Abstract
The mechanisms by which neighborhood environmental exposures influence health are poorly understood, although immune system dysregulation represents a potential biological pathway. While many neighborhood exposures have been investigated, there is little research on residential proximity to brownfield waste. Using biomarker data from 262 participants in the Detroit Neighborhood Health Study (DNHS), we estimated the association between proximity to brownfields and heavy traffic and signal-joint T-cell Receptor Excision Circles (sjTRECs, a measure of naïve T-cell production), C-reactive protein (CRP, a measure of systemic inflammation), and interleukin 6 (IL-6, a proinflammatory cytokine). We assessed residential proximity ≤200 meters from brownfields and highways on all three biomarkers using multivariate regression. We demonstrated that living ≤200m from a brownfield site was associated with a 0.30 (95% CI = 0.59, 0.02, p = 0.04) loge-unit decrease in sjTRECs per million whole blood cells, as well as non-significantly elevated levels of CRP and IL-6. Heavy traffic was not associated with any biomarker. Persons living in close proximity to brownfield sites had significantly lower naïve T-cell production, suggesting accelerated immune aging. Decreased T-cell production associated with brownfield proximity may be caused by toxicant exposure in brownfield sites, or may serve as a marker of other neighborhood stressors.
Keywords: epidemiology, population based studies, personal exposure, exposure modeling, environmental monitoring
INTRODUCTION:
Multiple neighborhood-level environmental exposures, including heavy metals, traffic, and air pollution, have been shown to influence health. While exposures like air pollution (1–4) and heavy metals (5–8) have been well studied, there is far less data on the health effects of exposure to brownfield sites in the United States. The majority of published brownfield literature focuses on identifying soil-based contaminants (9,10), technical remediation strategies (11,12), and land use redevelopment (13–15). Previous research on the health effects of brownfield sites is limited to a regional investigation of mortality related to ward-level brownfield concentration in England (16) and a single study of mortality related to tract-level brownfield concentration in Baltimore (17). No study, to our knowledge, has investigated associations between residential proximity to brownfield sites and biomarkers of immunity in the United States.
Much of the difficulty in studying the health impacts of domestic brownfield sites results from the sheer diversity of the label, “brownfield”. Since the passage of the Small Business Liability Relief and Brownfields Revitalization Act of 2002, the United States Environmental Protection Agency has defined a brownfield as any “real property, the expansion, redevelopment, or reuse of which may be complicated by the presence or potential presence of a hazardous substance, pollutant, or contaminant” (18, now codified at 42 U.S.C. § 9601(39)(A)). This definition is broad enough to include contamination from manufacturing, waste management, plastic and petroleum products, metals, etc. Thus, the “exposure” to brownfield sites can be difficult to quantify unless researchers have access to granular data on location-specific contaminants from air, water, and soil samples. Many state governments, however, collect site-specific brownfield information that may be leveraged to study the potential health effects of exposure to brownfield sites.
In contrast to the state of research on brownfields and health, multiple studies have linked exposure to high-traffic areas and motor vehicle exhaust to the incidence of such disparate outcomes as preterm birth/low-birth weight (19,20), female pubertal development (21), airway hyper-responsiveness (22,23), cardiovascular disease (24), rheumatoid arthritis (1), lung cancer (2), and more. Multiple studies in urban Chinese cohorts, which suffer from air pollution above levels experienced in most of the United States, have linked motor vehicle exhaust exposure with multiple biological markers of cardiovascular and metabolic disease, including elevated fasting insulin levels, hypertension, elevated heart rate, hypercholesterolemia, and DNA damage due to oxidative stress (25–28). Weaker associations between exposure to vehicle exhaust and C-reactive protein, a marker of systemic inflammation, have been noted in both the United States (29), and Europe (3,4,30), but not all domestic studies have found significant associations between traffic and C-reactive protein or interleukin-6, a proinflammatory cytokine (31).
Using data from the Detroit Neighborhood Health Study (DNHS), a representative sample of adults living in Detroit, Michigan, from 2008–2013, we sought to estimate the association of household proximity to brownfield sites and a global measure of T cell production and generalized inflammation. We used signal-joint T-cell receptor excision circles (sjTRECs) as a marker of naïve T-cell production and aging of the thymus, and C-reactive protein (CRP) and interleukin 6 (IL-6) as markers of inflammation. We used data on brownfield sites collected by the Michigan Department of Environmental Quality under the Michigan Natural Resources and Environmental Protection Act (NREPA) of 1994, Parts 201 (“Environmental Remediation”) and 213 (“Leaking Underground Storage Tanks”) (32). We also investigated the association between all three biomarkers and residential proximity to primary and secondary roadways and heavy traffic.
METHODS:
Study Population:
We used data from the Detroit Neighborhood Health Study (DNHS), a longitudinal observational study of adults aged 18 years and older living in Detroit, Michigan. The DNHS proceeded annually in five waves from 2008–2013. Wave 1 participants were recruited in 2008–2009 using a two-stage probability sample of all households in Detroit, with one adult from each household randomly selected for inclusion. Participants completed a 40-minute telephone survey on individual and household demographics, life-course stressors, physical and mental health, neighborhood attributes, and substance abuse. The response rate among all eligible contacted adults was 53.0% (33). The survey was pilot-tested before each wave and used validated scales from comparable past research (33,34). Two-tailed chi squared testing showed that the DNHS study population was representative of the Detroit population in age, gender, race, income, and educational attainment when compared to American Community Survey data (34).
Wave 1 (2008–2009) included 1,547 participants. 1054 participants (68%) from Wave 1 completed Wave 2 (2009–2010), and a supplemental group of 534 individuals was recruited during Wave 2 to increase the overall sample size. All participants were contacted annually to participate in Waves 3 through 5 (2010–2011, 2011–2012, and 2012–2013). 614 participants from the original 1,547 (39.7%), and 231 participants from the supplemental sample of 534 (43.3%), completed surveys in Wave 4 (when sjTRECs were assessed).
In addition to the telephone survey, participants could elect to provide a venous blood sample at their home during Waves 1, 2, 4, and 5. Previous work has shown that the sample of participants who provided blood was comparable to the overall DNHS population (34). All participants who provided a venous blood sample for assessment of CRP (Waves 1 and 2), IL-6 (Waves 1 and 2), and sjTRECs (Wave 4) were eligible for this analysis (n = 263). We used Wave 2 CRP and IL-6 measurements for participants who provided blood during both Waves 1 and 2, as Wave 2 measurements were more proximate to our environmental exposure data. Household address and GPS coordinates were recorded during each wave. After excluding missing data, a final n = 262 was available for this analysis.
Compared to all Wave 4 participants, participants in this study were slightly older (mean of 60 years versus 58 years in Wave 4 overall) and had slightly lower educational attainment (57% completed more than High School versus 66% in Wave 4 overall). Other sociodemographic characteristics (gender, race, income, etc.) were very similar between this population and the total Wave 4 cohort.
All participants provided informed consent and the study was approved by the Institutional Review Board at the University of Michigan and the University of North Carolina.
Biomarker Quantification:
Participant DNA was isolated from venous whole blood samples and shipped on dry ice to the Laboratory of Immunovirology at the University of Seville, Spain, for thymic function assessment as sjTRECs per million whole blood cells. sjTREC quantification was performed in a two-round quantitative polymerase chain reaction (qPCR) as previously described (35,36).
Serum CRP was measured at the University of Michigan using the CRPUltra Wide Range Reagent Kit (Genzyme, USA – lot 9CRPC45) following the manufacturer’s protocol. CRP values below the limit of detection (0.05 mg/L) were coded as 0.025 mg/L, as done previously by our research group (37).
Serum IL-6 was measured at the University of Michigan using the QuantiGlo Human IL-6 quantitative sandwich immunoassay kit (R&D Systems, USA – lot 241400) following the manufacturer’s protocol. IL-6 values below the limit of detection (0.50 pg/mL) were coded as 0.25 pg/mL, as done previously by our research group (37).
All three biomarkers of interest (sjTRECs, CRP, and IL-6) were modeled as continuous, loge-transformed variables in order to approximate a normal distribution.
Environmental Exposure Assessment:
Brownfield data were downloaded from the Michigan Department of Environmental Quality (MDEQ) portal (https://secure1.state.mi.us/FacilitiesInventoryQueries/) on November 13, 2014, by Data Driven Detroit (https://portal.datadrivendetroit.org/). The brownfield dataset includes locations with a known release of hazardous substances as defined by the MDEQ under the Michigan Natural Resources and Environmental Protection Act (NREPA) parts 201 (“Environmental Remediation”) and 213 (“Leaking Underground Storage Tanks”). Of the 34,085 sites present in the raw data from the MDEQ, we removed all sites (n = 31,340) outside of the City of Detroit using MDEQ-provided location data. We also removed all Baseline Environmental Assessments (BEAs) inside of Detroit (n = 1,566), which do not necessarily meet standards for remediation under NREPA parts 201 and 213. Thus, our final sample of 1,208 sites is a blend of sites qualifying for environmental remediation under parts 201 and 213, which we hereafter refer to as “brownfields.”
Of brownfield sites within Detroit’s city limits, we removed all locations missing latitude/longitude data from the MDEQ (n = 28), then removed all duplicated sites (n = 2) for a final dataset of 1,149 sites. This dataset does not necessarily include all contaminated areas in Detroit, as land owners are not required to notify the MDEQ of on-site contamination if they pursue site remediation independently. It does, however, contain all known active sites under NREPA Parts 201 and 213 in 2014, a good proxy for known contaminated sites during the study period.
In order to better understand the range of potential contaminants included in the MDEQ’s brownfield data, we investigated a random sample of 200 sites from our analysis using the MDEQ “Facility Name” associated with each site. Brownfields were manually coded as 1) “Industrial” when the facility name included terms such as “industrial,” “manufacturing,” “production,” “engine plant,” or other industry-related terms, 2) “Gas Station” when the facility name included terms such as “gas,” “petro,” “gas station,” or other gas station-related names/terms, 3) “Auto Shop” when the facility name included terms such as “auto center,” “automotive service,” “car service,” “oil change,” “auto repair,” “tire shop,” or other auto shop-related terms, 4) “Commercial” when the facility name included terms such as “restaurant,” “condominium,” “mini mart,” “bank,” “catering company,” “pharmacy,” or other commercial-related terms, 5) “Municipal” when the facility name included terms such as “water department,” “police station,” “school,” “mail service,” or other municipal government-related terms, 6) “Auto Wash” when the facility name included the phrase “car wash” or “auto wash,” or 7) “Unknown” when the facility name did not include any terms clearly identifying it as one of the above categories or any other type of facility.
We also investigated roadway proximity and heavy traffic as a proxy for air quality. Average annual daily traffic (AADT) rates for all primary and secondary highways in Detroit during 2011 were collected from the Michigan Department of Transportation (MDOT). Participant household environmental exposure was calculated as the Great Circle distance between a participant’s household GPS coordinates and, separately, the nearest brownfield or highway. Exposure to brownfields was coded “close” if the participant’s household was ≤200 meters from the nearest brownfield (n = 66) and “far” if > 200m (n = 196). Participant exposure to high-traffic areas was coded “high” if the household was ≤200m from a highway with AADT ≥50,000 vehicles/day (n = 18), “medium” if the household was ≤200m from a highway with AADT <50,000 vehicles/day (n = 31), and “low” if the household was >200m from the nearest highway (n = 213). We chose 200m as the cutoff for environmental exposure a priori based on previous work that shows a strong gradient of highway pollution ending within the 150–250 meter range (19,23,31,38,39). Proximity measures were calculated for each wave of biomarker assessment (Wave 1 or 2 for CRP and IL-6, Wave 4 for sjTRECs) using GPS coordinates to control for any changes in household address between waves.
In order to investigate potential exposure-response relationships between environmental exposure and biomarkers of interest, within the limits of our sample size, we further separated brownfield-proximate participants based on the number of brownfields within 200m of their residence (1 vs. 2 or more). We similarly stratified highway-proximate participants into those living 0–100m and 101–200m from the nearest highway, regardless of traffic.
Covariate Assessment:
We used directed acyclic graphs to assess the inclusion of potential confounders of the exposure-outcome relationship for both exposures (brownfields, high traffic areas) and all three biomarker outcomes (sjTRECs, CRP, IL-6). We included age, gender, race/ethnicity, income, educational attainment, BMI, and cigarette smoking status for all exposure-outcome relationships in our final models. All covariates for CRP and IL-6 were evaluated by telephone interview during Waves 1 and 2, and all covariates for sjTRECs were evaluated during Wave 4. BMI was assessed continuously in kg/m2 during Wave 3 and 4 blood draws; we used wave 4 BMI records for analyses regarding sjTRECs and wave 3 BMI records for analyses regarding CRP and IL-6. Age was assessed continuously in years. Gender was dichotomized as female or male. While race/ethnicity was assessed in the survey as Black or African American, American Indian/Alaskan Native, Native Hawaiian/other Pacific islander, Hispanic, Asian, White, or other, we dichotomized race/ethnicity as “non-Hispanic Black/African American” and “Other” for our analysis, due to the DNHS population being primarily non-Hispanic Black/African American. Income was categorized as <$15000, $15000–34999, $35000–74999, and $75000 and above. Educational attainment was dichotomized as high school education or less, or more than a high school education. Cigarette smoking was operationalized as current, former, or never smoker.
Statistical Analysis:
All data organization, statistical analysis, and image generation were conducted in R (version 3.5.2). Code and data cannot be made available due to protected health information in the DNHS. Participant characteristics were calculated as means and standard deviations (SD) for continuous variables. Categorical variables were calculated as counts and proportions.
We assessed the impact of household proximity to brownfields and high-traffic highways on the biomarkers of interest using generalized linear regression. For each biomarker and exposure of interest, we tested 4 models stepwise: Model 1 adjusted for age; Model 2 additionally adjusted for gender, and race; Model 3 additionally adjusted for income and education; and Model 4 additionally adjusted for BMI and smoking status. We conducted complete case analysis in all models, excluding 24 of 262 participants (9.2%) due to missing at least one covariate in the final model. All tests of significance were two-sided with α = 0.05.
RESULTS:
Sociodemographic and clinical characteristics from Wave 4 participants in this analysis are shown in Table 1. Participants were 60 years old on average (SD = 14), majority female (61%), and majority non-Hispanic African American (80%). The mean BMI was 32 (SD = 9.2), and 29% were current smokers. Individuals living ≤200m from the nearest brownfield were older, more likely to be female, earned less money, and more likely to be current or former smokers than individuals living >200m from the nearest brownfield. A similar pattern of high individual social and economic disadvantage existed for individuals living in close proximity to areas with heavy traffic. Mean levels of sjTRECs were lower, and mean levels of CRP and IL-6 higher, among participants living near brownfield sites and heavy traffic.
Table 1.
Sociodemographic and clinical characteristics of Wave 4 participants in this analysis (n = 262), Detroit Neighborhood Health Study. Note: SD = standard deviation, AADT = Average Annual Daily Traffic, column percentages may not sum to 100% due to rounding.
| Brownfield Proximity | Highway Proximity | ||||||
|---|---|---|---|---|---|---|---|
| Total (n=262) |
>200m (n=196) |
≤200m (n=66) |
>200m (n=213) |
≤200m, AADT <50,000 (n=31) |
≤200m, AADT ≥50,000 (n=18) |
||
| Age (years) | |||||||
| Mean (SD) | 60 (± 14) | 59 (± 15) | 63 (± 13) | 60 (± 15) | 61 (± 12) | 65 (± 14) | |
| Gender | |||||||
| Female | 159 (61 %) | 112 (57 %) | 47 (71 %) | 134 (63 %) | 16 (52 %) | 9 (50 %) | |
| Male | 103 (39 %) | 84 (43 %) | 19 (29 %) | 79 (37 %) | 15 (48 %) | 9 (50 %) | |
| Race | |||||||
| non-Hispanic African American | 209 (80 %) | 156 (80 %) | 53 (80 %) | 169 (79 %) | 24 (77 %) | 16 (89 %) | |
| Other | 53 (20 %) | 40 (20 %) | 13 (20 %) | 44 (21 %) | 7 (23 %) | 2 (11 %) | |
| Income Bracket | |||||||
| < $15,000 | 89 (34 %) | 61 (31 %) | 28 (42 %) | 66 (31 %) | 11 (35 %) | 12 (67 %) | |
| $15,000 – 34,999 | 77 (29 %) | 61 (31 %) | 16 (24 %) | 64 (30 %) | 8 (26 %) | 5 (28 %) | |
| $35,000 – 74,999 | 60 (23 %) | 48 (24 %) | 12 (18 %) | 51 (24 %) | 8 (26 %) | 1 (6 %) | |
| $75,000 or above | 21 (8 %) | 17 (9 %) | 4 (6 %) | 18 (8 %) | 3 (10 %) | 0 (0 %) | |
| Missing | 15 (5.7%) | 9 (4.6%) | 6 (9.1%) | 14 (6.6%) | 1 (3.2%) | 0 (0%) | |
| Educational Attainment | |||||||
| High School or less | 112 (43 %) | 82 (42 %) | 30 (45 %) | 90 (42 %) | 13 (42 %) | 9 (50 %) | |
| > High School | 150 (57 %) | 114 (58 %) | 36 (55 %) | 123 (58 %) | 18 (58 %) | 9 (50 %) | |
| BMI (kg/m2) | |||||||
| Mean (SD) | 32 (± 9.2) | 32 (± 8.8) | 31 (± 10) | 32 (± 8.8) | 30 (± 9.6) | 34 (± 12) | |
| Missing | 9 (3.4%) | 7 (3.6%) | 2 (3.0%) | 7 (3.3%) | 0 (0%) | 2 (11.1%) | |
| Smoking Status | |||||||
| Never Smoker | 85 (32 %) | 70 (36 %) | 15 (23 %) | 68 (32 %) | 9 (29 %) | 8 (44 %) | |
| Former Smoker | 102 (39 %) | 74 (38 %) | 28 (42 %) | 85 (40 %) | 10 (32 %) | 7 (39 %) | |
| Current Smoker | 75 (29 %) | 52 (27 %) | 23 (35 %) | 60 (28 %) | 12 (39 %) | 3 (17 %) | |
| loge(sjTRECs) | |||||||
| Mean (SD) | 8.0 (± 1.1) | 8.1 (± 1.1) | 7.8 (± 1.1) | 8.1 (± 1.1) | 8.1 (± 1.0) | 7.7 (± 1.2) | |
| loge(CRP) | |||||||
| Mean (SD) | 0.72 (± 1.6) | 0.66 (± 1.6) | 0.99 (± 1.3) | 0.69 (± 1.6) | 0.66 (± 1.7) | 1.2 (± 1.1) | |
| loge(IL-6) | |||||||
| Mean (SD) | 1.2 (± 0.92) | 1.2 (± 0.88) | 1.3 (± 1.1) | 1.2 (± 0.90) | 1.0 (± 1.0) | 1.5 (± 0.95) | |
The characteristics of a random sample of 200 brownfields is shown in Table 2. Sites with no clear identifying name (“Unknown”) were the most common in the dataset (41.5%), followed by industrial sites (21.5%) and gas stations (16.0%). Among the 43 industrial sites in our sample, a diverse set of automotive plants, metalworking facilities, and other manufacturing parks was represented. Municipal sites represented multiple police stations, the Highland Park Water Department, several Detroit schools, the Detroit Housing commission, and a mail delivery facility. Sites with a clear identifying name listed by the MDEQ under Part 201 of the NREPA were primarily industrial, whereas sites under Part 213 were more likely to be gas stations. This is in keeping with NREPA guidelines, which outlines Part 213 remediation “as a result of releases from underground storage tank systems,” whereas Part 201 refers to “environmental remediation” more generally (32). While these findings are not informative regarding relative concentrations of any specific toxicants in the soil, water, or air of sites included in this study, they provide a rough estimate of the types of environmental exposures that may be present in the brownfield dataset.
Table 2.
Characteristics of 200 randomly selected brownfields (out of 1149 brownfields in complete analysis). Data collected from Michigan Department of Environmental Quality “Facility Name” associated with each brownfield site. Facilities with a discernable name collected under NREPA Part 201 are primarily industrial sites, whereas facilities collected under Part 213 are primarily gas stations.
| Total (n = 200) |
Part 201 (n = 67) |
Part 213 (n = 133) |
|
|---|---|---|---|
| Brownfield Type | |||
| Unknown | 83 (41.5%) | 35 (52.2%) | 48 (36.1%) |
| Industrial | 43 (21.5%) | 19 (28.4%) | 24 (18.0%) |
| Gas Station | 32 (16.0%) | 1 (1.5%) | 31 (23.3%) |
| Auto Shop | 18 (9.0%) | 2 (3.0%) | 16 (12.0%) |
| Commercial | 14 (7.0%) | 7 (10.4%) | 7 (5.3%) |
| Municipal | 8 (4.0%) | 3 (4.5%) | 5 (3.8%) |
| Auto Wash | 2 (1.0%) | 0 (0%) | 2 (1.5%) |
Coefficients and 95% confidence intervals from regression analysis are shown in Table 3. We observed a significant negative association between household proximity to brownfields and thymic function in Model 4, which adjusts for age, gender, race, income, education, BMI, and smoking status. Participants living within 200 meters of the nearest brownfield had a 0.30 (95% CI = 0.59, 0.02, p = 0.04) loge-unit decrease in sjTRECs per million whole blood cells compared to their counterparts living more than 200m from the nearest brownfield. Previous work in the DNHS showed a 0.039 loge-unit decrease in sjTRECs with each year of age (37), so we estimate that household proximity to brownfields is associated with a roughly 7.7 year age decrement in thymic function. By stratifying participants based on the number of proximate brownfields in Model 4, we observed that individuals with one proximate brownfield had −0.26 (95% CI: −0.58, 0.06, p=0.11) loge-unit sjTRECs compared to non-proximate participants, while individuals with two or more proximate brownfields had −0.41 (95% CI: −0.90, 0.08, p=0.10) loge-unit sjTRECs compared to non-proximate participants. Neither of these associations was statistically significant, but suggest a possible exposure-response association between number of proximate brownfield sites and thymic function in this cohort. CRP and IL-6 were not significantly associated with brownfield sites in this analysis, although both showed consistently positive associations with brownfield-proximate households across all 4 models.
Table 3.
Regression coefficients (β) and 95% confidence intervals for regression analysis of residential proximity to brownfields or highways and loge-transformed sjTRECs, CRP, and IL-6. Model 1 adjusts for age, Model 2 additionally adjusts for gender and race, Model 3 additionally adjusts for income and education, and Model 4 additionally adjusts for BMI and smoking status. Tests of statistical significance were two-sided with α = 0.05.
| Loge(sjTRECs): | β (95% confidence interval) | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Brownfield Proximity | ||||
| >200m | REF | REF | REF | REF |
| ≤200m | −0.19 (−0.46, 0.09) | −0.24 (−0.51, 0.03) | −0.22 (−0.50, 0.05) | −0.30 (−0.59, −0.02) |
| p = 0.19 | p = 0.08 | p = 0.12 | p = 0.04 | |
| Highway Proximity | ||||
| >200m | REF | REF | REF | REF |
| ≤200m, AADT <50,000 | 0.09 (−0.28, 0.47) | 0.14 (−0.22, 0.51) | 0.19 (−0.17, 0.56) | 0.16 (−0.20, 0.53) |
| p = 0.63 | p = 0.45 | p = 0.31 | p = 0.39 | |
| ≤200m, AADT ≥50,000 | −0.15 (−0.63, 0.32) | −0.14 (−0.61, 0.32) | −0.11 (−0.59, 0.36) | 0.03 (−0.48, 0.53) |
| p = 0.54 | p = 0.55 | p = 0.64 | p = 0.93 | |
| Loge(CRP): | β (95% confidence interval) | |||
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Brownfield Proximity | ||||
| >200m | REF | REF | REF | REF |
| ≤200m | 0.34 (−0.10, 0.77) | 0.27 (−0.16, 0.70) | 0.24 (−0.20, 0.68) | 0.27 (−0.15, 0.70) |
| p = 0.13 | p = 0.22 | p = 0.29 | p = 0.22 | |
| Highway Proximity | ||||
| >200m | REF | REF | REF | REF |
| ≤200m, AADT <50,000 | −0.11 (−0.70, 0.49) | −0.03 (−0.61, 0.56) | −0.04 (−0.63, 0.54) | 0.03 (−0.51, 0.58) |
| p = 0.73 | p = 0.93 | p = 0.89 | p = 0.91 | |
| ≤200m, AADT ≥50,000 | 0.41 (−0.40, 1.21) | 0.49 (−0.30, 1.28) | 0.38 (−0.42, 1.18) | 0.41 (0.39, 1.21) |
| p = 0.33 | p = 0.23 | p = 0.36 | p = 0.32 | |
| Loge(IL-6): | β (95% confidence interval) | |||
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Brownfield Proximity | ||||
| >200m | REF | REF | REF | REF |
| ≤200m | 0.11 (−0.14, 0.37) | 0.09 (−0.16, 0.35) | 0.11 (−0.15, 0.37) | 0.07 (−0.20, 0.34) |
| p = 0.38 | p = 0.48 | p = 0.41 | p = 0.61 | |
| Highway Proximity | ||||
| >200m | REF | REF | REF | REF |
| ≤200m, AADT <50,000 | −0.23 (−0.57, 0.12) | −0.20 (−0.55, 0.14) | −0.20 (−0.55, 0.14) | −0.12 (−0.46, 0.21) |
| p = 0.20 | p = 0.25 | p = 0.26 | p = 0.48 | |
| ≤200m, AADT ≥50,000 | 0.09 (−0.37, 0.56) | 0.11 (−0.36, 0.58) | 0.08 (−0.40, 0.56) | 0.23 (−0.26, 0.73) |
| p = 0.70 | p = 0.65 | p = 0.75 | p = 0.37 | |
We found no significant associations between high-traffic areas and CRP, IL-6, or sjTRECs, with estimates of effect on both sides of the null for all three biomarkers and levels of exposure (Table 3). Participants living within 200m of the nearest highway with AADT <50,000 vehicles/day appear to experience a non-statistically significant protective effect associated with their highway proximity (i.e. increased thymic function and decreased inflammation), but participants living within 200m of the nearest highway with AADT ≥50,000 vehicles/day experience a non-statistically significant deleterious effect due to their highway proximity (i.e. decreased thymic activity and increased inflammation). Stratifying highway-proximate participants into individuals living 0–100m, 101–200m, and >200m from the nearest highway yielded no apparent patterns in any biomarker of interest and was challenged by limited precision due to low sample size at each level of highway exposure.
Regression coefficients and 95% confidence intervals for all model covariates are displayed in supplementary tables 1–6. In fully adjusted models, sjTRECs were strongly negatively associated with increasing age, male gender, and increasing BMI, CRP was positively associated with increased BMI and current smoking, and IL-6 was positively associated with increased age, increased BMI, and positive smoking history. Associations between these covariates and each biomarker of interest should not be interpreted causally, however, due to potential unresolved confounding by other covariates not included in our analysis (40).
DISCUSSION:
Using a community-based sample of residents from Detroit, Michigan, we found a robust negative association between household proximity to brownfield sites and thymic activity as measured by sjTRECs, representing an increased thymic “age” among individuals who lived closer to brownfield sites. We also found suggestive evidence of an exposure-response relationship between brownfield proximity and thymic age, with lower levels of loge-transformed sjTRECs among individuals living near two or more brownfields compared to individuals living near just one brownfield site. Systemic inflammation, as measured by CRP and IL-6, were not significantly associated with brownfield proximity. In our full model, CRP was positively associated with elevated BMI and smoking, and IL-6 was positively associated with BMI and increased age; all four associations have been described previously in other populations (41–47).
Overall, our findings mark the first study (to our knowledge) describing an association between brownfield proximity and a global measure of immunity, sjTREC production. Previous work has identified sjTRECs as sensitive markers of thymic involution (35), the age-related decline in naïve T-cell production that ultimately reduces the diversity of the T-cell repertoire in later life (48). The immune system’s ability to mount a T-cell response to new antigens throughout the life course relies almost entirely on the diversity of receptors present at any given time in the naïve T-cell pool (49,50). Any biological or stress-related process that decreases output of naïve T-cells from the thymus, therefore, may diminish the immune system’s ability to mount responses to novel pathogens or cancers.
Exposure to pollution is often conceptualized in one of two ways, either as an effect of actual environmental toxicants (e.g. contamination of soil, water, air, and food) or a manifestation of social and economic deprivation (e.g. a marker of poverty, transiency, or limited social and economic opportunity). Assuming the associations observed in this study are causal, however, we would argue that exposure to brownfield sites may reflect both toxicological and social mechanisms of increased thymic aging. Figure 1 presents this conceptual framework as two pathways leading from brownfield pollution to thymic function. While the present study cannot identify any specific agents that may directly contribute to the observed decrease in naïve T-cell production associated with brownfield exposure (Figure 1, pathway 1), the brownfields in our study area suggest many candidate chemicals that warrant further investigation. Potential soil-based contaminants that may impact thymic activity and immune function include polycyclic aromatic hydrocarbons, organic solvents, combustion products, industrial-use chlorinated hydrocarbons, and heavy metals such as lead, chromium, mercury, and arsenic (9,11,51,52). For example, hydrocarbons such as gasoline have been linked to changes in lymphohematopoietic stem cell-cycle regulation in children and adults in multiple epidemiologic studies, raising the possibility that we are capturing decreased stem cell activity due to hydrocarbon exposure in this cohort (53–55).
Figure 1.
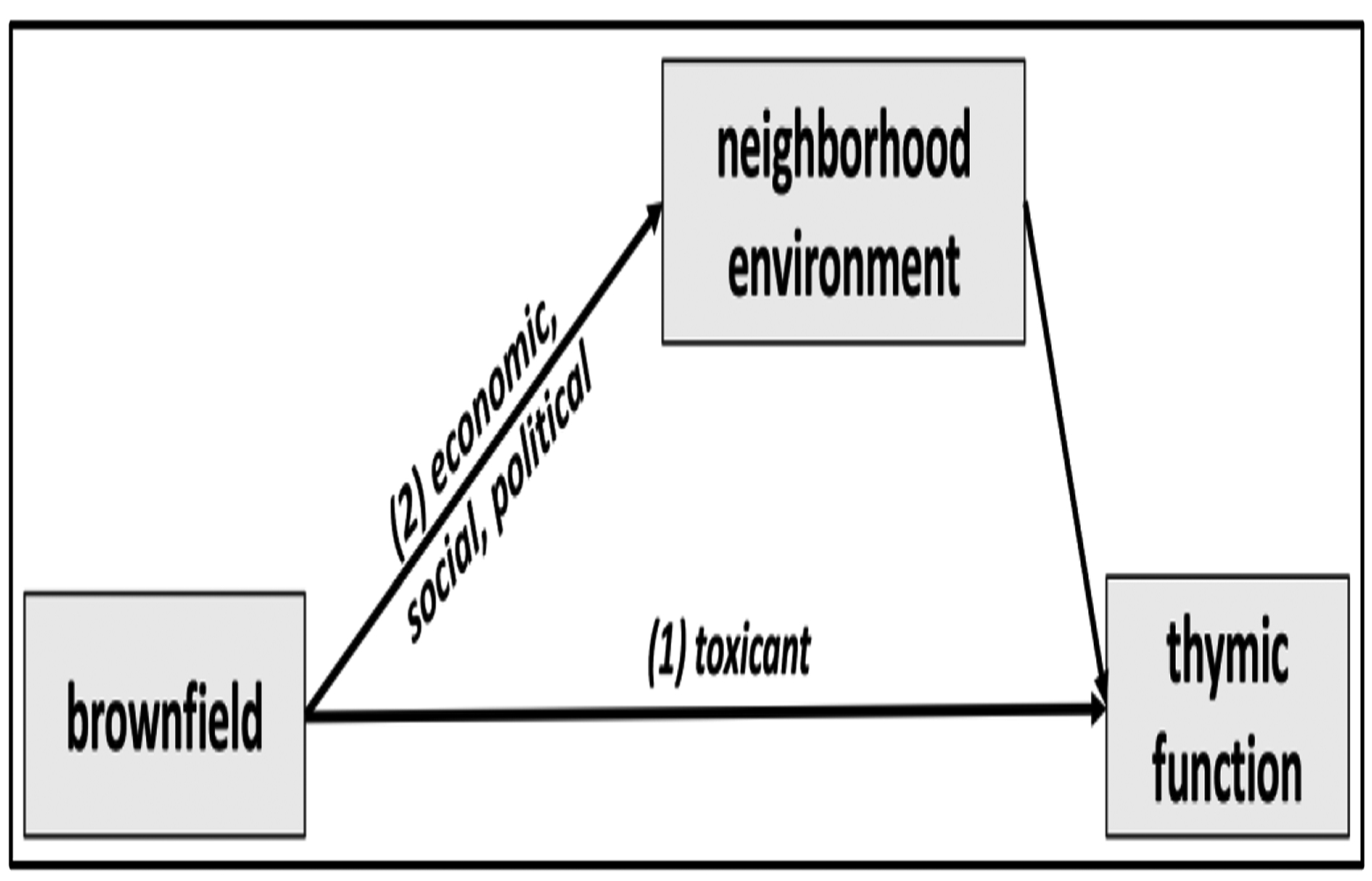
Simplified conceptual diagram of two pathways leading from brownfield proximity to thymic function (as measured by sjTRECs). Pathway (1) represents the direct effect of brownfields on immune function due to exposure to toxicants present in the soil, water, and air. Pathway (2) represents social and economic pathways that may influence neighborhoods that are proximate to brownfield-polluted areas.
In addition to actual toxicant exposure, however, the presence of one or more brownfield sites in a community puts demonstrable limits on neighborhood economic, social, and political opportunity (Figure 1, pathway 2) in the surrounding area (14,56). In many cases, brownfield sites represent absence of opportunity in the most literal sense, as many old manufacturing sites used to provide labor opportunities that are no longer available. The DNHS was coincidentally conducted during the Great Recession, when the presence of abandoned houses, vacant properties, and other remnants of a faltering economy served as physical reminders of accelerating economic losses in Detroit since the mid-20th century (57). Thus, the presence of brownfields in a neighborhood may also represent loss of economic mobility and income, which has been previously tied to immune aging and T-cell immunosenescence through various stress-related pathways in the DNHS cohort (58). This makes it challenging to disentangle the economic impacts of brownfield sites, with the available data, from the chemical hazards they contain. If complete environmental data were available (e.g. soil, water, and air samples combined with detailed historic land use information), it would be possible to investigate the direct effect of brownfield toxicants while controlling for neighborhood-level social, economic, and historical factors. Until then, however, critically evaluating brownfield sites as sources of mixed environmental and socioeconomic risk is crucial as efforts to remediate and revitalize polluted areas continue in the coming years.
Our study has several limitations that should be considered. Neighborhood economic factors and brownfield sites have a reciprocal and reinforcing relationship, as economically depressed neighborhoods attract industrial development because of the low costs associated with such locations, and the presence of industrial development further decreases neighborhood economic standing by depressing land and real estate value. This relationship raises the potential analytic concern that our fully-adjusted models, which control for participant-level income and education, may adjust for a mediator in the association between brownfields and immune function. While we cannot completely eliminate that possibility, we hypothesize that the majority of the industrial contamination in Detroit most likely predates the participants in our analysis, i.e. participants’ low SES is the cause of their residing near brownfield sites, but not vice versa. In such a setting, proxies for individual SES (income and education) should be controlled as confounders of the brownfield-immune function relationship, while brownfield-mediated neighborhood effects on immune function (Figure 1, pathway 2) should be left open.
Exposure misclassification for brownfields in this study is a concern. There are almost certainly numerous brownfield sites distributed throughout Detroit that were not captured in our study due to the reporting system designed by the MDEQ. However, it is unlikely that exposure misclassification is differential with respect to our outcomes of interest. In addition, the size of brownfield land parcels may vary dramatically. Given the available data, however, we are unable to evaluate the effect of this potential bias. Outcome misclassification is also possible, as the use of sjTRECs may mask participant-level differences in T cell division, T cell death, longevity of naïve T cells, and other markers (59). We expect that these largely stochastic biological processes will only introduce random error, not systematic bias, in our results. We were also unable to use the sj/β-TREC ratio, a potentially more reliable measure of thymic involution and aging (35), due to a high proportion of β-TREC results in the DNHS below the limit of detection (data not shown).
Due to limited sample size (262 participants overall), we were unable to investigate immune or inflammatory dysregulation associated with particular types of brownfields to gain a better understanding of brownfield toxicology. As an example, it would be possible to combine gas stations and auto shops together as areas with high potential gasoline vapor concentrations, a more specific exposure to petrochemical pollution. With only 66 brownfield-proximate participants with biomarker data, however, this was impractical in our analytic sample. In addition, future studies should include repeated sjTREC measurements to investigate temporal trends associated with brownfield exposure. While we had two measurements of CRP and IL-6 for some participants in this study, the sample size was too small to conduct any longitudinal analyses. As it is, our results should be used to generate new hypotheses about the potential immune impacts of brownfields in larger longitudinal population-based studies. Last, we are unable to survey or find specific information on the contaminants present at the brownfield sites in our sample. Therefore, we do not know whether chemical exposures, either volatile or in soil, are the reason for the observed associations between brownfields and thymic function in this study.
High-traffic highway exposure was not consistently associated with any biomarker in this study, with estimates on both sides of the null in most models. Our lack of consistent findings regarding exposure to high-traffic highways and inflammation as measured by CRP and IL-6 is consistent with a similar analysis in Seattle, Washington (31). While previous research has identified strong, statistically significant associations between exposure to traffic and biomarkers of cardiovascular disease (25–28) and inflammation (3,4,29,30), it may be that any pro-inflammatory effects of exposure to traffic and vehicle exhaust are simply too small to be statistically significant in our analysis. The direction of effect between the three levels of traffic exposure in our analysis does not hold with such an interpretation, however, as we observe anti-inflammatory estimates in participants living near highways with AADT <50,000 vehicles/day, but pro-inflammatory estimates in participants living near highways with AADT ≥50,000 vehicles/day (Table 3). A more specific approach may be modeling the effect of true residence-level air pollution (ex. particulate matter, NOx, ozone, etc.) on our outcomes of interest to better test the relationship between traffic-related air pollution and biomarkers of immune function and inflammation. Such an approach has been used in epigenetic (60) and cardiovascular epidemiology (61) to provide estimates of effect for household-level air quality.
In this study, we report a novel association between immune function and household proximity to brownfield sites in Detroit, Michigan. Using a representative sample of Detroit residents, we show that participants living in close proximity to brownfield sites in the city had accelerated thymic aging compared to their more distant peers. This study highlights the utility of sjTRECs in environmental epidemiology. Future studies are needed to replicate these findings and should include environmental sampling of brownfield sites to determine whether specific contaminant exposures affect thymic function in human populations.
Supplementary Material
NOVELTY:
Brownfield sites are extremely prevalent across the country, but the health risks associated with exposure to these sites are poorly understood and vastly under-studied. This is the first study, to our knowledge, reporting that residential proximity to brownfield sites is associated with accelerated thymic aging as quantified by signal joint T-cell receptor excision circles (sjTRECs). No other study has reported associations between brownfield sites and any biomarker. This study also serves to introduce environmental epidemiologists to sjTRECs as a sensitive marker of immune function and naïve T-cell production by the thymus.
ACKNOWLEDGEMENTS:
The Detroit Neighborhood Health Study received funding from the National Institutes of Health (R01 DA022720, R01 MD011728). The University of Michigan Nathan Shock Center provided funding for IL-6 and CRP testing (AG013283). Lodge was supported by the University of North Carolina at Chapel Hill Medical Scientist Training Program (T32 GM008719-18), the National Institute of Environmental Health Sciences (T32 ES007018), the National Institute of Child Health and Human Development (T32 HD007168), and a University of North Carolina at Chapel Hill Graduate School Doctoral Merit Assistantship. Data Driven Detroit (https://datadrivendetroit.org) provided data on brownfield sites in Detroit obtained by the Michigan Department of Environmental Quality (MDEQ) in 2014 with the help of N. Urban.
Footnotes
CONFLICT OF INTEREST:
The authors declare they have no actual or potential competing financial interests. Funders of this project played no role in data collection, study design, analysis, or manuscript preparation.
REFERENCES:
- 1.De Roos AJ, Koehoorn M, Tamburic L, Davies HW, Brauer M. Proximity to Traffic, Ambient Air Pollution, and Community Noise in Relation to Incident Rheumatoid Arthritis. Environ Health Perspect. 2014. October;122(10):1075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamra GB, Laden F, Cohen AJ, Raaschou-Nielsen O, Brauer M, Loomis D. Lung Cancer and Exposure to Nitrogen Dioxide and Traffic: A Systematic Review and Meta-Analysis. Environ Health Perspect. 2015. November;123(11):1107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hennig F, Fuks K, Moebus S, Weinmayr G, Memmesheimer M, Jakobs H, et al. Association between Source-Specific Particulate Matter Air Pollution and hs-CRP: Local Traffic and Industrial Emissions. Environ Health Perspect. 2014. July;122(7):703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann B, Moebus S, Dragano N, Stang A, Möhlenkamp S, Schmermund A, et al. Chronic Residential Exposure to Particulate Matter Air Pollution and Systemic Inflammatory Markers. Environ Health Perspect. 2009. August;117(8):1302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanna-Attisha M, LaChance JL, Sadler RC, Schnepp AC. Elevated Blood Lead Levels in Children Associated With the Flint Drinking Water Crisis: A Spatial Analysis of Risk and Public Health Response. American journal of public health. 2016;106(2):283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph CLM, Havstad S, Ownby DR, Peterson EL, Maliarik M, McCabe MJ, et al. Blood Lead Level and Risk of Asthma. Environ Health Perspect. 2005. July;113(7):900–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hicken MT, Gee GC, Connell C, Snow RC, Morenoff J, Hu H. Black–White Blood Pressure Disparities: Depressive Symptoms and Differential Vulnerability to Blood Lead. Environ Health Perspect. 2013. February;121(2):205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadler RC, LaChance J, Hanna-Attisha M. Social and Built Environmental Correlates of Predicted Blood Lead Levels in the Flint Water Crisis. Am J Public Health. 2017. March 21;107(5):763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian Y, Gallagher F, Deng Y, Wu M, Feng H. Risk assessment and interpretation of heavy metal contaminated soils on an urban brownfield site in New York metropolitan area. Environ Sci Pollut Res. 2017. October 1;24(30):23549–58. [DOI] [PubMed] [Google Scholar]
- 10.Pan L, Wang Y, Ma J, Hu Y, Su B, Fang G, et al. A review of heavy metal pollution levels and health risk assessment of urban soils in Chinese cities. Environ Sci Pollut Res. 2018. January 1;25(2):1055–69. [DOI] [PubMed] [Google Scholar]
- 11.Megharaj M, Naidu R. Soil and brownfield bioremediation. Microb Biotechnol. 2017. August 22;10(5):1244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pizzol L, Zabeo A, Klusáček P, Giubilato E, Critto A, Frantál B, et al. Timbre Brownfield Prioritization Tool to support effective brownfield regeneration. Journal of Environmental Management. 2016. January 15;166:178–92. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg MR. Reversing urban decay: brownfield redevelopment and environmental health. Environ Health Perspect. 2003. February;111(2):A74–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg M Should Housing Be Built on Former Brownfield Sites? Am J Public Health. 2002. May 1;92(5):703–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maantay JA, Maroko AR. Brownfields to Greenfields: Environmental Justice Versus Environmental Gentrification. Int J Environ Res Public Health [Internet]. 2018. October [cited 2019 Feb 16];15(10). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6210586/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bambra C, Cairns JM, Kasim A, Smith J, Robertson S, Copeland A, et al. This divided land: An examination of regional inequalities in exposure to brownfield land and the association with morbidity and mortality in England. Health & Place. 2015. July 1;34:257–69. [DOI] [PubMed] [Google Scholar]
- 17.Litt JS, Tran NL, Burke TA. Examining urban brownfields through the public health “macroscope”. Environ Health Perspect. 2002. April;110(Suppl 2):183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.United States Environmental Protection Agency. Summary of the Small Business Liability Relief and Brownfields Revitalization Act. 2002; Available from: https://www.epa.gov/brownfields/summary-small-business-liability-relief-and-brownfields-revitalization-act#title2
- 19.Généreux M, Auger N, Goneau M, Daniel M. Neighbourhood socioeconomic status, maternal education and adverse birth outcomes among mothers living near highways. Journal of Epidemiology & Community Health. 2008. August 1;62(8):695–700. [DOI] [PubMed] [Google Scholar]
- 20.Kingsley SL, Eliot MN, Whitsel EA, Huang Y-T, Kelsey KT, Marsit CJ, et al. Maternal residential proximity to major roadways, birth weight, and placental DNA methylation. Environ Int. 2016;92–93:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGuinn LA, Voss RW, Laurent CA, Greenspan LC, Kushi LH, Windham GC. Residential Proximity to Traffic and Female Pubertal Development. Environ Int. 2016. September;94:635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.J Keeler G, Dvonch T, Yip F, Parker E, A Isreal B, Marsik F, et al. Assessment of Personal and Community-Level Exposures to Particulate Matter among Children with Asthma in Detroit, Michigan, as Part of Community Action Against Asthma (CAAA). Environmental health perspectives. 2002. April 1;110 Suppl 2:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riley S, Wallace J, Nair P. Proximity to major roadways is a risk factor for airway hyper-responsiveness in adults. Can Respir J. 2012;19(2):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward-Caviness CK, Kraus WE, Blach C, Haynes CS, Dowdy E, Miranda ML, et al. Associations Between Residential Proximity to Traffic and Vascular Disease in a Cardiac Catheterization Cohort. Arterioscler Thromb Vasc Biol. 2018. January;38(1):275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang S, Bo L, Gong C, Du X, Kan H, Xie Y, et al. Traffic-related air pollution is associated with cardio-metabolic biomarkers in general residents. Int Arch Occup Environ Health. 2016. August 1;89(6):911–21. [DOI] [PubMed] [Google Scholar]
- 26.Dong Guang-Hui, Qian Zhengmin (Min), Xaverius Pamela K., Trevathan Edwin, Maalouf Salwa, Parker Jamaal, et al. Association Between Long-Term Air Pollution and Increased Blood Pressure and Hypertension in China. Hypertension. 2013. March 1;61(3):578–84. [DOI] [PubMed] [Google Scholar]
- 27.Liu C, Chen R, Zhao Y, Ma Z, Bi J, Liu Y, et al. Associations between ambient fine particulate air pollution and hypertension: A nationwide cross-sectional study in China. Science of The Total Environment. 2017. April 15;584–585:869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan C, Lu S, Wang Y, Zhu Y, Shi T, Lin M, et al. Long-term exposure to high air pollution induces cumulative DNA damages in traffic policemen. Science of The Total Environment. 2017. September 1;593–594:330–6. [DOI] [PubMed] [Google Scholar]
- 29.Diez Roux AV, Auchincloss AH, Astor B, Barr RG, Cushman M, Dvonch T, et al. Recent Exposure to Particulate Matter and C-reactive Protein Concentration in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2006. September 1;164(5):437–48. [DOI] [PubMed] [Google Scholar]
- 30.Lanki T, Hampel R, Tiittanen P, Andrich S, Beelen R, Brunekreef B, et al. Air Pollution from Road Traffic and Systemic Inflammation in Adults: A Cross-Sectional Analysis in the European ESCAPE Project. Environ Health Perspect. 2015. August;123(8):785–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams LA, Ulrich CM, Larson T, Wener MH, Wood B, Campbell PT, et al. Proximity to Traffic, Inflammation, and Immune Function among Women in the Seattle, Washington, Area. Environ Health Perspect. 2009. March;117(3):373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.State of Michigan Legislature. Act 451 of 1994: Natural Resources and Environmental Protection Act. 1994; Available from: http://www.legislature.mi.gov/(S(msqwoo5jvp5ypk31z5kywvyn))/mileg.aspx?page=print&objectname=mcl-Act-451-of-1994
- 33.Goldmann E, Aiello A, Uddin M, Delva J, Koenen K, Gant LM, et al. Pervasive Exposure to Violence and Posttraumatic Stress Disorder in A Predominantly African American Urban Community. J Trauma Stress. 2011. December;24(6):747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de los Santos R, et al. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2010. May 18;107(20):9470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrando-Martínez S, Franco JM, Ruiz-Mateos E, Hernández A, Ordoñez A, Gutierrez E, et al. A reliable and simplified sj/β-TREC ratio quantification method for human thymic output measurement. Journal of Immunological Methods. 2010. January 31;352(1):111–7. [DOI] [PubMed] [Google Scholar]
- 36.Ferrando-Martínez S, Romero-Sánchez MC, Solana R, Delgado J, de la Rosa R, Muñoz-Fernández MÁ, et al. Thymic function failure and C-reactive protein levels are independent predictors of all-cause mortality in healthy elderly humans. Age (Dordr). 2013. February;35(1):251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feinstein L, Ferrando-Martínez S, Leal M, Zhou X, Sempowski GD, Wildman DE, et al. Population Distributions of Thymic Function in Adults: Variation by Sociodemographic Characteristics and Health Status. Biodemography and Social Biology. 2016. May 3;62(2):208–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brugge D, Durant JL, Rioux C. Near-highway pollutants in motor vehicle exhaust: A review of epidemiologic evidence of cardiac and pulmonary health risks. Environ Health. 2007. August 9;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Y-C, Batterman SA. Proximity of schools in Detroit, Michigan to automobile and truck traffic. Journal of Exposure Science and Environmental Epidemiology. 2006. September;16(5):457–70. [DOI] [PubMed] [Google Scholar]
- 40.Westreich D, Greenland S. The Table 2 Fallacy: Presenting and Interpreting Confounder and Modifier Coefficients. Am J Epidemiol. 2013. February 15;177(4):292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-Reactive Protein Levels in Overweight and Obese Adults. JAMA. 1999. December 8;282(22):2131–5. [DOI] [PubMed] [Google Scholar]
- 42.Timpson NJ, Nordestgaard BG, Harbord RM, Zacho J, Frayling TM, Tybjærg-Hansen A, et al. C-reactive protein levels and body mass index: Elucidating direction of causation through reciprocal Mendelian randomization. Int J Obes (Lond). 2011. February;35(2):300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fried SK, Bunkin DA, Greenberg AS. Omental and Subcutaneous Adipose Tissues of Obese Subjects Release Interleukin-6: Depot Difference and Regulation by Glucocorticoid. J Clin Endocrinol Metab. 1998. March 1;83(3):847–50. [DOI] [PubMed] [Google Scholar]
- 44.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, et al. Subcutaneous Adipose Tissue Releases Interleukin-6, But Not Tumor Necrosis Factor-α, in Vivo. J Clin Endocrinol Metab. 1997. December 1;82(12):4196–200. [DOI] [PubMed] [Google Scholar]
- 45.Khaodhiar L, Ling P-R, Blackburn GL, Bistrian BR. Serum Levels of Interleukin-6 and C-Reactive Protein Correlate With Body Mass Index Across the Broad Range of Obesity. JPEN, Journal of Parenteral and Enteral Nutrition; Silver Spring. 2004. December;28(6):410–5. [DOI] [PubMed] [Google Scholar]
- 46.Tonstad S, Cowan JL. C-reactive protein as a predictor of disease in smokers and former smokers: a review. Int J Clin Pract. 2009. Nov;63(11):1634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of Ageing and Development. 2007. January 1;128(1):92–105. [DOI] [PubMed] [Google Scholar]
- 48.Goronzy JJ, Lee W-W, Weyand CM. Aging and T-cell diversity. Exp Gerontol. 2007. May;42(5):400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naylor K, Li G, Vallejo AN, Lee W-W, Koetz K, Bryl E, et al. The Influence of Age on T Cell Generation and TCR Diversity. The Journal of Immunology. 2005. June 1;174(11):7446–52. [DOI] [PubMed] [Google Scholar]
- 50.Lynch HE, Goldberg GL, Chidgey A, Van den Brink MRM, Boyd R, Sempowski GD. Thymic involution and immune reconstitution. Trends Immunol. 2009. July;30(7):366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petrová Š, Rezek J, Soudek P, Vaněk T. Preliminary study of phytoremediation of brownfield soil contaminated by PAHs. Science of The Total Environment. 2017. December 1;599–600:572–80. [DOI] [PubMed] [Google Scholar]
- 52.Litt JS, Burke TA. Uncovering the historic environmental hazards of urban brownfields. J Urban Health. 2002. December;79(4):464–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoon B-I, Hirabayashi Y, Kawasaki Y, Kodama Y, Kaneko T, Kim D-Y, et al. Mechanism of action of benzene toxicity: Cell cycle suppression in hemopoietic progenitor cells (CFU-GM). Experimental Hematology. 2001. March 1;291(3):278–85. [DOI] [PubMed] [Google Scholar]
- 54.Infante PF. Residential Proximity to Gasoline Stations and Risk of Childhood Leukemia. Am J Epidemiol. 2017. January 1;185(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glass DC, Gray CN, Jolley DJ, Gibbons C, Sim MR, Fritschi L, et al. Leukemia Risk Associated With Low-Level Benzene Exposure. Epidemiology. 2003. September;14(5):569. [DOI] [PubMed] [Google Scholar]
- 56.McAvoy PV, Driscoll MB, Gramling BJ. Integrating the Environment, the Economy, and Community Health: A Community Health Center’s Initiative to Link Health Benefits to Smart Growth. Am J Public Health. 2004. April 1;94(4):525–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farley R The Bankruptcy of Detroit: What Role did Race Play? City & Community. 2015;14(2):118–37. [Google Scholar]
- 58.Aiello AE, Feinstein L, Dowd JB, Pawelec G, Derhovanessian E, Galea S, et al. Income and markers of immunological cellular aging. Psychosom Med. 2016;78(6):657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hazenberg MD, Verschuren MC, Hamann D, Miedema F, Dongen JJ. T cell receptor excision circles as markers for recent thymic emigrants: basic aspects, technical approach, and guidelines for interpretation. J Mol Med. 2001. November 1;79(11):631–40. [DOI] [PubMed] [Google Scholar]
- 60.Dhingra R, Nwanaji-Enwerem JC, Samet M, Ward-Caviness CK. DNA Methylation Age—Environmental Influences, Health Impacts, and Its Role in Environmental Epidemiology. Curr Envir Health Rpt. 2018. September 1;5(3):317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shin J, Choi J, Kim KJ. Association between long-term exposure of ambient air pollutants and cardiometabolic diseases: A 2012 Korean Community Health Survey. Nutrition, Metabolism and Cardiovascular Diseases. 2019. February;29(2):144–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


