Abstract
Objective:
We evaluated the effect of chest compression fraction (CCF) on survival to hospital discharge and return of spontaneous circulation (ROSC) in out-of-hospital cardiac arrest (OHCA) patients with non-shockable rhythms.
Methods:
This is a retrospective analysis (completed in 2016) of a prospective cohort study which included OHCA patients from ten U.S. and Canadian sites (Resuscitation Outcomes Consortium Epistry and PRIMED study (2007-2011)). We included all OHCA victims of presumed cardiac aetiology, not witnessed by emergency medical services (EMS), without automated external defibrillator shock prior to EMS arrival, receiving > 1 minute of CPR with CPR process measures available, and initial non-shockable rhythm. We measured CCF using the first 5 minutes of electronic CPR records.
Results:
Demographics of 12,928 adult patients were: mean age 68; male 59.9%; public location 8.5%; bystander witnessed 35.2%; bystander CPR 39.3%; median interval from 911 to defibrillator turned on 10min:04sec; initial rhythm asystole 64.8%, PEA 26.0%, other non-shockable 9.2%; compression rate 80-120/min (69.1%); median CCF 74%; ROSC 25.6%; survival to hospital discharge 2.4%. Adjusted odds ratio (OR); 95% confidence intervals (95%CI) of survival for each CCF category were: 0-40% (2.00; 1.16, 3.32); 41-60% (0.83; 0.54, 1.24); 61-80% (1.02; 0.77, 1.35); and 81-100% (reference group). Adjusted (OR; 95%CI) of ROSC for each CCF category were: 0-40% (1.02; 0.79, 1.30); 41-60% (0.83; 0.72, 0.95); 61-80% (0.85; 0.77, 0.94); and 81-100% (reference group).
Conclusions:
We observed an incremental benefit from higher CCF on the incidence of ROSC, but not survival, among non-shockable OHCA patients with CCF higher than 40%.
Keywords: cardiopulmonary resuscitation, heart arrest, resuscitation
INTRODUCTION
Background
In North America, overall survival to hospital discharge for out-of-hospital cardiac arrest (OHCA) has reached 10%.1 Survival varies by community, and has improved over time.1-4 Bystander cardiopulmonary resuscitation (CPR) and early defibrillation are two modifiable factors clearly associated with increased survival for OHCA.5 Several large studies have sought to improve CPR quality and to determine the proper rate,6 depth,7 and ventilation to chest compression ratio.1 The 2015 International Consensus on CPR recommends that interruptions in chest compressions be minimized,8 including during the management of the victim’s airway,9 and during the period of time preceding and following shock delivery.10 Such interruptions in chest compressions directly affect the chest compression fraction (CCF), which is defined as the proportion of time spent providing chest compressions while the patient is pulseless and in cardiac arrest.
Importance
There currently exists conflicting evidence supporting efforts to achieve a greater CCF. Higher CCF has been associated with both higher11-13 and lower14,15 survival to hospital discharge for OHCA patients. However, most studies have involved patients with an initial shockable rhythm (ventricular fibrillation or tachycardia, VF/VT). Very little is known about the impact of CCF in a population of OHCA patients with initial non-shockable rhythms (asystole and pulseless electrical activity). This group now represents almost 75% of all OHCA victims, and has a substantially poorer prognosis compared to patients with an initial shockable rhythm.1 Importantly, cardiac arrest aetiology and physiology may differ in the non-shockable group such that optimal CPR, including CCF, may also differ.16 A single prior study of this rhythm group suggested that higher CCF could possibly be associated with a higher incidence of return of spontaneous circulation (ROSC) among non-shockable OHCA victims.17 This study, however, was relatively small (2,103 patients including 42 survivors) and was under-powered to robustly evaluate the impact of CCF on ROSC or clinical outcomes such as survival to hospital discharge.
Goal of this investigation
We sought to determine the association between CCF and survival to hospital discharge and ROSC in a much larger cohort of OHCA patients with a non-shockable initial rhythm.
METHODS
Design
This is a retrospective analysis (completed in 2016) of a cohort of OHCA patients prospectively enrolled either in the Resuscitation Outcomes Consortium (ROC) PRIMED study (from June 2007-November 2009) or in the ROC Epistry following the completion of the ROC PRIMED study (January 2010-April 2011). ROC is a clinical network of 11 regional centres distributed across North America conducting research in the fields of OHCA and life-threatening traumatic injury.18 The ROC PRIMED study used a cluster-randomized design to study the impact of early (after no more than 30 to 60 seconds of CPR) or delayed (after 180 seconds of CPR) first rhythm analysis on OHCA survival outcomes.19 The ROC Epistry has been collecting population-based prospective data on OHCA from more than 260 emergency medical service (EMS) agencies since December, 2005.20 Information collected by the ROC is subject to standardized operational definitions, and all data are managed by a central data coordinating centre. In addition to collecting the elements suggested by Utstein,21 ten of the eleven ROC regional centres also collected digitally recorded CPR process data (for example chest compression rate, depth, and pauses in compressions).
Setting
ROC regional centres from which CPR process data was available for this study included: Ottawa/OPALS, ON; Toronto/Rescu, ON; and Vancouver, BC in Canada; and Seattle/King County, WA; Pittsburgh, PA; Portland, OR; Dallas, TX; Iowa City, IA; Milwaukee, WI; and Birmingham, AL in the United States.
Population
Eligible patients for this study were adults (18 years of age and older) experiencing OHCA of presumed cardiac aetiology prior to EMS arrival for whom resuscitation was attempted, and initial rhythm was other than VF/VT (i.e. non-shockable). The initial rhythm was determined to be non-shockable if the initial automated external defibrillator (AED) did not recommend a shock, or if the EMS provider interpreted the initial rhythm to be other than VF/VT. All rhythm diagnoses were later confirmed by research staff. We excluded patients who received a shock from an AED prior to EMS arrival or preceding EMS CPR. We excluded patients for whom the statuses of out-of-hospital ROSC or survival to hospital discharge were missing. Patients were also excluded if they had less than one minute of analysable CPR process data available over the first five minutes, period during which CPR process data was most reliably available.
The ROC PRIMED study and the ROC Epistry both received approval from all the US Institutional Review Boards, Canadian Research Ethics Boards, and EMS Services Institutional Review Boards at each of the participating sites.
Methods of measurement
We used digital CPR recordings available from PhysioControl (Redmond, WA) defibrillators, Zoll (Chelmsford, MA) defibrillators, Philips (Andover, MA) defibrillators, and Laerdal (Stavanger, Norway) defibrillators following electrode application onto the patient’s chest, and measured the presence and frequency of chest compressions. This was achieved indirectly, either by changes in thoracic impedance recorded from external defibrillation electrodes 22 or via an accelerometer interface between the rescuer and the patient’s chest using commercially available defibrillators.
Outcome measures
The primary outcome measure was survival to hospital discharge. The secondary outcome measure was any out-of-hospital ROSC, which we determined to be present if there was any palpable pulse in any vessel for any length of time.
Predictor of interest
Our predictor of interest was CCF. CCF was calculated as the proportion of analysable time during which chest compressions were administered over the first five minutes of recorded CPR process data. This was calculated by analytic software which permitted identification of all interruptions greater than two to three seconds, which were considered time intervals without chest compression. Trained research staff reviewed the automated calculation of CCF at each site prior to entering CCF values. CPR process data was occasionally unanalysable due to circumstances such as when the patient was being moved, when cables were temporarily disconnected, or when the patient had ROSC.
Data analyses
We used descriptive statistics to compare the patient and system characteristics for our study population to those excluded due to missing CPR process data or having less than one minute of analysable CPR time. For our study population, we used descriptive statistics to compare the patient and system characteristics across categories of CCF (0-40%, 41-60%, 61-80%, and 81-100%). We combined the 0-20% and the 20-40% groups for analyses because there were too few subjects in the 0-20% group. For our primary outcome, we used multivariable logistic regression to estimate the adjusted odds ratio of survival to hospital discharge for each CCF category (0-40%, 41-60%, and 61-80%) relative to a CCF of 81-100%. For our secondary outcome, we used multivariable logistic regression to estimate the adjusted odds ratio of any out-of-hospital ROSC for each CCF category (0-40%, 41-60%, and 61-80%) relative to a CCF of 81-100%. In both multivariable logistic regression models, the adjustment variables were confounders that were selected a priori: age (continuous), sex (male; female), arrest location (public; private), bystander witness status (yes; no or unknown), bystander CPR status (yes; no or unknown), chest compression rate (80-120; <80 or >120), time from 911 call until the AED was turned on (continuous), initial rhythm (asystole; PEA; AED no-shock), and ROC site (10 sites). All analyses were conducted using R version 3.0.2. (Vienna, Austria).
RESULTS
Characteristics of study subjects
Figure 1 summarizes the refinement of our study population. Of the 30,139 adults with OHCA of cardiac aetiology with a resuscitation attempt, 26,233 were not EMS-witnessed or shocked prior to EMS arrival. Of these patients, 19,693 had a non-shockable initial rhythm. We excluded 28 patients with missing survival or ROSC status, 116 patients who received a defibrillator shock by EMS prior to CPR, and 6,540 patients who had missing CPR process data. Of the remaining 13,099 patients, 171 had less than one minute of analysable CPR process data, resulting in a study population of 12,928 patients.
Figure 1. Study Cohort and Exclusions.
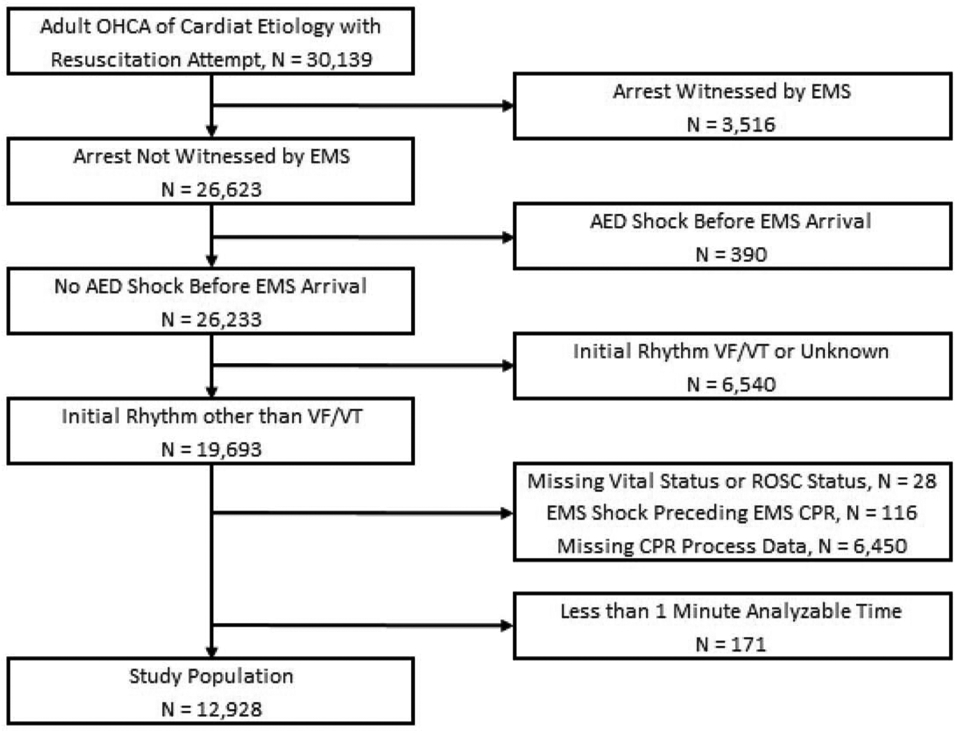
OHCA indicates out-of-hospital cardiac arrest; EMS, emergency medical services; AED, automated external defibrillator; VF/VT, ventricular fibrillation/ventricular tachycardia; ROSC, return of spontaneous circulation; and CPR, cardiopulmonary resuscitation.
We compare those excluded due to missing CPR process data (n=6,450) or due to having less than one minute of analysable time (n=171) to our study population (n=12,928) in Table 1. Of note, those excluded due to having less than one minute of CPR process data had, on average, better outcomes than those in our study sample. They were more likely to have ROSC within the first five minutes of CPR, and more likely to survive to hospital discharge. Those excluded due to having missing CPR process data had similar characteristics to our study population. In our study population, the mean age was 68, 59.9% were male, 8.5% arrested in a public location, 35.2% were witnessed by a bystander, and 39.3% received bystander CPR. The initial rhythms in our non-shockable population were asystole (64.8%), PEA (26.0%), and ‘AED no shock, no strip’ (9.2%; which refers to when the AED did not advise a shock to be given, but an ECG rhythm strip was not available). Of the 12,928 patients in our study population, 307 (2.4%) survived to hospital discharge and 3,304 (25.6%) had out-of-hospital ROSC. Of the 307 survivors, most (95.8%) had out-of-hospital ROSC.
Table 1.
Patient and System Characteristics Comparing Patients Included in the Analyses to Those Excluded Because of Insufficient CPR Process Measures.
|
Study population |
Excluded | Excluded | |
|---|---|---|---|
| <1 minute analysable CPR process data |
Missing CPR process data |
||
| (n=12,928) | (n=171) | (n=6,450) | |
| Age - mean (SD) | 68 (17) | 70 (16) | 68 (17) |
| Male - n (%) | 7,741 (59.9%) | 109 (63.7%) | 3,816 (59.2%) |
| Public location - n (%) | 1,096 (8.5%) | 13 (7.6%) | 597 (9.3%) |
| Bystander witnessed - n (%) | 4,555 (35.2%) | 70 (40.9%) | 2,321 (36.0%) |
| Bystander CPR - n (%) | 5,078 (39.3%) | 69 (40.4%) | 2,425 (37.6%) |
| No. of contributing agencies*- mean (SD) | 2.5 (0.6) | 2.5 (0.6) | 2.6 (0.5) |
| Site - n (%) | |||
| A | 163 (1.3%) | 12 (7.0%) | 274 (4.2%) |
| B | 1,310 (10.1%) | 25 (14.6%) | 373 (5.8%) |
| C | 881 (6.8%) | 0 (0%) | 545 (8.4%) |
| D | 1,745 (13.5%) | 21 (12.3%) | 1,031 (16.0%) |
| E | 696 (5.4%) | 1 (0.6%) | 133 (2.1%) |
| F | 596 (4.6%) | 5 (2.9%) | 669 (10.4%) |
| G | 931 (7.2%) | 4 (2.3%) | 439 (6.8%) |
| H | 1,643 (12.7%) | 34 (19.9%) | 551 (8.5%) |
| I | 3,520 (27.2%) | 36 (21.1%) | 1,763 (27.3%) |
| J | 1,443 (11.2%) | 33 (19.3%) | 672 (10.4%) |
| ALS first on scene - n (%) | 4,686 (36.3%) | 53 (31.0%) | 2,810 (44.6%) |
| ALS on scene - n (%) | 12,528 (96.9%) | 164 (95.9%) | 6,281 (97.4%) |
| Minutes from 9-1-1 call to scene - median (Q1, Q3) | 5.4 (4.2, 6.9) | 5.2 (4.3, 6.5) | 5.5 (4.2, 7) |
| Minutes from 9-1-1 call to first EMS rhythm interpretation - median (Q1, Q3) | 10 (8.2, 12) | 9.6 (7.9, 12) | 10 (7.9, 12) |
| Adrenaline (Epinephrine) use - n (%) | 11,059 (85.5%) | 121 (70.8%) | 5,029 (78.0%) |
| Initial rhythm - n (%) | |||
| Asystole | 8,378 (64.8%) | 107 (62.6%) | 3,627 (56.2%) |
| PEA | 3,366 (26.0%) | 49 (28.7%) | 1,380 (21.4%) |
| AED no shock, no strip | 1,184 (9.2%) | 15 (8.8%) | 1,443 (22.4%) |
| Any out-of-hospital shock - n (%) | 2,481 (19.2%) | 30 (17.5%) | 1,029 (16.0%) |
| ROSC within first 5 minutes of CPR - n (%) | 266 (2.1%) | 20 (11.7%) | 163 (2.5%) |
| Any out-of-hospital ROSC - n (%) | 3,304 (25.6%) | 50 (29.2%) | 1,643 (25.5%) |
| Survived to hospital discharge - n (%) | 307 (2.4%) | 13 (7.6%) | 226 (3.5%) |
SD indicates standard deviation; CPR, cardiopulmonary resuscitation; ALS, Advanced Life Support; Q1, Q3, the 1st and 3rd quantiles; EMS, emergency medical services; and ROSC, return of spontaneous circulation.
information was only available about the first four EMS units at the scene.
We compare patient and system characteristics of our study population by CCF category in Table 2. Those in the lowest CCF category were more likely to have a chest compression rate below 80, have an initial rhythm of PEA, and have ROSC within the first five minutes of CPR compared to those with higher CCF. For the CCF categories of 0-40%, 41-60%, 61-80%, and 81-100%, survival to hospital discharge was respectively 5.2%, 1.8%, 2.2%, and 2.5%, and any out-of-hospital ROSC was 27.0%, 23.0%, 24.3%, and 28.1% (Figure 2).
Table 2.
Patient and System Characteristics by Chest Compression Fraction Category.
| Chest Compression Fraction | |||||
|---|---|---|---|---|---|
| Total (n=12,928) |
0-40% (n=444) |
41-60% (n=2,059) |
61-80% (n=5,747) |
81-100% (n=4,678) |
|
| Age - mean (SD) | 68 (17) | 67 (18) | 68 (16) | 68 (16) | 67 (17) |
| Male - n (%) | 7,741 (59.9%) | 235 (52.9%) | 1,204 (58.8%) | 3,460 (60.2%) | 2,842 (60.8%) |
| Public location - n (%) | 1,096 (8.5%) | 46 (10.4%) | 178 (8.7%) | 480 (8.4%) | 392 (8.4%) |
| Bystander witnessed - n (%) | 4,555 (35.2%) | 180 (40.5%) | 725 (35.4%) | 2,061 (35.9%) | 1,589 (34.0%) |
| Bystander CPR - n (%) | 5,078 (39.3%) | 150 (33.8%) | 719 (35.1%) | 2,242 (39.0%) | 1,967 (42.1%) |
| ALS first on scene - n (%) | 4,686 (36.3%) | 201 (45.3%) | 655 (32.0%) | 1,882 (32.7%) | 1,948 (41.6%) |
| ALS on scene - n (%) | 12,528 (96.9%) | 435 (98.0%) | 1,994 (97.3%) | 5,567 (96.9%) | 4,532 (96.9%) |
| No. of contributing agencies* - mean (SD) | 2.5 (0.6) | 2.4 (0.5) | 2.6 (0.6) | 2.6 (0.6) | 2.5 (0.5) |
| Adrenaline (Epinephrine) use - n (%) | 11,059 (85.5%) | 357 (80.4%) | 1,689 (82.4%) | 4,896 (85.2%) | 4,117 (88.0%) |
| Chest compression fraction - median (Q1, Q3) Chest compression rate - n (%) |
0.74 (0.63, 0.84) | 0.31 (0.21, 0.37) | 0.54 (0.49, 0.57) | 0.71 (0.66, 0.76) | 0.87 (0.84, 0.91) |
| <80 | 439 (3.4%) | 181 (40.8%) | 103 (5.0%) | 90 (1.6%) | 65 (1.4%) |
| 80-120 | 8,933 (69.1%) | 192 (43.2%) | 1,512 (73.8%) | 4,073 (70.9%) | 3,156 (67.5%) |
| >120 | 3,530 (27.3%) | 67 (15.1%) | 443 (21.6%) | 1,565 (27.2%) | 1,455 (31.1%) |
| Initial rhythm - n (%) | |||||
| Asystole | 8,378 (64.8%) | 240 (54.1%) | 1,270 (62.0%) | 3,671 (63.9%) | 3,197 (68.3%) |
| PEA | 3,366 (26.0%) | 154 (34.7%) | 575 (28.1%) | 1,557 (27.1%) | 1,080 (23.1%) |
| AED no shock, no strip | 1,184 (9.2%) | 50 (11.3%) | 214 (10.4%) | 519 (9.0%) | 401 (8.6%) |
| Minutes from 9-1-1 call to scene - median (Q1, Q3) | 5.4 (4.2, 6.9) | 5 (4, 6.2) | 5.2 (4.1, 6.6) | 5.4 (4.3, 7) | 5.5 (4.2, 7) |
| Minutes from 9-1-1 call to AED on - median (Q1, Q3) | 8.5 (6.8, 11) | 8.7 (6.7, 11) | 8.5 (6.8, 10) | 8.5 (6.9, 11) | 8.4 (6.7, 11) |
| Minutes from 9-1-1 call to first EMS shock assessment - median (Q1, Q3) | 10 (8.2, 12) | 10 (8.2, 12) | 9.9 (8, 12) | 10 (8.2, 12) | 10 (8.3, 13) |
| Any out-of-hospital shock - n (%) | 2,481 (19.2%) | 79 (17.8%) | 387 (18.9%) | 1,168 (20.3%) | 847 (18.1%) |
| ROSC within first 5 minutes of CPR - n (%) | 266 (2.1%) | 25 (5.6%) | 51 (2.5%) | 106 (1.8%) | 84 (1.8%) |
| Any out-of-hospital ROSC - n (%) | 3,304 (25.6%) | 120 (27.0%) | 472 (23.0%) | 1,398 (24.3%) | 1,314 (28.1%) |
| Survived to hospital discharge - n (%) | 307 (2.4%) | 23 (5.2%) | 37 (1.8%) | 129 (2.2%) | 118 (2.5%) |
SD indicates standard deviation; CPR, cardiopulmonary resuscitation; ALS, Advanced Life Support; Q1, Q3, the 1st and 3rd quantiles; EMS, emergency medical services; and ROSC, return of spontaneous circulation.
information was only available about the first four EMS units at the scene.
Figure 2.
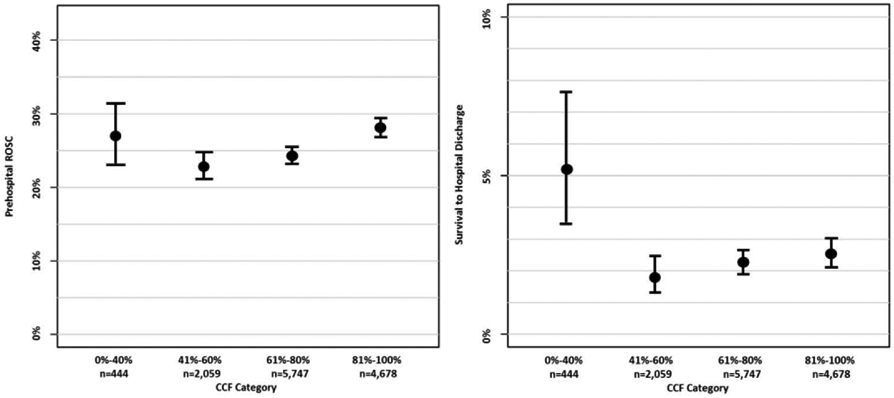
Percent Survival to Hospital Discharge and Return of Spontaneous Circulation (ROSC) by Category of Chest Compression Fraction (CCF) with 95% Confidence Intervals.
Main Results
Unadjusted and adjusted odds ratios of survival to hospital discharge and ROSC are presented in Table 3. The adjusted odds of survival for patients with CCF of 61-80% and 41-60% were not found to be statistically different from the odds for patients with CCF of 81-100%. The adjusted odds of survival in patients with 0-40% CCF were estimated to be 2.00 times larger (95% CI: 1.16-3.32) than those of patients with 81-100% CCF. The adjusted odds of ROSC for patients with CCF of 61-80% and 41-60% were estimated to be 0.85 (95% CI: 0.72-0.95) and 0.83 (95% CI: 0.77-0.94) times lower than the odds of ROSC for patients with CCF of 81-100%. The adjusted odds of ROSC in patients with 0-40% CCF were not found be statistically different from the odds for patients with CCF of 81-100%.
Table 3.
Predictors of Survival to Hospital Discharge and any Out-of-Hospital Return of Spontaneous Circulation.
| Survival to Discharge |
ROSC |
|||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)* | Unadjusted OR (95% CI) | Adjusted OR (95% CI)a | |
| CCF category | ||||
| 81-100% | Reference | Reference | Reference | Reference |
| 61-80% | 0.89 (0.69, 1.14) | 1.02 (0.77, 1.35) | 0.82 (0.75, 0.90) | 0.85 (0.77, 0.94) |
| 41-60% | 0.71 (0.48, 1.02) | 0.83 (0.54, 1.24) | 0.76 (0.67, 0.86) | 0.83 (0.72, 0.95) |
| 0-40% | 2.11 (1.30, 3.27) | 2.00 (1.16, 3.32) | 0.95 (0.76, 1.18) | 1.02 (0.79, 1.30) |
| Age (per ten-year increase) | 0.81 (0.76, 0.87) | 0.76 (0.71, 0.82) | 1.06 (1.04, 1.09) | 1.02 (0.997, 1.05) |
| Male | 0.73 (0.58, 0.92) | 0.66 (0.52, 0.84) | 0.74 (0.69, 0.81) | 0.71 (0.65, 0.78) |
| Public location | 2.15 (1.56, 2.90) | 1.60 (1.13, 2.23) | 1.00 (0.87, 1.16) | 0.85 (0.73, 0.996) |
| Bystander witnessed | 2.75 (2.18, 3.46) | 2.02 (1.57, 2.61) | 2.43 (2.24, 2.63) | 2.14 (1.95, 2.34) |
| Bystander CPR | 1.02 (0.81, 1.28) | 0.84 (0.65, 1.07) | 1.13 (1.04, 1.22) | 1.01 (0.92, 1.11) |
| Chest compression rate of 80-120 | 1.19 (0.93, 1.54) | 1.00 (0.75, 1.32) | 1.18 (1.09, 1.29) | 1.03 (0.93, 1.13) |
| Time from 9-1-1 call to AED turned on (per 10 min increase) | 0.86 (0.73, 1.02) | 0.95 (0.77, 1.05) | 0.95 (0.87, 1.00) | 0.98 (0.91, 1.02) |
| Initial rhythm | ||||
| Asystole | Reference | Reference | Reference | Reference |
| PEA | 5.36 (4.17, 6.93) | 4.77 (3.64, 6.29) | 2.70 (2.48, 2.95) | 2.27 (2.07, 2.50) |
| AED no shock, no strip | 2.29 (1.47, 3.44) | 2.29 (1.29, 3.86) | 1.38 (1.20, 1.59) | 1.55 (1.30, 1.85) |
OR indicates odds ratio; CI, confidence interval; CCF, chest compression fraction; CPR, cardiopulmonary resuscitation; and AED, automatic external defibrillator.
Adjusted for ROC site and all other covariates in the table.
DISCUSSION
In this retrospective analysis of data from a large multi-centre prospective cohort study, we describe the association of CCF with survival to hospital discharge and ROSC using an adult population of OHCA patients for whom the initial ECG rhythm was non-shockable. In this population, which represents the majority of OHCA cases, we found that increased CCF was associated with a significantly higher likelihood of ROSC among patients having a CCF exceeding 40%, but not with increased survival. These observations were independent of other known potential predictors of ROSC and survival to hospital discharge and highlight the potential importance of post ROSC care. We also found that survival was highest among the 0-40% CCF group.
We had to carefully define the study population included in this analysis in order to mitigate the impact of early survival among a group with very low CCF, as well as the impact of late death among a group with very high CCF and prolonged resuscitation efforts. We chose to study a cohort of patients where at least one minute of analysable CPR process data was available, up to and including the first five minutes of recorded data. In contrast, Christenson et al. only included available CPR process data up to and including the minute during which the first shock was provided (mean of 3.2 minutes included),11 whereas Cheskes et al. included the first ten minutes of CPR process data following the first documented EMS compression.14 Rea et al. noticed that CCF did not appear to be associated with increased ROSC or survival in a population of OHCA patients in ventricular fibrillation during the first 5 minutes of resuscitation, but those requiring greater than 10-20 minutes of CPR appeared to benefit from increased CCF.12
We also chose the group with a CCF of 81-100% as our reference group for the analyses. This is in contrast with prior studies by Christenson et al.11 and Vaillancourt et al.17 where the 0-20% and 0-40% CCF groups were respectively used as references. In our patient cohort, those with a CCF of 40% or less not only received a minimum amount of chest compressions, they were also more likely to be witnessed and treated in a public location, more likely to be in PEA, and more likely to receive a lower chest compression rate. Overall, this group had the highest survival rate despite receiving very little CPR. The reasons why that is are multifactorial, although unlikely the result of early conversion to a shockable rhythm.23 Instead, it is conceivable this group could have been in pseudo-PEA and mostly benefited from interventions other than CPR.24-26
Among patients for whom CCF exceeded 40%, our findings confirmed the hypothesis previously postulated by Vaillancourt et al. that higher CCF could result in higher ROSC,17 but could not confirm an association between CCF and survival for OHCA patients in an initial non-shockable rhythm. We believe even a much larger study would be unlikely to yield very different results. This is in contrast with the findings from Christenson et al. where such an association between CCF and survival existed,11 but consistent with the conclusions from Cheskes et al.,14 both in a population of OHCA with shockable rhythm. Our findings are also consistent with a study by Vadeboncoeur et al. which found an inverse relationship between CCF and survival in a population of OHCA patients presenting with any initial rhythm.15 Wik et al. suggested the inability to find an association between CCF and survival resulted from failing to take into account other resuscitation activities.13 This was fortunately not the case in the adjusted findings we are presenting.
In our adjusted analyses, an optimum chest compression rate of 80-120 compressions per minute was associated with neither ROSC nor survival. We interpret this as follows considering prior studies published by Idris et al. in 2012 and 2015.6,27 In their primary adjusted analyses, statistical modeling comparing pre-determined chest compression rate categories to a reference rate of 80-1406 or 100-11927 compressions per minute showed an association with neither ROSC (p=0.96 and p=0.93)6,27 nor survival (p=0.18 and p=0.19).6,27 A relationship between chest compression rate and ROSC was only found in post hoc exploratory analyses where an adjusted natural cubic spline curve show that greater ROSC was associated with a chest compression rate of 125 per minute before declining sharply (p = 0.012).6 Furthermore, an optimum chest compression rate of 100-119 was associated with survival in an adjusted analysis including a population where CCF and compression depth were both known (p=0.02).27
These findings may have implications for future cardiac arrest research. It appears that CCF is indeed a complex measure of overall CPR quality, one that seems to play a variable role based on the temporal evolution of a given resuscitation effort (early vs. late). Since out-of-hospital ROSC has not been shown to be a strong predictor of survival to hospital discharge in the past, the importance that should be given to CCF by rescuers over the other important components of CPR quality such as rate, depth, and peri-shock pauses remains unclear. As such, CCF may be better suited as an overall debriefing performance feedback measure rather than a metric that should be optimized during resuscitation efforts. Future similar analyses should consider including all available CPR process data, notwithstanding the decreasing survival rate associated with increasingly prolonged resuscitation efforts.12 Moreover, investigators conducting randomized controlled trials should carefully examine patients surviving in spite of receiving little to no CPR (low CCF group). Although these cases should be equally distributed between groups, we postulate they may be more likely to be in PEA with week-undetectable pulse (i.e. not truly in cardiac arrest), and less likely to benefit from the implemented intervention.
LIMITATIONS
This study has a number of limitations. First, because we used an observational cohort design, we can only report associations between increased CCF and the incidence of ROSC and survival to hospital discharge. We would need to adopt a controlled interventional study design to test whether a causal relationship exists. However, such a study would be extremely challenging to undertake. Second, the low survival rate from non-shockable OHCA reduces the statistical power to detect any impact of CCF on survival, our primary outcome. Nonetheless, with more than 12,000 cases available for analyses, we estimate we had sufficient power to detect a 1% increase in survival from increased CCF. In addition, the in-hospital care provided to cardiac arrest victims reaching the hospital with ROSC was not standardized and may have had a greater influence on survival to hospital discharge than CCF did during prehospital care. Third, we had to exclude a large number of potentially suitable cases because CPR process measures were not available. Nonetheless, these cases were very similar to those included in our study population. Forth, our data source did not include the time of adrenaline administration, and the total dose of adrenaline administered was often missing or not reliably documented. However, we could report accurately the proportion of cases who received adrenaline. Finally, it is possible that selection bias occurred based on differential patient contribution by study site, two of which were also using a continuous chest compression strategy. Irrespective, the resulting association of CCF with survival and ROSC was adjusted for several possible cofounders identified a priori, including study site.
CONCLUSIONS
In this large observational study of OHCA patients with non-shockable initial rhythm, increased CCF was independently associated with increased likelihood of ROSC among patients having a CCF exceeding 40%, but not survival. These findings are important since non-shockable rhythms constitute the majority of initial cardiac arrest rhythms observed in the out-of-hospital setting.
ACKNOWLEDGEMENTS
We would like to acknowledge the professional care provided by first responders and paramedics, the hard labour of research team members at all participating sites, and the diligent supervision provided by the central data coordinating centre study personnel, without which many more lives would be lost each day. We would also like to recognize the review and guidance offered by members of the ROC publications committee during the preparation of this work, and Mrs. Angela Marcantonio for her administrative support with this submission.
All authors provided substantial contributions to the concept and design of the work, or the acquisition, analysis, or interpretation of data for the word, and drafting of the work or revising it critically for important intellectual content, and gave final approval of the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
FUNDING SOURCES
The Resuscitation Outcomes Consortium (ROC) is supported by a series of cooperative agreements with10 regional clinical centres and one data Coordinating Centre (5U01HL077863, HL077881, HL077871 HL077872, HL077866 HL077908HL077867 HL077885 HL077887 HL077873 HL077865) from the National Heart, Lung, and Blood Institute in partnership with the National Institute of Neurological Disorders and Stroke, U.S. Army, Medical Research & Material Command, the Canadian Institutes of Health Research – Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, the Heart and Stroke Foundation of Canada, and the American Heart Association.
This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institutes of Health or any of the other stated funding agencies.
Appendix: Resuscitation Outcomes Consortium Investigators
Alabama Resuscitation Center, University of Alabama at Birmingham, Birmingham, AL: Jeffrey D. Kerby, MD, PhD, Principal Investigator
Core Investigators: Todd B. Brown, MD, MSPH, Thomas Terndrup, MD
Dallas Center for Resuscitation Research, University of Texas Southwestern Medical Center, Dallas, TX: Ahamed H. Idris, MD, Principal Investigator
Core Investigators: Fernando Benitez, MD, Raymond Fowler, MD, Dorothy Lemecha, MD, Joseph Minei, MD, Paul Pepe, MD, Michael Ramsay, MD, Robert Simonson, MD, Jane Wigginton, MD
University of Iowa Carver College of Medicine-Iowa Resuscitation Network, University of Iowa, Iowa City, IA: Richard Kerber, MD, Principal Investigator
Core Investigator: Steve Hata, MD, Dianne Atkins, MD
Milwaukee Resuscitation Research Center, Medical College of Wisconsin, Milwaukee, WI: Tom P. Aufderheide, MD, Principal Investigator
Core Investigators: Ronald G. Pirrallo, MD, MHSA, Karen J. Brasel, MD, MPH, Andrea L. Winthrop, MD, John P. Klein, PhD
Ottawa/OPALS/British Columbia RCC, Ottawa Health Research Institute, University of Ottawa, Ontario and St. Paul’s Hospital, University of British Columbia, British Columbia, Canada: Ian Stiell, MD, MSc Principal Investigator
Core Investigators: Jim Christenson, MD, Christian Vaillancourt, MD, MSc
Pittsburgh Resuscitation Network, the University of Pittsburgh, Pittsburgh, PA: Clifton Callaway, MD, PhD, Principal Investigator
Core Investigators: Samuel Tisherman, MD, Jon Rittenberger, MD, David Hostler, PhD
Portland Resuscitation Outcomes Consortium, Oregon Health and Science University, Portland, OR: Principal Investigator: Jerris R. Hedges, MD, MS,
Core Investigators: Craig D. Newgard, MD, MPH, Mohamud R. Daya, MD, MS, Robert A. Lowe, MD, MPH
UCSD-San Diego Resuscitation Research Center, University of California at San Diego, San Diego, CA: Daniel Davis, MD, Principal Investigator
Core Investigators: David Hoyt, MD, Raul Coimbra, MD, PhD, Gary Vilke, MD
Seattle-King County Center for Resuscitation Research at the University of Washington, University of Washington, Seattle, WA: Peter J. Kudenchuk, MD, Principal Investigator
Core Investigators: Tom D. Rea, MD, Eileen Bulger, MD, Mickey S. Eisenberg, MD, Michael Copass, MD
Toronto Regional Resuscitation Research out of hospital Network (Toronto Regional RESCUeNET), University of Toronto, Toronto, Ontario, Canada: Arthur Slutsky, MD, Principal Investigator
Core Investigators: Laurie Morrison, MD, MSc, Paul Dorian MD, PhD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
Dr. Cheskes has received investigator-initiated grant funding support and funding for educational speaking on CPR quality from Zoll Medical.
Dr. Nichol discloses salary support from University of Washington through Leonard A. Cobb Medic One Foundation Endowed Chair in Prehospital Emergency Care; Research support from ZOLL Medical Corp, Chelmsford, MA; Consultant to GE Healthcare Inc., Chicago, IL; Kestra Medical Technologies, Kirkland, WA; QOOL Therapeutics, Menlo Park, CA; and ZOLL Circulation Inc., San Jose, CA.
Contributor Information
Christian Vaillancourt, Department of Emergency Medicine and Ottawa Hospital Research Institute, University of Ottawa, ON, Canada.
Ashley Petersen, Division of Biostatistics, University of Minnesota, Minneapolis, MN.
Eric N. Meier, Department of Biostatistics, University of Washington, Seattle, WA, USA.
Jim Christenson, University of British Columbia, Vancouver, BC, Canada.
James J. Menegazzi, University of Pittsburgh, Pittsburgh, PA, USA.
Tom P. Aufderheide, Medical College of Wisconsin, Milwaukee, WI, USA.
Graham Nichol, University of Washington, Seattle, WA, USA.
Robert Berg, University of Pennsylvania, The Children's Hospital of Philadelphia, PA, USA.
Clifton W. Callaway, University of Pittsburgh, Pittsburgh, PA, USA.
Ahamed H. Idris, Department of Emergency Medicine, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Daniel Davis, Air Methods Corporation, Greenwood Village, CO, USA.
Raymond Fowler, Department of Emergency Medicine, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Debra Egan, National Center for Complementary and Integrative Health, National Institutes of Health, Bethesda, MD, USA.
Douglas Andrusiek, Doctors Without Borders, Toronto, ON, Canada.
Jason E. Buick, Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, ON, Canada.
T.J. Bishop, Lake Chelan Community Hospital EMS, Chelan, WA.
M. Riccardo Colella, Departments of Emergency Medicine, Pediatrics and the Institute for Health and Equity, Medical College of Wisconsin, Milwaukee, WI, USA.
Ritu Sahni, Clackamas County EMS, Oregon City, OR, USA.
Ian G. Stiell, Department of Emergency Medicine, and Ottawa Hospital Research Institute, University of Ottawa, ON, Canada.
Sheldon Cheskes, Department of Family and Community Medicine, Division of Emergency Medicine, University of Toronto, Toronto, ON, Canada.
REFERENCES
- 1.Nichol G, Leroux B, Wang H, et al. Trial of Continuous or Interrupted Chest Compressions during CPR. New England Journal of Medicine. December 03 2015;373(23):2203–2214. [DOI] [PubMed] [Google Scholar]
- 2.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. September 24 2008;300(12):1423–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaillancourt C, Stiell IG, Canadian Cardiovascular Outcomes Research Team (CCORT). Cardiac arrest care and emergency medical services in Canada. Can J Cardiol. 2004;20(11):1081–1090. [PubMed] [Google Scholar]
- 4.Grunau B, Kawano T, Dick W, et al. Trends in care processes and survival following prehospital resuscitation improvement initiatives for out-of-hospital cardiac arrest in British Columbia, 2006-2016. Resuscitation. 2018;125:118–125. [DOI] [PubMed] [Google Scholar]
- 5.Stiell IG, Wells GA, Field B, et al. Advanced cardiac life support in out-of-hospital cardiac arrest. New England Journal of Medicine. 2004;351(7):647–656. [DOI] [PubMed] [Google Scholar]
- 6.Idris AH, Guffey D, Aufderheide TP, et al. Relationship between chest compression rates and outcomes from cardiac arrest. Circulation. June 19 2012;125(24):3004–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stiell IG, Brown SP, Christenson J, et al. What is the role of chest compression depth during out-of-hospital cardiac arrest resuscitation? Crit Care Med. April 2012;40(4):1192–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perkins GD, Travers AH, Berg RA, et al. Part 3: Adult basic life support and automated external defibrillation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation. October 2015;95:e43–69. [DOI] [PubMed] [Google Scholar]
- 9.Kurz MC, Prince D, Christenson J, et al. Association of Advanced Airway Device with Chest Compression Fraction During Out-of-Hospital Cardiopulmonary Arrest. Resuscitation. 2016;98:35–40. [DOI] [PubMed] [Google Scholar]
- 10.Cheskes S, Schmicker RH, Verbeek PR, et al. The impact of peri-shock pause on survival from out-of-hospital shockable cardiac arrest during the Resuscitation Outcomes Consortium PRIMED trial. Resuscitation. March 2014;85(3):336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christenson J, Andrusiek D, Everson-Stewart S, et al. Chest compression fraction determines survival in patients with out-of-hospital ventricular fibrillation. Circulation. September 29 2009;120(13):1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rea T, Olsufka M, Yin L, Maynard C, Cobb L. The relationship between chest compression fraction and outcome from ventricular fibrillation arrests in prolonged resuscitations. Resuscitation. 2014;85(7):879–884. [DOI] [PubMed] [Google Scholar]
- 13.Wik L, Olsen J-A, Persse D, et al. Why do some studies find that CPR fraction is not a predictor of survival? Resuscitation. 2016;104:59–62. [DOI] [PubMed] [Google Scholar]
- 14.Cheskes S, Schmicker RH, Rea T, et al. Chest compression fraction: A time dependent variable of survival in shockable out-of-hospital cardiac arrest. Resuscitation. December 2015;97:129–135. [DOI] [PubMed] [Google Scholar]
- 15.Vadeboncoeur T, Stolz U, Panchal A, et al. Chest compression depth and survival in out-of-hospital cardiac arrest. Resuscitation. 2014;85(2):182–188. [DOI] [PubMed] [Google Scholar]
- 16.Mehta C, Brady W. Pulseless electrical activity in cardiac arrest: electrocardiographic presentations and management considerations based on the electrocardiogram. American Journal of Emergency Medicine. 2012;30(1):236–239. [DOI] [PubMed] [Google Scholar]
- 17.Vaillancourt C, Everson-Stewart S, Christenson J, et al. The impact of increased chest compression fraction on return of spontaneous circulation for out-of-hospital cardiac arrest patients not in ventricular fibrillation. Resuscitation. December 2011;82(12):1501–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis DP, Garberson LA, Andrusiek DL, et al. A descriptive analysis of Emergency Medical Service Systems participating in the Resuscitation Outcomes Consortium (ROC) network. Prehospital Emergency Care. Oct-Dec 2007;11(4):369–382. [DOI] [PubMed] [Google Scholar]
- 19.Stiell IG, Nichol G, Leroux BG, et al. Early versus later rhythm analysis in patients with out-of-hospital cardiac arrest. New England Journal of Medicine. September 1 2011;365(9):787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison LJ, Nichol G, Rea TD, et al. Rationale, development and implementation of the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Resuscitation. August 2008;78(2):161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa). Circulation. 2004 November 23 2004;110(21):3385–3397. [DOI] [PubMed] [Google Scholar]
- 22.Valenzuela TD, Kern KB, Clark LL, et al. Interruptions of chest compressions during emergency medical systems resuscitation. Circulation. August 30 2005;112(9):1259–1265. [DOI] [PubMed] [Google Scholar]
- 23.Thomas AJ, Newgard CD, Fu R, Zive DM, Daya MR. Survival in out-of-hospital cardiac arrests with initial asystole or pulseless electrical activity and subsequent shockable rhythms. Resuscitation. 2013;84(9):1261–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prosen G, Krizmaric M, Zavrsnik J, Grmec S. Impact of modified treatment in echocardiographically confirmed pseudo-pulseless electrical activity in out-of-hospital cardiac arrest patients with constant end-tidal carbon dioxide pressure during compression pauses. Journal of International Medical Research. 2010;38(4):1458–1467. [DOI] [PubMed] [Google Scholar]
- 25.Teran F, Dean AJ, Centeno C, et al. Evaluation of out-of-hospital cardiac arrest using transesophageal echocardiography in the emergency department. Resuscitation. 2019;137:140–147. [DOI] [PubMed] [Google Scholar]
- 26.Wu C, Zheng Z, Jiang L, et al. The predictive value of bedside ultrasound to restore spontaneous circulation in patients with pulseless electrical activity: A systematic review and meta-analysis. PLoS ONE [Electronic Resource]. 2018;13(1):Published online doi: 10.1371/journal.pone.0191636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Idris AH, Guffey D, Pepe PE, et al. Chest compression rates and survival following out-of-hospital cardiac arrest. Crit Care Med. April 2015;43(4):840–848. [DOI] [PubMed] [Google Scholar]


