Abstract
Maternal smoking during pregnancy (MSDP) remains one of the most common prenatal drug exposures in the US and worldwide. MSDP is associated with medical risk for the fetus and altered behavioral development in infants; however, fewer studies have examined the impact of MSDP on fetal behavior or newborn behavioral state. We investigated associations between MSDP and (a) fetal motor activity and (b) newborn behavioral state following handling. Participants were 79 healthy mother-fetus/newborn pairs (57% MSDP-exposed). MSDP was measured by maternal interview and verified by saliva biomarkers. Mothers completed an observational fetal ultrasound assessment between 24 and 37 weeks gestation (M=28 weeks), including baseline, vibro-acoustic stimulus and recovery periods. Total fetal motor activity and complex body movements were coded from ultrasound videos. Following delivery, newborn post-handling behavioral state was assessed by direct observational coding. MSDP exposure was associated with higher baseline fetal motor activity, particularly at younger gestational ages. Further, motor reactivity to stimulation emerged at later gestational ages in MSDP-exposed fetuses, while motor reactivity was consistent across gestational ages in unexposed fetuses. Finally, heavy MSDP exposure was associated with more arousal following handling and greater need for soothing interventions in the newborn period. Monitoring of fetal behavior via ultrasound may offer a unique opportunity to identify at-risk infants and provides data for stronger public health messaging regarding risks of MSDP. Associations between MSDP and increased newborn fussiness highlight opportunities for education and anticipatory guidance in the postpartum period.
Keywords: pregnancy, smoking, fetal, infant, behavior, ultrasound
1. Introduction
Despite widespread public education campaigns, maternal smoking during pregnancy (MSDP) continues to be a major public health concern (England et al., 2017; Haviland et al., 2004; Orleans et al., 2004). More than 7% of pregnant women continue to smoke in the United States, with rates greater than 15% in young, poor, and less-educated women (Drake et al., 2018; Tong et al., 2013; U.S. Department of Health and Human Services). Increasing use of new tobacco products such as electronic cigarettes and hookah in pregnant women may further increase rates of prenatal nicotine/tobacco use (Kurti et al., 2017; Liu et al., 2019). Decades of research have documented associations between MSDP and infant morbidity (e.g., low birth weight, preterm birth) and mortality (sudden infant death syndrome) that were sufficient to infer causality in Surgeon General’s reports (U.S. Department of Health and Human Services, 2004). MSDP has also been associated with behavioral alterations in the neonatal period. Specifically, MSDP-exposed neonates have shown increased irritability and excitability, and increased need for external soothing interventions (Law et al., 2003; Mansi et al., 2007; Stroud et al., 2009a; Stroud et al., 2009b). MSDP has also been associated with increased risk of infantile colic (Canivet et al., 2008; Milidou et al., 2012; Reijneveld et al., 2005; Sondergaard et al., 2001). Although MSDP is associated with medical risk for the fetus and altered infant behavior, fewer studies have investigated effects of MSDP on fetal behavioral development.
1.1. Characterization of Fetal Behavior
Characterization of fetal behavioral development was made possible by early observational ultrasound studies (Birnholz et al., 1978; Nijhuis et al., 1999), and has evolved along with technological refinements in fetal monitoring and ultrasonography (DiPietro et al., 2010; Kurjak et al., 2005; Salisbury, 2010). Validity of fetal behavioral measurements has been demonstrated across a number of dimensions, including: (a) continuity of fetal behavior over gestation (DiPietro, J., 2010), (b) continuity between fetal behavior and infant and child behavior (Dipietro et al., 2018), (c) associations between fetal behavior and fetal medical risk (e.g., intra-uterine growth retardation, preterm delivery) (Andonotopo and Kurjak, 2006; DiPietro, 2001), and (d) associations between fetal behavior and maternal medical and psychiatric conditions (Kainer et al., 1997; Kisilevsky et al., 2012; Monk et al., 2004).
Fetal responsivity to external stimuli is an important domain of fetal behavior that may differentiate healthy and at-risk fetuses; it is most frequently assessed via response to a vibro-acoustic stimulus (VAS; a vibratory and acoustic stimulus applied to the maternal abdomen) (Kisilevsky et al., 2012; Kisilevsky et al., 1990, 1992; Smith, 1994; Smith et al., 1988). Fetal response to stimuli is measured by behavioral and/or heart rate changes that occur within five to ten seconds of the stimulus presentation (Birnholz and Benacerraf, 1983) and reflect maturation and coordination of the central and peripheral nervous systems. A typical response to the VAS includes increased movement and heart rate (indicative of arousal); the initial response to the VAS may include a startle and has been reported to be related to fetal state and gestational age (Groome et al., 1993; Groome et al., 1995). The VAS is repeated in some studies as a measure of habituation and fetal well-being. Fetal behavioral (startle, general body movements) responses to a VAS begin as early as 24 gestational weeks, with an increase in reliable behavioral responses by 26–29 weeks, and concordant heart rate accelerations by 29 weeks (Das et al., 2019; Kisilevsky et al., 1992).
1.2. Maternal Smoking During Pregnancy and Fetal Behavior
Despite important implications for early identification of at-risk offspring and providing impetus for stronger public health messaging regarding risks of MSDP, only a small number of studies have investigated associations between MSDP and fetal behavior, including cardiovascular and autonomic regulation. Initial studies examined fetal response to acute smoking episodes. Acute cigarette exposure was associated with decreases in fetal heart rate, and fetal heart rate variability and reactivity, decreases in felt movements (Goodman et al., 1984; Graca et al., 1991; Lehtovirta et al., 1983; Thaler et al., 1980), and greater rates of maladaptive response to the ―non-stress test‖--a measure of fetal well-being (Oncken et al., 2002; Phelan, 1980). Chronic MSDP was associated with attenuated fetal heart rate variability and disruption of fetal autonomic regulation (Zeskind and Gingras, 2006). Finally, MSDP exposure was also associated with altered fetal heart rate response to the maternal voice early in third trimester (Cowperthwaite et al., 2007).
One prior study investigated behavioral response to repeated administration of the VAS in MSDP-exposed fetuses. Exposed fetuses demonstrated reduced behavioral habituation (or increased behavioral reactivity) to the repeated VAS (Gingras and O’Donnell, 1998). Most recently, our group demonstrated associations between MSDP and fetal activity before and after a single VAS administered late in 3rd trimester (35 weeks) (unadjusted analyses) as well as associations between fetal behavior and newborn neurobehavioral development (Stroud et al., 2018).
The present study adds to the literature in this area by investigating associations between MSDP and motor activity in 2nd through 3rd trimester fetuses in an independent cohort from (Stroud et al., 2018). In particular, we investigated links between MSDP and fetal motor activity before and after a VAS as well as the impact of fetal gestational age on these associations. The present study may provide additional data regarding differences between MSDP-exposed and unexposed fetuses that may be useful for future public education campaigns.
1.3. Maternal Smoking During Pregnancy and Newborn Behavioral State
Behavioral states emerge in late gestation, become increasingly stable, and are easily observed in the newborn as coalescing behavioral and physiological patterns that reflect internal processes and response to external stimuli (Junge, 1979; Thoman, 1990). Newborn behavioral state changes in response to stimuli are elicitors of external regulation/soothing, and are primary components of self-regulation. Newborn behavioral states include two main sleep states (quiet and active) and a range of wake states from sleep-wake transition to crying/high arousal; all map onto brain and physiologic systems (Brown, 1964; Thoman, 1990; Thoman et al., 1987; Wolff, 1973). Behavioral states measured as early as the first postnatal day were shown to serve as sensitive indices of later neurodevelopmental functions (Freudigman and Thoman, 1993). Prior studies of MSDP and newborn neurobehavior focused on newborn behaviors over the course of a mild stressor (neurobehavioral examination) (Law et al., 2003; Mansi et al., 2007; Stroud et al., 2009a; Stroud et al., 2009b). In this study, we extend prior studies of MSDP and newborn neurobehavior to investigate effects of MSDP on newborn behavioral state measured by direct observation following a mild stressor (neurobehavioral exam).
1.4. The Present Study
The primary aims of the present study were to investigate: (a) the association between MSDP and fetal motor activity at baseline and in response to a vibroacoustic stimulus in fetuses ranging in gestational age from 24 to 37 weeks, (b) the impact of fetal gestational age on associations between MSDP and fetal motor activity, and (c) the association of MSDP with newborn behavioral state following a mild stressor in a parallel and independent sample from (Stroud et al., 2018). Based on prior studies showing increased arousal in MSDP-exposed fetuses and infants (Gingras and O’Donnell, 1998; Law et al., 2003; Stroud et al., 2018; Stroud et al., 2009b), we hypothesized that MSDP would be associated with increased activity at baseline and in response to the VAS, and with increased newborn arousal/behavioral state following handling.
2. Material and Methods
2.1. Participants
Participants were a subsample from a larger, prospective study designed to investigate fetal health and behavior. Pregnant mothers carrying singletons were recruited through flyers posted at community sites and obstetric offices. All participants provided written informed consent; all procedures were reviewed and approved by the Women and Infants Hospital Institutional Review Board. One hundred thirty-nine pregnant women were enrolled in the larger prospective study. Measures pertaining to MSDP (e.g., nicotine biomarkers) were implemented part way through enrollment for the larger study. Exclusion criteria for the subsample included: maternal age <18 (n = 6), gestational diabetes (n = 3), psychotropic medication use (n = 6), illicit drugs besides marijuana (n = 5), and > 0.5 drinks/day after pregnancy recognition (n = 1). Biospecimen collection was initiated later in the study; thus, participants whose non-smoking status could not be confirmed by biomarker data (n = 39) were excluded from analyses. The final analytic sample included 79 English and Spanish-speaking pregnant mothers ages 18–39 (M = 25, SD = 5) enrolled between 24–37 weeks gestation (M = 28; SD = 4). Outcome measures were available for 65 fetuses and 58 infants. The excluded participants included fewer racial/ethnic minorities than the final sample (p < .0001); otherwise, no significant differences in maternal characteristics emerged between participants in the larger cohort (N = 139) who were subsequently excluded (N = 60) and those included in the present sample (N = 79) (ps ≥ .11). NOTE: The participant sample (including the parent sample and subsample) for the present study is independent from the participant sample (including the parent sample and subsample) in our prior published work (Stroud et al., 2018).
2.1.1. MSDP Exposure Classification Groups.
Participants (N = 21) who reported that they were currently smoking at the time of the ultrasound and/or had a salivary cotinine or nicotine assay of > 10 ng/mL were assigned to the active smoking group. Participants (N=24) who denied smoking at the time of the ultrasound but who had a salivary cotinine or nicotine assay of 10 ng/mL were assigned to the low-level smoking group. Participants (N = 34) who denied smoking during pregnancy and 3 months prior to conception and who had a negative cotinine/nicotine bioassay were assigned to the Non-smoking group.
2.2. Procedures
2.2.1. Overview.
Participants were recruited at any point during their pregnancy, then scheduled for an interview-ultrasound session between 24 to 37 weeks gestation (M=28, SD=4) based on (a) timing of recruitment/scheduling convenience and (b) following a reassuring anatomy ultrasound as part of routine obstetric care. The interview-ultrasound session included: (a) interview and self-report measures including measures of smoking behavior, depression, caffeine, and demographics, (2.2.2.), (b) a saliva sample for nicotine, cotinine, and caffeine determination (2.2.2., 3.1.), followed by (c) a fetal observational ultrasound (2.2.3). Following delivery, neonatal behavioral state was assessed between postnatal days 1 and 10 (2.2.4.).
2.2.2. Maternal interviews.
Women completed the following procedures immediately preceding the ultrasound observation: (a) Timeline Follow Back (TLFB), a structured calendar/event-based interview to cue recall regarding daily-level cigarette, drug, and alcohol use (Robinson et al., 2014), (b) socioeconomic status (SES) interview to derive the Hollingshead four-factor SES index (Gottfried, 1985), (c) hours of environmental tobacco smoke (ETS) exposure in the prior month assessed by detailed interview, and (d) the Beck Depression Inventory (Beck et al., 1988). The TLFB covered a time period that included all days across the three months prior to conception and each day of pregnancy up to the day of the interview. After completing the interview and self-report questions, women also provided a saliva sample for measurement of cotinine--a nicotine metabolite--and nicotine to verify their smoking status, and caffeine. They were not asked to abstain from smoking on the day of the study.
2.2.3. Fetal Neurobehavioral Assessment.
Ultrasounds were conducted between 12:00 and 4:00 pm to account for diurnal variability in fetal activity levels (Pillai et al., 1992). Women reclined at a 45-degree angle while the ultrasound recording was obtained using real-time ultrasound (Toshiba SSA-340A, Toshiba American Medical, Duluth, GA) with a single 3.50-MHz transducer focused on a longitudinal view of the fetal head, trunk, and upper limbs. Observation of baseline fetal behavior proceeded for at least 10 minutes until a period of 15 seconds with no gross fetal movements was observed, indicating a resting state. Once in the resting state, a single, 3-second vibroacoustic stimulus (VAS; Corometrics 146) was applied to the maternal abdomen, followed by a 5-minute post-VAS observation period (post-VAS). In addition, prior to the recording procedures, the VAS was placed on participants’ arms for a 3-second stimulus to demonstrate the vibration participants could expect on their abdomen during the VAS portion of the recording. Ultrasound video was recorded to a media file for later coding of fetal behavior. (See Measures 2.3.3.)
2.2.4. Newborn Behavioral State.
Postnatal newborn assessment took place within the first 10 postnatal days (M=2, SD=2) due to previous evidence for pervasive impact of MSDP on neurobehavior in the early postnatal days (Law et al., 2003; Stroud et al., 2009b), evidence for the predictive validity of behavioral state measured in the early postnatal days period (Freudigman and Thoman, 1993), evidence for associations between fetal behavior and newborn behavior measured in the early postnatal days (Kurjak et al., 2004; Salisbury et al., 2005), and due to a known developmental shift in circadian rhythms after ~14 days (Wolff, 1973). Data for fifty-eight newborns between the ages of 0 and 10 days were available for analyses. Given our interest in early postnatal state, the majority (76%; N=39) of infant state observations took place on postnatal day 1 or 2. However, due to scheduling convenience, some observations took place after the 2nd postnatal day (24%; N=19). 76% of infants were assessed in the hospital; 24% were assessed in participants’ homes. Infants were initially handled using the NICU Network Neurobehavioral Scale (NNNS (Lester et al., 2004)). Following the NNNS, infants were soothed to a quiet state if fussing or crying and then placed into a crib in a supine position with a light swaddle. Infants were then observed in real time for ten minutes by trained coders, blind to MSDP exposure condition. The presence of eye and body movements, regular vs. irregular respiration patterns, and muscle tone were coded in 10 second increments to determine behavioral states using a modification of Thoman’s classification (Thoman, 1990; Thoman et al., 1987). Infants with continuous fussing or crying were consoled with escalating and cumulative handling techniques.
2.3. Measures
2.3.1. Salivary Cotinine and Nicotine.
Nicotine exposure was measured using a saliva bioassay for nicotine and cotinine, the primary nicotine metabolite. Cotinine is a reliable biomarker for nicotine (Jarvis et al., 1987), and is readily passed from mother to fetus (Donnenfeld et al., 1993). Maternal saliva samples were assayed for cotinine and nicotine via gas chromatography-mass spectrometry conducted at Clinical Pharmacology Laboratories (University of California, San Francisco) (Jacob et al., 1981; Jacob et al., 1991); limits of quantitation (LOQ) were 10 ng/ml and 1 ng/ml, respectively (Jacob et al., 1991).
2.3.2. Salivary caffeine.
Maternal saliva caffeine concentrations were utilized as a biomarker for caffeine intake, assessed as a potential confounder/covariate in the analyses. Caffeine concentrations were quantified via gas liquid chromatography using nitrogen phosphorus detection, modified from the assay of Jacob et al. 1981 (Stehlik et al., 1982). LOQ for caffeine was 100 ng/ml.
2.3.3. Fetal Neurobehavioral Coding.
Fetal movements of limbs, head, and trunk were coded from recorded media files using the Action, Analysis, Coding, and Training Program (Intelligent Hearing Systems) by certified coders blind to MSDP group status. A total of 10 minutes of fetal ultrasound recording were coded including 5 minutes of baseline and 5 minutes of VAS/post-VAS behavior. Twenty percent of video recordings were double-coded to determine inter-rater reliability. Each fetal movement was tested for percent agreement across 4 coders, percent agreement was 87% for limb, 95% for head, and 93% for trunk movements; intraclass correlations (ICCs) also showed a high rate of reliability for each movement type, ranging from 0.85 to 0.95. Video recordings were marked into consecutive 15-second epochs, and then viewed frame-by-frame to determine duration of each fetal head, limb, and trunk movement. Duration was coded from the first second of movement until two consecutive seconds without movement were observed. Raw durations in seconds of fetal movements were then calculated into the relative proportion of each 15-second epoch the fetus spent in each of the 3 movement types (range of 0–15 seconds). Total Activity was the total duration of time that the fetus was moving within each 15-second interval; the interval data was then summed to determine the percentage of time the fetus was active during a given interval (e.g., baseline, VAS/post-VAS period). The coded files were then viewed in consecutive 15-second epochs to determine which epochs included activity with coincident head, limb, and trunk movements (Complex Body Movements (CBM)) versus movements that occurred in isolation. CBM is a measure of simultaneous movements that typically require some degree of motor coordination (e.g. change in body position) and are more frequently observed with an increase in fetal heart rate and variability suggestive of a higher arousal state (Nijhuis, 1986; Pillai et al., 1992) while isolated movements of the head or limbs (upper and lower extremities) are less likely to be associated with changes in fetal heart rate and more frequently observed during periods of relative quiescence (lower states of arousal (Pillai and James, 1990; Salisbury et al., 2009)). The proportion of fetal active time that included CBMs was calculated for the baseline and VAS/post-VAS periods. Percentage of time with Fetal Activity and the percentage of active time with CBMs served as fetal summary variables in analyses. There was a significant positive association between baseline fetal activity and CBMs at baseline (ρ = .64, p < .001), but no significant associations during the VAS/post-VAS period (ρ = .10, p = ns).
2.3.4. Newborn Behavioral State Coding.
During live observation, each 10-second epoch was assigned a behavioral state classification based on observed infant behaviors, eye movements, and respiration. Sleep states included: quiet sleep (QS; regular respiration, minimal body movements, and no rapid eye movement [REM]), active sleep (AS; intermittent movement, REM, and irregular respiration), and sleep-wake transition (SWT; periods of behavioral arousal from sleep to wake or drowsiness from wake to sleep). Waking states included quiet awake (QA), active awake (AA) and crying (C). The direct observation codes were then subjected to a 30-second moving window to determine the predominant state over consecutive epochs. The percentage of time spent in each state over the observation period was calculated. Additionally, behavioral states were assigned numerical codes to represent each state by the degree of arousal/activity generally observed within each state, QS=1 (minimal to no activity), AS=2, SWTr=3, QA=4, AA=5, C=6 (continuous activity/high arousal). A second time series was applied to obtain the mode of numerical state over 1-minute periods. Summary variables included: (a) behavioral state (1–6 scale) per minute, (b) mean state level over the 10-minute observation. Examiner interventions to soothe the infant were scored for each minute of the observation as: 0 = none, 1 = infant self-soothing, 2 = examiner talks softly to infant, 3 = hand holding/ventral pressure, 4= rock infant in crib, 5 = pacifier in crib, 6 = swaddle in crib, 7 = hold infant, 8 = hold and rock, 9 = hold, rock and pacifier.
2.4. Statistical Analysis
Summary variables from the TLFB covered the time period from conception through the interview-ultrasound session. Summary variables included: average cigarettes per day, average drinks per week, any marijuana use. Potential confounders/covariates included maternal demographic characteristics (maternal race and ethnicity, age, socioeconomic status, education), other substance use, environmental tobacco smoke, and depression (Table 1) for all analyses. Gestational age at birth, infant age at state observation, location of the state observation (hospital/home) and type of delivery were also evaluated for infant analyses. Potential covariates were evaluated for significant associations (p < .10) with MSDP group and/or fetal and infant outcomes, then tested individually in each model for significance (p < .10) and improved model fit. No potential covariates that showed statistically significant associations with MSDP (i.e., maternal age, second-hand smoke) were associated with fetal behavior; these covariates also did not reach significance in the full models. No potential covariates were associated with newborn state (including newborn age, location of observation, delivery mode). Given prior evidence for race/ethnicity as a moderator of links between MSDP and infant behavior (Yolton et al., 2009), we specifically tested race/ethnicity as a potential confounder in all analyses; inclusion of race/ethnicity as a covariate did not change the pattern nor significance of any findings.
Table 1.
Maternal and infant characteristics by smoking group and full sample
| Active Smokers (n=21) | Low-level Smokers (n=24) | Non-smokers (n=34) | Total (n=79) | |
|---|---|---|---|---|
| Mean (SD)/% | Mean (SD)/% | Mean (SD)/% | Mean (SD)/% | |
| Maternal Characteristics | ||||
| Maternal age (years) ** | 23 (4) | 24 (5) | 27 (6) | 25 (5) |
| Race/Ethnicity (% Non-Hispanic White) | 58% | 33% | 44% | 46% |
| Hollingshead SES1 | 4 (1) | 3 (1) | 3 (1) | 3 (1) |
| Parity | 2 (1) | 2 (1) | 2 (1) | 2 (1) |
| Living Children | 1 (1) | 1 (1) | 1 (1) | 1 (1) |
| Alcohol Use (>1 drink per week) | 0% | 0% | 0% | 0% |
| Any Marijuana Use in Pregnancy | 8% | 0% | 3% | 4% |
| Maternal Depression Symptoms2 | 9 (9) | 6 (4) | 7 (6) | 7 (7) |
| ETS Exposure (hours/day)3 ** | 21 (28) | 2 (3) | 5 (10) | 9 (18) |
| Cigarettes per day4 *** | 9 (9) | 0.5 (2) | 0 (0) | 3 (6) |
| Maternal saliva cotinine (ng/mL)5 *** | 55 (69) | 10 (2) | 0 (0) | 18 (43) |
| Maternal saliva nicotine (ng/mL)5 *** | 74 (104) | 2 (3) | 0 (0) | 22 (64) |
| Maternal saliva caffeine (ng/mL)5 | 1068 (1311) | 724 (741) | 1005 (1509) | 946 (1276) |
| Gestational Age at Ultrasound | 28 (4) | 29 (4) | 28 (3) | 28 (4) |
| Infant Characteristics | ||||
| Sex (% male) | 53% | 53% | 46% | 49% |
| Gestational age at birth (weeks) | 39 (2) | 39 (1) | 39 (1) | 39 (2) |
| Birth weight (grams) | 3256 (501) | 3335 (441) | 3328 (809) | 3311 (644) |
| Apgar score, 5 minutes | 9 (1) | 9 (1) | 9 (0) | 9 (1) |
| Age at newborn assessment (postnatal | 2 (2) | 2 (2) | 3 (2) | 2 (2) |
NOTE:
p<.01;
p<.001.
Based on a range of 1 to 5 on the Hollingshead Index (1=highest SES).
Score on Beck Depression Inventory (Beck et al., 1988).
Hours of Environmental Tobacco Smoke (ETS) exposure per week measured by structured interview.
Mean cigarettes per day across pregnancy.
Saliva cotinine, nicotine, and caffeine concentrations at ultrasound assessment. Although the values for either cotinine or nicotine in the low-level smoking group are exactly 10; because some participants had nicotine values of exactly 10 for nicotine but >10 for cotinine or vice versa, there is a range of cotinine and nicotine values.
Maternal and newborn characteristics were compared between exposure groups using F-and χ2 tests as appropriate. Independent variables for the fetal analyses included MSDP Group (High exposed, Low exposed, Unexposed) and continuous fetal gestational age (GA) at ultrasound assessment. Period (baseline, VAS/post-VAS periods) was included as a repeated measure. Fetal movement variables (Total Activity, CBMs) were the dependent measures. Generalized Linear Mixed Models (GLMM (McCulloch, 1997)) were utilized to examine (a) MSDP group and (b) MSDP group × continuous gestational age associations with total activity and CBMs over baseline and VAS/post-VAS periods. To determine the immediate VAS response by MSDP group, a GLMM analysis was conducted with (1) baseline: mean seconds active per epoch over the first 4.75 minutes of the baseline period, (2) the 15-second interval prior to the stimulus (targeted quiescence), and (3) each subsequent 15-second epoch from the VAS stimulus to the end of the coded observation as repeated measures. Significant interactions were followed by planned contrasts or pairwise comparisons with correction for multiple comparisons with the Benjamini-Hochberg false discovery rate approach (Benjamini and Hochberg, 1995). GLMM was also utilized in 3 models to investigate MSDP group associations with (1) the distribution of newborn behavioral states over the post-handling observation period, (2) the predominant state per minute, and (3) the level of soothing techniques per minute needed for crying/fussing newborn. All analyses were conducted using SPSS 25.0.
3. Results
3.1. Maternal and newborn characteristics
Characteristics of the sample overall and by exposure group are presented in Table 1. Racial breakdown was 62% White, 11% African American, 27% Other/Multi-Race; 35% of the sample was of Hispanic ethnicity. No significant differences between MSDP groups emerged for race, parity, live children, depression, alcohol or marijuana use, caffeine levels, or gestational age (GA) at the ultrasound assessment. However, active and low-level smokers were significantly younger than non-smokers. Active smokers reported an average of 9 cigarettes per day across pregnancy (SD = 9); low-level smokers reported an average of 0.5 cigarettes per day (SD = 2); average cotinine was 55 ng/ml (SD = 69) for active smokers and 10 ng/ml (SD = 2) for low-level smokers. Active smokers also showed higher levels of environmental tobacco smoke exposure than low-level and non-smokers. High and low MSDP-exposed and unexposed infants did not differ on GA at birth, birthweight, Apgar scores, or age at newborn exam.
3.2. Associations between maternal smoking and fetal motor activity
3.2.1. Fetal Activity.
To depict the immediate change in activity in response to the VAS, Figure 1 presents the percentage of time active for each group summed over the 4.75 minutes prior to the stimulus as well as in 15-second epochs immediately before, during and after the VAS presentation. Controlling for GA, there was a significant MSDP group by period effect (F (42, 207) = 7.07, p = .0001). There were no significant differences between the groups at the immediate pre-VAS epoch or at the time of the VAS stimulus (ps> 0.2). However, there were significant increases in activity between the immediate pre-VAS epoch and the VAS epoch for all groups (ts > 2.14, ps < .04) and between the 4.75 minute baseline and the VAS epoch for the low MSDP and unexposed groups (ts > 2.04, ps < .05).
Figure 1.
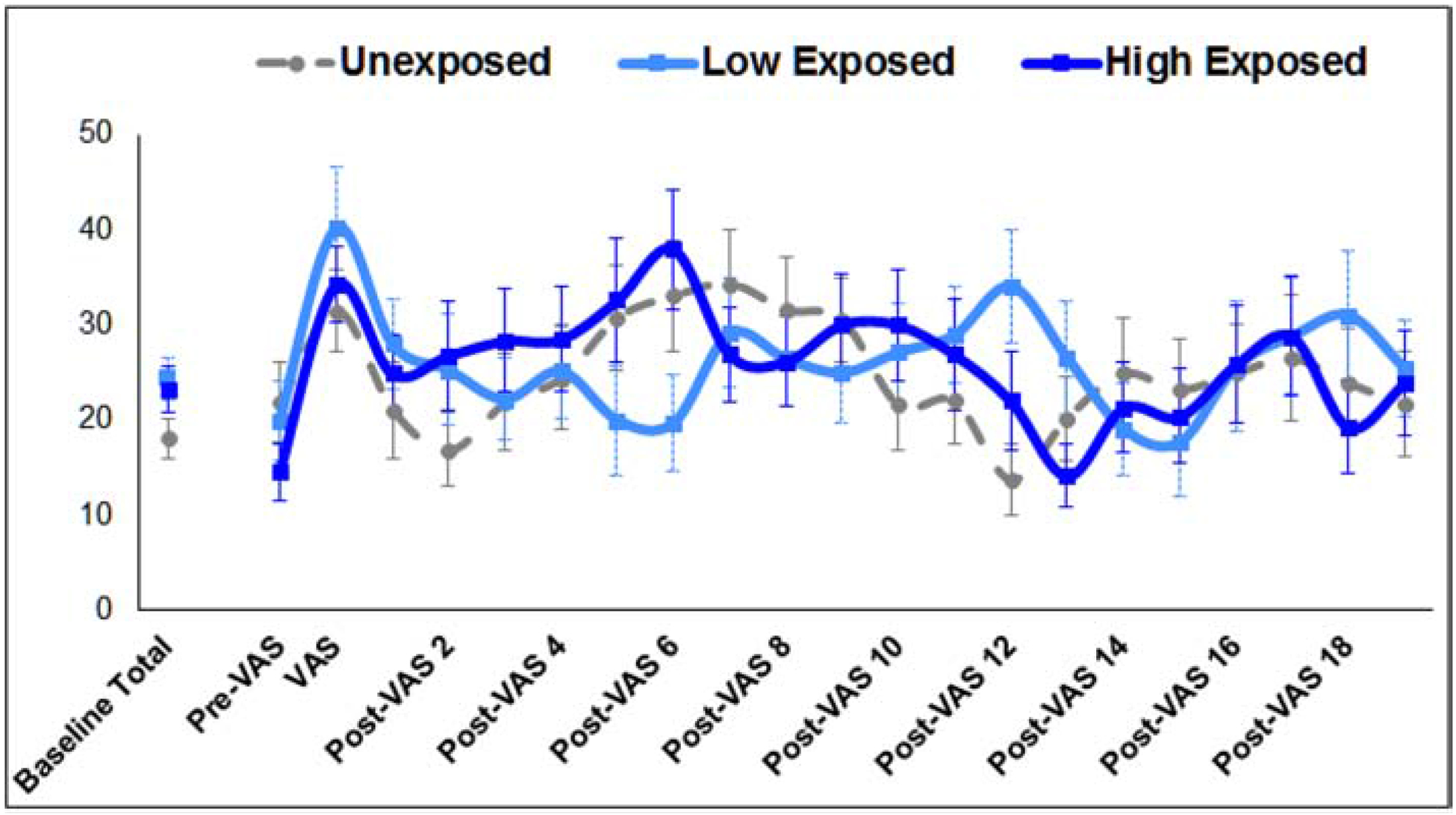
Mean percent time active for high MSDP-exposed, low MSDP-exposed and unexposed fetuses over mean baseline (4 minutes), and in 15-second epochs over immediate pre-VAS, VAS, and post-VAS periods.
To investigate the impact of GA on associations between MSDP and fetal activity, the GLMM model was run with the total % time with fetal activity during the baseline and VAS/post-VAS periods. Mean fetal activity in each period by group and summary statistics are shown in Table 2. A significant MSDP group by VAS period (baseline, post-VAS) interaction was found (F (2, 78) = 4.03, p = .022). Specifically, high- and low-MSDP exposed fetuses showed 5.1% and 6.2% greater activity at baseline versus unexposed fetuses (SEs = 3.3, 3.0%), although effects were not statistically significant for high-exposed fetuses (ps = .119 and .042, respectively). No significant group differences emerged for activity over the VAS/post-VAS period. Only unexposed fetuses showed a significant change in activity over the VAS/post-VAS period from baseline—showing a 6.9% (SE = 2.9%) increase in activity (t = 2.4, p < .03; ps for exposed groups > 0.17)). These associations were significantly moderated by GA (F (3, 91) = 8.21, p < .0001). As shown in Figure 2a, in the baseline period, there were no significant associations between baseline fetal activity levels and fetal GA (p = 0.25) for unexposed fetuses, however, high and low-exposed fetuses showed 3.0% and 2.9% decreased baseline fetal activity (SEs = 0.6, 0.7%) with each additional week fetal GA, respectively (ps <.0001). As shown in Figure 2b, in the post-stimulus period, only the high-exposed fetuses showed a significant association between fetal GA and fetal activity (p < .03); post-stimulus activity decreased by 1.4% (SE =0.6%) with each additional week GA. Finally, as shown in Figure 2c, while unexposed fetuses showed no change in reactivity to the VAS across fetal GA (p = 0.72), high and low MSDP-exposed fetuses showed 1.6% and 2.5% increased activity from baseline to post-VAS (SEs = 0.7, 0.8%) with each additional week fetal GA (ps < .02).
Table 2.
Descriptive statistics and general linear mixed models for fetal activity and complex body movements by smoking group. N=65.
| Fetal Activity (% Time Moving) | Complex Body (% Movement Movements Time) | |||
|---|---|---|---|---|
| Descriptive Statistics | ||||
| Baseline | VAS& Post-VAS | Baseline | VAS& Post-VAS | |
| Mean (SE, SD) | Mean (SE, SD) | Mean (SE, SD) | Mean (SE, SD) | |
| High-Exposed (n=22) | 23.2 (2.5, 11.7) | 26.4 (2.5,11.5) | 52.2 (5.0,23.5) | 62.0 (5.3, 24.9) |
| Low-Exposed (n=20) | 24.3 (2.2, 9.8) | 26.2 (2.4, 10.5) | 55.6 (5.2,23.3) | 56.1 (4.5,20.1) |
| Unexposed (n=23) | 18.0 (2.1, 10.1) | 24.9 (2.4,11.3) | 40.7 (5.1,24.5) | 53.3 (5.6, 26.9) |
| General Linear Mixed Models | ||||
| F (df) | P | F (df) | p | |
| Corrected Model | 10.12 (11, 118) | <.0001 | 7.21 (11,118) | <.0001 |
| MDSP group | 1.09 (2,89) | 0.341 | 0.37 (2, 74) | 0.691 |
| Gestation al Age (GA) | 28.95 (1,95) | <.0001 | 16.88 (1,60) | <.0001 |
| Vibro-Acoustic Stimulus (VAS) | 7.00 (1,78) | 0.010 | 4.08 (1,76) | 0.135 |
| MSDP * GA | 0.92 (2, 94) | 0.401 | 0.03 (2, 64) | 0.786 |
| MSDP * VAS | 4.03 (2, 78) | 0.022 | 10.57 (2, 78) | <.0001 |
| MSDP * VAS * GA | 8.21 (3,91) | <.0001 | 7.67 (3, 67) | <.0001 |
NOTE:MSDP = Maternal smoking during pregnancy; VAS = Vibro-acoustic stimulus; GA = gestational age.
Figure 2.

Percentage of fetal activity for high MSDP-exposed, low MSDP-exposed and unexposed fetuses by fetal gestational age during (A) Baseline (5 minutes), (B) VAS/post-VAS (5 minutes), and (C) change from baseline to VAS/post-VAS.
3.2.2. Complex Body Movements (CBM).
As shown in Table 2, significant associations were found between MSDP group and change in CBMs over mean baseline and mean VAS/post-VAS periods (F (2, 78) = 10.57, p < .0001). Specifically, high and low-MSDP exposed fetuses showed 14.8% and 11.4% greater percentage of CBMs at baseline versus unexposed fetuses (SEs = 7.1, 7.3%), although effects were not statistically significant for high-exposed fetuses (ps = .112 and .045, respectively). No significant group differences emerged in the VAS/post-VAS period. Only unexposed fetuses showed a change in CBMs in response to the VAS—showing a 12.6% (SE = 5.4%) increase in CBMs from baseline to VAS/post-VAS (t=2.3, p<.03)--while high- and low-MSDP exposed fetuses showed no change in CBMs in response to the VAS (ps > .12). These associations were significantly moderated by fetal GA (F (3, 67) = 11.83, p < .0001). As shown in Figure 3a, in the baseline period, there was no association between fetal GA and baseline CBMs for unexposed fetuses (p = 0.95), however, both high and low-exposed fetuses showed 6.4% and 5.2% decreased baseline fetal CBMs (SEs = 1.3, 1.4%) with each additional week fetal GA, respectively (ps <.001). As shown in Figure 3b, in the post-stimulus period, only the unexposed fetuses showed a significant association between fetal GA and CBMs (p < .02). Post-stimulus CBMs decreased by 5.3% (SE =1.8%) with each additional week GA. Finally, as shown in Figure 3c, high and low MSDP-exposed fetuses showed greater CBM reactivity with higher fetal GA, while unexposed fetuses showed decreased CBM reactivity with higher fetal GA. Specifically, high-exposed fetuses showed a 6.5% increase in CBM reactivity (SE = 1.4%) with each additional week fetal GA (p < .001), low-exposed fetuses showed a similar trend (3.1% increased CBM reactivity (SE = 1.6%) for each additional week fetal GA (p = .062)), and unexposed fetuses showed a 4.9% decrease in CBM reactivity (SE = 2.1%) for each additional week fetal GA (p < .03).
Figure 3.

Percentage of fetal CBMs for high MSDP-exposed, low MSDP-exposed and unexposed fetuses by fetal gestational age during (A) Baseline (5 minutes), (B) VAS/post-VAS (5 minutes), and (C) change from baseline to VAS/post-VAS.
3.3. Associations between maternal smoking and newborn behavioral state
Percentage of high and low MSDP-exposed and unexposed newborns in each behavioral state and the level of soothing required over the 10-minute post-handling assessment is shown in Figure 4. Mean behavioral state per minute and percent time in each state across the 10-minute post-handling period is shown in Table 3. For the first model focused on distribution of states across the 10-minute observation period, a significant MSDP group by state distribution interaction emerged (F (10, 220) = 1.9, p < .05). Relative to high MSDP-exposed newborns, (a) unexposed newborns spent a greater percentage of time in a quiet awake versus a crying state (B = 18.7, SE = 9.4, p < .05) and (b) low MSDP-exposed newborns spent a greater percentage of time in sleep-wake transition versus a crying state (B = 24.7, SE = 10.5, p < .02). In the second model focused on state over minutes of observation, a significant exposure group × time interaction emerged (F (18, 180) = 3.2, p = .0001): (a) high MSDP-exposed newborns showed higher scaled behavioral state than the low MSDP-exposed group in minutes 1 and 3 of the state observation period (ps < .05), (b) low MSDP-exposed showed lower scaled behavioral state than the unexposed group in minutes 3 and 4 of state observation (ps = .002, .056), (c) no differences in scaled behavioral state were evident after the first 4 minutes. However, while there were no group differences in state after minute 4, in the third model focused on the level of soothing techniques needed per minute, high MSDP-exposed newborns required increasing soothing techniques relative to unexposed newborns (F (18, 210) = 2.0, p < .01) from minute 4 (B = 1.4, SE = 0.7; t = 2.2, p < .04) through minute 10 (B = 2.7, SE= 0.9; t = 2.9, p < .004).
Figure 4.

Percentage of high and low MSDP-exposed and unexposed newborns in each behavioral state and mean level of soothing intervention required over the 10-minute post-handling observation period. N=58 (15 high exposed; 17 low exposed; 26 unexposed).
Table 3.
Mean behavioral state per minute and percent time in each state over the 10-minute post-handling observation period by smoking group. N=58.
| High-Exposed (n=15) Mean (SE, SD) | Low-Exposed (n=17) Mean (SE, SD) | Unexposed (n=26) Mean (SE, SD) | ||
|---|---|---|---|---|
| Mean State per Minute of Observation (Scaled 0–6) | ||||
| Minute 1 | 4.53 (0.25, 0.96) | 3.65 (0.26, 1.08) | 4.27 (0.20, 1.02) | |
| Minute 2 | 4.40 (0.26, 1.02) | 3.82 (0.31, 1.29) | 4.12 (0.20, 1.01) | |
| Minute 3 | 4.47 (0.36, 1.41) | 3.24 (0.20, 0.81) | 4.19 (0.20, 1.00) | |
| Minute 4 | 3.93 (0.29, 1.12) | 3.41 (0.28, 1.14) | 4.19 (0.17, 0.88) | |
| Minute 5 | 3.80 (0.34, 1.33) | 3.41 (0.22, 0.91) | 4.00 (0.20, 1.00) | |
| Minute 6 | 3.60 (0.31, 1.20) | 3.41 (0.24, 0.97) | 3.92 (0.20, 1.00) | |
| Minute 7 | 3.60 (0.35, 1.36) | 3.35 (0.18, 0.76) | 3.77 (0.19, 0.97) | |
| Minute 8 | 3.87 (0.26, 1.02) | 3.41 (0.25, 1.03) | 3.62 (0.20, 1.04) | |
| Minute 9 | 3.67 (0.40, 1.53) | 3.55 (0.21, 0.87) | 3.62 (0.20, 1.04) | |
| Minute 10 | 3.80 (0.36, 1.38) | 3.24 (0.18, 0.76) | 3.62 (0.24, 1.24) | |
| State Distribution over Observation Time (% Time in each State) | ||||
| Cry | 14.11 (4.49, 22.91) | 3.82 (1.57, 6.49) | 7.82 (2.67, 10.34) | |
| Active Awake | 18.67 (4.34, 22.15) | 7.94 (2.38, 9.79) | 17.05 (4.23, 16.39) | |
| Quiet Awake | 35.78 (6.22, 31.72) | 38.43 (8.37, 36.01) | 48.21 (6.81, 26.36) | |
| Sleep-Wake Transition | 19.44 (4.26, 21.70) | 33.88 (7.47, 30.78) | 16.79 (4.30, 16.67) | |
| Active Sleep | 8.56 (3.54, 18.07) | 15.63 (5.85, 24.14) | 9.68 (3.56, 13.80) | |
| Quiet Sleep | 3.44 (2.09, 10.67) | 0.29 (0.29, 1.18) | 0.45 (0.31, 1.19) | |
Analyses were also conducted restricting the sample to newborns ≤ 2 postnatal days (N=39). Restricting the sample did not alter pattern or statistical significance of results.
4. Discussion
MSDP remains one of the most common prenatal drug exposures (U.S. Department of Health and Human Services); growing availability of new tobacco products (hookah, electronic cigarettes) may further increase rates of exposure to prenatal nicotine/tobacco (Kurti et al., 2017; Liu et al., 2019). Some prior neurobehavioral studies of MSDP-exposed offspring suggest a profile of increased fussiness and difficulties with self-soothing in the neonatal period (Law et al., 2003; Mansi et al., 2007; Stroud et al., 2009a; Stroud et al., 2009b; U.S. Department of Health and Human Services). In the present study—an independent and parallel cohort from our group’s prior study of MSDP and fetal neurobehavior (Stroud et al., 2018)—we investigated whether MSDP-exposed fetuses would also show alterations in behavior—with a focus on fetal motor activity across a range of gestational ages. We also investigated the influence of MSDP on newborn behavioral state following handling. We found increased baseline fetal activity and altered reactivity to stimulation in MSDP-exposed fetuses, with evidence for differential patterns of fetal motor reactivity across gestational ages (GA) in MSDP-exposed versus unexposed fetuses. In newborns, heavy MSDP exposure was associated with increased arousal following handling and need for additional examiner soothing following handling.
We hypothesized that MSDP would be associated with increased fetal activity at baseline and following stimulation (VAS) based on prior studies showing increased arousal in MSDP-exposed fetuses and in the early newborn period (Gingras and O’Donnell, 1998; Law et al., 2003; Stroud et al., 2018; Stroud et al., 2009b). As hypothesized, we found increased motor activity at baseline in MSDP-exposed versus unexposed fetuses. However, contrary to our hypothesis, MSDP exposure was not associated with increased motor activity during stimulation. In general, all groups showed a robust, immediate motor response to the VAS. However, changes in motor activity from baseline to post-stimulus were dependent upon fetal GA and showed differing patterns by MSDP exposure. Unexposed fetuses showed significant motor reactivity to the VAS across the range of GA, while MSDP-exposed fetuses only showed motor reactivity to the VAS at later GAs. These effects may be due, in part, to differences in maturation of baseline motor activity; MSDP-exposed fetuses showed significantly lower baseline activity with increasing GA while unexposed fetuses showed no change in baseline activity with increasing GA. The pattern for CBMs was similar, with MSDP-exposed fetuses showing a lower proportion of movements that were large and complex with increasing GA, while unexposed fetuses did not show significant changes in CBMs with increasing GA.
Findings of associations between MSDP exposure and greater baseline fetal motor activity measured by ultrasound coding in the present study complement our group’s prior study documenting associations between MSDP exposure and increased fetal activity measured by actocardiograph at 35 weeks GA (Stroud et al., 2018). Links between MSDP and increased activity and body movements may be due to differences in structural or functional neurological maturation, in fetal behavioral states, and/or in rest-activity patterns related to MSDP. In our prior study, associations with MSDP were evident both before and following stimulation, whereas in the present study effects were only evident prior to stimulation. Further, in the present study, exposed fetuses showed increased complex body movements in the baseline period, while in our prior study, MSDP-exposed fetuses showed increased isolated but not complex movements. Differential results may be due to differences in the range of gestational ages in the two samples. Our prior study focused on later 3rd trimester fetuses only and did not include 2nd trimester fetuses (range 32–37 weeks; M = 35 weeks GA), while the present study included a wider range of GA (24–37 weeks, M = 28 weeks).
Whereas unexposed fetuses showed consistent response to the VAS across GA, exposed fetuses only showed increased motor reactivity to the VAS at later GAs--driven by a decreasing baseline activity but similar post-VAS activity over gestation. These findings contrast with our prior study, which revealed associations between MSDP and increased motor activity before and after the VAS in late 3rd trimester fetuses (unadjusted analyses). One potential explanation for differences in later gestation between our prior study and the present study is that the shorter coded observation period in the present study (10 versus 60 minutes) may have limited the range of behaviors observed in 3rd trimester. Because fetal rest-activity patterns develop into more organized behavioral states during 3rd trimester, fetal motor activity may be more likely to be state-dependent. Thus, a shorter fetal observation period may be sufficient to reveal exposure effects in later 2nd trimester, while a longer observation period may be needed to detect effects of MSDP on motor activity after 28 weeks GA. Future larger, longitudinal studies are needed to investigate patterns of basal and reactive fetal behavior across gestation in relation to MSDP.
Our study also revealed associations between MSDP and increased arousal in the newborn period using a rigorous observational coding system. As hypothesized, exposure to MSDP was associated with increased arousal following handling in the more heavily exposed MSDP group. Specifically, as hypothesized, more heavily MSDP-exposed infants showed higher levels of arousal and spent a greater proportion of time in an active awake or crying state in the first several minutes after handling. They also required more intensive soothing techniques in the later minutes of observation after handling. Our findings extend prior research demonstrating associations between MSDP and increased fussiness and need for external soothing during handling (Espy et al., 2011; Stroud et al., 2009a; Stroud et al., 2009b; Yolton et al., 2009). Evidence for increased arousal and need for external soothing following handling in the more heavily exposed infants may indicate prolonged arousal during recovery from daily stressors (handling). Prolonged periods of arousal in the infant are associated with poorer state stability, which may impact learning and brain development (Becker and Thoman, 1982; Scher, 2005). In addition, given associations between MSDP and poverty and other contextual stressors (Allen et al., 2019; Drake et al., 2018; Tong et al., 2013), the combination of a stressed mother and a highly reactive, difficult-to-soothe infant may lead to disruptions in parental-infant attachment. Contrary to our hypotheses, light MSDP-exposed infants showed attenuated arousal and spent a greater proportion of time in sleep-wake transition following handling. Findings regarding differing arousal levels (higher versus lower) in the high versus low MSDP-exposed infants are worthy of replication and highlight the importance of quantifying dose-response associations between MSDP and offspring behavior.
Strengths of the present study included: (a) detailed coding of fetal motor activity using ultrasound recordings, (b) MSDP measured by daily-level maternal interview and biochemical verification, (c) comparability of exposure groups on gravida, parity, other substance use, and infant characteristics, and (d) the low-income, ethnically-diverse sample. With respect to study limitations, associations between MSDP fetal motor activity over gestation were examined in a cross-sectional design in a relatively small sample that was a subset of a larger study. Ongoing studies by our group are investigating patterns of fetal behavior in a within-participants design across gestation in a larger sample. Additional limitations include: the lack of sham VAS condition to control for the felt effects of the VAS by the mother, the wide range of fetal gestational ages (24–37 weeks) and infant ages (postnatal days 1–10). Furthermore, although there is evidence that early behavioral state (e.g., first postnatal day) is a uniquely sensitive indicator of later neurodevelopment (Freudigman and Thoman, 1993), questions have been raised regarding the validity of neurobehavioral exams conducted prior to 20 hours (Xu et al., 2011). There may also be influences of labor and delivery on early measures of infant state. Finally, although we evaluated multiple relevant confounders, it is possible that associations between MSDP and fetal motor activity are related to other maternal characteristics that differed between smoking groups or other unmeasured confounders. Relatedly, although race/ethnicity was not found to be a significant covariate in our analyses, prior studies have found differential links between by MSDP and newborn neurobehavior by race (Yolton et al., 2009). Given well-documented differences in nicotine metabolism by race/ethnicity (Perez-Stable et al., 1998; Piliguian et al., 2014), future studies should investigate race as a moderator of associations between MSDP and fetal motor activity and newborn behavioral state. Future studies are also needed that integrate fetal physiological response with behavioral response and fetal state and that harness 3D and 4D ultrasound technology to investigate fetal behavioral development (Kurjak et al., 2008).
4.1. Conclusions
Study findings suggest that associations between MSDP and infant behavior may be evident prior to birth. Specifically, the profile of a smoking-exposed fetus may include increased basal motor activity suggestive of increased arousal as well as diminished and delayed motor response to stimulation. Results add to an emerging body of research highlighting the promise of direct measures of fetal behavior for identifying subtle effects of prenatal exposures. In the newborn period, high levels of MSDP exposure were associated with increased arousal following handling that may portend increased fussiness and difficulty soothing. Data from the present study may provide additional useful information for future public education campaigns. There is also a need for continued focus on resources and interventions to help pregnant and postpartum smokers quit and education efforts regarding fussiness and soothing techniques for families of smoking-exposed infants.
HIGHLIGHTS.
Investigated impact of maternal smoking on fetal motor activity and infant state.
Smoking-exposed fetuses are more active at baseline.
Motor response to stimulation emerges later in gestation for exposed fetuses.
Exposed newborns show higher arousal and need more soothing after handling.
Ultrasound monitoring of fetal behavior may identify at-risk infants.
ACKNOWLEDGMENTS
We are grateful to the families who contributed to this study, to Alana Corey and Katelyn Borba for their assistance with figures, and to Pamela Borek for administrative assistance.
ROLE OF THE FUNDING SOURCE
Preparation for this manuscript was supported by NIH grant R01 DA036999, R01 DA045492 and R01 DA019558, R01DA019558–02S1, and a Flight Attendant Medical Research Institute (FAMRI) Clinical Innovator Award to LRS and K23 MH65479 to ALS. The funding agencies had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Non-standard Abbreviations:
- MSDP
Maternal Smoking During Pregnancy
- VAS
Vibro-Acoustic Stimulus
- SES
socioeconomic status
- GA
Gestational Age
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors have no biomedical financial interests or potential conflicts of interest.
5. References
- Allen AM, Jung AM, Lemieux AM, Alexander AC, Allen SS, Ward KD, al’Absi M, 2019. Stressful life events are associated with perinatal cigarette smoking. Prev Med 118, 264–271. doi: 10.1016/j.ypmed.2018.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andonotopo W, Kurjak A, 2006. The assessment of fetal behavior of growth restricted fetuses by 4D sonography. J Perinat Med 34(6), 471–478. doi: 10.1515/JPM.2006.092 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG, 1988. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review 8(1), 77–100. [Google Scholar]
- Becker PT, Thoman EB, 1982. ‘Waking activity’: the neglected state of infancy. Brain Res 256(4), 395–400. [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B 57(1), 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Birnholz JC, Benacerraf BR, 1983. The development of human fetal hearing. Science 222(4623), 516–518. doi: 10.1126/science.6623091 [DOI] [PubMed] [Google Scholar]
- Birnholz JC, Stephens JC, Faria M, 1978. Fetal movement patterns: a possible means of defining neurologic developmental milestones in utero. AJR Am J Roentgenol 130(3), 537–540. doi: 10.2214/ajr.130.3.537 [DOI] [PubMed] [Google Scholar]
- Brown JL, 1964. States in newborn infants. Merrill Palmer Q 10, 313–327. [Google Scholar]
- Canivet CA, Ostergren PO, Jakobsson IL, Dejin-Karlsson E, Hagander BM, 2008. Infantile colic, maternal smoking and infant feeding at 5 weeks of age. Scand J Public Health 36(3), 284–291. doi: 10.1177/1403494807086981 [DOI] [PubMed] [Google Scholar]
- Cowperthwaite B, Hains SM, Kisilevsky BS, 2007. Fetal behavior in smoking compared to non-smoking pregnant women. Infant Behav Dev 30(3), 422–430. doi: 10.1016/j.infbeh.2006.12.004 [DOI] [PubMed] [Google Scholar]
- Das R, Jana N, Arora N, Sengupta S, 2019. Ultrasound assessment of fetal hearing response to vibroacoustic stimulation. J Matern Fetal Neonatal Med 10.1080/14767058.2018.1548600, 10.1080/14767058.2018.1548600, 1–7. doi: 10.1080/14767058.2018.1548600 [DOI] [PubMed] [Google Scholar]
- DiPietro J, 2001. Fetal Neurobehavioral Assessment, in: Zeskind PS, Singer JE (Eds.), Biobehavioral Assessment. In Press. [Google Scholar]
- DiPietro J, 2010. In the beginning, in: Zimmerman AW, Connors SL (Eds.), Maternal Influences on Fetal Neurodevelopment: Clinical and Research Aspects. Springer LLC. [Google Scholar]
- DiPietro JA, 2010. In the beginning, in: Zimmerman AW, Connors SL (Eds.), Maternal Influences on Fetal Neurodevelopment: Clinical and Research Aspects. Springer Science + Business Media. [Google Scholar]
- DiPietro JA, Ghera MM, Costigan KA, 2008. Prenatal origins of temperamental reactivity in early infancy. Early Hum Dev 84(9), 569–575. doi: 10.1016/j.earlhumdev.2008.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Hodgson DM, Costigan KA, Hilton SC, Johnson TR, 1996. Development of fetal movement--fetal heart rate coupling from 20 weeks through term. Early Hum Dev 44(2), 139–151. doi: 10.1016/0378-3782(95)01704-6 [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Kivlighan KT, Costigan KA, Rubin SE, Shiffler DE, Henderson JL, Pillion JP, 2010. Prenatal antecedents of newborn neurological maturation. Child Dev 81(1), 115–130. doi: 10.1111/j.1467-8624.2009.01384.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipietro JA, Voegtline KM, Pater HA, Costigan KA, 2018. Predicting child temperament and behavior from the fetus. Dev Psychopathol 30(3), 855–870. doi: 10.1017/S0954579418000482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenfeld AE, Pulkkinen A, Palomaki GE, Knight GJ, Haddow JE, 1993. Simultaneous fetal and maternal cotinine levels in pregnant women smokers. Am J Obstet Gynecol 168(3 Pt 1), 781–782. doi: 10.1016/s0002-9378(12)90818-2 [DOI] [PubMed] [Google Scholar]
- Drake P, Driscoll AK, Mathews TJ, 2018. Cigarette Smoking During Pregnancy: United States, 2016. NCHS Data Brief(305), 1–8. [PubMed] [Google Scholar]
- England L, Tong VT, Rockhill K, Hsia J, McAfee T, Patel D, Rupp K, Conrey EJ, Valdivieso C, Davis KC, 2017. Evaluation of a federally funded mass media campaign and smoking cessation in pregnant women: a population-based study in three states. BMJ Open 7(12), e016826. doi: 10.1136/bmjopen-2017-016826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy KA, Fang H, Johnson C, Stopp C, Wiebe SA, 2011. Prenatal tobacco exposure: developmental outcomes in the neonatal period. Dev Psychol 47(1), 153–156. doi: 10.1037/a0020724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudigman KA, Thoman EB, 1993. Infant sleep during the first postnatal day: an opportunity for assessment of vulnerability. Pediatrics 92(3), 373–379. [PubMed] [Google Scholar]
- Gingras JL, O’Donnell KJ, 1998. State Control in the Substance-Exposed: Fetus I. The Fetal Neurobehavioral Profile: An Assessment of Fetal State, Arousal, and Regulation Competency. Ann N Y Acad Sci 846(1), 262–276. doi: 10.1111/j.1749-6632.1998.tb09743.x [DOI] [PubMed] [Google Scholar]
- Goodman JD, Visser FG, Dawes GS, 1984. Effects of maternal cigarette smoking on fetal trunk movements, fetal breathing movements and the fetal heart rate. Br J Obstet Gynaecol 91(7), 657–661. doi: 10.1111/j.1471-0528.1984.tb04826.x [DOI] [PubMed] [Google Scholar]
- Gottfried AW, 1985. Measures of socioeconomic status in child development research: Data and recommendations. Merrill-Palmer Quarterly 31, 85–92. [Google Scholar]
- Graca LM, Cardoso CG, Clode N, Calhaz-Jorge C, 1991. Acute effects of maternal cigarette smoking on fetal heart rate and fetal body movements felt by the mother. J Perinat Med 19(5), 385–390. doi: 10.1515/jpme.1991.19.5.385 [DOI] [PubMed] [Google Scholar]
- Groome LJ, Bentz LS, Singh KP, Mooney DM, 1993. Behavioural state change in normal human fetuses following a single vibroacoustic stimulus: effect of duration of quiet sleep prior to stimulation. Early Hum Dev 33(1), 21–27. doi: 10.1016/0378-3782(93)90170-y [DOI] [PubMed] [Google Scholar]
- Groome LJ, Singh KP, Burgard SL, Neely CL, Deason MA, 1995. Motor responsivity during habituation testing of normal human fetuses. J Perinat Med 23(3), 159–166. doi: 10.1515/jpme.1995.23.3.159 [DOI] [PubMed] [Google Scholar]
- Haviland L, Thornton AH, Carothers S, Hund L, Allen JA, Kastens B, Wojciak A, Hamasaka L, Healton C, 2004. Giving infants a great start: launching a national smoking cessation program for pregnant women. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 6 Suppl 2, S181–188. doi: 10.1080/14622200410001669114 [DOI] [PubMed] [Google Scholar]
- Jacob P 3rd, Wilson M, Benowitz NL, 1981. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr 222(1), 61–70. doi: 10.1016/s0378-4347(00)81033-6 [DOI] [PubMed] [Google Scholar]
- Jacob P 3rd, Yu L, Wilson M, Benowitz NL, 1991. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3’,3’-d2 in humans. Biol Mass Spectrom 20(5), 247–252. doi: 10.1002/bms.1200200503 [DOI] [PubMed] [Google Scholar]
- Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y, 1987. Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health 77(11), 1435–1438. doi: 10.2105/ajph.77.11.1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge HD, 1979. Behavioral states and state related heart rate and motor activity patterns in the newborn infant and the fetus antepartum -- a comparative study. I. Technique, illustration of recordings, and general results. J Perinat Med 7(2), 85–107. doi: 10.1515/jpme.1979.7.2.85 [DOI] [PubMed] [Google Scholar]
- Kainer F, Prechtl HF, Engele H, Einspieler C, 1997. Assessment of the quality of general movements in fetuses and infants of women with type-I diabetes mellitus. Early Hum Dev 50(1), 13–25. doi: 10.1016/s0378-3782(97)00089-3 [DOI] [PubMed] [Google Scholar]
- Kisilevsky BS, Gilmour A, Stutzman SS, Hains SM, Brown CA, 2012. Atypical fetal response to the mother’s voice in diabetic compared with overweight pregnancies. J Dev Behav Pediatr 33(1), 55–61. doi: 10.1097/DBP.0b013e31823e791e [DOI] [PubMed] [Google Scholar]
- Kisilevsky BS, Muir DW, Low JA, 1990. Maturation of responses elicited by a vibroacoustic stimulus in a group of high-risk fetuses. Matern Child Nurs J 19(3), 239–250. [PubMed] [Google Scholar]
- Kisilevsky BS, Muir DW, Low JA, 1992. Maturation of human fetal responses to vibroacoustic stimulation. Child Dev 63(6), 1497–1508. [PubMed] [Google Scholar]
- Kurjak A, Carrera J, Medic M, Azumendi G, Andonotopo W, Stanojevic M, 2005. The antenatal development of fetal behavioral patterns assessed by four-dimensional sonography. J Matern Fetal Neonatal Med 17(6), 401–416. doi: 10.1080/14767050400029657 [DOI] [PubMed] [Google Scholar]
- Kurjak A, Stanojevic M, Andonotopo W, Salihagic-Kadic A, Carrera JM, Azumendi G, 2004. Behavioral pattern continuity from prenatal to postnatal life--a study by four-dimensional (4D) ultrasonography. J Perinat Med 32(4), 346–353. doi: 10.1515/JPM.2004.065 [DOI] [PubMed] [Google Scholar]
- Kurjak A, Tikvica A, Stanojevic M, Miskovic B, Ahmed B, Azumendi G, Di Renzo GC, 2008. The assessment of fetal neurobehavior by three-dimensional and four-dimensional ultrasound. J Matern Fetal Neonatal Med 21(10), 675–684. doi: 10.1080/14767050802212166 [DOI] [PubMed] [Google Scholar]
- Kurti AN, Redner R, Lopez AA, Keith DR, Villanti AC, Stanton CA, Gaalema DE, Bunn JY, Doogan NJ, Cepeda-Benito A, Roberts ME, Phillips J, Higgins ST, 2017. Tobacco and nicotine delivery product use in a national sample of pregnant women. Prev Med 104, 50–56. doi: 10.1016/j.ypmed.2017.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law KL, Stroud LR, LaGasse LL, Niaura R, Liu J, Lester BM, 2003. Smoking during pregnancy and newborn neurobehavior. Pediatrics 111(6 Pt 1), 1318–1323. doi: 10.1542/peds.111.6.1318 [DOI] [PubMed] [Google Scholar]
- Lehtovirta P, Forss M, Rauramo I, Kariniemi V, 1983. Acute effects of nicotine on fetal heart rate variability. Br J Obstet Gynaecol 90(8), 710–715. doi: 10.1111/j.1471-0528.1983.tb09299.x [DOI] [PubMed] [Google Scholar]
- Lester BM, Tronick EZ, Brazelton TB, 2004. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics 113(3 Pt 2), 641–667. [PubMed] [Google Scholar]
- Liu B, Xu G, Rong S, Santillan DA, Santillan MK, Snetselaar LG, Bao W, 2019. National Estimates of e-Cigarette Use Among Pregnant and Nonpregnant Women of Reproductive Age in the United States, 2014–2017. JAMA Pediatr 173(6), 600–602. doi: 10.1001/jamapediatrics.2019.0658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansi G, Raimondi F, Pichini S, Capasso L, Sarno M, Zuccaro P, Pacifici R, Garcia-Algar O, Romano A, Paludetto R, 2007. Neonatal urinary cotinine correlates with behavioral alterations in newborns prenatally exposed to tobacco smoke. Pediatr Res 61(2), 257–261. doi: 10.1203/pdr.0b013e31802d89eb [DOI] [PubMed] [Google Scholar]
- McCulloch CE, 1997. Maximum likelihood algorithms for generalized linear mixed models. Journal of the American Statistical Association 92(437), 162–170. doi:DOI: 10.1080/01621459.1997.10473613 [DOI] [Google Scholar]
- Milidou I, Henriksen TB, Jensen MS, Olsen J, Sondergaard C, 2012. Nicotine replacement therapy during pregnancy and infantile colic in the offspring. Pediatrics 129(3), e652–658. doi: 10.1542/peds.2011-2281 [DOI] [PubMed] [Google Scholar]
- Monk C, Sloan RP, Myers MM, Ellman L, Werner E, Jeon J, Tager F, Fifer WP, 2004. Fetal heart rate reactivity differs by women’s psychiatric status: an early marker for developmental risk? Journal of the American Academy of Child and Adolescent Psychiatry 43(3), 283–290. doi: 10.1097/00004583-200403000-00009 [DOI] [PubMed] [Google Scholar]
- Nijhuis IJ, ten Hof J, Nijhuis JG, Mulder EJ, Narayan H, Taylor DJ, Visser GH, 1999. Temporal organization of fetal behavior from 24-weeks gestation onwards in normal and complicated pregnancies. Dev Psychobiol 34(4), 257–268. doi: [DOI] [PubMed] [Google Scholar]
- Nijhuis JG, 1986. Behavioural states: concomitants, clinical implications and the assessment of the condition of the nervous system. Eur J Obstet Gynecol Reprod Biol 21(5–6), 301–308. doi: 10.1016/0028-2243(86)90008-0 [DOI] [PubMed] [Google Scholar]
- Oncken C, Kranzler H, O’Malley P, Gendreau P, Campbell WA, 2002. The effect of cigarette smoking on fetal heart rate characteristics. Obstet Gynecol 99(5 Pt 1), 751–755. doi: 10.1016/s0029-7844(02)01948-8 [DOI] [PubMed] [Google Scholar]
- Orleans T, Melvin C, Marx J, Maibach E, Vose KK, National Partnership to Help Pregnant Smokers, Q., 2004. National action plan to reduce smoking during pregnancy: the National Partnership to Help Pregnant Smokers Quit. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 6 Suppl 2, S269–277. doi: 10.1080/14622200410001669105 [DOI] [PubMed] [Google Scholar]
- Perez-Stable EJ, Herrera B, Jacob P 3rd, Benowitz NL, 1998. Nicotine metabolism and intake in black and white smokers. JAMA 280(2), 152–156. doi: 10.1001/jama.280.2.152 [DOI] [PubMed] [Google Scholar]
- Phelan JP, 1980. Diminished fetal reactivity with smoking. Am J Obstet Gynecol 136(2), 230–233. doi: 10.1016/0002-9378(80)90602-x [DOI] [PubMed] [Google Scholar]
- Piliguian M, Zhu AZ, Zhou Q, Benowitz NL, Ahluwalia JS, Sanderson Cox L, Tyndale RF, 2014. Novel CYP2A6 variants identified in African Americans are associated with slow nicotine metabolism in vitro and in vivo. Pharmacogenet Genomics 24(2), 118–128. doi: 10.1097/FPC.0000000000000026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai M, James D, 1990. Development of human fetal behavior: a review. Fetal Diagn Ther 5(1), 15–32. doi: 10.1159/000263530 [DOI] [PubMed] [Google Scholar]
- Pillai M, James DK, Parker M, 1992. The development of ultradian rhythms in the human fetus. Am J Obstet Gynecol 167(1), 172–177. doi: 10.1016/s0002-9378(11)91654-8 [DOI] [PubMed] [Google Scholar]
- Reijneveld SA, Lanting CI, Crone MR, Van Wouwe JP, 2005. Exposure to tobacco smoke and infant crying. Acta Paediatr 94(2), 217–221. doi: 10.1111/j.1651-2227.2005.tb01894.x [DOI] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, Leo GI, 2014. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav 28(1), 154–162. doi: 10.1037/a0030992 [DOI] [PubMed] [Google Scholar]
- Salisbury AL, 2010. Before Infant Assessment Fetal Neurobehavior, in: Lester BM, Sparrow JD (Eds.), Nurturing children and families: Building on the legacy of T. Berry Brazelton John Wiley & Sons, West Sussex, UK, pp. 29–39. [Google Scholar]
- Salisbury AL, Fallone MD, Lester B, 2005. Neurobehavioral assessment from fetus to infant: the NICU Network Neurobehavioral Scale and the Fetal Neurobehavior Coding Scale. Ment Retard Dev Disabil Res Rev 11(1), 14–20. doi: 10.1002/mrdd.20058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury AL, McFarland J, DiPietro J, 2009. Fetal behavioral indicators for state classification in Development Psychobiology., International Society for Development Psychobiology, 42nd Annual Meeting Chicago, IL, pp. 579–604. [Google Scholar]
- Scher A, 2005. Infant sleep at 10 months of age as a window to cognitive development. Early Hum Dev 81(3), 289–292. doi: 10.1016/j.earlhumdev.2004.07.005 [DOI] [PubMed] [Google Scholar]
- Smith CV, 1994. Vibroacoustic stimulation for risk assessment. Clin Perinatol 21(4), 797–808. [PubMed] [Google Scholar]
- Smith CV, Phelan JP, Broussard P, Paul RH, 1988. Fetal acoustic stimulation testing. III. Predictive value of a reactive test. J Reprod Med 33(2), 217–218. [PubMed] [Google Scholar]
- Sondergaard C, Henriksen TB, Obel C, Wisborg K, 2001. Smoking during pregnancy and infantile colic. Pediatrics 108(2), 342–346. doi: 10.1542/peds.108.2.342 [DOI] [PubMed] [Google Scholar]
- Stehlik G, Kainzbauer J, Tausch H, Richter O, 1982. Improved method for routine determination of nicotine and its main metabolites in biological fluids. J Chromatogr 232(2), 295–303. doi: 10.1016/s0378-4347(00)84169-9 [DOI] [PubMed] [Google Scholar]
- Stroud LR, McCallum M, Salisbury AL, 2018. Impact of maternal prenatal smoking on fetal to infant neurobehavioral development. Dev Psychopathol 30(3), 1087–1105. doi: 10.1017/S0954579418000676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Paster RL, Goodwin MS, Shenassa E, Buka S, Niaura R, Rosenblith JF, Lipsitt LP, 2009a. Maternal smoking during pregnancy and neonatal behavior: a large-scale community study. Pediatrics 123(5), e842–848. doi: 10.1542/peds.2008-2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Paster RL, Papandonatos GD, Niaura R, Salisbury AL, Battle C, Lagasse LL, Lester B, 2009b. Maternal smoking during pregnancy and newborn neurobehavior: effects at 10 to 27 days. J Pediatr 154(1), 10–16. doi: 10.1016/j.jpeds.2008.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler I, Goodman JD, Dawes GS, 1980. Effects of maternal cigarette smoking on fetal breathing and fetal movements. Am J Obstet Gynecol 138(3), 282–287. doi: 10.1016/0002-9378(80)90249-5 [DOI] [PubMed] [Google Scholar]
- Thoman EB, 1990. Sleeping and waking states in infants: a functional perspective. Neurosci Biobehav Rev 14(1), 93–107. doi: 10.1016/s0149-7634(05)80165-4 [DOI] [PubMed] [Google Scholar]
- Thoman EB, Davis DH, Denenberg VH, 1987. The sleeping and waking states of infants: correlations across time and person. Physiol Behav 41(6), 531–537. doi: 10.1016/0031-9384(87)90307-6 [DOI] [PubMed] [Google Scholar]
- Tong VT, Dietz PM, Morrow B, D’Angelo DV, Farr SL, Rockhill KM, England LJ, Centers for Disease, C., Prevention, 2013. Trends in smoking before, during, and after pregnancy--Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000–2010. MMWR Surveill Summ 62(6), 1–19. [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA, 2014; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [Google Scholar]
- U.S. Department of Health and Human Services, 2004. The Health Consequences of Smoking: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA. [Google Scholar]
- Wolff PH, 1973. Organization of behavior in the first three months of life. Res Publ Assoc Res Nerv Ment Dis 51, 132–153. [PubMed] [Google Scholar]
- Xu Y, Yolton K, Khoury J, 2011. Earliest appropriate time for administering neurobehavioral assessment in newborn infants. Pediatrics 127(1), e69–75. doi: 10.1542/peds.2010-1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton K, Khoury J, Xu Y, Succop P, Lanphear B, Bernert JT, Lester B, 2009. Low-level prenatal exposure to nicotine and infant neurobehavior. Neurotoxicol Teratol 31(6), 356–363. doi: 10.1016/j.ntt.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeskind PS, Gingras JL, 2006. Maternal cigarette-smoking during pregnancy disrupts rhythms in fetal heart rate. J Pediatr Psychol 31(1), 5–14. doi: 10.1093/jpepsy/jsj031 [DOI] [PubMed] [Google Scholar]


