Abstract
Neurogenic bowel following spinal cord injury (SCI) leads to decreased colonic motility, remodeling of the neuromuscular compartment and results in chronic evacuation difficulties. The distal colon of the rat serves a dual role for fluid absorption and storage that is homologous to the descending colon of humans. Dysmotility of the descending colon is one component of neurogenic bowel. We investigated the integrity of the enteric neuromuscular transmission responsible for the generation of excitatory and inhibitory junction potentials (EJPs and IJPs, respectively) in the distal colon of rats. We previously demonstrated a chronic reduction in colonic enteric neurons from rats with acute and chronic high-thoracic (T3) SCI and hypothesized that neurogenic bowel following T3-SCI results from diminished enteric neuromuscular transmission.
Immunohistochemical labeling for myenteric neuronal nitric oxide synthase (nNOS) and choline acetyltransferase (ChAT) neurons demonstrated a significant loss of presumptive nitric oxide (NO) and acetylcholine (ACh) immunoreactive neurons in both 3-day and 3-week injured animals. Colonic neuromuscular transmission in response to transmural electrical stimulation of the colon was significantly reduced 3-days and 3-weeks following SCI in male rats. Specifically, cholinergic-mediated excitatory junction potentials (EJPs) and nitrergic-mediated slow inhibitory junction potentials (IJPs) were significantly reduced while ATP-mediated fast IJPs remained unaffected.
We conclude that a reduction in excitatory and inhibitory enteric neuromuscular transmission contributes to neurogenic bowel observed following SCI, and that these loss-of-function changes involve enteric-mediated cholinergic and nitrergic pathways.
Keywords: Neurogenic bowel, colonic motility, myenteric plexus, junction potentials, enteric nervous system, constipation, colokinetics, nitric oxide, ATP, acetylcholine
Introduction
Neurogenic bowel is one of the most common comorbidities associated with spinal cord injury (SCI). Frequently manifested as a reduction in colorectal transit time, neurogenic bowel provokes significant side effects, including impaction, hemorrhoids, rectal bleeding, prolapse, formation of anal fissures, fecal incontinence, and chronic constipation (Lynch et al., 2001). For many injured individuals, the time requirements of a bowel program, loss of both convenience and independence, and potential episodes of incontinence are emotionally distressing aspects of SCI that profoundly impact quality of life. Furthermore, GI complications are associated with 11% of hospitalizations in the SCI population (Middleton et al., 2004).
The mechanism for neurogenic bowel is frequently attributed to the loss of descending input to the lumbosacral spinal cord innervating the bowel. This relationship has frequently led to an emphasis directed at damaged supraspinal regulation of somatic (Holmes et al., 1998;Holmes et al., 2005) and autonomic circuitry of the spinal cord (Chung & Emmanuel, 2005;Enck et al., 2005;Holmes et al., 2017). However, in addition to the sympathetic and parasympathetic neural control that regulates all other visceral organs, the gastrointestinal (GI) tract is unique in that it has its own extensive intrinsic nervous system, known as the enteric nervous system (ENS). The ENS is a complex network responsible for the quasi-autonomous regulation of secretory, segmentation and propulsive functions along the entire GI tract (Furness et al., 2014). For example, the ENS elicits the coordinated peristaltic reflex necessary for the orthograde movement of luminal contents. This circuit has an intrinsic polarity whereby the ascending limb ultimately innervates excitatory motor neurons and the descending limb innervates inhibitory motor neurons. A stimulus distending a point in the gut lumen elicits a contraction of smooth muscle proximal to the stimulus and relaxation of smooth muscle distal to the stimulus, thereby generating a pressure gradient that allows for the movement of luminal contents. Although the ENS can function independently, the extrinsic sympathetic and parasympathetic systems modulate the intrinsic reflexes and coordinate GI activity with the homeostatic requirements of the whole organism (Furness et al., 2014) and the complex integrated activities requiring both voluntary and reflexive muscular control, such as defecation, are significantly impaired by SCI (Lynch et al., 2001).
The essential neuromuscular responses during reflex colonic contraction and relaxation can be experimentally recorded intracellularly as smooth muscle excitatory and inhibitory junction potentials (EJPs and IJPs, respectively; Furness, 1969;Strong et al., 2010). Electrophysiological studies have shown nitrergic and purinergic signaling to be the major inhibitory neurotransmission pathways within most GI tissues, including colonic circular muscle (Keef et al., 1993;Serio et al., 2003;Serio et al., 1995;Spencer et al., 1998). Both excitatory and inhibitory junction potentials are important to facilitate peristalsis, but unlike EJPs, IJPs rely on two different types of neurotransmitters. Waterman and Costa (1994) demonstrated that inhibiting both nitrergic and purinergic neurotransmitters has consequences on peristalsis, but peristaltic contractions are present when either inhibitory pathway is preserved, albeit significantly diminished (Waterman & Costa, 1994). This excitatory and inhibitory output from the ENS to the intestinal smooth muscle is controlled by roughly 20% of the total number of enteric neurons (Bornstein et al., 2004). Studies examining neuromuscular transmission at areas of colitis-induced ulceration have found that, while there is no change in the EJP, there is a significant decrease in the amplitude of the IJP, specifically in the amplitude of the fast, purinergic component of the IJP (Roberts et al., 2013;Strong et al., 2010). These studies further demonstrated that the decreased purinergic transmission may play a significant role in the slowed motility seen in colitis.
We have previously demonstrated that following experimental T3-SCI, the colon undergoes changes to the neuromuscular compartment that have been seen in similar human bowel disease states (den Braber-Ymker et al., 2017). These changes include increased collagen content and thickening of the muscularis propria as well as increasing neuronal loss within the myenteric plexus (White & Holmes, 2018). Furthermore, cholinergic sensitivity of colonic smooth muscle strips is reduced after T3 spinal transection (Frias et al., 2019). To date, a fully developed model of colonic dysmotility following a clinically relevant contusion model of traumatic SCI has been lacking.
In our rodent model, we tested the hypothesis that T3-SCI provokes changes in neuromuscular transmission and a reduction in colonic contractility. We investigated if T3-SCI alters excitatory and inhibitory neuromuscular transmission ex-vivo, and identified the neurochemical phenotype of the enteric neurons with immunohistochemistry in order to specify the excitatory and inhibitory populations lost following T3-SCI.
Materials and methods
Animals
All procedures followed National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee at the Pennsylvania State University College of Medicine. Male Wistar rats ≥6 weeks of age (Hsd:WI, Stock 001, Envigo, Indianapolis, IN, USA) upon entrance into the experiment, initially weighing 150–200 g; were double housed in a room maintained at 21–24 °C and a 12:12-h light–dark cycle with food and water provided ad libitum.
Surgical procedures and post-operative care
Rats (n=45) were randomly assigned for SCI or surgical control surgery and each group was further divided into 3-day and 3-week postsurgical study groups. After being deeply anesthetized with 3–5% mixture of Isoflurane with oxygen (400–600ml/min), surgery for T3-contusion SCI was performed using established surgical techniques as previously described in detail (White et al., 2018). Briefly, upon completion of surgical exposure of the T2 laminectomy site, the adjacent T1 and T3 vertebrae were clamped into the Infinite Horizons SCI device (Precision Systems and Instrumentation, Lexington KY) and a 300 kDyne impact (15 sec dwell time) was initiated. Surgical controls underwent all procedures except for the spinal impact.
Post-operatively, rats were administered supplemental fluids by injection of 5cc warmed lactated Ringer’s solution (s.c.) and stabilized in an incubator (37°C) until fully recovered from anesthesia. Animals were monitored daily for any signs of infection or complications from surgery. Body weight (bw) and food intake were measured daily for all animals as an index of well-being and rats were administered post-operative care as previously described (White et al., 2018).
Colonic and spinal tissue harvest
Deeply anesthetized rats were exsanguinated via decapitation. For each animal, colonic tissue (proximal and distal) was isolated and immediately placed in ice-cold aerated Krebs solution (10 ml min−1; mmol l−1: NaCl, 121; KCl, 5.9; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 25; NaH2PO4, 1.2; and glucose 8; aerated with 95%O2/5%CO2; all chemicals purchased from Sigma, St Louis, MO, USA). Nifedipine (5 μM; Sigma-Aldrich) was added to all bath solutions to block smooth muscle contractile activity. The distal colon was rapidly dissected in cold Krebs by cutting along the mesenteric border and pinned flat. Next, the mucosal and submucosal layers were removed to expose the circular muscle layer. A 2–3cm portion of the smooth muscle tissue was excised and transferred to a Sylgard-lined recording chamber of our own design. The smooth muscle was then perfused with warmed (ca. 35°C) aerated Krebs and remained undisturbed for 1 hour before recordings were initiated.
An additional 5mm intact section of distal and proximal colon tissue was immediately post-fixed in 4% paraformaldehyde for 24 hours and then transferred to 70% ethanol for an additional 24 hours prior to paraffin embedding. For whole mount preparations for immunohistochemistry, the remaining distal and proximal tissue samples were immediately placed in ice-cold aerated Krebs before being opened along the mesenteric border and pinned flat. The mucosal and submucosal layers were removed and the tissue fixed in situ in 4% paraformaldehyde.
The vertebrae and spinal cord at the level of the lesion were removed en bloc and postfixed in 4% paraformaldehyde. Following fixation, the spinal cord was extracted from the vertebrae for sectioning and histological processing of T3-SCI lesion extent.
Electrophysiological recording
Intracellular recordings from individual smooth muscle cells were obtained using glass microelectrodes pulled with a Flaming/Brown Micropipette puller (Sutter Instruments, p-97, Novato, CA), filled with 2 M KCl, and that had resistances ranging from 80 to 120 MΩ. A Nikon TMZ inverted microscope was used at 200× magnification to visualize the smooth muscle cells of the circular muscle layer. Voltage recordings were obtained with an Axoclamp-2A amplifier (Axon Instruments, Union City, CA, USA), and signals digitized and analyzed with Spike2 (version 8.01; Cambridge Electronic Design, Cambridge, England). Smooth muscle cells were impaled at random. Impalements were accepted if 1) the membrane potential was −35 mV or lower and 2) if the recording was stable for at least 5 minutes.
For intracellular recordings of IJPs, the tissue was pinned so that the oral aspect of the colon was positioned perpendicularly relative to two parallel silver/silver chloride wire electrodes connected to a stimulus isolation unit (SIU5, Grass Instruments, Quincy, MA) driven by a two-channel stimulator (S88, Grass Instruments). Due to the polarity of the descending inhibitory motor neurons, evoked inhibitory junction potentials (IJPs) were recorded aborally to the transmural stimulating electrodes. The aboral aspect was pinned flat within the recording region of the chamber to expose the circular muscle layer for recording electrodes. The tissue was immediately and continuously exposed to warmed and aerated circulating Krebs solution and allowed to equilibrate for 1 hour. For intracellular recordings of EJPs, the tissue was pinned so that the aboral aspect of the tissue was positioned over the stimulating electrodes and the oral aspect was pinned flat within the recording region of the chamber. The recording region of the chamber for all intracellular recordings was 7–10mm from the stimulating electrodes.
For IJP recording experiments, atropine (1 μM; Sigma-Aldrich) was added to the circulating Krebs solution to block spontaneous and evoked cholinergic activation of smooth muscle. In order to isolate the nitrergic (slow IJP) and purinergic (fast IJP) components, two separate solutions were prepared. Both preparations comprised of 200 mL of Krebs solution containing nifedipine (5 μM) and atropine (1 μM). For the isolation of the nitrergic component, one Krebs solution also contained MRS-2179 (3 μM; Sigma-Aldrich) to block the P2Y1 purinergic receptors. To isolate the purinergic component, the Krebs solution contained N-nitro-L-arginine (L-NNA; 100 μM; Sigma-Aldrich) to block nitric oxide synthase.
For EJP recording experiments, separate stock solutions of MRS-2179 (3 μM) or L-NNA (100 μM; both from Sigma-Aldrich) were prepared with the addition of nifedipine to the circulating Krebs solution to block spontaneous and evoked purinergic and nitrergic activation, respectively, of smooth muscle.
Transmural stimuli (55 ms pulse duration, 0.1 Hz, 50V) were used to activate junction potentials. For IJPs, once consistent baseline responses were established and recorded, the circulating Krebs solution was changed to the MRS-2179 containing solution. Effects of the MRS-2179 could be seen approximately 2–3 minutes after its circulation began. Once the fast component was fully blocked, the slow nitrergic IJP response (sIJP) was recorded for approximately 10 minutes. At completion of recording the sIJP signal, the bath solution containing MRS-2179 was then returned to the normal recording Krebs solution. After return of the complete IJP response (10–30 minutes) the normal recording Krebs solution was switched for the L-NNA containing solution. Due to the irreversible inhibition of nitric oxide (NO) synthesis by L-NNA (Dwyer et al., 1991), the effects of L-NNA were always tested last. Effects of the L-NNA could be seen starting within 1–2 minutes of its circulation, however, its full effect could not be appreciated until approximately 10 minutes. As with the MRS-2179, once it was determined that the sIJP component was fully blocked, the purinergic response was recorded for approximately 10 minutes. During the recording of EJPs, the stock Krebs solution was first utilized in order to record IJPs as evidence of tissue viability. Once the tissue was deemed acceptable, the MRS-2179 + L-NNA solution was introduced and EJPs were randomly recorded. Due to the nature of EJP recordings (in which all other activity is blocked), tissue recordings were only attempted across a two-hour period of time. In order to validate that the tissue remained viable after two-hours, the stock Krebs solution was returned to the chamber. Following sufficient washout of the MRS-2179, the tissue was deemed viable if a fIJP could be elicited.
At the completion of the recordings, the maximum amplitude for the appropriate junction potential was calculated. For each animal, 10 data points for each component were randomly selected. The 10 randomly selected data points were then averaged for each animal.
Histological processing of spinal cord
The spinal cord encompassing the lesion epicenter was embedded in OCT and sectioned at 40 μm thick with a cryostat. Alternate coronal sections were mounted onto gelatin-coated slides. To verify lesion severity or control, spinal cord sections were stained with luxol fast blue (LFB) to visualize myelinated fibers.
LFB-stained slides were digitally imaged on a Zeiss Axioscope light microscope and Axiocam CCD camera, imported into Adobe Photoshop and contrast digitally enhanced to allow identification and threshold measurements of LFB-stained (i.e., spared) white matter. For individual images, the boundaries of the tissue slice were outlined to determine cross-sectional area. A separate threshold histogram was generated and the pixels corresponding to LFB staining above background were selected. These pixels were quantified and expressed per unit cross-sectional area (Noble & Wrathall, 1985). The proximity of the T3 lesion center to the cervical enlargement precluded an appropriate determination of spinal cord cross-sectional area in undamaged tissue rostral to the injury (i.e., damaged tissue extended into the cervical enlargement as described in Tong & Holmes, 2009). Therefore, it was necessary that the cross-sectional area of the intact spinal cords at T3 of comparably sized animals be determined for normalization purposes. LFB-stained myelin in injured tissue was then expressed as a percent of the total spinal cord cross-sectional area as would be predicted by the intact tissue. Based upon previous reports (Scheff et al., 2003;Tong et al., 2011;Totoiu & Keirstead, 2005) we determined a priori that animals sacrificed 3 days following surgery in which LFB staining at the lesion epicenter accounted for ≤ 25% of the region occupied by white matter would be categorized as having received a severe spinal injury; those with ≥ 25% LFB staining were excluded from further analysis (n=1 T3-SCI rat met this criterion). This criterion is based upon the observation that considerable LFB-staining remains within the lesion center in the 1–3 days following injury, though the majority of the LFB-stained tissue likely consists of remaining myelinated axons as well as myelin debris in a loose fibrous matrix as reported previously (Totoiu et al., 2005). Historically, our animals with the same 300 kDyne injury that are sacrificed 3 weeks after injury display ≤ 5% of LFB staining above threshold as the lesion center is clear of cellular debris. After 3 weeks any remaining LFB staining is usually confined to a thin band within the ventrolateral white matter in a manner consistent with previous reports characterizing a 200 kDyne injury level (Scheff et al., 2003).
Colonic tissue processing for immunohistochemistry
Fixed tissue samples from the colon were processed in an automated Tissue-Tek VIP processor and paraffin-embedded with a Tissue-Tek TEC embedding station (Sakura Finetek USA, Torrance, CA). Sections were serially cross-sectioned at 6 μm and processed for enteric neuronal immunohistochemistry. Following deparaffinization and rehydration (including a peroxidase wash to quench endogenous peroxidases), samples were taken and subjected to heat-mediated antigen retrieval in 10 mM sodium citrate buffer for 20 minutes. Non-specific antibody binding was reduced by incubation in UltraVision Protein Block (TA060PBQ; Thermo Scientific) for 5 minutes at room temperature. The sections were incubated for 2 hours at room temperature in a primary antibody cocktail containing either Hu/CD, nNOS or ChAT antibody and 2% bovine serum albumin (BSA; dilutions in Table 1). Following the incubation, the tissue underwent three 2 minute washes in wash buffer and then incubated in a secondary antibody cocktail (Table 1) for 1 hour at room temperature. Labeling was localized using diaminobenzadine (DAB) and sections were counterstained with hematoxylin.
Table 1.
Primary and secondary antibodies, and their respective dilutions, used for immunohistochemistry
| Host species | Dilution | Source | ||
|---|---|---|---|---|
| Primary antibodies | nNOS | Goat | 1:1000 | Abcam |
| ChAT | Rabbit | 1:2000 | Abcam | |
| Secondary antibodies | Anti-goat IgG-Peroxidase |
Rabbit | 1:100 | Sigma-Aldrich |
| Anti-rabbit IgG-Peroxidase |
Goat | 1:100 | Sigma-Aldrich |
Using a previously described protocol to determine the number of neurons per enteric ganglion area (Rivera et al., 2014), images of ChAT and nNOS positive ganglia from each tissue section were analyzed using Image J software (NIH, Bethesda, MD, USA). Immunopositive neurons were determined by the density of staining above a pre-determined threshold intensity. All specimens were scored according to the same threshold level and were scored blinded to group identity. Individual ganglia demonstrate variable cross-sectional size within the intact myenteric plexus and this might be reflected in what region of the ganglion appears in the plane of section. While reduction of ganglion area could be expected as a result of neuronal loss, differences in cross sectional area are deemed to be a greater source of variability. To normalize quantification of the number of neurons, total ganglion area was measured (as μm2) and the number of ChAT or nNOS positive neurons within the designated ganglion area were counted and calculated as the number of neurons per unit area.
Statistical analysis
The occurrence of outliers was tested using a Grubbs test macro written in GraphPad (GraphPad Software, La Jolla, CA). No significant outliers were identified for removal from further analysis. Tissue unresponsive to transmural electrical field stimulation was considered to be a non-viable smooth muscle recording preparation (n=4 preparations).
All subsequent statistical calculations were performed using SigmaPlot software (version 12.5; Systat Software, Inc., San Jose, CA, USA). Between groups results from anatomical or ex vivo studies were compared by Student’s T-test or ANOVA followed by Tukey post hoc analysis as appropriate. Results are expressed as means ± SEM with significance defined as P<0.05.
Results
Verification of 300 kdyne injury level
Our 300 kdyne lesion, with a 15 sec dwell time, historically produces very little white matter sparing at the lesion epicenter which is necessary to affect the diffuse distribution of descending supraspinal inputs to autonomic preganglionic centers (Besecker et al., 2017;Swartz & Holmes, 2014). The size of the lesion epicenter was determined by calculating the reduction of LFB-stained white matter at the T3 spinal cord segment (Fig. 1). The white matter calculated as percent area of total spinal cord cross-sectional area at the lesion epicenter of 3-day T3-SCI rats was significantly reduced in comparison to T3-control animals (4.3±0.7% vs. 71.1±1%, respectively; p < 0.05). At 3-weeks, the post-injury progression of the lesion epicenter approaches a plateau and the lesion boundaries are more clearly established (Hill et al., 2001), the percent area of spared white matter at the lesion epicenter of 3-week T3-SCI rats was significantly reduced in comparison to age-matched T3-control animals (4.3±1.0% vs. 74.6±0.3%, respectively; p < 0.05). These data are comparable to the injury extent reported previously and indicate the severity of our injury model (Qualls-Creekmore et al., 2010;Swartz et al., 2014;Tong et al., 2009;Tong et al., 2011). One T3-SCI rat exhibited only unilateral damage and was excluded from further analysis.
Figure 1.
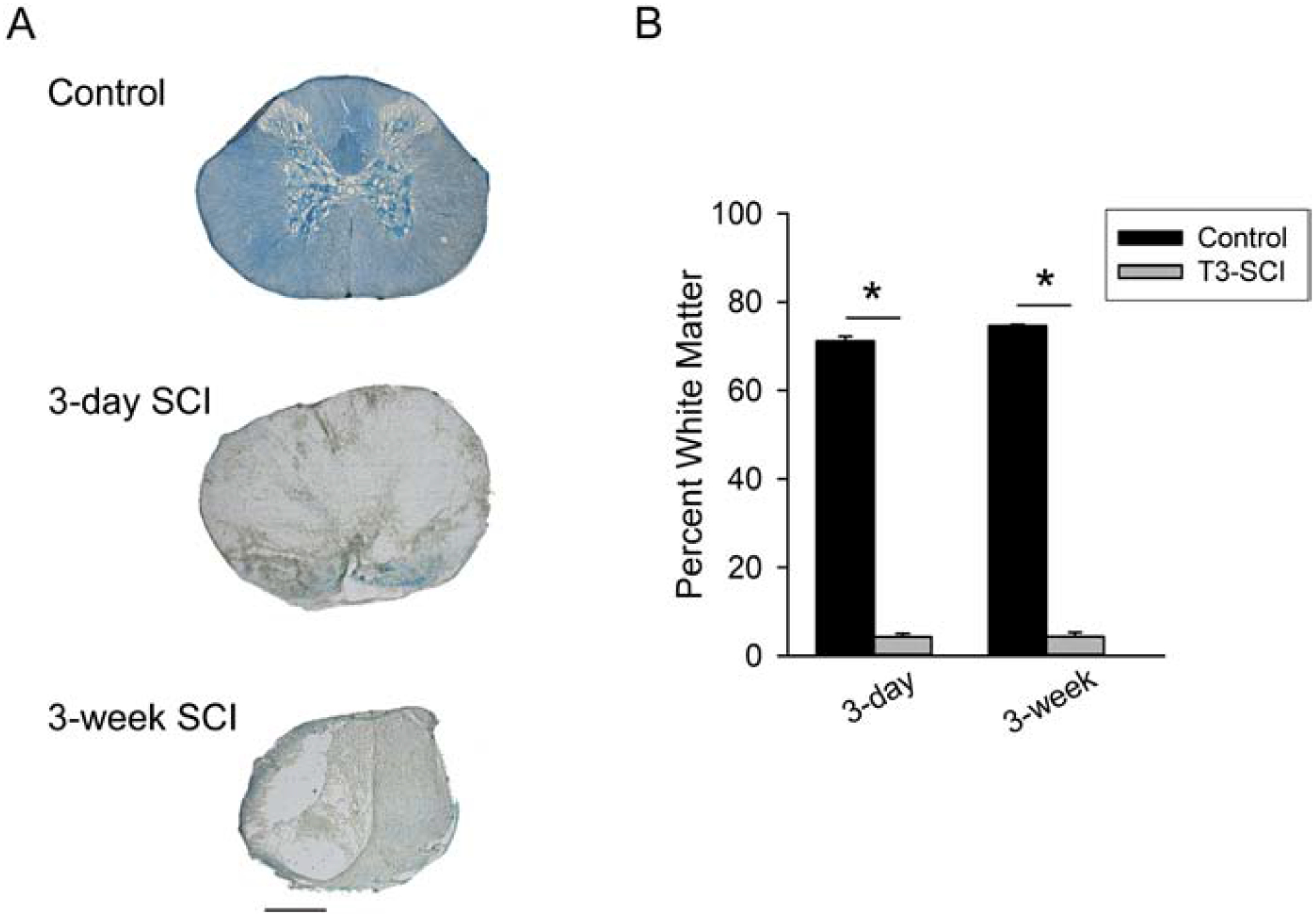
Luxol-stained white matter from T3-level of the spinal cord (A) from surgical control; 3-day postoperative T3-SCI; and 3-week postoperative T3-SCI rats (calibration = 500 μm). B) graphic summary of the percent sparing of white matter at the lesion epicenter of control, 3-day, or 3-week rats following a 300-kdyn contusion spinal cord injury (SCI) (*p < 0.05 vs. post-operative time-matched controls).
nNOS-immunopositive neuron populations of the distal colon are reduced following T3-SCI
Following T3-SCI there is a significant decrease of overall enteric neuron populations as measured by HuC/D-positive cells (White et al., 2018). The reduced slow IJP amplitudes suggested a reduction of nNOS specific neuron populations within the distal colon (Fig. 2). The number of nNOS-positive neuronal populations within the myenteric ganglia of the distal colon were significantly decreased in 3-day and 3-week T3-SCI animals (Fig. 2B; p < 0.002). There was no difference between the two groups of injured animals or between the two groups of surgical controls.
Figure 2.
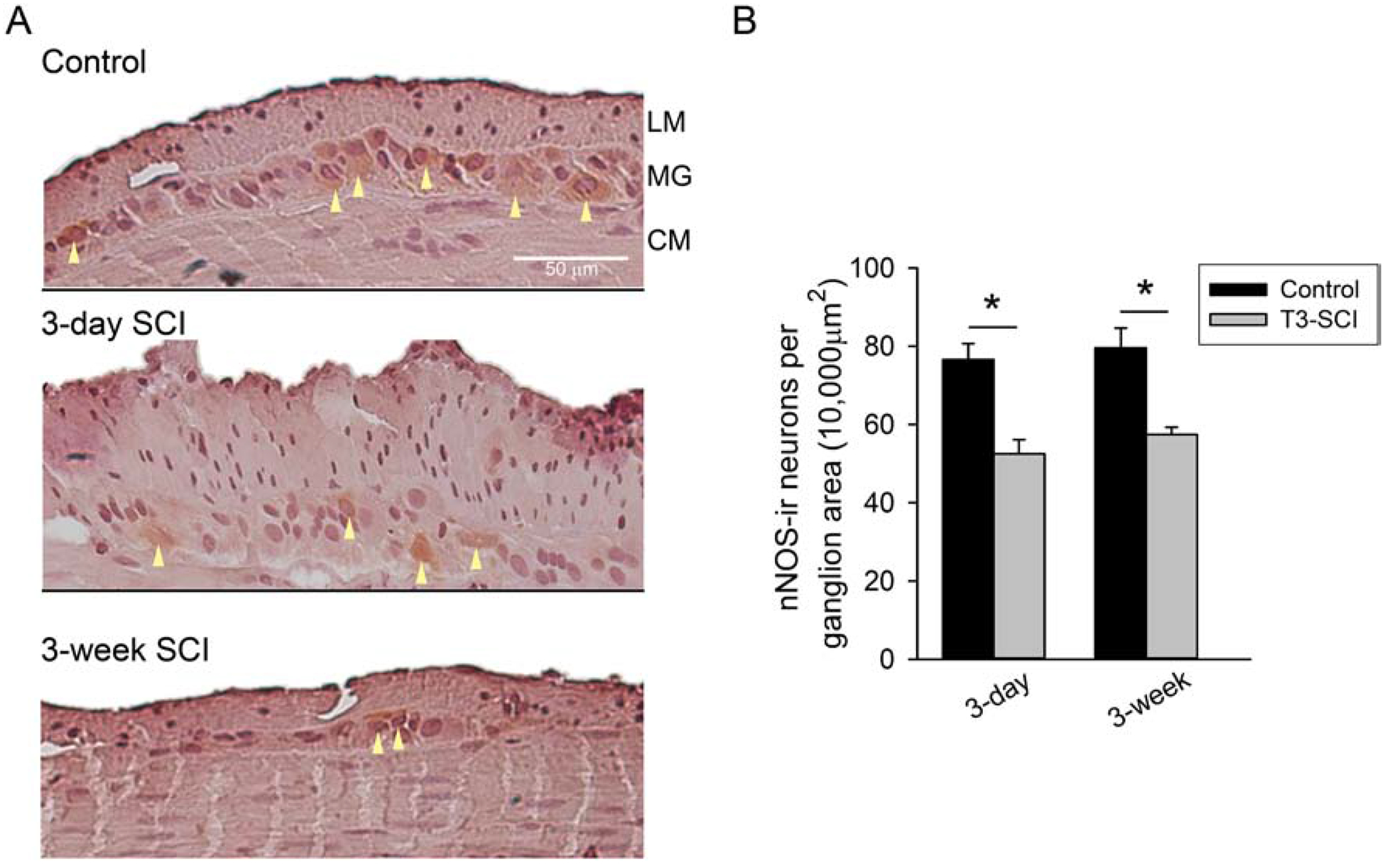
A) nNOS-immunoreactive tissue sections from the distal colon of control (top), 3-day T3-SCI (middle) or 3-week T3-SCI (bottom). Representative cells exceeding staining detection threshold denoted by arrowheads. Tissues sections are from distal colon samples adjacent to those harvested from rats used for intracellular recordings (X400, scale bar = 50 μm). B) T3-SCI provokes a significant reduction in nNOS immunoreactivity in the myenteric ganglia (Values expressed as mean ± SEM, *p < 0.05 vs. post-operative time-matched controls, n = 24).
These results suggest that enteric neuron populations are not only reduced acutely following SCI, but neuronal numbers do not immediately recover. It is unclear if this loss of nNOS-positive neurons, and the subsequent loss of inhibitory neural input to the smooth muscle of the colon, is sufficient to provoke impaired motility following SCI.
ChAT neuron populations of the distal colon are reduced following T3-SCI
Myenteric ganglia from the distal colon of T3-SCI rats displayed less ChAT immunoreactivity (Fig. 3). ChAT-positive neuronal cell counts were significantly decreased in 3-day and 3-week T3-SCI animals (Fig. 3B; p < 0.002). There was no difference between the two groups of injured animals or between the two groups of surgical controls.
Figure 3.
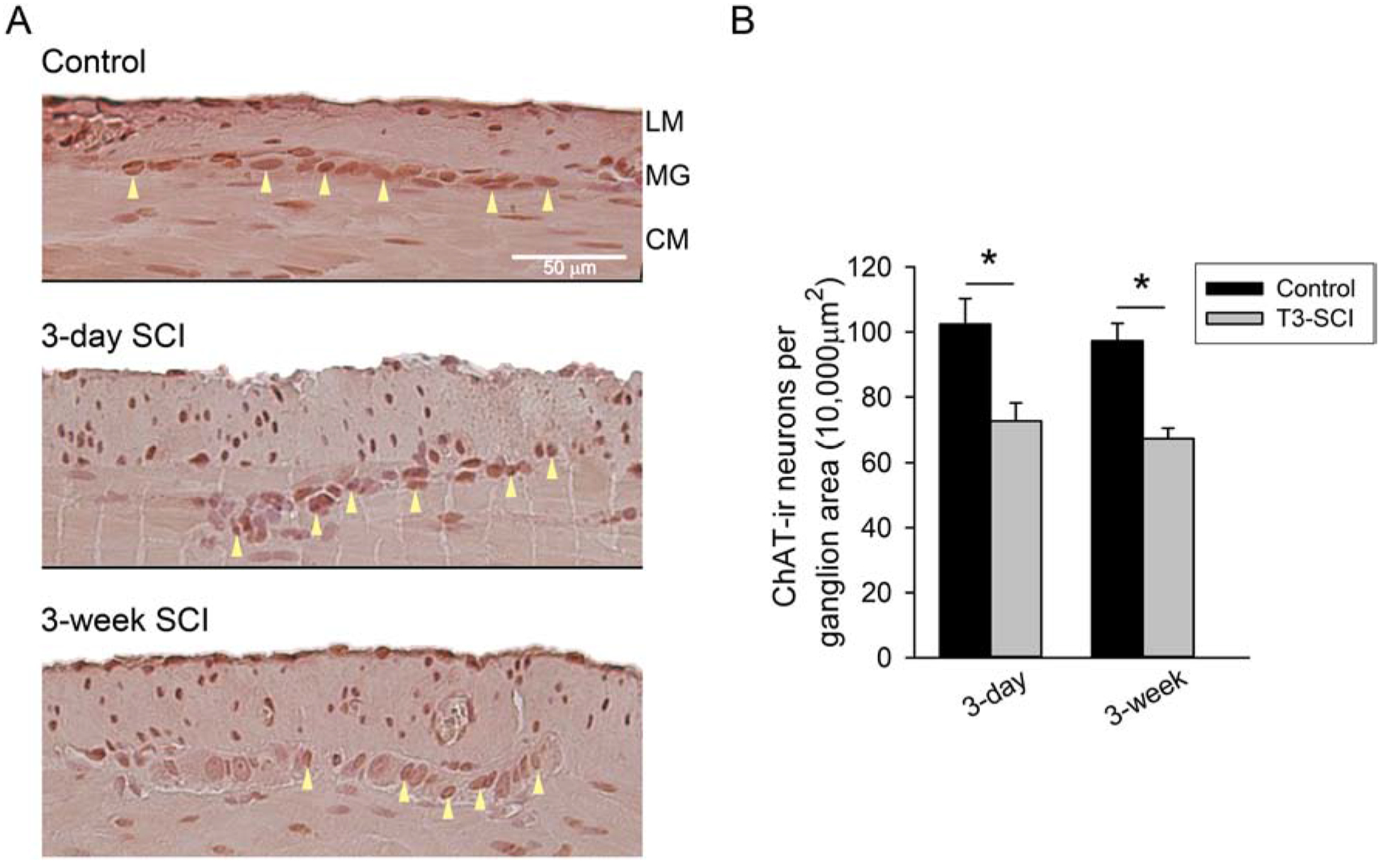
A) ChAT-immunoreactive tissue sections from the distal colon control (top), 3-day T3-SCI (middle) or 3-week T3-SCI (bottom). Tissues sections are from separate distal colon samples harvested from rats used for intracellular recordings (X400, scale bar, 50 μm). B) ChAT-positive neurons were reduced following T3-SCI in the distal colon (Values expressed as mean ± SEM, *p < 0.05 vs. post-operative time-matched controls, n = 24).
These results, combined with our findings for nNOS-immunopositive neurons, suggest that two enteric neural populations that are critical for the coordinated contraction and receptive-relaxation of the colonic smooth muscle in order to achieve proper colonic propulsion are not only reduced acutely following SCI, but remain diminished long-term. It is unclear if the remaining purinergic inhibitory component is sufficient for receptive relaxation in colonic propulsive reflexes following SCI. Since transit of fecal pellets do continue to occur in injured rats, albeit in a reduced manner, it is unclear if compensatory enteric or extrinsic mechanisms develop to mediate colonic propulsion or if the residual cholinergic/nitrergic neural circuits are adequate once an “overflow” condition is reached within the rodent colon.
Membrane properties of distal colon smooth muscle cells
The essential neuromuscular building blocks of reflex colonic contraction and relaxation can be experimentally provoked by electrical stimulation and recorded intracellularly as smooth muscle excitatory and inhibitory junction potentials (EJPs and IJPs, respectively; Furness, 1969;Strong et al., 2010). Upon successfully impaling a smooth muscle cell and establishing a stable recording, the resting membrane potential (RMP) was established. The RMP of smooth muscle cells from the distal colon was not significantly different between T3-SCI and control rats at either 3 days or 3 weeks after surgery (Table 2). Since the bath solution for detecting EJPs is also devoid of atropine, statistical comparisons were only for the resting membrane potential of tissues in identical bath solutions.
Table 2.
Resting membrane potential of distal colon circular muscle
| Control | T3-SCI | ||
|---|---|---|---|
| IJP | 3 day | −0.50 ± 0.02mV | −0.53 ± 0.02mV |
| 3 week | −0.54 ± 0.02mV | −0.53 ± 0.03mV | |
| EJP | 3 day | −0.45 ± 0.01mV | −0.42 ± 0.01mV |
| 3 week | −0.44 ± 0.02mV | −0.45 ± 0.03mV |
The nitrergic component of the IJP is selectively reduced in the distal colon following T3-SCI
In the colon, the IJP is comprised of an initial fast and following slow component. The fast component (fIJP) is mediated by ATP at P2Y1 receptors (Mutafova-Yambolieva et al., 2007;Wang et al., 2007) and is sensitive to potent selective P2Y1 receptor antagonists such as MRS-2179 (Strong et al., 2010). The slow component (sIJP) is nitrergic (see (Sanders & Ward, 2019) and can be blocked by nitric oxide synthase inhibitors, such as N-nitro-L-arginine (L-NNA; Gil et al., 2010). To determine whether one or both of these components were decreased in the T3-SCI animals, IJPs were evaluated in the presence of atropine in addition to either MRS-2179 or L-NNA to isolate the nitrergic and purinergic components, respectively.
A total of 86 smooth muscle cell recordings were made from the distal colons of 3-day control animals and transmural electrical stimulation evoked IJPs in 79 cells, whereas 76 smooth muscle cell recordings were made from 3-day T3-SCI animals and 66 cell responded with IJPs (92% vs 87%, respectively). For recordings from control animals 3-weeks following surgery 55/56 smooth muscle cells responded with evoked IJPs and 60/66 cells from 3-week T3-SCI animals responded with IJPs (98% vs 90%, respectively).
In the presence of MRS-2179, the remaining nitrergic component (Fig. 4) of the sIJP demonstrated a significant reduction in the distal colon of both 3-day and 3-week T3-SCI animals (Fig. 4B; p < 0.05). In the presence of L-NNA, the fIJP remained evident (Fig. 5). There was a significant difference in the fIJP between the 3-day and 3-week T3-SCI animal groups; however, when compared to surgical controls, there was no difference detected in the purinergic component of the IJP (Fig. 5B; p < 0.05).
Figure 4.
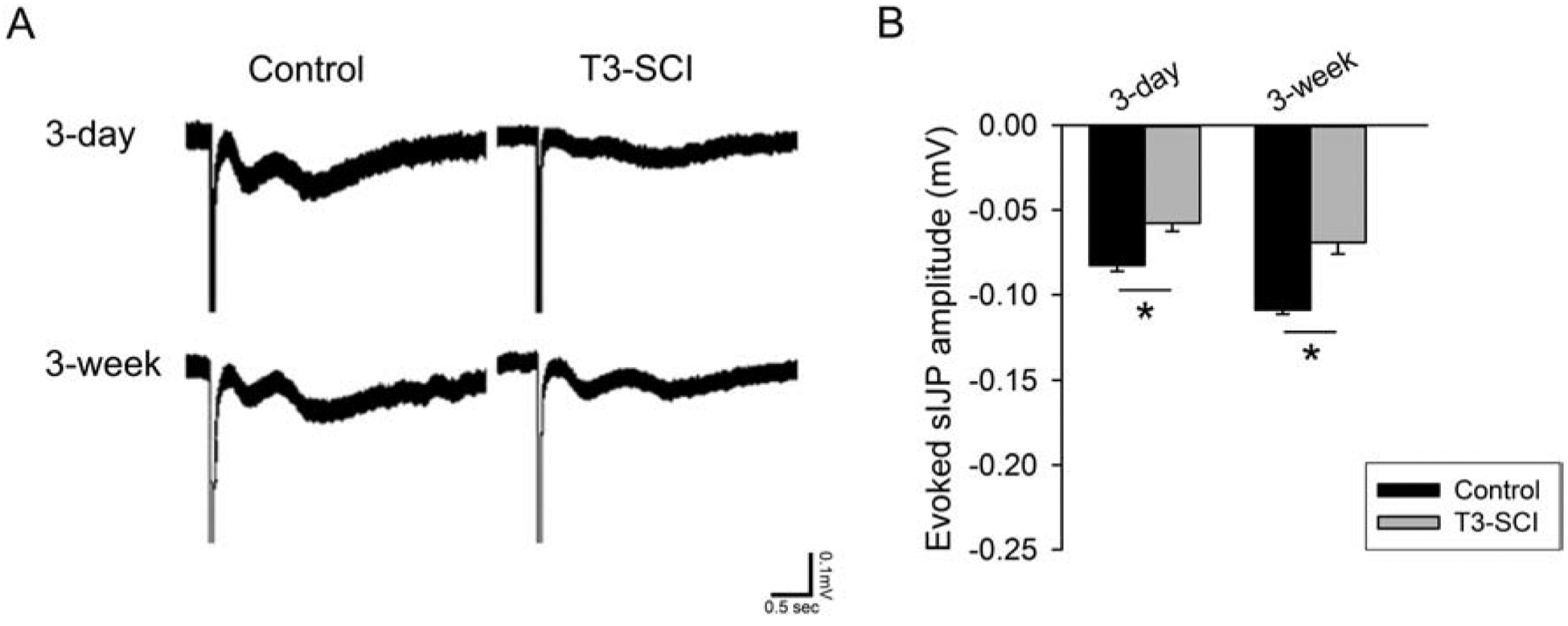
A) The nitrergic component of the IJP is selectively reduced following T3-SCI in the presence of MRS-2179. B) Graph of evoked nitrergic IJPs in the presence of MRS-2179 shows a significant reduction in T3-SCI rats at both 3-days or 3-weeks as well as representative recordings of IJPs in the presence of MRS-2179. (Values expressed as mean ± SEM, *p < 0.05 vs. post-operative time-matched controls, n = 20).
Figure 5.
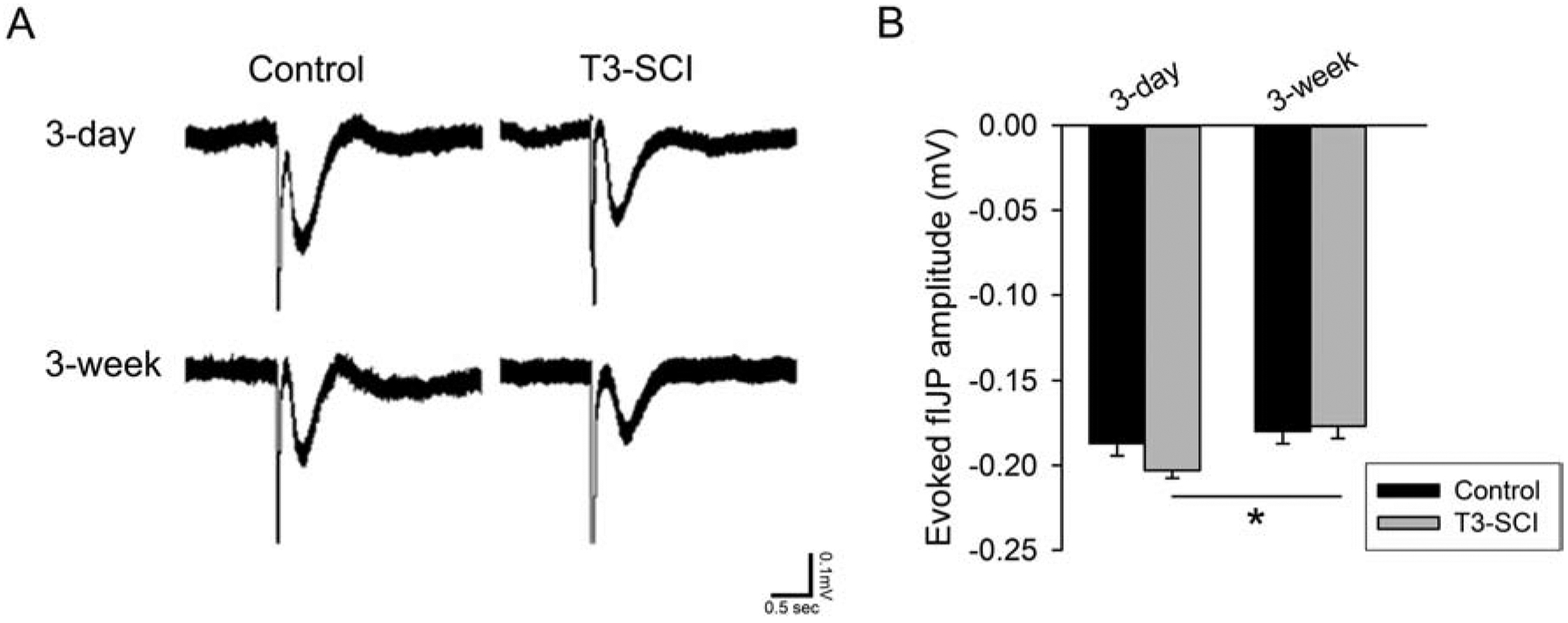
A) The purinergic fIJP recorded in the presence of L-NNA is unaffected following T3-SCI. B) Graph of evoked purinergic fIJPs in the presence of MRS-2179 shows a significant reduction between T3-SCI rats at 3-days or 3-weeks (Values expressed as mean ± SEM, *p < 0.05, n = 20).
These results indicate that T3-SCI in the rat pathophysiologically change the slow nitrergic component of the IJP. While altered IJPs are not uncommon in disease states, the selective loss of the nitrergic sIJP suggests that the overall changes in neuromuscular transmission within the distal colon and colonic dysmotility due to reduced smooth muscle inhibition are through a different mechanism than inflammatory bowel conditions.
The cholinergic EJP is reduced in the distal colon following T3-SCI
The colonic EJP is predominantly mediated by acetylcholine acting at muscarinic receptors (Holzer et al., 1993;Spencer & Smith, 2001). This cholinergic component acts upon M2 and M3 receptors (Gómez et al., 1992). Our complementary studies of EJPs were evaluated in the presence of MRS-2179 and L-NNA to isolate the cholinergic smooth muscle response, exclusively.
A total of 47 smooth muscle cell recordings were made from the aborally-oriented distal colons of 3-day control animals and transmural electrical stimulation evoked EJPs in 36 cells, whereas 57 smooth muscle cell recordings were made from 3-day T3-SCI animals and only 16 cell responded with EJPs (77% vs 28%, respectively). For recordings from control animals 3-weeks following surgery 21/21 smooth muscle cells responded with evoked EJPs and 2/23 cells from 3-week T3-SCI animals responded with EJPs (100% vs 9%, respectively). Successful smooth muscle cell impalements from aborally-oriented distal colons that produced an IJP in response to electrical stimulation were not included in the total and were not analyzed.
In the combined presence of MRS-2179 and LNNA to block the generation of inhibitory signals, the isolated EJP (Fig. 6) was significantly reduced in the distal colon of both 3-day and 3-week T3-SCI animals (Fig. 6B; p ≤ 0.05). These results indicate that T3-SCI in the rat concurrently reduces the cholinergic excitatory EJP. Altered EJPs, in addition to the diminished slow IJPs, suggest there are distinct mechanisms resulting in the diminished colonic propulsion associated with SCI.
Figure 6.
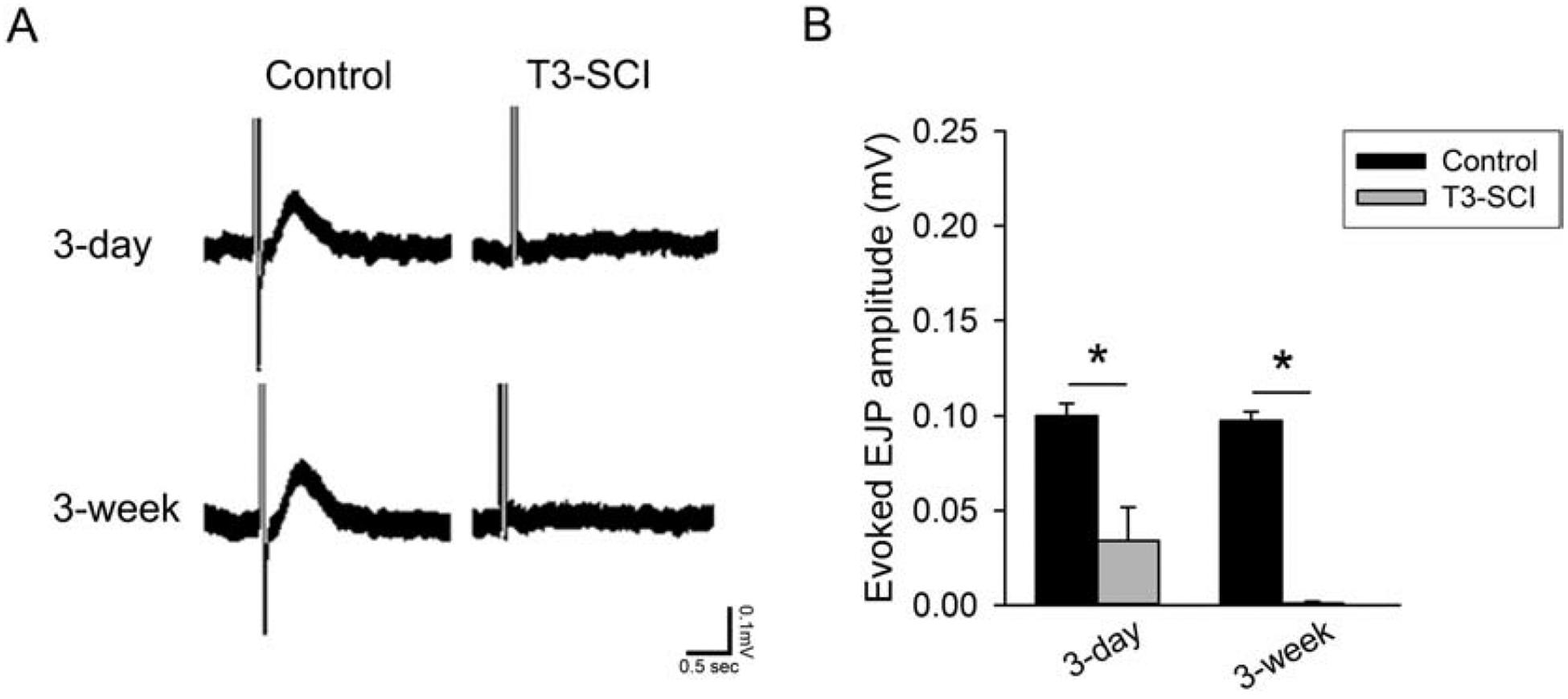
A) Evoked EJPs in the presence of MRS-2179 and L-NNA and recorded with the distal colon oriented aborally to the stimulating electrodes. B) Graph of shows a significant reduction in T3-SCI rats at both 3-days or 3-weeks. (Values expressed as mean ± SEM, *p < 0.05 vs. post-operative time-matched controls, n = 17).
Discussion
Spinal cord injury results in profound changes to colonic function. Previous studies noted architectural abnormalities in the colonic wall and myenteric neurons of SCI patients (den Braber-Ymker et al., 2017). However, the findings of that study were limited by the lack of specific information regarding patient demographic details, functional deficits and therapeutic interventions. Our previous animal study controlled for the duration and severity of SCI in the absence of any intervention and confirmed the anatomical changes to the neuromuscular compartment of the colon but did not evaluate the expression or residual function of key neurocircuitry; namely smooth muscle junction potentials mediated by ACh, ATP or NO (White 2018). These new data indicate that associated pathophysiological changes occur within the enteric neural circuitry after SCI. Specifically: 1) overall myenteric neuron density for nNOS- and ChAT-immunopositive populations within the distal colon are reduced over 3 weeks after T3-SCI; 2) the resting membrane potential of untreated smooth muscle preparations remains the same between surgical control and T3-SCI rats; 3) nitrergic inhibitory neuromuscular transmission is diminished following T3-SCI; 4) purinergic inhibitory neuromuscular transmission remains similar; and 5) excitatory cholinergic neuromuscular transmission is also diminished by T3-SCI.
The principal neurochemistry for distal muscle inhibition of the colonic enteric neuromuscular interface is predominantly through NO (Sanders & Ward, 1992) and ATP (Mutafova-Yambolieva et al., 2007;Wang et al., 2007). As previously stated, the colonic IJP is comprised of an initial fast and following slow component. The fast component (fIJP) is mediated by ATP, while the slow component (sIJP) is nitrergic. No prior functional studies of nitrergic or purinergic neurotransmission appear in the literature, but clinical reports offer some insight to potential inhibitory deficits. The rectal wall tone of patients with acute or chronic SCI in response to stimulation exhibited a reduced amplitude of the rectoanal inhibitory reflex, though baseline tone was higher (Krogh et al., 2002). These observations could be interpreted as reflecting a diminished inhibitory component to reflexes, though multiple variables remain plausible; including diminished sensory detection of stimuli by the intrinsic primary afferent neurons (IPANS) involved in the peristaltic reflex (Furness, 2012). Anatomical loss of presumptive IPANs have been observed in patients with neurogenic bowel (den Braber-Ymker et al., 2017). Our current immunohistochemical findings demonstrate a decline in nNOS-immunopositive neurons within the myenteric ganglia. We infer that these anatomical observations are responsible for the functional decline in circular muscle sIJPs, but a reduction in smooth muscle IPANS would not be revealed by our experimental design. An additional limitation to our studies concerns the inhibitory role of the abundantly-expressed neuropeptide, vasoactive intestinal peptide (VIP; Sarna & Shi, 2006). Previous electrophysiological analysis of antral and duodenal smooth muscle IJPs following T10-SCI revealed a reduction in the purinergic-mediated fIJP 2 days following SCI while the nitrergic-mediated sIJP was almost completely eliminated (Kabatas et al., 2008). These studies further reported significant reductions in gastrointestinal vasoactive intestinal polypeptide (VIP) and neuronal nitric oxide synthase (nNOS) immunoreactivity. Since VIP co-mediates slow IJPs along with nNOS those authors speculated that SCI disrupted small intestine enteric neurotransmission. Our observed decrease in IJP signaling did reveal a small residual IJP of unknown neuropharmacological origin, and VIP is a leading candidate for this residual function. Our original hypothesis was based upon the findings in colitis studies that have observed a reduction in IJP via reduced purinergic neurotransmission, not nitrergic (Roberts et al., 2013;Strong et al., 2010). Our present data, and that previously reported (Kabatas et al., 2008), suggest this may reflect differences from other disease models and in turn may reflect a difference in mechanism for the resultant colonic dysmotility.
Conversely, the principal neurochemistry of the colonic enteric neuromuscular interface for proximal reflex contraction is predominantly through acetylcholine and tachykinins. Acetylcholine appears to mediate reflex contraction in response to low intensity stimulation while tachykinins appear to augment the response during high intensity stimulation (Holzer et al., 1993;Spencer et al., 2001). Previous studies in spinally transected rats concluded that diminished contractility of colonic muscle strips was due to failure of local cholinergic neuromuscular transmission by means of reduced M3 receptor sensitivity (Frias et al., 2019). Those prior findings demonstrated the effects on tension of isolated muscle strips by bath application of acetylcholine and the M3 cholinergic receptor antagonist 4-DAMP, but inherently only tested the postsynaptic integrity of longitudinal muscle contraction. Our current findings complement and extend those findings by anatomically demonstrating a decline in ChAT-immunopositive neurons within the myenteric ganglia. We infer that these anatomical observations are responsible for the functional decline in circular muscle EJPs, though a reduction in smooth muscle cholinergic receptors cannot be ruled out by our recording technique. A functional role for systemic administration of tachykinin receptor agonists, particularly the neurokinin 2 receptor (NK2R), in an animal model of SCI is emerging (Kullmann et al., 2017;Marson et al., 2020;Marson et al., 2018). The balance of effect between cholinergic and tachykinergic at the enteric neuromuscular interface remains to be determined.
The diminished colonic contractions, combined with the morphological changes shown in our previous studies (White et al., 2018) is consistent with other functional disorders such as colitis (Auli et al., 2008;Sanovic et al., 1999), slow-transit constipation (Wedel et al., 2002), ischemia and reperfusion (Palombit et al., 2013), and general aging (Phillips et al., 2004;Saffrey, 2013). Some studies indicate a generalized pathology of the ENS or of the extrinsic innervation of the gastrointestinal tract, but numerous studies have shown that neurons that contain neuronal nitric oxide synthase are more susceptible to damage than other enteric neurons (Choi et al., 2008;Jo et al., 2014;Rivera et al., 2011). One potential causative mechanism for the observed enteric dysfunction may involve the systemic inflammatory response that occurs following SCI (Dewar et al., 2009). Multiorgan failure after SCI frequently focuses upon pulmonary, cardiac, renal and hepatic dysfunction as well as the recognition that SCI provokes gut barrier permeability (Liu et al., 2004) and dysbiosis (Kigerl et al., 2016). Emerging evidence further expands the multivariate nature of this response to a hepatic feedforward response (Goodus & McTigue, 2020) as well as the role of gut dysbiosis (Wallace et al., 2019). Unfortunately, the cause-and-effect of dysbiosis and altered GI integrity and physiology remains unclear.
Several limitations remain to be addressed by future studies. Sex differences in clinical and animal model populations have long been recognized, but infrequently explored particularly in regard to gastrointestinal dysfunction following SCI. These studies were performed in male SCI rats and differential distribution of estrogen receptors have been identified between females and males (Kawano et al., 2004) with purported effects upon motility (Liu et al., 2019;Zielinskaet al., 2017). Additionally, the neuroprotective effects of sex steroids and SCI have received attention (Evgeni et al., 2016;Sengelaub et al., 2018;Sengelaub & Xu, 2018;Webb et al., 2006) but the possible enteric protection offered in other disease models (Cóté et al., 2015) remains to be investigated for neurogenic bowel.
Functionally, the proximal-most region of the colon consists of the cecum, which serves as an important site of microbial digestion in hindgut fermenters such as rats (Sakaguchi, 2003). In addition to microbial fermentation, the proximal colon serves to mix and store liquid feces, absorb excess fluid and electrolytes; whereas, the distal colon serves mainly to propel and expel dehydrated feces [reviewed in (Sarna et al., 2006)]. These processes are not homogeneous, and the regional complexity of enteric neurocircuitry varies along the proximal and distal intestine; whereby, the distal colon demonstrates a limited portfolio of motility patterns that are consistent with a propulsive movement of luminal contents (Li et al., 2019). It is for this reason that our recordings were limited to the distal colon. Furthermore, this current approach and our previous pressure recording studies (White et al., 2018) do not distinguish between either the propagation of a pressure wave along the colon that would be indicative of longitudinal muscle-mediated propulsive contractions or localized, bi-directional, contractions that would be indicative of circular muscle segmentation. Our current data was limited to recordings of the functionality of the distal colonic circular muscle and should be expanded further to include the proximal colon.
It is apparent that neurogenic bowel following SCI is extremely complex and multifactorial. In healthy individuals, the propulsive reflexes of the distal colon and rectum are regulated by central control centers that maintain fecal continence and, when it is appropriate, trigger defecation through central commands that are relayed through the defecation center in the lumbosacral spinal cord (Furness, 2012). Voluntary controls of defecation are lost if cortico-spinal connections to the defecation center are interrupted following SCI, and these extrinsic functions must ultimately be considered in therapeutic options. Several neural prosthesis are commercially available that are intended to mimic activation within the defecation center (Coggrave & Norton, 2013). However, this present study urges caution regarding invasive therapeutic neuromodulatory interventions after an injury. Failure within the enteric circuitry coupled with structural changes to the colon ultimately compromise what is the final common pathway to colonic smooth muscle functionality. Compromise of the colonic neuromuscular compartment may limit the efficacy of surgically-invasive devices or pharmacologic interventions.
Highlights.
Inhibitory myenteric nNOS immunoreactive cells are reduced by injury
Excitatory neuromuscular signaling is diminished following spinal cord injury
Nitrergic slow inhibitory neuromuscular signaling is diminished following SCI
Purinergic fast inhibitory neuromuscular signaling was unaffected by SCI
Excitatory myenteric choline acetyltransferase immunopositive cells are reduced by injury
Acknowledgements
The authors wish to extend their gratitude for the support across multiple capacities and the input provided by Emily N. Blanke, Lisa B. Willing and Gina Marcucci.
Funding sources
This work was supported by National Institute of Neurological Disorders and Stroke (NINDS) Grant R01-NS-105987 and Craig H. Neilsen Foundation Senior Research Award (295319)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that no conflicts of interest exist
REFERENCES
- 1.Auli M, Nasser Y, Ho W, Burgueno JF, Keenan CM, Romero C, Sharkey KA, Fernandez E, 2008. Neuromuscular changes in a rat model of colitis. Auton Neurosci 141, 10–21. [DOI] [PubMed] [Google Scholar]
- 2.Besecker EM, Deiter GM, Pironi N, Cooper TK, Holmes GM, 2017. Mesenteric vascular dysregulation and intestinal inflammation accompanies experimental spinal cord injury. Am J Physiol Regul Integr Comp Physiol 312, 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bornstein JC, Costa M, Grider JR, 2004. Enteric motor and interneuronal circuits controlling motility. Neurogastroenterol Motil 16, 34–38. [DOI] [PubMed] [Google Scholar]
- 4.Choi KM, Gibbons SJ, Nguyen TV, Stoltz GJ, Lurken MS, Ordog T, Szurszewski JH, Farrugia G, 2008. Heme Oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology 135, 2055–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung EA, Emmanuel AV, 2005. Gastrointestinal symptoms related to autonomic dysfunction following spinal cord injury. Prog. Brain Res 152, 317–333. [DOI] [PubMed] [Google Scholar]
- 6.Coggrave M, Norton C, 2013. Management of faecal incontinence and constipation in adults with central neurological diseases. Cochrane. Database. Syst. Rev 12, CD002115. [DOI] [PubMed] [Google Scholar]
- 7.Cóté M, Bourque M, Poirier A, Aubé B, Morissette M, Di Paolo T, Soulet D, 2015. GPER1-mediated immunomodulation and neuroprotection in the myenteric plexus of a mouse model of Parkinson’s disease. Neurobiol. Dis 82, 99–113. [DOI] [PubMed] [Google Scholar]
- 8.den Braber-Ymker M, Lammens M, van Putten MJAM, Nagtegaal ID, 2017. The enteric nervous system and the musculature of the colon are altered in patients with spina bifida and spinal cord injury. Virchows Arch. 470, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewar D, Moore FA, Moore EE, Balogh Z, 2009. Postinjury multiple organ failure. Injury 40, 912–918. [DOI] [PubMed] [Google Scholar]
- 10.Dwyer MA, Bredt DS, Snyder SH, 1991. Nitric oxide synthase: Irreversible inhibition by L-NG-Nitroarginine in brain in vitro and in vivo. Biochem. Biophys. Res. Commun 176, 1136–1141. [DOI] [PubMed] [Google Scholar]
- 11.Enck P, Greving I, Klosterhalfen S, Wietek B, 2005. Upper and lower gastrointestinal motor and sensory dysfunction after human spinal cord injury. Prog. Brain Res 152, 373–384. [DOI] [PubMed] [Google Scholar]
- 12.Evgeni B, Shaun E.Gruenbaum, Matthew B, Ruslan K, Alexander Zlotnik and Moti Klein, 2016. Neuroprotection by Estrogen and Progesterone in Traumatic Brain Injury and Spinal Cord Injury. Curr Neuropharm 14, 641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frias B, Phillips AA, Squair JW, Lee AHX, Laher I, Krassioukov AV, 2019. Reduced colonic smooth muscle cholinergic responsiveness is associated with impaired bowel motility after chronic experimental high-level spinal cord injury. Auton Neurosci 216, 33–38. [DOI] [PubMed] [Google Scholar]
- 14.Furness JB, 1969. An electrophysiological study of the innervation of the smooth muscle of the colon. J Physiol 205, 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furness JB, 2012. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9, 286–294. [DOI] [PubMed] [Google Scholar]
- 16.Furness JB, Callaghan BP, Rivera LR, Cho HJ, 2014. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med. Biol 817, 39–71. [DOI] [PubMed] [Google Scholar]
- 17.Gil V, Gallego D, Grasa L, Martín MT, Jiménez M, 2010. Purinergic and nitrergic neuromuscular transmission mediates spontaneous neuronal activity in the rat colon. Am J Physiol Gastrointest Liver Physiol 299, G158–G169. [DOI] [PubMed] [Google Scholar]
- 18.Gómez A, Martos F, Bellido I, Marquez E, Garcia AJ, Pavia J, de la Cuesta FS, 1992. Muscarinic receptor subtypes in human and rat colon smooth muscle. Biochem. Pharmacol 43, 2413–2419. [DOI] [PubMed] [Google Scholar]
- 19.Goodus MT, McTigue DM, 2020. Hepatic dysfunction after spinal cord injury: A vicious cycle of central and peripheral pathology? Exp Neurol, 113160. [DOI] [PubMed] [Google Scholar]
- 20.Hill CE, Beattie MS, Bresnahan JC, 2001. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp. Neurol 171, 153–169. [DOI] [PubMed] [Google Scholar]
- 21.Holmes GM, Rogers RC, Bresnahan JC, Beattie MS, 1998. External anal sphincter hyper-reflexia following spinal transection in the rat. J Neurotrauma 451, 451–457. [DOI] [PubMed] [Google Scholar]
- 22.Holmes GM, Van Meter MJ, Bresnahan JC, Beattie MS, 2005. Serotonergic fiber sprouting to external anal sphincter motoneurons after spinal cord contusion. Exp. Neurol 193, 29–42. [DOI] [PubMed] [Google Scholar]
- 23.Holmes GM, Hudson TR, Filart R, 2017. Neurogastroenterology in Spinal Cord Dysfunction In: Weidner N, Rupp R, Tansey KE (Eds.), Neurological Aspects of Spinal Cord Injury Springer, New York, pp. 397–437. [Google Scholar]
- 24.Holzer P, Schluet W, Maggi CA, 1993. Ascending enteric reflex contraction: roles of acetylcholine and tachykinins in relation to distension and propagation of excitation. J Pharmacol Exp Ther 264, 391–396. [PubMed] [Google Scholar]
- 25.Jo HJ, Kim N, Nam RH, Kang JM, Kim JH, Choe G, Lee HS, Park JH, Chang H, Kim H, Lee MY, Kim YS, Kim JS, Jung HC, 2014. Fat deposition in the tunica muscularis and decrease of interstitial cells of Cajal and nNOS-positive neuronal cells in the aged rat colon. Am J Physiol Gastrointest Liver Physiol 306, G659–G669. [DOI] [PubMed] [Google Scholar]
- 26.Kabatas S, Yu D, He XD, Thatte HS, Benedict D, Hepgul KT, Black PM, Sabharwal S, Teng YD, 2008. Neural and anatomical abnormalities of the gastrointestinal system resulting from contusion spinal cord injury. Neuroscience 154, 1627–1638. [DOI] [PubMed] [Google Scholar]
- 27.Kawano N, Koji T, Hishikawa Y, Murase K, Murata I, Kohno S, 2004. Identification and localization of estrogen receptor α- and β-positive cells in adult male and female mouse intestine at various estrogen levels. Histochem. Cell Biol 121, 399–405. [DOI] [PubMed] [Google Scholar]
- 28.Keef KD, Du C, Ward SM, McGregor B, Sanders KM, 1993. Enteric inhibitory neural regulation of human colonic circular muscle: role of nitric oxide. Gastroenterology 105, 1009–1016. [DOI] [PubMed] [Google Scholar]
- 29.Kigerl KA, injury, Wang L, Mo X, Yu Z, Popovich PG, 2016. Gut dysbiosis impairs recovery after spinal cord. J Exp Med 213, 2603–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krogh K, Mosdal CF, Gregersen HF, Laurberg S, 2002. Rectal wall properties in patients with acute and chronic spinal cord lesions. Dis Colon Rectum 45, 641–649. [DOI] [PubMed] [Google Scholar]
- 31.Kullmann FA, Katofiasc M, Thor KB, Marson L, 2017. Pharmacodynamic evaluation of Lys5, MeLeu9, Nle10-NKA(4–10) prokinetic effects on bladder and colon activity in acute spinal cord transected and spinally intact rats. Naunyn Schmiedebergs Arch. Pharmacol 390, 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, Hao MM, Van den Haute C, Baekelandt V, Boesmans W, Vanden Berghe P, 2019. Regional complexity in enteric neuron wiring reflects diversity of motility patterns in the mouse large intestine. eLife 8, e42914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, An H, Jiang D, Huang W, Zou H, Meng C, Li H, 2004. Study of bacterial translocation from gut after paraplegia caused by spinal cord injury in rats. Spine 29, 164–169. [DOI] [PubMed] [Google Scholar]
- 34.Liu JYH, Lin G, Fang M, Rudd JA, 2019. Localization of estrogen receptor ERα, ERβ and GPR30 on myenteric neurons of the gastrointestinal tract and their role in motility. Gen. Comp. Endocrinol 272, 63–75. [DOI] [PubMed] [Google Scholar]
- 35.Lynch AC, Antony A, Dobbs BR, Frizelle FA, 2001. Bowel dysfunction following spinal cord injury. Spinal Cord 39, 193–203. [DOI] [PubMed] [Google Scholar]
- 36.Marson L, Piatt RK, Katofiasc MA, Bobbitt C, Thor KB, 2020. Chronic, Twice-Daily Dosing of an NK2 Receptor Agonist [Lys5,MeLeu9,Nle10]-NKA(4–10), Produces Consistent Drug-Induced Micturition and Defecation in Chronic Spinal Rats. J Neurotrauma 37, 868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marson L, Thor KB, Katofiasc M, Burgard EC, Rupniak NMJ, 2018. Prokinetic effects of neurokinin-2 receptor agonists on the bladder and rectum of rats with acute spinal cord transection. Eur J Pharmacol 819, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Middleton JW, Lim K, Taylor L, Soden R, Rutkowski S, 2004. Patterns of morbidity and rehospitalisation following spinal cord injury. Spinal Cord 42, 359–367. [DOI] [PubMed] [Google Scholar]
- 39.Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM, 2007. Beta-nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci 104, 16359–16364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noble LJ, Wrathall JR, 1985. Spinal cord contusion in the rat: morphometric analyses of alterations in the spinal cord. Exp Neurol. 88, 135–149. [DOI] [PubMed] [Google Scholar]
- 41.Palombit K, Mendes C.b., Tavares-de-Lima W, Silveira M.v., Castelucci P, 2013. Effects of ischemia and reperfusion on subpopulations of rat enteric neurons expressing the P2X7 receptor. Dig Dis Sci 58, 3429–3439. [DOI] [PubMed] [Google Scholar]
- 42.Phillips RJ, Kieffer EJ, Powley TL, 2004. Loss of glia and neurons in the myenteric plexus of the aged Fischer-344 rat. Anat. Embryol 209, 19–30. [DOI] [PubMed] [Google Scholar]
- 43.Qualls-Creekmore E, Tong M, Holmes GM, 2010. Time-course of recovery of gastric emptying and motility in rats with experimental spinal cord injury. Neurogastroenterol. Motil 22, 62–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rivera LR, Leung C, Pustovit RV, Hunne BL, Andrikopoulos S, Herath C, Testro A, Angus PW, Furness JB, 2014. Damage to enteric neurons occurs in mice that develop fatty liver disease but not diabetes in response to a high-fat diet. Neurogastroenterol. Motil 26, 1188–1199. [DOI] [PubMed] [Google Scholar]
- 45.Rivera LR, Poole DP, Thacker M, Furness JB, 2011. The involvement of nitric oxide synthase neurons in enteric neuropathies. Neurogastroenterol. Motil 23, 980–988. [DOI] [PubMed] [Google Scholar]
- 46.Roberts JA, Durnin L, Sharkey KA, Mutafova-Yambolieva VN, Mawe GM, 2013. Oxidative stress disrupts purinergic neuromuscular transmission in the inflamed colon. J Physiol 591, 3725–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saffrey MJ, 2013. Cellular changes in the enteric nervous system during ageing. Dev. Biol 382, 344–355. [DOI] [PubMed] [Google Scholar]
- 48.Sakaguchi E, 2003. Digestive strategies of small hindgut fermenters. Anim Sci 74, 327–337. [Google Scholar]
- 49.Sanders KM, Ward SM, 1992. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am. J. Physiol 262, G379–G392. [DOI] [PubMed] [Google Scholar]
- 50.Sanders KM, Ward SM, 2019. Nitric oxide and its role as a non-adrenergic, noncholinergic inhibitory neurotransmitter in the gastrointestinal tract. British Journal of Pharmacology 176, 212–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanovic S, Lamb DP, Blennerhassett MG, 1999. Damage to the enteric nervous system in experimental colitis. Am J Pathol 155, 1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarna SK, Shi XZ, 2006. Function and Regulation of Colonic Contractions in Health and Disease In: Johnson LR, Barrett KE, Ghishan FK, Merchant JL, Said HM, Wood JD (Eds.), Physiology of the Gastrointestinal Tract Elsevier Academic Press, New York, pp. 965–994. [Google Scholar]
- 53.Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp J-EJ, 2003. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma 20, 179–193. [DOI] [PubMed] [Google Scholar]
- 54.Sengelaub DR, Han Q, Liu NK, Maczuga MA, Szalavari V, Valencia SA, Xu XM, 2018. Protective effects of estradiol and dihydrotestosterone following spinal cord injury. J Neurotrauma 35, 825–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sengelaub DR, Xu XM, 2018. Protective effects of gonadal hormones on spinal motoneurons following spinal cord injury. Neural Regen Res 13, 971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serio R, Alessandro M, Zizzo MG, Tamburello MP, Mulè F, 2003. Neurotransmitters involved in the fast inhibitory junction potentials in mouse distal colon. Eur J Pharmacol 460, 183–190. [DOI] [PubMed] [Google Scholar]
- 57.Serio R, Mulè F, Postorino A, 1995. Noradrenergic, noncholinergic inhibitory junction potentials in rat proximal colon: role of nitric oxide. Can. J. Physiol. Pharmacol 73, 79–84. [DOI] [PubMed] [Google Scholar]
- 58.Spencer NJ, Bywater RAR, Holman ME, Taylor GS, 1998. Spontaneous and evoked inhibitory junction potentials in the circular muscle layer of mouse colon. J Auton Nerv Sys 69, 115–121. [DOI] [PubMed] [Google Scholar]
- 59.Spencer NJ, Smith TK, 2001. Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J Physiol 533, 787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strong DS, Cornbrooks CF, Roberts JA, Hoffman JM, Sharkey KA, Mawe GM, 2010. Purinergic neuromuscular transmission is selectively attenuated in ulcerated regions of inflamed guinea pig distal colon. J Physiol 588, 847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swartz E, Holmes G, 2014. Gastric vagal motoneuron function is maintained following experimental spinal cord injury. Neurogastroenterol. Motil 27, 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tong M, Holmes GM, 2009. Gastric dysreflexia after acute experimental spinal cord injury in rats. Neurogastroenterol. Motil 21, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tong M, Qualls-Creekmore E, Browning KN, Travagli RA, Holmes GM, 2011. Experimental spinal cord injury in rats diminishes vagally-mediated gastric responses to cholecystokinin-8s. Neurogastroenterol. Motil 23, e69–e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Totoiu MO, Keirstead HS, 2005. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol 486, 373–383. [DOI] [PubMed] [Google Scholar]
- 65.Wallace DJ, Sayre NL, Patterson T, Nicholson SE, Hilton D, Grandhi R, 2019. Spinal cord injury and the human microbiome: beyond the brain-gut axis. Neurosurg Focus 46, E11. [DOI] [PubMed] [Google Scholar]
- 66.Wang GD, Wang XY, Hu HZ, Liu S, Gao N, Fang X, Xia Y, Wood JD, 2007. Inhibitory neuromuscular transmission mediated by the P2Y1 purinergic receptor in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol 292, G1483–G1489. [DOI] [PubMed] [Google Scholar]
- 67.Waterman SA, Costa M, 1994. The role of enteric inhibitory motoneurons in peristalsis in the isolated guinea-pig small intestine. J Physiol 477, 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Webb AA, Chan CB, Brown A, Saleh TM, 2006. Estrogen reduces the severity of autonomic dysfunction in spinal cord-injured male mice. Behav. Brain Res 171, 338–349. [DOI] [PubMed] [Google Scholar]
- 69.Wedel T, Spiegler J, Soellner S, Roblick UJ, Schiedeck THK, Bruch HP, Krammer HJ, 2002. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology 123, 1459–1467. [DOI] [PubMed] [Google Scholar]
- 70.White A, Holmes GM, 2018. Anatomical and functional changes to the colonic neuromuscular compartment after experimental spinal cord injury. J Neurotrauma 35, 1079–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zielinska M, Fichna J, Bashashati M, Habibi S, Sibaev A, Timmermans JP, Storr M, 2017. G protein-coupled estrogen receptor and estrogen receptor ligands regulate colonic motility and visceral pain. Neurogastroenterol Motil 29, e13025. [DOI] [PubMed] [Google Scholar]


