Summary
Background:
Equine parvovirus-hepatitis (EqPV-H) has been proposed as the etiologic cause of Theiler’s disease, also known as serum hepatitis. EqPV-H-associated Theiler’s disease has not been previously reported in Europe.
Objectives:
To determine whether EqPV-H infection was associated with a 2018–2019 outbreak of Theiler’s disease in four horses on a stud farm.
Study design:
Descriptive case series.
Methods:
The medical records of four horses from the same farm diagnosed with fatal Theiler’s disease were examined retrospectively. Information collected included a clinical history, physical examination findings, tetanus antitoxin exposure, serum biochemistry, and necropsy reports. Liver tissue from all four horses were tested for EqPV-H using PCR and in situ hybridisation (ISH) assays.
Results:
Three of the horses had a history of recent (7–11 weeks) tetanus antitoxin administration. Liver tissue from all four horses tested positive for EqPV-H with PCR. In situ hybridisation revealed a widespread distribution of viral nucleic acid in hepatocytes in one case, and a more sporadic distribution in the remaining three cases.
Main limitations:
Case controls were not available from the farm in question given the retrospective nature of analysis.
Conclusions:
This case series documents the first reported EqPV-H-associated Theiler’s disease in Europe and the first use of ISH to visualise the viral nucleic acid in liver tissues of horses with Theiler’s disease.
Keywords: horse, serum hepatitis, liver, Copiparvovirus, equine parvovirus, in situ hybridisation
Introduction
Theiler’s disease, also known as equine serum hepatitis or idiopathic acute hepatitis, is an often-fatal disease of adult horses that was first described in 1918 in South Africa by Sir Arnold Theiler [1]. Since the original report, serum hepatitis has been described in horses worldwide and is associated with the administration of a number of equine biologic products including tetanus antitoxin [2–8], botulinum antitoxin [9], pregnant mare’s serum [3], equine plasma [10], Streptococcus equi antiserum [3,11], and more recently, allogeneic stem cell preparations [12]. Horses that are in contact with equine biologic-associated Theiler’s disease cases have also been reported to develop disease, suggesting that it is both an infectious and contagious disease [1,13].
Equine parvovirus-hepatitis (EqPV-H), a novel equine parvovirus of the genus Copiparvovirus, was recently discovered in a case of biologic-associated Theiler’s disease [14]. The virus was identified in the serum and liver of the affected horse and in the administered tetanus antitoxin. Experimental infection of two horses with this antitoxin resulted in EqPV-H viremia and hepatitis [14]. A follow-up prospective study on Theiler’s disease demonstrated the detection of EqPV-H in 18 consecutive cases of equine serum hepatitis [12]. The authors also described the detection of EqPV-H in 9 out of 10 cases of suspected Theiler’s disease that occurred in the absence of any recent equine origin blood biologic product administration [13]. Another study examining 18 batches of commercial equine serum demonstrated the presence of EqPV-H DNA in samples from multiple countries of origin including the USA, Canada, New Zealand, Italy, and Germany [15]. EqPV-H has also been identified in the serum of horses in China, suggesting a broad geographical distribution [16].
Our first aim was to determine whether these four cases of Theiler’s disease were associated with EqPV-H infection, which has not been reported in Europe to date. The second aim was to use in situ hybridisation (ISH) to demonstrate the presence of EqPV-H nucleic acid within the diseased tissue.
Materials and Methods
Case collection
Within a period of 11 months, four cases of Theiler’s disease were diagnosed and treated on a single stud farm. Only the first two cases were housed in-contact with each other. The remaining two horses were on the same farm but housed separate from each other. Case inclusion criteria was a diagnosis of Theiler’s disease based on the acute onset of clinical signs associated with fulminant hepatic failure, clinicopathologic evidence of hepatitis, and post-mortem findings characteristic of Theiler’s disease [12]. The diagnosis of Theiler’s disease was made prior to submission of samples for viral testing. Post-mortem and histopathologic examinations were performed on all four cases. Liver samples were transported as formalin-fixed, paraffin-embedded (FFPE) tissue blocks for further analysis.
Polymerase Chain Reaction Testing and Serum Biochemistry
Viral nucleic acids were extracted from FFPE liver of all 4 horses with Thermo Fisher RecoverAll™ Total Nucleic Acid Isolation Kit for FFPE (Catalogue no. AM1975)a. Real-time PCR for EqPV-H DNA was performed using the primer pair EqPV-3218/3386R, [12,13] using a Path-ID RT-PCR kit (Catalogue no. 4442137)a with cycling conditions of 10 min at 48°C, then 10 min at 95°C, followed by 40 cycles of 15 sec at 95°C and 60 sec at 60°C. Testing FFPE liver for non-primate hepacivirus (NPHV) which is also called equine hepacivirus (EqHV) or hepacivirus A, Theiler’s disease-associated virus (TDAV, aka pegivirus D), and equine pegivirus (EPgV, aka pegivirus E) was also performed using real-time PCR, as previously described [12,13]. Three different lots of the commercial tetanus antitoxin were used by veterinarians on the affected premises, during the case period, and were available for testing. Information on the specific antitoxin lot that was administered to each horse was not recorded. Tetanus antitoxin samples were tested for EqPV-H using the same PCR protocol as described above. To generate amplicons for sequencing, a nested PCR for EqPV-H DNA was performed with initial primer set EqPV-H ak5/ak6 and second round EqPV-H ak7/ak8 primers [14] using Q5 High-Fidelity 2X master mix (New England Biolabs Catalogue no MO494S)b. First round cycling conditions were 98°C for 30 sec, followed by 40 cycles of 98°C for 10 sec, 56°C for 30 sec and 72°C for 90 sec. Second round cycling was identical with exception of an increase in annealing temperature to 60°C. Sanger sequencing was performed at the Cornell University Biotechnology Resource Center. Partial VP1 sequences generated from cases 2 and 3, and TAT lots 1154, 1951, and 2558, are available in GenBank (MN991308–MN991312).
To assess viral load in the FFPE livers, a new PCR was developed. The first PCR detected both RNA and DNA. Here, we detected EqPV-H DNA copies only and normalised to cell number using primers EqPV-H qVP1 F15/R15 (CACGGTCCCAGGACATTTAC/ TCACAGATCGTCCCTACCAC) and EqB2M qF/R (CAGCAGGCAAAGAAGAATCC/ CTCTATCCCGTCACCACACC) at 0.3 μM. Samples were amplified with Bio-Rad iTaqTM Universal SYBR® Green Supermix (Bio-Rad Laboratories, Inc. Catalog no. 1725120)c and 2 μl of NA in a 20 μl reaction volume. Serial dilution of plasmids containing EqPV-H VP1 and beta-2 microglobulin (B2M) amplicons were used as standards and had linear ranges of 101 - 106 GE/PCR reaction for EqPV-H and 103 - 106 copies/PCR reaction for B2M. Cell count was determined as B2M copies / 2.
Serum biochemistry was performed using an Abaxis VETSCAN VS2 analyserd. The key liver-associated serum biochemistry findings are summarised in Table 1.
Table 1.
Signalment, clinical signs and clinical pathologic findings of 4 cases of Theiler's Disease
| Case | Signalment | Clinical signs | TAT administration | AST (U/L) Reference range 175–340 | Total Bilirubin (μmol/L) Reference range 9–39 | GGT (U/L) Reference range 5–24 |
|---|---|---|---|---|---|---|
| 1 | 27-year-old LZ M | Depression, impacted large colon | + | Not reported† | 309 | 110 |
| 2 | 12-year-old LZ M | Depression, ataxia | − | 1537 | 212 | 81 |
| 3 | 10-year-old LZ S | Depression, ataxia | + | 1319 | 189 | 49 |
| 4 | 12-year-old LZ M | Depression, anorexia | + | 1345 | 74 | 165 |
Not reported by the biochemistry analyser due to severe icterus.
M = Mare; S = Stallion; LZ = Lipizzaner; TAT = tetanus antitoxin; AST = Aspartate aminotransferase; GGT = gamma-glutamyltransferase.
In situ hybridisation (ISH)
In situ hybridisation (ISH) probes were designed by Advanced Cell Diagnostics (ACD)e to detect a proprietary sequencec of DNA and RNA in the 3001–4177 base pair region of the EqPV-H genome (GenBank MG136722) [17]. The 20 probe pairs were designed to target the DNA and/or the RNA of VP1 capsid portion of the EqPV-H genome (ACD catalog no 559991, RNAscope® Probe- V-EqPV-H-VP1)e. The equine gene PPIB probe (ref. 462351, lot 18038A)e was used as a positive control for the ISH procedure (Figure S1). The labelling was performed according to the manufacturer’s protocol for colorimetric ISH for liver tissue (RNAscope® 2.5 HD Detection Kit – Red)e. Briefly, the 4 μm sections of FFPE tissue were cut, deparaffinised in xylene, washed with ethanol, and dried. Slides were treated with H2O2e to block endogenous peroxides for 10 min. Slides were boiled for 30 min in antigen retrieval solutionc and then treated with protease plusc for 30 min. Slides were incubated for 2 h with V-EqPV-H-VP1 probese, followed by 6 amplification steps with ACD reagents. Slides were developed for 10 min and counterstained with Mayer’s haematoxylin (Dako)f. Liver tissue from horses experimentally infected with EqPV-H were used as positive control tissue for the EqPV-H probe (Figure S1). Liver tissue from horses that tested negative for EqPV-H via PCR were used as negative controls (Figure S1).
Results
Clinical data and case history
Case 1
A 27-year-old Lipizzaner mare was examined for depression and not following the herd in May 2018. She was kept in loose housing with 44 other mares with free access to paddock and pasture. She was treated for a premolar (408) fracture 11 weeks (February) prior to the onset of illness. The tooth was subsequently extracted. Treatment after simple tooth extraction included tetanus antitoxin from a European manufacturer.
On presentation, an impaction colic was suspected due to reduced intestinal motility, and the presence of distended colon and dry faeces on rectal examination. The mare was treated with intravenous lactated Ringer’s solution (B Braun)g and flunixin meglumine (Finadyne, MSD)h. Haematology was consistent with dehydration, whereas serum biochemistry was indicative of liver disease and failure (Table 1). AST activity was not reported by the biochemistry analyser due to severe icterus. The mare rapidly deteriorated and was euthanised approximately 24 h after first clinical signs were noted.
Case 2
A 12-year-old Lipizzaner mare was examined for severe neurological signs that included depression and ataxia. This mare had been housed in-contact with Case 1 and several other mares on the farm. The disease developed in October, five months after Case 1. Medical history on the mare for the previous two years was available. In this period, she was treated for distal leg lameness, which was managed with nonsteroidal anti-inflammatory drugs, shock wave and laser therapy. No tetanus antitoxin treatment was recorded during this period. The mare was vaccinated against equine influenza and tetanus (ProteqFlu-Te, Merial S.A.S.)i one and thirteen months prior to the onset of clinical signs.
The serum biochemistry results were consistent with liver disease and failure (Table 1). Haematology was consistent with dehydration. The mare was treated with lactated Ringers solution (B Braun)g and vitamin/mineral/amino-acid solution (Duphalyte, Zoetis Belgium SA)j. Further treatment included dexamethasone (Rapidexone, Eurovet Animal Health B.V)k and marbofloxacin (Marbocyl 10%, Vetoquinol SA)l. The clinical condition deteriorated rapidly, and the mare was euthanised 24 h after the first clinical signs were noted.
Case 3
A 10-year-old Lipizzaner stallion was presented for depression and ataxia in November. The stallion was housed in a loose box with a daily paddock turn out, and had not been in direct contact with Case 1 or 2. Seven weeks prior to the onset of neurological signs (September), the stallion was treated for head trauma and resulting maxillary fracture. Treatment of the head trauma also included tetanus antitoxin from the same European manufacturer as Case 1.
The stallion died soon after the initial clinical examination was performed. Abnormally high serum activity of AST, GGT and concentration of bilirubin were detected on a biochemistry profile (Table 1).
Case 4
A 12-year-old Lipizzaner mare was initially examined for anorexia and depression in April. She was kept in a loose housing with eight other mares with free access to a paddock and had not been in direct contact with Cases 1–3. The mare had her teeth floated in January, nine weeks before the onset of illness, and was given tetanus antitoxin from the same manufacturer as Case 1 and 3 after her dentistry procedure.
The initial haematological and biochemical profiles were within normal ranges, with the exception of AST activity, which was mildly increased at 380 U/L (reference interval 175–340) (data not shown). The mare was treated with penicillin-streptomycin (Sustrepen, Dechra veterinary products)m and flunixin meglumine (Finadyne, MSD)h, and improved clinically within three days. However; severe anorexia and depression reoccurred two weeks later. At that time, the serum biochemistry profile was indicative of liver disease and failure (Table 1). The mare was treated with lactated Ringers solution (B Braun)g, vitamin/mineral/amino-acid solution (Duphalyte, Zoetis Belgium SA)j, dexamethasone (Rapidexone, Eurovet Animal Health B.V)k, marbofloxacin (Marbocyl 10%, Vetoquinol SA)l, flunixin meglumine (Finadyne, MSD)h, and lactulose (Portalac, Belupo)n. The mare developed neurological signs including ataxia and head pressing, which progressively worsened over the period of four days, and she was no longer able to stand on day 5 of treatment. Consecutive biochemistry profiles showed that total bilirubin concentrations remained increased at about 300 μmol/L (reference interval 9–39), whereas additional AST and GGT values were not reported by the biochemistry analyser due to severe icterus. The mare was euthanised on day five of treatment.
Necropsy and histopathological examination
All four horses were subjected to necropsy examination. Gross lesions, including moderate icterus and ascites, were similar in all affected horses. Numerous petechial and ecchymotic haemorrhages were scattered on serous membranes of the thoracic cavities. The livers were small, flattened and flabby, brown to dark brown, and discoloured by bile pigments. The cut surfaces were severely congested with an enhanced lobular pattern. The other internal organs had no remarkable gross lesions.
Liver samples were collected during post-mortem examination for histopathology analysis. They were fixed in a 10 % neutral buffered formalin, embedded in paraffin, sectioned at 4 μm, and stained with haematoxylin and eosin (HE).
Microscopic changes were mostly characterised by the distortion of hepatic lobular architecture and massive loss of hepatocytes in centrilobular areas (Fig 1a). Additional histochemical stains used to evaluate areas of hepatocyte loss revealed collapse of the reticulin framework and no evidence of lobular fibrosis (Figure S2). The remaining hepatocytes, mainly scattered in the portal areas, had loss of cellular detail and swollen cytoplasm with vacuolation (Fig 1b). Scattered throughout the lobules and portal areas were small numbers of lymphocytes, macrophages, plasma cells and fewer neutrophils (data not shown).
Fig 1: Histological and in situ hybridisation results from Case 1 (a and b) and Case 3 (c and d).
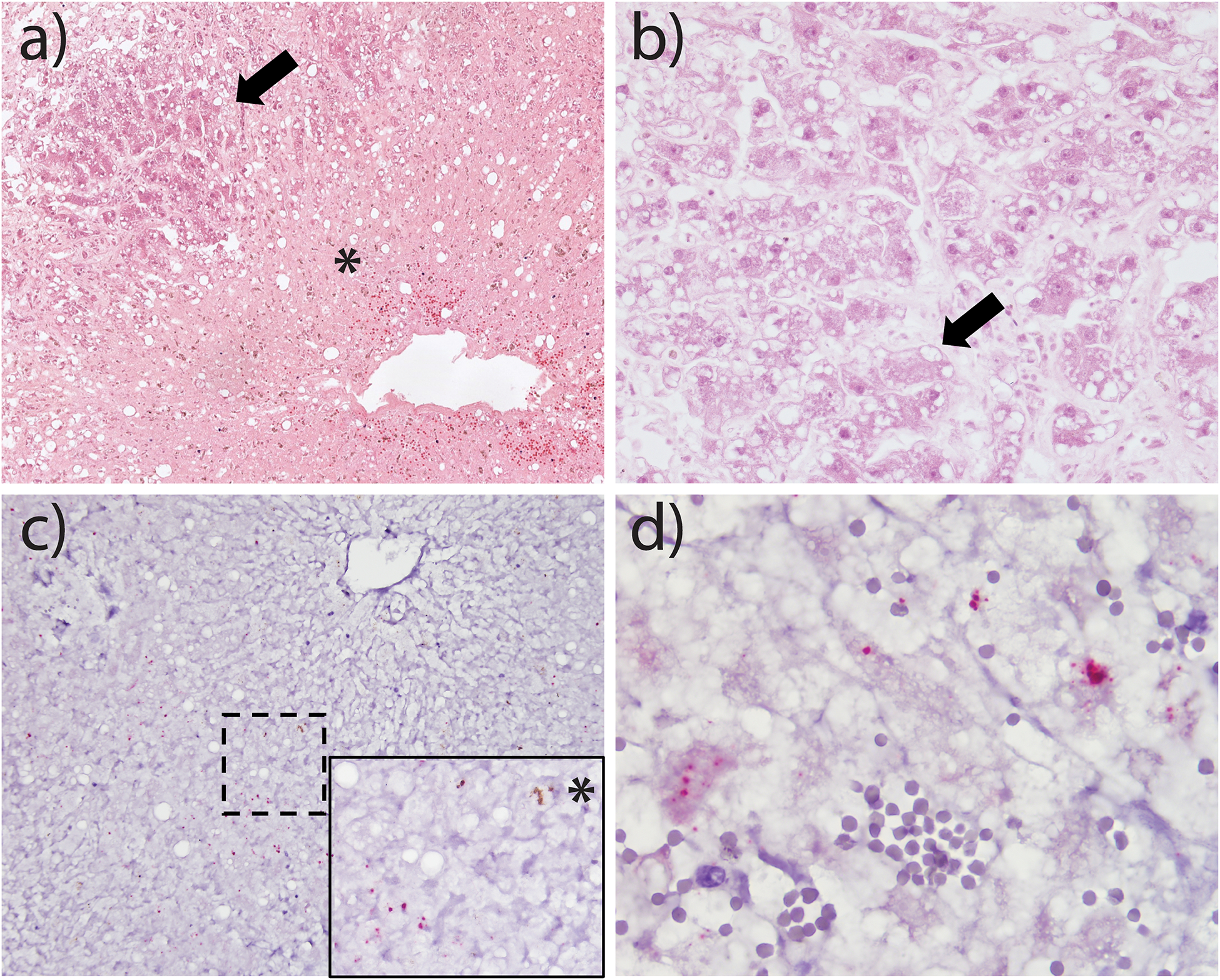
Loss of centrilobular hepatocytes (asterisk) and an island of remnant hepatocytes (arrow), 200x, HE.
Cytoplasmic vacuolation of hepatocytes (arrow), 400x, HE.
Hybridisation of viral nucleic acid is highest in periportal regions and reduced in centrilobular regions where hepatocytes are lost (asterisk in inset), 200x, 1000x inset, EqPV-H in situ hybridisation.
Red puncta of hybridisation within the cytoplasm of multiple hepatocytes, 600x, EqPV-H in situ hybridisation.
Virology
EqPV-H nucleic acid was detected via real-time PCR [13] in the FFPE liver of all four horses. Viral loads were 3.52 ×105, 2.89 ×104, 2.04 ×109, and 1.26 104 GE/ million cells for Cases 1–4, respectively. All liver samples were negative for NPHV/EqHV, TDAV, and EPgV. EqPV-H viral nucleic acid was consistently detected throughout the liver tissue of all four horses using the ISH probes designed to detect the EqPV-H VP1 genome or transcript. Positive punctate hybridisation was most commonly identified within hepatocytes in periportal areas and was reduced in the severely necrotic centrilobular regions (Fig 1c). Due to extensive autolytic changes in the tissue, differentiation of specific cytoplasmic versus nuclear hybridisation was difficult in some cases (Fig 1d). The distribution of the hybridisation was interpreted to reflect a higher quantity of viral nucleic acid in regions with remnant hepatocytes and reduced amounts in areas of massive hepatocyte loss, as correlated with the H&E findings.
Three lots of tetanus antitoxin that were reportedly used on the farm during the case period tested positive for EqPV-H using real time PCR. A 541 base pair region of the EqPV-H VP1 gene was amplified and sequenced for each lot of antitoxin and for Cases 2 and 3. Despite repeated attempts using a nested-set conventional PCR, we were unable to amplify VP1 sequences from cases 1 and 4, presumably due to the inhibitory effects of formalin fixation. Two of the three antitoxin lots sequenced matched each other identically (lots 2558 and 1951). The second lot (1951) was most likely given to Cases 3 and 4 according to the attending veterinarians. The sequence of this lot (1951) had 99.3% identity (3 of 541 nucleotides different) to Case 3. These sequence differences were confounding, and multiple amplifications and sequencing runs were performed on lot 1951 to attempt to resolve this discrepancy. The algorithm base call sequences were identical for all sequencing runs (n=4). However, on close inspection of the 1951 sequence electropherograms we observed minor secondary peaks, specifically corresponding to 2 of the 3 positions in difference and matching the base calls in Case 3, although these observations were inconsistent from one sequencing run to another. We interpret this as an indication that antitoxin lot 1951 contains multiple strains of EqPV-H, as might be reasonably expected as commercial anti-serum products typically contain sera from more than one donor horse. The third antitoxin lot (1154) was most likely given to Case 1 and had an identical sequence to Case 2. Although Case 2 was not given tetanus antitoxin, it was housed with Case 1. These findings are summarised graphically in Figure S3.
Discussion
The cause of Theiler’s disease had eluded clinicians and researchers since its first description over 100 years ago. While a blood-borne virus had long been suspected, it was not until the advent of advanced sequencing technologies that a candidate was identified. Recent studies have demonstrated strong evidence that Theiler’s disease is caused by the novel equine parvovirus, EqPV-H [12–14]. This descriptive case series demonstrated EqPV-H infection in four horses that died of Theiler’s disease.
All four horses in this cases series had clinical signs, serum biochemistry changes, and post-mortem findings consistent with previously reported cases of Theiler’s disease [1–8, 10–14]. Commonly reported histopathological features of Theiler’s disease include centrilobular hepatic necrosis, cytoplasmic vacuolation of surviving periportal hepatocytes, sinusoidal congestion, small numbers of mixed inflammatory cells scattered throughout the parenchyma, and the inconsistent presence of ductular reaction and fibroplasia in portal areas [1–8,10–14]. All of these changes were appreciated in the cases examined in this series with the exception of a ductular reaction. This feature, however, could easily be masked by the autolysis present in the examined sections.
In recent years, four viruses have been implicated in equine hepatitis including EqPV-H [12–14], NPHV/EqHV [18–21], TDAV [9], and EPgV [22]. Importantly, all four horses in our case series were positive for EqPV-H and negative for NPHV/EqHV, TDAV, and EPgV. Three lots of tetanus antitoxin produced by a European manufacturer and used on the farm during this outbreak were tested for EqPV-H and were PCR positive, demonstrating the presence of EqPV-H nucleic acid within the European equine biologic supply. This is consistent with the findings of Meister et al. that described the presence of EqPV-H DNA in European equine serum pools [15]. Based on sequence analysis, an unequivocal link between tetanus antitoxin 1951 and case 3 could not be established. However, we also found evidence for multiple EqPV-H strains within antitoxin 1951 leading to the hypothesis that a minor variant dominated in the recipient horse. Alternative explanations include that the lot of antitoxin used was mis-identified, the horses acquired EqPV-H through other means, PCR amplification or formalin fixation introduced sequence errors [23,24], or that the virus mutated in the recipient horse. Given the relatively long duration between infection and disease, it is reasonable that a virus could acquire mutations in that time period.
One of the four horses in this case series (Case 2) did not receive an equine biologic but was in direct contact with a horse that had received tetanus antitoxin (Case 1). Although the horse was vaccinated within the preceding year, the ProteqFlu-Te vaccine (Merial S.A.S.)g used with Case 2 does not contain equine biological material and has not been associated with Theiler’s disease [25]. Non-biologic-associated Theiler’s disease has been reported previously [12], and further highlights the contagious nature of EqPV-H and the potential for outbreaks on farms with Theiler’s disease cases. One study in North America found that all 10 non-biologic-associated Theiler’s disease cases reported occurred during the summer to fall season [12]. Vector-borne mechanical transmission was proposed as a possible means of natural horizontal transmission given the seasonal distribution and the ability of EqPV-H to be transmitted through blood products [12]. The horse in Case 2 became ill in October, which is consistent with the proposed summer/fall infection and disease pattern [12].
In this study, liver infection with EqPV-H was further characterised using a novel ISH technique that has become increasingly useful for associating newly discovered viruses with pathological changes [26]. A modified form of Koch’s postulates was developed in 1996 to address the problems of isolation, reinfection, and re-isolation when using sequence-based approaches that have become increasingly common in the era of high throughput next generation sequencing [27]. This new postulate stipulates that the nucleic acid sequence should be present intralesionally. Here, the use of ISH demonstrated the presence of EqPV-H nucleic acid within the liver tissue of affected horses. While the amount of hybridisation varied between cases, all were positive. Importantly, control horses without EqPV-H infection were negative for hybridisation and liver samples from EqPV-H experimentally infected horses were positive (Figure S1). These data further support EqPV-H as the likely cause of Theiler’s disease. However, a limitation of our study is that the sample size is small, only limited to one farm, and that we could not include case controls from the same farm given the retrospective nature of analysis. As such, further case control studies are needed to demonstrate the presence of EqPV-H within the affected liver tissue of new and historical Theiler’s disease cases in comparison to both normal horses and horses with liver diseases other than Theiler’s disease.
While Theiler’s disease cases have been described in Europe previously, recent published reports of the disease in Europe were not found with a literature search [4]. Our study documents the first reported cases of EqPV-H-associated Theiler’s disease in Europe and the first use of ISH to confirm the presence of this virus within the diseased liver tissue.
Supplementary Material
Acknowledgements
The authors would like to thank Irena Marc, DVM, for her assistance with case data collection.
Source of funding
Harry M. Zweig Memorial Fund for Equine Research, The Niarchos Family, Slovenian Research Agency Fund No. P4–0053, National Institute of Food and Agriculture Grant/Award number: 2016-67015-24765, and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K08AI141767. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authors’ declaration of interests
No competing interests have been declared.
Ethical animal research
Research ethics committee oversight not currently required by this journal: procedures were performed as part of clinical investigations and post-mortem examination.
Owner informed consent
Informed consent was given for every horse included in this study.
Data accessibility statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Thermo Fisher Scientific, Waltham, Massachusetts, USA.
New England Biolabs, Ipswich, Massachusetts, USA.
Bio-Rad Laboratories, Inc., Hercules, California, USA.
Abaxis Europe GmbH, Griesheim, Germany.
Advanced Cell Diagnostics, Newark, California, USA.
Dako (Agilent), Santa Clara, California, USA.
B. Braun Melsungen AG, Melsungen, Germany.
MSD Europe, Westdijk, Belgium.
Merial S.A.S., Lyon, France.
Zoetis Belgium SA, Ottignies-Louvain-la-Neuve, Belgium.
Eurovet Animal Health B.V, Bladel, Netherlands.
Vetoquinol SA, Lure, France.
Dechra veterinary products, Shrewsbury, Shropshire, UK.
Belupo, Koprivnica, Croatia.
References
- 1.Theiler A (1918) Acute liver-atrophy and parenchymatous hepatitis in horses. Union of South Africa. Dept. of Agriculture. 5th and 6th Repts. of the Director of Veterinary Research; [Google Scholar]
- 2.Hjerpe CA (1964) Serum Hepatitis in the Horse. J. Am. Vet. Med. Assoc 144, 734–740. [PubMed] [Google Scholar]
- 3.Rose J, Immenschuh R, Rose E. (1974) Serum hepatitis in the horse. Proceedings of the annual convention of the American Association of Equine Practitioners 175–185. [Google Scholar]
- 4.Thomsett LR (1971) Acute Hepatic Failure in the Horse. Equine Veterinary Journal 3, 15–19. [DOI] [PubMed] [Google Scholar]
- 5.Step DL, Blue JT and Dill SG (1991) Penicillin-induced hemolytic anemia and acute hepatic failure following treatment of tetanus in a horse. Cornell Vet 81, 13–18. [PubMed] [Google Scholar]
- 6.Messer NT and Johnson PJ (1994) Serum hepatitis in two brood mares. J. Am. Vet. Med. Assoc 204, 1790–1792. [PubMed] [Google Scholar]
- 7.Guglick MA, MacAllister CG, Ely RW and Edwards WC (1995) Hepatic disease associated with administration of tetanus antitoxin in eight horses. J. Am. Vet. Med. Assoc 206, 1737–1740. [PubMed] [Google Scholar]
- 8.Messer NT and Johnson PJ (1994) Idiopathic acute hepatic disease in horses: 12 cases (1982–1992). J. Am. Vet. Med. Assoc 204, 1934–1937. [PubMed] [Google Scholar]
- 9.Chandriani S, Skewes-Cox P, Zhong W, Ganem DE, Divers TJ, Van Blaricum AJ, Tennant BC and Kistler AL (2013) Identification of a previously undescribed divergent virus from the Flaviviridae family in an outbreak of equine serum hepatitis. Proc. Natl. Acad. Sci. U.S.A 110, E1407–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aleman M, Nieto JE, Carr EA and Carlson GP (2005) Serum Hepatitis Associated with Commercial Plasma Transfusion in Horses. Journal of Veterinary Internal Medicine, 19: 120–122. [DOI] [PubMed] [Google Scholar]
- 11.Panciera RJ (1969) Serum hepatitis in the horse. J. Am. Vet. Med. Assoc 155, 408–410. [PubMed] [Google Scholar]
- 12.Tomlinson JE, Tennant BC, Struzyna A, Mrad D, Browne N, Whelchel D, Johnson PJ, Jamieson C, Löhr CV, Bildfell R, McKenzie EC, Laverack M, Renshaw RW, Dubovi E, Kapoor A, Meirs RS, Belgrave R, Engiles J, Van de Walle GR and Divers TJ (2019) Viral testing of 10 cases of Theiler’s disease and 37 in-contact horses in the absence of equine biologic product administration: A prospective study (2014–2018). Journal of Veterinary Internal Medicine 33, 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomlinson JE, Kapoor A, Kumar A, Tennant BC, Laverack MA, Beard L, Delph K, Davis E, Schott II H, Lascola K, Holbrook TC, Johnson P, Taylor SD, McKenzie E, Carter-Arnold J, Setlakwe E, Fultz L, Brakenhoff J, Ruby R, Trivedi S, Van de Walle GR, Renshaw RW, Dubovi EJ and Divers TJ (2019) Viral testing of 18 consecutive cases of equine serum hepatitis: A prospective study (2014–2018). Journal of Veterinary Internal Medicine 33, 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Divers TJ, Tennant BC, Kumar A, McDonough S, Cullen J, Bhuva N, Jain K, Chauhan LS, Scheel TKH, Lipkin WI, Laverack M, Trivedi S, Srinivasa S, Beard L, Rice CM, Burbelo PD, Renshaw RW, Dubovi E and Kapoor A (2018) New Parvovirus Associated with Serum Hepatitis in Horses after Inoculation of Common Biological Product. Emerging Infectious Diseases 24, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meister TL, Tegtmeyer B, Postel A, Cavalleri J-MV, Todt D, Stang A and Steinmann E (2019) Equine Parvovirus-Hepatitis Frequently Detectable in Commercial Equine Serum Pools. Viruses 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu G, Sun L, Ou J, Xu H, Wu L and Li S (2018) Identification and genetic characterization of a novel parvovirus associated with serum hepatitis in horses in China. Emerg Microbes Infect 7, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F, Flanagan J, Su N, Wang L-C, Bui S, Nielson A, Wu X, Vo H-T, Ma X-J and Luo Y (2012) RNAscope. J Mol Diagn 14, 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramsay JD, Evanoff R, Jr TEW, Divers TJ, Knowles DP and Mealey RH (2015) Experimental Transmission of Equine Hepacivirus in Horses as a Model for Hepatitis C Virus. 61, 14. [DOI] [PubMed] [Google Scholar]
- 19.Pfaender S, Cavalleri JMV, Walter S, Doerrbecker J, Campana B, Brown RJP, Burbelo PD, Postel A, Hahn K, Anggakusuma, Riebesehl N, Baumgärtner W, Becher P, Heim MH, Pietschmann T, Feige K and Steinmann E (2015) Clinical course of infection and viral tissue tropism of hepatitis C virus-like nonprimate hepaciviruses in horses. Hepatology 61, 447–459. [DOI] [PubMed] [Google Scholar]
- 20.Pfaender S, Walter S, Grabski E, Todt D, Bruening J, Romero-Brey I, Gather T, Brown RJP, Hahn K, Puff C, Pfankuche VM, Hansmann F, Postel A, Becher P, Thiel V, Kalinke U, Wagner B, Bartenschlager R, Baumgärtner W, Feige K, Pietschmann T, Cavalleri JMV and Steinmann E (2017) Immune protection against reinfection with nonprimate hepacivirus. Proc Natl Acad Sci U S A 114, E2430–E2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheel TKH, Kapoor A, Nishiuchi E, Brock KV, Yu Y, Andrus L, Gu M, Renshaw RW, Dubovi EJ, McDonough SP, Van de Walle GR, Lipkin WI, Divers TJ, Tennant BC and Rice CM (2015) Characterization of nonprimate hepacivirus and construction of a functional molecular clone. Proc. Natl. Acad. Sci. U.S.A 112, 2192–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapoor A, Simmonds P, Cullen JM, Scheel TKH, Medina JL, Giannitti F, Nishiuchi E, Brock KV, Burbelo PD, Rice CM and Lipkin WI (2013) Identification of a Pegivirus (GB Virus-Like Virus) That Infects Horses. J Virol 87, 7185–7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hedegaard J, Thorsen K, Lund MK, Hein A-MK, Hamilton-Dutoit SJ, Vang S, Nordentoft I, Birkenkamp-Demtröder K, Kruhøffer M, Hager H, Knudsen B, Andersen CL, Sørensen KD, Pedersen JS, Ørntoft TF and Dyrskjøt L (2014) Next-Generation Sequencing of RNA and DNA Isolated from Paired Fresh-Frozen and Formalin-Fixed Paraffin-Embedded Samples of Human Cancer and Normal Tissue. PLoS ONE 9, e98187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spencer DH, Sehn JK, Abel HJ, Watson MA, Pfeifer JD and Duncavage EJ (2013) Comparison of Clinical Targeted Next-Generation Sequence Data from Formalin-Fixed and Fresh-Frozen Tissue Specimens. The Journal of Molecular Diagnostics 15, 623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anonymous (2018) ProteqFlu-Te. European Medicines Agency [WWW document]. URL https://www.ema.europa.eu/en/medicines/veterinary/EPAR/proteqflu-te [Accessed December 20, 2019]. [Google Scholar]
- 26.Pfankuche V, Hahn K, Bodewes R, Hansmann F, Habierski A, Haverkamp A-K, Pfaender S, Walter S, Baechlein C, Postel A, Steinmann E, Becher P, Osterhaus A, Baumgärtner W and Puff C (2018) Comparison of Different In Situ Hybridization Techniques for the Detection of Various RNA and DNA Viruses. Viruses 10, 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fredericks DN and Relman DA (1996) Sequence-based identification of microbial pathogens: a reconsideration of Koch’s postulates. Clin Microbiol Rev 9, 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


