Abstract
Endothelial progenitor cells (EPCs) are important to tissue repair and regeneration especially after ischemic injury, and very heterogeneous in phenotypes and biological features. Reactive oxygen species are involved in regulating EPC number and function. N-acetylcysteine (NAC) inhibits ischemia-induced reactive oxygen species formation and promotes ischemic limb recovery. This study was to evaluate the effect of NAC on EPC subpopulations in bone marrow (BM) and blood in mice with limb ischemia. Limb ischemia was induced by femoral artery ligation in male C57BL/6 mice with or without NAC treatment. EPC subpopulations, intracellular reactive oxygen species production, cell proliferation and apoptosis in BM and blood cells were analyzed at baseline, day 3 (acute ischemia) and 21 (chronic) after ligation. c-Kit+/CD31+, Sca-1+/Flk-1+, CD34+/CD133+, and CD34+/Flk-1+ were used to define EPC subpopulations. Limb blood flow, function, muscle structure, and capillary density were evaluated with laser Doppler perfusion imaging, treadmill test, and immunohistochemistry, respectively, at day 3, 7, 14 and 21 post ischemia. Reactive oxygen species production in circulating and BM mononuclear cells and EPCs populations were significantly increased in BM and blood in mice with acute and chronic ischemia. NAC treatment effectively blocked ischemia-induced reactive oxygen species production in circulating and BM mononuclear cells, and selectively increased EPC population in circulation, not BM, with preserved proliferation in mice with chronic ischemia, and enhanced limb blood flow and function recovery, while preventing acute ischemia-induced increase in BM and circulating EPCs. These data demonstrated that NAC selectively enhanced circulating EPC population in mice with chronic limb ischemia.
Keywords: NAC, EPCs, angiogenesis, limb ischemia
1. Introduction
Bone marrow (BM)-derived endothelial progenitor cells (EPCs) are potential sources for cell-based therapy for tissue repair and regeneration after injuries especially ischemic tissue injury (Bianconi et al., 2018; Parikh et al., 2017). The therapeutic efficacy of EPCs could be largely determined by their number and/or function. A variety of factors, disease conditions, and pharmacological agents are involved in the regulation of the number and/or function of EPCs, including hypoxia, cytokines, growth factors, inflammation, diabetes mellitus, ischemia, and statins (Hu et al., 2018b; Steinhoff et al., 2017).
Reactive oxygen species are critically involved in the regulation of the number and/or function of EPCs (Cui et al., 2015b; Hu et al., 2018a). Excessive reactive oxygen species production could impair the effectiveness of progenitor cell therapy following limb ischemia (Higashi et al., 2006). reactive oxygen species -dependent mechanism was involved in the reduction of EPCs in mice exposed to PM2.5 or treated with ox-LDL or high-fat diet (Cui et al., 2015a; Cui et al., 2015b; Cui et al., 2015d). N-acetylcysteine (NAC) could effectively inhibit ischemia-induced reactive oxygen species production both in vitro and in vivo in various organ systems like kidney, lung, heart and neurological system (Cakir et al., 2003; Cui et al., 2015c; Holzapfel et al., 2007; Takhtfooladi et al., 2016; Wang et al., 2011). NAC enhances recovery of limb ischemia, decreases inflammation in human subjects and mice (de Medeiros et al., 2018; Martinez de Lizarrondo et al., 2017), partially prevents ischemia-induced reactive oxygen species production, and preserves endothelial cell proliferation (Wang et al., 2016). However, it is unclear if NAC could increase the number and/or function of EPCs that may contribute to the beneficial effect of NAC on the recovery of ischemic limb.
EPCs are a group of very heterogeneous cell populations with different phenotypes and biological characteristics (Wojakowski and Tendera, 2005). There are different origins for EPCs with blood and BM as the major sources. The number and function of EPCs are reduced in a variety of cardiovascular diseases, including hypertension, atherosclerosis, diabetes, coronary artery disease, and heart failure, as well as cardiovascular comorbid conditions such as aging, hypercholesterolemia, cigarette smoking and air pollution (Cui et al., 2016; Sirrs et al., 2018; Steinhoff et al., 2017). All of these conditions are associated with increased risk for ischemic cardiovascular injuries and increased reactive oxygen species production. However, changes in EPC subpopulations in response to ischemia-induced oxidative stress varies significantly (Liu et al., 2019). The present study was designed to test the hypothesis that NAC could enhance the recovery of ischemic limb in association with increased number of EPCs. There were two objectives for the present study: 1) to evaluate the effect of NAC on EPC subpopulations in BM and blood in mice with limb ischemia; 2) to determine the effect of NAC on ischemia-induced reactive oxygen species production and mononuclear cell proliferation and apoptosis.
2. Materials and methods
2.1. Animal models
All animal experiments were performed in accordance with the “Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health”. All animal work was reviewed and approved by the Institutional Animal Care and Use Committee at the University of Missouri-Columbia (protocol number 9227). Limb ischemia model was produced in wild-type male C57BL/6 mice (6-8 weeks old, Jackson Lab, ME, USA) by ligation of left femoral artery as described below. To evaluate the effect of NAC on limb ischemia, a group of mice were pretreated with NAC (1 mg/ml in the drinking water, Sigma-Aldrich, St. Louis, MO, A7250) as described (Cui et al., 2015c) for 24 hours before limb ischemia surgery and continuously for the rest of the experiments with PBS as control. NAC was used for the present study since it was a FDA-approved drug with long standing safety record and readily available for clinical use.
2.2. Hind limb ischemia and blood flow measurement
Acute hind limb ischemia was produced by femoral artery ligation (FAL) as described (Faber et al., 2011). Briefly, mice were anesthetized with 1.25% isoflurane/O2, and the hind limbs were depilated with body temperature maintained at 37±0.5 °C. Left femoral artery was exposed through a 2-mm incision, and transected at the site between the origin of lateral caudal femoral and superficial epigastric arteries and genu artery after ligation. After closure of the incision, mice were allowed to recover with continuous monitoring until suture removal (7-10 days). Recovery of blood flow in the hind limbs was determined with Laser Doppler perfusion imaging (LDPI) pre-operatively, immediately post-operatively, and at day 3, 7, 14, and 21 post ischemia. Mice were placed on a heating pad at 37 °C to minimize temperature variation during imaging. Right limb blood flow was also measured as control. The ratio of blood flow (left ischemic limb blood flow/right normal limb blood flow) was used to monitor blood flow recovery.
2.3. Treadmill study
Mice with NAC or PBS treatment were evaluated with a treadmill test for limb function recovery as described (Massett and Berk, 2005). Briefly, mice were placed on a rodent treadmill equipped with an electric grid at the rear and allowed to run continuously until exhaustion (indicated by falling on the electric grid twice) at day 7, 14, and 21 after limb ischemia. The running time on the treadmill for each mouse was recorded. Two separate trials with 8 mice with NAC treatment and 8 with PBS treatment per trial were conducted. All mice in each group were able to run more than 400 min before limb ischemia at baseline.
2.4. CD31 immunohistochemistry and angiogenesis analysis
For CD31 immunohistochemistry (ICH) analysis, limb tibialis anterior muscle was collected at day 21 after limb ischemia, fixed with 4% paraformaldehyde in PBS, sectioned with 5 μm thickness, and incubated with rat anti-mouse biotinylated CD31 antibody (1:200, BD Biosciences) followed by a biotin-conjugated secondary antibody (goat anti-rat, dilution 1:300; AbCam) (Gounis et al., 2005; McClung et al., 2016). Hind limb muscle before ligation was used as control. The number of cells positive for CD31 was counted on at least 4 pictures per mouse. Vessels with a diameter of and over 15 μm were eliminated from analysis to exclude arteries and arterioles (Tadeo et al., 2016). Each section was viewed with the pictures taken both by 20x and 40x under microscope. For vascular density quantification, non-overlapping 5-8 fields were captured. Capillaries were counted using Image J software, and the capillary density was analyzed as previously described (Brechot et al., 2008; Dai et al., 2009).
2.5. Intracellular reactive oxygen species detection
BM and blood cells were harvested from mice with or without NAC treatment after limb ischemia at day 3 and 21. Mice without limb ischemia were used as control. Red blood cells (RBC) were eliminated using RBC lysis as described (Houlihan et al., 2012). Intracellular reactive oxygen species level in BM and blood mononuclear cells after limb ischemia was determined using H2DCFDA (Invitrogen, Carlsbad, California, D399) as described (Bilski et al., 2002). After incubation with the reagent for 10 min at 37°C, the cells were washed twice with PBS, and then suspended in warm PBS for analysis using flow cytometry. Fluorescence-positive cells were quantitatively evaluated using a LSRII system (BD Bioscience, CA, USA) at the wavelength of 525nm as described (Robinson et al., 1988).
2.6. EPCs analysis
To determine the acute and chronic effect of limb ischemia on the populations of EPCs, BM and blood cells were harvested from mice after limb ischemia at day 3 and 21, and from control animals (without limb ischemia). After elimination of RBC with RBC lysis buffer, multicolor analysis for BM and circulating EPCs was performed using a LSRII system (BD Bioscience, CA, USA). A variety of cell markers and their combinations, including CD34+/Flk-1+, Sca-1+/Flk-1, c-Kit+/CD31+ and CD34+/CD133+, were used to identify EPCs as described (Feng et al., 2012; Liu et al., 2013; Perin et al., 2012; Traverse, 2013; Westerweel et al., 2013; Yang et al., 2011; Zhao et al., 2013). The presence of endothelial markers, such as Flk-1, CD31, endothelial nitric oxide synthase (eNOS), VE-cadherin, and vWF, was used as indication of functional EPCs (Nguyen et al., 2015). All antibodies were from Biolegend (San Diego, CA, U.S, 108127, 105812, 102418, 141204) except Flk-1 APC-Cy7 (from Becton Dickinson Biosciences, NJ, USA, 561252), and CD34 FITC (from eBioscience, San Diego, CA, USA, 11-0341).
2.7. Evaluation of bone marrow and blood mononuclear cell apoptosis and proliferation
BM and blood cell apoptotic rate was determined with FACS using apoptosis kit from BD Pharmingen (CA, USA, 556547). Early apoptotic cells were defined as Annexin V FITC positive cells, and the late apoptotic cells was defined as Annexin V FITC and propidium iodide (PI) double positive cells as described (Cui et al., 2015d). For in vivo BM and blood mononuclear cell proliferation analysis, mice were injected (i.p.) with 1 mg BrdU 12h before cell collection. BM and blood mononuclear cells were collected, permeabilized, and stained with anti-BrdU FITC using BrdU Flow Kit as per manufacture’s instruction (Becton Dickinson and Company BD Biosciences, San Jose, CA, 559619) as described (Cui et al., 2015a).
2.8. Statistical analysis
All the data were presented as means ± standard deviation (S.D.), and statistically analyzed using unpaired Student t-test (two-sided) for two groups of data or one way ANOVA (analysis of variance) (PRISM Version 4.0; GraphPad Software, Inc., San Diego, CA) followed by post hoc conservative Tukey’s test for three or more groups of data to minimize type I error. Two way ANOVA (PRISM Version 4.0; GraphPad Software, Inc., San Diego, CA) followed by Bonferroni post-test was used for comparing the subgroups of data from mice with or without NAC treatment to minimize type I error. Differences were considered statistically significant when a two-tailed P < 0.05.
3. Result
3.1. NAC treatmant enhanced the recovery of blood flow and function of ischemic limb
After acute limb ischemia procedure, there was no measurable blood flow in the ischemic limb as expected (Fig. 1A and 1B), confirming successful creation of acute ischemia model. Blood flow in the ischemic limb slowly recovered to 66% of the limb without ligation at day 21 after ischemia. Limb funciton as reflected by the running time on treadmill also slowly improved after limb ischemia (Fig. 1C). The slow recovery of limb blood flow and function was accompanied with limited angiogenesis as shown with CD31 immunohistochemical staining in Fig. 2.
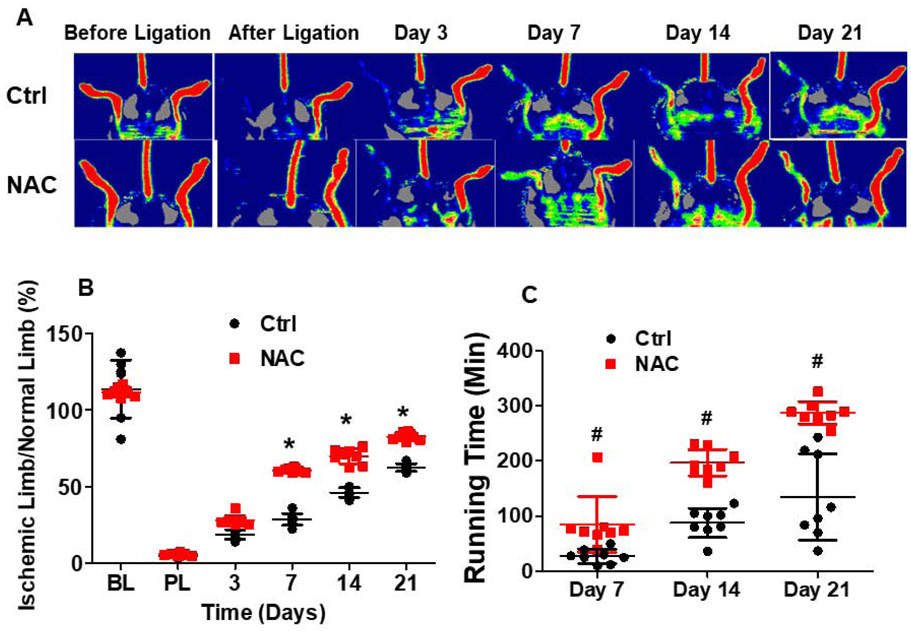
Fig.1. Mice with NAC treatment exhibited enhanced recovery of blood flow and limb function after limb ischemia.
Limb ischemia was induced in C57BL/6 mice with or without NAC treatment by left hind limb femoral artery ligation. Mouse hind limb blood flow was measured using LDPI before, after ligation, and at day 3, 7, 14, and 21 after limb ischemia procedure. Mouse limb function was evaluated using the running time on treadmill at baseline and at day 7, 14, and 21 after limb ischemia. No measurable blood flow was present in the limb immediately after femoral artery ligation (A), confirming successful creation of the limb ischemia model. The recovery of blood flow (B) and limb function (C) was significantly enhanced in mice with NAC treatment compared to the control. NAC: C57BL/6 mice with NAC treatment; Ctrl: C57BL/6 mice with PBS control. Ischemic limb/normal limb: ratio of mouse ischemic left hind limb blood flow to normal right hind limb blood flow. *Day14 or 21 vs day7, P <0.01, n=8; #ctrl vs NAC, P <0.01, n=8.
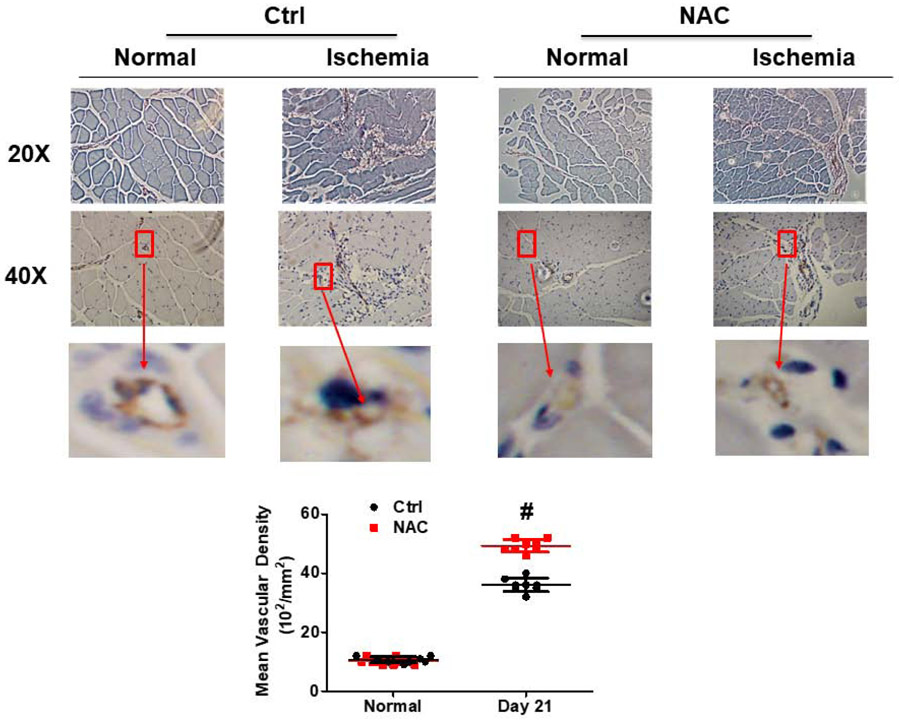
Fig. 2. NAC treatment promoted angiogenesis after limb ischemia.
CD31 immunohistochemistry staining was performed on isolated TA muscle at day 21 after limb ischemia to evaluate angiogenesis. Each section was viewed with pictures taken by both 20X and 40X under microscope. The level of angiogenesis in the limb muscle after ischemia as reflected by capillary vessel density was significantly higher in the mice with NAC treatment than the control. Normal: normal right limb; Ischemia: ischemic left limb. #ctrl vs NAC, P <0.01, n=8.
Blood flow ratio in the ischemic limb was significantly higher in the mice with NAC treatment at day 7 (61%) after ischemia, and reached almost 82.3% of the blood flow in limb without ischemia at day 21 compared to the ischemic limb of control group (66%) (Fig. 1A and 1B). Capillary density in the ischemic limb was significantly increased in NAC-treated mice at day 21 (48.8±2.3; 102/mm2) as compared with the control (36.2±3.0; 102/mm2) (Fig. 2). Limb function as reflected by the running time was significantly improved in mice with NAC treatment as compared with the control (Fig. 1C).
3.2. NAC blocked ischemia-induced reactive oxygen species generation, and selectively incrased the population of circulating EPCs in mice with chronic limb ischemia
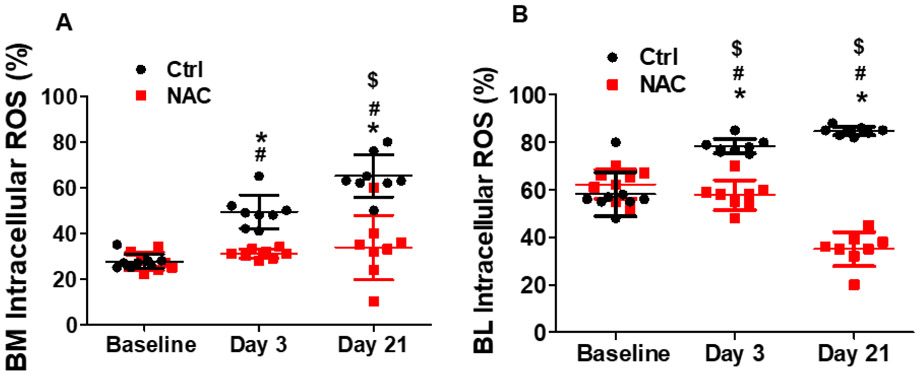
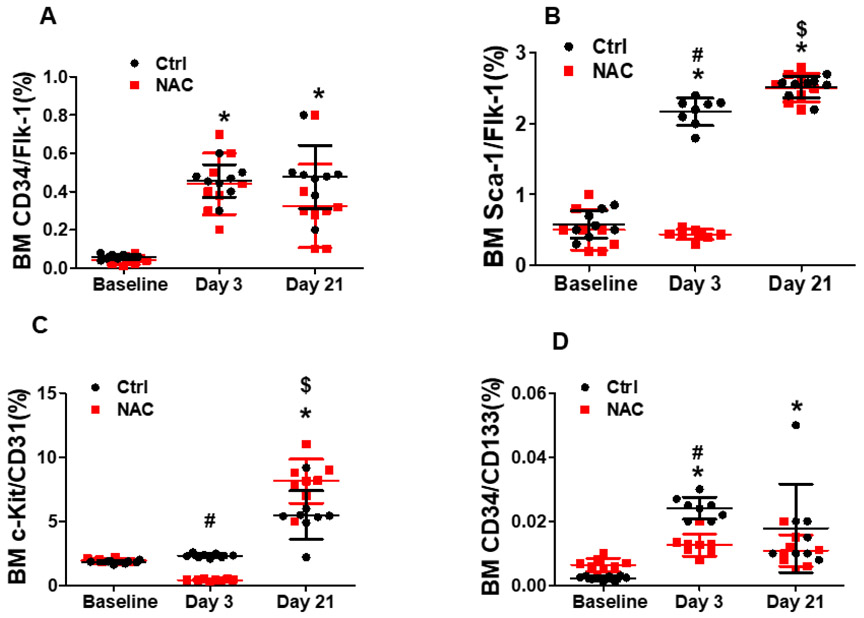
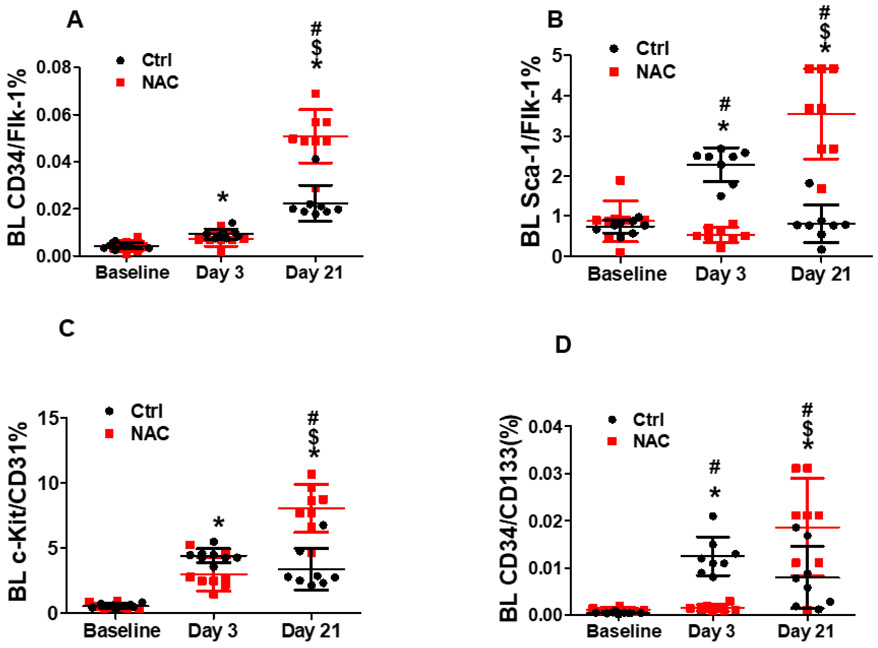
Flow cytometry analysis showed that both BM and blood intracellular reactive oxygen species were significantly increased shortly after limb ischemia that lasted for up to 21 days. NAC treatment effectively blocked ischemia-induced reactive oxygen species production in mice as expected (Fig. 3A and 3B). BM cells positive for CD34+/Flk-1+ and Sca-1+/Flk-1+ (EPCs) were significantly increased up to 7 and 4 folds, respectively, at day 3 after ischemia, and remained at the same level at day 21 after ischemia (Fig. 4A and 4B). However, BM c-Kit+/CD31+ cell population did not change at day 3 after ischemia, but increased at day 21 (Fig. 4C). BM CD34+/CD133+ cell population slightly increased at day 3, then remained at this level at day 21 (Fig. 4D). Circulating Sca-1+/Flk-1+ cell population was initially increased significantly at day 3, then returned to baseline level at day 21 (Fig. 5B and 5D). CD34+/Flk-1+ cell population in blood remained at low level at day 3, and only slightly increased at day 21 (Fig. 5A). The levels of all circulating EPCs were significantly increased by 2-3 folds at day 21 in mice treated with NAC compared to control mice (Fig. 5A-5D), while no significant difference was observed for the population of BM EPCs in the mice with limb ischemia with NAC treatment as compared to the control (Fig. 4A-4D). In contrast, NAC treatment significantly prevented ischemia-induced increase in the populations of BM Sca-1+/Flk-1+ cells, c-Kit+/CD31+ cells, and CD34+/CD133+ cells, as well as circulating Sca-1+/Flk-1+ cells and CD34+/CD133+ cells in mice at day 3 after limb ischemia. Of note, based on previous report and our published data (Liu et al., 2019; Takahashi et al., 1999), the EPC levels peaked at day 3 after limb ischemia, and remained at the same level at day 7 for both circulating EPCs and BM EPCs. Thus, the data on EPC populations at day 7 after limb ischemia was not shown in the present study.
Fig.3. NAC treatment attenuated reactive oxygen species production in BM and blood cells following limb ischemia in mice.
Murine BM and blood cells were collected for intracellular reactive oxygen species test after limb ischemia at day3 and 21. Mice without limb ischemia were used as control. Limb ischemia significantly increased intracellular reactive oxygen species production in BM and blood mononuclear cells that was effectively prevented by NAC treatment in mice. *Day3 or 21 vs Baseline, P <0.05, n≥8; $Day21 vs 3, P <0.05, n≥8; #ctrl vs NAC, P <0.05, n≥8.
Fig. 4. Effect of NAC treatment on the population of BM EPCs in mice following limb ischemia.
BM cells were collected for EPCs analysis at day 3 and 21 after limb ischemia. Mice without limb ischemia were used as control. Flow cytometry analysis showed that BM cells positive for CD34+/Flk-1+ and Sca-1+/Flk-1+ (EPCs) were significantly increased up to 7 and 4 folds, respectively, at day 3 after ischemia, and remained at the same level at day 21 after ischemia (A and B). However, BM c-Kit+/CD31+ cell population did not change at day 3 after ischemia, but increased at day 21 (C). BM CD34+/CD133+ cell population slightly increased at day 3, and remained at this level at day 21 (D). No significant difference in the population of BM CD34+/Flk-1+, Sca-1+/Flk-1+, c-Kit+/CD31+ and CD34+/CD133+ cell population were observed between the mice treated with NAC at day 21 after limb ischemia and the control mice; however, NAC treatment significantly prevented ischemia-induced increases in the populations of BM Sca-1+/Flk-1+ cells, c-Kit+/CD31+ cells, and CD34+/CD133+ cells in mice at day 3 after limb ischemia (A-D). BM: bone marrow; Ctrl: mice without limb ischemia; Day3: 3 days after limb ischemia; Day 21: 21 days after limb ischemia. *Day3 or 21 vs Ctrl, P <0.05, n≥8; $Day21 vs 3, P <0.05, n≥8; #ctrl vs NAC, P <0.05, n≥8.
Fig. 5. NAC treatment resulted in sustained elevation of circulating EPCs population in mice with chronic limb ischemia.
Blood cells were collected for EPCs analysis after limb ischemia at day 3 and 21. Mice without limb ischemia were used as control. Flow cytometry analysis demonstrated that circulating Sca-1+/Flk-1+ cell population was initially increased significantly at day 3, then returned to baseline level at day 21 (B and D). CD34+/Flk-1+ cell population in blood was at low level at day 3, and only slightly increased at day 21 (A). The levels of all circulating EPCs were significantly increased by 2-3 folds at day 21 in mice treated with NAC compared to the control mice (A-D). On the other hand, NAC treatment resulted in a sustained elevation of circulating EPCs population (CD34+/Flk-1+, Sca-1+/Flk-1+, c-Kit+/CD31+ and CD34+/CD133+) in mice at day 21 of limb ischemia compared to the control, while leading to a significant reduction in the number of circulating Sca-1+/Flk-1+ cells and CD34+/CD133+ cells in mice at day 3 after limb ischemia. *Day 3 or 21 vs Ctrl, P <0.05, n≥8; $Day21 vs 3, P <0.05, n≥8; #ctrl vs NAC, P <0.05, n≥8.
3.4. NAC treatment preserved the proliferation of blood mononuclear cells following limb ischemia
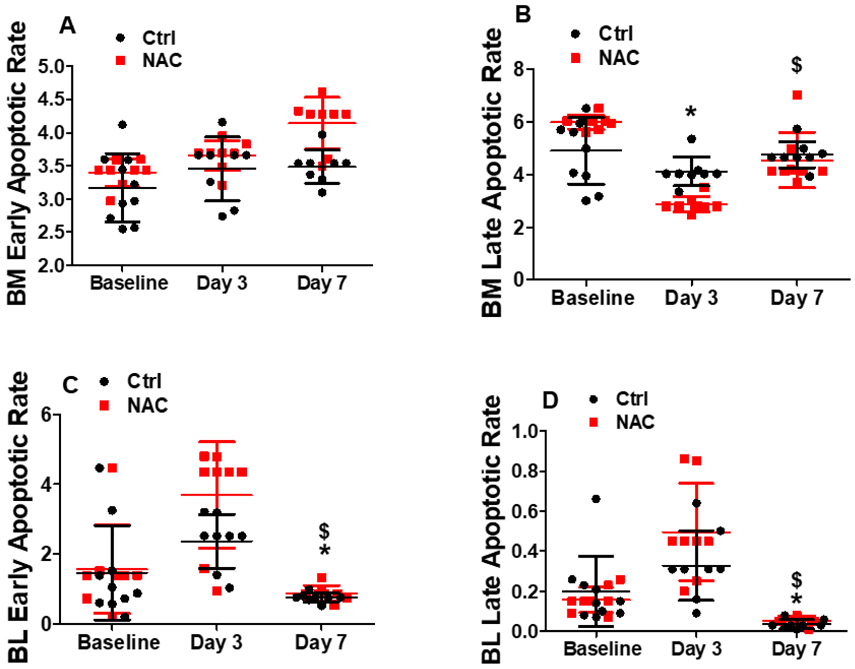
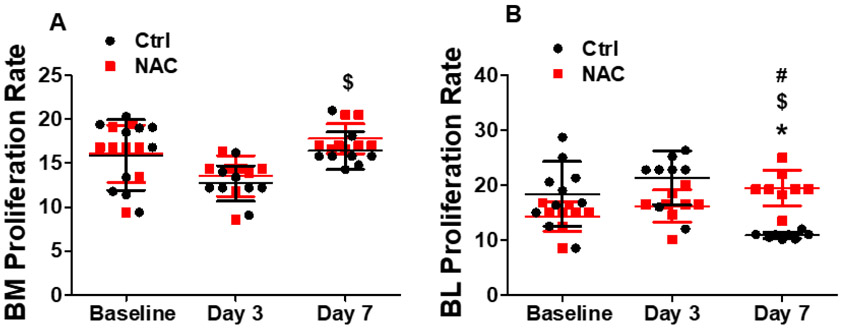
We evaluated BM and blood mononuclear cell apoptosis and proliferation. As shown in Fig. 6A, 6B and 7A, there was no significant change in BM cell apoptotic or proliferation rate at day 3 and day 7 after limb ischemia. However, both apoptotic and proliferation rate of blood mononuclear cells were significantly decreased at day 7 following limb ischemia (Fig. 6C, 6D and 7B). When the mice were pre-treated with NAC, there were no significant changes in BM mononuclear cell apoptotic and proliferation rate as well as blood mononuclear cell apoptotic rate after limb ischemia compared with the control except decreased late apoptosis for BM mononuclear cells with NAC treatment. At day 7, blood mononuclear cell proliferation was much higher than that at day 3 and baseline in NAC-treated mice, which was much higher than that in the control (Fig. 7B).
Fig. 6. Effect of NAC treatment on apoptosis of mononuclear cells in mice with limb ischemia.
Murine BM and blood cells were collected to determine the apoptotic rate at day3 and 7 using flow cytometry analysis. Mice without limb ischemia were used as control. There was no significant change in the BM cell apoptotic rate at day 3 and day 7 after limb ischemia. However, the apoptotic rate of blood mononuclear cells was significantly decreased at day 7 following limb ischemia. When the mice were pre-treated with NAC, there was no significant change in the apoptotic rate in BM and blood mononuclear cells after limb ischemia compared with the control except decreased late apoptosis for BM mononuclear cells with NAC treatment. *Day3 or 7 vs Baseline, P <0.05, n≥8; $Day7 vs 3, P <0.05, n≥8.
Fig. 7. NAC treatment preserved blood mononuclear cell proliferation in mice with limb ischemia.
Murine BM and blood cells were collected to determine the proliferation rate at day3 and 7 using flow cytometry analysis. Mice without limb ischemia were used as control. There was no significant change in the BM cell proliferation rate at day 3 and day 7 after limb ischemia. However, the proliferation rate of blood mononuclear cells were significantly decreased at day 7 following limb ischemia. When the mice were pre-treated with NAC, there was no significant change in the BM mononuclear cell proliferation rate after limb ischemia compared with the control. At day 21, the blood mononuclear cell proliferation was preserved to the same level as day 3 and 7 in NAC-treated mice, which was much higher than that in the control. *Day3 or 7 vs Baseline, P <0.05, n≥8; $Day7 vs 3, P <0.05, n≥8; #ctrl vs NAC, P<0.05, n≥8.
4. Disscusion
In the present study, we demonstrated that both acute and chronic limb ischemia increased reactive oxygen species production in circulating and BM mononuclear cells, and significantly increased EPCs levels in both BM and blood. NAC treatment effectively blocked ischemia-induced reactive oxygen species production in circulating and BM mononuclear cells, and selectively increased the population of circulating EPCs (not BM EPCs) in mice with chronic limb ischemia (at day 21 after ischemia), and significantly enhanced the recovery of blood flow and function of the ischemic limb. The data also showed that NAC treatment significantly prevented ischemia-induced increases in the sub-populations of BM Sca-1+/Flk-1+ cells, c-Kit+/CD31+ cells, and CD34+/CD133+ cells, as well as circulating Sca-1+/Flk-1+ cells and CD34+/CD133+ cells in mice with acute limb ischemia at day 3 after limb ischemia. This was the first time to report that NAC significantly and selectively increased the populations of circulating EPCs (not BM EPCs) in mice with chronic (not acute) limb ischemia. The present study also demonstrated that changes in the subpopulations of EPCs in BM and blood were dynamic and variable in response to limb ischemia, and NAC treatment had very different effects on the same subpopulations of EPCs in BM and blood in mice with acute vs chronic limb ischemia.
NAC, as a potent antioxidant, improves tissue recovery following ischemic injury, including central nerves system, kidney, lung, heart, and limb in mouse, rat and human (Cakir et al., 2003; Cui et al., 2015c; Takhtfooladi et al., 2016; Wang et al., 2011). The mechanisms are largely believed to be related to its ability to prevent reactive oxygen species production and attenuate inflammation (Dludla et al., 2018). Other mechanisms include promoting arterial thrombolysis mainly through interruption of vWF that cross-links platelets in arterial thrombi without significant damage of normal hemostasis (Martinez de Lizarrondo et al., 2017), and preventing Bach1-induced endothelial cell apoptosis, and suppression of cell proliferation (Wang et al., 2016). Pre-reperfusion administration of NAC could decrease ischemia/reperfusion injury-induced allodynia through inhibiting N-methyl-D-aspartate receptor phosphorylation. NAC could restore reactive oxygen species -impaired cell adhesion, and recover the decreased function of human umbilical vein endothelial cells (HUVECs) under oxidative stress (Huang et al., 2016). Additionally, NAC accelerates the healing of amputation stumps in the setting of diabetes and ischemia through stimulating HUVEC migration and proliferation with a phospholipase C β-dependent mechanism and decreased Gαq palmitoylation (Zayed et al., 2017).
EPCs play an important role in cardiovascular repair and regeneration after injuries like limb ischemia, and are associated with cardiovascular morbidity and mortality (Bianconi et al., 2018; Inampudi et al., 2018; Wojakowski and Tendera, 2005). EPCs could repair endothelium via direct differentiation and proliferation at the injured sites and release of various pro-angiogenic molecules (Bianconi et al., 2018). Reactive oxygen species formation and inflammation following limb ischemia could limit the survival and function of EPCs (Higashi et al., 2006). Antioxidant could prevent reactive oxygen species production in EPCs following limb ischemia (Bolcal et al., 2007; Iacobazzi et al., 2014). NAC could protect EPCs from hostile in vivo microenvironment with excessive oxidative stress in conditions like hyperlipidemia and diabetes (Cui et al., 2015b; Hayes et al., 2018). The finding from the present study that NAC significantly increased the population of circulating EPCs in mice with chronic limb ischemia could provide another mechanism that might contribute to the beneficial effect of NAC on the functional recovery of ischemic limb. However, future studies are needed to address the following important questions: 1) how could increase circulating EPCs contribute to the recoveries of blood flow and function of the ischemic limb; 2) does differentiation of circulating EPCs into endothelial cells in the place of ischemia play an important role in mediating the beneficial effects on the recoveries of blood flow and function of ischemic limb? 3) how could growth factors secreted from circulating EPCs contribute to the growth of endothelial cells and capillary vessels after limb ischemia? 4) does NAC has direct impact on muscle cells and/or endothelial cells at the ischemic site such as apoptosis or reactive oxygen species production that may contribute to the recoveries of blood flow and limb function after ischemia? And 5) do antioxidants other than NAC have similar effects on blood flow and limb function recoveries?
The present study showed that NAC effectively blocked limb ischemia (acute and chronic)-induced reactive oxygen species production in both BM and blood cells, yet, only significantly increased the populations of circulating EPCs in mice with chronic ischemia, and interestingly, prevented ischemia-induced increase in EPC numbers in both BM and blood in mice with acute limb ischemia. We previously demonstrated that the role of reactive oxygen species could be very different in the regulation of EPCs populations in different conditions. Short-term treatment with oxidized low-density lipoproteins or hyperlipidemia significantly increased reactive oxygen species production and resulted in a reactive oxygen species-dependent increase in circulating CD34+/Flk-1+ cell population in mice (Cui et al., 2015b). On the other hand, fine particulate matter (PM2.5) exposure could lead to a significant reactive oxygen species-dependent reduction in the population of circulating CD34+/CD133+ cells in mice (Cui et al., 2015d). In the present study, NAC treatment effectively blocked ischemia-induced reactive oxygen species formation in the cells in both BM and blood in mice with acute and chronic limb ischemia, and yet, had very different effects on the sub-populations of EPCs in BM and blood in acute and chronic ischemia. The reason(s) for the dramatic difference in the role of reactive oxygen species in the regulation of the same cell population in different locations and different duration of ischemia is unknown as yet. Clearly the local microenvironment (such as local oxygen level in BM and blood) could be an important factor that contributes significantly to the impact of reactive oxygen species on individual cell populations like BM EPCs. Indeed, different oxygen level has different effects on EPCs (Csete, 2005; Gallagher et al., 2006). It is known that BM is a very hypoxic environment with oxygen saturation between 4% and 7% (Lennon et al., 2001) even as low as 1-2% (Cipolleschi et al., 1993; Ma et al., 2009), while the average oxygen saturation is about 70% in mixed venous blood, and 95.5% in arterial blood (Collins et al., 2015). Hypoxic condition in the BM is also suitable for EPCs survival with decreased apoptosis (Zhou et al., 2017), and enhanced mobilization into peripheral circulation (Gallagher et al., 2006). The differential effects of oxygen at different levels on EPCs are believed to be related to different reactive oxygen species levels in the local environment, and naturally, higher oxygen level leads to more reactive oxygen species production (Hao et al., 2011). These observations are consistent with the findings from the present study that the basal reactive oxygen species level in BM is much lower than that in blood (Fig. 3A and 3B). Two EPCs subpopulations (Sca-1+/Flk-1+ and CD34+/CD133+) were significantly lower in blood than the control after inhibiting reactive oxygen species production by NAC shortly after limb ischemia (3 days). However, at day 21 after ischemia, all circulating EPCs populations in mice with limb ischemia with NAC treatment were significantly increased over the control. It is certainly possible that increased reactive oxygen species level may promote EPCs migration from BM to peripheral blood in the early phase after limb ischemia, while sustained increase in reactive oxygen species level could decrease the number of EPCs in the late phase after limb ischemia. Clearly, further studies are needed to define the mechanism(s) on the selective role of reactive oxygen species in the regulation of circulating EPCs at different anatomical locations (BM vs blood) at early and late phase of limb ischemia.
Some disease and pathological conditions like hyperlipidemia, diabetes mellitus, smoking, and air pollution are associated with increased reactive oxygen species formation and decreased EPC number and function (Cui et al., 2015a; Cui et al., 2015b; Cui et al., 2015d). Patients with these conditions especially diabetes mellitus have significantly increased risk for developing severe PAD with critical limb ischemia and unhealing chronic ulcers. Treatment options are often very limited for these patients with poor outcome, and amputation is frequently required (Gulanti et al., 2015; Forsythe and Hinchliffe 2016). The present study showed that NAC significantly increased circulating EPCs and enhanced the recoveries of blood flow and function of chronic ischemic limb in mice. Thus, the findings in the present study could have substantial clinical significance and therapeutic value for patients with chronic critical limb ischemia. A clinical study is needed to confirm the findings of the mouse model in patients with significant PAD and critical limb ischemia who are not candiadates for re-vasculizaiton.
In conclusion, the data from the present study demonstrated that NAC effectively attenuated ischemia-induced reactive oxygen species production in both BM and blood cells, and selectively enhanced the populations of circulating EPCs in mice with chronic limb ischemia. Further studies are needed to investigate the mechanisms for the differential role of ischemia-induce reactive oxygen species in regulating the number and function of EPCs.
6. Acknowledgements
This work was supported by a US NIH grants to ZL (NIH HL148196 and ES026200).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement
No competing financial interests exist.
8. References
- Bianconi V, Sahebkar A, Kovanen P, Bagaglia F, Ricciuti B, Calabro P, Patti G, Pirro M, 2018. Endothelial and cardiac progenitor cells for cardiovascular repair: A controversial paradigm in cell therapy. Pharmacology & therapeutics 181, 156–168. [DOI] [PubMed] [Google Scholar]
- Bilski P, Belanger AG, Chignell CF, 2002. Photosensitized oxidation of 2 ',7 '-dichlorofluorescin: Singlet oxygen does not contribute to the formation of fluorescent oxidation product 2 ',7 '-dichlorofluorescein. Free Radical Bio Med 33, 938–946. [DOI] [PubMed] [Google Scholar]
- Bolcal C, Yildirim V, Doganci S, Sargin M, Aydin A, Eken A, Ozal E, Kuralay E, Demirkilic U, Tatar H, 2007. Protective effects of antioxidant medications on limb ischemia reperfusion injury. The Journal of surgical research 139, 274–279. [DOI] [PubMed] [Google Scholar]
- Brechot N, Gomez E, Bignon M, Khallou-Laschet J, Dussiot M, Cazes A, Alanio-Brechot C, Durand M, Philippe J, Silvestre JS, Van Rooijen N, Corvol P, Nicoletti A, Chazaud B, Germain S, 2008. Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin-1-deficient mice. PLoS One 3, e3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir O, Erdem K, Oruc A, Kilinc N, Eren N, 2003. Neuroprotective effect of N-acetylcysteine and hypothermia on the spinal cord ischemia-reperfusion injury. Cardiovascular surgery 11, 375–379. [DOI] [PubMed] [Google Scholar]
- Cipolleschi MG, Dello Sbarba P, Olivotto M, 1993. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood 82, 2031–2037. [PubMed] [Google Scholar]
- Collins JA, Rudenski A, Gibson J, Howard L, O'Driscoll R, 2015. Relating oxygen partial pressure, saturation and content: the haemoglobin-oxygen dissociation curve. Breathe 11, 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csete M, 2005. Oxygen in the cultivation of stem cells. Annals of the New York Academy of Sciences 1049, 1–8. [DOI] [PubMed] [Google Scholar]
- Cui Y, Jia F, He J, Xie X, Li Z, Fu M, Hao H, Liu Y, Liu DZ, Cowan PJ, Zhu H, Sun Q, Liu Z, 2015a. Ambient Fine Particulate Matter Suppresses In Vivo Proliferation of Bone Marrow Stem Cells through Reactive Oxygen Species Formation. PLoS One 10, e0127309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Narasimhulu CA, Liu L, Li X, Xiao Y, Zhang J, Xie X, Hao H, Liu JZ, He G, Cowan PJ, Cui L, Zhu H, Parthasarathy S, Liu Z, 2015b. Oxidized low-density lipoprotein alters endothelial progenitor cell populations. Frontiers in bioscience 20, 975–988. [DOI] [PubMed] [Google Scholar]
- Cui Y, Narasimhulu CA, Liu L, Zhang Q, Liu PZ, Li X, Xiao Y, Zhang J, Hao H, Xie X, He G, Cui L, Parthasarathy S, Liu Z, 2015c. N-acetylcysteine inhibits in vivo oxidation of native low-density lipoprotein. Scientific reports 5, 16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Sun Q, Liu Z, 2016. Ambient particulate matter exposure and cardiovascular diseases: a focus on progenitor and stem cells. J Cell Mol Med 20, 782–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Xie X, Jia F, He J, Li Z, Fu M, Hao H, Liu Y, Liu JZ, Cowan PJ, Zhu H, Sun Q, Liu Z, 2015d. Ambient fine particulate matter induces apoptosis of endothelial progenitor cells through reactive oxygen species formation. Cell Physiol Biochem 35, 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, He Y, Zhang H, Yu L, Wan T, Xu Z, Jones D, Chen H, Min W, 2009. Endothelial-specific expression of mitochondrial thioredoxin promotes ischemia-mediated arteriogenesis and angiogenesis. Arteriosclerosis, thrombosis, and vascular biology 29, 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Medeiros WA, da Silva LA, Dall'Igna DM, Michels M, Manfredini A, Dos Santos Cardoso J, Constantino L, Scaini G, Vuolo F, Streck EL, Ritter C, Dal-Pizzol F, 2018. N-acetylcysteine effects on a murine model of chronic critical limb ischemia. Biochimica et biophysica acta 1864, 454–463. [DOI] [PubMed] [Google Scholar]
- Dludla PV, Dias SC, Obonye N, Johnson R, Louw J, Nkambule BB, 2018. A Systematic Review on the Protective Effect of N-Acetyl Cysteine Against Diabetes-Associated Cardiovascular Complications. American journal of cardiovascular drugs : drugs, devices, and other interventions. [DOI] [PubMed] [Google Scholar]
- Faber JE, Zhang H, Lassance-Soares RM, Prabhakar P, Najafi AH, Burnett MS, Epstein SE, 2011. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arteriosclerosis, thrombosis, and vascular biology 31, 1748–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Schouteden S, Geenens R, Van Duppen V, Herijgers P, Holvoet P, Van Veldhoven PP, Verfaillie CM, 2012. Hematopoietic stem/progenitor cell proliferation and differentiation is differentially regulated by high-density and low-density lipoproteins in mice. PloS one 7, e47286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher KA, Goldstein LJ, Thom SR, Velazquez OC, 2006. Hyperbaric oxygen and bone marrow-derived endothelial progenitor cells in diabetic wound healing. Vascular 14, 328–337. [DOI] [PubMed] [Google Scholar]
- Gounis MJ, Spiga MG, Graham RM, Wilson A, Haliko S, Lieber BB, Wakhloo AK, Webster KA, 2005. Angiogenesis is confined to the transient period of VEGF expression that follows adenoviral gene delivery to ischemic muscle. Gene therapy 12, 762–771. [DOI] [PubMed] [Google Scholar]
- Hao Y, Cheng D, Ma Y, Zhou W, Wang Y, 2011. The relationship between oxygen concentration, reactive oxygen species and the biological characteristics of human bone marrow hematopoietic stem cells. Transplantation proceedings 43, 2755–2761. [DOI] [PubMed] [Google Scholar]
- Hayes KL, Messina LM, Schwartz LM, Yan J, Burnside AS, Witkowski S, 2018. Type 2 diabetes impairs the ability of skeletal muscle pericytes to augment postischemic neovascularization in db/db mice. American journal of physiology. Cell physiology 314, C534–C544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y, Nishioka K, Umemura T, Chayama K, Yoshizumi M, 2006. Oxidative stress, endothelial function and angiogenesis induced by cell therapy and gene therapy. Current pharmaceutical biotechnology 7, 109–116. [DOI] [PubMed] [Google Scholar]
- Holzapfel K, Neuhofer W, Bartels H, Fraek ML, Beck FX, 2007. Role of focal adhesion kinase (FAK) in renal ischaemia and reperfusion. Pflugers Archiv : European journal of physiology 455, 273–282. [DOI] [PubMed] [Google Scholar]
- Houlihan DD, Mabuchi Y, Morikawa S, Niibe K, Araki D, Suzuki S, Okano H, Matsuzaki Y, 2012. Isolation of mouse mesenchymal stem cells on the basis of expression of Sca-1 and PDGFR-alpha. Nature protocols 7, 2103–2111. [DOI] [PubMed] [Google Scholar]
- Hu C, Zhao L, Peng C, Li L, 2018a. Regulation of the mitochondrial reactive oxygen species: Strategies to control mesenchymal stem cell fates ex vivo and in vivo. Journal of cellular and molecular medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Dai SC, Luan X, Chen J, Cannavicci A, 2018b. Dysfunction and Therapeutic Potential of Endothelial Progenitor Cells in Diabetes Mellitus. Journal of clinical medicine research 10, 752–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Pan WY, Tseng MT, Lin KJ, Yang YP, Tsai HW, Hwang SM, Chang Y, Wei HJ, Sung HW, 2016. Enhancement of cell adhesion, retention, and survival of HUVEC/cbMSC aggregates that are transplanted in ischemic tissues by concurrent delivery of an antioxidant for therapeutic angiogenesis. Biomaterials 74, 53–63. [DOI] [PubMed] [Google Scholar]
- Iacobazzi D, Mangialardi G, Gubernator M, Hofner M, Wielscher M, Vierlinger K, Reni C, Oikawa A, Spinetti G, Vono R, Sangalli E, Montagnani M, Madeddu P, 2014. Increased antioxidant defense mechanism in human adventitia-derived progenitor cells is associated with therapeutic benefit in ischemia. Antioxidants & redox signaling 21, 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inampudi C, Akintoye E, Ando T, Briasoulis A, 2018. Angiogenesis in peripheral arterial disease. Current opinion in pharmacology 39, 60–67. [DOI] [PubMed] [Google Scholar]
- Lennon DP, Edmison JM, Caplan AI, 2001. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. Journal of cellular physiology 187, 345–355. [DOI] [PubMed] [Google Scholar]
- Liu L, Cui Y, Li X, Que X, Xiao Y, Yang C, Zhang J, Xie X, Cowan PJ, Tian J, Hao H, Liu Z, 2019. Concomitant overexpression of triple antioxidant enzymes selectively increases circulating endothelial progenitor cells in mice with limb ischaemia. J Cell Mol Med 23, 4019–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Kakiuchi-Kiyota S, Arnold LL, Johansson SL, Wert D, Cohen SM, 2013. Pathogenesis of human hemangiosarcomas and hemangiomas. Human pathology 44, 2302–2311. [DOI] [PubMed] [Google Scholar]
- Ma T, Grayson WL, Frohlich M, Vunjak-Novakovic G, 2009. Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnology progress 25, 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez de Lizarrondo S, Gakuba C, Herbig BA, Repesse Y, Ali C, Denis CV, Lenting PJ, Touze E, Diamond SL, Vivien D, Gauberti M, 2017. Potent Thrombolytic Effect of N-Acetylcysteine on Arterial Thrombi. Circulation 136, 646–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massett MP, Berk BC, 2005. Strain-dependent differences in responses to exercise training in inbred and hybrid mice. American journal of physiology. Regulatory, integrative and comparative physiology 288, R1006–1013. [DOI] [PubMed] [Google Scholar]
- McClung JM, McCord TJ, Southerland K, Schmidt CA, Padgett ME, Ryan TE, Kontos CD, 2016. Subacute limb ischemia induces skeletal muscle injury in genetically susceptible mice independent of vascular density. Journal of vascular surgery 64, 1101–1111 e1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MP, Lee D, Lee SH, Lee HE, Lee HY, Lee YM, 2015. Deguelin inhibits vasculogenic function of endothelial progenitor cells in tumor progression and metastasis via suppression of focal adhesion. Oncotarget 6, 16588–16600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh PP, Liu ZJ, Velazquez OC, 2017. A Molecular and Clinical Review of Stem Cell Therapy in Critical Limb Ischemia. Stem cells international 2017, 3750829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DXM, Silva GV, Lai DJ, Thomas JD, Kronenberg MW, Martin AD, Anderson RD, Traverse JH, Penn MS, Anwaruddin S, Hatzopoulos AK, Gee AP, Taylor DA, Cogle CR, Smith D, Westbrook L, Chen J, Handberg E, Olson RE, Geither C, Bowman S, Francescon J, Baraniuk S, Piller LB, Simpson LM, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD, Networ CCTR, 2012. Effect of Transendocardial Delivery of Autologous Bone Marrow Mononuclear Cells on Functional Capacity, Left Ventricular Function, and Perfusion in Chronic Heart Failure The FOCUS-CCTRN Trial. Jama-J Am Med Assoc 307, 1717–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JP, Bruner LH, Bassoe CF, Hudson JL, Ward PA, Phan SH, 1988. Measurement of Intracellular Fluorescence of Human-Monocytes Relative to Oxidative-Metabolism. J Leukocyte Biol 43, 304–310. [DOI] [PubMed] [Google Scholar]
- Sirrs S, Hannah-Shmouni F, Nantel S, Neuberger J, Yoshida EM, 2018. Transplantation as disease modifying therapy in adults with inherited metabolic disorders. Journal of inherited metabolic disease. [DOI] [PubMed] [Google Scholar]
- Steinhoff G, Nesteruk J, Wolfien M, Grosse J, Ruch U, Vasudevan P, Muller P, 2017. Stem cells and heart disease - Brake or accelerator? Advanced drug delivery reviews 120, 2–24. [DOI] [PubMed] [Google Scholar]
- Tadeo I, Bueno G, Berbegall AP, Fernandez-Carrobles MM, Castel V, Garcia-Rojo M, Navarro S, Noguera R, 2016. Vascular patterns provide therapeutic targets in aggressive neuroblastic tumors. Oncotarget 7, 19935–19947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T, 1999. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nature medicine 5, 434–438. [DOI] [PubMed] [Google Scholar]
- Takhtfooladi HA, Hesaraki S, Razmara F, Takhtfooladi MA, Hajizadeh H, 2016. Effects of N-acetylcysteine and pentoxifylline on remote lung injury in a rat model of hind-limb ischemia/reperfusion injury. Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia 42, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse JH, 2013. Effect of the Use and Timing of Bone Marrow Mononuclear Cell Delivery on Left Ventricular Function After Acute Myocardial Infarction: The TIME Randomized Trial (vol 308, pg 2380, 2012). Jama-J Am Med Assoc 309, 343–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhang X, Ding Q, Hu B, Xie Y, Li X, Yang Q, Xiong L, 2011. Limb remote postconditioning alleviates cerebral reperfusion injury through reactive oxygen species-mediated inhibition of delta protein kinase C in rats. Anesthesia and analgesia 113, 1180–1187. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu J, Jiang L, Wei X, Niu C, Wang R, Zhang J, Meng D, Yao K, 2016. Bach1 Induces Endothelial Cell Apoptosis and Cell-Cycle Arrest through ROS Generation. Oxidative medicine and cellular longevity 2016, 6234043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerweel PE, Teraa M, Rafii S, Jaspers JE, White IA, Hooper AT, Doevendans PA, Verhaar MC, 2013. Impaired endothelial progenitor cell mobilization and dysfunctional bone marrow stroma in diabetes mellitus. PloS one 8, e60357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojakowski W, Tendera M, 2005. Mobilization of bone marrow-derived progenitor cells in acute coronary syndromes. Folia histochemica et cytobiologica 43, 229–232. [PubMed] [Google Scholar]
- Yang J, Ii M, Kamei N, Alev C, Kwon SM, Kawamoto A, Akimaru H, Masuda H, Sawa Y, Asahara T, 2011. CD34+ cells represent highly functional endothelial progenitor cells in murine bone marrow. PloS one 6, e20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayed MA, Wei X, Park KM, Belaygorod L, Naim U, Harvey J, Yin L, Blumer K, Semenkovich CF, 2017. N-Acetylcysteine accelerates amputation stump healing in the setting of diabetes. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 31, 2686–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yu P, Wu R, Ge Y, Wu J, Zhu J, Jia R, 2013. Renal cell carcinoma-adjacent tissues enhance mobilization and recruitment of endothelial progenitor cells to promote the invasion of the neoplasm. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 67, 643–649. [DOI] [PubMed] [Google Scholar]
- Zhou P, Tan YZ, Wang HJ, Wang GD, 2017. Hypoxic preconditioning-induced autophagy enhances survival of engrafted endothelial progenitor cells in ischaemic limb. Journal of cellular and molecular medicine 21, 2452–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]