health CHOICES for Young Adults with Sickle Cell Disease or Trait: Randomized Controlled Trial Outcomes over Two Years. Journal of genetic counseling. 2016;25:325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciske DJ, Haavisto A, Laxova A, Rock LZ, Farrell PM. Genetic counseling and neonatal screening for cystic fibrosis: an assessment of the communication process. Pediatrics. 2001;107:699–705. [DOI] [PubMed] [Google Scholar]
- 4.Gurian EA, Kinnamon DD, Henry JJ, Waisbren SE. Expanded newborn screening for biochemical disorders: the effect of a false-positive result. Pediatrics. 2006;117:1915–21. [DOI] [PubMed] [Google Scholar]
- 5.Morrison DR, Clayton EW. False positive newborn screening results are not always benign. Public Health Genomics. 2011;14:173–7. [DOI] [PubMed] [Google Scholar]
- 6.DeLuca JM, Kearney MH, Norton SA, Arnold GL. Parents’ experiences of expanded newborn screening evaluations. Pediatrics. 2011;128:53–61. [DOI] [PubMed] [Google Scholar]
- 7.Ulph F, Cullinan T, Qureshi N, Kai J. Parents’ responses to receiving sickle cell or cystic fibrosis carrier results for their child following newborn screening. Eur J Hum Genet. 2015;23:459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chudleigh J, Buckingham S, Dignan J, O’Driscoll S, Johnson K, Rees D, et al. Parents’ Experiences of Receiving the Initial Positive Newborn Screening (NBS) Result for Cystic Fibrosis and Sickle Cell Disease. J Genet Couns. 2016;25:1215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayeems RZ, Miller FA, Barg CJ, Bombard Y, Kerr E, Tam K, et al. Parent Experience With False-Positive Newborn Screening Results for Cystic Fibrosis. Pediatrics. 2016;138. [DOI] [PubMed] [Google Scholar]
- 10.Moran J, Quirk K, Duff AJ, Brownlee KG. Newborn screening for CF in a regional paediatric centre: the psychosocial effects of false-positive IRT results on parents. J Cyst Fibros. 2007;6:250–4. [DOI] [PubMed] [Google Scholar]
- 11.Tluczek A, Koscik RL, Modaff P, Pfeil D, Rock MJ, Farrell PM, et al. Newborn screening for cystic fibrosis: parents’ preferences regarding counseling at the time of infants’ sweat test. Journal of genetic counseling. 2006;15:277–91. [DOI] [PubMed] [Google Scholar]
- 12.Waisbren SE, Albers S, Amato S, Ampola M, Brewster TG, Demmer L, et al. Effect of expanded newborn screening for biochemical genetic disorders on child outcomes and parental stress. JAMA : the journal of the American Medical Association. 2003;290:2564–72. [DOI] [PubMed] [Google Scholar]
- 13.Lipstein EA, Perrin JM, Waisbren SE, Prosser LA. Impact of false-positive newborn metabolic screening results on early health care utilization. Genetics in medicine : official journal of the American College of Medical Genetics. 2009;11:716–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarini BA, Clark SJ, Pilli S, Dombkowski KJ, Korzeniewski SJ, Gebremariam A, et al. False-positive newborn screening result and future health care use in a state Medicaid cohort. Pediatrics. 2011;128:715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hampton ML, Anderson J, Lavizzo BS, Bergmen AB. Sickle cell “nondisease”. A potentially serious public health problem. American journal of diseases of children (1960). 1974;128:58–61. [PubMed] [Google Scholar]
- 16.Farrell MH, Sims A, Farrell PM, Tarini BA. Vulnerable Baby syndrome and NBS. Submitted manuscript. 2019. [Google Scholar]
- 17.Tluczek A, McKechnie AC, Brown RL. Factors associated with parental perception of child vulnerability 12 months after abnormal newborn screening results. Research in nursing & health. 2011;34:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tluczek A, Henriques JB, Brown RL. Support for the reliability and validity of a six-item state anxiety scale derived from the State-Trait Anxiety Inventory. J Nurs Meas. 2009;17:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wade CH, Wilfond BS, McBride CM. Effects of genetic risk information on children’s psychosocial wellbeing: a systematic review of the literature. Genetics in medicine : official journal of the American College of Medical Genetics. 2010;12:317–26. [DOI] [PubMed] [Google Scholar]
- 20.Cavanagh L, Compton CJ, Tluczek A, Brown RL, Farrell PM. Long-term evaluation of genetic counseling following false-positive newborn screen for cystic fibrosis. Journal of genetic counseling. 2010;19:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsh VM, Kamuya DM, Molyneux SS. ‘All her children are born that way’: gendered experiences of stigma in families affected by sickle cell disorder in rural Kenya. Ethn Health. 2011;16:343–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tluczek A, Orland KM, Cavanagh L. Psychosocial consequences of false-positive newborn screens for cystic fibrosis. Qual Health Res. 2011;21:174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laird L, Dezateux C, Anionwu EN. Neonatal screening for sickle cell disorders: what about the carrier infants? Bmj. 1996;313:407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorenson JR, Levy HL, Mangione TW, Sepe SJ. Parental response to repeat testing of infants with ‘false-positive’ results in a newborn screening program. Pediatrics. 1984;73:183–7. [PubMed] [Google Scholar]
- 25.Tluczek A, Chevalier McKechnie A, Lynam PA. When the cystic fibrosis label does not fit: a modified uncertainty theory. Qual Health Res. 2010;20:209–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tluczek A, Koscik RL, Farrell PM, Rock MJ. Psychosocial risk associated with newborn screening for cystic fibrosis: parents’ experience while awaiting the sweat-test appointment. Pediatrics. 2005;115:1692–703. [DOI] [PubMed] [Google Scholar]
- 27.Sveger T, Thelin T. A future for neonatal alpha1-antitrypsin screening? Acta Paediatr. 2000;89:628–31. [DOI] [PubMed] [Google Scholar]
- 28.Oliver S, Dezateux C, Kavanagh J, Lempert T, Stewart R. Disclosing to parents newborn carrier status identified by routine blood spot screening. Cochrane Database Syst Rev. 2004:CD003859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayeems RZ, Miller FA, Barg CJ, Bombard Y, Carroll JC, Tam K, et al. Psychosocial Response to Uncertain Newborn Screening Results for Cystic Fibrosis. The Journal of pediatrics. 2017;184:165–71 e1. [DOI] [PubMed] [Google Scholar]
- 30.Miller FA, Robert JS, Hayeems RZ. Questioning the consensus: managing carrier status results generated by newborn screening. American journal of public health. 2009;99:210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farrell MH, Christopher SA. Frequency of high-quality communication behaviors used by primary care providers of heterozygous infants after newborn screening. Patient education and counseling. 2013;90:226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.La Pean A, Farrell MH, Eskra KL, Farrell PM. Effects of immediate telephone follow-up with providers on sweat chloride test timing after cystic fibrosis newborn screening identifies a single mutation. The Journal of pediatrics. 2013;162:522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farrell MH, Christopher SA, Tluczek A, Kennedy-Parker K, La Pean A, Eskra K, et al. Improving communication between doctors and parents after newborn screening. WMJ : official publication of the State Medical Society of Wisconsin. 2011;110:221–7. [PMC free article] [PubMed] [Google Scholar]
- 34.Collins JL, La Pean A, O’Tool F, Eskra KL, Roedl SJ, Tluczek A, et al. Factors that influence parents’ experiences with results disclosure after newborn screening identifies genetic carrier status for cystic fibrosis or sickle cell hemoglobinopathy. Patient education and counseling. 2013;90:378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.La Pean A, Collins JL, Christopher SA, Eskra KL, Roedl SJ, Tluczek A, et al. A qualitative secondary evaluation of statewide follow-up interviews for abnormal newborn screening results for cystic fibrosis and sickle cell hemoglobinopathy. Genetics in medicine : official journal of the American College of Medical Genetics. 2012;14:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradford L, Roedl SJ, Christopher SA, Farrell MH. Use of social support during communication about sickle cell carrier status. Patient education and counseling. 2012;88:203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christopher SA, Collins JL, Farrell MH. Effort required to contact primary care providers after newborn screening identifies sickle cell trait. Journal of the National Medical Association. 2012;104:528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmad NY, Farrell MH. Linguistic markers of emotion in mothers of sickle cell carrier infants: what are they and what do they mean? Patient education and counseling. 2014;94:128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farrell MH, Christopher SA, Kirschner AL, Roedl SJ, O’Tool FO, Ahmad NY, et al. Improving the quality of physician communication with rapid-throughput analysis and report cards. Patient education and counseling. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patterson R, Roedl SJ, Farrell MH. Internet Searching after Parents Receive Abnormal Newborn Screening Results. Journal of Communication in Healthcare: Strategies, Media and Engagement in Global Health. 2015;8:303–15. [Google Scholar]
- 41.Garrick MD, Dembure P, Guthrie R. Sickle-cell anemia and other hemoglobinopathies. Procedures and strategy for screening employing spots of blood on filter paper as specimens. N Engl J Med. 1973;288:1265–8. [DOI] [PubMed] [Google Scholar]
- 42.Gregg RG, Wilfond BS, Farrell PM, Laxova A, Hassemer D, Mischler EH. Application of DNA analysis in a population-screening program for neonatal diagnosis of cystic fibrosis (CF): comparison of screening protocols. American journal of human genetics. 1993;52:616–26. [PMC free article] [PubMed] [Google Scholar]
- 43.Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol. 1992;31 ( Pt 3):301–6. [DOI] [PubMed] [Google Scholar]
- 44.Spielberger C, Gorsuch R, Lushene R, Vagg P, Jacobs G. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- 45.National Institutes of Health. Racial and Ethnic Categories and Definitions for NIH Diversity Programs and for Other Reporting Purposes. 2018. [Available from: https://grants.nih.gov/grants/guide/notice-files/not-od-15-089.html. [Google Scholar]
- 46.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Family medicine. 2004;36:588–94. [PubMed] [Google Scholar]
- 47.Nelson DA, Deuster PA, Kurina LM. Sickle Cell Trait and Rhabdomyolysis among U.S. Army Soldiers. The New England journal of medicine. 2016;375:1696. [DOI] [PubMed] [Google Scholar]
- 48.Podduturi V, Guileyardo JM. Sickle cell trait as a contributory cause of death in natural disease. J Forensic Sci. 2015;60:807–11. [DOI] [PubMed] [Google Scholar]
- 49.Edwards JK. Risk factors: sickle cell trait increases the risk of chronic kidney disease. Nat Rev Nephrol. 2015;11:65. [DOI] [PubMed] [Google Scholar]
- 50.Bucknor MD, Goo JS, Coppolino ML. The risk of potential thromboembolic, renal and cardiac complications of sickle cell trait. Hemoglobin. 2014;38:28–32. [DOI] [PubMed] [Google Scholar]
- 51.Austin H, Lally C, Benson JM, Whitsett C, Hooper WC, Key NS. Hormonal contraception, sickle cell trait, and risk for venous thromboembolism among African American women. American journal of obstetrics and gynecology. 2009;200:620 e1–3. [DOI] [PubMed] [Google Scholar]
- 52.Baker MW, Atkins AE, Cordovado SK, Hendrix M, Earley MC, Farrell PM. Improving newborn screening for cystic fibrosis using next-generation sequencing technology: a technical feasibility study. Genetics in medicine : official journal of the American College of Medical Genetics. 2016;18:231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.