Abstract
Background:
Asthmatic patients who are allergic are uniquely susceptible to acute wheezing episodes provoked by rhinovirus. However, the underlying immune mechanisms and interaction between RV and allergy remain enigmatic, and current paradigms are controversial.
Objective:
To perform a comprehensive analysis of type 1 and type 2 innate and adaptive responses in allergic asthmatics infected with rhinovirus.
Methods:
Circulating virus-specific Th1 cells and allergen-specific Th2 cells were precisely monitored before and after rhinovirus challenge in allergic asthmatics (total IgE 133–4692 IU/mL; n=28) and healthy non-allergic controls (n=12) using peptide/MHCII tetramers. T cells were sampled for up to 11 weeks to capture steady state and post-infection phases. T-cell responses were analyzed in parallel with 18 cytokines in the nose, upper and lower airway symptoms, and lung function. The influence of in vivo IgE blockade was also examined.
Results:
In uninfected asthmatics, higher numbers of circulating virus-specific PD-1+ Th1 cells, but not allergen-specific Th2 cells, were linked to worse lung function. Rhinovirus infection induced an amplified anti-viral Th1 response in asthmatics versus controls, with synchronized allergen-specific Th2 expansion, and production of type 1 and 2 cytokines in the nose. By contrast, Th2 responses were absent in infected asthmatics who had normal lung function, and in those receiving anti-IgE. Across all subjects, early induction of a minimal set of nasal cytokines that discriminated high responders (G-CSF, IFN-γ, TNF-α), correlated with both egress of circulating virus-specific Th1 cells and worse symptoms.
Conclusions:
Rhinovirus induces robust Th1 responses in allergic asthmatics that may promote disease, even after infection resolves.
Keywords: rhinovirus, asthma, interferon, cytokines, T cells, Th1, Th2, tetramers, anti-IgE
Graphical Abstract

Capsule Summary:
Comprehensive analysis of type 1 and type 2 immune components in allergic asthmatics infected with rhinovirus supports a preferential role for anti-viral Th1 responses in promoting airway disease.
Introduction
Human rhinovirus (RV) is a major cause of the common cold. Repeated infections caused by multiple RV strains result in up to a billion cases annually in the US alone, with children bearing the brunt1. Children who have allergic asthma are susceptible to acute wheezing episodes induced by RV2–4. These types of episodes account for ~500,000 hospitalizations annually and can be life-threatening5. The prevalence of RV-induced wheezing will likely continue to increase given that asthma now affects 1 in 13 people in the US6, with allergic asthma accounting for the majority. Sensitization to inhalant allergens is a hallmark of allergic asthma. In this context, IgE production is thought to reflect underlying type 2 inflammation in the lower airways mediated by allergen-specific Th2 cells that secrete IL-4, IL-5, and IL-13. These cytokines exert pleiotropic and overlapping functions related to IgE production, mucus secretion, eosinophil recruitment, and airway hyperreactivity.
The interactions between RV and allergy that promote asthma exacerbations remain ill-resolved; however, type 2 inflammation in the lower airways appears to be a key contributor. In accord with this notion, elevated IgE antibodies, including IgE specific for dust mite allergens, are associated with worse cold symptoms, and increased risk and severity of wheeze during RV infection4,7,8. The reduced number of asthma exacerbations in children treated with anti-IgE, lends further credence to this theory9. However, the in vivo influence of anti-IgE on the immune response to RV infections in asthmatics is unknown.
Studies on the mechanisms of RV-induced asthma have been framed according to the mutually inhibitory actions of type 1 and type 2 responses. Type 1 responses are critical to anti-viral responses, and interferons (IFNs) are an integral component. Secretion of IFNs is triggered during early infection in airway epithelial cells and plasmacytoid dendritic cells (pDCs), by interactions between RV and pattern recognition receptors. Several studies have reported impaired expression of IFNs, or else a diminished response to ligands that bind RV-sensing receptors in primary bronchial epithelial cells, cells from the lower airways, and circulating pDCs and PBMCs of allergic asthmatics10–19. These observations imply the attenuation of type 1 responses by pre-existing type 2 disease20–22. By contrast, other work has either failed to corroborate these findings23–26, or else found that expression of IFNs or IFN response genes is actually enhanced in the airways of asthmatics infected with respiratory viruses27–31. Moreover, the ability to eliminate virus from the airways is not hampered in asthmatics32. From a different perspective, other studies have implicated RV pathways in promoting type 2 responses in the airways by augmenting secretion of the epithelial cell-derived alarmins TSLP and IL-3333,34. Together, these studies imply that RV infection modulates downstream T-cell responses and resulting airway inflammation by inhibiting virus-specific Th1 responses, enhancing Th2 responses, or both. Despite this, no studies have directly tested the relative contributions of Th1 and Th2 cells to the in vivo response to RV in asthmatics.
To address this, we precisely tracked circulating virus-specific and allergen-specific CD4+ T cells using peptide/MHCII tetramers in asthmatics experimentally infected with RV. Type 1 and type 2 responses were assessed by analyzing T-cell numbers and phenotypes relevant to lung pathology, and by testing relationships of T cells to cytokine profiles and disease in the airways. The ability for anti-IgE to modulate the immune response to RV was also examined. Our multi-faceted approach enabled comprehensive longitudinal sampling of the blood and airways in allergic asthmatics across a broad range of serum IgE levels, and comparisons with healthy non-allergic controls35. Our findings support the coordinated actions of Th1 and Th2 cells in driving asthma in response to RV.
Methods
Human Subjects
Subjects were adults with mild allergic asthma (n =31, total IgE >125 IU/ml) and healthy non-allergic controls (n =13, total IgE <50 IU/mL) who tested seronegative for RV-A1635. All asthmatics had airway hyperreactivity based on positive methacholine challenge, were allergic by positive skin prick test, and had specific IgE (>0.70 kUA/L, CAP class 2) to one or more common aeroallergen (Fig. E1, Table E1). Subjects who received RV challenge alone included 11 asthmatics and 13 healthy controls (Fig. E2). Additional asthmatics who had total IgE levels within the dosing range for anti-IgE, received Omalizumab (Genentech, USA, Inc., San Francisco, CA) beginning 8 weeks prior to RV challenge in a double-blind placebo-controlled trial (DBPC) (Treatment group, n =10; Placebo group, n =10) (Fig. E2). Studies were approved by the FDA, the NIAID safety committee, and the University of Virginia Human Investigations Committee (ClinicalTrials.gov ID: NCT02111772 & NCT02388997). All subjects gave written informed consent. See this article’s online repository for additional details, including full inclusion/exclusion criteria.
Experimental Infection Models
Subjects were challenged with live RV-A16 by intranasal administration of 1 mL inoculum containing 300 TCID50 of virus (0.5 mL per nostril)7,35,36 (Fig. E2a). Subjects were considered infected based on the following criteria: (1) ≥ four-fold increase in RV-A16 serum neutralizing antibody titer at day 21 post-inoculation, (2) one or more positive RV-A16 cultures from nasal washes on days 1–4, and (3) one or more positive RV qPCR tests from nasal washes on days 1–4. Viral titers were determined using standard culture methods, and by pan-RV qPCR, as previously described32. Study Design: The design of the study is summarized in Figure E2. Briefly, monitoring of symptom severity, lung function, and airway inflammation was performed at specified time points before and after RV challenge using the modified Jackson criteria, spirometry, and FeNO, respectively, in addition to complete blood counts (CBCs) (Fig. E2)37,38. Venous blood samples were obtained for cell analyses before and after RV challenge as follows: RV challenge alone – immediately before RV challenge (day 0) and on days 4, 7, and 21 post-challenge; Anti-IgE trial – 8 weeks before RV challenge, and then on days 0, 4, 7 and 21 (Fig. E2a).
Flow Cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated from venous blood by density gradient centrifugation, and cryopreserved for later analysis. Aliquots of whole blood were reserved for fresh analysis of dendritic cell populations.
Antigen-specific CD4+ T cells:
RV-A16-specific and allergen-specific CD4+ T cells were identified by dual tetramer staining using a modification of established methods and available tetramers39–41. Subjects included in tetramer studies were those with compatible HLA types and who had serum specific IgE directed against allergens for which tetramers were available (Tables E1 & E2)39,40. To identify antigen-specific T cells, cryopreserved PBMCs from all time points were thawed and analyzed in a single experiment for each subject. PBMCs were stained with PE-conjugated RV pMHCII tetramers and PE-CF594-conjugated allergen tetramers within the same sample. Tetramer+ cells were next enriched using an AutoMACS Pro with anti-PE magnetic beads (Miltenyi Biotec, Auburn, CA). Cells were then counterstained for viability and surface proteins (CD3, CD4, CD14, CD19, CD45RO, CD127, CCR4, CCR5, CCR7, CXCR3, CRTH2, PD-1) to exclude non-T cells and determine T-cell phenotypes by multi-color flow cytometry. Numbers of tetramer-positive T cells were expressed as n/N, where n is the number of tetramer+ cells in the enriched fraction, and N is the total number of CD4+ T cells in a pre-enrichment aliquot of cells from the same specimen39–41. (See this article’s online repository).
Dendritic Cells and Basophils:
Cells were analyzed using whole blood specimens (See this article’s online repository).
Nasal Cytokines
See this article’s online repository.
Statistical Analysis
See this article’s online repository.
Results
Activated PD-1+ RV-specific T cells are linked to asthma in the absence of infection.
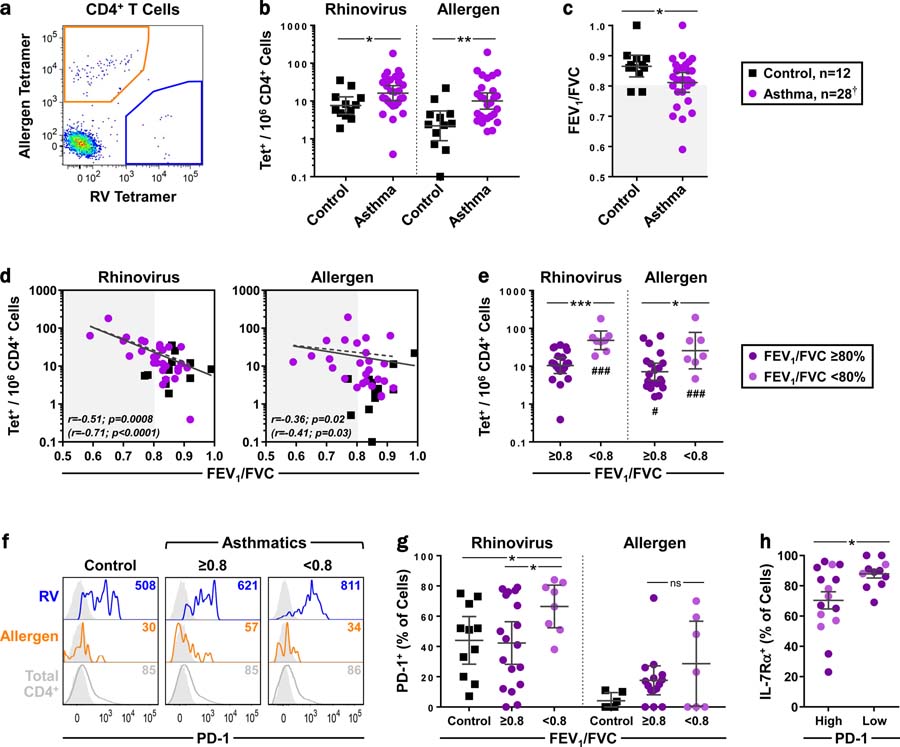
Initially, circulating antigen-specific T cells were analyzed in allergic asthmatics (n =28) and healthy controls (n =12) prior to any intervention, to assess their relevance to disease in the absence of infection 39,41. We employed a method of dual tetramer staining to simultaneously detect virus- and allergen-specific CD4+ T cells within the same specimen (Tables E1 & E2). This strategy identified higher numbers of both virus- and allergen-specific CD4+ T cells in asthmatics versus controls (geometric mean [GM]: RV =16/106 vs. 8/106, p =0.013; allergen =10/106 vs. 2/106, p =0.002) (Figs. 1a–b & E3). As expected, asthmatics had worse lung function compared with controls based on lower FEV1/FVC ratio (p =0.04) (Fig. 1c). Unexpectedly, higher numbers of virus-specific T cells were more strongly correlated with worse lung function than allergen-specific T cells (r =−0.11, p =0.0008 and r =−0.36, p =0.02, respectively) (Fig 1d). Furthermore, numbers of virus-specific T cells were higher in asthmatics who had reduced lung function (FEV1/FVC <80%; p =0.001) (Fig. 1e). These findings were not influenced by the number or type of tetramers used (Fig. E4). Analysis of PD-1, a marker implicated in lung fibrosis42, revealed a higher percentage of PD-1+ virus-specific T cells in asthmatics with worse lung function, compared with other asthmatics and controls (p <0.05) (Figs. 1f–g). Virus-specific T cells from asthmatics that were classified as PD-1high (≥50% PD-1+) were more activated based on lower expression of IL-7Rα (Fig. 1h)43. Together, these data identify a link between activated PD-1+ RV-specific T cells and airway inflammation in asthmatics who are nonetheless uninfected.
Figure 1. Activated PD-1+ RV-specific T cells are linked to asthma in the absence of infection.
(a) Representative plot of antigen-specific cells in an uninfected asthmatic. (b) Comparison of RV-specific and allergen-specific T-cell numbers in uninfected asthmatics and controls (geometric mean [GM] ± 95% CI, Mann-Whitney). (c) Comparison of FEV1/FVC between groups at the time of subject screening (GM ± 95% CI, Mann-Whitney). Shaded region denotes decreased lung function73. (d) Spearman correlation between antigen-specific T cell-numbers and FEV1/FVC. Dashed regression lines and values in parentheses depict asthmatics only. (e) Numbers of antigen-specific T cells in asthmatics, classified by lung function (GM ± 95% CI, Kruskal-Wallis). #Compared with healthy controls in panel b. (f) Representative histograms of PD-1 expression on antigen-specific T cells, with FMO controls (grey). Values denote geometric mean fluorescence intensity (MFI). (g) Percentage of PD-1+ antigen-specific T cells, by lung function. Samples with low cell counts were excluded from analyses (mean ± 95% CI, Kruskal-Wallis). (h) Expression of IL-7Rα on PD-1high (≥50% positive) and PD-1low (<50% positive) RV-specific T cells in asthmatics (mean ± 95% CI, Mann-Whitney). Panels e, g, & h: Light purple symbols indicate asthmatics with worse lung function. *p≤0.05, **p≤0.01, ***p≤0.001 (#p≤0.05, ###p≤0.001). † n=27 for allergen-specific cells.
Antigen-specific Th1 and Th2 effectors are armed for airway recruitment in asthmatics.
Next, we tested whether virus-specific and allergen-specific T helper (Th) cells in uninfected asthmatics were equipped for tissue homing and rapid response. Circulating RV-specific Th1 memory cells are known to be present in healthy subjects. These likely arise from priming by previous exposures to related RV strains39,40. In the present study, RV-specific T cells in asthmatics were highly enriched for memory cells (CD45RO+; median =90.0%) and included both central memory (TCM, CD45RO+CCR7+; median =52.5%) and effector memory (TEM, CD45RO+CCR7–; median =30.0%) subsets, similar to controls (Figs. 2a–c). RV-specific memory cells in asthmatics displayed the Th1-associated chemokine receptors CXCR3 and CCR5, and lacked the Th2 markers CCR4 and CRTH2 (Figs. 2d–f)44,45. Furthermore, expression of CXCR3 was higher on RV-specific TCM versus TEM, whereas the opposite was true for CCR5 (Figs. 2e–f). Hence, TEM cells resembled those CCR5+CXCR3– T cells that were previously found to be most activated in the nose during RV infection (Fig. E5)40. As expected, allergen-specific cells contained more total memory cells in asthmatics versus controls (median =22.6% vs. 58.5%), as well as more TEM cells (median =21.5% vs. 5.6%), consistent with priming by allergen exposure (p ≤0.05) (Figs. 2a–c). Both allergen-specific TCM and TEM subsets were Th2-like (CCR4+, CXCR3–), and expression of CRTH2, which is expressed on pathogenic effector Th2 cells46, was highest on the TEM subset (p =0.012) (Figs. 2d–f). These findings, coupled with established roles for CCR5 and CRTH2 in tissue-homing44,45, confirm that antigen-specific Th1 and Th2 effectors are armed for rapid recruitment to the airways in uninfected asthmatics.
Figure 2. Antigen-specific Th1 and Th2 effectors are armed for airway recruitment in asthmatics.
(a) Representative contour plots of RV-specific T cells overlaid on total CD4+ T cells, analyzed for CCR7 and CD45RO expression. (b & c) Percentage of RV-specific, allergen-specific, and total CD4+ T cells expressing CD45RO (b) or TCM and TEM phenotypes (c). Control, n ≥6; asthma, n ≥22. (Box-and-whiskers, Kruskal-Wallis & Mann-Whitney). (d) Representative plots showing marker expression on antigen-specific and total CD4+ T cells in an asthmatic subject. (e) Comparison of RV- and allergen-specific TCM and TEM surface markers within asthmatic subjects (Box-and-whiskers, Wilcoxon). (f) SPICE plots showing average signatures of RV-specific and allergen-specific TCM and TEM cells in asthmatics and controls. Asterisks denote significant differences for TCM vs TEM subsets within each group. Samples with insufficient numbers of tetramer+ cells were excluded from phenotypic analyses. nd, not determined. *p ≤0.05, **p ≤0.01, ***p ≤0.001.
Rhinovirus induces amplified anti-viral Th1 responses with bystander Th2 expansion in asthmatics.
Next, to test how T cells in allergic asthmatics respond during RV infection, asthmatics with high total IgE (>590 IU/ml, n =11) were experimentally infected with RV-A16, along with healthy controls (n =13) (Figs. 3a–b). Allergic asthmatics experienced increased symptoms versus controls during both acute (days 1–7) and chronic (days 15–21) phases; however, viral clearance was similar between groups, with a return to baseline levels by day 14 (Fig. E6)35. Circulating antigen-specific T cells were monitored during acute infection (day 4), at the T-cell effector phase (day 7), and at convalescence (day 21). Results showed that asthmatics mounted a more rapid and robust T-cell response to RV compared with controls, based on higher numbers of virus-specific T cells during acute infection (Day 4: GM =13/106 vs. 4/106, p ≤0.001) and in the effector phase (Day 7: 45/106 vs. 19/106, p =0.003) (Figs. 3c–f). In asthmatics only, numbers of allergen-specific T cells also increased in the effector phase, but to a lesser degree than RV-specific T cells (Day 7 fold change =2.8x vs. 5.2x). A more marked expansion of total lymphocytes was also observed in asthmatics versus controls during acute infection (p <0.001 on day 2) (Fig. 3g).
Figure 3. Change in numbers of antigen-specific T cells during RV infection.
(a) RV challenge model. (b) Serum total IgE (left) and FEV1/FVC ratio (right) in asthmatics and controls at the time of subject screening (GM ± 95% CI, Mann-Whitney). Shaded region denotes decreased lung function. (c) Representative flow plots showing tetramer staining for RV-specific and allergen-specific (Der p 1) CD4+ T cells at days 0 and 7 in an asthmatic. (d) Change in antigen-specific T-cell numbers after RV challenge (GM ± 95% CI, generalized estimating equations [GEE]). (e) Fold change in T-cell numbers (Log2-transformed) during RV infection (mean ± SEM, Mann-Whitney). (f) Change in T-cell numbers on day 7. Numbers denote fold change. (g) Change in blood lymphocyte counts during RV infection (mean ± SEM, mixed effects models). T-cell assays: Control, n =12; asthma, n ≥10. Within-group and between-group comparisons for panels d, e, and g are denoted by (*) and (#) respectively: *p ≤0.05, **p ≤0.01, ***p ≤0.001 (#p ≤0.05, ##p ≤0.01, ###p ≤0.001).
To better understand transitions in antigen-specific T cells in asthmatics, their molecular signatures were next examined during infection. The mean percentage of RV-specific TEM and PD-1+ cells decreased on day 4 versus day 0 (p <0.05) (Figs. 4a–c); however, absolute numbers of RV-specific TEM remained unchanged (GM: 6/106 vs. 5/106). By contrast, on day 7, the percentage of RV-specific TCM increased from day 0 (p =0.002) (Figs 4a–b), as well as their absolute numbers (GM: 30/106 vs. 6/106, p =0.01). This coincided with increased expression of CCR5 and lower IL-7Rα on RV-specific T cells (Figs. 4c–d ). Together, these findings were consistent with egress and sequestration of PD-1+ TEM cells after infection, along with systemic mobilization of an expanded and activated TCM subset. These changes were accompanied by increased percentages of allergen-specific TCM and decreased naïve cells (day 4, p ≤0.01); however, IL-7Rα was unchanged on these cells (Figs. 4b–d). RV infection did not alter the distinct Th phenotypes of antigen-specific T cells within TEM or TCM subsets (Figs. 4e & E7). In controls, transitions in TEM and TCM subsets of RV-specific T cells, as well as their absolute numbers, trended in a similar fashion to those in asthmatics, but results were not significant at any time point (p >0.05) (Fig. E8). Furthermore, changes in allergen-specific phenotypes were unremarkable. Together, data in Figures 3 and 4 demonstrate that asthmatics mount a rapid and amplified Th1 response to RV along with a coordinated expansion of allergen-specific Th2 cells that does not trigger downregulation of IL-7Rα. This latter phenomenon suggests a mechanism of Th2 activation occurring independent of T-cell receptor ligation, consistent with bystander activation.
Figure 4. Phenotypic transitions in antigen-specific T cells in asthmatics during RV infection.
(a) Representative contour plots showing transitions in memory subsets during RV infection. (b) Change in the relative percentages of antigen-specific TN, TCM, and TEM cells compared with day 0 (mean, generalized linear model [GLM]). (c) Change in percentages of antigen-specific T cells expressing PD-1, IL-7Rα, and CCR5 (mean ± SEM, GLM & Kruskal-Wallis). (d) Representative histograms showing change in IL-7Rα expression on antigen-specific T cells (FMO controls shaded in grey). Values denote MFIs. (e) SPICE charts showing signatures of antigen-specific TEM cells. Sample size was determined by numbers of tetramer+ cells sufficient for SPICE analysis. Asthma, n ≥10. *p ≤0.05, **p ≤0.01, ***p ≤0.001.
Rhinovirus induces robust type 1 and type 2 responses in the nose of asthmatics.
Next, we tested whether the cytokine milieu in the nose of infected asthmatics was conducive to the recruitment and activity of both Th1 and Th2 cells. Cytokines were assayed in nasal lining fluid (to optimize the detection of mediators that are typically secreted at low levels), and in nasal washes. Analysis of nasal lining fluid revealed induction of the innate interferon, IFN-α on day 4 at similar levels in asthmatics and controls (p <0.05 vs. day 0). The Th1 effector cytokine, IFN-γ, was also induced in both groups, albeit to a lesser degree (Fig. 5a). Analysis of the kinetics of IFN responses in nasal washes of asthmatics revealed peak secretion at days 3–4, and the persistence of higher levels of IFN-γ on day 7 versus controls (Fig. E9). This was preceded by expansion of circulating pDCs, which are pivotal to anti-viral Th1 responses (Fig. E10). These changes also coincided with more robust induction of ligands for CCR5 and CXCR3 (MIP-1β/CCL4 and IP-10/CXCL10, respectively) in nasal washes from asthmatics (Figs. 5b). High levels of IL-17A, a cytokine expressed by Th17 cells and some Th1 cells, were also detected in a subset of asthmatics (Fig. 5a). Analysis of type 2 cytokines in nasal lining fluid revealed the induction of IL-4 (and to a lesser degree, IL-5) on day 4 in asthmatics, in parallel with marked induction of the CCR4 ligand MDC/CCL22 in nasal wash (Figs. 5a–b). Although nasal IL-13 levels were higher in asthmatics versus controls before infection, they remained unchanged after RV challenge (Fig 5a). In controls, there was also a modest induction of Th2 cytokines on day 4; however, this effect was only significant for IL-5, and levels remained lower than for asthmatics before and after RV challenge. These results confirmed the robust induction of both type 1 and type 2 cytokines in the nose of asthmatics, including T-cell chemoattractants known to recruit Th1 and Th2 cells.
Figure 5. Rhinovirus induces robust type 1 and type 2 responses in the nose of asthmatics.
(a) Levels of Th-associated cytokines in nasal lining fluid (GM ± 95% CI, Mann-Whitney). All groups, n ≥5 for each time point (“dry” samples owing to insufficient secretions were not tested). (b) Levels of T-cell chemoattractants in nasal wash fluid (top, GM, mixed effects models), and Log2 fold change in levels from day 0 (bottom, mean, Mann-Whitney). Asterisks in panel b denote between-group comparisons only. *p ≤0.05, **p ≤0.01, ***p ≤0.001. † n =11 and ‡ n =10 for MIP-1β.
Bystander Th2 responses are absent in infected asthmatics with normal lung function or with IgE blockade.
A prevailing view is that type 2 inflammation in the airways of allergic asthmatics promotes RV-induced disease. This theory is supported by reduced asthma exacerbations in asthmatics treated with omalizumab9. Thus, we tested whether anti-IgE might attenuate both type 1 and type 2 responses in infected asthmatics. Asthmatics with suitable IgE levels (133–493 IU/mL) participated in a DBPC trial of omalizumab starting 8 weeks before RV-A16 challenge (n =10 per group) (Figs. 6a–b)35. At study entry, subjects receiving anti-IgE had lung function similar to asthmatics outside the trial (FEV1/FVC =0.77 vs. 0.80), whereas the placebo group had normal lung function (FEV1/FVC =0.87) (Fig. 6b). This difference was reflected in the higher numbers of RV-specific T cells (p =0.01) and their activated signature (↑PD-1 and ↓IL-7Rα) in the anti-IgE vs. placebo group at the same time point (Fig. E11a).
Figure 6. Effect of anti-IgE on the immune response to rhinovirus.
(a) Model of anti-IgE trial with RV challenge. (b) Serum total IgE (left) and FEV1/FVC (right) at the time of subject screening. Shaded areas denote the IgE range suitable for omalizumab, or decreased lung function (GM ± 95% CI, Kruskal-Wallis). (c) Effect of anti-IgE on FcεRIα on basophils and DCs (mean ± SEM, Kruskal-Wallis). (d & e) Transitions in antigen-specific T-cell numbers (d; GM ± 95% CI, mixed-effects models) and their Log2 fold change (e; mean ± SEM, Mann-Whitney). (f) Change in blood lymphocytes counts during infection. (g) Cytokine levels in nasal lining fluid on day 4 (GM ± 95% CI, Kruskal-Wallis). Dry specimens were not tested. Data shown in gray is for asthmatics and controls outside the trial, for comparison purposes. T-cell assays: n ≥8; Cytokine assays: n ≥7. Within-group and between-group comparisons for panels c and d are denoted by (*) and (#), respectively: *p ≤0.05, **p ≤0.01, ***p ≤0.001 (##p ≤0.01).
Following RV challenge, subjects receiving anti-IgE experienced a reduced drop in lung function on days 1–4 versus placebo, whereas symptoms remained similar between groups 35. Analysis of immune cells confirmed decreased expression of the high affinity IgE receptor (FcεRIα) on circulating basophils and dendritic cells in the anti-IgE group (p <0.05) (Fig. 6c). After infection, anti-IgE and placebo groups experienced a similar fold increase in circulating RV-specific T cells (day 7 fold change: 2.1x and 1.5x, respectively) (Figs. 6d–e), and Th1 signatures were maintained (Fig. E11b); however, the response was lower than in asthmatics with high IgE (5.2x), and expansion at day 4 was not evident. Moreover, there was no expansion of allergen-specific Th2 cells in anti-IgE or placebo groups, and expansion of total lymphocytes was also blunted (Figs. 6d–f).
Previous work found that cells isolated from asthmatics and stimulated with RV in vitro produced more IFN-α after subjects were treated with anti-IgE47. Thus, given that nasal IFNs were not deficient in infected asthmatics (Figs. 5a & E9), we queried whether anti-IgE might boost IFNs in response to RV. Within the anti-IgE group, levels of nasal IFN-α were higher versus placebo during acute infection. Moreover, IFN-γ levels were the highest of all asthmatics in the study (Fig. 6g & E12). By contrast, no differences in IL-17A or type 2 cytokines, including IL-4 and IL-13, were identified (Figs. 6g & E12). Together, these findings confirmed that systemic anti-viral T-cell responses are attenuated, and bystander Th2 responses are absent, in asthmatics who have normal lung function, or else when IgE is blocked.
Local mediators, T-cell responses in the blood, and respiratory disease are connected.
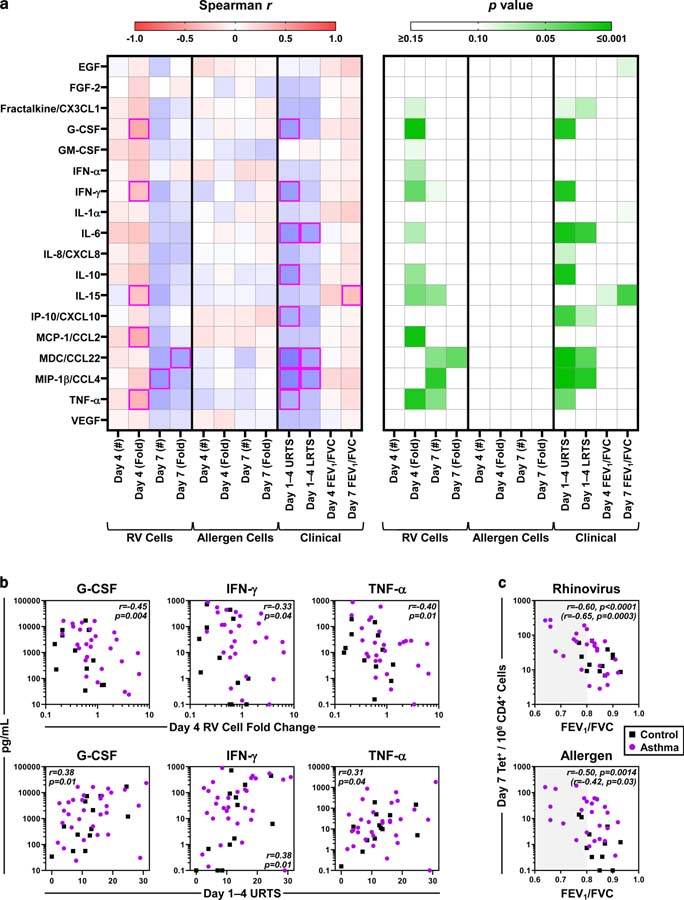
Given the heterogeneity of subjects in our study, we leveraged the clinical and immune parameters tested across all 44 subjects (31 asthmatics and 13 controls) to gain insight into factors orchestrating T-cell responses, and to assess immune relevance to disease. As expected, analysis of 18 nasal mediators confirmed that RV infection induced the secretion of multiple cytokines that significantly correlated with each other during the peak response (Fig. E13a). Induction of a narrower set of cytokines, including type 1 and pro-inflammatory mediators (G-CSF, IFN-γ, IL-15, MCP-1/CCL2 [CCR5 ligand], TNF-α), related to the decrease in RV-specific T cells at day 4 (r =−0.32 to −0.45, p ≤0.046) (Figs. 7a–b). By contrast, no relationships between cytokines and allergen-specific T cells were identified. Significant positive correlations with upper respiratory symptoms were also identified for multiple cytokines (G-CSF, IFN-γ, IL-6, IL-10, IP-10/CXCL10 [CXCR3 ligand], MDC/CCL22 [CCR4 ligand], MIP-1β/CCL4 [CCR5 ligand], TNF-α) (p ≤ 0.043) (Fig. 7a). Of these, the pro-inflammatory cytokine IL-6, and Th1 and Th2 chemoattractants (MIP-1β and MDC, respectively) also related to lower respiratory symptoms (r ≥0.34, p ≤0.034). Notably, G-CSF, IFN-γ, and TNF-α correlated with both T-cell responses and respiratory symptoms (Fig. 7b). Similar to results in steady state, higher numbers of both virus- and allergen-specific T cells during infection linked to reduced lung function (Day 7: r =−0.60 and −0.50 respectively, p ≤0.002) (Figs. 1d, 7c, & E13b).
Figure 7. Nasal cytokines, T-cell responses, and respiratory disease are connected.
(a) Spearman r (left) and p values (right) for correlations between peak levels of 18 cytokines in nasal washes (median: days 3–4) and antigen-specific T-cell responses (numbers and fold change), upper respiratory (URTS) and lower respiratory (LRTS) symptoms, and lung function. Magenta boxes denote significant correlations. (b) Spearman correlations of G-CSF, IFN-γ, and TNF-α with T-cell and symptom outcomes. (c) Spearman correlation between the numbers of antigen-specific T cells and FEV1/FVC ratio on day 7. Values in parentheses refer to asthmatics only. Shaded region denotes decreased lung function. n ≥38 (Control, n ≥11; asthma, n ≥26). *p ≤0.05.
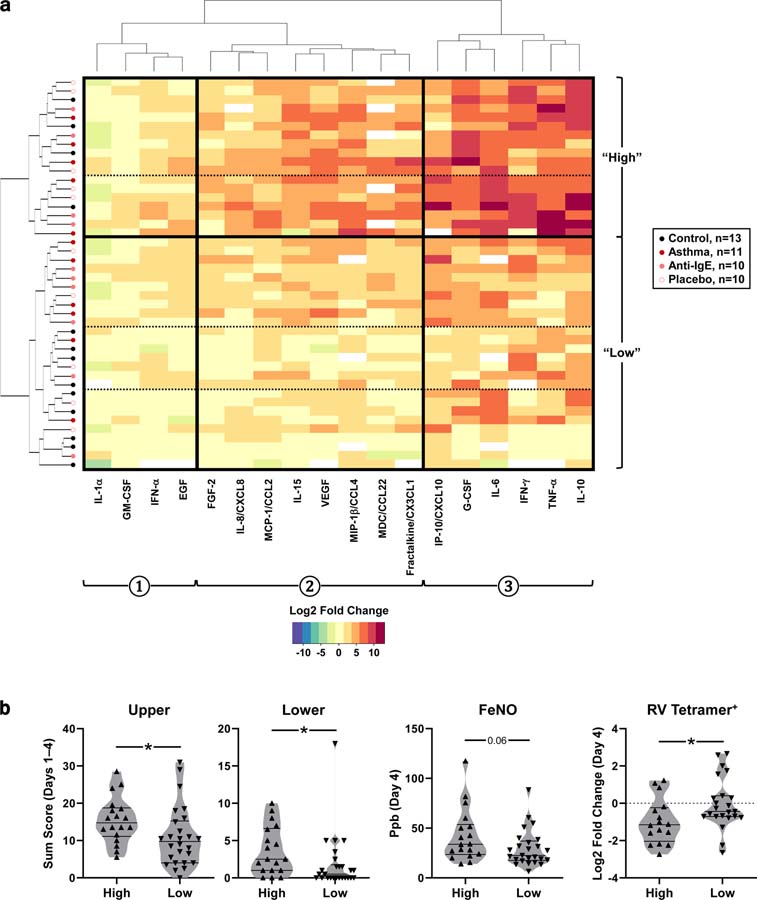
Hierarchical clustering identified 18 “high” responders based on differential expression of 3 sets of cytokines. The cytokine set most highly induced during infection (#3) included mediators linked to T-cell responses and respiratory measures, including G-CSF, IFN-γ, TNF-α, IL-6, and IP-10 (Fig. 8a). A greater percentage of asthmatics fell within the high responder group compared with controls (45. 2% vs. 30.8%), and most controls fell within the lowest tiers of low responders (69.2%). High responders had more severe respiratory symptoms, elevated exhaled nitric oxide (FeNO), and a more pronounced decrease in circulating RV-specific, but not allergen-specific, T cells during acute infection versus low responders (Fig. 8b). Taken together, these findings link the induction of a discrete set of mediators at the infection site to the recruitment of RV-specific T cells from the blood and to respiratory disease.
Figure 8. Identification of high responders to RV by hierarchical clustering.
(a) Heat map of the acute fold change (day 4) for 18 cytokines present in nasal washes from 44 subjects. Hierarchical clustering identified distinct cytokine sets (denoted 1, 2, and 3) that were differentially expressed across subject groups (“high” and “low” responding subjects; solid line) and subsets thereof (dashed lines). (b) Respiratory symptoms, exhaled nitric oxide (FeNO; parts-per-billion, ppb), and fold change in RV-specific T cells in high (n =18) versus low (n =26) responders during the acute phase (Mann-Whitney). Fold change values are Log2-transformed. T-cell assays: n =39 (High, n =16; low, n =23).
Discussion
The general view is that allergic asthma is perpetuated by type 2 inflammatory responses. Here, by precisely tracking antigen-specific Th1 and Th2 cells during experimental RV infection, we report a paradoxical amplification of anti-viral Th1 responses in asthmatics with high IgE, with synchronized bystander activation of Th2 cells. Those RV-specific T cells identified in the present study target peptide epitopes of capsid proteins that are highly conserved across multiple RV-A strains, and thus, are poised to respond rapidly owing to priming by previous RV infections39. This was borne out in their tissue-homing and memory signatures in steady state, as well as their numbers and functional profiles after RV challenge. A notable feature of our study was the connection between T-cell responses, respiratory disease, and the robust early induction of a unique set of mediators comprising G-CSF, IFN-γ, TNF-α, IL-6, and IP-10. These findings echo a new report linking an enhanced IFN response in primary bronchial epithelial cells to reduced FEV1 in infected asthmatics30. Thus, it seems unlikely that the Th1 response in asthmatics reflects a compensatory adaptive mechanism for a deficient innate response, a view that is bolstered by the efficient viral clearance in asthmatics24,32.
In addition to an exaggerated Th1 response during infection, our results implicate RV-specific T cells in maintaining airway inflammation even in the absence of virus. Specifically, the link between higher numbers of PD-1+ RV-specific T cells and worse lung function in uninfected asthmatics was an unexpected and novel finding. PD-1 is expressed on the surface of activated T cells, and is known to inhibit T-cell activation. However, recent studies suggest that PD-1 can also promote pulmonary fibrosis via the PD-1/PD-L1 pathway through a process involving cross-talk between T cells and lung fibroblasts, resulting in collagen production42,48. Hence, the ability for PD-1+ RV-specific T cells to promote airway remodeling in asthma warrants investigation.
Our results point to the coordinated actions of Th1 and Th2 cells in virus-induced asthma. Higher numbers of allergen-specific Th2 cells were present in uninfected asthmatics. During infection, these cells expanded without the loss of surface IL-7Rα, perhaps indicating virus-induced bystander activation occurring independent of TCR ligation or IL-743. Bystander T-cell activation is a well-recognized feature of anti-microbial responses that involves surface microbial sensors, and cytokines such as IL-2 and IL-1549–53. Our data recapitulate much earlier work in animal models of asthma that reported cooperation between Th1 and Th2 cells in airway inflammation. In that work, respiratory viral infections and Th1 cells aided in the recruitment of resting allergen-specific Th2 cells54–56. The concept of “Th1 on Th2” inflammation is further evidenced in the present study by the robust induction of type 1 and type 2 mediators in the upper airways of infected asthmatics with high IgE.
Together, our results suggest that a single RV exposure in allergic asthmatics agitates and sustains airway inflammation via Th1-mediated mechanisms. Younger asthmatics, who are most at risk of RV-induced exacerbations, typically experience 6–8 colds annually57,58. Thus, we propose a model wherein multiple RV infections occurring in close succession, and in the context of pre-existing type 2 inflammation, induce recurring cycles of dysregulated anti-viral Th1 responses that drive persistent airway inflammation through coordinated actions of Th1 and Th2 cells that endure long after virus has cleared.
Questions remain regarding the mechanisms of Th1 amplification and coordination with Th2 cells in allergic asthma. The robust induction of type 1 and type 2 mediators in the asthmatic airways in response to RV is likely pivotal in this regard. Many cytokines that are integral to Th2 responses are also pivotal to Th1 responses. One such example is IL-33, which is highly expressed in the airways of both children and adults with severe asthma59–62. This cytokine is induced by RV33, directly activates Th1 cells in inflamed settings63, and has been linked to IgE production to prototypical Th1 antigens in asthma59,62. However, we were unable to detect IL-33 in the nose during RV infection (data not shown), likely owing to the dilutional effects of the nasal wash procedure. Similarly in the absence of infection, type 2 mediators present in the asthmatic airways, such as MDC/CCL2264,65, might sustain RV-specific Th1 cells, thereby accounting for their increased numbers in the blood.
Our study analyzed T-cell responses to RV in asthmatics receiving anti-IgE. In these subjects, bystander Th2 responses were absent, whereas anti-viral Th1 responses resembled those of non-asthmatic controls. Within the nose, type 2 cytokines did not appear to be inhibited by anti-IgE in the acute phase. While this was surprising, inspection of the literature indicates that the effects of anti-IgE on type 2 cytokines in the human airways are unknown. By contrast, nasal IFNs were enhanced in asthmatics receiving anti-IgE, and were actually higher than for all other asthmatics in our study. Consideration of the newer literature is informative in this regard. There is evidence to suggest that in certain contexts, the IgE pathway can provide a “brake” on RV-induced IFNs. This comes from: (1) in vitro cellular data showing that cross-linking of IgE receptor on pDCs inhibits IFN secretion18; and (2) a link between anti-IgE treatment in vivo and enhanced IFN responses in pDCs stimulated ex vivo with RV47. However, different mechanisms may regulate the production of IFNs in the airways. Several newer studies indicate that IFN responses are not deficient at the epithelial barrier in asthmatics infected with RV, or in those experiencing cold symptoms27,30,66. One report suggests that mast cells, which are enriched in the allergic airways, may contribute to IFN production when type 2 inflammation is present. This is based on the observation that IL-4 enhanced virus-induced IFN production by mast cells, and this phenomenon was augmented by IgE-mediated activation.67. Hence, IFNs detected in nasal washes in the present study likely reflect the culmination of complex regulatory mechanisms that differ according to cell type and context, and cannot be predicted based on in vitro systems.
A major differentiating feature of our study from prior work is that it is the first to link in vivo treatment with omalizumab in asthmatics to the in vivo production of nasal IFNs during RV infection. Considering why IFNs are enhanced in this scenario, if IgE pathway regulates IFN production in pDCs and anti-IgE releases this “brake”, then the IFN boost in asthmatics who are not deficient in IFN secretion to begin with, is arguably not surprising. Such a mechanism could involve direct effects of anti-IgE on IgE receptor+ cells residing in the airway mucosa that secrete IFNs, or else indirect effects on cell and signaling networks triggered by RV66.
A key question is why enhanced nasal IFNs do not translate to augmented Th1 responses in the blood of asthmatics treated with anti-IgE. Complex spatio-temporal factors in the immune response to RV are likely an important factor in this regard. This concept was borne out by our recent work that delineated differential involvement of RV-specific B cells and antibody isotypes according to the anatomic site and time of infection68. Thus, the axis linking IFNs secreted in the nose to Th1 responses in the blood is not necessarily linear. Given that the actions of anti-IgE likely extend beyond the IgE pathway, we speculate that modulation of immune networks both locally and systemically could result in reduced activation, or else re-routing of Th1 cells. Looking forward, systems biology methods will provide a useful tool for assessing the global impact of anti-IgE on cell and cytokine networks operating in RV infection. This type of approach has revealed complex interactions between type 1 and 2 networks during viral exacerbations of asthma66.
Our study established that Th2 responses were absent in asthmatics in the placebo group of the anti-IgE trial, who had normal lung function. Thus, we could not definitively prove whether the absence of Th2 responses in the anti-IgE group (who had worse lung function similar to asthmatics outside the trial) was attributable to IgE blockade. Nonetheless, our findings highlighted differences in T-cell responses across a broad IgE spectrum in asthmatics. There were additional limitations of our study. First, all RV challenges were performed in young adults since children cannot be challenged for safety and ethical reasons69. Nonetheless, several features point to commonalities between children with acute asthma and adults in our model. These include (1) similar mediator profiles, RV receptor (ICAM-1) profiles, and viral loads in the nose during infection7,32; and (2) increased RV-induced symptoms with higher total IgE4,7. Second, it was not possible to directly assess the contributions of Th1 and Th2 cells to responses in the lungs, since sampling of the lower airways was not feasible owing to regulatory constraints. Third, limitations on specimen availability precluded a more detailed analysis of RV-specific T cells, including the determination of additional Th types not accounted for in our phenotyping panel, as well as their cytokine profiles. This was the result of the high cell numbers required to attain reliable tetramer signals by flow cytometry, the blood volumes required for longitudinal sampling, and multi-parameter limitations at the start of the study. Our previous work demonstrated that RV-specific T cells in healthy uninfected subjects are predominantly IFN-γ+, and to a lesser degree IL-17A+, and that IL-17A+IFN-γ+ T cells are a feature of severe asthma39,40,59. In the present study, nasal secretion of high levels of IL-17A during acute infection in a subset of asthmatics, suggests that Th17 cells or else IL-17A+ IFN-γ+ cells are also a feature of RV infected asthmatics. The links identified between Th17-associated nasal cytokines (notably IL-6 and G-CSF), T cells, and respiratory disease, as well as observations by other groups, lend further credence to this notion70–72. Work is ongoing to elucidate the nature of RV-specific T cells in asthmatics using high-dimensional single-cell analytical methods.
In summary, our findings imply a key role for Th1 responses to RV in promoting chronic airway inflammation in allergic asthma, and for their coordinated actions with allergen-specific Th2 cells. Our results challenge existing paradigms of type 2 asthma, and shift the spotlight to virus-induced Th1 cells as an integral component of asthma pathogenesis in allergic patients.
Supplementary Material
Key Messages:
Th1 cells that target rhinovirus are a prominent feature of allergic asthma both in the presence and absence of infection.
Coordinated allergen-specific Th2 responses are absent in asthmatics who have normal lung function, or those receiving anti-IgE.
Early local secretion of type 1 and pro-inflammatory cytokines links to anti-viral T-cell responses and respiratory illness.
Acknowledgments
The authors thank: Mark Conaway, PhD (University of Virginia), for his assistance with statistical analyses in the parent clinical study; Joanne Lannigan, MS, Michael Solga, MS, Claude Chew, BS, and Alexander Wendling, BS (University of Virginia) for assistance with flow cytometry and multiplex bead assays. Graphical abstract was created using BioRender.
Funding: Work was supported by Novartis Pharmaceuticals, NIH/NIAID U01 AI100799, NIH/NIAID R01 AI020565, NIH/NIAID U01 AI125056, and NIH/NIAID T32 AI007496.
Abbreviations:
- HLA
human leukocyte antigen
- Ig
immunoglobulin
- IFN
interferon
- IL
interleukin
- mDC
myeloid dendritic cell
- MHC
major histocompatibility complex
- pDC
plasmacytoid dendritic cell
- RV
rhinovirus
- TCM
central memory
- TCR
T-cell receptor
- TEM
effector memory
- Th
T helper
- TN
naïve
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflicts of interest
References
- 1.Gwaltney JM Jr. Acute community-acquired sinusitis. Clin Infect Dis 1996;23:1209–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heymann PW, Carper HT, Murphy DD, Platts-Mills TAE, Patrie J, McLaughlin AP, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol 2004;114:239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care IgE and eosinophil analyses. Am J Respir Crit Care Med 1999;159:785–90. [DOI] [PubMed] [Google Scholar]
- 4.Soto-Quiros M, Avila L, Platts-Mills TAE, Hunt JF, Erdman DD, Carper HT, et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J Allergy Clin Immunol 2012;129:1499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dougherty RH, Fahy JV. Acute exacerbations of asthma: Epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy 2009;39:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asthma’s Impact on the Nation: Data from the CDC National Asthma Control Program [Internet]. 2010.
- 7.Zambrano JC, Carper HT, Rakes GP, Patrie JT, Murphy DD, Platts-Mills TAE, et al. Experimental rhinovirus challenges in adults with mild asthma: Response to infection in relation to IgE. J Allergy Clin Immunol 2003;111:1008–16. [DOI] [PubMed] [Google Scholar]
- 8.Kantor DB, Stenquist N, McDonald MC, Schultz BJ, Hauptman M, Smallwood CD, et al. Rhinovirus and serum IgE are associated with acute asthma exacerbation severity in children. J Allergy Clin Immunol 2016;138:1467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med 2011;364:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 2005;201:937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PAB, Bartlett NW, et al. Role of deficient type III interferon-λ production in asthma exacerbations. Nat Med 2006;12:1023–6. [DOI] [PubMed] [Google Scholar]
- 12.Bratke K, Prieschenk C, Garbe K, Kuepper M, Lommatzsch M, Virchow JC. Plasmacytoid dendritic cells in allergic asthma and the role of inhaled corticosteroid treatment. Clin Exp Allergy. 2013;43:312–21. [DOI] [PubMed] [Google Scholar]
- 13.Sykes A, Edwards MR, Macintyre J, del Rosario A, Bakhsoliani E, Trujillo-Torralbo M-B, et al. Rhinovirus 16-induced IFN-α and IFN-β are deficient in bronchoalveolar lavage cells in asthmatic patients. J Allergy Clin Immunol 2012;129:1506–14. [DOI] [PubMed] [Google Scholar]
- 14.Hatchwell L, Collison A, Girkin J, Parsons K, Li J, Zhang J, et al. Toll-like receptor 7 governs interferon and inflammatory responses to rhinovirus and is suppressed by IL-5-induced lung eosinophilia. Thorax 2015;70:854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, et al. Counterregulation between the FcεRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol 2010;184:5999–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. Rhinovirus-induced alterations on peripheral blood mononuclear cell phenotype and costimulatory molecule expression in normal and atopic asthmatic subjects. Clin Exp Allergy. 2002;32:537–42. [DOI] [PubMed] [Google Scholar]
- 17.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and TH1/2 cytokine and IL-10 production. Proc Natl Acad Sci USA. 2008;105:13562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durrani SR, Montville DJ, Pratt AS, Sahu S, DeVries MK, Rajamanickam V, et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol 2012;130:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu J, Message SD, Mallia P, Kebadze T, Contoli M, Ward CK, et al. Bronchial mucosal IFN-α/β and pattern recognition receptor expression in patients with experimental rhinovirus-induced asthma exacerbations. J Allergy Clin Immunol 2019;143:114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch JP, Werder RB, Simpson J, Loh Z, Zhang V, Haque A, et al. Aeroallergen-induced IL-33 predisposes to respiratory virus-induced asthma by dampening anti-viral immunity. J Allergy Clin Immunol 2016;138:1326–37. [DOI] [PubMed] [Google Scholar]
- 21.Werder RB, Zhang V, Lynch JP, Snape N, Upham JW, Spann K, et al. Chronic IL-33 expression predisposes to virus-induced asthma exacerbations by increasing type 2 inflammation and dampening antiviral immunity. J Allergy Clin Immunol 2018;141:1607–19. [DOI] [PubMed] [Google Scholar]
- 22.Contoli M, Ito K, Padovani A, Poletti D, Marku B, Edwards MR, et al. TH2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy. 2015;70:910–20. [DOI] [PubMed] [Google Scholar]
- 23.Patel DA, You Y, Huang G, Byers DE, Kim HJ, Agapov E, et al. Interferon response and respiratory virus control are preserved in bronchial epithelial cells in asthma. J Allergy Clin Immunol 2014;134:1402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeMore JP, Weisshaar EH, Vrtis RF, Swenson CA, Evans MD, Morin A, et al. Similar colds in subjects with allergic asthma and nonatopic subjects after inoculation with rhinovirus-16. J Allergy Clin Immunol 2009;124:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleming HE, Little FF, Schnurr D, Avila PC, Wong H, Liu J, et al. Rhinovirus-16 colds in healthy and in asthmatic subjects: Similar changes in upper and lower airways. Am J Respir Crit Care Med 1999;160:100–8. [DOI] [PubMed] [Google Scholar]
- 26.Sykes A, Edwards MR, Macintyre J, del Rosario A, Gielen V, Haas J, et al. TLR3, TLR4 and TLRs7–9 induced interferons are not impaired in airway and blood cells in well controlled asthma. PLoS One. 2013;8:e65921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansel TT, Tunstall T, Trujillo-Torralbo M-B, Shamji B, del Rosario A, Dhariwal J, et al. A comprehensive evaluation of nasal and bronchial cytokines and chemokines following experimental rhinovirus infection in allergic asthma: Increased interferons (IFN-γ and IFN-λ) and type 2 inflammation (IL-5 and IL-13). EBioMedicine. 2017;19:128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altman MC, Reeves SR, Parker AR, Whalen E, Misura KM, Barrow KA, et al. Interferon response to respiratory syncytial virus by bronchial epithelium from children with asthma is inversely correlated with pulmonary function. J Allergy Clin Immunol 2018;142:451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwantes EA, Manthei DM, Denlinger LC, Evans MD, Gern JE, Jarjour NN, et al. Interferon gene expression in sputum cells correlates with the Asthma Index Score during virus-induced exacerbations. Clin Exp Allergy. 2014;44:813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravi A, Koster J, Dijkhuis A, Bal SM, Sabogal Piñeros YS, Bonta PI, et al. Interferon-induced epithelial response to rhinovirus-16 in asthma relates to inflammation and FEV1. J Allergy Clin Immunol 2019;143:442–7. [DOI] [PubMed] [Google Scholar]
- 31.Sykes A, Macintyre J, Edwards MR, del Rosario A, Haas J, Gielen V, et al. Rhinovirus-induced interferon production is not deficient in well controlled asthma. Thorax 2014;69:240–6. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy JL, Shaker M, McMeen V, Gern J, Carper H, Murphy D, et al. Comparison of viral load in individuals with and without asthma during infections with rhinovirus. Am J Respir Crit Care Med 2014;189:532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson DJ, Makrinioti H, Rana BMJ, Shamji BWH, Trujillo-Torralbo M-B, Footitt J, et al. IL-33–dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med 2014;190:1373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uller L, Leino M, Bedke N, Sammut D, Green B, Lau L, et al. Double-stranded RNA induces disproportionate expression of thymic stromal lymphopoietin versus interferon-β in bronchial epithelial cells from donors with asthma. Thorax 2010;65:626–32. [DOI] [PubMed] [Google Scholar]
- 35.Heymann PW, Platts-Mills TA, Woodfolk JA, Borish L, Murphy DD, Carper HT, et al. Understanding the Asthmatic Response to an Experimental Rhinovirus Infection: Exploring the Effects of Blocking IgE. J Allergy Clin Immunol In press. [DOI] [PMC free article] [PubMed]
- 36.Agrawal R, Wisniewski J, Yu MD, Kennedy JL, Platts-Mills T, Heymann PW, et al. Infection with human rhinovirus 16 promotes enhanced IgE responsiveness in basophils of atopic asthmatics. Clin Exp Allergy. 2014;44:1266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson GG, Dowling HF. Transmission of the common cold to volunteers under controlled conditions. IV. Specific immunity to the common cold. J Clin Invest 1959;38:762–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spergel JM, Fogg MI, Bokszczanin-Knosala A. Correlation of exhaled nitric oxide, spirometry and asthma symptoms. J Asthma 2005;42:879–83. [DOI] [PubMed] [Google Scholar]
- 39.Muehling LM, Mai DT, Kwok WW, Heymann PW, Pomés A, Woodfolk JA. Circulating memory CD4+ T cells target conserved epitopes of rhinovirus capsid proteins and respond rapidly to experimental infection in humans. J Immunol 2016;197:3214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muehling LM, Turner RB, Brown KB, Wright PW, Patrie JT, Lahtinen SJ, et al. Single-cell tracking reveals a role for pre-existing CCR5+ memory TH1 cells in the control of rhinovirus-A39 after experimental challenge in humans. J Infect Dis 2018;217:381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwok WW, Roti M, DeLong JH, Tan V, Wambre E, James EA, et al. Direct ex vivo analysis of allergen-specific CD4+ T cells. J Allergy Clin Immunol 2010;125:1407–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Celada LJ, Kropski JA, Herazo-Maya JD, Luo W, Creecy A, Abad AT, et al. PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci Transl Med 2018;10:eaar8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schluns KS, Lefrançois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol 2003;3:269–79. [DOI] [PubMed] [Google Scholar]
- 44.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu Rev Immunol 2014;32:659–702. [DOI] [PubMed] [Google Scholar]
- 45.Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med 2001;193:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wambre E, Bajzik V, DeLong JH, O’Brien K, Nguyen QA, Speake C, et al. A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gill MA, Liu AH, Calatroni A, Krouse RZ, Shao B, Schiltz A, et al. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J Allergy Clin Immunol 2018;141:1735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geng Y, Liu X, Liang J, Habiel DM, Kulur V, Coelho AL, et al. PD-L1 on invasive fibroblasts drives fibrosis in a humanized model of idiopathic pulmonary fibrosis. JCI Insight 2019;4:e125326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tough DF, Sun S, Sprent J. T cell stimulation in vivo by lipopolysaccharide (LPS). J Exp Med 1997;185:2089–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Unutmaz D, Pileri P, Abrignani S. Antigen-independent activation of naive and memory resting T cells by a cytokine combination. J Exp Med 2004;180:1159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chakir H, Lam DKY, Lemay AM, Webb JR. “Bystander polarization” of CD4+ T cells: Activation with high-dose IL-2 renders naive T cells responsive to IL-12 and/or IL-18 in the absence of TCR ligation. Eur J Immunol 2003;33:1788–98. [DOI] [PubMed] [Google Scholar]
- 52.Avice M-N, Demeure CE, Delespesse G, Rubio M, Armant M, Sarfati M. IL-15 promotes IL-12 production by human monocytes via T cell-dependent contact and may contribute to IL-12-mediated IFN-γ secretion by CD4+ T cells in the absence of TCR ligation. J Immunol 1998;161:3408–15. [PubMed] [Google Scholar]
- 53.Bangs SC, Baban D, Cattan HJ, Li CK-F, McMichael AJ, Xu X-N. Human CD4+ memory T cells are preferential targets for bystander activation and apoptosis. J Immunol 2009;182:1962–71. [DOI] [PubMed] [Google Scholar]
- 54.Randolph DA, Stephens R, Carruthers CJL, Chaplin DD. Cooperation between TH1 and TH2 cells in a murine model of eosinophilic airway inflammation. J Clin Invest 1999;104:1021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen-specific TH1 cells fail to counterbalance TH2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest 1999;103:175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stephens R, Randolph DA, Huang G, Holtzman MJ, Chaplin DD. Antigen-nonspecific recruitment of TH2 cells to the lung as a mechanism for viral infection-induced allergic asthma. J Immunol 2002;169:5458–67. [DOI] [PubMed] [Google Scholar]
- 57.Monto AS, Ullman BM. Acute respiratory illness in an American community: The Tecumseh study. JAMA 1974;227:164–9. [PubMed] [Google Scholar]
- 58.Byington CL, Ampofo K, Stockmann C, Adler FR, Herbener A, Miller T, et al. Community surveillance of respiratory viruses among families in the Utah Better Identification of Germs-Longitudinal Viral Epidemiology (BIG-LoVE) study. Clin Infect Dis 2015;61:1217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wisniewski JA, Muehling LM, Eccles JD, Capaldo BJ, Agrawal R, Shirley DA, et al. TH1 signatures are present in the lower airways of children with severe asthma, regardless of allergic status. J Allergy Clin Immunol 2018;141:2048–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Préfontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, et al. Increased expression of IL-33 in severe asthma: Evidence of expression by airway smooth muscle cells. J Immunol 2009;183:5094–103. [DOI] [PubMed] [Google Scholar]
- 61.Préfontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, et al. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol 2010;125:752–4. [DOI] [PubMed] [Google Scholar]
- 62.Castanhinha S, Sherburn R, Walker S, Gupta A, Bossley CJ, Buckley J, et al. Pediatric severe asthma with fungal sensitization is mediated by steroid-resistant IL-33. J Allergy Clin Immunol 2015;136:312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baumann C, Bonilla WV, Fröhlich A, Helmstetter C, Peine M, Hegazy AN, et al. T-bet-and STAT4-dependent IL-33 receptor expression directly promotes antiviral TH1 cell responses. Proc Natl Acad Sci 2015;112:4056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ying S, O’Connor B, Ratoff J, Meng Q, Fang C, Cousins D, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol 2008;181:2790–8. [DOI] [PubMed] [Google Scholar]
- 65.Fitzpatrick AM, Higgins M, Holguin F, Brown LAS, Teague WG. The molecular phenotype of severe asthma in children. J Allergy Clin Immunol 2010;125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Altman MC, Gill MA, Whalen E, Babineau DC, Shao B, Liu AH, et al. Transcriptome networks identify mechanisms of viral and nonviral asthma exacerbations in children. Nat Immunol 2019; [DOI] [PMC free article] [PubMed]
- 67.Portales-Cervantes L, Crump OM, Dada S, Liwski CR, Gotovina J, Haidl ID, et al. IL-4 Enhances Interferon Production by Virus-Infected Human. J Allergy Clin Immunol In Press. [DOI] [PubMed] [Google Scholar]
- 68.Eccles JD, Turner RB, Kirk NA, Muehling LM, Borish L, Steinke JW, et al. T-bet+ Memory B Cells Link to Local Cross-Reactive IgG upon Human Rhinovirus Infection. Cell Rep 2020;30:351–366.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heymann PW, Platts-Mills TAE, Johnston SL. Role of viral infections, atopy and antiviral immunity in the etiology of wheezing exacerbations among children and young adults. Pediatr Infect Dis J 2005;24:S217–22. [DOI] [PubMed] [Google Scholar]
- 70.Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med 2000;162:2226–31. [DOI] [PubMed] [Google Scholar]
- 71.Jarjour NN, Gern JE, Kelly EAB, Swenson CA, Dick CR, Busse WW. The effect of an experimental rhinovirus 16 infection on bronchial lavage neutrophils. J Allergy Clin Immunol 2000;105:1169–77. [DOI] [PubMed] [Google Scholar]
- 72.Hou W, Kang HS, Kim BS. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J Exp Med 2009;206:313–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.National Heart Lung and Blood Institution. National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. 2007;08–5846.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.