Abstract
Medial patellofemoral ligament (MPFL) reconstruction is currently the primary surgical procedure for treating recurrent lateral patellar instability. The understanding of graft function has largely been based on studies performed with normal knees. The current study was performed to characterize graft function following MPFL reconstruction, focusing on the influence of pathologic anatomy on graft tension, variations with knee flexion, and the influence on patellar tracking. Knee squatting was simulated with fifteen multibody dynamic simulation models representing knees being treated for recurrent lateral patellar instability. Squatting was simulated in a pre-operative condition and following MPFL reconstruction with a hamstrings tendon graft set to allow 0.5 quadrants of lateral patellar translation with the knee at 30° of flexion. Linear regressions were performed to relate maximum tension in the graft to parameters of knee anatomy. Repeated measures comparisons evaluated variations in patellar tracking at 5° increments of knee flexion. Maximum graft tension was significantly correlated with a parameter characterizing lateral position of the tibial tuberosity (maximum lateral tibial tuberosity to posterior cruciate ligament attachment distance, r2 = 0.73, p < 0.001). No significant correlations were identified for parameters related to trochlear dysplasia (lateral trochlear inclination) or patella alta (Caton-Deschamps index, patellotrochlear index). Graft tension peaked at low flexion angles and was minimal by 30° of flexion. MPFL reconstruction decreased lateral patellar shift (bisect offset index) compared to pre-operative tracking at all flexion angles from 0° to 50° of flexion, except 45°. At 0°, the average bisect offset index decreased from 0.81 for the pre-operative condition to 0.71. The results indicate that tension within an MPFL graft increases with the lateral position of the tibial tuberosity. The graft tension peaks at low flexion angles and decreases lateral patellar maltracking. The factors that influence graft function following MPFL reconstruction need to be understood to limit patellar maltracking without overloading the graft or over constraining the patella.
Keywords: patellar instability, MPFL reconstruction, patellar tracking, computational simulation
Introduction
Medial patellofemoral ligament (MPFL) reconstruction is currently the primary surgical procedure for treating recurrent lateral patellar instability1,2. While rates of recurrent instability following MPFL reconstruction are generally reported at less than 4%2,3, rates as high as 8% to 20% have also been reported4–8. Poor outcomes following MPFL reconstruction are typically attributed to anatomical risk factors, in particular, trochlear dysplasia or a laterally positioned tibial tuberosity4,9–13. A non-anatomic MPFL graft attachment on the femur and over-tensioning of the graft have been reported to contribute to poor outcomes11. To limit the risk of over-tensioning, the graft length is typically set to allow the patella to glide laterally within the trochlear groove14,15.
Numerous biomechanical studies have evaluated the function of MPFL grafts. Studies utilizing in vitro and computational simulation of knee function have indicated that MPFL reconstruction limits lateral patellar tracking with respect to the femur16–20, although measures of patellar tracking relative to the trochlear groove would be more valuable for clinical interpretation. Trochlear dysplasia and a lateral position of the tibial tuberosity have been shown to increase lateral patellar tracking following MPFL reconstruction21,22, but the influence of knee pathology on graft function has yet to be thoroughly characterized. Computational simulation of knee function has also indicated that tension within an MPFL graft peaks at low flexion angles, with minimal graft tension beyond 30° of flexion17,18, but other studies focused on variations in graft tension with knee flexion angle have been inconsistent. The path length between standardized graft attachment points on the femur and patella has been measured in vitro as an indicator of graft tension. Some studies indicated an MPFL graft would be nearly isometric during function23– 26. Others indicated graft tension would be expected to decrease27 or increase28 with knee flexion. Similar studies using computational models reconstructed from imaging data of subjects indicated that graft tension would be expected to decrease with flexion29, decrease in early flexion followed by an increase30, or increase in early flexion followed by a decrease31. The studies based on path length between graft attachment points did not account for all of the anatomical factors that contribute to patellar instability or patellar glide allowed intra-operatively.
The current study was performed to provide a more thorough characterization of graft function following MPFL reconstruction for recurrent patellar instability than is currently available. The study focuses on the influence of pathologic anatomy on graft tension, variations with knee flexion, and the influence of a graft on patellar tracking relative to the trochlear groove. The study is based on multibody dynamic simulation of knee function with computational models representing knees being treated for recurrent patellar instability.
Methods
Knee function was simulated with computational models representing the symptomatic knees of fifteen subjects being treated for recurrent lateral patellar instability. The subjects ranged in age from 12 to 34 years (mean = 19 years). One of the subjects had previously been treated with a lateral release and medial imbrication that subsequently failed, and one had previously been treated with tibial tuberosity realignment prior to the latest instability episode. The institutional review boards of the two treating institutions (Johns Hopkins University, Akron Children’s Hospital) provided approval for the study.
The accuracy of simulated patellar tracking was characterized previously for the original nine models. For each model, a functional activity performed by the corresponding subject during a diagnostic scan was simulated. The root mean square errors between simulated patellar kinematics and kinematics measured from the subjects over all flexion angles were 2.7 mm and 3.7° for lateral patellar shift and tilt, respectively17. The surgical procedure performed on each subject (MPFL reconstruction or tibial tuberosity realignment) was also represented with each model to simulate a post-operative functional activity performed by the corresponding subject. For the subject-derived kinematics, the average (± standard deviation) patellar lateral shift and tilt decreased 1.4 ± 3.8 mm and 2.5° ± 4.1°, respectively, from the pre-operative to post-operative condition, compared to 2.4 ± 4.8 mm and 2.3° ± 5.9° for the simulated motions17.
Computational Reconstruction of Knee Motion for Accuracy Assessment
The accuracy assessment was expanded to include the six new models developed for the current study. Seven of the fifteen subjects performed knee extension against gravity within a dynamic CT scanner (Aquilion ONE, Canon Medical Systems, Ōtawara, Japan)32,33. The other eight subjects positioned the injured knee at multiple positions of flexion within an MRI scanner (Magnetom Skyra, Siemens Healthcare GmbH, Erlangen, Germany), with quadriceps activation induced by a loading frame applying an external force at the foot34. The self-selected force that subjects could resist during the scan (approximately one minute) without pain, apprehension, or movement ranged from 60 to 85 N.
Post-operative imaging was not available for the six models added to the current study, so the accuracy assessment was only updated for pre-operative patellar tracking. For five subjects, the scans were performed for clinical evaluation (dynamic CT of knee extension plus an MRI scan), with the imaging retrospectively utilized to develop models. One subject prospectively participated in isometric knee extension at multiple flexion angles within an MRI scanner, but was lost to follow up.
A computational model of each knee was reconstructed (3D Doctor, Able Software Corp, Lexington, MA and Mimics, Materialise, Leuven, Belgium) from an MRI scan of the extended and unloaded knee (fourteen 3.0 T and one 1.5 T, proton density weighted, slice thickness ranging from 0.5 mm to 3.0 mm). The femur, tibia and patella were also reconstructed at 3 to 5 positions spanning the flexion range from the CT or MRI scans acquired during knee function. For each subject, a single model of each bone was shape matched to all flexed positions using an iterative closest point algorithm35. Anatomical coordinate systems were created for the femur, patella and tibia32 to characterize knee kinematics based on the floating axis convention36.
Simulation of Knee Motion
The multibody dynamic simulation models (RecurDyn, FunctionBay, Seongnam, Korea) and representation of knee function have been described in detail previously17,18,21,37. Bones, cartilage surfaces and soft tissue attachment points were reconstructed from the unloaded MRI scans (Fig. 1). The femur, tibia, and patella were represented as rigid bodies. Simplified Hertzian contact determined the reaction forces at the articulating cartilage surfaces. Tension-only springs with properties assigned for stiffness, damping, and pre-strain at full extension represented bundles of the medial and lateral collateral ligaments (3 each), anterior and posterior cruciate ligaments (2 each), patellar tendon (5), posterior capsule (4), lateral retinaculum (2), and medial retinaculum minus the native MPFL (1). Quadriceps forces were applied through springs representing the quadriceps tendon and divided among the vastus medialis obliquus, vastus lateralis, and combination of the vastus intermedius, rectus femoris, and vastus medialis longus. A weak vastus medialis obliquus was represented by applying 5% of the total quadriceps force based on subjects with lateral patellar malalignment38.
Figure 1.
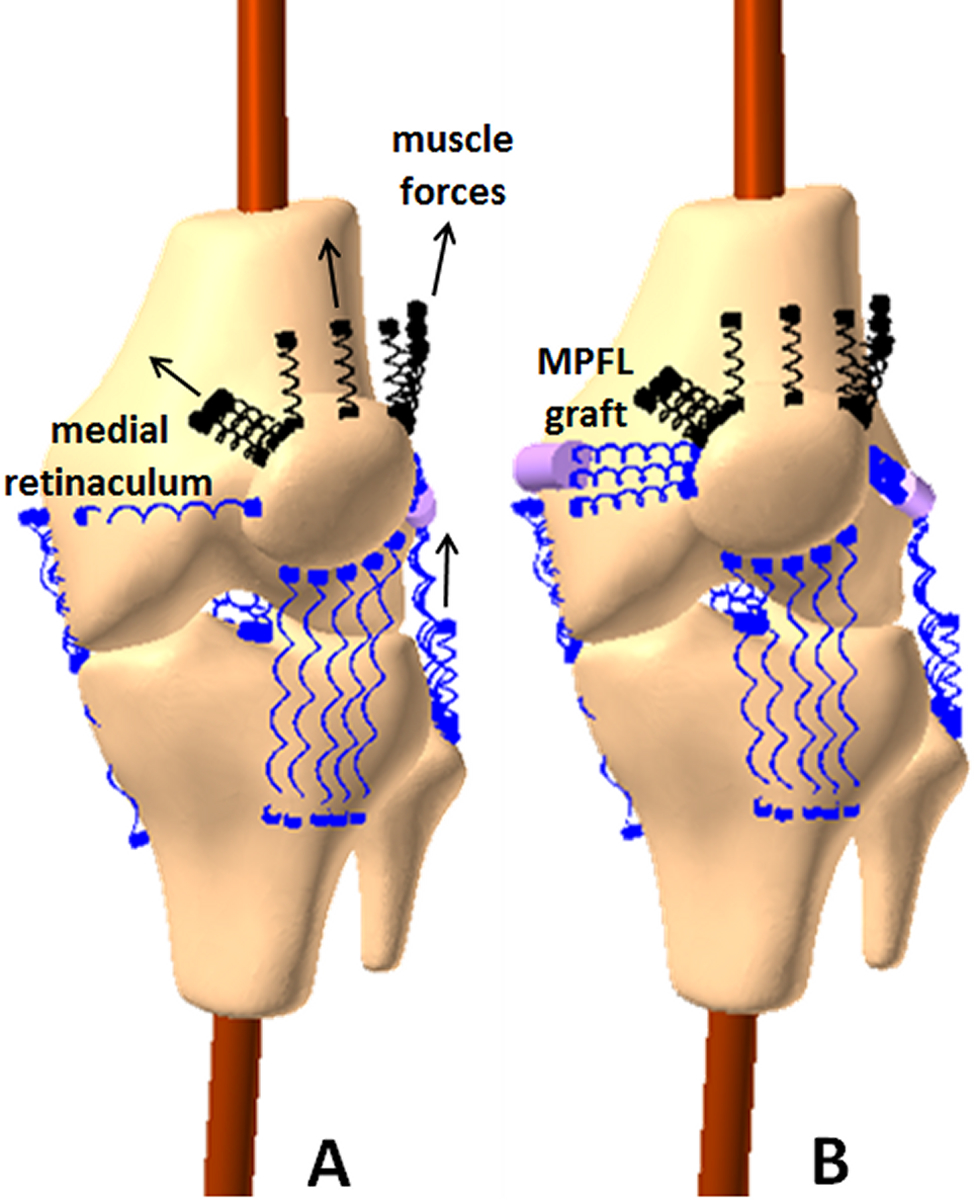
Computational model for multibody dynamic simulation for one knee. The model is shown for the pre-operative condition (A) and for the post-operative condition with an MPFL graft (B) at 15° of flexion.
For the accuracy assessment, the motion each subject performed within a scanner was simulated. For the models of subjects who performed dynamic knee extension against gravity, the femur was fixed in space, and mass was added to the tibia to represent the moment of the lower limb about the flexion axis18. A quadriceps loading profile that initiated and maintained extension from maximum flexion in the CT scanner (approximately 50°) to 0° was applied. For the models of subjects who performed isometric knee extension, the models were set at each flexion angle from the MRI scans. The femur was fixed in place, and a force was applied proximally at the foot to match the force applied to each subject. The quadriceps force that maintained the flexion angle was applied17. Knee kinematics were quantified using the same anatomical coordinate systems used for computational reconstruction of the functional activities performed by the subjects for calculation of root mean square errors with respect to measurements from the subjects.
Function of MPFL grafts was assessed while simulating a dual limb knee squat with all fifteen models. Three rotational degrees of freedom were allowed at the ankle and flexion/extension, varus/valgus rotation, and superior/inferior translation allowed at the hip. The total quadriceps force increased from 42 N at full extension to 300 N at 50° of flexion. Medial and lateral hamstrings forces combined to apply one-third of the total quadriceps force. Body weight of 200 N was represented. A hip flexion moment applied over the first few degrees of flexion initiated the squatting motion.
Squatting was simulated for the pre-operative condition and for simulated MPFL reconstruction. The Schöttle point was identified on each femur to represent the intra-operative target for femoral attachment of the graft39 (Fig. 1). The patellar attachment was positioned between the medial edge of the vastus medialis obliquus attachment and the medial edge of the patella. The grafts wrapped around the femoral condyle, with the portion from the femoral attachment to the wrapping surface represented by a rigid cylinder. The graft resting length was set while allowing 0.5 quadrants of patellar lateral glide with the knee at 30° of flexion. A quadrant represents one-quarter of the medial-lateral width of the patella. A previous simulation study that compared multiple tensioning protocols found this to provide the most resistance to lateral patellar maltracking without over constraining the patella17. A dual strand gracilis tendon graft for MPFL reconstruction was represented by two springs with a total stiffness of 20 N/mm40. The total force within the two springs, or graft tension, was quantified throughout the motion.
Measures of patellar tracking and anatomy were quantified throughout the full range of knee flexion based on landmarks identified on the models (Fig. 2) with automated algorithms (Matlab, Mathworks, Natick, MA). Anatomical measures were based on parameters previously correlated with patellar tracking for computational reconstruction of function and simulation of function21,33,34, and parameters commonly used for clinical evaluation. Patellar tracking was based on measures used for assessment of patellar position with respect to the trochlear groove. Patellar tracking was characterized based on the bisect offset index, equal to the ratio of the patellar width lateral to the deepest point of the groove to the total width. The lateral direction was determined by the posterior condylar axis of the femur. The lateral position of the tibial tuberosity was quantified based on the lateral tibial tuberosity to posterior cruciate ligament attachment (TT-PCL) distance, which is the lateral distance from the patellar tendon attachment on the tibial tuberosity to the medial border of the posterior cruciate ligament attachment on the tibia. Trochlear dysplasia was based on the lateral trochlear inclination, the maximum slope of the lateral ridge of the trochlear groove with respect to the femoral posterior condylar axis. Patellar tracking, tibial tuberosity position and trochlear dysplasia measurements were made within a plane normal to the long axis of the patella. Identification of the deepest point of the trochlear groove and measurement of the lateral trochlear inclination occurred within an axial slice of the model including the most anterior point of the lateral ridge of the groove. Patella alta was characterized by the Caton-Deschamps index, the ratio of the distance from the distal point of the patellar cartilage to the anterior–superior border of the tibia to the articular length along the patella. Patellotrochlear index41, a ratio of the articular overlap between the patellar and trochlear cartilage to the total length of the patellar cartilage, was also quantified to characterize patella alta with a parameter more closely related to articulation of the patella within the trochlear groove. The measures of patella alta were made with the landmarks projected onto a sagittal plane.
Figure 2.
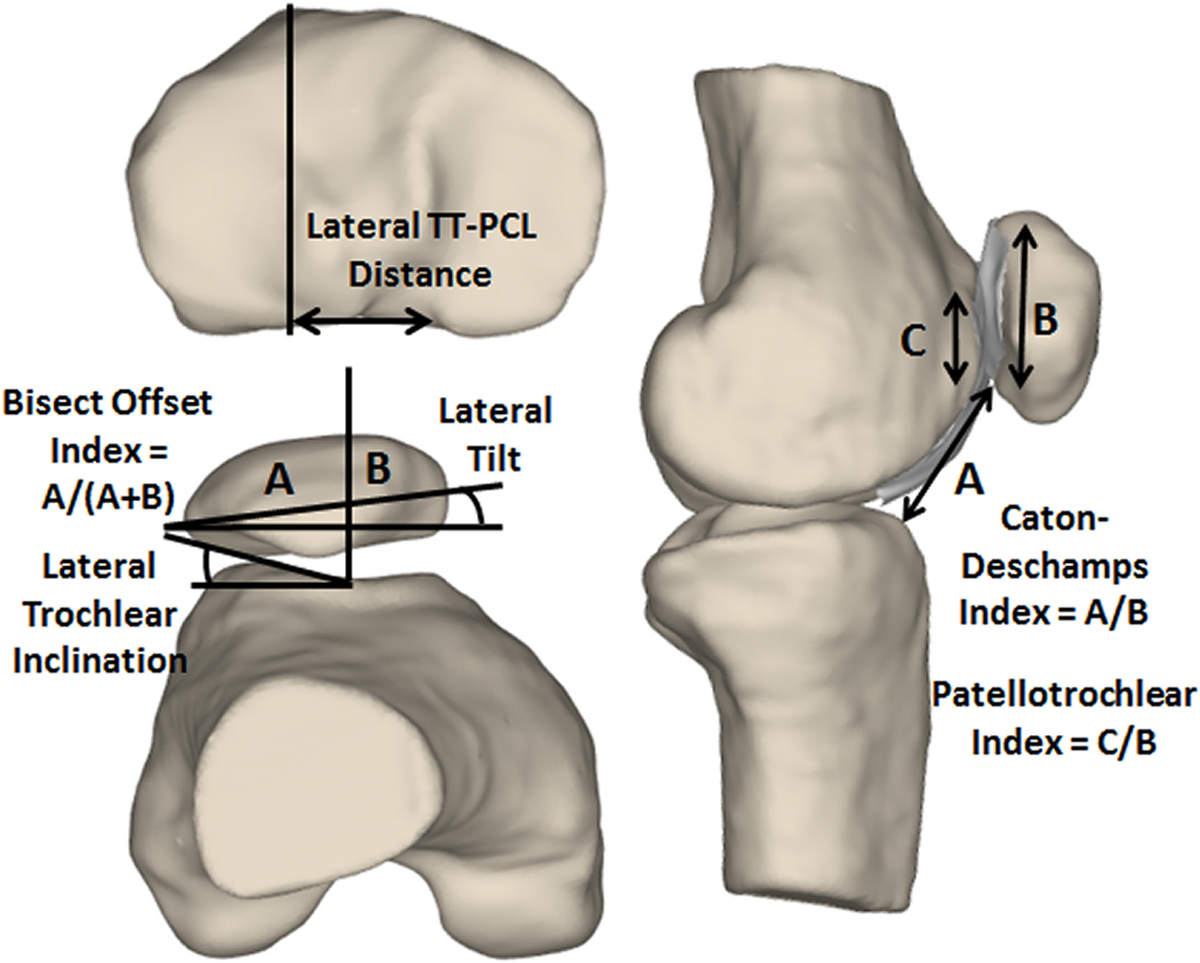
Measurements from the computational models to characterize patellar tracking (bisect offset index and lateral tilt), trochlear dysplasia (lateral trochlear inclination), tibial tuberosity position (lateral TT-PCL distance), and patella alta (Caton-Deschamps index and patellotrochlear index).
Statistical Analysis
Statistical analysis focused on correlations between anatomical parameters and graft tension and changes in patellar tracking following MPFL reconstruction. Step-wise multivariable linear regression was used to correlate graft tension with measures of anatomy. Statistical significance was set at p < 0.05, with variables included in the regression for p < 0.10. The correlations focused on maximum graft tension, with comparisons to the most pathologic condition of each anatomical parameter throughout the range of flexion: maximum lateral TT-PCL distance, minimum lateral trochlear inclination, maximum Caton-Deschamps index, and minimum patellotrochlear index. At 5° intervals of knee flexion, bisect offset index and lateral patellar tilt were compared between the pre-operative and post-operative conditions. For each comparison, normal distribution of the standardized residuals was evaluated with Shapiro-Wilk tests. Comparisons were performed with a paired t-test or a Wilcoxon signed rank test, as appropriate based on the tests for normality.
Results
For all fifteen models, the root mean square errors for comparisons between the simulated patellar kinematics and kinematics measured from reconstruction of function from the subjects were similar to the previously reported values for nine models. The root mean square errors over all flexion angles were 4.0° and 2.5 mm for patellar tilt and lateral shift, respectively.
The maximum tension within an MPFL graft was correlated with the maximum lateral TT-PCL distance. The ranges for the maximum lateral TT-PCL distance, minimum lateral trochlear inclination, maximum Caton-Deschamps index, and minimum patellotrochlear index were 13 to 31 mm, 3° to 20°, 0.8 to 1.5, and 0.0 to 0.7, respectively. The maximum graft tension was significantly correlated with the maximum lateral TT-PCL distance (r2 = 0.73, p < 0.001, Fig. 3). The maximum Caton-Deschamps index, minimum lateral trochlear inclination, and minimum patellotrochlear index were not significantly correlated with maximum graft tension in univariate regressions (p > 0.05) or maintained within multivariate regressions (p > 0.10). Tension within the MPFL grafts was largest at low flexion angles and was minimal by 30° of flexion (Fig. 4).
Figure 3.
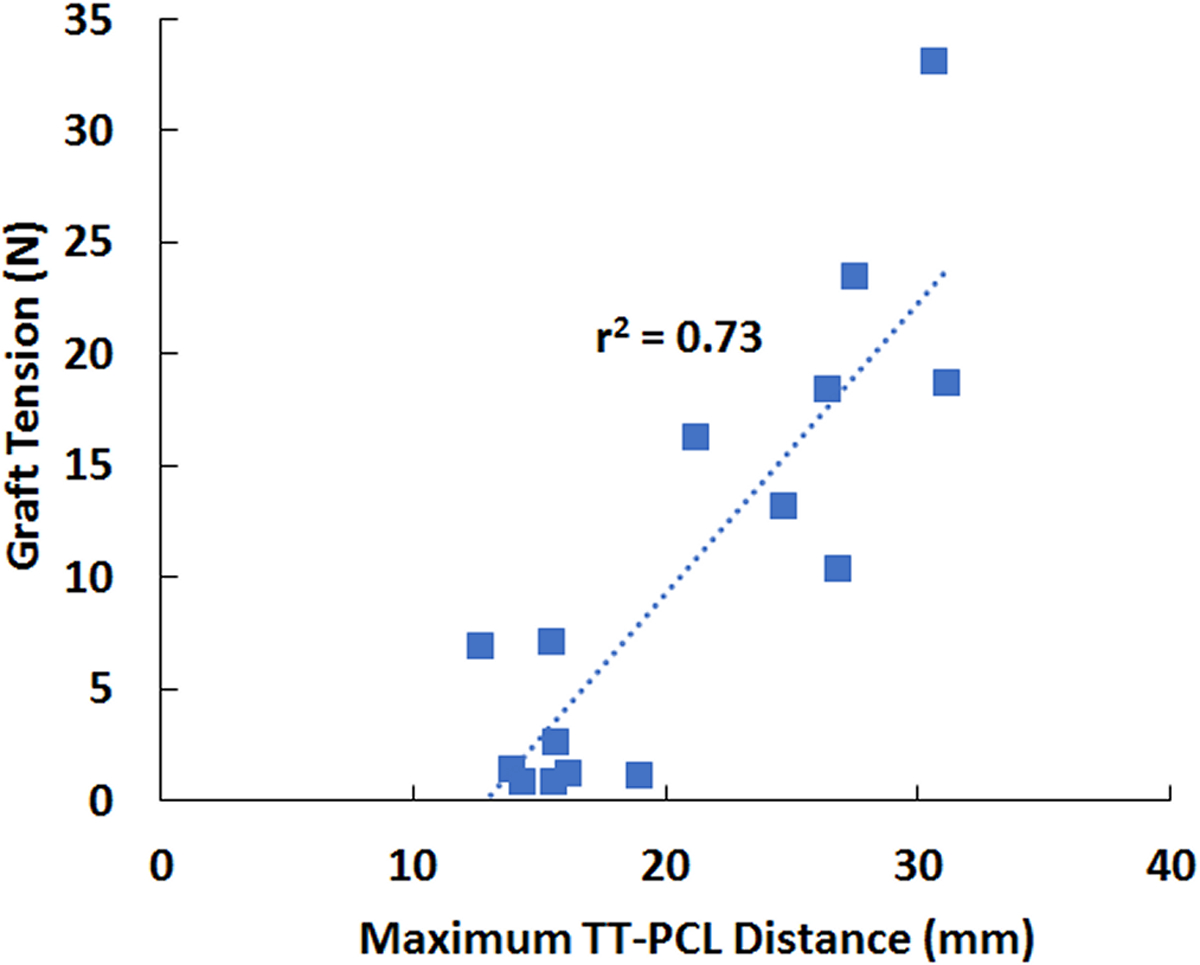
MPFL graft tension vs. maximum lateral TT-PCL distance for all 15 computational models. The best fit line and the square of the correlation coefficient are shown.
Figure 4.
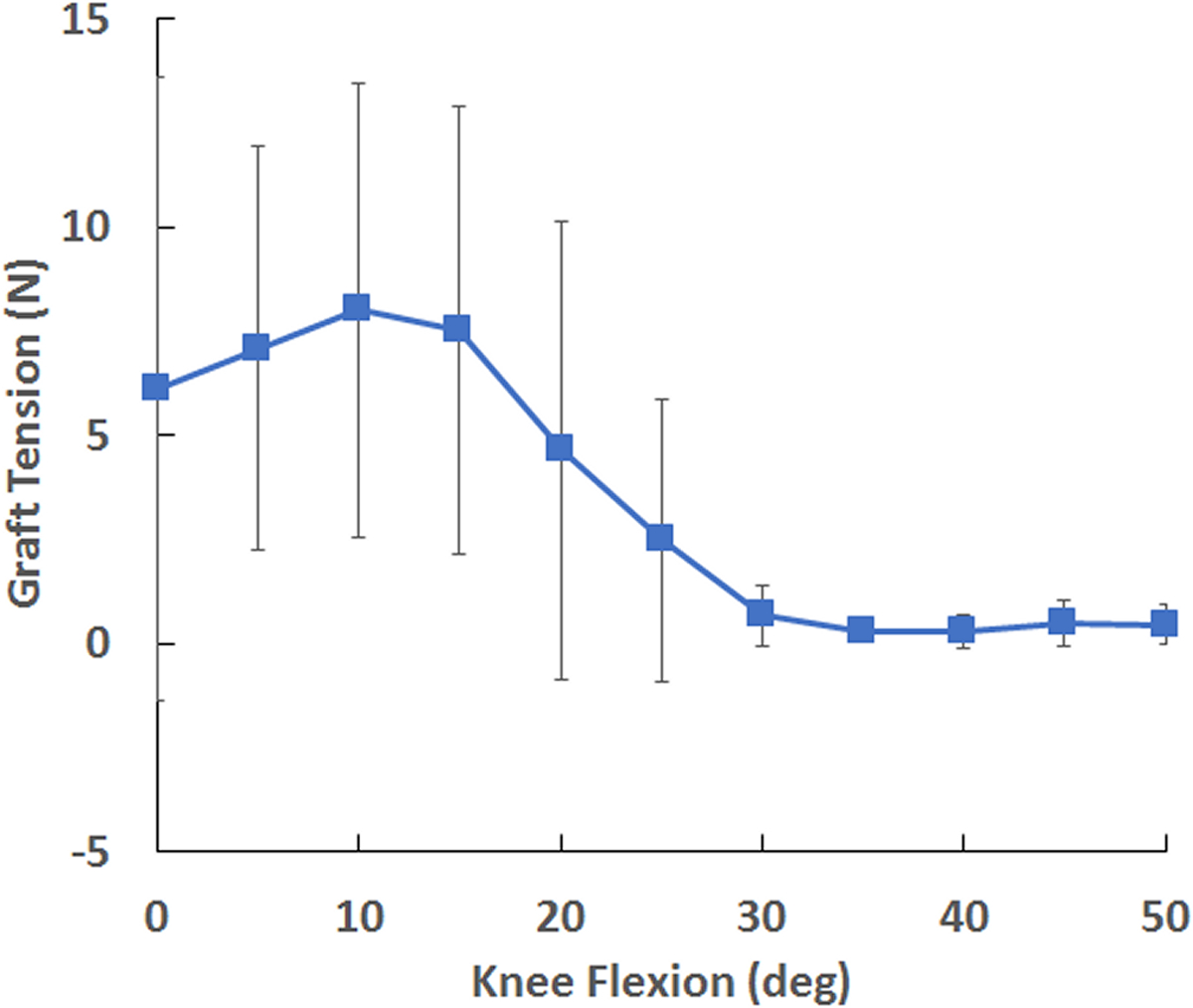
Average (± standard deviation) MPFL graft tension vs. knee flexion angle, showing a peak at 10° and minimal graft tension by 30°.
MPFL reconstruction decreased lateral patellar tracking. MPFL reconstruction significantly decreased bisect offset index compared to the pre-operative condition at every flexion angle from 0° to 50° of flexion (Fig. 5), except 45°. At 0° of flexion, the position with the largest lateral tracking, average bisect offset index decreased from a pre-operative value of 0.81 to 0.71 following MPFL reconstruction. MPFL reconstruction significantly decreased lateral patellar tilt compared to the pre-operative condition from 0° to 30° of flexion (Fig. 6). At 0°, MPFL reconstruction decreased the average lateral tilt by 3°.
Figure 5.
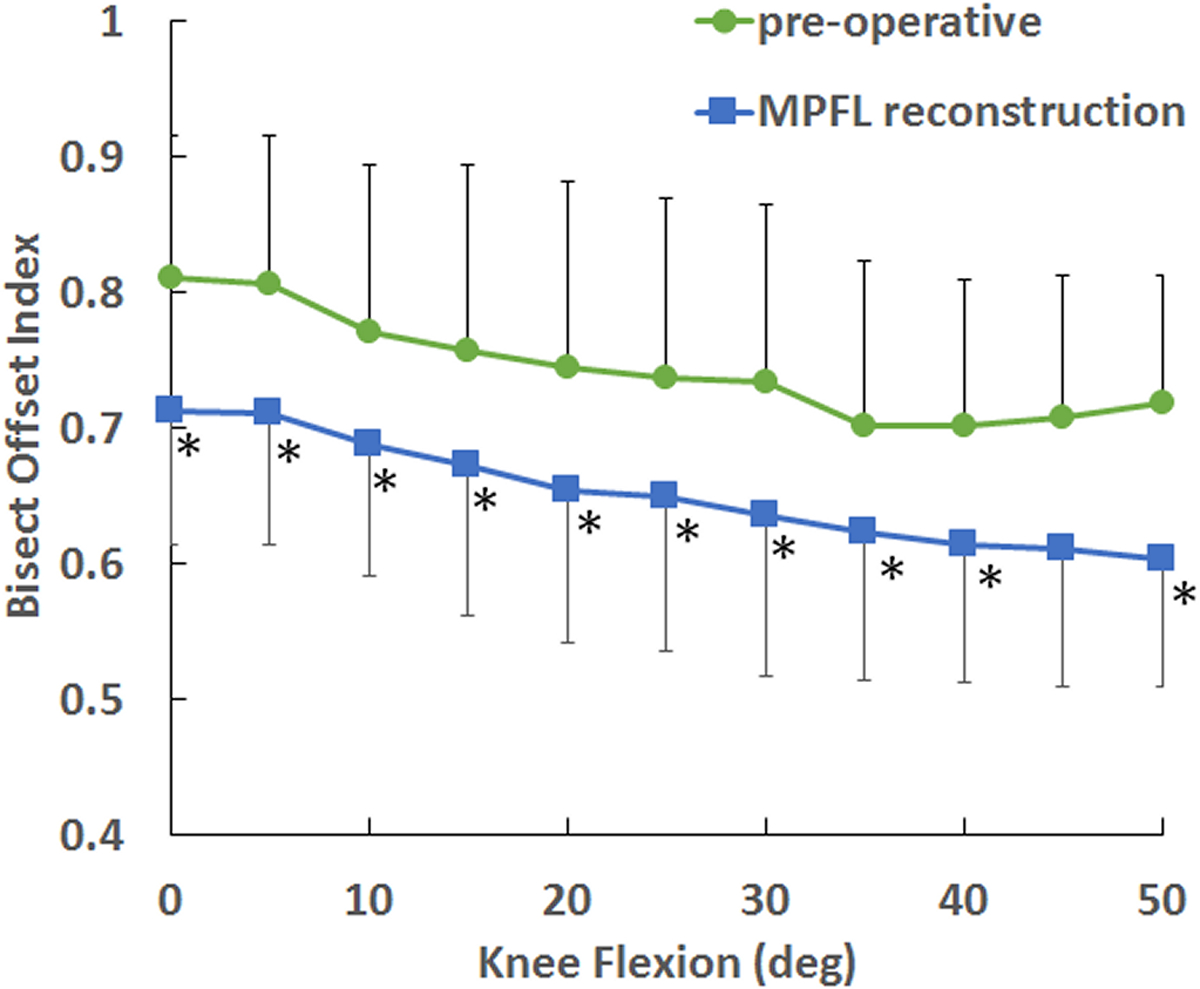
Average (± standard deviation) bisect offset index vs. knee flexion angle for the pre-operative condition and following MPFL reconstruction. MPFL reconstruction significantly decreased bisect offset index at nearly all flexion angles (*).
Figure 6.
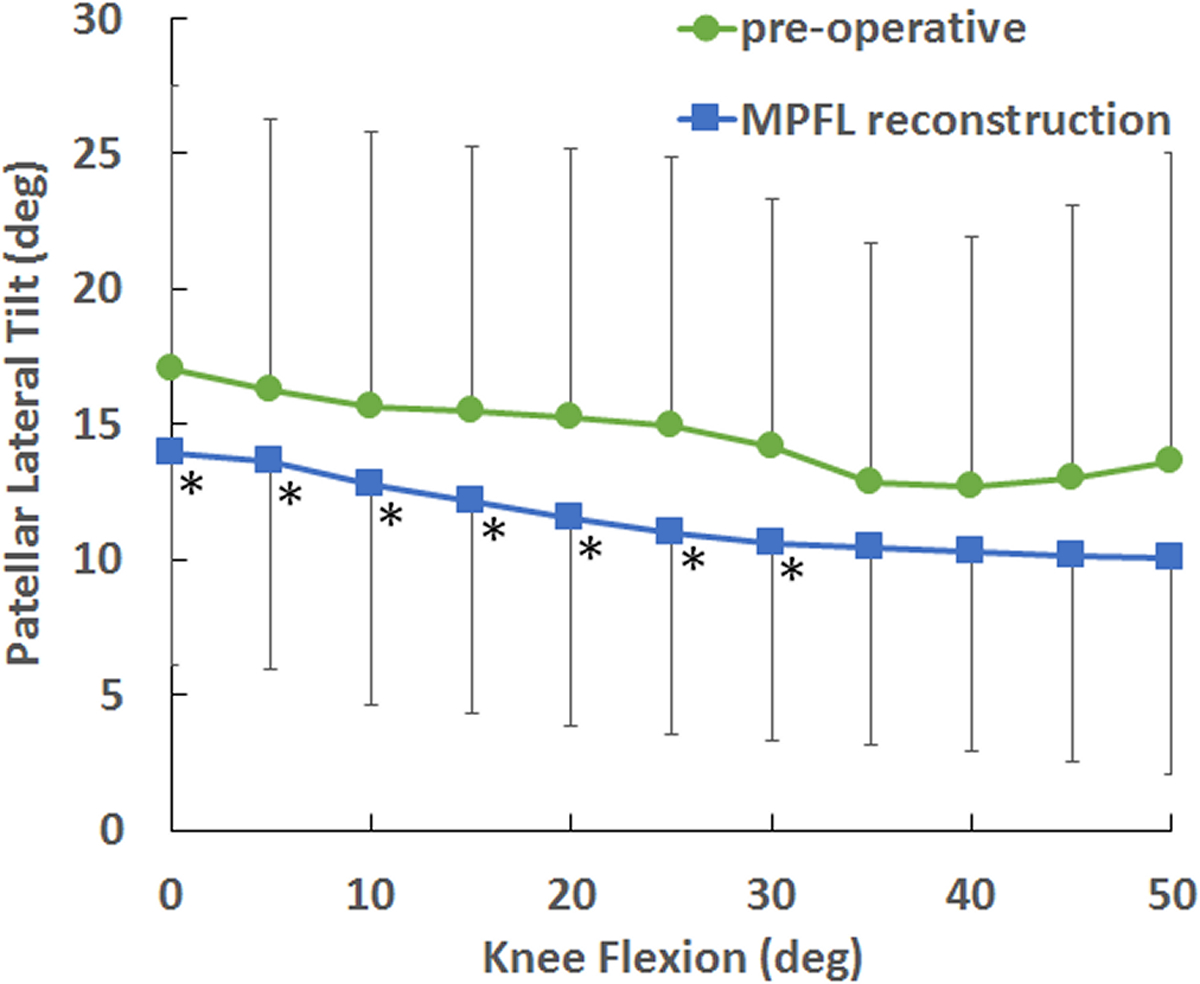
Average (± standard deviation) patellar lateral tilt vs. knee flexion angle for the pre-operative condition and following MPFL reconstruction. MPFL reconstruction significantly decreased patellar lateral tilt up to 30° of flexion (*).
Discussion
The results indicate that the maximum tension within a MPFL graft is correlated with the most lateral position of the tibial tuberosity during function. The graft tension was not significantly correlated with any parameters related to trochlear dysplasia or patella alta. The authors are not aware of any previous study that has related MPFL graft tension to knee anatomy. A previous simulation study identified the lateral TT-PCL distance as the primary factor correlated with patellar lateral maltracking during dynamic squatting21, although trochlear dysplasia was correlated to a lesser degree. In addition to influencing pre-operative patellar tracking, a lateral position of the tibial tuberosity creates a force vector through the patellar tendon that is opposed by the MPFL graft. The results could potentially vary with the activity, as previous simulations indicated that lateral maltracking is correlated more strongly with trochlear dysplasia than lateral position of the tibial tuberosity for knee extension against gravity21.
The results also indicate that MPFL grafts primarily function at low flexion angles, limiting lateral maltracking when the patella is proximal to the trochlear groove and guiding the patella into the groove, with minimal tension once the patella is engaged with the groove. A previous simulation study similarly showed that graft tension peaks at 10° of flexion and begins to decrease as the patella enters the trochlear groove, with minimal tension by 30° of knee flexion17. The in vitro studies that measured the path length between femoral and patellar attachment points for a graft showed a wide variety of results, including a decrease in graft length with flexion27, isometric function over the flexion range23–26, and an increase in graft length with flexion28. Previous studies that measured the path length with computational models reconstructed from imaging data of subjects indicated that graft length decreases with flexion29, decreases in early flexion followed by an increase30, and increases in early flexion followed by a decrease31 Differences with respect to the previously published data are likely related to the experimental approach. The previous studies did not directly measure tension within the graft or consider the intra-operative graft tensioning protocol included in the current study. The previous studies were also based on normal knees that did not display the pathology or maltracking associated with recurrent patellar instability, although one study created patella alta and a laterally positioned tibial tuberosity in a cadaveric model26. Both conditions caused the graft length to decrease with increasing flexion.
Graft tension that occurred at low flexion angles decreased lateral patellar tracking throughout the full range of flexion. Previous computational and experimental studies showed that MPFL reconstruction decreases lateral patellar maltracking17–20, although only the current study characterized patellar tracking based on alignment relative to the trochlear groove for comparison to clinical evaluation. The decrease in the average peak bisect offset index from 0.8 for the pre-operative condition to 0.7 for MPFL reconstruction is considered a change from maltracking to tracking within normal parameters42. The decrease in patellar tilt on the order of 3° is relatively small compared to differences of approximately 15° measured between patients with patellar instability and controls43,44; however, a significant difference of 3° in patellar tilt was identified between initial instability patients who went on to a second dislocation within two years compared to those who did not45. MPFL reconstruction maintained normal levels of lateral tracking even with minimal tension in the graft beyond 30° of flexion due to the graft guiding the patella into the trochlear groove at low flexion angles, and the subsequent articular constraint provided by the groove.
The graft tension values recorded for the current study would not be expected to induce failure. All tension values were less than 35 N. In vitro testing has identified failure loads of greater than 100 N for MPFL grafts40,46. The current study represented a simple squatting motion to maintain stability throughout the full range of flexion for the pre-operative condition to allow statistical comparisons to the post-operative condition. Future modeling studies can represent a motion more likely to cause an instability episode, such as a cutting motion with internal femoral rotation47, which would likely produce higher graft tension due to larger quadriceps forces and a more lateral position of the tibial tuberosity. Biomechanical studies have also associated MPFL graft tension with elevated pressure applied to patellofemoral cartilage, particularly cartilage on the medial facet that may be damaged following an instability episode16–18,20. The graft tension associated with elevated medial cartilage pressures is primarily associated with a poorly positioned attachment point on the femur or intra-operative over-tensioning. A previous computational study indicated that allowing 0.5 quadrants of lateral glide when setting the graft length did not overload medial cartilage17, so it was not considered a risk for the current study. The computational models optimized conditions for identifying the femoral attachment site and setting the graft length, however. Errors in either parameter could occur intra-operatively11, increasing graft tension and inducing the risk of failure or overloading medial cartilage, particularly when combined with a lateral position of the tibial tuberosity.
The models used for the current study included pathology associated with patellar instability. The average of the maximum lateral TT-PCL distances was 21 mm. A value of 21 mm is considered the threshold for pathology for the TT-PCL distance48, although for a measure along the tibial medial-lateral axis rather than the femoral posterior condylar axis. The average of the maximum Caton-Deschamps indexes was 1.2, which is considered a borderline value for patella alta49. The average of the minimum lateral trochlear inclination values was 15°. The value was less than 11° for six knees, indicating trochlear dysplasia50. The lateral trochlear inclination values may also have been slightly higher than would be measured clinically due to the computational measurement based on the maximum slope.
Limitations
The modeling approach does include limitations. Material properties and loading conditions assigned to the models were generalized based on previously published data, rather than individualized for the subjects. The assumed properties emphasize the importance of the continued assessment of accuracy compared to patellar kinematics measured for the subjects being treated for patellar instability represented by the models. The accuracy assessment was described in terms of lateral shift in mm, instead of the bisect offset index used for the current analysis, in order to utilize identical coordinate systems for both reconstruction of the subjects’ function and simulated motions. The analysis with respect to MPFL reconstruction was based on bisect offset index for the benefit of clinical interpretation. Simulated patellar tracking for subjects being treated for recurrent instability and changes due to MPFL reconstruction were expressed in terms of lateral shift in a previous publication17. The simulated activity was chosen to maintain patellar stability across the full range of motion, even for the pre-operative condition. This approach limited the graft tension, and did not provide characterization of graft function for conditions in which the graft is preventing a patellar dislocation.
Conclusion
Based on the current simulations of dynamic knee squatting, the tension within an MPFL graft is largest when reconstruction is performed on knees with a laterally positioned tibial tuberosity. For motion patterns less stable than the one simulated or with errors in intra-operative graft placement, elevated graft tension related to a laterally positioned tuberosity could potentially induce the risk of failure or over constrain the patella. For the simulated squatting motion, graft tension peaks at low flexion angles and limits lateral patellar maltracking, while tension becomes minimal once the patella becomes fully engaged within the bony constraint of trochlea.
Acknowledgements
Funding was provided by a Research Grant from the Arthroscopy Association of North America and the National Institute of Arthritis And Musculoskeletal And Skin Diseases of the National Institutes of Health under Award Number R21AR069150.
Footnotes
Conflicts of Interest
Dr. Elias received a research grant from NIH/NIAMS related to the study. Dr. Cosgarea received a research grant from the Arthroscopy Association of North America related to the study. Dr. Cosgarea also reports textbook royalties from Elsevier and membership on the board of directors of the Patellofemoral Foundation. Dr. Tanaka received a research grant from the Arthroscopy Association of North America related to the study, and is a committee member for the American Academy of Orthopedic Surgeons and on the electronic media editorial board for the American Journal of Sports Medicine. Jared Forman has no potential conflicts of interest to declare.
Contributor Information
Miho J. Tanaka, Department of Orthopaedic Surgery, Massachusetts General Hospital Harvard Medical School, Boston, MA.
Andrew J. Cosgarea, Department of Orthopaedic Surgery, Johns Hopkins University, Baltimore, MD.
Jared M. Forman, Department of Orthopaedic Surgery, Johns Hopkins University, Baltimore, MD.
John J. Elias, Department of Research, Cleveland Clinic Akron General, Akron, OH.
REFERENCES
- 1.Liu JN, Steinhaus ME, Kalbian IL, et al. Patellar Instability Management: A Survey of the International Patellofemoral Study Group. Am J Sports Med 2018;46:3299–3306 [DOI] [PubMed] [Google Scholar]
- 2.Schneider DK, Grawe B, Magnussen RA, et al. Outcomes After Isolated Medial Patellofemoral Ligament Reconstruction for the Treatment of Recurrent Lateral Patellar Dislocations: A Systematic Review and Meta-analysis. Am J Sports Med 2016;44:2993–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah JN, Howard JS, Flanigan DC, Brophy RH, Carey JL, Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med 2012;40:1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopper GP, Leach WJ, Rooney BP, Walker CR, Blyth MJ. Does degree of trochlear dysplasia and position of femoral tunnel influence outcome after medial patellofemoral ligament reconstruction? Am J Sports Med 2014;42:716–722 [DOI] [PubMed] [Google Scholar]
- 5.Lind M, Enderlein D, Nielsen T, Christiansen SE, Faunø P. Clinical outcome after reconstruction of the medial patellofemoral ligament in paediatric patients with recurrent patella instability. Knee Surg Sports Traumatol Arthrosc 2016;24:666–671 [DOI] [PubMed] [Google Scholar]
- 6.Lippacher S, Dreyhaupt J, Williams SR, Reichel H, Nelitz M. Reconstruction of the Medial Patellofemoral Ligament: Clinical Outcomes and Return to Sports. Am J Sports Med 2014;42:1661–1668 [DOI] [PubMed] [Google Scholar]
- 7.Ronga M, Oliva F, Longo UG, Testa V, Capasso G, Maffulli N. Isolated medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med 2009;37:1735–1742 [DOI] [PubMed] [Google Scholar]
- 8.Schiphouwer L, Rood A, Tigchelaar S, Koëter S. Complications of medial patellofemoral ligament reconstruction using two transverse patellar tunnels. Knee Surg Sports Traumatol Arthrosc 2017;25:245–250 [DOI] [PubMed] [Google Scholar]
- 9.Hiemstra LA, Kerslake S, Loewen M, Lafave M. Effect of Trochlear Dysplasia on Outcomes After Isolated Soft Tissue Stabilization for Patellar Instability. Am J Sports Med 2016;44:1515–1523 [DOI] [PubMed] [Google Scholar]
- 10.Kita K, Tanaka Y, Toritsuka Y, et al. Factors Affecting the Outcomes of Double-Bundle Medial Patellofemoral Ligament Reconstruction for Recurrent Patellar Dislocations Evaluated by Multivariate Analysis. Am J Sports Med 2015;43:2988–2996 [DOI] [PubMed] [Google Scholar]
- 11.Nelitz M, Williams RS, Lippacher S, Reichel H, Dornacher D. Analysis of failure and clinical outcome after unsuccessful medial patellofemoral ligament reconstruction in young patients. Int Orthop 2014;38:2265–2272 [DOI] [PubMed] [Google Scholar]
- 12.Valkering KP, Rajeev A, Caplan N, Tuinebreijer WE, Kader DF. An evaluation of the effectiveness of medial patellofemoral ligament reconstruction using an anatomical tunnel site. Knee Surg Sports Traumatol Arthrosc 2017;25:3206–3212 [DOI] [PubMed] [Google Scholar]
- 13.Wagner D, Pfalzer F, Hingelbaum S, Huth J, Mauch F, Bauer G. The influence of risk factors on clinical outcomes following anatomical medial patellofemoral ligament (MPFL) reconstruction using the gracilis tendon. Knee Surg Sports Traumatol Arthrosc 2013;21:318–324 [DOI] [PubMed] [Google Scholar]
- 14.Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med 2010;38:2248–2254 [DOI] [PubMed] [Google Scholar]
- 15.Sampatacos NE, Getelman MH. Medial patellofemoral ligament reconstruction using a modified “reverse-loop” technique. Arthrosc Tech 2013;2:e175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck P, Brown NA, Greis PE, Burks RT. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med 2007;35:1557–1563 [DOI] [PubMed] [Google Scholar]
- 17.Elias JJ, Jones KC, Lalonde MK, Gabra JN, Rezvanifar SC, Cosgarea AJ. Allowing one quadrant of patellar lateral translation during medial patellofemoral ligament reconstruction successfully limits maltracking without overconstraining the patella. Knee Surg Sports Traumatol Arthrosc 2018;26:2883–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elias JJ, Kelly MJ, Smith KE, Gall KA, Farr J. Dynamic simulation of the effects of graft fixation errors during medial patellofemoral ligament reconstruction. Orthop J Sports Med 2016;4:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philippot R, Boyer B, Testa R, Farizon F, Moyen B. Study of patellar kinematics after reconstruction of the medial patellofemoral ligament. Clin Biomech 2012;27:22–26 [DOI] [PubMed] [Google Scholar]
- 20.Stephen JM, Kittl C, Williams A, et al. Effect of Medial Patellofemoral Ligament Reconstruction Method on Patellofemoral Contact Pressures and Kinematics. Am J Sports Med 2016;44:1186–1194 [DOI] [PubMed] [Google Scholar]
- 21.Elias JJ, Jones KC, Cyrus Rezvanifar S, Gabra JN, Morscher MA, Cosgarea AJ. Dynamic tracking influenced by anatomy following medial patellofemoral ligament reconstruction: Computational simulation. Knee 2018;25:262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephen JM, Dodds AL, Lumpaopong P, Kader D, Williams A, Amis AA. The ability of medial patellofemoral ligament reconstruction to correct patellar kinematics and contact mechanics in the presence of a lateralized tibial tubercle. Am J Sports Med 2015;43:2198–2207 [DOI] [PubMed] [Google Scholar]
- 23.Burrus MT, Werner BC, Cancienne JM, Gwathmey FW, Diduch DR. MPFL graft fixation in low degrees of knee flexion minimizes errors made in the femoral location. Knee Surg Sports Traumatol Arthrosc 2017;25:3092–3098 [DOI] [PubMed] [Google Scholar]
- 24.Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee 2003;10:221–227 [DOI] [PubMed] [Google Scholar]
- 25.Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med 2012;40:1871–1879 [DOI] [PubMed] [Google Scholar]
- 26.Redler LH, Meyers KN, Brady JM, Dennis ER, Nguyen JT, Shubin Stein BE. Anisometry of Medial Patellofemoral Ligament Reconstruction in the Setting of Increased Tibial Tubercle-Trochlear Groove Distance and Patella Alta. Arthroscopy 2018;34:502–510 [DOI] [PubMed] [Google Scholar]
- 27.Gobbi RG, Pereira CA, Sadigursky D, et al. Evaluation of the isometry of different points of the patella and femur for medial patellofemoral ligament reconstruction. Clin Biomech 2016;38:8–12 [DOI] [PubMed] [Google Scholar]
- 28.McCulloch PC, Bott A, Ramkumar PN, et al. Strain within the native and reconstructed MPFL during knee flexion. J Knee Surg 2014;27:125–31 [DOI] [PubMed] [Google Scholar]
- 29.Oka S, Matsushita T, Kubo S, et al. Simulation of the optimal femoral insertion site in medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 2014;22:2364–71 [DOI] [PubMed] [Google Scholar]
- 30.Kernkamp WA, Wang C, Li C, H et al. The Medial Patellofemoral Ligament Is a Dynamic and Anisometric Structure: An In Vivo Study on Length Changes and Isometry. Am J Sports Med 2019;47:1645–1653 [DOI] [PubMed] [Google Scholar]
- 31.Song SY, Pang CH, Kim CH, Kim J, Choi ML, Seo YJ. Length change behavior of virtual medial patellofemoral ligament fibers during in vivo knee flexion. Am J Sports Med 2015;43:1165–1171 [DOI] [PubMed] [Google Scholar]
- 32.Elias JJ, Carrino JA, Saranathan A, Guseila LM, Tanaka MJ, Cosgarea AJ. Variations in kinematics and function following patellar stabilization including tibial tuberosity realignment. Knee Surg Sports Traumatol Arthrosc 2014;22:2350–2356 [DOI] [PubMed] [Google Scholar]
- 33.Elias JJ, Soehnlen NT, Guseila LM, Cosgarea AJ. Dynamic tracking influenced by anatomy in patellar instability. Knee 2016;23:450–455 [DOI] [PubMed] [Google Scholar]
- 34.Biyani R, Elias JJ, Saranathan A, Feng H, Guseila LM, Morscher MA, Jones KC. Anatomical factors influencing patellar tracking in the unstable patellofemoral joint. Knee Surg Sports Traumatol Arthrosc 2014;22:2334–2341 [DOI] [PubMed] [Google Scholar]
- 35.Besl PJ, McKay HD. A method for registration of 3-D shapes. IEEE Trans Pattern Anal Mach Intel 1992;14:239–256 [Google Scholar]
- 36.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng 1983;105:136–144 [DOI] [PubMed] [Google Scholar]
- 37.Elias JJ, Jones KC, Copa AJ, Cosgarea AJ. Computational simulation of medial versus anteromedial tibial tuberosity transfer for patellar instability. J Orthop Res 2018;36:3231–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elias JJ, Kilambi S, Goerke DR, Cosgarea AJ. Improving vastus medialis obliquus function reduces pressure applied to lateral patellofemoral cartilage. J Orthop Res 2009;27:578–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med 2007;35:801–804 [DOI] [PubMed] [Google Scholar]
- 40.Saper MG, Meijer K, Winnier S, Popovich J Jr, Andrews JR, Roth C. Biomechanical Evaluation of Classic Solid and All-Soft Suture Anchors for Medial Patellofemoral Ligament Reconstruction. Am J Sports Med 2017;45:1622–1626 [DOI] [PubMed] [Google Scholar]
- 41.Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc 2006;14:707–712 [DOI] [PubMed] [Google Scholar]
- 42.Tanaka MJ, Elias JJ, Williams AA, Demehri S, Cosgarea AJ. Characterization of patellar maltracking using dynamic kinematic CT imaging in patients with patellar instability. Knee Surg Sports Traumatol Arthrosc 2016;24:3634–3641 [DOI] [PubMed] [Google Scholar]
- 43.Charles MD, Haloman S, Chen L, Ward SR, Fithian D, Afra R. Magnetic resonance imaging-based topographical differences between control and recurrent patellofemoral instability patients. Am J Sports Med 2013;41:374–84 [DOI] [PubMed] [Google Scholar]
- 44.Van Haver A, Mahieu P, Claessens T, Li H, Pattyn C, Verdonk P, Audenaert EA. A statistical shape model of trochlear dysplasia of the knee. Knee 2014;21:518–23 [DOI] [PubMed] [Google Scholar]
- 45.Balcarek P, Oberthür S, Hopfensitz S, et al. Which patellae are likely to redislocate? Knee Surg Sports Traumatol Arthrosc 2014;22:2308–14 [DOI] [PubMed] [Google Scholar]
- 46.Joyner PW, Bruce J, Roth TS, et al. Biomechanical tensile strength analysis for medial patellofemoral ligament reconstruction. Knee 2017;24:965–976 [DOI] [PubMed] [Google Scholar]
- 47.Smith TO, Donell ST, Chester R, Clark A, Stephenson R. What activities do patients with patellar instability perceive makes their patella unstable? Knee 2011;18:333–339 [DOI] [PubMed] [Google Scholar]
- 48.Boutris N, Delgado DA, Labis JS, McCulloch PC, Lintner DM, Harris JD. Current evidence advocates use of a new pathologic tibial tubercle-posterior cruciate ligament distance threshold in patients with patellar instability. Knee Surg Sports Traumatol Arthrosc 2018;26:2733–2742 [DOI] [PubMed] [Google Scholar]
- 49.Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc 2014;22:2545–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Batailler C, Neyret P. Trochlear dysplasia: imaging and treatment options. EFORT Open Rev 2018;3:240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]


