Abstract
Magnetic resonance (MR) imaging studies have demonstrated reduced global and regional brain volumes in infants with congenital heart disease (CHD). This study aimed to provide a more detailed evaluation of altered structural brain development in newborn infants with CHD compared to healthy controls using tensor-based morphometry (TBM). We compared brain development in 64 infants with CHD to 192 age- and sex-matched healthy controls. T2-weighted MR images obtained prior to surgery were analysed to compare voxel-wise differences in structure across the whole brain between groups. Cerebral oxygen delivery (CDO2) was measured in infants with CHD (n = 49) using phase contrast MR imaging and the relationship between CDO2 and voxel-wise brain structure was assessed using TBM. After correcting for global scaling differences, clusters of significant volume reduction in infants with CHD were demonstrated bilaterally within the basal ganglia, thalami, corpus callosum, occipital, temporal, parietal and frontal lobes, and right hippocampus (p < 0.025 after family-wise error correction). Clusters of significant volume expansion in infants with CHD were identified in cerebrospinal fluid spaces (p < 0.025). After correcting for global brain size, there was no significant association between voxel-wise brain structure and CDO2. This study localizes abnormal brain development in infants with CHD, identifying areas of particular vulnerability.
Subject terms: Magnetic resonance imaging, Brain, Congenital heart defects, Paediatric research
Introduction
Congenital heart disease (CHD) is the most common congenital abnormality, with a reported prevalence of over 9 per 1,000 live births worldwide1. As advances in management of CHD lead to improved mortality rates2, morbidity and long-term outcomes have become of increasing concern. Infants and children with CHD may have impaired neurodevelopmental outcomes compared to healthy peers, even in the absence of known genetic syndromes3–10. Impaired neurodevelopment in CHD leads not only to short-term impact on early childhood development, but also long-term impact, including poorer school performance and academic achievement, and an increased need for special education services11–14.
The neurodevelopmental abnormalities observed in survivors of CHD may be related to abnormal brain development. Magnetic resonance (MR) imaging studies have identified brain injury and abnormal brain development in infants with CHD including impaired cortical folding and white and grey matter microstructural abnormalities15–26. In addition, foetuses and infants with CHD have impaired brain growth, including reductions in total and regional brain volumes, compared to healthy controls22–34. These abnormalities are evident prior to surgery and have been associated with reduced cerebral oxygen delivery24,25,33.
Tensor-based morphometry (TBM) is an MR analysis approach that enables the comparison of variation in shape and volume between the brains of individuals on a voxel-wise basis across the whole brain35,36. This method enables increased precision in localizing structural variation between groups compared to methods that involve segmentation of pre-selected brain regions37. Of note, TBM is able to identify structural differences that are independent of global scaling differences, ensuring observed differences do not merely reflect differences in global brain volume and head size.
The primary aim of this study was to assess differences in brain structure between infants with CHD and healthy controls using TBM in order to identify those areas at risk of abnormal development in infants with CHD. Our secondary aim was to assess the relationship between structural brain development at a voxel-wise level and cerebral oxygen delivery (CDO2) in infants with CHD.
Results
Demographics
We studied 64 infants with CHD (35 male) and 192 healthy controls (105 male). There were no significant differences in gestational age (GA) at birth, post-menstrual age (PMA) at scan, sex, birthweight, head circumference at birth, head circumference at scan, or mode of delivery between infants with CHD and healthy controls (Table 1).
Table 1.
Demographic details of infants with congenital heart disease (CHD) and healthy controls.
| Variable | Infants with CHD (n = 64) | Healthy controls (n = 192) | p-value |
|---|---|---|---|
| Gestational age at birth, weeks | 38.57 (35.29–41.57) | 38.86 (35.14–40.43) | 0.06† |
| Post-menstrual age at scan, weeks | 39.29 (36.43–42.29) | 39.43 (36.43–42.86) | 0.23† |
| Male, no. (%) | 35 (55) | 105 (55) | 1.00# |
| Birth weight, kg | 3.11 (1.81–4.29) | 3.11 (1.87–4.80) | 0.37* |
| Birth weight z-score | − 0.83 (− 4.32 to 1.58) | − 0.65 (− 3.57 to 3.12) | 0.23* |
| Birth head circumference, cm | 33.70 (29.00–38.50) | 34.00 (30.00–37.00) | 0.48† |
| Birth head circumference z-score | − 0.60 (− 4.92 to 3.09) | − 0.27 (− 3.96 to 2.75) | 0.24† |
| Head circumference at scan, cm | 34.00 (29.50–37.40) | 34.00 (28.50–37.50) | 0.35† |
| Head circumference at scan z-score | − 0.81 (− 4.60 to 2.15) | − 0.48 (− 5.61 to 2.70) | 0.30† |
| Mode of delivery, no. (%) | |||
| Spontaneous/induced vaginal delivery | 24 (38) | 58 (30) | 0.07# |
| Instrumental delivery | 11 (17) | 38 (20) | |
| Elective C-section | 7 (11) | 48 (25) | |
| Emergency C-section | 22 (34) | 48 (25) | |
| Cardiac diagnosis, no. (%) | |||
| Transposition of the great arteries | 29 (45) | – | – |
| Coarctation of the aorta | 15 (23) | – | – |
| Hypoplastic left heart syndrome | 3 (5) | – | – |
| Pulmonary atresia | 2 (3) | – | – |
| Tricuspid atresia | 2 (3) | – | – |
| Tetralogy of Fallot | 7 (11) | – | – |
| Pulmonary stenosis | 4 (6) | – | – |
| Truncus arteriosus | 1 (2) | – | – |
| Large ventricular septal defect | 1 (2) | – | – |
Values shown are median (range), except where indicated.
*Student’s t-test used.
†Mann–Whitney U test used.
#Chi-squared test used.
Of the 64 infants with CHD, white matter injury was identified in 19 infants and cerebellar haemorrhage was identified in 4 infants on MR imaging (Supplementary Table S1). Sixty of the 64 infants were diagnosed with CHD antenatally.
Tensor-based morphometry
CHD versus healthy controls
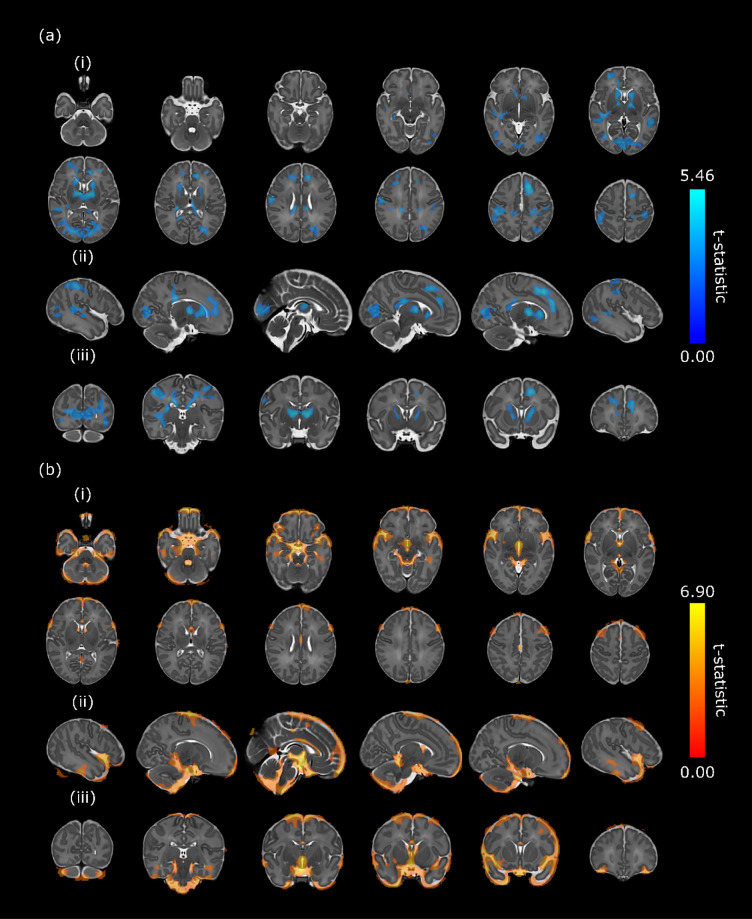
After factoring out global scaling differences, infants with CHD had clusters of significantly reduced volumes within the deep grey matter, corpus callosum, temporal, parietal, occipital and frontal lobes, compared to controls (Fig. 1a). Specifically, significant reductions in volume were identified bilaterally in the caudate nuclei, globus pallidi and anterior and medial thalami; in the right hippocampus; in the isthmus and splenium of the corpus callosum; the posterior cingulate gyri; within the frontal lobes bilaterally; the postcentral gyri; the medial occipital lobes, around the calcarine fissures and along the optic radiations; bilaterally within the middle and posterior parts of the superior temporal gyri and in the posterior part of the medial and inferior temporal gyri.
Figure 1.
Map of t-statistic values of areas of significant (a) reductions and (b) expansions in volumes in infants with congenital heart disease compared to healthy controls (family-wise-error-corrected p < 0.025). Images in 3 planes are shown: (i) axial, (ii) sagittal, (iii) coronal. t-statistic range is shown on the colour bars. Results are overlaid on the template image with post-menstrual age at scan of 40 weeks. Left–right orientation follows radiological convention. Sagittal views are presented from right to left.
There were clusters of significantly expanded volumes in cerebrospinal fluid (CSF) spaces compared to controls (Fig. 1b). Significantly expanded volumes were identified within the cisterna magna, cisterna pontis, and interpenduncular cistern, as well as bilaterally in the ambient cisterns, and extra-axial spaces around the sylvian fissures, frontal lobes, parietal lobes and inferior poles of the temporal lobes. Symmetrical clusters of significant expansions were also found in the third ventricle, fourth ventricle and in the caudothalamic notches bilaterally.
On comparison of total brain and regional brain volumes, we found that infants with CHD had significantly smaller total brain, cortical grey matter, white matter, deep grey matter, and cerebellum volumes than controls (Supplementary Table S2). There was no significant difference in relative regional volumes between groups (Supplementary Table S2). The results of subgroup analysis of abnormal mixing cardiac lesions (n = 31), left-sided cardiac lesions (n = 18), and right-sided cardiac lesions (n = 15) compared to matched controls are included in Supplementary Figure S1.
Brain development and cerebral oxygen delivery
Forty-nine infants with CHD underwent phase contrast flow imaging and cerebral blood flow (CBF) was measured and used to calculate CDO2. The median CBF was 84.58 mL/min with range 45.58–123.16 mL/min and the median CDO2 was 1657 mLO2/min with range 1,106–3,023 mLO2/min. There were no significant differences between infants in whom CDO2 was measured, and those in whom CDO2 was not (Supplementary Table S3). No significant association between voxel-wise brain structure and CDO2 or CBF was found on TBM analysis.
There were no significant differences in CBF or CDO2 between cardiac subgroups (Supplementary Figure S2). A significant positive correlation was observed between CDO2 and total brain, cortical grey matter, and deep grey matter volumes (Supplementary Table S4).
Discussion
This study identified regions of abnormal brain development on preoperative MR imaging in infants with CHD compared to healthy age- and sex-matched controls after taking into account differences in overall brain size between groups. However, after accounting for global scaling, we did not identify any significant associations between CDO2 and structural brain development at a voxel-wise level.
Previous studies have shown reductions in total brain volume, regional brain volumes23–29 and biometric measurements of brain size30,31, and expansions in extra-axial CSF volume24 in infants with CHD prior to surgery. Similar to our previous reports in a subsample of this cohort, we observed smaller total brain, white matter, deep grey matter and regional cortical grey matter volumes in infants with CHD compared to controls24,25. However, we did not observe any significant differences in relative regional volumes between infants with CHD and controls, suggesting similar volume reductions across all brain regions at this coarse level of analysis. Our study used TBM to extend these analyses in order to identify specific locations of altered brain development in infants with CHD, taking into account global size differences, without the need for a priori definition of regions of interest or image segmentation.
In the analysis of different CHD subgroups, we observed different patterns of abnormal brain development in infants with different cardiac physiologies. However, there were no significant differences in measures of cerebral haemodynamics between cardiac subgroups. It is not clear whether the smaller regions of volume differences in the left- and right-sided lesion groups compared to controls are due to cardiac physiology or the small sample sizes of these 2 groups.
Assessing brain development may be useful in understanding the basis of impaired long-term neurodevelopment in survivors of CHD. Previous studies have demonstrated significant associations between brain volume and impaired neurodevelopment in this high-risk group. Reduced subcortical grey matter and increased CSF volumes in the neonatal period are associated with impaired behavioural state regulation and visual orienting in infants with CHD prior to surgery10. In addition, smaller cortical grey matter, white matter, cerebellar28, basal ganglia and thalami, and brainstem38 volumes in newborns with CHD after surgery are associated with poorer cognitive and language outcome scores28 and below-average IQ38 in later infancy28 and childhood38. These relationships persist into adolescence where reduced white matter, cerebellar39, and hippocampal volumes39,40 are associated with impaired total IQ and other measures of cognitive, motor and executive functions39,40.
In this study, we identified significant clusters of reduced volume in regions that are important for cognitive, behavioural and motor function including the caudate nuclei, globus pallidi, thalami, posterior cingulate gyri, frontal lobes, and the hippocampus. The basal ganglia and thalami are associated with cognitive, affective, somatosensory and motor function41,42 and reductions in volume within the thalami and basal ganglia have been linked to adverse neurodevelopment in CHD38 and prematurity43,44. In particular, the anterior thalami, where we localized volume reduction, have been associated with information processing and attention42, memory42,45 and spatial navigation45. The hippocampi play a crucial role in intellectual function and memory46–48 and significant correlations have been observed between hippocampal volume and working memory40, verbal comprehension40, perceptual reasoning39 and total IQ in adolescents with CHD39,40. In addition, we localized volume reductions to the posterior cingulate gyri, regions which are linked to memory49,50, emotional response51, attention52 and spatial orientation49. We also observed clusters of significant reductions in the frontal lobes. The frontal lobes are associated with many higher cognitive functions, including attention, executive function, impulse control, language, and memory53, functions which may be impaired in children with CHD54–56.
We localized significant volume expansions in CSF spaces, including the basal cisterns, third and fourth ventricles and caudothalamic notches bilaterally. Increased CSF volume has been found in children with specific language impairment compared to controls57 and has been linked to autism spectrum disorder in infants at high genetic risk58, and to moderate-severe neurodevelopmental disability59 and decreased working memory performance60 in preterm infants.
There are a number of potential mechanisms underlying abnormal brain development in infants with CHD including altered cerebral haemodynamics24,61–63, reduced substrate delivery to the brain64, genetic factors65–67 and impaired placental development68,69. We previously reported a correlation between CDO2 and preoperative neonatal total brain and cortical grey matter volumes in our CHD cohort24. In this study, we also observed significant associations between CDO2 and total brain, cortical grey matter, and deep grey matter volumes. However, after controlling for global scaling, we did not identify additional clusters of altered development on a voxel-wise level associated with CDO2. This suggests that either (i) reduced CDO2 impairs growth across the whole brain and there are no regions that are specifically vulnerable to low CDO2 or (ii) there was insufficient statistical power to assess voxel-wise differences after correcting for multiple comparisons. Of note, the measurement of CDO2 at a single postnatal timepoint may not reflect the full burden of hypoxia experienced by these infants. Indeed, reduced cerebral oxygenation has been associated with reduced brain volume in utero33. While the reduction of CDO2 is probably global, intrinsic vulnerabilities, such as high metabolic demand, could play a role in the impact on specific brain structures. In other at-risk populations of neonates, such as in those experiencing acute hypoxic-ischaemic events, patterns of injury involving structures with higher metabolic demand, including the basal ganglia and thalami, and hippocampus, have been observed70,71.
However, in this study, we observed clusters of volume reductions, in regions including both the grey and white matter. This is presumably because the effect of chronic hypoxia differs from that of acute events, and different regions of the brain have varying metabolic demands at different stages of development72,73. Furthermore, cerebral autoregulatory mechanisms play an important role61, and different regions of the brain may be affected by different degrees of “brain sparing”74, particularly as the cerebral vasculature is itself developing during mid-late gestation75.
A limitation of this study is that, as yet, we do not have neurodevelopmental outcome data for all of the infants. Further studies are required to examine the relationship between regions of altered brain development identified on voxel-wise analyses and neurodevelopmental outcome.
Conclusions
Using tensor-based morphometry, this study identified clusters of localized volume alterations in regions important for subsequent cognitive, behavioural and motor function in infants with CHD compared to healthy control infants. Whilst cerebral oxygen delivery was significantly associated with total brain and regional brain volumes, no significant relationship was found between cerebral oxygen delivery and voxel-wise brain volume after controlling for global size differences. Assessing brain structural abnormalities using a voxel-wise approach, such as tensor-based morphometry, refines our understanding of abnormal brain development in infants with CHD.
Methods
The project was approved by the National Research Ethics Service West London committee (CHD: 07/H0707/105; Controls: 14/LO/1169). Informed written parental consent was obtained before imaging. All methods and experiments were carried out in compliance with relevant guidelines and regulations.
Participants
A prospective cohort of 74 infants born with critical or serious CHD requiring surgery within one year, based on previously published UK classification76,77, was recruited for MR neuroimaging from the Neonatal Intensive Care Unit at St Thomas’ Hospital, London. Exclusion criteria were known or suspected genetic syndromes, major abnormalities on MR imaging and GA at birth < 35 weeks. Sixty-four infants were included in the analysis (Fig. 2). Healthy controls were obtained from the Developing Human Connectome Project (dHCP) database78 and were matched for GA at birth, PMA at scan, and sex, to each infant in the CHD group in a ratio of three healthy controls per one infant with CHD. Matching of healthy controls to infants with CHD was carried out in R, version 3.2.379, using an automated method based on the daisy dissimilarity matrix calculation25,80.
Figure 2.
Flowchart showing number of cases excluded from further analysis.
MR imaging
High-resolution MR imaging was performed on a Philips Achieva 3-T system (Best, The Netherlands) using a 32-channel neonatal head coil and neonatal positioning device81. T2-weighted, T1-weighted, and diffusion-weighted MR images were acquired using the dHCP protocol optimized for neonatal scanning78,81,82. Phase contrast flow imaging was also acquired for the CHD cases24.
T2-weighted images were used for TBM analysis and were acquired using a multi-slice turbo spin echo sequence in 2 stacks of 2-dimensional slices in sagittal and axial planes, with pulse repetition time 12 s, echo time 156 ms, flip angle 90°, slice thickness 1.6 mm with 0.8 mm overlap, in-plane resolution 0.8 mm × 0.8 mm. Quantitative flow imaging was acquired using velocity-sensitized phase contrast imaging, with a single-slice spoiled gradient echo sequence, with field of view 100 mm × 100 mm, acquisition resolution 0.6 mm × 0.6 mm × 4.0 mm, repetition time 6.4 ms, echo time 4.3 ms, flip angle 10°, 20 cardiac phases, maximal encoding velocity 140 cm/s, and scan time 71 s83. Other imaging parameters have been previously described25. Infants were scanned in natural sleep without sedation, in the presence of a paediatrician experienced in MR imaging procedures. All images were reviewed for abnormalities by a paediatric neuroradiologist.
Infants had physiological monitoring (electrocardiography, respiratory rate, oxygen saturations and temperature) throughout the scan. Ear protection was used to minimize impact of scanner noise: ear plugs moulded from putty (President Putty, Coltene Whaledent, Mahwah, NJ, USA) placed in the external auditory meatus and covered with neonatal earmuffs (MiniMuffs, Natus Medical Inc., San Carlos, CA, USA), with an acoustic foam hood for noise absorption positioned over the infant in the scanner.
Structural image processing
Motion-correction and slice-to-volume image reconstruction were carried out retrospectively using a dedicated algorithm to obtain 0.8 mm3 isotropic T2-weighted images84,85. These were segmented into tissue type (white matter, grey matter, cerebrospinal fluid (CSF), cerebellum, deep grey matter) using a multi-structure expectation–maximization-based segmentation technique in a neonatal-specific automated pipeline described previously86–88.
Cerebral oxygen delivery
For infants with CHD, cerebral blood flow (CBF) was calculated from phase contrast MR imaging acquired in a plane perpendicular to both internal carotids and basilar arteries at the level of the sphenoid bone, using a previously described method24,83. Haemoglobin (Hb) measurements were performed as part of routine clinical care, at a median (range) of 2 (− 1 to 10) days before the scan. Arterial oxygen saturation (SaO2) was measured at the time of scan using a Masimo Radical-7 monitor (Masimo Corp, Irvine, CA) applied to the right hand. Cerebral oxygen delivery (CDO2) was calculated using the following formula89:
where 1.36 is the amount of oxygen bound per gram of haemoglobin at 1 atmosphere (Hüfner’s constant)29.
Tensor-based morphometry
Neonatal templates constructed for each week of gestation created from the dHCP neuroimaging database were used as templates for registration90. Subject T2-weighted images were registered to an age-at-scan-matched template image using the Symmetric Normalization (SyN) algorithm from Advanced Normalization Tools (ANTs), version 3.091. Segmented T2-weighted images were included in the algorithm to improve the quality of registration.
Following rigid and affine transformations of the image, the non-linear transformations from the SyN algorithm were used to create deformation tensor fields within the template space. By using only non-linear transformations, global volume differences are factored out. The resulting tensor fields describing the voxel-wise shape and volume change from the template to each subject image were used to calculate scalar Jacobian determinants, which were subject to logarithm transformation, using ANTs92.
Log-Jacobian determinant maps were smoothed with a sigma of 3.5 mm full width at half maximum Gaussian filter. To facilitate processing, the log-Jacobian determinant maps were re-sized to a voxel size of 1 mm3 isotropic prior to statistical analysis.
Statistical analysis
Clinical data were compared between groups using Student’s t-test or Mann–Whitney U for continuous variables, with prior normality testing using the Shapiro–Wilk test. Chi-squared test was used for categorical variables. A p-value of less than 0.05 was taken as significant. Missing values for clinical data were dealt with through pairwise deletion. Statistical analysis was performed with IBM SPSS Statistics for Windows, version 24 (IBM Corp., Armonk, N.Y., USA). Z-scores for birth weight and head circumference were calculated using the GrowthChartsUK-WHO application, version 2.0.193, based on UK-WHO 2006 population reference data94.
For TBM analysis, the 64 infants with CHD were first compared to age- and sex-matched healthy controls. Voxel-wise t-tests of log-Jacobian determinants between CHD and control groups were carried out using FSL Randomise (FSL, version 6.01)95,96. Threshold-free cluster enhancement was used with a random permutation method with 10 000 permutations, based on a General Linear Model (GLM) matrix95,97. Additionally, for CHD infants with successful phase contrast flow imaging (n = 49), voxel-wise regression analyses of log-Jacobian determinants with CDO2 and CBF were performed. A brain mask for the template image at a PMA of 40-weeks was used in all analyses to include only brain tissue and CSF in the comparisons90. GA at birth and sex were included as nuisance variables in each model. P-values were corrected for multiple comparisons. A family-wise-error-corrected p-value of less than 0.025 was considered significant, to account for testing differences in two directions (i.e. CHD > controls and CHD < controls, or in the case of CDO2, a positive and negative correlation with CDO2).
Further supplementary methods are included within the supplementary information.
Supplementary information
Acknowledgements
We are indebted to the families who supported this study. We thank the staff from the St Thomas’ Neonatal Intensive Care Unit; the Evelina London Children's Hospital Foetal and Paediatric Cardiology Departments; the Evelina London Paediatric Intensive Care Unit; the Centre for the Developing Brain at King's College London; our research radiologists, including Sophie Arulkumaran, Kelly Pegoretti, and Olivia Carney; our research radiographers, including Joanna Allsop, Ana Dos Santos Gomes, and Elaine Green; and our neonatal scanning team including Katy Vecchiato, Claire Caldwell, Julia Wurie, José Bueno Conde, Maryann Sharma, Beatriz Santamaria, Camilla O'Keeffe, and Jacqueline Brandon. This research was funded by the Medical Research Council UK (MR/L011530/1), the British Heart Foundation (FS/15/55/31649), and Action Medical Research (GN2630). This work received funding from the European Research Council under the European Union’s Seventh Framework Program (FP7/20072013)/ERC grant agreement no. 319456 (dHCP project), and was supported by the Wellcome Engineering and Physical Sciences Research Council Centre for Medical Engineering at Kings College London (WT 203148/Z/16/Z), MRC strategic grant (MR/K006355/1), Medical Research Council Centre grant (MR/N026063/1), and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and Kings College London. I.H.X.N. is supported by the NIHR Academic Clinical Fellowship. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Author contributions
I.H.X.N. processed, analysed and interpreted the data and drafted the manuscript, A.F.B. assisted with collection and analysis of the data, C.J.K. collected the data, L.C.-G. developed the motion correction methods and processed the structural images, E.J.H. supervised and conducted image acquisition, A.N.P., J.H. and J.V.H. developed the neonatal-specific MRI sequences, S.V. provided clinical assistance, A.S. and D.R. assisted with analysis of the data, J.S. provided clinical assistance and cardiology input, A.D.E. assisted with interpretation of the data, M.A.R. reviewed all images, D.B. supervised image processing and analysis of the data, S.J.C. supervised imaging, image processing, analysis and interpretation of the data. All authors reviewed and approved the manuscript.
Data availability
Anonymised data pertaining to the Developing Human Connectome Project have been made publicly available at the Developing Human Connectome Project repository and can be accessed at https://www.developingconnectome.org/. The remaining data, analytic methods, and study materials will be available to other researchers for the purposes of reproducing the results or replicating the procedure on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-72009-3.
References
- 1.van der Linde D, et al. Birth prevalence of congenital heart disease worldwide. J. Am. Coll. Cardiol. 2011;58:2241–2247. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122:2254–2263. doi: 10.1161/CIRCULATIONAHA.110.947002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snookes SH, et al. A systematic review of motor and cognitive outcomes after early surgery for congenital heart disease. Pediatrics. 2010;125:e818–827. doi: 10.1542/peds.2009-1959. [DOI] [PubMed] [Google Scholar]
- 4.Mussatto KA, et al. Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics. 2014;133:e570–577. doi: 10.1542/peds.2013-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clancy, T., Jordan, B., de Weerth, C. & Muscara, F. Early emotional, behavioural and social development of infants and young children with congenital heart disease: A systematic review. J. Clin. Psychol. Med. Settings (2019). [DOI] [PubMed]
- 6.Marino BS, et al. Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management. Circulation. 2012;126:1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 7.Latal B. Neurodevelopmental outcomes of the child with congenital heart disease. Clin. Perinatol. 2016;43:173–185. doi: 10.1016/j.clp.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Calderon J, Bellinger DC. Executive function deficits in congenital heart disease: Why is intervention important? Cardiol. Young. 2014;25:1238–1246. doi: 10.1017/S1047951115001134. [DOI] [PubMed] [Google Scholar]
- 9.Bellinger DC. Are children with congenital cardiac malformations at increased risk of deficits in social cognition? Cardiol. Young. 2008;18:3–9. doi: 10.1017/S104795110700176X. [DOI] [PubMed] [Google Scholar]
- 10.Owen M, et al. Brain volume and neurobehavior in newborns with complex congenital heart defects. J. Pediatr. 2014;164:1121–1127.e1. doi: 10.1016/j.jpeds.2013.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farr SL, Downing KF, Riehle-Colarusso T, Abarbanell G. Functional limitations and educational needs among children and adolescents with heart disease. Congenit. Heart Dis. 2018;13:633–639. doi: 10.1111/chd.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulkey SB, et al. Academic proficiency in children after early congenital heart disease surgery. Pediatr. Cardiol. 2014;35:344–352. doi: 10.1007/s00246-013-0781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulkey SB, et al. School-age test proficiency and special education after congenital heart disease surgery in infancy. J. Pediatr. 2016;178:47–54.e1. doi: 10.1016/j.jpeds.2016.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riehle-Colarusso T, et al. Congenital heart defects and receipt of special education services. Pediatrics. 2015;136:496–504. doi: 10.1542/peds.2015-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller SP, et al. Abnormal brain development in newborns with congenital heart disease. N. Engl. J. Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 16.Licht DJ, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–537. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimitropoulos A, et al. Brain injury and development in newborns with critical congenital heart disease. Neurology. 2013;81:241–248. doi: 10.1212/WNL.0b013e31829bfdcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McQuillen PS, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736–741. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 19.Guo T, et al. White matter injury in term neonates with congenital heart diseases: Topology & comparison with preterm newborns. Neuroimage. 2019;185:742–749. doi: 10.1016/j.neuroimage.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulkey SB, et al. White matter injury in newborns with congenital heart disease-A diffusion tensor imaging study. Pediatr. Neurol. 2014;51:377–383. doi: 10.1016/j.pediatrneurol.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortinau C, et al. Cortical folding is altered before surgery in infants with congenital heart disease. J. Pediatr. 2013;163:1507–1510. doi: 10.1016/j.jpeds.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clouchoux C, et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb. Cortex. 2013;23:2932–2943. doi: 10.1093/cercor/bhs281. [DOI] [PubMed] [Google Scholar]
- 23.Claessens NHP, et al. Delayed cortical gray matter development in neonates with severe congenital heart disease. Pediatr. Res. 2016;80:668–674. doi: 10.1038/pr.2016.145. [DOI] [PubMed] [Google Scholar]
- 24.Kelly CJ, et al. Impaired development of the cerebral cortex in infants with congenital heart disease is correlated to reduced cerebral oxygen delivery. Sci. Rep. 2017;7:15088. doi: 10.1038/s41598-017-14939-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly CJ, et al. Abnormal microstructural development of the cerebral cortex in neonates with congenital heart disease is associated with impaired cerebral oxygen delivery. J. Am. Heart Assoc. 2019;8:e009893. doi: 10.1161/JAHA.118.009893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagmann C, Singer J, Latal B, Knirsch W, Makki M. Regional microstructural and volumetric magnetic resonance imaging (MRI) abnormalities in the corpus callosum of neonates with congenital heart defect undergoing cardiac surgery. J. Child Neurol. 2016;31:300–308. doi: 10.1177/0883073815591214. [DOI] [PubMed] [Google Scholar]
- 27.von Rhein M, et al. Severe congenital heart defects are associated with global reduction of neonatal brain volumes. J. Pediatr. 2015;167:1259–1263.e1. doi: 10.1016/j.jpeds.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Meuwly E, et al. Postoperative brain volumes are associated with one-year neurodevelopmental outcome in children with severe congenital heart disease. Sci. Rep. 2019;9:10885. doi: 10.1038/s41598-019-47328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim JM, et al. Cerebral oxygen delivery is reduced in newborns with congenital heart disease. J. Thorac. Cardiovasc. Surg. 2016;152:1095–1103. doi: 10.1016/j.jtcvs.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 30.Ortinau C, et al. Regional alterations in cerebral growth exist preoperatively in infants with congenital heart disease. J. Thorac. Cardiovasc. Surg. 2012;143:1264–1270.e2. doi: 10.1016/j.jtcvs.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortinau C, et al. Congenital heart disease affects cerebral size but not brain growth. Pediatr. Cardiol. 2012;33:1138–1146. doi: 10.1007/s00246-012-0269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Limperopoulos C, et al. Brain volume and metabolism in fetuses with congenital heart disease: Evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun L, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. 2015;131:1313–1323. doi: 10.1161/CIRCULATIONAHA.114.013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andescavage N, et al. 3-D volumetric MRI evaluation of the placenta in fetuses with complex congenital heart disease. Placenta. 2015;36:1024–1030. doi: 10.1016/j.placenta.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashburner J, Friston KJ. Voxel-based morphometry - The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 36.Ashburner J, et al. Identifying global anatomical differences: Deformation-based morphometry. Hum. Brain Mapp. 1998;6:348–357. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<348::AID-HBM4>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanson JL, et al. Early stress is associated with alterations in the orbitofrontal cortex: A tensor-based morphometry investigation of brain structure and behavioral risk. J. Neurosci. 2010;30:7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Claessens NHP, et al. Perioperative neonatal brain injury is associated with worse school-age neurodevelopment in children with critical congenital heart disease. Dev. Med. Child Neurol. 2018;60:1052–1058. doi: 10.1111/dmcn.13747. [DOI] [PubMed] [Google Scholar]
- 39.Von Rhein M, et al. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain. 2014;137:268–276. doi: 10.1093/brain/awt322. [DOI] [PubMed] [Google Scholar]
- 40.Latal B, et al. Hippocampal volume reduction is associated with intellectual functions in adolescents with congenital heart disease. Pediatr. Res. 2016;80:531–537. doi: 10.1038/pr.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arsalidou M, Duerden EG, Taylor MJ. The centre of the brain: Topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Hum. Brain Mapp. 2013;34:3031–3054. doi: 10.1002/hbm.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fama R, Sullivan EV. Thalamic structures and associated cognitive functions: Relations with age and aging. Neurosci. Biobehav. Rev. 2015;54:29–37. doi: 10.1016/j.neubiorev.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boardman JP, et al. A common neonatal image phenotype predicts adverse neurodevelopmental outcome in children born preterm. Neuroimage. 2010;52:409–414. doi: 10.1016/j.neuroimage.2010.04.261. [DOI] [PubMed] [Google Scholar]
- 44.Loh WY, et al. Neonatal basal ganglia and thalamic volumes: Very preterm birth and 7-year neurodevelopmental outcomes. Pediatr. Res. 2017;82:970–978. doi: 10.1038/pr.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jankowski, M. M. et al. The anterior thalamus provides a subcortical circuit supporting memory and spatial navigation. Front. Syst. Neurosci.7 (2013). [DOI] [PMC free article] [PubMed]
- 46.Isaacs, Elizabeth B Lucas, A. et al. Hippocampal volume and everyday memory in children of very low birth weight. Pediatr. Res.47, 713–720 (2000). [DOI] [PubMed]
- 47.Isaacs EB, et al. Brain morphometry and IQ measurements in preterm children. Brain. 2004;127:2595–2607. doi: 10.1093/brain/awh300. [DOI] [PubMed] [Google Scholar]
- 48.Shohamy D, Turk-Browne NB. Mechanisms for widespread hippocampal involvement in cognition. J. Exp. Psychol. Gen. 2013;142:1159–1170. doi: 10.1037/a0034461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beckmann M, Johansen-Berg H, Rushworth MFS. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J. Neurosci. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- 51.Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum. Brain Mapp. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chayer C, Freedman M. Frontal lobe functions. Curr. Neurol. Neurosci. Rep. 2001;1:547–552. doi: 10.1007/s11910-001-0060-4. [DOI] [PubMed] [Google Scholar]
- 54.Miatton M, De Wolf D, François K, Thiery E, Vingerhoets G. Neuropsychological performance in school-aged children with surgically corrected congenital heart disease. J. Pediatr. 2007;151:73–78. doi: 10.1016/j.jpeds.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 55.Cassidy AR, White MT, DeMaso DR, Newburger JW, Bellinger DC. Executive function in children and adolescents with critical cyanotic congenital heart disease. J. Int. Neuropsychol. Soc. 2015;21:34–49. doi: 10.1017/S1355617714001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howell HB, et al. Neurodevelopmental outcomes of children with congenital heart disease: A review. Curr. Probl. Pediatr. Adolesc. Health Care. 2019;49:100685. doi: 10.1016/j.cppeds.2019.100685. [DOI] [PubMed] [Google Scholar]
- 57.Girbau-Massana D, Garcia-Marti G, Marti-Bonmati L, Schwartz RG. Gray-white matter and cerebrospinal fluid volume differences in children with specific language impairment and/or reading disability. Neuropsychologia. 2014;56:90–100. doi: 10.1016/j.neuropsychologia.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Shen MD, et al. Increased extra-axial cerebrospinal fluid in high-risk infants who later develop autism. Biol Psychiatry. 2017;82:186–193. doi: 10.1016/j.biopsych.2017.02.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inder TE, Warfield SK, Wang H, Hüppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 60.Woodward LJ, Edgin JO, Thompson D, Inder TE. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain. 2005;128:2578–2587. doi: 10.1093/brain/awh618. [DOI] [PubMed] [Google Scholar]
- 61.Donofrio MT, et al. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: The brain sparing effect. Pediatr. Cardiol. 2003;24:436–443. doi: 10.1007/s00246-002-0404-0. [DOI] [PubMed] [Google Scholar]
- 62.Kaltman JR, Di H, Tian Z, Rychik J. Impact of congenital heart disease on cerebrovascular blood flow dynamics in the fetus. Ultrasound Obstet. Gynecol. 2005;25:32–36. doi: 10.1002/uog.1785. [DOI] [PubMed] [Google Scholar]
- 63.Modena A, et al. Fetuses with congenital heart disease demonstrate signs of decreased cerebral impedance. Am. J. Obstet. Gynecol. 2006;195:706–710. doi: 10.1016/j.ajog.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 64.Rudolph AM. Impaired cerebral development in fetuses with congenital cardiovascular malformations: Is it the result of inadequate glucose supply? Pediatr. Res. 2016;80:172–177. doi: 10.1038/pr.2016.65. [DOI] [PubMed] [Google Scholar]
- 65.Russell MW, Chung WK, Kaltman JR, Miller TA. Advances in the understanding of the genetic determinants of congenital heart disease and their impact on clinical outcomes. J. Am. Heart Assoc. 2018;7:e006906. doi: 10.1161/JAHA.117.006906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Homsy J, et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science. 2015;350:1262–1266. doi: 10.1126/science.aac9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruneau BG. The developmental genetics of congenital heart disease. Nature. 2008;451:943–948. doi: 10.1038/nature06801. [DOI] [PubMed] [Google Scholar]
- 68.Schlatterer SD, et al. Placental pathology and neuroimaging correlates in neonates with congenital heart disease. Sci. Rep. 2019;9:4137. doi: 10.1038/s41598-019-40894-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matthiesen NB, et al. Congenital heart defects and indices of placental and fetal growth in a nationwide study of 924 422 liveborn infants. Circulation. 2016;134:1546–1556. doi: 10.1161/CIRCULATIONAHA.116.021793. [DOI] [PubMed] [Google Scholar]
- 70.Okereafor A, et al. Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics. 2008;121:906–914. doi: 10.1542/peds.2007-0770. [DOI] [PubMed] [Google Scholar]
- 71.Mañeru C, et al. Residual hippocampal atrophy in asphyxiated term neonates. J. Neuroimaging. 2003;13:68–74. [PubMed] [Google Scholar]
- 72.Kinnala A, et al. Cerebral metabolic rate for glucose during the first six months of life: An FDG positron emission tomography study. Arch. Dis. Child. Fetal Neonatal Ed. 1996;74:F153–F157. doi: 10.1136/fn.74.3.f153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abrams RM, et al. Local cerebral glucose utilization in fetal and neonatal sheep. Am. J. Physiol. 1984;246:R608–R618. doi: 10.1152/ajpregu.1984.246.4.R608. [DOI] [PubMed] [Google Scholar]
- 74.Dubiel M, Gunnarsson GÖ, Gudmundsson S. Blood redistribution in the fetal brain during chronic hypoxia. Ultrasound Obstet. Gynecol. 2002;20:117–121. doi: 10.1046/j.1469-0705.2002.00758.x. [DOI] [PubMed] [Google Scholar]
- 75.Kuban KC, Gilles FH. Human telencephalic angiogenis. Ann. Neurol. 1985;17:539–548. doi: 10.1002/ana.410170603. [DOI] [PubMed] [Google Scholar]
- 76.Kelly CJ, et al. Neuroimaging findings in newborns with congenital heart disease prior to surgery: An observational study. Arch. Dis. Child. 2019;104:1042–1048. doi: 10.1136/archdischild-2018-314822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ewer AK, et al. Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): A test accuracy study. Lancet. 2011;378:785–794. doi: 10.1016/S0140-6736(11)60753-8. [DOI] [PubMed] [Google Scholar]
- 78.The Developing Human Connectome Project. (2019). https://www.developingconnectome.org/.
- 79.R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, Vienna, 2015). https://www.r-project.org/.
- 80.Kaufman, L. & Rousseeuw, P. J. Finding Groups in Data: An Introduction to Cluster Analysis. (Wiley, 1990).
- 81.Hughes EJ, et al. A dedicated neonatal brain imaging system. Magn. Reson. Med. 2017;78:794–804. doi: 10.1002/mrm.26462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hutter J, et al. Time-efficient and flexible design of optimized multishell HARDI diffusion. Magn. Reson. Med. 2018;79:1276–1292. doi: 10.1002/mrm.26765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Varela M, Groves AM, Arichi T, Hajnal JV. Mean cerebral blood flow measurements using phase contrast MRI in the first year of life. NMR Biomed. 2012;25:1063–1072. doi: 10.1002/nbm.2771. [DOI] [PubMed] [Google Scholar]
- 84.Cordero-Grande L, Hughes EJ, Hutter J, Price AN, Hajnal JV. Three-dimensional motion corrected sensitivity encoding reconstruction for multi-shot multi-slice MRI: Application to neonatal brain imaging. Magn. Reson. Med. 2018;79:1365–1376. doi: 10.1002/mrm.26796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuklisova-Murgasova M, Quaghebeur G, Rutherford MA, Hajnal JV, Schnabel JA. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Med. Image Anal. 2012;16:1550–1564. doi: 10.1016/j.media.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Makropoulos A, et al. Automatic whole brain MRI segmentation of the developing neonatal brain. IEEE Trans. Med. Imaging. 2014;33:1818–1831. doi: 10.1109/TMI.2014.2322280. [DOI] [PubMed] [Google Scholar]
- 87.Makropoulos A, et al. Regional growth and atlasing of the developing human brain. Neuroimage. 2016;125:456–478. doi: 10.1016/j.neuroimage.2015.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Makropoulos A, et al. The developing human connectome project: A minimal processing pipeline for neonatal cortical surface reconstruction. Neuroimage. 2018;173:88–112. doi: 10.1101/125526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McLellan, S. A. & Walsh, T. S. Oxygen delivery and haemoglobin. Contin. Educ. Anaesth. Crit. Care Pain4, 123–126 (2004).
- 90.Andreas Schuh et al. Unbiased construction of a temporally consistent morphological atlas of neonatal brain development. bioRxiv (2018). 10.1101/251512
- 91.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Avants B, Gee JC. Geodesic estimation for large deformation anatomical shape averaging and interpolation. Neuroimage. 2004;23:S139–150. doi: 10.1016/j.neuroimage.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 93.Kelly, C. Growth Charts UK-WHO. (2017).
- 94.World Health Organisation. WHO Child Growth Standards. (2006). www.who.int/childgrowth/en.
- 95.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 97.Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymised data pertaining to the Developing Human Connectome Project have been made publicly available at the Developing Human Connectome Project repository and can be accessed at https://www.developingconnectome.org/. The remaining data, analytic methods, and study materials will be available to other researchers for the purposes of reproducing the results or replicating the procedure on reasonable request.