Eosinophilic esophagitis (EoE) is estimated to affect 34.4/100,000 individuals,(1) and the incidence appears to be increasing.(2) However, previous prevalence studies used administrative data that did not include those insured by Medicaid, a federally-funded program available to low-income Americans. Furthermore, while disparities in asthma morbidity(3) and food allergy (4) have been seen by socioeconomic status, our understanding of the association between EoE, poverty, and urbanization is poor. Our objective was to estimate the overall prevalence of EoE among children enrolled in Medicaid, and to examine this prevalence by gender, race/ethnicity, urban/rural status, and neighborhood-level poverty.
This was a cross-sectional study of individuals aged 0–17 years enrolled in Medicaid in 2012. Individuals were included if they were enrolled for the full 12-month period. EoE was defined as having at least one inpatient or outpatient visit with an International Classification of Diseases, Ninth Revision (ICD-9) code for EoE (530.13) during the 12-month period. Period prevalence was estimated by dividing the number of EoE cases by the total number of included individuals. Prevalence estimates were stratified by age, race/ethnicity, gender, rural/urban classifications, and poverty status. Multivariable logistic regression, adjusted for the above covariates and state of residence, was used to assess the relationship between poverty, urban/rural status, and EoE. Further details regarding the study methodology can be found in the Online Repository Text and Figure E1.
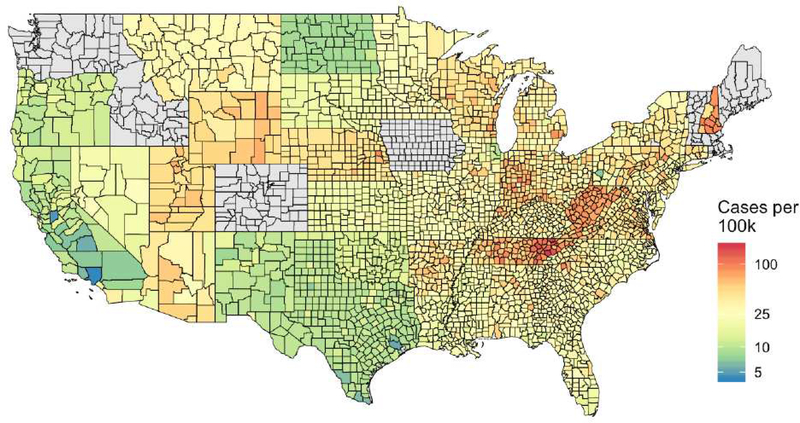
A total of 18,452,886 individuals were included. Population characteristics and EoE prevalence estimates are presented in Table E1. Among this population, 4,836 cases of EoE were identified, for an overall prevalence of 26.21/100,000. The prevalence estimates were highest among males (35.68/100,000), those with self-reported “White” (43.97/100,000) and “Unknown” (62.85/100,000) race/ethnicity, those living in large fringe-metro (suburban) areas (32.03/100,000) and those living in ZCTAs with 0–5% of the population below the poverty level (42.91/100,000). The five states with the highest prevalence of EoE were New Hampshire, West Virginia, Tennessee, Utah, and Arizona (Figure 1).
Figure 1.
County-level prevalence estimates of EoE among 2012 enrollees in the U.S., smoothed within state. States in gray were excluded from all analyses due to concerns about data quality (ME, ID, RI) or ≥ 15% missing data for race/ethnicity (CO, IA, MA, VT, WA).
In unadjusted models, children living in large-central metro areas had the lowest odds of EoE (Table 1). However, when adjusting for potential confounders, this relationship changed, and living in rural areas was protective for EoE diagnosis when compared to large-central metro areas (small metro: OR 0.78; 95% CI 0.69–0.88); micropolitan: OR 0.79; 95% CI 0.70–0.89; non-core (rural): OR 0.68; 95% CI 0.59–0.78, all p<0.001). This inversion appeared to be driven by adjustment for race/ethnicity rather than age or neighborhood-level poverty (Table E2). There was also a negative relationship between poverty and EoE diagnosis, with less neighborhood poverty conveying a significantly higher odds of EoE diagnosis (OR 3.09; 95% CI 2.69–3.54; p<0.001), which persisted in adjusted models (Table 1). These results did not change in a sensitivity analysis including states that were previously excluded due to missing data on race/ethnicity (Table E3).
Table 1:
Association Between ZCTA-Level Poverty, Urban/Rural Status and EoE
| Crude OR | 95% CI | Adjusted OR* | 95% CI | |
|---|---|---|---|---|
| Gender | ||||
| Female | REF | REF | ||
| Male | 2.19 | 2.06 – 2.33 | 2.14 | 2.01 – 2.27 |
| Age Categories | ||||
| 0–2 y | REF | REF | ||
| 3–5 y | 0.99 | 0.90 – 1.09 | 0.99 | 0.90 – 1.09 |
| 6–8 y | 1.00 | 0.91 – 1.11 | 0.99 | 0.89 – 1.09 |
| 9–11 y | 1.07 | 0.97 – 1.18 | 1.03 | 0.94 – 1.14 |
| 12–14 y | 1.02 | 0.92 – 1.13 | 0.98 | 0.89 – 1.09 |
| 15–17 y | 0.89 | 0.79 – 1.00 | 0.87 | 0.77 – 0.98 |
| Race/Ethnicity | ||||
| White | REF | REF | ||
| Black | 0.36 | 0.33 – 0.39 | 0.41 | 0.38 – 0.45 |
| Asian | 0.30 | 0.23 – 0.39 | 0.37 | 0.28 – 0.49 |
| Hispanic | 0.20 | 0.18 – 0.22 | 0.31 | 0.27 – 0.35 |
| Grouped§ | 0.32 | 0.28 – 0.37 | 0.40 | 0.34 – 0.47 |
| Unknown | 1.43 | 1.30 – 1.57 | 1.64 | 1.48 – 1.80 |
| Urban/Rural Status | ||||
| Large Central Metro | REF | REF | ||
| Large Fringe Metro | 1.62 | 1.47 – 1.79 | 0.93 | 0.84 – 1.02 |
| Medium Metro | 1.46 | 1.32 – 1.61 | 0.95 | 0.87 – 1.05 |
| Small Metro | 1.45 | 1.28 – 1.64 | 0.78 | 0.69 – 0.88 |
| Micropolitan | 1.55 | 1.37 – 1.74 | 0.79 | 0.70 – 0.89 |
| Noncore (rural) | 1.36 | 1.18 – 1.55 | 0.68 | 0.59 – 0.78 |
| ZCTA-Level Poverty | ||||
| < 5% | 3.09 | 2.69 – 3.54 | 2.00 | 1.75 – 2.28 |
| 6 – 10% | 2.58 | 2.27 – 2.94 | 1.78 | 1.57 – 2.00 |
| 11 – 15% | 2.00 | 1.75 – 2.29 | 1.50 | 1.33 – 1.69 |
| 16 – 20% | 1.49 | 1.29 – 1.74 | 1.30 | 1.14 – 1.49 |
| 21 – 25% | 1.02 | 0.86 – 1.21 | 0.99 | 0.84 – 1.16 |
| 26 – 100% | REF | REF |
Values expressed as odds ratios (OR) and 95% CIs
Models adjusted for gender, age, race/ethnicity, urban/rural status, state of residence, and ZCTA-level poverty
Defined as the combination of “Native American/Alaskan,” “>1 race (Hispanic), “>1 race (non-Hispanic),” and “Hawaiian” 147 ZCTA: Zip-code tabulation area
In this study of 18,452,886 children enrolled in Medicaid in 2012, we found that the overall prevalence of EoE was 26.21/100,000. In addition, we found that living in rural areas (compared to large-central metro) and in areas with more neighborhood poverty were associated with a lower odds of being diagnosed with EoE. Finally, we found that there was notable spatial variation in the prevalence of EoE, with some states (i.e. NH, WV, and TN) showing markedly higher rates than others (CA, ND, and TX), (Figure 1). Future research should explore environmental factors that may explain these differences, such as exposure to fine and coarse particulate matter.
Previous studies examining the association between EoE and urbanization have yielded conflicting results. Using a national pathology database, Jensen et al found that the odds of having esophageal eosinophilia was higher among individuals living in areas with low population density.(5) While we found similar results in unadjusted models, when we adjusted for potential confounders, the odds of being diagnosed with EoE was lower in rural areas. This inversion is likely due to confounding by race/ethnicity, as the proportion of non-Hispanic Whites living in rural areas is high (>40%), and the prevalence of EoE is higher among this population (Table E1). (7)It is also possible that the discrepancy in our findings and that of Jensen et al may be due differences in the definition of urbanization or the age of the study populations. as <3% of the biopsies in the previous study were from pediatric patients. Further research into whether there are unique protective factors in rural environments, and whether the determinants of EoE are different among children and adults, is clearly needed.
While disparities in asthma morbidity and food allergy diagnosis by poverty have been seen,(3, 4) very little is known about the effect of poverty on EoE. One previous case-control study found that individuals with EoE were more affluent and more educated than control populations, but this association did not persist when adjusting for race/ethnicity, and the study was limited by selection bias.(6) Using a cohort in Southern California, Kim et al, demonstrated that EoE was more prevalent among households with higher annual income, however this study did not assess whether this was due to confounding by gender or race/ethnicity.(7) In our study examining a broader U.S. population, we similarly found that children living in areas with more neighborhood poverty were less likely to be diagnosed with EoE, despite adjusting for potential confounders. Whether this is due to unique environmental exposures that are protective for the development of EoE, under-diagnosis and/or under-perception of EoE, or lack of access to care in impoverished populations is unknown and warrants further study.
This study is primarily limited by the utilization of ICD-9 coding to define EoE, which has been shown to be specific (99%), but not sensitive (60%),(8) for identifying patients with EoE. Furthermore, this data was collected prior to the revised EoE diagnosis guidelines,(9) and thus the ICD-9 code may have been reserved for individuals who completed a PPI trial. Thus, it is possible that the prevalence of EoE was underestimated in this population. This study is further limited by the lack of individual-level data on income, potentially leading to residual confounding and ecological fallacy. In addition, a large number of individuals with EoE had “Unknown” race/ethnicity, which limits our ability to make meaningful conclusions about the differences in EoE prevalence by race/ethnicity. Finally, the results of this study may not be generalizable to all socioeconomic groups. These limitations, however, are balanced by the fact that this study included over 18 million children enrolled in a nationwide database, which enables us to identify associations with low-income children across the U.S., rather than relying on a specific geographic region. Furthermore, this is the first study to examine the association between neighborhood-level poverty and EoE.
In summary, our findings suggest that EoE is less prevalent among children living in impoverished areas. Whether the prevalence of EoE is truly lower in this population, and if so, whether this is due to unique environmental exposures or under-diagnosis and under-treatment remains unknown and warrants further study.
Online Repository Text
Participants
As previously described, the data were centrally compiled and cleaned by the Research Data Assistance Center (ResDAC, University of Minnesota, Minneapolis, MN)(3) and obtained by the authors in August 2018. Medicaid data were collected and aggregated on the state level and then processed by the Centers for Medicare and Medicaid into the Medicaid Analytic Extract (MAX), with individuals grouped by zip-code tabulation area (ZCTA). All individuals aged 0–17 who were enrolled for the entirety of 2012 were eligible for inclusion in these analyses. The ZCTA data was then linked to the 2013 NCHS Urban-Rural Classification scheme for Counties, based on the containing county, and the 2011 American Community Survey. Use of the data was approved by the Johns Hopkins School of Medicine Institutional Review Board, and all analyses were performed in R.
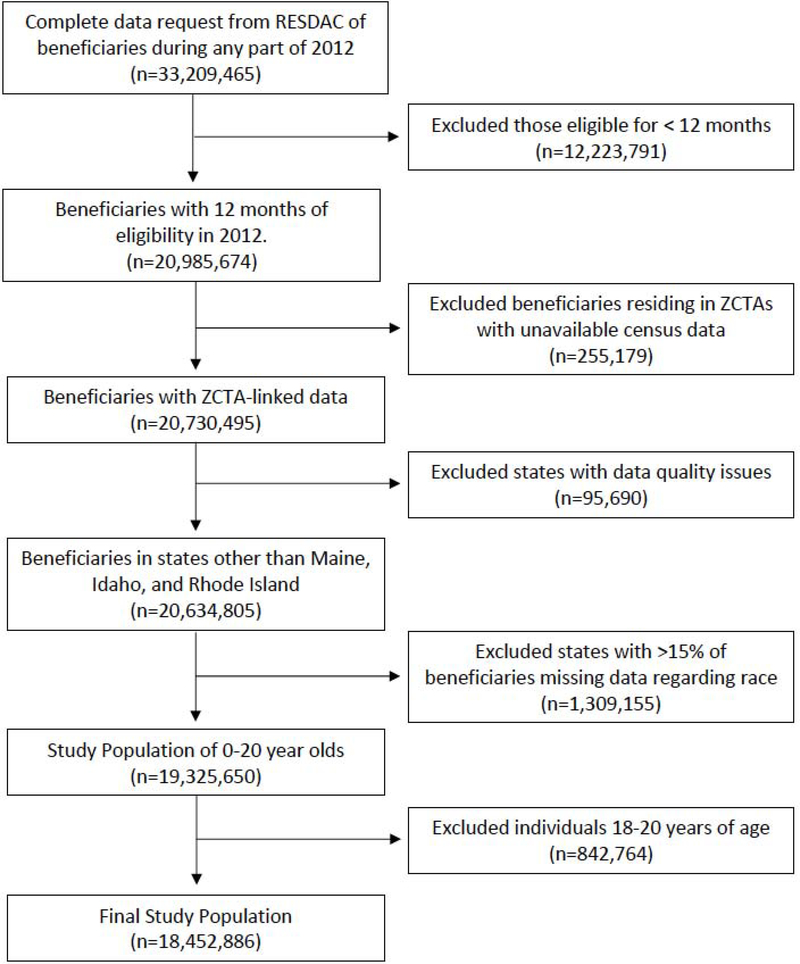
Three states were excluded from the analysis because of concerns about data quality: Maine, which had incomplete utilization data, and Idaho and Rhode Island, which had abnormally low levels of enrollment (<1000 individuals). In addition, five states were excluded because greater than 15% of subjects had missing data for race/ethnicity: Colorado (n=241,722), Iowa (n=178,524), Massachusetts (n=356,927), Vermont (n=42,066), and Washington (n=489,916). This data linkage and exclusion process is depicted in Figure E1.
Definition of Covariates
Urbanization was categorized according to this scheme as “large central metro,” “large fringe metro (suburban)” “medium metro” (250,000–999.999 population), “small metro” (50,000 –249,999 population), “micropolitan” (10,000 – 49,999 population, and “noncore” (rural, <10,000 population). Neighborhood-level poverty was defined as the proportion of families at or below the poverty level in the ZCTA. Race/ethnicity was defined by self-report, and categories included “White,” “Black,” “Asian,” “Hispanic,” “North American Native,” “>1 race (Hispanic), >1 race (non-Hispanic), Hawaiian, and “Unknown.” For the purpose of analysis, “Native American/Alaskan,” “>1 race (Hispanic), “>1 race (non-Hispanic),” and “Hawaiian” were combined into “Grouped.”
Statistical Analysis
Age, race, gender, ZCTA-level poverty, and county urban-rural code were all treated as categorical variables in the multivariable logistic regression models. In addition to the analyses described in the main manuscript, sensitivity analyses were performed to a) examine whether the inversion of the association between urban/rural status and EoE diagnosis was due to confounding by race/ethnicity or neighborhood-level poverty and to b) assess whether the exclusion of States due to missing data on race/ethnicity influenced the overall results. In order to graphically depict the prevalence of EoE, county-level estimates were plotted and adjusted for the statewide average (“smoothed”) in order to account for differences in the number of enrollees in each county. In all analyses, correlation within zip-code tabulation area (ZCTA) was accounted for using generalized estimating equations.
Supplemental Results
In order to further examine whether the inversion of the association between urban/rural status and EoE diagnosis was due to confounding by race/ethnicity or neighborhood-level poverty, we first adjusted the analyses for only gender and age (Table E2, Model 1), and the results did not change. We then adjusted the model for gender, age, and race/ethnicity, and the results were similar to the inversion seen in the full model (Table E2, Model 2). Finally, we then further adjusted for neighborhood-level poverty (Table E2, Final Model), and the results were unchanged. In the sensitivity analysis examining the exclusion of States with missing data, the trends and conclusions depicted in the main manuscript did not change (Table E3).
Acknowledgments
Funding: This work was funded by the NIH through the following grants: (NIAID) 1K23AI103187, (NIAID) 1K23AI123596, Johns Hopkins Pediatrics Innovation Award
Appendix
Table E1:
EoE Prevalence by Demographic Characteristics
| Overall Medicaid Population | EOE Cases | EOE Prevalence (per 100,000) | |
|---|---|---|---|
| Overall | 18,452,886 | 4,836 | 26.21 |
| Gender | |||
| Male | 9,439,267 (51%) | 3,368 | 35.68 |
| Female | 9,013,619 (49%) | 1,468 | 16.29 |
| Age Categories | |||
| 0–2 y | 2,869,959 (16%) | 751 | 26.17 |
| 3–5 y | 4,084,601 (22%) | 1,059 | 25.93 |
| 6–8 y | 3,463,024 (19%) | 911 | 26.31 |
| 9–11 y | 3,173,084 (17%) | 890 | 28.05 |
| 12–14 y | 2,796,751 (15%) | 745 | 26.64 |
| 15–17 y | 2,065,467 (11%) | 480 | 23.24 |
| Race/Ethnicity | |||
| White | 6,531,027 (35%) | 2,872 | 43.97 |
| Black | 4,775,776 (26%) | 748 | 15.66 |
| Asian | 483,620 (3%) | 64 | 13.22 |
| Hispanic | 4,261,405 (23%) | 369 | 8.66 |
| Grouped§ | 1,492,556 (8%) | 212 | 14.20 |
| Unknown | 908,502 (5%) | 571 | 62.85 |
| Urban/Rural | |||
| Large Central Metro | 6,603,911 (36%) | 1,308 | 19.81 |
| Large Fringe Metro | 3,327,678 (18%) | 1,066 | 32.03 |
| Medium Metro | 3,976,658 (22%) | 1,147 | 28.84 |
| Small Metro | 1,588,802 (9%) | 456 | 28.70 |
| Micropolitan | 1,733,698 (9%) | 531 | 30.63 |
| Noncore (rural) | 1,222,139 (7%) | 328 | 26.84 |
| ZCTA-Level Poverty | |||
| 0 – 5% | 1,931,817 (11%) | 829 | 42.91 |
| 6 – 10% | 4,057,501 (22%) | 1,458 | 35.93 |
| 11 – 15% | 4,235,865 (23%) | 1,180 | 27.86 |
| 16 – 20% | 3,151,910 (17%) | 657 | 20.84 |
| 21 – 25% | 1,977,825 (11%) | 281 | 14.21 |
| 26 – 100% | 3,097,968 (17%) | 431 | 13.91 |
Values expressed as n (%)
Defined as the combination of “Native American/Alaskan,” “>1 race (Hispanic), “>1 race (non-Hispanic),” and “Hawaiian”ZCTA: Zip-code tabulation area 60
Table E2:
Association Between ZCTA-Level Poverty, Urban/Rural Status and EoE
| Model #1 | 95% CI | Model #2 | 95% CI | Final Model | 95% CI | |
|---|---|---|---|---|---|---|
| Urban/Rural Status | ||||||
| Large Central Metro | REF | REF | REF | |||
| Large Fringe Metro | 1.42 | 1.29 – 1.56 | 1.07 | 0.98 – 1.18 | 0.93 | 0.84 – 1.02 |
| Medium Metro | 1.32 | 1.19 – 1.46 | 1.01 | 0.92 – 1.12 | 0.95 | 0.86 – 1.05 |
| Small Metro | 1.16 | 1.02 – 1.32 | 0.82 | 0.73 – 0.93 | 0.78 | 0.69 – 0.88 |
| Micropolitan | 1.22 | 1.08 – 1.37 | 0.81 | 0.72 – 0.92 | 0.79 | 0.70 – 0.89 |
| Noncore (rural) | 1.03 | 0.89 – 1.19 | 0.68 | 0.59 – 0.78 | 0.68 | 0.59 – 0.78 |
Model #1: Adjusted for state, gender, and age
Model #2: Adjusted for state, gender, age, and race/ethnicity
Final Model: Adjusted for state, gender, age, race/ethnicity, and neighborhood-level poverty
Table E3:
Sensitivity Analysis including States previously excluded due to missing data
| Model #1 | 95% CI | Model #2 | 95% CI | |
|---|---|---|---|---|
| Gender | ||||
| Female | REF | REF | ||
| Male | 2.14 | 2.01 – 2.27 | 2.08 | 1.98 – 2.23 |
| Age Categories | ||||
| 0–2 y | REF | REF | ||
| 3–5 y | 0.99 | 0.90 – 1.09 | 0.99 | 0.90 – 1.08 |
| 6–8 y | 0.99 | 0.89 – 1.09 | 1.01 | 0.92 – 1.11 |
| 9–11 y | 1.03 | 0.94 – 1.14 | 1.05 | 0.96 – 1.16 |
| 12–14 y | 0.98 | 0.89 – 1.09 | 1.01 | 0.92 – 1.12 |
| 15–17 y | 0.87 | 0.77 – 0.98 | 0.90 | 0.81 – 1.01 |
| Race/Ethnicity | ||||
| White | REF | REF | ||
| Black | 0.41 | 0.38 – 0.45 | 0.41 | 0.38 – 0.45 |
| Asian | 0.37 | 0.28 – 0.49 | 0.38 | 0.29 – 0.49 |
| Hispanic | 0.31 | 0.27 – 0.35 | 0.31 | 0.27 – 0.35 |
| Grouped§ | 0.40 | 0.34 – 0.47 | 0.45 | 0.39 – 0.52 |
| Unknown | 1.64 | 1.48 – 1.80 | 1.43 | 1.30 – 1.58 |
| Urban/Rural Status | ||||
| Large Central Metro | REF | REF | ||
| Large Fringe Metro | 0.93 | 0.84 – 1.02 | 0.97 | 0.89 – 1.07 |
| Medium Metro | 0.95 | 0.87 – 1.05 | 0.97 | 0.88 – 1.06 |
| Small Metro | 0.78 | 0.69 – 0.88 | 0.76 | 0.68 – 0.86 |
| Micropolitan | 0.79 | 0.70 – 0.89 | 0.80 | 0.71 – 0.90 |
| Noncore (rural) | 0.68 | 0.59 – 0.78 | 0.67 | 0.59 – 0.77 |
| ZCTA-Level Poverty | ||||
| < 5% | 2.00 | 1.75 – 2.28 | 1.98 | 1.74 – 2.26 |
| 6 – 10% | 1.78 | 1.57 – 2.00 | 1.78 | 1.58 – 2.00 |
| 11 – 15% | 1.50 | 1.33 – 1.69 | 1.52 | 1.34 – 1.71 |
| 16 – 20% | 1.30 | 1.14 – 1.49 | 1.34 | 1.17 – 1.52 |
| 21 – 25% | 0.99 | 0.84 – 1.16 | 1.00 | 0.86 – 1.17 |
| 26 – 100% | REF | REF |
Values expressed as odds ratios (OR) and 95% CIs
Model #1 is the final adjusted model defined in the manuscript, excluding States with >15% missing data for race/ethnicity
Model #2 is the final adjusted model defined in the manuscript, including States with >15% missing data for race/ethnicity
Defined as the combination of “Native American/Alaskan,” “>1 race (Hispanic), “>1 race (non-Hispanic),” and “Hawaiian” ZCTA: Zip-code tabulation area
Figure E1:
Flow Diagram of Medicaid 2012 Beneficiaries Included in Study
Footnotes
Disclosure of potential conflicts of interest: E. McGowan has received grants from the National Institutes of Health (NIH), the American Academy of Allergy, Asthma and Immunology (AAAAI), and Food Allergy Research and Education (FARE), and served as a consultant for Shire, Inc. J. Keller and E.S. Dellon do not report any conflicts that are relevant to this paper. R. Peng receives grant support from the NIH and serves as a consultant for Health Effect Institute. C.A. Keet receives grant support from the NIH.
Clinical Implications: Among 18,452,886 children enrolled in Medicaid in 2012, children living in areas with higher neighborhood-level poverty were less likely to be diagnosed with EoE. Whether this is due to unique environmental exposures or under-diagnosis and under-treatment remains unknown.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Emily C. McGowan, University of Virginia School of Medicine, Division of Allergy and Immunology, Charlottesville, VA; Johns Hopkins University School of Medicine, Division of Allergy and Clinical Immunology, Baltimore, MD.
Joshua P. Keller, Colorado State University, Department of Statistics, Fort Collins, CO.
Evan S. Dellon, University of North Carolina School of Medicine, Division of Gastroenterology, Chapel Hill, NC.
Roger Peng, Johns Hopkins Bloomberg School of Public Health, Department of Biostatistics, Baltimore, MD.
Corinne A. Keet, Johns Hopkins University School of Medicine, Division of Pediatric Allergy and Immunology, Baltimore, MD.
References
- 1.Navarro P,Arias A,Arias-Gonzalez L,Laserna-Mendieta EJ,Ruiz-Ponce M,Lucendo AJ. Systematic review with meta-analysis: the growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. 2019;49(9):1116–25. [DOI] [PubMed] [Google Scholar]
- 2.Dellon ES,Hirano I.Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018;154(2):319–32 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keet CA,Matsui EC, McCormack MC,Peng RD.Urban residence,neighborhood poverty, race/ethnicity, and asthma morbidity among children on Medicaid. J Allergy Clin Immunol. 2017;140(3):822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGowan EC,Matsui EC, McCormack MC,Pollack CE,Peng R,Keet CA.Effect of poverty, urbanization, and race/ethnicity on perceived food allergy in the United States. Ann Allergy Asthma Immunol. 2015;115(1):85–6 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen ET,Hoffman K,Shaheen NJ,Genta RM,Dellon ES.Esophageal eosinophilia is increased in rural areas with low population density: results from a national pathology database. Am J Gastroenterol. 2014;109(5):668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franciosi JP,Tam V,Liacouras CA,Spergel JM. A case-control study of sociodemographic and geographic characteristics of 335 children with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2009;7(4):415–9. [DOI] [PubMed] [Google Scholar]
- 7.Kim S,Kim S,Sheikh J.Prevalence of eosinophilic esophagitis in a population-based cohort from Southern California. J Allergy Clin Immunol Pract. 2015;3(6):978–9. [DOI] [PubMed] [Google Scholar]
- 8.Robson J,Korgenski K,Parsons K,McClain A,Barbagelata C,Allen-Brady K,et al. Sensitivity and Specificity of Administrative Medical Coding for Pediatric Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr. 2019;69(2):e49–e53. [DOI] [PubMed] [Google Scholar]
- 9.Dellon ES,Liacouras CA, Molina-Infante J,Furuta GT,Spergel JM,Zevit N,et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology. 2018;155(4):1022–33 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]