Abstract
Systemic inflammation resulting from the release of pro-inflammatory cytokines and the chronic activation of the innate immune system remains a major cause of morbidity and mortality in the United States. After having demonstrated that Fyn, a Src family kinase, regulates microglial neuroinflammatory responses in cell culture and animal models of Parkinson's disease, we investigate here its role in modulating systemic inflammation using an endotoxic mouse model. Fyn knockout (KO) and their wild-type (WT) littermate mice were injected once intraperitoneally with either saline or 5 mg/kg lipopolysaccharide (LPS) and were killed 48 h later. LPS-induced mortality, endotoxic symptoms and hypothermia were significantly attenuated in Fyn KO, but not WT, mice. LPS reduced survival in Fyn WT mice to 49% compared to 84% in Fyn KO mice. Fyn KO mice were also protected from LPS-induced deficits in horizontal and vertical locomotor activities, total distance traveled and stereotypic movements. Surface body temperatures recorded at 24 h and 48 h post-LPS dropped significantly in Fyn WT, but not in KO, mice. Importantly, endotoxemia-associated changes to levels of the serum pro-inflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6), splenocyte apoptosis and inducible nitric oxide synthase (iNOS) production in hepatocytes were also significantly attenuated in Fyn KO mice. Likewise, pharmacologically inhibiting Fyn with 10 mg/kg dasatinib (oral) significantly attenuated LPS-induced increases in plasma TNF-α and IL-6 protein levels and hepatic pro-IL-1β messenger ribonucleic acids (mRNAs). Collectively, these results indicate that genetic knockdown or pharmacological inhibition of Fyn dampens systemic inflammation, demonstrating for the first time that Fyn kinase plays a critical role in mediating the endotoxic inflammatory response.
Keywords: Endotoxemia, systemic inflammation, LPS, cytokines, apoptosis, sepsis, locomotor deficits, Parkinson’s disease
1. Introduction
Systemic inflammatory responses triggered by the release of pro-inflammatory cytokines and the chronic activation of the innate immune system is an important contributor to many human diseases, yet the key mediators linking systemic inflammation to specific pathophysiologic processes have yet to be elucidated. One classic animal model for investigating systemic inflammation is through systemic administration of lipopolysaccharide (LPS), a Gram-negative bacterial toxin. LPS activates the Toll-like receptor 4 (TLR4)-signaling cascade in a range of immune cells, leading to the production of reactive oxygen species (ROS), reactive nitrite species (RNS), and further exaggeration of inflammatory responses by increased production of pro-inflammatory cytokines and chemokines (Deutschman and Tracey, 2014). ROS damages host cell organelles, and the hypersecretion of cytokines recruits more inflammatory cells, thereby amplifying the immune response. In response to pro-inflammatory cytokines and LPS, inducible nitric oxide synthase (iNOS) expression is induced resulting in high levels of nitric oxide (NO) production. Although NO is produced primarily to combat infection, excessive NO production damages host cells, resulting in endothelial and smooth muscle dysfunction and dilatation, a dramatic drop in blood pressure and hyporeactivity to catecholamines. Despite the above biochemical and pathological changes attributed to systemic inflammation, key cell-signaling mediators driving its pro-inflammatory response are not well characterized.
Recently, we demonstrated that Fyn kinase, a key member of Src (sarcoma) family kinases (SFK), phosphorylates protein kinase C delta (PKCδ) at tyrosine residue 311 (Tyr-311), which serves as a priming post-translational modification that mediates its pro-apoptotic function in brain cells during neurotoxic stress (Saminathan et al., 2011). While studying the role of Fyn in brain inflammation in an LPS-induced neuroinflammation model, herein we report our serendipitous findings that Fyn knockout (KO) significantly reduces the systemic inflammatory response.
2. Materials and methods.
2.1. Chemicals.
LPS (strain 0111:B4) was purchased from Sigma. Dasatinib (CAS 302962-49-8) was purchased from Santa Cruz Biotechnology (sc-358114).
2.2. Animals.
Heterozygous Fyn KO mice (Fyn (+/−) were a kind gift from Prof. Dorit Ron (University of California, San Francisco), and upon arrival, we established a successful breeding colony in our animal facility at Iowa State University. We generated Fyn (+/+) and Fyn (−/−) mice from breeding heterozygous Fyn (+/−) mice and then bred them as homozygous. Fyn (+/+) mice were used as wild-type controls (Fyn-WT). The Fyn KO mice (129-Fyntm1Sor/J, strain 129S7/SvEvBrd-Hprt<+>) are now available from The Jackson Laboratory under stock number 002271. Use of animals and all animal-related procedures in this study (Protocol ID 12-05-6042-M) were approved and supervised by the Institutional Animal Care and Use Committee at Iowa State University.
2.3. Animal treatment Paradigms
2.3.1. LPS administration:
Six- to 8-week-old Fyn +/+ (WT) male mice and Fyn −/− (KO) male weighing 25 to 30 g were housed under standard conditions: constant temperature (22 ± 1°C), relative humidity (30%), and a 12-h light cycle. Mice were allowed ad libitum access to food and water. A single intraperitoneal injection of LPS (5 mg/kg, i.p.) or equal volumes of normal saline were administered to WT and Fyn KO mice. Mice were subject to behavior tests at 24 hrs post-LPS injection. Animals were also monitored for body temperature and mortality. At the end of the 48 h treatment, animals were killed, tissues and serum were collected and subject to immunohistochemistry (IHC) and cytokine (Luminex) assays. The number of mice per group was 3-9 mice, and each experiment was repeated at least twice
2.3.2. LPS and Dasatinib treatment:
In a separate set of experiments, naïve C57BL6 mice were co-administered LPS (5 mg/kg, i.p. once) and the pharmacological Src kinase inhibitor dasatinib (10 mg/kg, oral gavage) administered at 0, 6, 12 and 18 h. Dasatinib was dissolved 1:18 DMSO: Myglyol. Saline and LPS treated animals received DMSO: Myglyol as vehicle controls. The rational for dose and route of Dasatinib administration was determined from published literature (Schubert et al., 2017; Duyvestyn et al., 2014; Wang M, et al., Mol Cancer Ther, 2017). Several Serum samples were collected at 1 h, 3h and 24 h and subject to cytokine (Luminex) assays. At the end of the 24 h treatment, animals were killed, tissues were collected and subject to immunohistochemistry (IHC), qRT-PCR and RNA scope. The number of mice per group was 3-9 mice, and each experiment was repeated at least twice.
2.4. Automated analysis of LPS-induced motor activity deficits.
Behavioral deficits induced by LPS were quantified using the VersaMax automated animal activity monitors (model RXYZCM-16; AccuScan Instruments Inc, Columbus, OH) as described in our publication (Langley et al., 2017). The activity monitor consisted of a clear Plexiglas chamber (40 x 40 x 30.5 cm) covered with a ventilated Plexiglas lid. Infrared monitoring sensors were located every 2.54 cm along the perimeter of the chamber (16 infrared beams along each side) and 2.54 cm above the floor. A second, identical array of sensors was located 8.0 cm above the floor. LPS-induced behavior deficits, expressed as changes in horizontal, vertical, and stereotypic (repetitive) locomotor movements, recorded as infrared beam breaks, were analyzed by a computer-controlled VersaMax analyzer (model CDA-8 AccuScan Instruments Inc). Data are expressed as mean ± SEM (n = 6-8) where the vehicle-treated group served as the control. Behavior observations were obtained during 10-min sessions 24 h after exposure to LPS.
2.5. Immunohistochemistry (IHC).
For both experiments involving LPS-administration to Fyn +/+ and Fyn −/− male and LPS/Dasatinib treatment to C57Bl6 mice, animals were anesthetized spleen and liver tissues were collected from mice were post-fixed in 4% paraformaldehyde (PFA) solution and embedded in paraffin.
2.5.1. Hematoxylin and eosin (H&E)–staining and spleenocyte apoptosis:
Paraffin-embedded spleen tissues were processed and sectioned at 5 μM for microscopic analysis. Apoptotic lymphocytes were quantified in a blinded manner by a board-certified veterinary pathologist at our institution reading hematoxylin and eosin (H&E)–stained sections by light microscopy. The criteria used to identify apoptotic lymphocytes was the presence of small or fragmented lymphocytes with hyperchromatic nuclei. The scoring system for evaluating the degree of spleen injury was based on the scale of splenocyte apoptosis: 0, no remarkable lesions; 1, earliest detectable regions of apoptosis of lymphocytes (<10% of lymphocytes) in one or several follicles; 2, up to 30% apoptosis of lymphocytes in all follicles; 3, up to 60% apoptosis of lymphocytes in all follicles; and 4, more than 60% apoptosis of lymphocytes in all follicles. Total scores were calculated for each animal.
2.5.2. iNOS 3,3′-diaminobenzidine (DAB) staining:
Before staining, 5μM liver sections were deparaffinized and rehydrated. For 3,3′-diaminobenzidine (DAB) staining, deparaffinized tissue sections were treated with 3% H2O2 for 30 min, and triple-rinsed with phosphate-buffered saline (PBS). Non-specific binding sites were blocked with 5% bovine serum albumin (BSA) in PBS. Tissue sections were incubated overnight at room temperature (RT) with primary antibodies against iNOS (rabbit polyclonal antibody from Santa Cruz Biotechnology, CA, 1:500). After rinsing in PBS, tissue sections were further incubated with biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA, 1:100) for 1 h and with the avidin/biotin system (Vector Laboratories) for 1 h before being visualized using DAB solution (0.05% DAB and 0.003% H2O2 in 0.1 M PBS). Sections were dried overnight, dehydrated and mounted using DPX mountant. DAB-stained sections were examined under a bright-field microscope (Olympus Optical, BX51, Tokyo, Japan).
2.5.3. iNOS and cleaved Caspase-3 immunofluorescence
Paraffin-embedded spleen and liver sections (5μM) were also subjected to cleaved caspase-3 (a marker of apoptosis) and iNOS immunostaining using antigen retrieval techniques using citrate buffer (10 mM sodium citrate, pH 8.5) at 90°C for 30 min. Sections were then washed with PBS and permeabilized with blocking buffer (2% BSA, 0.1% Triton X-100 and 0.05% Tween-20 in PBS) for 1 h at RT. Antibodies directed against cleaved caspase-3 (rabbit monoclonal, 1:500) and iNOS (rabbit polyclonal, 1:500) were incubated with the microscopic sections overnight at 4°C. After several washes with PBS, sections were incubated with Alexa fluor-555 conjugated secondary antibody (Thermo-Fisher Scientific) for 1 h at RT. Rabbit IgG (Cell Signaling Technology) was used as an isotypic control. Sections were then mounted on slides using the ProLong Gold antifade mounting medium (Molecular Probes) according to the manufacturer’s instructions. A SPOT digital camera attached to an inverted fluorescence microscope (Nikon TE2000-U) was used to capture photomicrographs of sections at 20X magnification. Quantification methods were performed as described (Langley et al., 2017).
2.6. Cytokine assays.
Multiplex analysis was performed using multi-analyte profiles (MAPs) based on Luminex xMAP (Luminex Corporation, Austin, TX) technology to detect multiple cytokine levels from single samples. Equal volumes of serum samples were thawed at RT, vortexed, and spun at 13,000 × g for 5 min to remove any precipitates/cell debris. An aliquot of each sample was introduced into one of the capture microsphere multiplexes of the antibodies for cytokines TNF-α, IL-10, IL-1β and IL-6. These mixtures of sample and capture microspheres were thoroughly mixed and incubated at RT for 1 h. Multiplexed cocktails of biotinylated reporter antibodies for each multiplex were then added robotically and were thoroughly mixed. The mixture was then incubated for an additional 1 h at RT. Multiplexes developed using streptavidin-phycoerythrin solution were thoroughly mixed into each multiplex and incubated further for 1 h at RT. The volume of each multiplexed reaction was reduced by vacuum filtration and then increased by diluting in matrix buffer for analysis, which was performed in a Luminex 100. For Dasatinib experiments described in Fig. 7B, we used the Bio-Plex Pro Mouse Cytokine Th17 Panel A 6-plex kit from Bio-Rad. The assay was performed with 25 μl of plasma sample as per protocol provided by the manufacturer and fluorescence value was read on a Bio-Plex reader (Bio-Rad). For each multiplex, serial dilutions of standards were included on each microtiter plate. Unknown values for each of the analytes localized in a specific multiplex were determined using curve fitting algorithms included in the data analysis package.
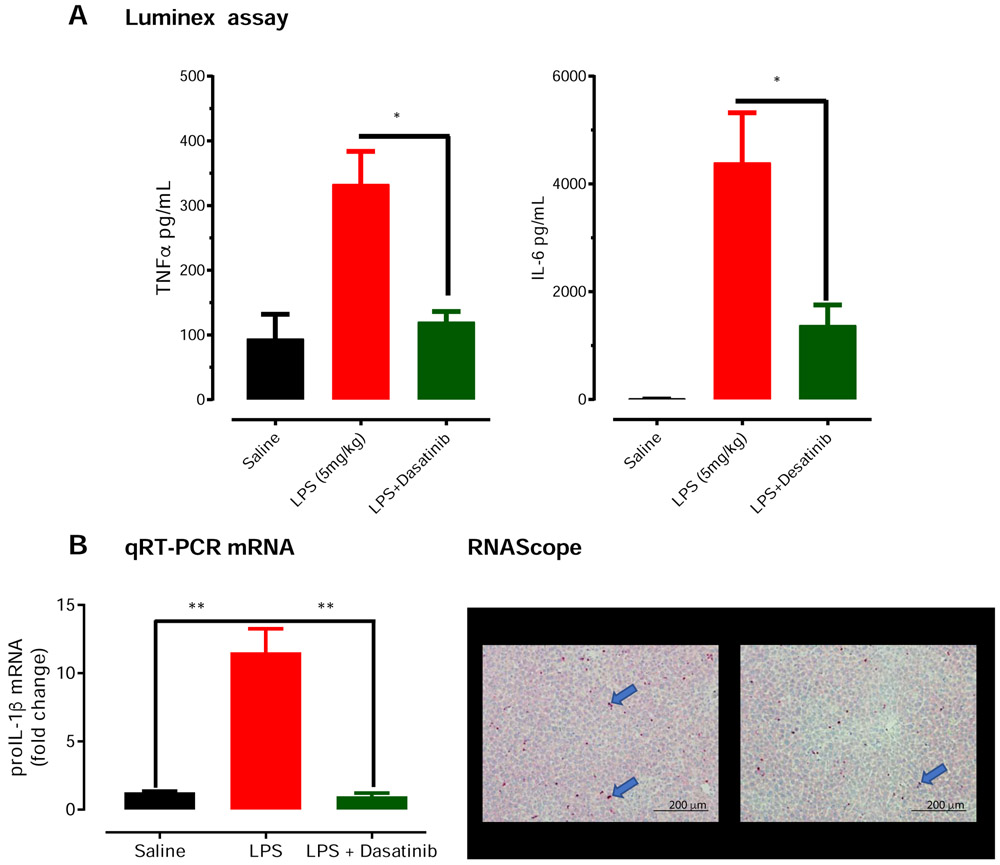
Fig 7. Co-treatment with dasatinib, a tyrosine kinase inhibitor, protects against LPS-induced pro-inflammatory cytokines.
Wild-type C57BL/6 mice were randomly separated into 3 groups (n =3). The control group received only saline, the LPS group received 1 dose of LPS at 5mg/kg via subcutaneous administration, and the third group received 1 dose of LPS and 4 doses of dasatinib (10 mg/kg) at the indicated time points. (A) Luminex assay revealed that dasatinib treatment reduced LPS-induced TNF-α and IL-6 proinflammatory cytokine induction in the plasma to near control levels at 1 hour post-LPS treatment. (B). Quantitative PCR and RNA scope. Our qRT-PCR revealed dasatinib significantly reduced transcripts levels of pro-IL-1β in liver tissue (B). Finally, representative images from RNAscope assay probing for pro-IL-1β transcripts (blue arrows) found reduced pro-IL-1β transcripts in liver sections of dasatinib-treated mice Significant differences between groups indicated by *, P ≤ 0.05. Scale bar: 200 μm.
2.7. Quantitative reverse transcription polymerase chain reaction (qRT-PCR).
RNA extraction and qRT-PCR were performed as described previously (Gordon et al., 2016, Neal et al., 2018). Briefly, dissected animal tissues were homogenized. TRIzol reagent was used to isolate total RNA from the tissues and the concentration was measured using NanoDrop. Affinity Script qPCR complementary deoxyribonucleic acid (cDNA) synthesis system (Agilent Technologies) was used to convert total RNA to cDNA. Real-time PCR was performed with the PowerUp SYBR Green master mix (Thermo Fisher). The following genes from QuantiTect Primer Assay (Qiagen) were used for qRT-PCR: pro-IL-1β. The housekeeping gene 18s ribosomal (r)RNA (Qiagen #PPM57735E) was used as the reference for all qRT-PCR experiments. The results are reported as fold change in gene expression, which was determined via the ΔΔCt method using the threshold cycle (Ct) value for the housekeeping gene and for the respective gene of interest in each sample.
2.8. RNAScope In Situ Hybridization
Five-micron sections of formalin-fixed paraffin-embedded liver were prepared by the Comparative Pathology Core Service facility at Iowa State University. In situ hybridization was conducted using the RNAscope technology (Advanced Cell Diagnostics – Newark NJ). RNAscope is a new technology that produces highly sensitive and specific results with little to no background staining (Liu et al., 2018; Yu et al., 2017). The probe for murine IL-1β was purchased from ACD. The stain was performed using a one-color/one-probe staining kit (2.5 HD – Red purchased from ACD) following the manufacture’s detailed instructions (user manuals 322452-USM and 322350-USM). Slides were evaluated on an Olympus BX53 microscopic. Images were captured using a DP73 digital camera and were processed using cell-Sense imaging software (Olympus).
2.9. Data analysis.
Using Prism 4.0 Software (GraphPad Prism, San Diego, CA), data were first analyzed using Student’s t-test or one-way ANOVA and then Tukey’s post-hoc test to compare all treatment groups. Kaplan-Meier survival curves for LPS-challenged WT and Fyn KO groups were compared via the log-rank test. Differences with P ≤ 0.05 were reported as significant.
3. Results
3.1. Fyn KO mice are protected from endotoxic shock-induced mortality
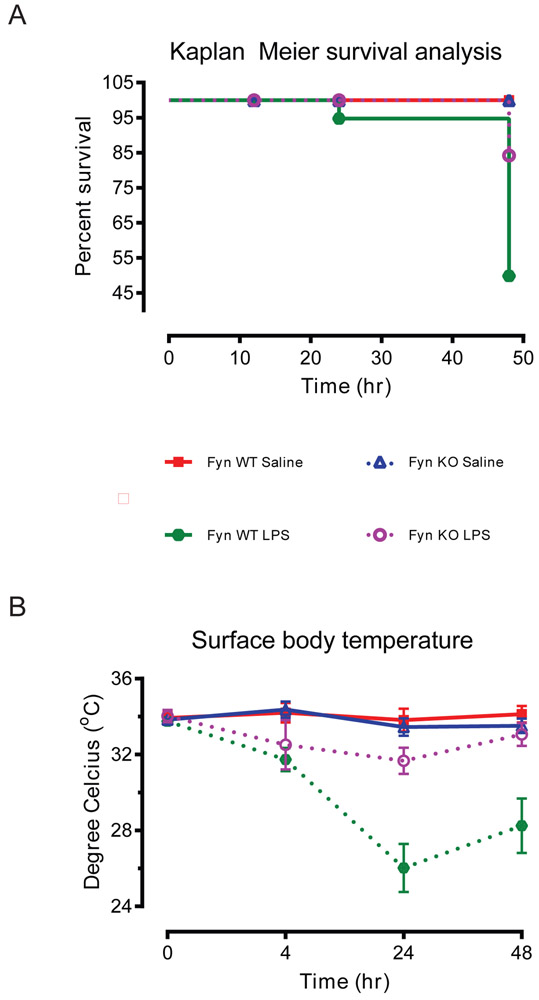
Most published in vivo studies using LPS to induce septic shock in mice used a dose of 1-25 mg/kg body weight (Warren, 2009), which is much higher than the amount needed to elicit severe shock in humans (Taveira da Silva et al., 1993). It also approximates the amount of LPS needed to achieve a 50% mortality rate. To investigate whether Fyn KO mice were resistant to LPS-induced endotoxic shock symptoms, we first examined the survival rates of mice systemically exposed to LPS. Fyn WT or KO mice intraperitoneally administered 5 mg/kg LPS were observed for 48 h before sacrificing for survival analysis. Systemic LPS administration reduced survival in Fyn WT mice to 49% while the survival rate in the Fyn KO animals was significantly greater at 84% (Fig. 1A). In addition to mortality, we also measured body temperature as an endpoint of systemic shock manifestation, based on evidence that hypothermia precedes LPS-induced shock (Krakauer et al., 2010). Injection of LPS in Fyn WT mice induced a significant drop in surface body temperatures recorded at 24 h and 48 h post-injection when compared to LPS-injected Fyn KO mice (Fig. 1B). Collectively, these results demonstrate that Fyn KO mice were resistant to LPS-induced endotoxicity.
Fig. 1. Fyn KO mice are protected from LPS-induced endotoxic shock in mice.
(A) Survival analysis of Fyn wild-type (WT) and knockout (KO) mice for up to 48 h following 5 mg/kg of intraperitoneal lipopolysaccharide (LPS) administration. Control mice were challenged with saline alone (n = 18 to 21). (B) Surface body temperature of the Fyn WT and KO mice after LPS administration (n = 6). Significant difference between the Fyn WT LPS and the Fyn KO LPS groups indicated by *, p ≤ 0.05.
3.2. Systemic LPS-induced peripheral inflammatory cytokines are negligible in Fyn KO mice
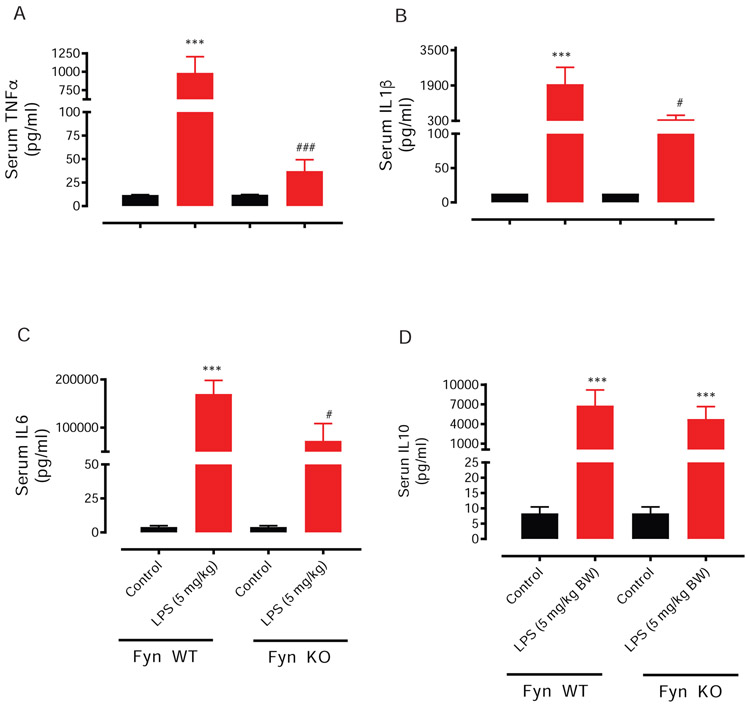
Pro-inflammatory mediators can cause critical pathophysiological effects, including septic shock following an exaggerated release of pro-inflammatory cytokines during LPS-induced endotoxicity. Just a single dose of LPS (5 mg/kg) injected intraperitoneally to male WT mice markedly increases circulatory levels of many peripheral pro-inflammatory cytokines, including TNF-α, IL-10, IL-1β and IL-6 (Fig 2A-D). The main mediators of sepsis are TNF-α, IL10, IL-1β and IL-6 (Delsesto and Opal, 2011). We, therefore, investigated the response of serum cytokine levels in Fyn WT and Fyn KO mice to intraperitoneal LPS injections (5 mg/kg). Interestingly, levels of TNF-α (Fig. 2A), IL-1β (Fig 2B), IL-6 (Fig. 2C) and but not IL-10 (Fig. 2D) were significantly attenuated in the Fyn KO mice 48 h following LPS exposure. Taken together, a lowered production of these cytokines in Fyn KO mice following LPS injections explains the earlier findings of improved survivability (Fig. 1A) and the subdued manifestation of hypothermia (Fig. 1B).
Fig. 2. Fyn KO dampens LPS-induced serum levels of proinflammatory cytokines.
Fyn wild-type (WT) and Fyn knockout (KO) mice were treated with saline or lipopolysaccharide (LPS, 5 mg/kg) via i.p. injection, and then killed 48 h later. Analysis of TNF-α (A), IL-1β (B), IL-6 (C) and IL-10 (D) was conducted via a multiplex Luminex cytokine assay. Significant differences between the Fyn WT control and LPS groups indicated by ***, P < 0.001. Significant differences between the Fyn WT LPS and the Fyn KO LPS groups indicated by #, P < 0.01; ###, P < 0.001.
3.7. Fyn KO mice are protected from LPS-induced behavior deficits
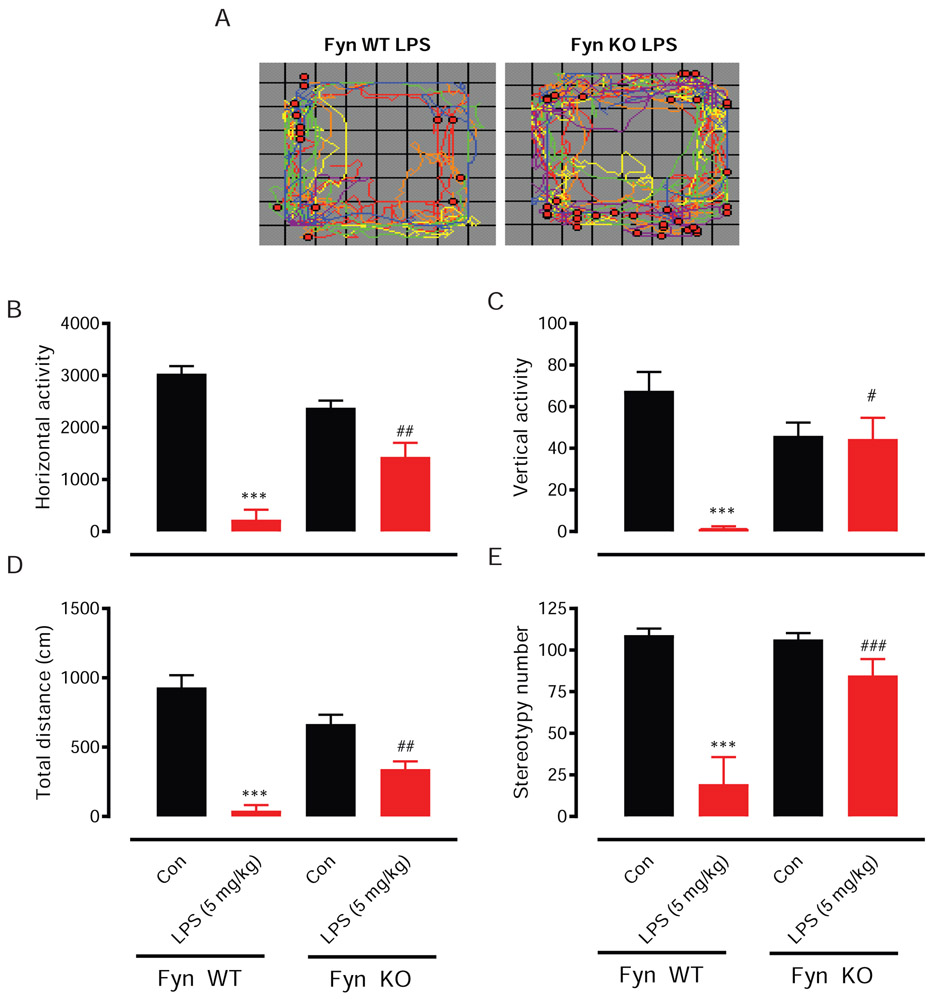
LPS toxicity activates macrophages and microglia, producing inflammatory cytokines and secondary messengers to modulate behavioral responses. Since Fyn KO mice produced lesser amounts of the inflammatory cytokines TNF-α and IL-6 than did Fyn WT mice in response to a peripheral LPS insult, we next examined if reduced cytokine levels were associated with any alterations in the behavior of LPS-treated mice. Representative locomotor activity maps (Fig. 3A) revealed that the LPS-induced deficits in the number of horizontal and rearing movements were greater in Fyn WT mice than in Fyn KO mice. As expected, LPS sharply decreased movements in both the horizontal (Fig. 3B) and vertical (Fig. 3C) directions for Fyn WT mice. However, in Fyn KO mice, LPS-induced locomotor deficits were dramatically attenuated. This same pattern of effects was also seen for total distance traveled (Fig. 3D), as well as, the number of stereotypic movements (Fig. 3E).
Fig. 3. LPS-induced behavior deficits were attenuated in Fyn KO mice.
Fyn wild-type (WT) and knockout (KO) mice were treated with saline or lipopolysaccharide (LPS, 5 mg/kg) via i.p. injection. Locomotor activity was measured using the VersaMax analyzer 1 to 2 days before sacrificing the animals. The vehicle-treated group served as control (Con). (A) Locomotor activity map showing both horizontal (colored traces) and rearing (red dots) movements, (B) horizontal activity, (C) vertical activity, (D) total distance and (E) stereotypy number. Significant differences between the Fyn WT control (Con) and LPS groups indicated by ***, P < 0.001. Significant differences between the Fyn WT LPS and Fyn KO LPS groups indicated by #, P ≤ 0.05; ##, P < 0.01; ###, P < 0.001.
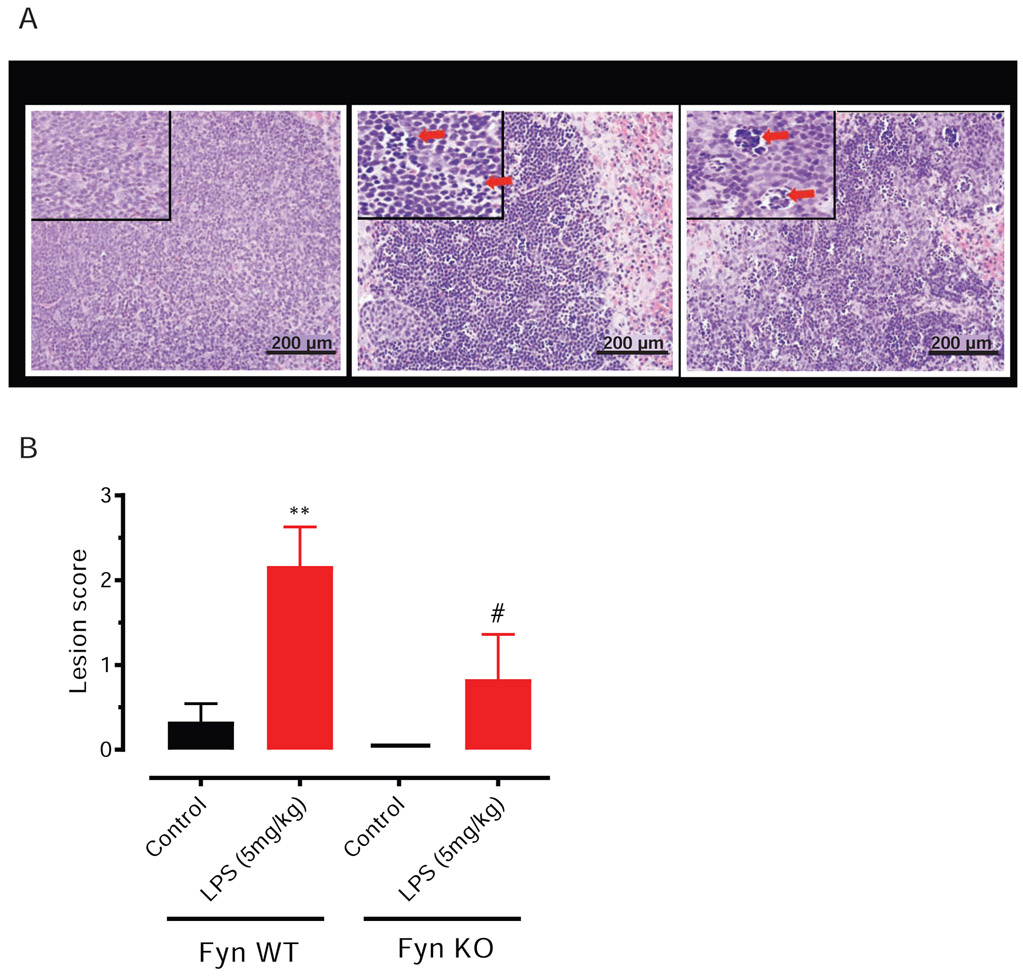
3.4. LPS-induced apoptosis and caspase-3 activation are significantly attenuated in the splenocytes of Fyn KO mice
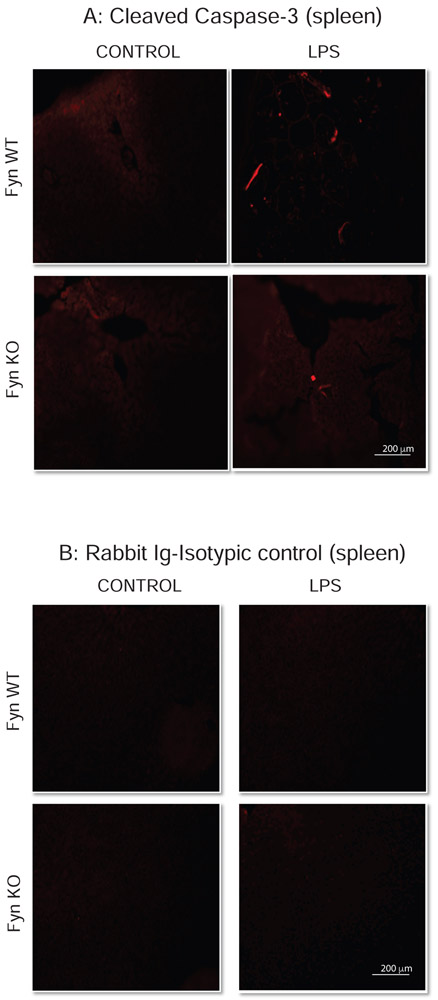
Extensive lymphocyte apoptosis is evident in the spleen and lymphoid organs of patients (Hotchkiss et al., 2005) and in mice during an LPS-induced endotoxic inflammatory response (Gautier et al., 2008). Since Fyn KO mice showed significant protection against LPS-induced endotoxic markers such as inflammatory cytokine induction and behavior deficits, we also examined histopathologically for any accompanying changes in peripheral tissues. Caspase-3 has been extensively studied and implicated to play an important role in apoptosis. Caspase-3 is proteolytically activated during apoptosis, producing active cleaved caspase-3. To investigate such changes in vital organs, we administered LPS at 5 mg/kg body weight intraperitoneally to WT and Fyn KO mice. At 48 h post-injection, each euthanized animal’s spleen and liver were examined for histo-morphologic alterations. Microscopic evaluation of H&E sections of the spleen showed apoptotic changes (small or fragmented lymphocytes with hyperchromatic nuclei) in the splenic lymphoid follicles of both WT LPS- and Fyn KO LPS-treated mice. Lesions were scored morphometrically based on their severity as indicated by arrows (Fig. 4A): 0, no discernible lesions; 1, smallest detectable region of apoptosis (<10% of lymphocytes) in one or several follicles; 2, moderate degree of apoptosis (>10% but <30% of lymphocytes) in all follicles; 3, advanced apoptosis (30-60% of lymphocytes) in all follicles; and 4, the most severe stage of apoptosis (>60% of lymphocytes) in all follicles. Quantitative analysis revealed Fyn KO mice were significantly protected from LPS-induced lymphocyte apoptosis in splenic follicles (Fig. 4B), but we did not notice any apoptotic injury in the lymphocytes from saline-treated Fyn WT and Fyn KO mice. Immunostaining with an antibody specific for cleaved caspase-3 confirmed that LPS increased cleaved caspase-3 in spleen sections and that the increase was significantly higher in Fyn WT animals than in Fyn KO animals (arrows in Fig. 5A). No caspase-3 cleaved immunostaining was observed in spleen sections from either saline-treated Fyn WT or Fyn KO animals. Spleen sections stained with control rabbit IgG isotype antibody followed by secondary Alexa Fluor 555 antibody did not result in any staining in either Fyn WT or KO mice (Fig. 5B). Taken together, these results suggest that Fyn KO animals are more resistant than Fyn WT, attributing an important role for Fyn in LPS-induced splenocyte apoptosis and caspase-3 activation.
Fig. 4. Fyn KO mice are resistant to LPS-induced splenocyte apoptosis.
Fyn wild-type (WT) and Fyn knockout (KO) mice (n = 6 males) treated with saline or lipopolysaccharide (LPS, 5 mg/kg) via i.p. injections were killed 48 h post-injection. (A) Representative H&E images showing how lesions were scored. Arrows indicate focal areas of splenocyte apoptosis. Scale bar = 200 μm. (B) Lesion scoring of splenocyte apoptosis was compared between saline and LPS-treated groups. Significant differences between the Fyn WT LPS and the Fyn KO LPS groups indicated by #, P ≤ 0.05, and between Fyn WT LPS and control by **, P < 0.01. Scale bar = 200 μm.
Fig. 5. Fyn KO mice are resistant to LPS-induced increases in cleaved caspase-3 in the spleen.
Fyn wild-type (WT) and Fyn knockout (KO) mice (n = 6 males) treated with saline or LPS as described in methods sections. (A) Representative immunofluorescence images indicate increased cleaved caspase-3 staining. (B) Representative images of sections immunostained with rabbit IgG isotype control. Arrows in (A) indicate increased iNOS immunoreactivity. Scale bar = 200 μm.
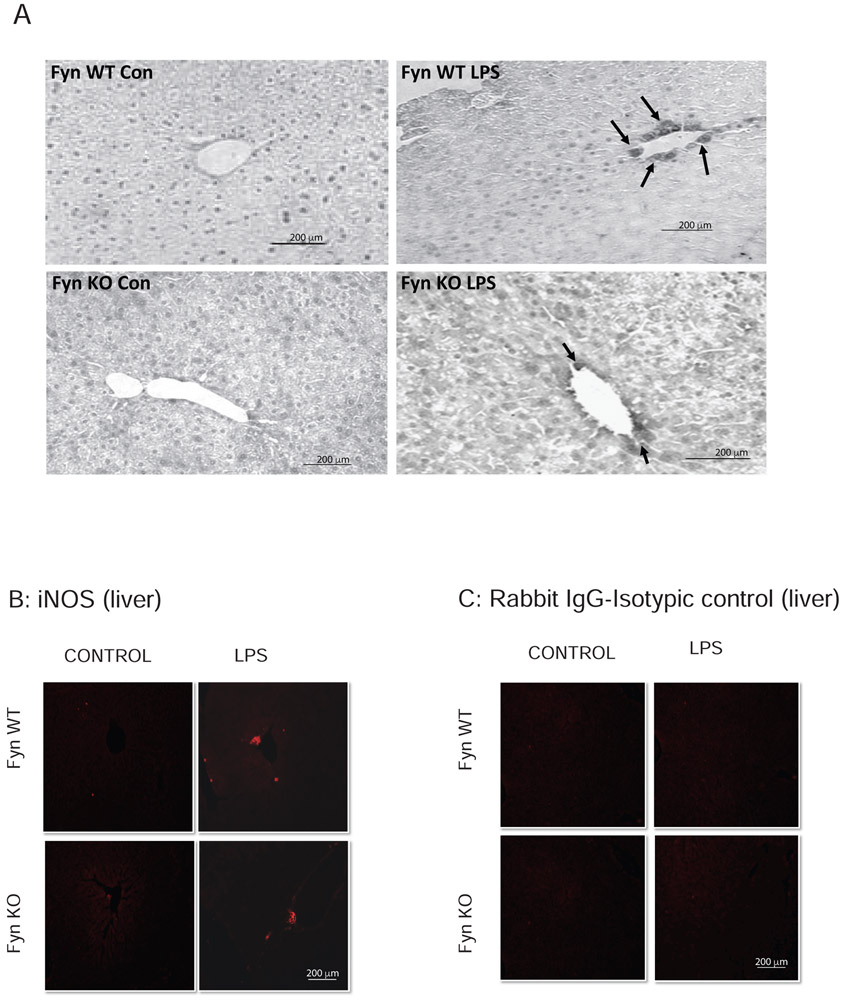
3.5. LPS-induced increases in iNOS are significantly attenuated in the liver of Fyn KO mice
Since NO, produced by the enzyme iNOS, is a key mediator of inflammation, we performed DAB immunostaining to determine Fyn’s role in LPS-induced iNOS expression and inflammation in the liver. The iNOS immunoreactivity in DAB-stained liver sections increased significantly in LPS-treated Fyn WT mice, but only increased marginally in Fyn KO mice (arrows in Fig. 6A). These results were confirmed in immunofluorescence experiments. LPS-administered Fyn WT animals exhibited increased iNOS expression in liver sections (arrows in Fig. 6B) that was significantly higher than that observed in Fyn KO animals. No iNOS immunostaining was observed in liver sections from either saline-treated Fyn WT or Fyn KO animals. Liver sections stained with control rabbit IgG isotype antibody followed by secondary Alexa Fluor 555 antibody did not result in any staining in either Fyn WT or KO mice (Fig. 6C). Taken together, these findings demonstrate that Fyn KO mice were protected from LPS-induced splenocyte apoptosis and liver inflammation, suggesting an important role for Fyn in endotoxin-induced pathological manifestations.
Fig. 6. Fyn KO mice are resistant to LPS-induced hepatic iNOS production.
Fyn wild-type (WT) and Fyn knockout (KO) mice (n = 6 males) treated with saline or LPS as described in methods sections. (A) DAB immunochemistry for iNOS in Fyn WT and Fyn KO liver sections from saline (Con)- or LPS-injected groups. (B) Representative immunofluorescence images indicate increased iNOS staining. (C) Representative images of sections immunostaining with rabbit IgG isotype control. Arrows in (A) and (B) indicate increased iNOS immunoreactivity. Scale bar = 200 μm.
3.6. Dasatinib, a pharmacological inhibitor of Fyn kinase, protects against systemic LPS-induced increases in TNF-α, IL-6 and pro-IL-1β
Since Fyn KO mice were resistant to LPS-induced inflammation, we next sought to pharmacologically inhibit Fyn kinase with an FDA approved cancer drug, dasatinib (Sprycel) (Araujo and Logothetis, 2010) to demonstrate the clinical relevance of Fyn inhibition in the LPS-induced sepsis model. Previous studies have shown that dasatinib treatment may provide a balanced immune response by preventing an overshooting inflammatory reaction on the one side and bacterial overgrowth on the other side (Goncalves-de-Albuquerque et al., 2018). Dasatinib has also been used as a salvage therapy for chronic myeloid leukemia with blast crisis and central nervous system involvement in cancer patients (Lai et al., 2015). Dasatinib is a broad Src kinase inhibitor with a good IC50 value of 10-50 nM for Fyn kinase (Wang et al., 2017). We have previously shown the pro-inflammatory process starts within hours of LPS-induced endotoxemia (Gordon et al., 2016, Panicker et al 2015), therefore, we sought to determine if dasatinib treatment could attenuate LPS-induced TNF-α and IL-6 production as early as 1 h. Naive C57BL/6 mice were co-treated with Dasatinib (10 mg/kg, oral gavage) and LPS (5 mg/kg, i.p.). The dasatinib dose used in this study was less than that used in mouse models of cancer (Scott et al., 2017; Song et al., 2013). Blood samples were collected at 1 h and 24 h and processed for plasma and animals were killed at 24 h. As shown in Fig. 7A, dasatinib treatment significantly suppressed LPS-induced increases in plasma TNF-α and IL-6 levels during early stages (1 h). Dasatinib treatment also attenuated LPS-induced increases in liver pro IL-1β in qRT-PCR and RNAScope experiments (Fig. 7B). These findings demonstrate, mice treated with, dasatinib protected against endotoxin-induced increases in proinflammatory cytokine plasma protein and liver mRNA levels.
4.0. Discussion
The present study demonstrates a novel role in systemic inflammatory mechanisms contributed by Fyn kinase following a systemic exposure to the endotoxin LPS. We find that knocking out Fyn not only improves survival rates in a mouse model of endotoxemia, but also normalizes the hypothermia often associated with LPS exposure in rodents. Notably, the protective effects manifested in Fyn KO mice were accompanied by a marked attenuation of LPS-induced behavioral deficits. Less severe LPS-induced endotoxic symptoms were positively correlated with a reduced production of pro-inflammatory cytokines and chemokines in Fyn KO mice. Our studies further demonstrate that Fyn KO mice were protected from endotoxin-induced pathological lesions and iNOS in the liver and spleen when systemically injected with LPS. In addition to the protective effects manifested in the absence of Fyn, LPS-induced behavioral deficits were markedly attenuated in the Fyn KO mice. The subdued endotoxic symptoms following the genetic attenuation of Fyn kinase were further corroborated by pharmacological inhibitor - dasatinib. Co-treatment of mice with dasatinib significantly protected from LPS-induced increases in plasma TNF-α and IL6 levels, as well as, pro-IL-1β mRNA levels. To our knowledge, we are the first to demonstrate that Fyn contributes directly to pathogenic mechanisms underlying endotoxic symptoms. We also report for the first time the attenuation of systemic cytokine production following endotoxic insult in Fyn-deficient mice.
Fyn is a 59-kDa cytoplasmic tyrosine kinase belonging to the SFK (Roskoski, 2005). Fyn is a widely expressed SFK member in both neuronal and non-neuronal cells, and therefore this kinase assumes multiple physiological roles. Fyn’s function is essential for brain development, myelination, oligodendrocyte maturation, axonal growth and neuronal plasticity throughout development from embryo to adult (Cooke and Perlmutter, 1989; Relucio et al., 2009). Fyn’s activity is essential for the migration of neuronal precursors in the developing brain (Nishimura et al.). In the nervous system, Fyn also controls the brain centers that regulate fear and memory (Miyakawa et al., 2001; Yagi et al., 1993), and its absence impairs apoptosis in the developing cerebellum. Besides multiple functional roles attributed to Fyn in the nervous system, its role in the immune system is also of physiological significance. One of the earliest studies showed that prostaglandin E2 (PGE2) modulates Fyn in T-lymphocytes during sepsis in rats (Choudhry et al., 1998), induced by implanting fecal pellets containing Escherichia coli (150 cfu) and Bacteroides fragilis (104 cfu) into the abdominal cavity in the presence or absence of indomethacin, a PGE2 inhibitor demonstrating that Fyn is essential for T-lymphocyte maturation, T-cell receptor signal transduction and lymphocyte activation (Appleby et al., 1992; Davidson et al., 1994; Palacios and Weiss, 2004). Fyn is also required for anaphylactic shock and mast cell function (Parravicini et al., 2002; Yamashita et al., 2007). Fyn deficiency impairs sphingosine kinase-dependent mast cell degranulation (Rivera et al., 2008).
Two different Fyn isoforms have been described in the mammalian system (Appleby et al., 1992; Davidson et al., 1994). The Fyn isoform expressed in the immune system has key roles in modulating both physiological and pathological conditions. In T-lymphocytes, Fyn was shown to be essential for T-cell receptor signal transduction and lymphocyte activation (Groves et al., 1996; Palacios and Weiss, 2004). Fyn also modulates the immunomodulatory functions of leptins such as insulin receptor substrate-1 (IRS-1) and phosphatidylinositol 3-Kinase (PI3-kinase) activation (Girasol et al., 2009). Furthermore, anaphylactic shock mechanisms, mast cell activation/degranulation and TNF-α release require Fyn function (Parravicini et al., 2002). During stimuli for mast cell degranulation, Fyn-deficient mast cells were defective in the production of inflammatory eicosanoid products, such as the leukotrienes B4 and C4, the cytokines IL-6 and TNF-α and the chemokines CCL2 (or monocyte chemoattractant protein 1, MCP-1) and CCL4 (MCP-1β), thereby contributing to anaphylactic pathways (Hernandez-Hansen et al., 2005). Fyn-deficient mast cells also showed a marked decrease in c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK), IκB kinase B activation and IκBα phosphorylation. Additionally, the nuclear factor kappa B (NFκB) DNA-binding activity that drives IL-6 and TNF-α production was lacking in Fyn-deficient mast cells (Gomez et al., 2005). Fyn deficiency results in impaired mast cell degranulation, less activation of sphingosine kinases and associated mast cell degranulation (Rivera et al., 2008). Recently, we reported that the upregulation of pro-neuroinflammatory responses involving microglia is mediated by Fyn kinase (Panicker et al., 2015). While Fyn’s multiple roles during both physiological and pathological states involving the immune system have been characterized, we report for the first time that Fyn also plays a critical role during endotoxic inflammatory responses.
Since the roles of Fyn during endotoxic insults remain unresolved, we examined the systemic changes in pro-inflammatory cytokines in Fyn KO mice after peripheral LPS injection. Derived from a gram-negative bacterium, LPS acts through the membrane-bound, TLR4 (Pålsson-McDermott and O'Neill, 2004) found in immune cells and microglia. LPS mediates its endotoxic effect through nuclear translocation of the transcription factor NF-κB (Al-Hanbali et al., 2009), leading to the upregulated expression of pro-inflammatory genes. Peripheral inflammation induced by LPS can initiate brain inflammation and associated events such as the loss of reactive astrocytes, an increase in pro-inflammatory cytokines and microglial activation, which are followed by degeneration of dopaminergic neurons in rodents (Qin et al., 2007). In this study, we demonstrate that serum levels of the pro-inflammatory cytokines TNF-α and IL-6 were significantly attenuated in LPS-treated Fyn KO mice. Co-treatment with a broad specific Fyn kinase inhibitor dasatinib, significantly suppressed LPS induced increases in plasma TNF-α and IL-6 levels and liver pro-IL-1β mRNA levels.
Previously, Hwang et al. (2009) had shown that Fyn regulates TNF-α-induced cyclooxygenase-2 (COX-2) expression in mouse epidermal cells. Also, Fyn-deficient mast cells in asthma and allergy models exhibited attenuated production of the pro-inflammatory cytokines IL-4, IL-6, IL-13 and other chemokines (Hernandez-Hansen et al., 2005). While Fyn’s role in regulating the production of pro-inflammatory cytokines in asthma and allergy models have been well investigated, we also showed recently (Panicker et al., 2015) that genetic knockdown of Fyn significantly attenuated striatal levels of the pro-inflammatory cytokines TNF-α and pro-IL-1β. Taken together, these findings lend compelling evidence of Fyn’s pathogenic roles in modulating both peripheral and CNS inflammatory pathways.
The massive increase in circulatory cytokine and chemokine levels upon LPS insult is always associated with behavior deficits and mortality in rodents (Dantzer et al., 1998). In agreement with previous investigations, significant locomotor deficits and poor survival rates were exactly what we observed in the present study when naive mice were treated with LPS. However, when Fyn KO mice were challenged with LPS, we show that Fyn deficiency led to an increase in post-LPS survival by nearly 2-fold and vastly improved exploratory locomotor activity as measured by horizontal and vertical movements, as well as distance traveled. Fyn KO mice were also more active when relatively stationary (e.g., grooming) as measured by stereotypy counts.
Massive lymphocytic apoptosis is central to the endotoxin-induced inflammatory response. In line with this view, our results reveal that mice lacking Fyn kinase have significantly less LPS-induced lymphocyte apoptosis and proteolytic activation of caspase-3, a marker of apoptosis in splenic follicles, when compared to WT mice. This difference may partly explain the protective phenotype observed in Fyn KO mice, which was both longer-lived and more active. In addition, MAPKs, especially p38, and mitochondrial injury are known to have roles in mediating lymphocyte apoptosis (Liu et al., 2013). We plan to establish the conditional microglia-specific Fyn knockout mice in future studies and using the tamoxifen-inducible approach of brain cell type-specific Fyn KO in adult mice to determine any potential confounding developmental effects in Fyn global KO. Study outcomes in these two model systems will further confirm the role of Fyn kinase in LPS-induced lymphocyte apoptosis and sepsis.
5.0. Conclusions
In summary, we provide evidence that Fyn plays an integral role in systemic inflammatory events during endotoxic insult. Genetic ablation or pharmacological inhibition of Fyn proved to be beneficial in mitigating multiple adverse effects associated with LPS exposure, including mortality, behavioral, pro-inflammatory mediators, and splenocyte apoptosis. Together, our data demonstrate for the first time a major role for Fyn kinase in the endotoxic inflammatory response.
Acknowledgments:
We thank Colleen Hogan, Piyush Padhi and Gary Zenitsky for their assistance with manuscript preparation.
Funding:
This work was funded by the National Institutes of Health (NIH) grants NS088206, ES026892 and NS100090. Other sources include Lloyd endowed chair and Eminent Scholar and Armbrust Endowment to AGK and Salsbury endowed chair to AK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Availability of data and materials:
The datasets used and/or analyzed for the current study are available from the corresponding author on reasonable request.
Ethics approval:
Use of animals and all animal-related procedures in this study were approved and supervised by the Institutional Animal Care and Use Committee at Iowa State University.
Consent for publication:
Not applicable
Competing interests:
AGK and VA have an equity interest in PK Biosciences Corporation located in Ames, IA. The terms of this arrangement have been reviewed and approved by Iowa State University in accordance with its conflict of interest policies. Other authors declare no actual or potential competing financial interests.
References
- Al-Hanbali M, Ali D, Bustami M, Abdel-Malek S, Al-Hanbali R, Alhussainy T, Qadan F, Matalka KZ, 2009. Epicatechin suppresses IL-6, IL-8 and enhances IL-10 production with NF-kappaB nuclear translocation in whole blood stimulated system. Neuro Endocrinol Lett 30, 131–138. [PubMed] [Google Scholar]
- Appleby MW, Gross JA, Cooke MP, Levin SD, Qian X, Perlmutter RM, 1992. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell 70, 751–763. [DOI] [PubMed] [Google Scholar]
- Araujo J, Logothetis C, 2010. Dasatinib: a potent SRC inhibitor in clinical development for the treatment of solid tumors. Cancer treatment reviews 36, 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry MA, Uddin S, Sayeed MM, 1998. Prostaglandin E2 Modulation of p59 Tyrosine Kinase in T Lymphocytes During Sepsis. The Journal of Immunology 160, 929–935. [PubMed] [Google Scholar]
- Cooke MP, Perlmutter RM, 1989. Expression of a novel form of the fyn proto-oncogene in hematopoietic cells. New Biol 1, 66–74. [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Laye S, Bret-Dibat JL, Parnet P, Kelley KW, 1998. Cytokines and sickness behavior. Ann N Y Acad Sci 840, 586–590. [DOI] [PubMed] [Google Scholar]
- Davidson D, Viallet J, Veillette A, 1994. Unique catalytic properties dictate the enhanced function of p59fynT, the hemopoietic cell-specific isoform of the Fyn tyrosine protein kinase, in T cells. Mol Cell Biol 14, 4554–4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delsesto D, Opal SM, 2011. Future perspectives on regulating pro-and anti-inflammatory responses in sepsis. Contributions to microbiology 17, 137–156. [DOI] [PubMed] [Google Scholar]
- Deutschman CS, Tracey KJ, 2014. Sepsis: current dogma and new perspectives. Immunity 40, 463–475 [DOI] [PubMed] [Google Scholar]
- Duyvestyn JM, Taylor SJ, Dagger SA, Orandle M, Morse HC 3rd, Thien CB, Langdon WY, 2014. Dasatinib targets B-lineage cells but does not provide an effective therapy for myeloproliferative disease in c-Cbl RING finger mutant mice. PloS one 9, e94717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier EL, Huby T, Saint-Charles F, Ouzilleau B, Chapman MJ, Lesnik P, 2008. Enhanced Dendritic Cell Survival Attenuates Lipopolysaccharide-Induced Immunosuppression and Increases Resistance to Lethal Endotoxic Shock. The Journal of Immunology 180, 6941–6946. [DOI] [PubMed] [Google Scholar]
- Girasol A, Albuquerque GG, Mansour E, Araujo EP, Degasperi G, Denis RG, Carvalheira JB, Saad MJ, Velloso LA, 2009. Fyn mediates leptin actions in the thymus of rodents. PLoS One 4, e7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez G, Gonzalez-Espinosa C, Odom S, Baez G, Cid ME, Ryan JJ, Rivera J, 2005. Impaired FcepsilonRI-dependent gene expression and defective eicosanoid and cytokine production as a consequence of Fyn deficiency in mast cells. Journal of immunology 175, 7602–7610. [DOI] [PubMed] [Google Scholar]
- Goncalves-de-Albuquerque CF, Rohwedder I, Silva AR, Ferreira AS, Kurz ARM, Cougoule C, Klapproth S, Eggersmann T, Silva JD, de Oliveira GP, Capelozzi VL, Schlesinger GG, Costa ER, Estrela Marins RCE, Mocsai A, Maridonneau-Parini I, Walzog B, Macedo Rocco PR, Sperandio M, de Castro-Faria-Neto HC, 2018. The Yin and Yang of Tyrosine Kinase Inhibition During Experimental Polymicrobial Sepsis. Frontiers in immunology 9, 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves T, Smiley P, Cooke MP, Forbush K, Perlmutter RM, Guidos CJ, 1996. Fyn can partially substitute for Lck in T lymphocyte development. Immunity 5, 417–428. [DOI] [PubMed] [Google Scholar]
- Hernandez-Hansen V, Bard JD, Tarleton CA, Wilder JA, Lowell CA, Wilson BS, Oliver JM, 2005. Increased expression of genes linked to FcepsilonRI Signaling and to cytokine and chemokine production in Lyn-deficient mast cells. J Immunol 175, 7880–7888. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE, 2005. Accelerated Lymphocyte Death in Sepsis Occurs by both the Death Receptor and Mitochondrial Pathways. The Journal of Immunology 174, 5110–5118. [DOI] [PubMed] [Google Scholar]
- Hwang MK, Kang NJ, Heo YS, Lee KW, Lee HJ, 2009. Fyn kinase is a direct molecular target of delphinidin for the inhibition of cyclooxygenase-2 expression induced by tumor necrosis factor-alpha. Biochem Pharmacol 77, 1213–1222. [DOI] [PubMed] [Google Scholar]
- Krakauer T, Buckley MJ, Fisher D, 2010. Proinflammatory mediators of toxic shock and their correlation to lethality. Mediators of inflammation 2010, 517594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SW, Huang TC, Chen JH, Wu YY, Chang PY, 2015. Dasatinib as the salvage therapy for chronic myeloid leukemia with blast crisis and central nervous system involvement: A case report. Oncology letters 9, 1957–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley M, Ghosh A, Charli A, Sarkar S, Ay M, Luo J, Zielonka J, Brenza T, Bennett B, Jin H, Ghaisas S, Schlichtmann B, Kim D, Anantharam V, Kanthasamy A, Narasimhan B, Kalyanaraman B, Kanthasamy AG, 2017. Mito-Apocynin Prevents Mitochondrial Dysfunction, Microglial Activation, Oxidative Damage, and Progressive Neurodegeneration in MitoPark Transgenic Mice. Antioxidants & redox signaling 27, 1048–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Huang NS, Qu H, O'Connell JF, Gonzalez-Mariscal I, Santa-Cruz-Calvo S, Doyle ME, Xi ZX, Wang Y, Onaivi ES, Egan JM, 2018. Identification of novel mouse and rat CB1R isoforms and in silico modeling of human CB1R for peripheral cannabinoid therapeutics. Acta pharmacologica Sinica. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZG, Ni SY, Chen GM, Cai J, Guo ZH, Chang P, Li YS, 2013. Histones-mediated lymphocyte apoptosis during sepsis is dependent on p38 phosphorylation and mitochondrial permeability transition. PloS one 8, e77131. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Miyakawa T, Yagi T, Takao K, Niki H, 2001. Differential effect of Fyn tyrosine kinase deletion on offensive and defensive aggression. Behav Brain Res 122, 51–56. [DOI] [PubMed] [Google Scholar]
- Nishimura YV, Sekine K, Chihama K, Nakajima K, Hoshino M, Nabeshima Y, Kawauchi T, Dissecting the factors involved in the locomotion mode of neuronal migration in the developing cerebral cortex. J Biol Chem 285, 5878–5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios EH, Weiss A, 2004. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene 23, 7990–8000. [DOI] [PubMed] [Google Scholar]
- Pålsson-McDermott EM, O'Neill LAJ, 2004. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 113, 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker N, Saminathan H, Jin H, Neal M, Harischandra DS, Gordon R, Kanthasamy K, Lawana V, Sarkar S, Luo J, Anantharam V, Kanthasamy AG, Kanthasamy A, 2015. Fyn Kinase Regulates Microglial Neuroinflammatory Responses in Cell Culture and Animal Models of Parkinson's Disease. The Journal of Neuroscience 35, 10058–10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, Saitoh S, Samelson LE, O'Shea JJ, Rivera J, 2002. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunol 3, 741–748. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT, 2007. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55, 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relucio J, Tzvetanova ID, Ao W, Lindquist S, Colognato H, 2009. Laminin alters fyn regulatory mechanisms and promotes oligodendrocyte development. J Neurosci 29, 11794–11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J, Fierro NA, Olivera A, Suzuki R, 2008. New insights on mast cell activation via the high affinity receptor for IgE. Advances in immunology 98, 85–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R Jr., 2005. Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun 331, 1–14. [DOI] [PubMed] [Google Scholar]
- Saminathan H, Asaithambi A, Anantharam V, Kanthasamy AG, Kanthasamy A, 2011. Environmental neurotoxic pesticide dieldrin activates a non receptor tyrosine kinase to promote PKCdelta-mediated dopaminergic apoptosis in a dopaminergic neuronal cell model. Neurotoxicology 32, 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert C, Chatain N, Braunschweig T, Schemionek M, Feldberg K, Hoffmann M, Dufva O, Mustjoki S, Brummendorf TH, Koschmieder S, 2017. The SCLtTAxBCR-ABL transgenic mouse model closely reflects the differential effects of dasatinib on normal and malignant hematopoiesis in chronic phase-CML patients. Oncotarget 8, 34736–34749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AJ, Song EK, Bagby S, Purkey A, McCarter M, Gajdos C, Quackenbush KS, Cross B, Pitts TM, Tan AC, Eckhardt SG, Fenton H, Arcaroli J, Messersmith WA, 2017. Evaluation of the efficacy of dasatinib, a Src/Abl inhibitor, in colorectal cancer cell lines and explant mouse model. PLoS One 12, e0187173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Sun X, Bai WL, Ji WY, 2013. Antitumor effects of Dasatinib on laryngeal squamous cell carcinoma in vivo and in vitro. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery 270, 1397–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveira da Silva AM, Kaulbach HC, Chuidian FS, Lambert DR, Suffredini AF, Danner RL, 1993. Brief report: shock and multiple-organ dysfunction after self-administration of Salmonella endotoxin. The New England journal of medicine 328, 1457–1460. [DOI] [PubMed] [Google Scholar]
- Wang M, Kommidi H, Tosi U, Guo H, Zhou Z, Schweitzer ME, Wu LY, Singh R, Hou S, Law B, Ting R, Souweidane MM, 2017. A Murine Model for Quantitative, Real-Time Evaluation of Convection-Enhanced Delivery (RT-CED) Using an (18)[F]-Positron Emitting, Fluorescent Derivative of Dasatinib. Molecular cancer therapeutics 16, 2902–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren HS, 2009. Editorial: Mouse models to study sepsis syndrome in humans. Journal of leukocyte biology 86, 199–201. [DOI] [PubMed] [Google Scholar]
- Yagi T, Aizawa S, Tokunaga T, Shigetani Y, Takeda N, Ikawa Y, 1993. A role for Fyn tyrosine kinase in the suckling behaviour of neonatal mice. Nature 366, 742–745. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Charles N, Furumoto Y, Odom S, Yamashita T, Gilfillan AM, Constant S, Bower MA, Ryan JJ, Rivera J, 2007. Cutting edge: genetic variation influences Fc epsilonRI-induced mast cell activation and allergic responses. J Immunol 179, 740–743. [DOI] [PubMed] [Google Scholar]
- Yu X, Guo S, Song W, Xiang T, Yang C, Tao K, Zhou L, Cao Y, Liu S, 2017. Estrogen receptor alpha (ERalpha) status evaluation using RNAscope in situ hybridization: a reliable and complementary method for IHC in breast cancer tissues. Human pathology 61, 121–129. [DOI] [PubMed] [Google Scholar]