Abstract
Background & Aims:
Pancreatic ductal adenocarcinomas (PDACs) frequently metastasize to the lymph nodes; strategies are needed to identify patients at highest risk for lymph node metastases. We performed genome-wide expression profile analyses of PDAC specimens, collected during surgery or endoscopic ultrasound fine-need aspiration (EUS-FNA), to identify a microRNA (miRNA) signature associated with metastasis to lymph nodes.
Methods:
For biomarker discovery, we analyzed miRNA expression profiles of primary pancreatic tumors from 3 public datasets (TCGA, GSE24279, and GSE32688). We then analyzed 157 PDAC specimens (83 from patients with lymph node metastases and 74 without) from Japan, collected from 2001 through 2017, for the training cohort and a 107 PDAC specimens (63 from patients with lymph node metastases and 44 without) from a different medical center in Japan, from 2002 through 2016, for the validation cohort. We also analyzed samples collected by EUS-FNA before surgery from 47 patients (22 patients with lymph node metastases and 25 without; 17 for the training cohort and 30 from the validation cohort), and 62 specimens prior to any treatment from patients who received neoadjuvant chemotherapy (9 patients with lymph node metastasis and 53 without) for additional validation. Multivariate logistic regression analyses were used to evaluate the statistical differences in miRNA expression between patients with vs without metastases.
Results:
We identified a miRNA expression pattern associated with diagnosis of PDAC metastasis to lymph nodes. Using logistic regression analysis, we optimized and trained a 6-miRNA risk prediction model for training cohort; this model discriminated patients with vs without lymph node metastases with an area under the curve (AUC) of 0.84 (95% CI. 0.77–0.89). In the validation cohort, the model identified patients with vs without lymph node metastases with an AUC of 0.73 (95% CI. 0.64–0.81). In EUS-FNA biopsies, the model identified patients with vs without lymph node metastases with an AUC of 0.78 (95% CI. 0.63–0.89). The miRNA expression pattern was an independent predictor of PDAC metastasis to lymph node in the validation cohort (odds ratio, 17.05; 95% CI. 2.43–119.57), and in the EUS-FNA cohort (95% CI. 0.65–0.87).
Conclusions:
Using data and tumor samples from 3 independent cohorts, we identified a miRNA signature that identifies patients at risk for PDAC metastasis to lymph nodes. The signature has similar levels of accuracy in analysis of resected tumor specimens and EUS-FNA biopsies. This model might be used to select treatment and management strategies for patients with PDAC.
Keywords: LNM, cancer progression, prognostic factor, prognosis
Graphical Abstract
LAY SUMMARY:
The authors identified genes that are expressed at higher levels in pancreatic tumors from patients with vs without metastases. This pattern of gene expression might be used to identify patients at higher risk for pancreatic tumor metastasis.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is an extremely lethal malignancy, and is projected to become the second leading cause of cancer-related deaths in the US by 20301–3. While complete surgical resection for the localized tumors is the only treatment option available for a cure or long-term survival, 80–90% of patients at initial diagnosis has an unresectable or borderline resectable disease- leading to poor survival outcomes. Consequently, the 5-year survival rates in PDAC patients following surgical resection is only 10 to 25% at best, because of the high risk of local and distant recurrence2, 4–6. Considering that the surgery alone has minimal survival benefits, in the recent years, multidisciplinary treatment strategies for a more effective therapeutic targeting in PDAC patients has aggressively been explored4, 7–13. The National Comprehensive Cancer Network (NCCN) guidelines recommend neoadjuvant therapy (NAT) in patients with borderline resectable disease; however, in PDAC patients with a resectable cancer, the guidelines suggest that NAT may only be considered in a limited subset of patients with specific high-risk features, including: large regional lymph nodes (LNs), elevated CA-19–9 levels, large primary tumors, excessive weight loss, and extreme pain.
Among all these risk factors, lymph node metastasis (LNM) status remains one of the most important predictors of survival in patients undergoing curative resection, and is considered to be of tremendous clinical significance for risk stratification and therapeutic decision-making in PDAC patients10, 14–21. Several recent studies have reported that PDAC patients with potentially resectable cancers who underwent NAT followed by curative surgery exhibited improved survival and longer time to recurrence; especially those with LNM4, 10, 11, 22–26; highlighting the fact that a pre-treatment diagnosis of LNM is critical determinant for developing a more personalized treatment strategy in PDAC patients11, 13, 20. However, pre-treatment diagnosis for the presence of LNM in PDAC patients is clinically challenging. Currently, such diagnosis is often made by computed tomography (CT), magnetic resonance imaging (MRI), endoscopic ultrasonography (EUS), and 18F-fluorodeoxyglucose positron emission tomography (PET). Unfortunately however, all of these methodologies are inadequate for evaluating the LNM status in PDAC patients due to their poor sensitivity and specificity20, 27–30; highlighting the need to develop potential molecular biomarkers that can overcome challenges of imaging-based methods for such diagnosis. Very limited research effort has been made on this front. A couple of studies reported that preoperative neutrophil-to-lymphocyte ratio and serum MMP7 levels could help predict LNM in PDAC20, 31, but the accuracy of these biomarkers was insufficient for their clinical use.
Recent technological advances have now enabled innovative analysis of genomic and epigenomic profiling in various malignancies and have facilitated identification of previously unrecognized molecular biomarkers32–40. MicroRNAs (miRNAs), which belong to the group of small non-coding RNAs, are 18 to 25 nucleotides long, single-stranded RNAs, that play key roles in post-transcriptional gene repression, oncogenesis and tumor metastasis, and are frequently dysregulated in various human cancers including PDAC32, 33, 35–40. Importantly, due to their short length, miRNAs are emerging as important biomarker candidates by virtue of their ability to resist RNAase-mediated degradation and their intact expression in a variety of bodily fluids, formalin-fixed paraffin-embedded (FFPE), as well as biopsy tissues32, 35, 39–41. More specifically, several miRNAs, including miR-21, have been reported to associate with early diagnosis and prognosis in PDAC. However, the clinical significance as various miRNAs to serve as biomarkers to identify LNM pre-operatively in PDAC, through a systematic and genomewide comprehensive analysis in large, multiple independent patient cohorts has not been attempted32, 33, 38, 39, 42–49. Availability of such biomarkers will facilitate physicians in making more informed clinical decision-making and developing individualized treatment strategies for improved management of PDAC patients.
Herein, for the first time, we performed a genome-wide, systematic and comprehensive biomarker discovery to identify and establish a novel miRNA expression signature for the detection of LNM in PDAC patients. This signature was initially confirmed in multiple, large, publicly-available datasets, followed by rigorous validation and performance evaluation in two independent, large, clinical cohorts. Finally, in order to translate our findings into a clinically-viable scenario, we were able to confirm the robustness of this biomarker signature in pre-treatment endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) biopsy specimens, highlighting the clinical significance of these biomarkers for the management of PDAC patients.
MATERIALS AND METHODS
MiRNA biomarker discovery
To perform a comprehensive biomarker discovery, we analyzed miRNA expression profiling results of primary tumor tissues from three, large, publicly-available datasets (TCGA, GSE24279, and GSE32688), to identify and establish a miRNA signature for the identification of LNM in PDAC patients, as illustrated in supplementary figure S1. TCGA miRNA expression profiling data (level 3 miRNA-sequencing data) were downloaded from the UCSC Xena Browser (https://xenabrowser.net, accessed on July 12, 2018). Likewise, GSE24279 and GSE32688 datasets (both normalized non-coding RNA profiling and clinical data) were downloaded from the GEO database in its processed form (https://www.ncbi.nlm.nih.gov, accessed on July 12, 2018) and from a previously published article (https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0034151)50. The cases where patients had distant metastasis, insufficient pathological LNM information, and those who received NAT (because pathological LNM can be modified by such treatment) were excluded. In total, miRNA-expression profiling data from total of 269 PDAC patients, including miRNA-sequencing data from TCGA cohort (167 patients, 121 LNM-positive [LNP] and 46 LNMnegative [LNN]), and the GSE24279 (77 patients, 69 LNP and 8 LNN) and GSE32688 (25 patients, 17 LNP and 8 LNN) cohorts, were analyzed to identify an miRNA signature in the discovery and internal validation phases, respectively (Table S1). To evaluate the diagnostic potential of the discovered miRNA signature, we first established a multivariate logistic regression model using the selected biomarkers, and subsequently determined the area under the curve (AUC) values for each of the receiver operator characteristic (ROC) plots41, 51.
Patient cohorts
For the clinical training and validation of the identified miRNA signature, we analyzed biospecimens from two large, and independent patient cohorts. A total of 264 FFPE specimens were examined, which included a training cohort (n = 157; 83 LNP and 74 LNN) of PDAC patients enrolled at the Nara Medical University between 2001 and 2017, and a validation cohort (n = 107; 63 LNP and 44 LNN) of patients enrolled at the Kumamoto University, Japan between 2002 and 2016. None of these patients received preoperative cancer treatment, and all tumors were diagnosed as PDAC. Matched FFPE EUS-FNA biopsy samples from 47 patients (22 LNP and 25 LNN) in both cohorts (training cohort: 17, validation cohort: 30) were also obtained. Additionally, EUS-FNA biopsy samples from 62 PDAC patients (9 LNP and 53 LNN) who received NAT followed by surgery were also collected for additional validation. These EUS-FNA biopsy specimens were obtained prior to initiation of any treatment, and the specimens were collected and processed as per the standard diagnostic procedures using endoscopic and cytological techniques52, 53. Tumors were classified according to the TNM staging system of the International Union Against Cancer (UICC) version 7. The LNM status was determined from histopathologic examination of resected LNs. The patients who had positive peritoneal washing cytology or para-aortic LNM-without other distant metastases were included in this study54. Exclusion criteria included macroscopically incomplete resection or a tumor histology other than diagnosis of PDAC. All patients were followed until death or June 2018. The study was conducted in accordance with the Declaration of Helsinki. A written informed consent was obtained from all patients, and the study was approved by the institutional review boards of all participating institutions.
RNA extraction and RT-qPCR
Total RNA was isolated from 10-mm-thick FFPE surgical tissues and EUS-FNA biopsy specimens by microdissection to enrich for neoplastic cells, using AllPrep DNA/RNA FFPE Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Synthesis of complementary DNA from total RNA was performed using Taqman MicroRNA Reverse Transcription Kit (ThermoFisher Scientific, Waltham, MA). Real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) analysis was performed using the SensiFAST™ probe Lo-ROX Kit (Bioline, London, UK) on the Quantstudio 7 Flex Real Time PCR System (Applied Biosystems, Foster City, CA), and expression levels were evaluated with Applied Biosystems QuantStudio 7 Flex Real Time PCR System Software. The relative abundance of target transcripts was evaluated and normalized to the expression levels of snRNA U6 as an internal control using the 2−ΔDCt method. Normalized values were further log10 transformed41, 55.
MicroRNA Regulatory Network
The miRNA:mRNA regulatory network was constructed using the validated miRNAs to elucidate pathways perturbed, through data analysis using miRWalk version 3.056–58. The miRNA:mRNA network was constructed wherein the target genes were consistently expressed in at least 2 of the three sources -TargetScan, miRDB, and miRTarBase. The pathway enrichment analysis for selected target genes was performed using KEGG pathways and Gene Ontology41, 59, 60.
Statistical analysis
Unpaired t-test was used to evaluate the statistical differences in miRNA expression between LNP and LNN patients in the public datasets. Pearson’s correlation analysis was performed to test multicollinearity. Recursive feature elimination using random forest method was performed to select important features miRNAs. For all cohorts, ROC curves and AUC values were determined using Medcalc statistical software V.16.2.0. Univariate and multivariate logistic regression were employed to evaluate various clinicopathological variables, including age, gender, carbohydrate antigen 19–9 (CA19–9), tumor location, tumor size evaluated by CT, and the miRNA signature for the detection of LNM status. The cutoff thresholds for continuous variables were divided by median value in the total participants. The overall survival (OS) time and relapse-free survival (RFS) times were calculated from the date of surgery to the date of death from any cause or recurrence, or last follow-up date. We estimated OS and RFS using the Kaplan-Meier method. We analyzed the primary endpoint with a stratified log-rank test7, 61. The median follow-up was calculated by the reverse Kaplan-Meier method7, 62. A multivariate Cox proportional hazard regression model was established and a P value < 0.05 was considered statistically significant. The statistical analyses were performed using the Medcalc statistical software V.16.2.0 (Medcalc Software bvba, Ostend, Belgium), GraphPad Prism V7.0 (GraphPad Software, San Diego, CA), and R (3.5.0, R Development Core Team, https://cran.r-project.org/).
RESULTS
Genome-wide miRNA expression profiling led to the identification of a novel 7-miRNA signature for the detection of lymph node metastasis in PDAC patients
We performed a genome-wide, unbiased, comprehensive biomarker discovery analysis in three independent miRNA expression profiling datasets (TCGA, GSE24279 and GSE32688) to identify a miRNA signature for the detection of LNM in PDAC patients. We first compared miRNA expression profiles between LNP and LNN patients in the TCGA and GSE24279 cohort, which included patients who had undergone curative surgery without NAT and identified 13 differentially expressed (P < 0.05) candidate targets with data availability in at least >50% of all cases, excluded highly correlated miRNAs and with consistent expression profiles in both cohorts. The random forest-based recursive feature elimination with a 10-fold cross validation on this dataset reduced this to a signature of 10 miRNAs. Among these, 7 miRNAs exhibited consistent expression profiles in all three datasets: miR-155–5p, miR-196b-5p, miR-365a-5p, miR-629–5p, miR-675–3p, miR-92b-3p and, let-7d-5p. Finally, a logistic regression model with these 7 miRNAs in the TCGA cohort resulted in an AUC of 0.76 (95% confidence interval (CI) = 0.66–0.82). Furthermore, the diagnostic ability of this 7-miRNA signature was significantly validated in two additional datasets (GSE24279: AUC = 0.75; 95% CI = 0.64–0.84, GSE32688: AUC = 0.92; 95% CI = 0.74–0.99; Fig. 1A–C); highlighting the diagnostic performance of this signature for the identification of LNM in PDAC patients.
Figure 1.
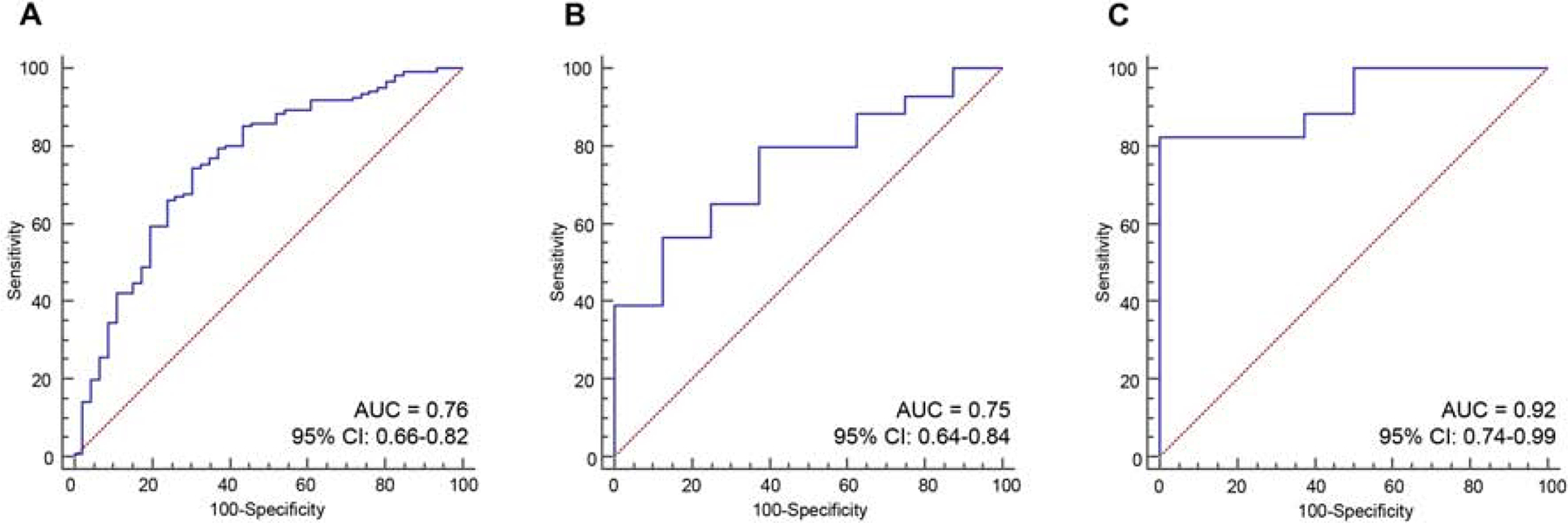
Genome-wide discovery and validation of a novel miRNA signature to detect lymph node metastasis in PDAC patients.
(A-C) The ROC curves demonstrate the diagnostic performance of the 7-miRNA signature for distinguish the patients with lymph node metastasis in (A) TCGA, (B) GSE24279 and (C) GSE32688 cohorts.
Clinical training and validation resulted in the establishment of a miRNA signature for detecting Lymph node metastasis in PDAC patients
In order to confirm the diagnostic robustness of our discovered miRNA signature, we next performed training and validation of these biomarkers in 2 large, independent clinical cohorts (Table 1). First, the performance of the 7-miRNA signature was evaluated by RT-qPCR assays in a training cohort of 157 PDAC patients (83 LNP and 74 LNN). In the training cohort, we excluded one miRNA (let-7d-5p) as it exhibited inconsistent expression in the internal validation cohort, resulting in a final signature of 6 miRNAs (miR-155–5p, miR-196b-5p, miR-365a-5p, miR-629–5p, miR-675–3p and miR-92b-3p). We successfully reconfirmed the diagnostic accuracy of this 6-miRNA model for its ability to detect LNM in PDAC patients from the three public datasets (Fig. S2). Subsequently, we trained a 6-miRNA risk-prediction model using logistic regression analysis in the training cohort, which robustly identified PDAC patients with LNM (AUC = 0.84, 95%CI = 0.77–0.89, Fig. 2A, C). The risk score model was developed based on the coefficients of individual miRNAs and the constant derived from this analysis as follows: 2.50737 + (0.50693*miR-155–5p) + (0.082317*miR-196b-5p) + (0.014458 *miR-365a-5p) + (1.74439*miR629–5p) + (2.71643*miR-675–3p) + (−5.15058*miR-92b-3p). Subsequently, we assessed the robustness and accuracy of this 6-miRNA signature by applying the same statistical model into a large, independent validation cohort (63 LNP and 44 LNN cases). Once again, our miRNA biomarkers exhibited remarkable diagnostic accuracy for the identification of LNM in PDAC patients in this validation cohort as well (AUC = 0.73, 95%CI = 0.64–0.81, Fig. 2B, D); underscoring the clinical significance of our miRNA signature in identifying presence of LNM in PDAC patients.
Table 1:
Clinicopathological characteristics of clinical cohorts
| Characteristics | Surgical specimens, n (%) | Matched FNA biopsy specimens, n (%) (n = 47) | Total participants (n = 264) | |
|---|---|---|---|---|
| Training cohort (n = 157) | Validation cohort (n = 107) | |||
| Gender | ||||
| Male | 93 (59.2) | 55 (51.4) | 29 (61.7) | 148 (56.1) |
| Female | 64 (40.8) | 52 (48.6) | 18 (39.3) | 116 (43.9) |
| Age (years) | ||||
| median (range) | 70 (32–87) | 70 (37–90) | 69 (51–82) | 70 (32–90) |
| Preoperative CA19–9 (U/mL) | ||||
| median (range) | 93 (1–19296) | 57.7 (0.1–3722) | 60 (0.6–1714) | 71.5 (0.1–19296) |
| Tumor location | ||||
| Ph | 100 (63.7) | 71 (66.4) | 33 (70.2) | 171 (64.8) |
| Pbt | 57 (36.3) | 36 (33.6) | 14 (29.8) | 93 (35.2) |
| Tumor size (mm) | ||||
| median (range) | 27 (5–90) | 30 (4–65) | 26 (10–90) | 28 (4–90) |
| T status | ||||
| T1–2 | 15 (9.6) | 15 (14.0) | 2 (4.3) | 30 (11.4) |
| T3–4 | 142 (90.4) | 92 (86.0) | 45 (95.7) | 234 (88.6) |
| Lymph node metastases | ||||
| Negative | 74 (47.1) | 44 (41.1) | 25 (53.2) | 118 (44.7) |
| Positive | 83 (52.9) | 63 (58.9) | 22 (46.8) | 146 (55.3) |
| UICC stage (ver.7) | ||||
| IA, IB | 11 (7.0) | 12 (11.2) | 2 (4.3) | 13 (4.9) |
| IIA | 60 (38.2) | 31 (29.0) | 22 (46.8) | 91 (34.5) |
| IIB | 70 (44.6) | 57 (53.3) | 19 (40.4) | 127 (48.1) |
| III | 0 (0.0) | 1 (0.9) | 1 (2.1) | 1 (0.4) |
| IV | 16 (10.2) | 6(5.6) | 3 (6.4) | 22 (8.3) |
| Adjuvant therapy | ||||
| Yes | 120 (76.4) | 86 (80.4) | 41 (87.2) | 206 (78.0) |
| No | 37 (23.6) | 21 (19.6) | 6 (12.8) | 58 (22.0) |
FNA, Fine needle aspiration; UICC, International Union Against Cancer
Plus-minus values are means standard error of the mean.
Figure 2.
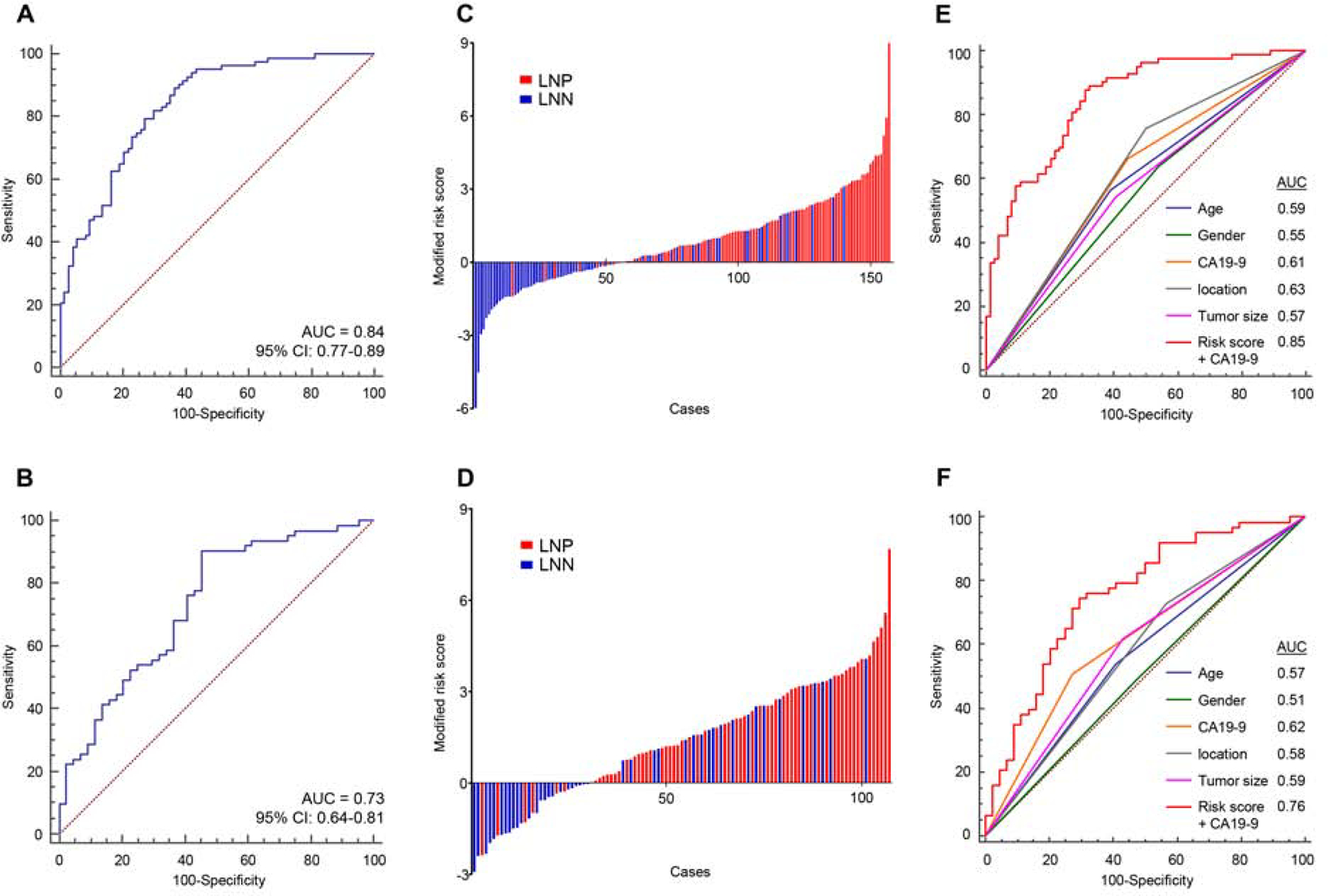
Training and validation of a miRNA signature for identifying Lymph node metastasis in PDAC patients in two independent clinical cohorts.
(A, B) A ROC curve of the 6-miRNA signature in the (A) training cohort (LNP = 83, LNN = 74, AUC = 0.84) and (B) validation cohort (LNP = 63, LNN = 44, AUC = 0.73). (C, D) Risk score distribution plot in (C) training cohort and (D) validation cohort. Modified risk score was obtained from subtracting individual risk score from Youden’s index value of risk model. (E, F) The new combination model, miRNA signature and CA19–9, outperformed the detection accuracy in both clinical cohorts (E: training cohort, F: validation cohort). LNN: lymph node metastasis negative, LNP: lymph node metastasis positive.
A combination signature of miRNA biomarkers and CA-19–9 levels demonstrated a significantly higher accuracy for detecting LNM in PDAC patients
Since, CA19–9 is a widely established and important biomarker in PDAC, we next examined whether a combination model of our miRNA signature and this glycoprotein levels might further improve the diagnostic accuracy for detecting LNM in PDAC patients. Interestingly, indeed this new combination signature demonstrated a significantly superior diagnostic accuracy for LNM in both training and validation cohorts (AUC= 0.85 and, 0.76, respectively, Fig. 2E, F). Furthermore, this new combination signature also demonstrated a significantly improved diagnostic accuracy compared to other classic preoperative clinicopathological features, including tumor location and size (Fig. 2E, F). We next categorized all patients into high- and low-risk groups using the cutoff thresholds derived by Youden’s index from this 6-miRNA signature model, and subsequently performed logistic regression for univariate and multivariate analyses63. Of interest, the multivariate analysis revealed that our newly established developed signature emerged as an independent predictor of LNM in PDAC patients, in both clinical cohorts [training cohort: odds ratio (OR) = 16.97; 95% CI = 6.78–42.50; P < 0.01, validation cohort: OR = 9.95; 95% CI = 3.49–28.34; P < 0.01, Table 2).
Table 2:
Univariate and multivariate logistic regression analysis for lymph node metastasis in training and validation cohorts
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Characteristics | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Training cohort (n = 157) | ||||||
| Age (≥70 vs. <70 years) | 0.55 | 0.29–1.04 | 0.07 | 0.53 | 0.23–1.23 | 0.14 |
| Gender (Female vs. Male) | 0.67 | 0.35–1.26 | 0.21 | 0.45 | 0.19–1.06 | 0.07 |
| CA19–9 (≥ 71.5 vs. < 71.5 U/mL) | 2.44 | 1.28–4.66 | < 0.01 | 2.18 | 0.92–5.14 | 0.08 |
| Location (Pbt vs. Ph) | 0.32 | 0.16–0.63 | < 0.01 | 0.21 | 0.09–0.53 | < 0.01 |
| Tumor size (≥ 28 vs. < 28 mm) | 1.75 | 0.91–3.38 | 0.01 | 1.85 | 0.78–4.41 | 0.17 |
| 6-miRNA signature (High vs. Low risk) | 33.36 | 9.57–116.23 | < 0.01 | 19.93 | 7.55–52.62 | < 0.01 |
| Validation cohort (n = 107) | ||||||
| Age (≥70 vs. <70 years) | 0.59 | 0.27–1.29 | 0.19 | 1.00 | 0.39–2.60 | 0.99 |
| Gender (Female vs. Male) | 0.94 | 0.44–2.04 | 0.88 | 1.11 | 0.43–2.83 | 0.83 |
| CA19–9 (≥ 71.5 vs. < 71.5 U/mL) | 2.75 | 1.20–6.30 | 0.02 | 1.55 | 0.58–4.13 | 0.38 |
| Location (Pbt vs. Ph) | 0.49 | 0.22–1.10 | 0.08 | 0.50 | 0.19–1.29 | 0.15 |
| Tumor size (≥ 28 vs. < 28 mm) | 2.14 | 0.98–4.68 | 0.06 | 1.75 | 0.66–4.65 | 0.26 |
| 6-miRNA signature (High vs. Low risk) | 11.09 | 4.09–30.04 | < 0.01 | 10.05 | 3.42–29.59 | < 0.01 |
OR, odds ratio; CI, confidence interval
Prognostic potential of the miRNA signature for PDAC patients in the clinical cohorts
Since LNM is often associated with poor patient survival in PDAC patients, we next were curious to inquire the prognostic potential of our miRNA signature. Reassuringly, consistent with previous reports, the presence of LNM had significant impact on patients’ prognosis in the training and validation cohorts (Fig. S3). In order to evaluate the prognostic potential of our miRNA biomarkers, we performed survival analysis for OS and RFS. The median follow-up times were 65.7 months (95% CI: 61.01–80.81) in the training cohort and 32.94 months (95% CI = 31.52–47.27) in the validation cohort. Importantly, PDAC patients within the high-risk group demonstrated a significantly worse prognosis in the training cohort (OS [P < 0.01]; RFS [P < 0.01]) and the validation cohort (OS [P < 0.01]; RFS [P = 0.03], Fig. 3A, B, D and E). In addition, in multivariate analysis using the Cox’s proportional hazard model along with other clinicopathological factors, high-risk patients defined by the miRNA signature associated with a significantly worse OS in both independent cohorts [training cohort: Hazard ratio (HR) = 1.78; 95% CI = 1.16–2.73; P < 0.01, validation cohort: HR = 2.41; 95% CI = 1.08–5.40; P = 0.03, Fig. 3C and F]. These results highlight that in addition to the diagnostic ability of our signature in detecting LNM in PDAC patients, it has a significant prognostic potential as well.
Figure 3.
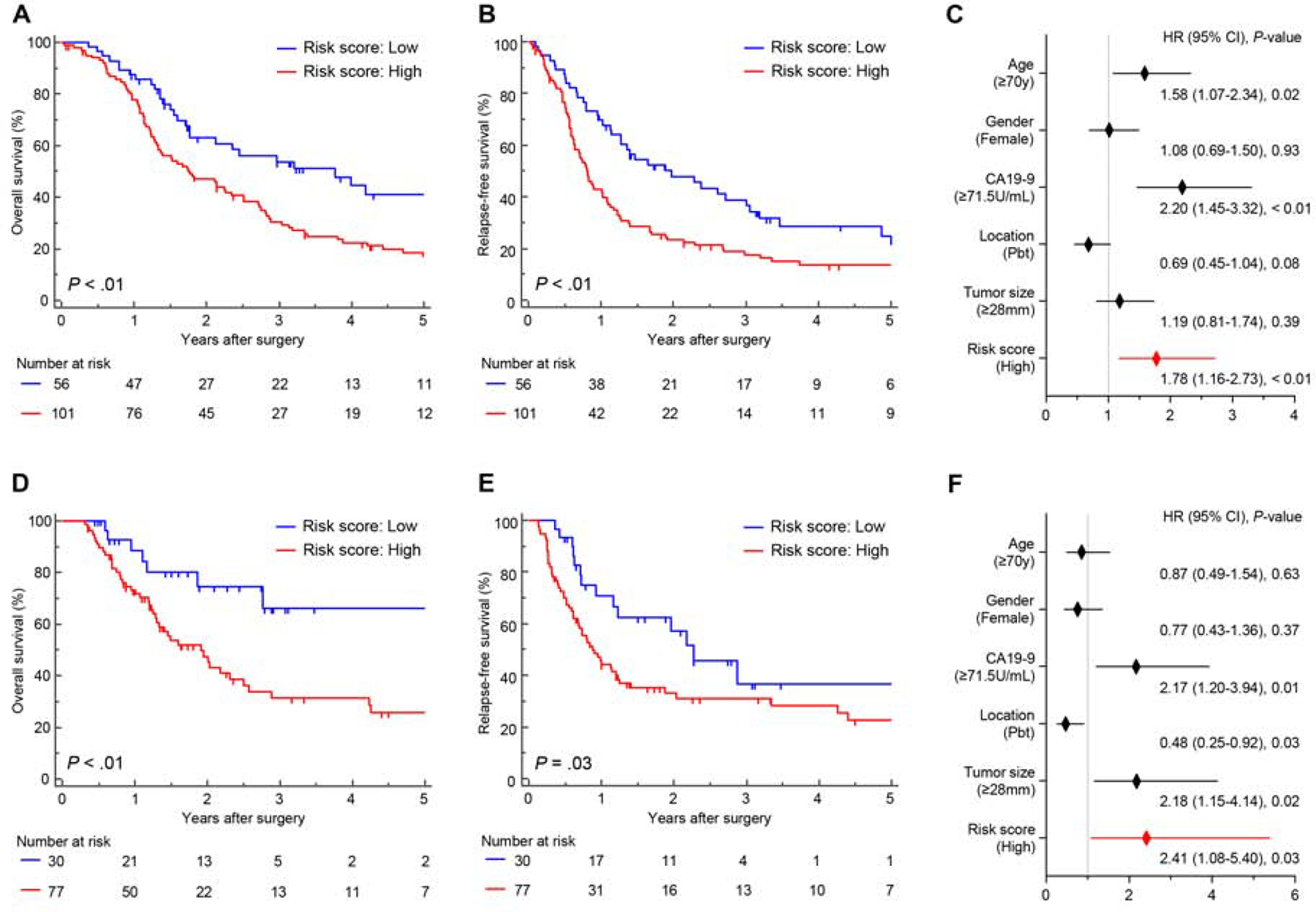
Prognostic potential of the miRNA signature for PDAC patients in the clinical cohorts. (A, B) A comparison of (A) OS and (B) RFS between high and low-risk group estimated by 6-miRNA signature model in the training cohort. (C) Forest plot with hazard ratio of clinicopathological variables and signature risk score status in multivariate cox proportional analysis of OS in training cohort. (D, E) A comparison of (D) OS and (E) RFS between high and low-risk group estimated by 6-miRNA signature model in the training cohort. (F) Forest plot with hazard ratio of clinicopathological variables and signature risk score status in multivariate cox proportional analysis of OS in validation cohort.
Higher-order validation of the miRNA signature in EUS-FNA biopsy specimens from PDAC patients
While the validation of biomarkers using surgically resected tissue specimens was necessary for constructing this LNM signature, we believe that validation of these biomarkers in pre-treatment biopsy specimens would pave a path for an easier translation of our miRNA signature in clinical settings. The underlying rationale is that if such a validation in biopsy specimens is feasible, the physicians are able to make a more informed decision for offering NAT to high-risk patients categorized that are deemed otherwise ‘resectable’ and improve their survival. Based on this hypothesis, we collected matched EUS-FNA biopsy specimens from a subset of 47 PDAC patients from the training and validation cohorts (Table 1). All 47 patients were classified as resectable based on NCCN resectability status. We evaluated the diagnostic accuracy of our miRNA biomarkers in these biopsy specimens and were enthused to observe that we were successfully able to confirm it yielded a satisfactory AUC value of 0.78 (95% CI = 0.63–0.89) for distinguishing LNM (Fig. 4A and B). Consistent with the training and validation cohorts, the performance of this model was improved by combination with CA19–9 (AUC = 0.81, 95% CI = 0.67–0.91) and demonstrated superior diagnostic potential than other clinicopathological factors (Fig. 4C). Furthermore, multivariate logistic regression analysis for LNM detection revealed that our 6-miRNA signature was an exclusive significant detective marker in FNA biopsy cohort (OR = 17.05, 95% CI = 2.43–119.57, Fig. 4D).
Figure 4.
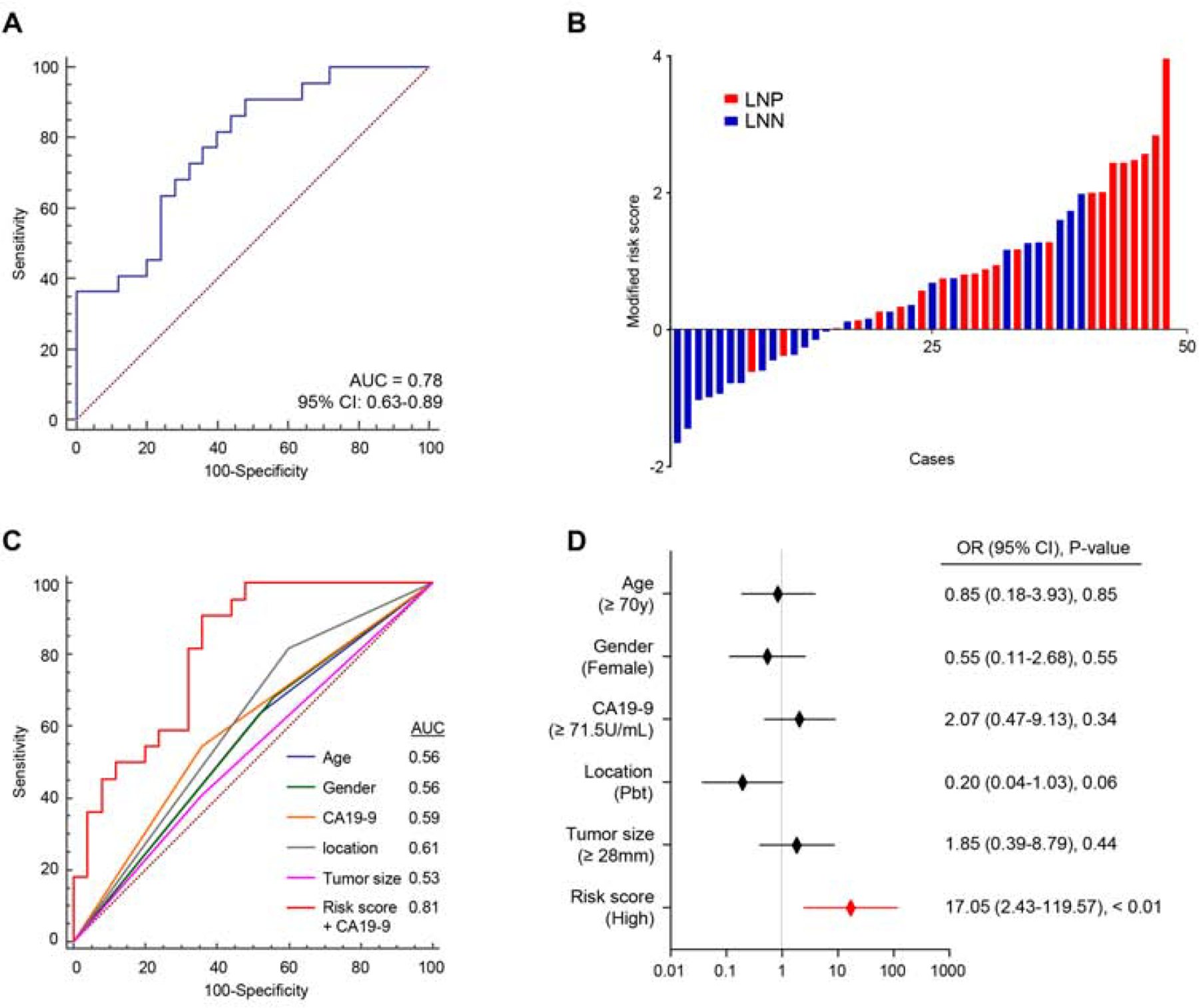
Higher-order validation of the miRNA signature in EUS-FNA biopsy specimens from PDAC patients.
(A) A ROC curve of the 6-miRNA signature in EUS-FNA biopsy cohort (LNP = 22, LNN = 25, AUC = 0.78). (B) Risk score distribution plot in EUS-FNA biopsy cohort. (C) The ROC curves of each clinicopathological factors and the risk model constructed with 6-miRNA signature and CA19–9 (AUC = 0.81). (D) Forest plot with odds ratio of clinicopathological variables and signature risk score status in multivariate logistic regression analysis of LNM in additional validation cohort. LNN: lymph node metastasis negative, LNP: lymph node metastasis positive.
Performance validation of the miRNA signature for predicting residual nodal involvement following neoadjuvant therapy in EUS-FNA biopsy specimens
Currently, multidisciplinary treatment strategies including NAT for resectable and borderline-resectable PDAC patients are actively being explored and becoming more common, especially in western countries. Moreover, pathological nodal involvement status which is modified by the effect of NAT (ypN), the residual LNM status following NAT, is often considered as one of the most important prognostic factors in PDAC patients undergoing NAT following surgery4, 10, 22–26. We hypothesized that our miRNA signature might also be able to identify ypN status before any treatment, which could potentially inform physicians for a more appropriate treatment selection based upon their pre-operative LNM status. Accordingly, for an easier translation of our miRNA signature in clinical settings, we enrolled a cohort of 62 patients who underwent NAT followed by surgery (9 ypN positive and 53 ypN negative, Table S2), from whom pre-treatment EUS-FNA specimens were available. Importantly, we applied the same statistical risk-model to this pre-treatment EUS-FNA cohort, which once again successfully confirmed the robustness of our risk-stratification in identifying LNM-positive patients with an excellent AUC value of 0.78 (95% CI 0.65–0.87, Fig. 5A and B). When we assessed the distribution of risk scores and ypN status, we observed that the patients with ypN positive had significantly higher risk scores than those who were ypN negative (P < 0.01, Mann Whitney test, Fig. 5C). Moreover, in multivariate analysis of logistic regression model, our miRNA signature emerged as an independent feature for ypN prediction before treatment (OR = 18.54; 95% CI = 2.45–140.33; P < 0.01, Fig. 5D).
Figure 5.
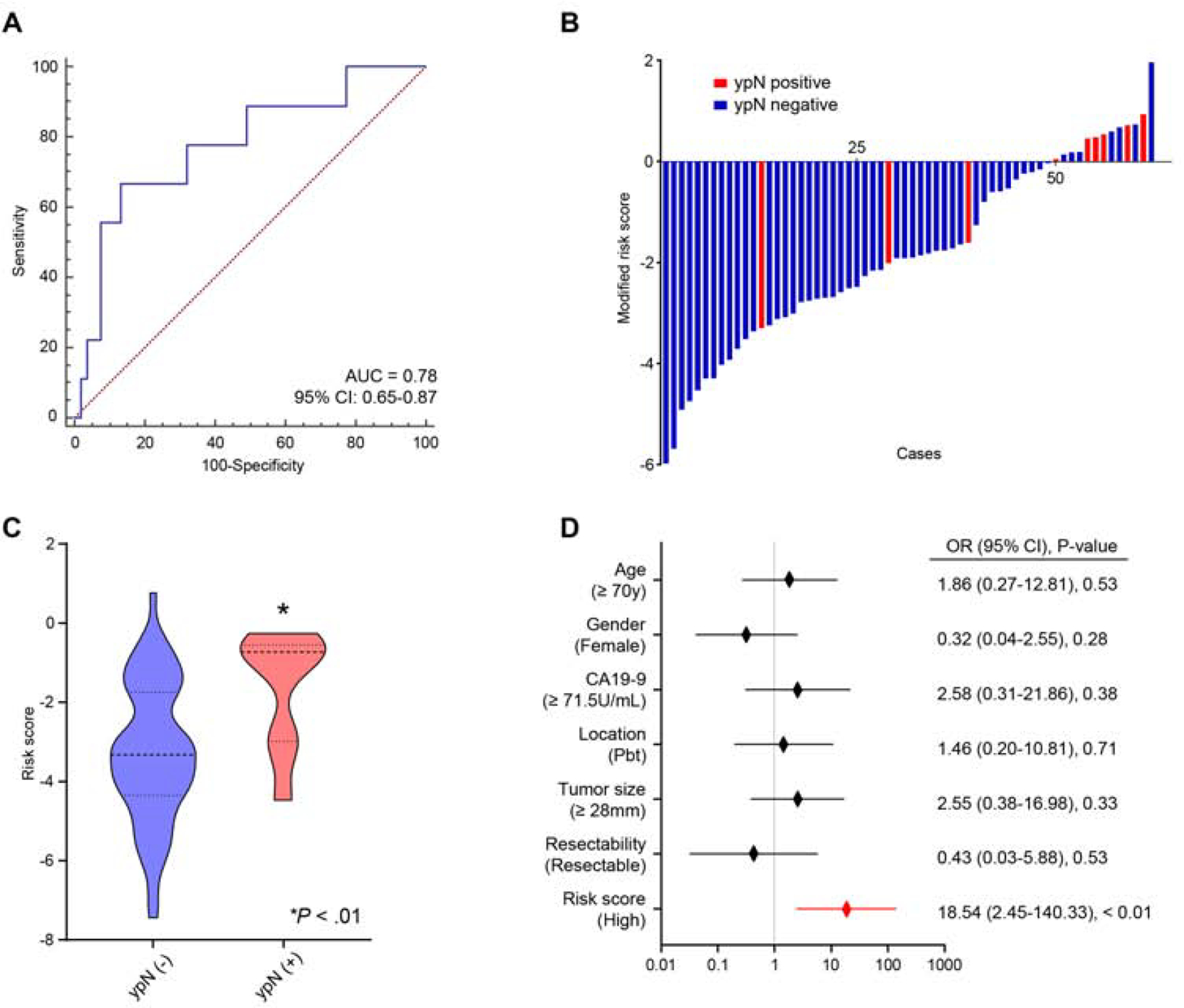
Additional validation of performance of the miRNA signature for predicting residual nodal involvement following neoadjuvant therapy in EUS-FNA biopsy specimens.
(A) A ROC curve of the 6-miRNA signature in additional validation cohort (pre-NAT EUS-FNA biopsies; ypN positive = 9, ypN negative = 53, AUC = 0.78). (B) Risk score distribution plots in an additional validation cohort. (C) The distribution of risk scores according to ypN status (P < 0.01, Mann Whitney test. (D) Forest plots with odds ratio for clinicopathological variables and risk scores in multivariate logistic regression analysis of ypN status in an additional validation cohort.
DISCUSSION
As cancer treatment has entered a new era of precision-medicine, development of individualized treatment strategies is essential for cancer patients. In the context of PDAC patients, presence of LNM is considered as high-risk feature; however, its diagnosis presets a clinical challenge. Our study was a step in this direction, wherein we undertook a systemic and comprehensive biomarker discovery and validation approach and successfully identified a novel miRNA signature that robustly identifies LNM in PDAC patients. These findings were validated in resected tissue specimens from two independent clinical cohorts. Furthermore, we were able to confirm the diagnostic potential of this signature even in pre-treatment EUS-FNA biopsy specimens, which was comparable to the performance of these biomarkers in surgically resected specimens; highlighting the potential significance of these findings for their clinical translation for improved risk-assessment and survival in PDAC patients. More interestingly, our miRNA signature was a robust predictor of the ypN status, which is considered as an important risk factor in patients who undergo NAT followed by surgery. These results highlight the potential clinical significance of our novel miRNA signature for identification of LNM in PDAC patients.
Several previous reports have favored the importance of multidisciplinary treatment including NAT in PDAC patients4, 10–13, 15, 16, 52. The NCCN guidelines indicate that NAT for resectable PDAC should be particularly considered in patients with high-risk features, with LNM being one such critical risk factor. On the other hand, in clinical settings, NAT has been actively explored and is becoming a common treatment option regardless of resectability status in PDAC patients. These findings highlight the need to develop robust biomarkers for LNM prior to any treatments, which offer superior risk stratification vis-à-vis other clinicopathological factors in PDAC patients. Our ability to successfully validate our miRNA signature in pre-treatment biopsy specimens underscores its clinical significance for improved treatment strategies in PDAC patients, especially the ones with LNM, and often worst survival outcomes. Several previous studies have similarly highlighted the clinical use of EUS-FNA biopsies for diagnostic purposes, as well as for drug response determination in PDAC patients; however, none of the previous studies have directly used these for diagnosing LNM and ypN status which can have profound impact in the selection of treatment strategies52, 53, 64, 65. Our findings for comparable performance of our biomarker signature in resected tissues and FNA biopsies in line with previous evidence52, 65, and highlights the clinical significance of such pre-treatment specimens for personalized treatment of cancer patients.
The Cancer Genome Atlas Research Network performed miRNA clustering on the TCGA dataset from high-purity 76 PDAC cases. However, the scope of this study was very limited and did not include enough LNM cases. Nonetheless, among the 31 miRNAs reported in the consensus clustering, one of our identified miRNAs, miR-365a-5p, was reported to be downregulated in clusters 1 and 3 and upregulated in cluster-266. Subsequently, we searched additional studies that have reported the clinical significance of our 6 candidate miRNAs. As illustrated in Table S3, we observed that high-expression of miR-155–5p and low-expression of miR-92b-3p associated with LNM in PDAC and gastric cancer; which are consistent with our study67, 68. In addition, we constructed a miRNA:mRNA regulatory network for the 6 miRNAs and identified 176 gene targets that provide support to their mechanistic involvement in gene regulatory pathways (Table S4). Subsequent pathway analysis of the validated downstream gene targets revealed significant biologically meaningful pathways associated with cancer cell biology (Table S5), providing an evidence for their involvement in key cancer-signaling pathways.
We would like to acknowledge a few potential limitations to our present study. First, the study was a retrospective design and we analyzed our miRNA signature in a moderately-sized clinical cohort; and future, prospective studies using larger patient cohorts are required before consideration of these biomarkers in clinical settings. Second, molecular profiles from PDAC tissue specimens potentially possess uneven tumor cellularity. In order to minimalize this bias, we evaluated the performance of our miRNA signature using independent multiple public datasets and clinical cohorts, surgically resected as well as EUS-FNA-biopsy specimens. Lastly, we did not have access to matched blood plasma specimens from the patient cohorts available to us; which, otherwise would be a most ideal scenario for exploring the liquid-biopsy approach for our discovered biomarkers. Nonetheless, our present study provides compelling evidence for the clinical significance of our miRNA signature for detecting LNM in PDAC patients, is potentially an important major step towards availability of robust molecular biomarkers for the risk-assessment and management of a lethal malignancy such as PDAC.
In conclusion, using a genomewide miRNA expression profiling effort, we have identified and developed a novel miRNA signature that was successfully validated in resected tissue specimens and EUS-FNA biopsies for the identification of LNM in PDAC patients. Pending validation in future prospective studies, our findings highlight the potential clinical impact of this signature in a more appropriate patient selection and institution of improved individualized treatment strategies for PDAC patients.
Supplementary Material
Supplementary Table S1: Clinicopathological characteristics in public datasets N/A, Not available; UICC, International Union Against Cancer
Supplementary Table S2: Clinicopathological characteristics of Pre-Neoadjuvant therapy EUS-FNA biopsy cohort EUS-FNA, Endoscopic ultrasound - guided fine needle aspiration
UICC, International Union Against Cancer
Supplementary Table S3: Recent studies reporting the clinical significance of 6 candidate miRNAs as cancer biomarkers
Supplementary Table S4: miRNA-mRNA network analysis of 6 candidate miRNAs
Supplementary Table S5: Pathway enrichment analysis
WHAT YOU NEED TO KNOW:
Background and Context:
Pancreatic ductal adenocarcinomas (PDACs) frequently metastasize to the lymph nodes, but it is a challenge to identify patients at highest risk for development of lymph node metastases.
New Findings:
Using data and tumor samples from 3 independent cohorts, the authors identified a microRNA expression pattern that identifies patients at risk for PDAC metastasis to lymph nodes. The signature has similar levels of accuracy in analysis of resected tumor specimens and EUS-FNA biopsies.
Limitations:
This was a retrospective study of data and samples from 3 cohorts. Larger, prospective studies are needed to test the prognostic ability of this miRNA expression pattern.
Impact:
This model might be used to select treatment and management strategies for patients with PDAC. It might also be studied to identify therapeutic targets for pancreatic cancer.
ACKNOWLEDGEMENTS
We thank Raju Kandimalla, Tadanobu Shimura, Tatsuhiko Kakisaka, Takeo Toshima, Goretti Hernandez, Priyanka Sharma, and Souvik Ghatak for discussing the experiments and analysis. We thank Naoya Ikeda, Kenji Nakagawa, Minako Nagai and Tadataka Takagi for collecting clinical samples and information.
Funding: The present work was supported by the CA72851, CA187956, CA202797 and CA214254 grants from the National Cancer Institute, National Institute of Health; and institutional grants from the Sammons Cancer Center and Baylor Foundation, as well as funds from the Baylor Scott & White Research Institute, Dallas, TX, USA. In addition, this work was also supported by a pilot research award from the City of Hope Ludwig Cancer Research-Hilton Foundation Partnership award.
Abbreviations:
- AUC
Area under the curve
- CT
Computed tomography
- EUS-FNA
Endoscopic ultrasound-guided fine needle aspiration
- FFPE
Formalin-fixed paraffin-embedded
- LNs
lymph nodes
- LNM
Lymph node metastasis
- LNN
Lymph node metastasis-negative
- LNP
Lymph node metastasis-positive
- miRNA
MicroRNA
- MRI
Magnetic resonance imaging
- NAT
Neoadjuvant therapy
- NCCN
The National Comprehensive Cancer Network
- OS
Overall survival
- PDAC
Pancreatic ductal adenocarcinoma
- PET
18F-fluorodeoxyglucose positron emission tomography
- RFS
Relapse-free survival
- ROC
Receiver operator characteristic
- RT-qPCR
Real-time quantitative reverse transcription polymerase chain reaction
- UICC
The International Union Against Cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None of the authors has any potential conflicts to disclose.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Groot VP, Gemenetzis G, Blair AB, et al. Defining and Predicting Early Recurrence in 957 Patients With Resected Pancreatic Ductal Adenocarcinoma. Ann Surg 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi H, Ohigashi H, Ishikawa O, et al. Perineural invasion and lymph node involvement as indicators of surgical outcome and pattern of recurrence in the setting of preoperative gemcitabine-based chemoradiation therapy for resectable pancreatic cancer. Ann Surg 2012;255:95–102. [DOI] [PubMed] [Google Scholar]
- 5.Masuda T, Dann AM, Elliott IA, et al. A Comprehensive Assessment of Accurate Lymph Node Staging and Preoperative Detection in Resected Pancreatic Cancer. J Gastrointest Surg 2018;22:295–302. [DOI] [PubMed] [Google Scholar]
- 6.Khorana AA, Mangu PB, Berlin J, et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34:2541–56. [DOI] [PubMed] [Google Scholar]
- 7.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. The Lancet 2017;389:1011–1024. [DOI] [PubMed] [Google Scholar]
- 8.Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). The Lancet 2016;388:248–257. [DOI] [PubMed] [Google Scholar]
- 9.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473–81. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi H, Akita H, Tomokuni A, et al. Preoperative Gemcitabine-based Chemoradiation Therapy for Borderline Resectable Pancreatic Cancer: Impact of Venous and Arterial Involvement Status on Surgical Outcome and Pattern of Recurrence. Ann Surg 2016;264:1091–1097. [DOI] [PubMed] [Google Scholar]
- 11.Roland CL, Yang AD, Katz MH, et al. Neoadjuvant therapy is associated with a reduced lymph node ratio in patients with potentially resectable pancreatic cancer. Ann Surg Oncol 2015;22:1168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conroy T, Bachet J-B, Ayav A, et al. Current standards and new innovative approaches for treatment of pancreatic cancer. European Journal of Cancer 2016;57:10–22. [DOI] [PubMed] [Google Scholar]
- 13.Li D, O’Reilly EM. Adjuvant and Neoadjuvant Therapy for Pancreatic Cancer. Surg Oncol Clin N Am 2016;25:311–26. [DOI] [PubMed] [Google Scholar]
- 14.Lowder CY, Metkus J, Epstein J, et al. Clinical Implications of Extensive Lymph Node Metastases for Resected Pancreatic Cancer. Ann Surg Oncol 2018;25:4004–4011. [DOI] [PubMed] [Google Scholar]
- 15.Hackert T Surgery for Pancreatic Cancer after neoadjuvant treatment. Ann Gastroenterol Surg 2018;2:413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang JY, Han Y, Lee H, et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann Surg 2018;268:215–222. [DOI] [PubMed] [Google Scholar]
- 17.Tol JA, Gouma DJ, Bassi C, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014;156:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakami Y, Uemura K, Sudo T, et al. Prognostic impact of para-aortic lymph node metastasis in pancreatic ductal adenocarcinoma. World J Surg 2010;34:1900–7. [DOI] [PubMed] [Google Scholar]
- 19.Basturk O, Saka B, Balci S, et al. Substaging of Lymph Node Status in Resected Pancreatic Ductal Adenocarcinoma Has Strong Prognostic Correlations: Proposal for a Revised N Classification for TNM Staging. Ann Surg Oncol 2015;22 Suppl 3:S1187–95. [DOI] [PubMed] [Google Scholar]
- 20.Tao L, Zhang L, Peng Y, et al. Preoperative neutrophil-to-lymphocyte ratio and tumor-related factors to predict lymph node metastasis in patients with pancreatic ductal adenocarcinoma (PDAC). Oncotarget 2016;7:74314–74324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morales-Oyarvide V, Rubinson DA, Dunne RF, et al. Lymph node metastases in resected pancreatic ductal adenocarcinoma: predictors of disease recurrence and survival. Br J Cancer 2017;117:1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugimoto M, Takahashi N, Farnell MB, et al. Survival benefit of neoadjuvant therapy in patients with non-metastatic pancreatic ductal adenocarcinoma: A propensity matching and intention-to-treat analysis. J Surg Oncol 2019;120:976–984. [DOI] [PubMed] [Google Scholar]
- 23.Klaiber U, Schnaidt ES, Hinz U, et al. Prognostic Factors of Survival After Neoadjuvant Treatment and Resection for Initially Unresectable Pancreatic Cancer. Annals of Surgery 2019:1. [DOI] [PubMed] [Google Scholar]
- 24.Aoki S, Motoi F, Murakami Y, et al. Decreased serum carbohydrate antigen 19–9 levels after neoadjuvant therapy predict a better prognosis for patients with pancreatic adenocarcinoma: a multicenter case-control study of 240 patients. BMC Cancer 2019;19:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurahara H, Shinchi H, Ohtsuka T, et al. Significance of neoadjuvant therapy for borderline resectable pancreatic cancer: a multicenter retrospective study. Langenbecks Arch Surg 2019;404:167–174. [DOI] [PubMed] [Google Scholar]
- 26.Nagakawa Y, Sahara Y, Hosokawa Y, et al. Clinical Impact of Neoadjuvant Chemotherapy and Chemoradiotherapy in Borderline Resectable Pancreatic Cancer: Analysis of 884 Patients at Facilities Specializing in Pancreatic Surgery. Ann Surg Oncol 2019;26:16291636. [DOI] [PubMed] [Google Scholar]
- 27.Prenzel KL, Holscher AH, Vallbohmer D, et al. Lymph node size and metastatic infiltration in adenocarcinoma of the pancreatic head. Eur J Surg Oncol 2010;36:993–6. [DOI] [PubMed] [Google Scholar]
- 28.Hidalgo M Pancreatic cancer. N Engl J Med 2010;362:1605–17. [DOI] [PubMed] [Google Scholar]
- 29.Tseng DS, van Santvoort HC, Fegrachi S, et al. Diagnostic accuracy of CT in assessing extra-regional lymphadenopathy in pancreatic and peri-ampullary cancer: a systematic review and meta-analysis. Surg Oncol 2014;23:229–35. [DOI] [PubMed] [Google Scholar]
- 30.Nawaz H, Fan CY, Kloke J, et al. Performance characteristics of endoscopic ultrasound in the staging of pancreatic cancer: a meta-analysis. JOP 2013;14:484–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang SC, Parekh JR, Porembka MR, et al. A Pilot Study Evaluating Serum MMP7 as a Preoperative Prognostic Marker for Pancreatic Ductal Adenocarcinoma Patients. J Gastrointest Surg 2016;20:899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szafranska AE, Doleshal M, Edmunds HS, et al. Analysis of microRNAs in pancreatic fine-needle aspirates can classify benign and malignant tissues. Clin Chem 2008;54:1716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daoud AZ, Mulholland EJ, Cole G, et al. MicroRNAs in Pancreatic Cancer: biomarkers, prognostic, and therapeutic modulators. BMC Cancer 2019;19:1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung G, Hernandez-Illan E, Moreira L, et al. Epigenetics of colorectal cancer: biomarker and therapeutic potential. Nat Rev Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kandimalla R, Gao F, Matsuyama T, et al. Genome-wide Discovery and Identification of a Novel miRNA Signature for Recurrence Prediction in Stage II and III Colorectal Cancer. Clin Cancer Res 2018;24:3867–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993;75:855–62. [DOI] [PubMed] [Google Scholar]
- 37.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993;75:843–54. [DOI] [PubMed] [Google Scholar]
- 38.Bloomston M, Frankel WL, Petrocca F, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA 2007;297:1901–8. [DOI] [PubMed] [Google Scholar]
- 39.Schultz NA, Dehlendorff C, Jensen BV, et al. MicroRNA Biomarkers in Whole Blood for Detection of Pancreatic Cancer. Jama 2014;311:392. [DOI] [PubMed] [Google Scholar]
- 40.Shimura T, Toden S, Kandimalla R, et al. Genomewide Expression Profiling Identifies a Novel miRNA-Based Signature for the Detection of Peritoneal Metastasis in Patients With Gastric Cancer. Ann Surg 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ozawa T, Kandimalla R, Gao F, et al. A MicroRNA Signature Associated With Metastasis of T1 Colorectal Cancers to Lymph Nodes. Gastroenterology 2018;154:844–848 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karmakar S, Kaushik G, Nimmakayala R, et al. MicroRNA regulation of K-Ras in pancreatic cancer and opportunities for therapeutic intervention. Semin Cancer Biol 2019;54:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mikamori M, Yamada D, Eguchi H, et al. MicroRNA-155 Controls Exosome Synthesis and Promotes Gemcitabine Resistance in Pancreatic Ductal Adenocarcinoma. Sci Rep 2017;7:42339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Preis M, Gardner TB, Gordon SR, et al. MicroRNA-10b expression correlates with response to neoadjuvant therapy and survival in pancreatic ductal adenocarcinoma. Clin Cancer Res 2011;17:5812–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schultz NA, Andersen KK, Roslind A, et al. Prognostic microRNAs in cancer tissue from patients operated for pancreatic cancer--five microRNAs in a prognostic index. World J Surg 2012;36:2699–707. [DOI] [PubMed] [Google Scholar]
- 46.Yu J, Ohuchida K, Mizumoto K, et al. MicroRNA, hsa-miR-200c, is an independent prognostic factor in pancreatic cancer and its upregulation inhibits pancreatic cancer invasion but increases cell proliferation. Mol Cancer 2010;9:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asangani IA, Rasheed SA, Nikolova DA, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 2008;27:2128–36. [DOI] [PubMed] [Google Scholar]
- 48.Giovannetti E, Funel N, Peters GJ, et al. MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res 2010;70:4528–38. [DOI] [PubMed] [Google Scholar]
- 49.Abue M, Yokoyama M, Shibuya R, et al. Circulating miR-483–3p and miR-21 is highly expressed in plasma of pancreatic cancer. Int J Oncol 2015;46:539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bauer AS, Keller A, Costello E, et al. Diagnosis of pancreatic ductal adenocarcinoma and chronic pancreatitis by measurement of microRNA abundance in blood and tissue. PLoS One 2012;7:e34151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sonohara F, Gao F, Iwata N, et al. Genome-wide Discovery of a Novel Gene-expression Signature for the Identification of Lymph Node Metastasis in Esophageal Squamous Cell Carcinoma. Annals of Surgery 2017:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dreyer SB, Pinese M, Jamieson NB, et al. Precision Oncology in Surgery: Patient Selection for Operable Pancreatic Cancer. Ann Surg 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berry W, Algar E, Kumar B, et al. Endoscopic ultrasound-guided fine-needle aspirate-derived preclinical pancreatic cancer models reveal panitumumab sensitivity in KRAS wild-type tumors. Int J Cancer 2017;140:2331–2343. [DOI] [PubMed] [Google Scholar]
- 54.Satoi S, Murakami Y, Motoi F, et al. Reappraisal of peritoneal washing cytology in 984 patients with pancreatic ductal adenocarcinoma who underwent margin-negative resection. J Gastrointest Surg 2015;19:6–14; discussion 14. [DOI] [PubMed] [Google Scholar]
- 55.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 56.Kalhori MR, Arefian E, Fallah Atanaki F, et al. miR-548x and miR-4698 controlled cell proliferation by affecting the PI3K/AKT signaling pathway in Glioblastoma cell lines. Sci Rep 2020;10:1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peng W, Li J, Chen R, et al. Upregulated METTL3 promotes metastasis of colorectal Cancer via miR-1246/SPRED2/MAPK signaling pathway. J Exp Clin Cancer Res 2019;38:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dweep H, Sticht C, Pandey P, et al. miRWalk--database: prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform 2011;44:839–47. [DOI] [PubMed] [Google Scholar]
- 59.Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 2000;25:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogata H, Goto S, Sato K, et al. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 1999;27:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peto RPJ. Asymptotically efficient rank invariant test procedures. J R Stat Soc Ser A Stat Soc 1972;135:185–207. [Google Scholar]
- 62.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343–6. [DOI] [PubMed] [Google Scholar]
- 63.Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- 64.Bournet B, Pointreau A, Souque A, et al. Gene expression signature of advanced pancreatic ductal adenocarcinoma using low density array on endoscopic ultrasound-guided fine needle aspiration samples. Pancreatology 2012;12:27–34. [DOI] [PubMed] [Google Scholar]
- 65.Frampton AE, Krell J, Prado MM, et al. Prospective validation of microRNA signatures for detecting pancreatic malignant transformation in endoscopic-ultrasound guided fine-needle aspiration biopsies. Oncotarget 2016;7:28556–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cancer Genome Atlas Research Network. Electronic address aadhe, Cancer Genome Atlas Research N. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2017;32:185–203 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim BH, Hong SW, Kim A, et al. Prognostic implications for high expression of oncogenic microRNAs in advanced gastric carcinoma. J Surg Oncol 2013;107:505–10. [DOI] [PubMed] [Google Scholar]
- 68.Long M, Zhan M, Xu S, et al. miR-92b-3p acts as a tumor suppressor by targeting Gabra3 in pancreatic cancer. Mol Cancer 2017;16:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Clinicopathological characteristics in public datasets N/A, Not available; UICC, International Union Against Cancer
Supplementary Table S2: Clinicopathological characteristics of Pre-Neoadjuvant therapy EUS-FNA biopsy cohort EUS-FNA, Endoscopic ultrasound - guided fine needle aspiration
UICC, International Union Against Cancer
Supplementary Table S3: Recent studies reporting the clinical significance of 6 candidate miRNAs as cancer biomarkers
Supplementary Table S4: miRNA-mRNA network analysis of 6 candidate miRNAs
Supplementary Table S5: Pathway enrichment analysis


