Abstract
Objective:
An ethanolic extract of Artemisia scoparia (SCO) improves adipose tissue function and reduces negative metabolic consequences of high-fat feeding. A scoparia has a long history of medicinal use across Asia, and has anti-inflammatory effects in various cell types and disease models. The objective of the current study was to investigate SCO’s effects on inflammation in cells relevant to metabolic health.
Methods:
Inflammatory responses were assayed in cultured adipocytes, macrophages, and insulinoma cells, by quantitative PCR, immunoblotting, and NF-κB reporter assays.
Results:
In TNFα-treated adipocytes, SCO mitigated ERK and NF-κB signaling, as well as transcriptional responses, but had no effect on fatty acid-binding protein 4 (FABP4) secretion. SCO also reduced levels of deleted in breast cancer 1 (DBC1) protein in adipocytes, and inhibited inflammatory gene expression in stimulated macrophages. Finally, in pancreatic β-cells, SCO decreased NF-κB-responsive promoter activity induced by IL-1β treatment.
Conclusions:
SCO’s ability to promote adipocyte development and function is thought to mediate its insulin-sensitizing actions in vivo. Our findings that SCO inhibits inflammatory responses through at least two distinct signaling pathways (ERK and NF-κB) in three cell types known to contribute to metabolic disease, reveal that SCO may act more broadly than previously thought to improve metabolic health.
Keywords: adipose tissue, botanical, inflammation, beta cells, macrophages, Type 2 diabetes
INTRODUCTION
The obesity epidemic and its associated metabolic disorders are among the major health challenges of our time (1). Adipose tissue plays a crucial role in these metabolic disease states, and disruptions in adipocyte development and function negatively impact whole-body insulin sensitivity and systemic metabolic health (2). Adipocyte differentiation and adipose tissue expansion are often impaired in obese states, leading to insulin resistance and metabolic dysregulation (3, 4). In addition, obesity and insulin resistance are associated with enhanced basal lipolysis rates, which exacerbate metabolic disease (reviewed in (5)). Inflammatory processes in adipose tissue are also a prominent feature of obesity and the metabolic syndrome, and there is extensive crosstalk between inflammation, adipogenesis, endocrine function, and lipid metabolism in adipose tissue (5, 6). Because of its importance in obesity and diabetes, adipose tissue is considered a valuable target for therapeutic intervention (2).
Botanicals have an extensive history of medicinal use across most, if not all cultures the world over, and plants have long been an important source of pharmaceutical compounds. Notably, metformin, the first-line drug in treating type 2 diabetes mellitus (T2DM), was derived from galegine, a bioactive first isolated from Galega officinalis, also known as goat’s rue or French lilac (7). Screening efforts in our laboratory identified an extract of Artemisia scoparia (SCO) as a potent enhancer of adipocyte differentiation in vitro. Subsequent studies in a diet-induced obesity (DIO) mouse model have shown that SCO also has metabolically beneficial effects on adipose tissue in vivo (8, 9). In addition, SCO supplementation improves whole-body insulin sensitivity, and reduces circulating levels of fatty acids and triglycerides (8–10). More recently, we have demonstrated that SCO can act on adipocytes in vitro to reduce lipolysis induced by the inflammatory cytokine, TNFα (11).
A. scoparia has a well-documented history of medicinal use (12, 13), and it has been shown to have a wide range of effects in disease models related to Alzheimer’s, renal oxidative stress, hepatotoxicity, hypertension, and others (14–17). More specifically, anti-inflammatory effects of A. scoparia have been described in a wide range of cell types and contexts (18–20). In adipose tissue, TNFα secreted from resident macrophages is a principal mediator of obesity-associated inflammation (6, 21, 22). Given the important role of adipose tissue inflammation in obesity-related metabolic dysfunction, we examined the ability of SCO to regulate TNFα action and inflammatory gene expression in adipocytes. We observed that SCO reduced TNFα’s effects on inflammatory cytokine expression and NF-κB activation. SCO also reduced nuclear levels of deleted in breast cancer 1 (DBC1, also known as cell cycle and apoptosis regulator 2 or CCAR2), a protein associated with impaired metabolic function (23–26). Although SCO reduced lipolysis induced by TNFα, it had no effect on TNFα-induced secretion of FABP4, which has been shown to be enhanced by lipolytic stimuli and reduced by interventions that inhibit lipolysis. (27, 28). Finally, we observed anti-inflammatory effects of SCO in two other cell types critical in metabolic disease states: macrophages and pancreatic β-cells. Taken together, data presented herein, as well as previously published studies, support further investigation of SCO as a potential therapeutic in the treatment of metabolic disease.
MATERIALS AND METHODS
Botanical extract source and preparation.
Artemisia scoparia plants were grown and harvested, and ethanolic extracts were prepared as previously reported (8, 11). SCO extracts were dissolved in DMSO at 1000X final concentration (10 mg/ml and 50 mg/ml respectively for 10 or 50 μg/ml treatments).
Adipocyte cell culture and treatments.
3T3-L1 preadipocytes were grown and differentiated as previously described (8). Briefly, cells were grown in high-glucose DMEM supplemented with 10% bovine calf serum. Cells were induced to differentiate two days after reaching confluence, in DMEM plus 10% fetal bovine serum (FBS) containing 0.5 mM IBMX, 1 μM dexamethasone, and 1.7 μM insulin. Cells were fed 48-72 hours later with DMEM plus 10% FBS and 0.425 μM insulin, then every 48-72 hours with DMEM plus 10% FBS. DMEM, IBMX, dexamethasone, and insulin were obtained from Sigma-Aldrich (St. Louis, MO); calf serum and FBS were purchased from Hyclone (GE Life Sciences, Logan, UT). Treatments with SCO were initiated between 6 and 13 days after induction of differentiation. Murine TNFα was purchased from Life Technologies (Carlsbad, CA) and dissolved at 0.5 μM in PBS containing 0.1% bovine serum albumin BSA, and added to cell culture media at the final concentrations indicated in figure legends.
Macrophage cell culture and treatments.
RAW 264.7 macrophages were cultured in high-glucose DMEM supplemented with 10% FBS. Cells were pretreated for 2 hours with SCO, then lipopolysaccharide from E. coli 0111:B4 strain (LPS) (InvivoGen, San Diego, CA) was added for an additional 5.5-hour incubation before harvest.
Insulinoma cell culture and treatments.
832/13 rat insulinoma cells were cultured as described previously (29) and transfected with an adenoviral luciferase reporter construct under the control of 5 copies of a consensus NF-κB element (vector obtained from Vector Biolabs, Malvern, PA: catalog number 1740). 12 hours after transfection, cells were treated overnight with 5 or 10 μg/ml of A. scoparia (SCO) or A. santolinaefolia (SAN), then for 4 hours with 1 ng/ml IL-1β. Luciferase reporter activity was assessed by luminometry and normalized to total protein content.
RNA isolation and gene expression analysis.
Treated cells were harvested in RLT cell lysis buffer (Qiagen, Hilden, Germany). Lysates were stored at −80°C prior to RNA extraction using the RNeasy Mini kit (Qiagen). The High-Capacity Reverse Transcription kit (Applied Biosystems, Foster City, CA) was used to reverse transcribe the RNA samples, and gene expression was assayed by qPCR with Takara SYBR premix (Takara Bio USA, Mountain View, CA) on the Applied Biosystems 7900HT system (cycling conditions: 2 min @ 50°C; 10 min @ 95°C; 40 cycles of 15s @ 95°C and 1 min @ 60°C; dissociation stage: 15 s @ 95°C, 15 s @ 60°C, and 15s @ 95°C with end step ramp rate of 2%). Data were analyzed using SDS 2.3 or 2.4 software. Target gene data were normalized to the reference gene non-POU-domain-containing, octamer-binding protein (Nono). Primers were obtained from Integrated DNA Technologies (IDT, Skokie, IL), and sequences were as follows: Nono forward 5’-CATCATCAGCATCACCACCA-3’, reverse 5’-TCTTCAGGTCAATAGTCAAGCC-3’; Ccl2 (Mcp1) forward 5’-GCAGAGAGCCAGACGGGAGGA-3’, reverse 5’-TGGGGCGTTAACTGCATCTGG-3’; Il6 forward 5’-TCCTCTCTGCAAGAGACTTCCATCC-3’, reverse 5’-AAGCCTCCGACTTGTGAAGTGGT-3’; Lcn2 forward 5’-TGCAAGTGGCCACCACGGAC-3’, reverse 5’-GCATTGGTCGGTGGGGACAGAGA-3’, Nos2 (iNos) forward 5’-CCCTCCTGATCTTGTGTTGGA-3’, reverse 5’-TCAACCCGAGCTCCTGGAA-3’; Tnfa forward 5’-AGACCCTCACACTCAGATCA-3’, reverse 5’-TCTTTGAGATCCATGCCGTTG-3’.
Protein samples and immunoblotting.
For whole-cell extract preparation, adipocyte monolayers were harvested in radioimmunoprecipitation assay (RIPA) buffer (30) containing the following protease and phosphatase inhibitors: 1 mM phenylmethylsulfonyl fluoride, 1 μM pepstatin, 50 trypsin inhibitory milliunits of aprotinin, 10 μM leupeptin, 1 mM 10-phenanthroline, 0.2 mM sodium orthovanadate, and 100 μM sodium fluoride. Lysates were stored at −80°C, then thawed, passed through a 20G needle three times and clarified by centrifugation at 13000g. Supernatants were recovered, and protein concentrations were determined by bicinchoninic acid (BCA) assay (Sigma-Aldrich). Equal amounts of protein from each sample were loaded onto polyacrylamide gels, subjected to electrophoresis, and transferred to nitrocellulose. Standard immunoblotting techniques were applied to probe for target proteins. Primary antibodies were purchased from Cell Signaling Technologies (Danvers, MA) for ERK 1/2, CCAR2 (DBC1), β-actin and NF-κB p65; from R&D Systems (Bio-Techne, Minneapolis, MN) for lipocalin 2 (LCN2), Promega (Madison, WI) for phosphorylated ERK 1/2 (active MAPK), and Abeam (Cambridge, MA) for FABP4. Detection was performed with horseradish peroxidase-conjugated antibodies from Jackson Immunoresearch (West Grove, PA). Autoradiography films were scanned and densitometry analysis performed using ImageStudio software from Li-Cor Biosciences (Lincoln, NE).
Subcellular fractionation.
After treatment, adipocytes were harvested in nuclear homogenization buffer (NHB) (20 mM Tris pH 7.4, 10 mM NaCl, and 3 mM MgCl2). IGEPAL CA-630 (Sigma-Aldrich) was added to the cell suspension at a final concentration of 0.15% prior to Dounce homogenization on ice. The nuclear fraction was pelleted by centrifugation at 517g, washed in NHB, and resuspended in IP buffer. NHB and IP buffer were supplemented with protease and phosphatase inhibitors. Protein concentrations were determined by BCA assay.
FABP4 secretion and lipolysis assays.
Cells pretreated for three days with SCO or DMSO vehicle, were treated overnight with or without 0.75 nM TNFα. The following morning, culture medium was replaced with lipolysis incubation medium (low-glucose DMEM + 2% BSA). Cells treated with TNFα overnight were treated with the same concentration of TNFα for the lipolysis/FABP4 secretion analysis. The remaining cells were treated with either vehicle (controls), or 2 nM or 10 μM of isoproterenol. Conditioned media samples were collected after four hours and analyzed for FAPB4 content by immunoblotting. Lipolysis was also assessed by measuring glycerol concentrations in the samples, using free glycerol reagent from Sigma-Aldrich.
RESULTS
SCO inhibits TNFα-induced inflammatory gene expression in 3T3-L1 adipocytes
Our previous studies have demonstrated that SCO could reduce protein levels of the inflammatory cytokine C-C motif chemokine ligand 2 (CCL2) (also known as monocyte chemoattractant protein 1, MCP-1) in the adipose tissue of high-fat diet-fed mice, and inflammatory gene expression in cultured adipocytes (9). Hence, we have sought to characterize the cell-autonomous anti-inflammatory effects of SCO in 3T3-L1 adipocytes treated with TNFα, a predominant mediator of adipose tissue inflammation that increases the expression of several inflammatory genes in adipocytes (21). As shown in Figure 1, pretreatment of 3T3-L1 adipocytes with 50 μg/ml SCO significantly diminished TNFα-induced expression of Ccl2 and interleukin 6 (Il6), consistent with previously published studies (8), as well as lipocalin 2 (Lcn2) and nitric oxide synthase 2, inducible (Nos2 or iNos). We also examined the time course for induction of these genes and for the effects of SCO. As shown in Figure 2, the temporal patterns of induction by TNFα and inhibition by SCO were different for all four genes assayed. While Ccl2 and Lcn2 both increased gradually over the 8-hour TNFα treatment, the effects of SCO were distinct. In the case of Ccl2, SCO significantly inhibited TNFα-induced gene expression at all time points. Yet, for Lcn2, SCO increased expression in basal conditions at all time points and at the early time points of TNFα treatment (1 and 2 hours). After 4 hours of TNFα treatment, SCO-treated cells had equivalent Lcn2 expression to the controls, and after 8 hours, expression was significantly lower in SCO-treated cells. Induction of Il6 gene expression by TNFα was observed at all time points, but the response was biphasic. Specifically, expression strongly increased at 1 hour, subsided at 2 and 4 hours, then substantially increased again at 8 hours. This same pattern of TNFα induction was observed in SCO-treated cells, but with lower Il6 expression levels than without SCO at all time points. SCO attenuation of the TNFα effect on Il6 was significant at 1, 4, and 8 hours, but not at 2 hours. Finally, Nos2 expression and nitric oxide production induced by TNFα have been implicated in the regulation of adipocyte lipolysis (31). TNFα induced Nos2 expression starting at the 2-hour time point, and SCO significantly but modestly inhibited TNFα’s effects, consistent with its previously described anti-lipolytic actions (11).
Figure 1. SCO inhibits TNFα-induced expression of inflammatory genes in 3T3-L1 adipocytes.
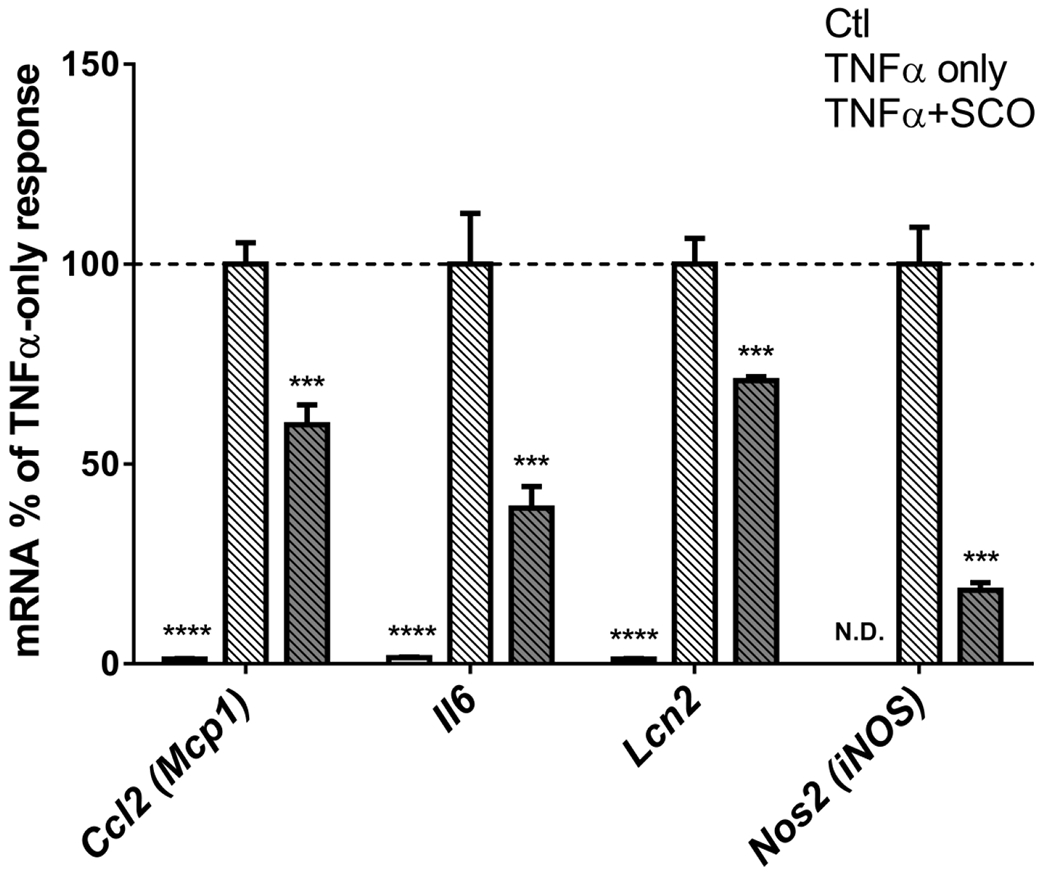
Mature 3T3-L1 adipocytes were pretreated with 50 μg/ml SCO for 3-days, then with 0.75 nM TNFα for 18 hours. Cells were harvested for RNA isolation, and gene expression was assayed by qPCR. Target gene data were normalized to the reference gene Nono. Fold-change was calculated versus the mean of TNFα-only controls for each gene. Data are shown as means +/− SE; n=4 biological replicates per condition. N.D.: Nos2 was below detection level for the qPCR assay. Data for each gene were analyzed by one-way ANOVA (for Ccl2, Il6, and Lcn2) or t-test (for Nos2). Significance expressed as ***P<0.001, ****P<0.0001 vs. TNFα-only controls. Results were replicated in two additional experiments on independent batches of cells.
Figure 2. Time course of TNFα- and SCO-induced changes in inflammatory gene expression.
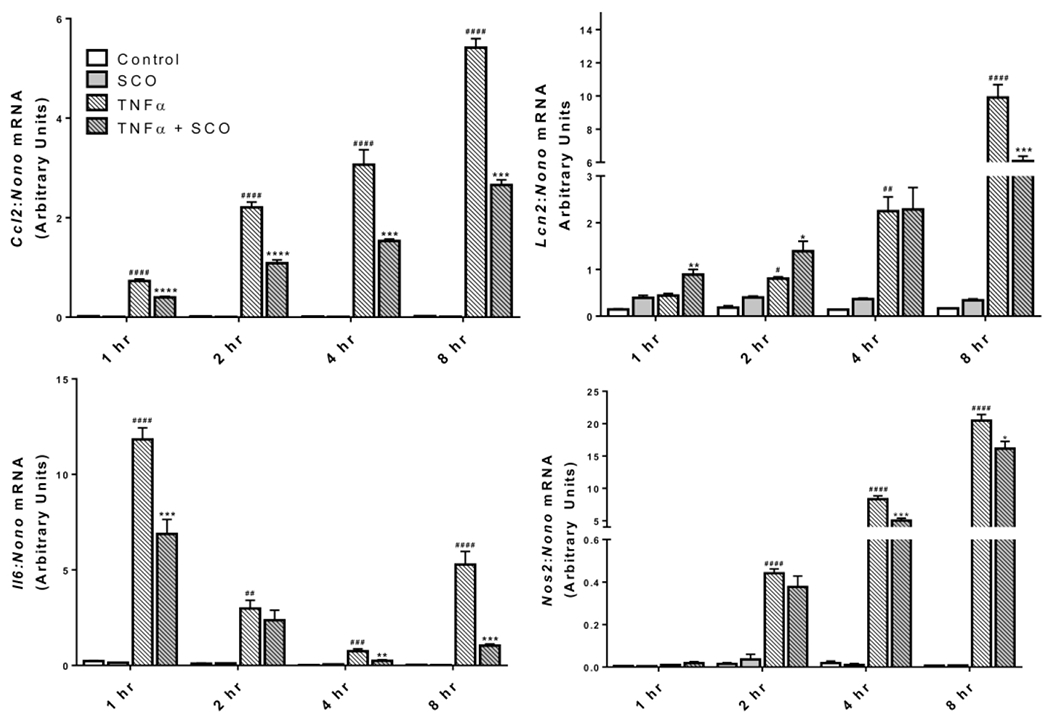
Mature 3T3-L1 adipocytes were pretreated with 50 μg/ml SCO or DMSO vehicle for 3 days prior to harvest. Media was replaced with low-glucose DMEM supplemented with 2% BSA, and cells were treated with 0.75 nM TNFα or 0.1 % BSA vehicle for the times indicated and harvested for RNA isolation. Expression of the inflammatory genes was assessed by qPCR and normalized to the reference gene Nono. One-way ANOVA was performed for each time point. ##P<0.01, ###P<0.001, and ####P<0.0001 for effect of TNFα vs CON; *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001 for effect of SCO vs. respective controls (CON or TNFα only). Results were replicated on an independent batch of cells.
SCO inhibits TNFα-induced lipocalin 2 secretion in 3T3-L1 adipocytes
Lipocalin 2 (LCN2) is a pro-inflammatory mediator secreted by adipocytes (reviewed in (32)). We have previously shown that LCN2 expression and secretion are induced by TNFα in 3T3-L1 adipocytes (33). As shown in Figure 3, the TNFα-induced expression and secretion of LCN2 from adipocytes was substantially reduced in the presence of SCO. These observations are consistent with the ability of SCO to impair TNFα induction of Lcn2 gene expression (Figures 1 and 2).
Figure 3. SCO inhibits TNFα-induced cellular levels and secretion of LCN2 in adipocytes.
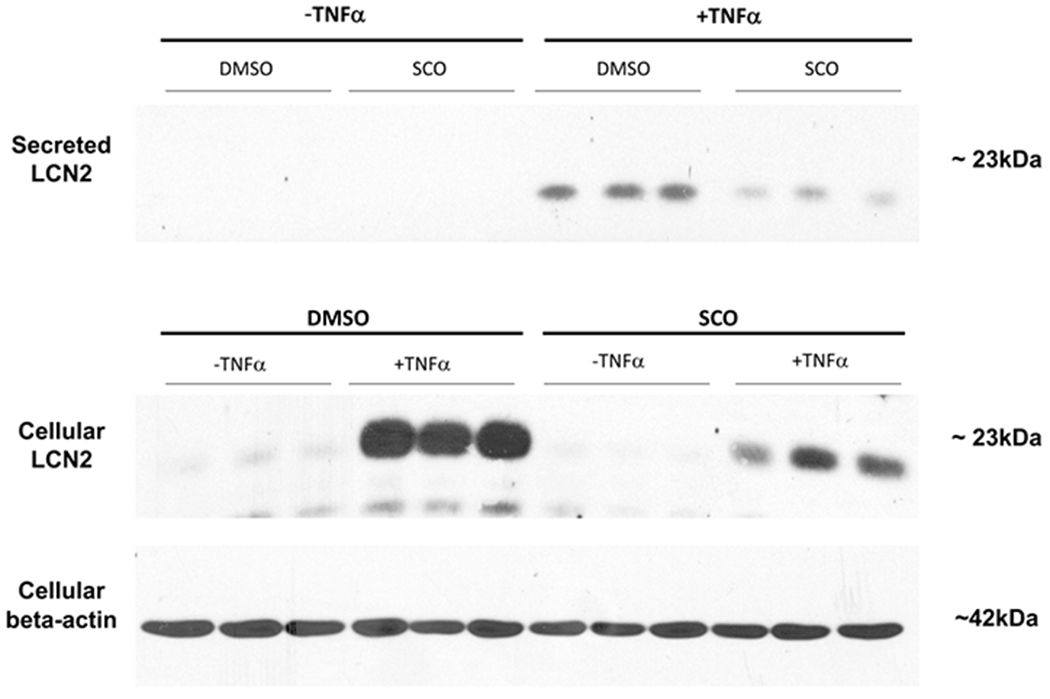
Mature 3T3-L1 adipocytes were pretreated with 50 μg/ml SCO or DMSO vehicle for 3 days prior to harvest. On the last day of SCO treatment, cells were treated with 0.75nM TNFα or 0.1% BSA vehicle for 18 hours. Medium on all cells was then replaced with low-glucose phenol-red-free DMEM containing either vehicle or 0.75 nM TNFα. After 4 hours, samples of conditioned media were collected and cells were harvested in RIPA buffer for immunoblotting. 125 μg (media) or 75 μg (whole-cell extracts) of total protein were loaded in each well.
SCO reduces total and phosphorylated ERK levels
In adipocytes, TNFα signaling mediates transcriptional regulation of numerous target genes involved in adipocyte function (inflammation, insulin signaling, lipolysis, endocrine function, stress responses), through the activation of several signaling pathways (reviewed in (21)), including the MAP kinase, ERK. To further characterize the effects of SCO in the context of TNFα action in adipocytes, we examined ERK activation in the presence or absence of SCO. As shown in Figure 4, TNFα regulates both the expression of ERK, and its activation, as judged by phosphorylation. TNFα induces ERK phosphorylation, and this activation is reduced in the presence of SCO. We also observed that total ERK1/2 levels were modulated by both TNFα (increased) and SCO (decreased). Comparison of the ratios of phosphorylated ERK 1/2 to total ERK 1/2 revealed that the effect of SCO on relative ERK activation was not significant.
Figure 4. SCO reduces total and phosphorylated ERK levels in 3T3-L1 adipocytes.
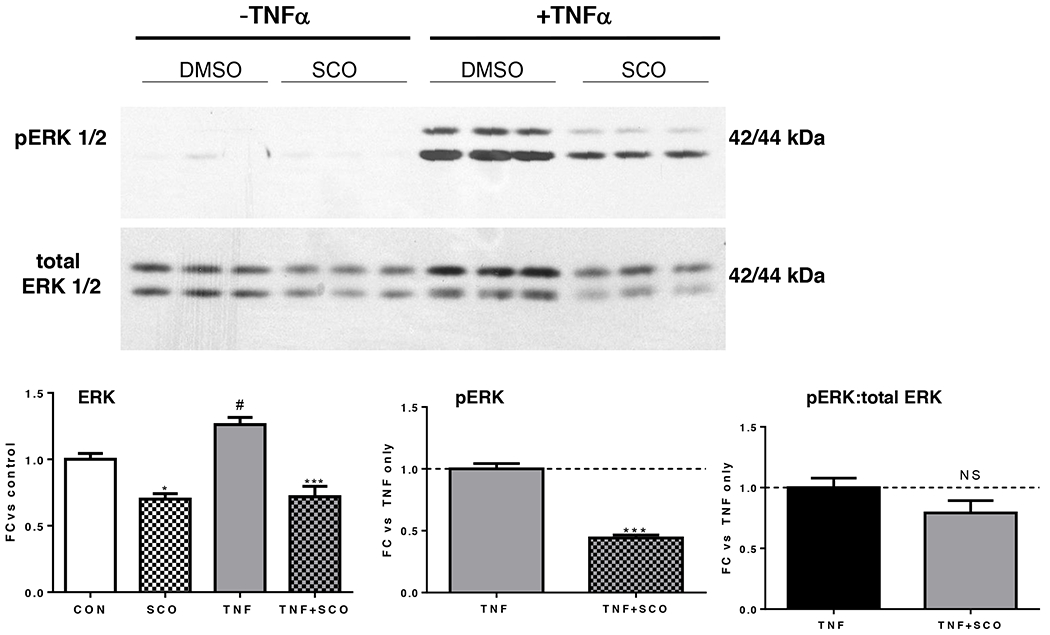
Mature 3T3-L1 adipocytes were pretreated with 50 μg/ml SCO or DMSO vehicle for 3 days, with 18-hour (overnight) serum deprivation (0.3% BSA) and 0.5 nM TNFα treatment on the last day. Cells were harvested in RIPA buffer and analyzed by Western blot. Blot images and quantitation data are shown. *P<0.05 and ***P<0.001 for effect of SCO vs. respective controls (CON or TNFα only); #P<0.05 for effect of TNFα vs CON; NS: not significant. Results were replicated in two additional experiments on independent batches of cells.
SCO inhibits TNFα-induced nuclear translocation of NF-κB and reduces nuclear levels of Deleted in breast cancer 1 (DBC1) in 3T3-L1 adipocytes.
Another major signaling pathway engaged by TNFα in adipocytes is the IKK/NF-κB pathway. TNFα causes phosphorylation of the IKK complex, as well as phosphorylation and degradation of its target, inhibitor of nuclear factor-κB (IκB), resulting in the translocation of NF-κB to the nucleus. In order to determine whether SCO could modulate the TNFα activation of NF-κB in fat cells, we treated 3T3-L1 adipocytes with TNFα, with or without a 3-day SCO pretreatment. The cytosolic and nuclear compartments were analyzed by immunoblotting. As shown in Figure 5, TNFα treatment produced a very robust increase in nuclear levels of the NF-κB p65 subunit, and SCO pretreatment greatly inhibited this response, indicating the ability of SCO to interfere with a major inflammatory signaling event.
Figure 5. SCO reduces nuclear DBC1 and TNFα-induced p65 nuclear translocation in 3T3-L1 adipocytes.
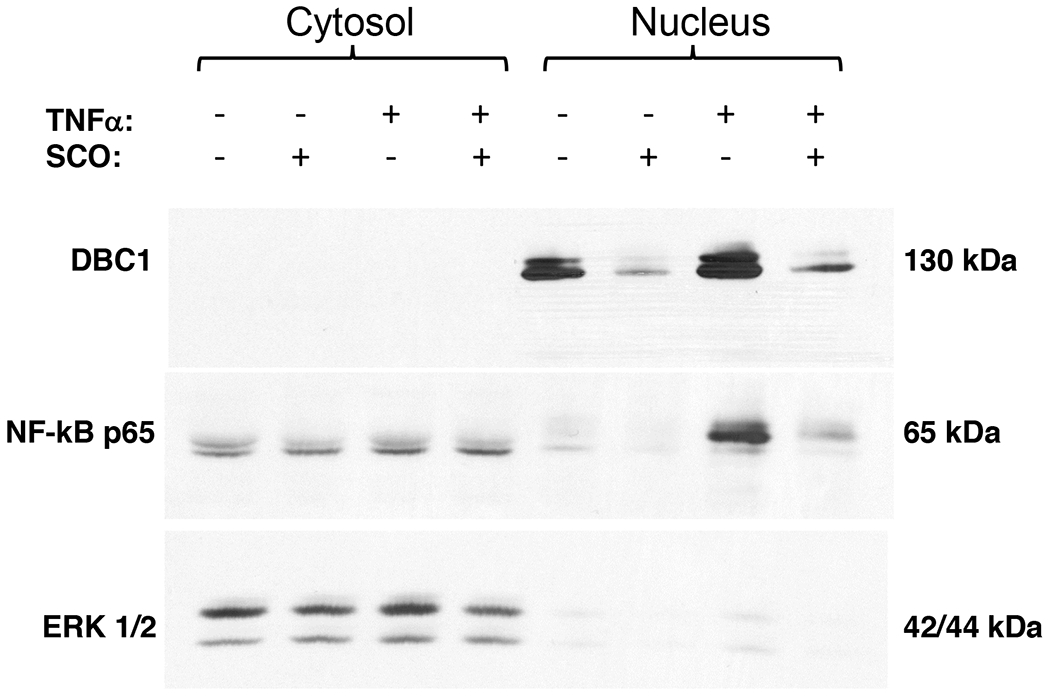
Mature 3T3-L1 adipocytes were pretreated for 3 days with 50 μg/ml SCO or DMSO vehicle, then with 0.5 nM TNFα for 20 minutes. Cells were harvested, and the cytosolic and nuclear compartments were isolated. Immunoblotting was performed to detect DBC1, p65 phosphorylated at Ser536. ERK 1/2 was included as a loading control for the cytoplasmic fractions.
The nuclear protein, Deleted in breast cancer 1 (DBC1), also known as cell cycle and apoptosis regulator 2 (CCAR2), has been implicated in several processes related to inflammation, adipocyte biology, and insulin resistance (23–25). Recent work in our laboratory has described a role for DBC1 in regulating TNFα-induced lipolysis in 3T3-L1 adipocytes (26). We examined DBC1 protein levels in cytosolic and nuclear fractions in TNFα-treated cells in the presence and absence of SCO (Figure 5) and found that SCO-treated adipocytes had less nuclear DBC1 in both basal and TNFα-stimulated conditions. As previously reported, DBC1 is not detected in the cytosol of cultured adipocytes (26).
SCO does not reduce TNFα- or isoproterenol-induced FABP4 secretion
In addition to inducing expression of inflammatory mediators in adipocytes, TNFα also stimulates lipolysis, thereby increasing circulating fatty acid levels and promoting further metabolic dysregulation in obese and insulin-resistant states (5). We have previously shown that SCO inhibits TNFα-induced, but not adrenergic-stimulated lipolysis in adipocytes (11). Fatty acid-binding protein 4 (FABP4) is secreted from adipocytes and adipose tissue in response to lipolytic conditions, including exposure to forskolin, cyclic AMP, or isoproterenol (27, 28). To our knowledge, the effects of TNFα on FABP4 secretion have not been reported. Therefore, we induced lipolysis in adipocytes using TNFα or isoproterenol, with or without SCO pretreatment, and measured FABP4 levels in the conditioned medium to assess whether TNFα could induce secretion of FABP4 in 3T3-L1 adipocytes under prolipolytic conditions. Consistent with previous studies, both doses of isoproterenol tested stimulated FABP4 secretion (Figure 6A). We also made the novel observation that TNFα also promoted secretion of FABP4 in the presence and absence of SCO (Figure 6A). Additionally, we examined lipolysis, as measured by glycerol release, in these adipocytes. As shown in Figure 6B, SCO did not attenuate isoproterenol-induced lipolysis, but did attenuate TNFα-induced lipolysis. SCO had no effect on FABP4 secretion in any of the treatments (Figure 6A). Of note, SCO significantly reduced lipolysis, but not FABP4 secretion, in TNFα-treated adipocytes.
Figure 6. SCO does not reduce TNFα- or isoproterenol-induced FABP4 secretion.
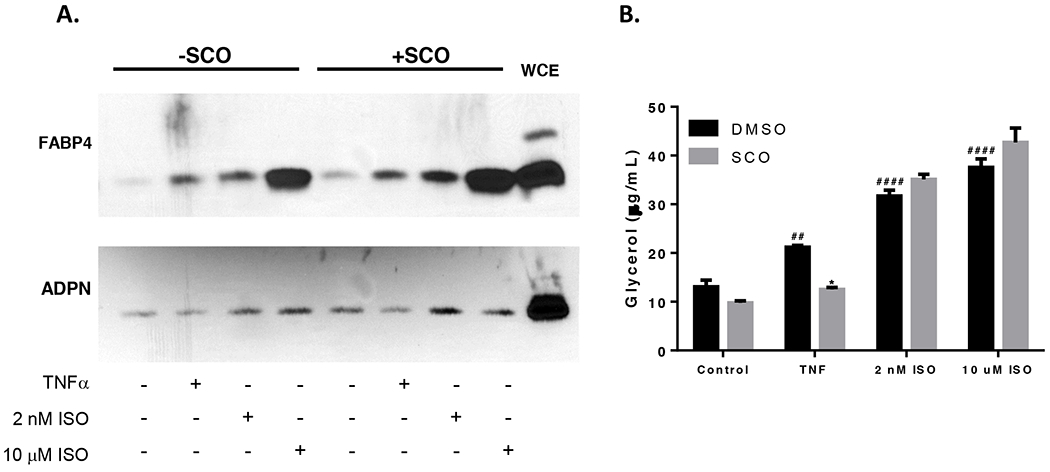
Mature 3T3-L1 adipocytes were pretreated with 50 μg/ml SCO or DMSO vehicle for 3 days prior to harvest. On the last day of SCO treatment, cells were treated with 0.75nM or 0.1% BSA vehicle overnight (18 hours). The following day, medium on all cells was replaced with low-glucose phenol-red-free DMEM containing either vehicle, 0.75 nM TNFα, 2 nM or 10 μM isoproterenol (ISO). Samples of conditioned media were collected after 4 hours. (A) 75 μg of total protein were loaded in each well and analyzed with standard immunoblotting techniques. Membranes were probed for FABP4. Blot images are shown. WCE: whole-cell extract control lysate. (B) 50 μl of conditioned media were also assayed for glycerol content. Effects of TNFα and isoproterenol versus control in the DMSO condition were analyzed by one-way ANOVA: ## P<0.01; #### P<0.0001 vs DMSO control. The effect of SCO was assessed in each condition versus the respective DMSO condition by t-tests. * P<0.05. All other interactions were non-significant.
SCO reduces LPS-induced expression of Il1b and Nos2 (iNos), but not Tnfa in macrophages.
Adipose tissue macrophages promote inflammation and contribute to adipocyte dysfunction in obese and insulin resistant states (6, 22). Flence, we examined the ability of SCO to modulate inflammatory gene expression in RAW 264.7 cells treated with LPS. As shown in Figure 7, LPS elicited a robust induction of Tnfa, Il1b, and Nos2. SCO pretreatment inhibited the LPS effect on Il1b and Nos2 in a dose-dependent manner, but had no effect on the induction of Tnfa gene expression by LPS, revealing a gene-specific anti-inflammatory effect of SCO in these cultured macrophages.
Figure 7. SCO reduces LPS-induced expression of Il1b and Nos2 (iNOS), but not Tnfa in RAW 264.7 macrophages.
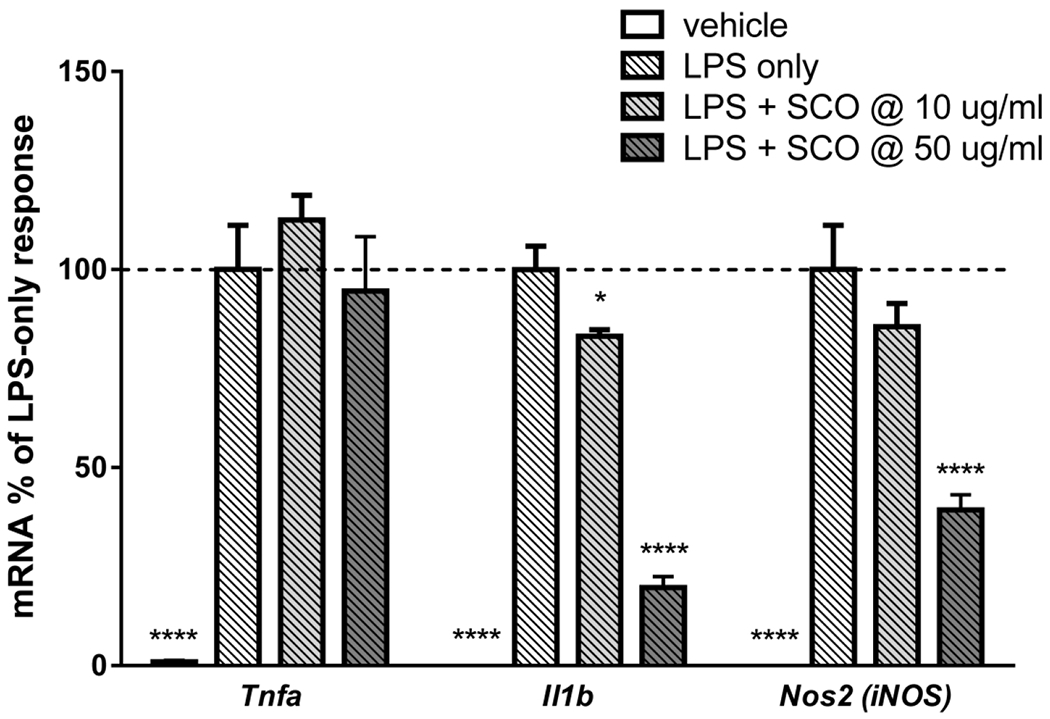
RAW macrophages were pretreated with 10 or 50 μg/ml SCO for 2 hours, then with 1 μg/ml LPS for 5.5 hours. RNA was isolated and reverse transcribed. Gene expression was assayed by qPCR, using Nono as the reference gene, and fold-change was calculated versus the average of the LPS-only condition for each gene. Data are shown as means +/− SE; n=4 biological replicates per condition. Data for each gene were analyzed by one-way ANOVA. Significance expressed as *P<0.05, ****P<0.0001 vs. LPS-only controls. Results were replicated in three independent experiments.
SCO reduces IL-1β-induced NF-κB activation in cultured pancreatic beta cells.
NF-κB signaling is crucial in mediating the inflammatory transcriptional responses which contribute to pancreatic β-cell dysfunction in diabetes (34–38). To determine whether SCO could regulate NF-κB activation in β-cells, we transduced 823/13 rat insulinoma cells with a NF-κB luciferase reporter, then treated cells with SCO at 5 or 10 μg/ml, or vehicle overnight before stimulating cells with IL-1β for 4 hours. IL-1β treatment induced NF-κB promoter activation 28.6-fold over untreated controls. As shown in Figure 8, we observed a dose-dependent reduction in NF-κB promoter activity in the presence of SCO. The higher dose of SCO resulted in a statistically significant decrease in IL-1β-induced NF-κB promoter activity. This effect was not observed with an extract from Artemisia santolinaefolia, a different Artemisia species also known to have adipogenic effects in vitro, as well as some metabolically favorable effects in a mouse DIO model (9).
Figure 8. SCO inhibits IL-1β-stimulated NF-κB promoter activity in rat insulinoma cells.
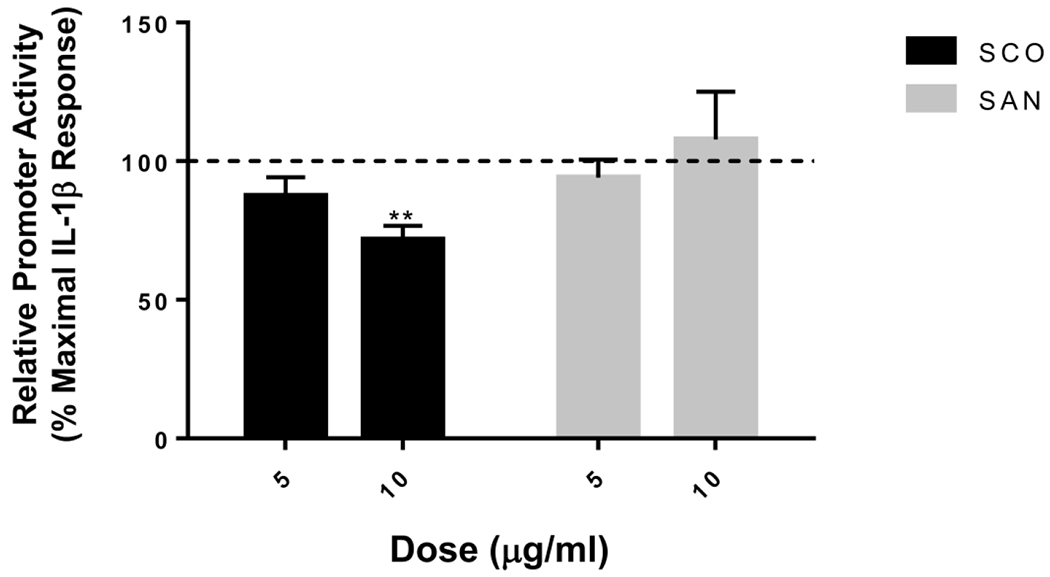
832/13 cells were transduced with an adenovirus overexpressing a luciferase reporter construct driven by a promoter containing 5 copies of a consensus κB element (5xNF-κB-Luc). 12h post-transduction, cells were untreated, or treated overnight with 5 and 10 μg/mL of SCO or an extract of Artemisia santolinaefolia (SAN). Cells were then stimulated for 4h with 1 ng/mL IL-1β. NF-kB promoter activity was induced 28.6-fold (for the SCO experiment) or 31.1-fold (for the SAN experiment) by IL-1b treatment (not shown). Promoter activity in cells pretreated with the extracts is expressed as % of this maximal induction. Data are means ± SEM of 4 individual experiments, each performed in triplicate. **, P < 0.01 versus control.
DISCUSSION
The impacts of obesity, metabolic syndrome, and T2DM clearly justify the search for novel therapeutic approaches, and adipose tissue is intensely studied as a target for such interventions. Obesity is considered a pro-inflammatory state, and the importance of adipose tissue inflammation in the progression of insulin resistance is well documented. In obesity, infiltration and activation of pro-inflammatory immune cells (particularly macrophages) in adipose tissue contribute to impaired adipocyte differentiation and function, as well as to systemic insulin resistance, mediated in large part by the paracrine actions of macrophage-derived TNFα on adipocytes (6, 21, 22). The present study demonstrates that SCO can impede inflammatory processes through at least two different signaling pathways (MAP kinase and NF-κB), and in three cell types relevant to metabolic health (adipocytes, macrophages, and pancreatic β-cells).
In adipocytes, we discovered gene-specific temporal patterns of induction by TNFα and inhibition by SCO. For example, three of the four genes we examined showed a steady increase over an 8-hour TNFα treatment, while the fourth gene, Il6, showed a biphasic response (Figure 2). Also, while SCO reduced expression of Il6, Mcp1, and iNOS in all TNFα-treated conditions, SCO pretreatment increased Lcn2 expression in basal conditions and at the early time points of TNFα treatment, but was inhibitory with longer TNFα treatments. Given the low levels of Lcn2 expression in basal conditions and at early time points of TNFα induction, SCO’s effects on these inflammatory genes under basal conditions are not likely to have physiological relevance. Likewise, SCO’s inhibition of Nos2 expression is modest after 2,4, or 8 hours of TNFα treatment compared to the robust induction with TNFα, and may not be of any biological significance. Taken together, these observations underscore the complexity of inflammatory signaling and suggest that SCO may be acting through distinct mechanisms on different genes.
Activation of the MAP kinase ERK by phosphorylation mediates some of TNFα’s effects in adipocytes (21). Although phosphorylated:total ERK1/2 ratios were not significantly altered by SCO pretreatment, TNFα-treated cells had lower absolute levels of active phosphorylated ERK 1/2 when pretreated with SCO (Figure 4), indicating suppression of MAPK signaling. This effect on ERK activation could contribute to the SCO-mediated reductions in inflammatory gene expression we observed, particularly that of Lcn2, whose expression has been shown to be dependent on ERK 1/2 activation (39). The ability of SCO to decrease total levels of ERK 1/2 implies that SCO pretreatment may alter its transcription, translation or stability. Avenues for further investigation of this observation could include studies of protein stability, unfolded protein response and endoplasmic reticulum stress, among others. At this time, we do not know whether the effect of SCO on ERK expression is required for its anti-inflammatory actions.
The NF-κB pathway is also well characterized and known to mediate TNFα’s effects on inflammatory cytokine gene transcription through nuclear translocation of the p65 (RelA) subunit of NF-κB, and NF-κB signaling events are involved in inflammation-related metabolic disease (reviewed in (40)). A study in LPS-stimulated macrophages has shown that the total flavonoid fraction of A. scoparia could inhibit inflammatory signaling through MAP kinases and NF-κB (19). Our studies in adipocytes reveal that SCO caused substantial inhibition of both pathways in adipocytes, and provide further evidence that SCO impedes inflammatory signaling pathways in fat cells.
DBC1 is a multi-functional protein proposed to be at the interface between aging, cancer, and metabolism (41). Loss of DBC1 in adipocytes inhibits TNFα-induced lipolysis (26), and DBC1 knockout mice are protected from several metabolic effects of DIO (23). In addition, DBC1 knockdown in adipocytes promotes adipogenesis and reduces inflammatory cytokine expression (24, 25), while in B cells, DBC1 has been shown to interact with proteins of the IKK complex, which directly regulates NF-kB signaling (42). Taken together, these studies link DBC1 inhibition with favorable metabolic effects. We have shown that SCO reduces DBC1 protein levels in adipocytes (Figure 5), which could contribute to its anti-inflammatory, anti-lipolytic, and antidiabetic properties. However, future studies will be needed to determine if loss of DBC1 plays an important role in the ability of SCO to promote metabolic health.
Elevated plasma levels of FABP4 are associated with obesity and insulin resistance in both mice and humans, and inhibition of plasma FABP4 activity improves glucose homeostasis in DIO mice (43). Acute activation of cAMP- or cGMP-dependent protein kinases (PKA and PKG) by increased intracellular cAMP or cGMP levels, respectively, stimulates lipolysis, and has been shown to promote FABP4 secretion from adipocytes. (28, 44). The mechanisms involved in the lipolytic effects of TNFα have not been fully elucidated, but they are distinct from those induced by fasting or adrenergic stimulation (5, 21). We report here that induction of lipolysis by TNFα is associated with increased FABP4 secretion (Figure 6A). While suppression of lipolysis with insulin or through pharmacological inhibition of PKA, PKG, or hormone-sensitive lipase has been shown to reduce FABP4 secretion in PKA- or PKG-stimulated conditions (27), we observed that SCO had no effect of TNFα-induced FABP4 secretion (Figure 6B) despite its ability to inhibit lipolysis, indicating that FABP4 secretion is not necessarily coupled to lipolysis. These data also argue against the involvement of FABP4 secretion in SCO’s insulin-sensitizing effects.
In LPS-treated RAW 264.7 macrophages, SCO significantly inhibited Il6 and Nos2 expression, but had no effect on Tnfa expression (Figure 7). This gene-specific response is not entirely unexpected. Toll-like receptor 4 (TLR4) signaling, which mediates the effects of LPS in macrophages, engages two main pathways which utilize distinct subsets of adaptor proteins. The so-called myeloid differentiation primary response 88 (MyD88)-dependent arm results in early-phase NF-κB activation and induction of inflammatory genes, including interleukins. The MyD88-independent arm involves activation of TIR domain-containing adaptor protein inducing interferon beta (TRIF) and interferon regulatory factor 3 (IRF3), and induction of genes such as the Type 1 interferons. Although TNFα is an NF-κB target gene, its regulation by TLR4 signaling is complex (45–47). It has been shown that activation of the NF-κB-independent TRIF/IRF3 pathway can induce early TNFα production and secretion, which in turn lead to a later-phase secondary autocrine activation of NF-κB signaling through TNFα receptor 1 (TNFR1) (48). In addition, transcriptional activation of NF-κB target genes is subject to complex regulation by many factors, including chromatin structure and epigenetic status of the target genes, as well as by various post-translational modifications of NF-κB subunits and variable subunit combinations of its dimers (46, 47, 49).
Interestingly, in a similar study in macrophages using a preparation of total flavonoids from A. scoparia, LPS-induced levels of Il6 and Tnfa were both inhibited (19). Although the reason for this discrepancy cannot be ascertained, LPS treatment in this study was much longer than ours (20 versus 5 hours). Under these more chronic conditions, regulation of Tnfa expression is more likely to be NF-κB dependent and thus susceptible to inhibition by SCO. Alternatively, differences in the composition of both extracts could explain these results. Analysis of our SCO extract by chromatography and mass spectrometry has revealed a complex mixture of compounds, many of which are not flavonoids (50). In addition, plants grown in different locations and conditions yield extracts with distinct chemical constituents and bioactivities. While SCO did not inhibit Tnfa gene expression in macrophages (Figure 7) it did attenuate the response to TNFα treatment in adipocytes (Figures 1 and 2), which could contribute to improving adipose tissue metabolic function in the presence of inflammatory stimuli. Clearly, further studies will be needed to fully elucidate the gene-specific effects of SCO in this context.
Finally, we have shown that SCO reduces NF-κB-responsive promoter activity in pancreatic β-cells. Recruitment of immune cells and activation of inflammatory pathways are major contributors to the pancreatic β-cell dysfunction that occurs with obesity and insulin resistance, and NF-κB is known to mediate these processes (36). The finding that SCO attenuates a measure of NF-κB activation in cultured β-cells (Figure 8) indicates that it can exert anti-inflammatory and metabolically favorable effects beyond adipose tissue.
Our laboratory has studied the effects of SCO on adipocyte differentiation and function, as well as in a DIO mouse model, for several years. We have now shown that SCO significantly impairs inflammatory signaling and transcriptional responses in three cell types that play critical roles in obesity, diabetes, and metabolic syndrome. In addition, we have described effects of SCO on adipocyte levels of DBC1, a protein that has garnered attention in recent years for its involvement in adipogenesis, inflammation, and metabolic dysfunction. We have also discovered that FABP4 secretion, known to be induced by various lipolytic agents, is stimulated by TNFα, although SCO did not modulate this response. Taken together, our findings reveal that SCO interferes with inflammatory signaling to attenuate responses that promote metabolic dysfunction, and demonstrate, for the first time, that SCO has favorable effects in pancreatic β-cells. These results support further investigation of SCO as a potential nutritional supplement to promote metabolic health in the context of obesity and insulin resistance.
STUDY IMPORTANCE QUESTIONS.
What is already known about this subject?
Adipose tissue inflammation plays a critical role in whole-body metabolic function in the context of obesity.
An ethanolic extract of Artemisia scoparia (SCO) improves measures of adipocyte and adipose tissue function, as well as whole-body insulin sensitivity in a mouse model of diet-induced obesity.
What are the new findings in our manuscript?
SCO inhibits inflammatory gene expression in cultured adipocyte and macrophages.
SCO inhibits ERK signaling in adipocytes, as well as NF-kB signaling in adipocytes and pancreatic β-cells.
How might our results change the direction of research or the focus of clinical practice?
SCO’s effects on adipocyte function and development have been well studied. Our current results indicate that SCO may be acting on various cell types, through several mechanisms, to mitigate obesity-related metabolic dysfunction; this opens up new directions for the investigation of SCO’s properties.
Our new data support the further study of SCO as a dietary supplement to promote metabolic resilience.
Acknowledgments
Support: This work was supported by the National Center for Complementary and Integrative Health, and the Office of Dietary Supplements of the National Institutes of Health (NIH): Award number P50AT002776, which funds the Botanical Dietary Supplements Research Center of the Pennington Biomedical Research Center (PBRC) and the Department of Plant Biology and Pathology in the School of Environmental and Biological Sciences of Rutgers University. Genomics Core facilities at PBRC used for this project are supported in part by COBRE and NORC center grants (NIH 8P20GM103528 and NIH 2P30DK072476, respectively).
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
REFERENCES
- 1.Engin A The definition and prevalence of obesity and metabolic syndrome In: Advances in Experimental Medicine and Biology. Vol 960 Springer New York LLC, 2017, pp 1–17. [DOI] [PubMed] [Google Scholar]
- 2.Kusminski CM, Bickel PE, Scherer PE. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat Rev Drug Discov 2016;15:639–660. [DOI] [PubMed] [Google Scholar]
- 3.Danforth E Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet 2000;26:13–13. [DOI] [PubMed] [Google Scholar]
- 4.Kim J-Y, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 2007;117:2621–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie 2016;125:259–266. [DOI] [PubMed] [Google Scholar]
- 6.Engin A The pathogenesis of obesity-associated adipose tissue inflammation. In: Advances in experimental medicine and biology. Vol 960, 2017, pp 221–245. [DOI] [PubMed] [Google Scholar]
- 7.Thomas I, Gregg B. Metformin; a review of its history and future: from lilac to longevity. Pediatr Diabetes 2017;18:10–16. [DOI] [PubMed] [Google Scholar]
- 8.Richard AJ, Fuller S, Fedorcenco V, et al. Artemisia scoparia Enhances Adipocyte Development and Endocrine Function In Vitro and Enhances Insulin Action In Vivo Dixit VD (ed.). PLoS One 2014;9:e98897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richard AJ, Burris TP, Sanchez-lnfantes D, Wang Y, Ribnicky DM, Stephens JM. Artemisia extracts activate PPARy, promote adipogenesis, and enhance insulin sensitivity in adipose tissue of obese mice. Nutrition 2014;30:S31–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang ZQ, Zhang XH, Yu Y, et al. Artemisia scoparia extract attenuates non-alcoholic fatty liver disease in diet-induced obesity mice by enhancing hepatic insulin and AMPK signaling independently of FGF21 pathway. Metabolism 2013;62:1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boudreau A, Richard AJ, Burrell JA, et al. An ethanolic extract of Artemisia scoparia inhibits lipolysis in vivo and has antilipolytic effects on murine adipocytes in vitro. Am J Physiol Metab 2018;315:E1053–E1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain W, Ullah M, Dastagir G, Badshah L. Quantitative ethnobotanical appraisal of medicinal plants used by inhabitants of lower Kurram, Kurram agency, Pakistan. Avicenna J phytomedicine 2018;8:313–329. [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain W, Badshah L, Ullah M, AN M, AN A, Hussain F. Quantitative study of medicinal plants used by the communities residing in Koh-e-Safaid Range, northern Pakistani-Afghan borders. J Ethnobiol Ethnomed 2018;14:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Promyo K, Cho JY, Park KH, Jaiswal L, Park SY, Ham KS. Artemisia scoparia attenuates amyloid β accumulation and tau hyperphosphorylation in spontaneously hypertensive rats. Food Sci Biotechnol 2017;26:775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sajid M, Rashid Khan MR, Shah NA, et al. Evaluation of Artemisia scoparia for hemostasis promotion activity. Pak J Pharm Sci 2017;30:1709–1713. [PubMed] [Google Scholar]
- 16.Gilani AH, Janbaz KH. Hepatoprotective effects of artemisia scoparia against carbon tetrachloride: an environmental contaminant. J Pak Med Assoc 1994;44:65–8. [PubMed] [Google Scholar]
- 17.Cho J-Y, Park K-H, Hwang D, et al. Antihypertensive Effects of Artemisia scoparia Waldst in Spontaneously Hypertensive Rats and Identification of Angiotensin I Converting Enzyme Inhibitors. Molecules 2015;20:19789–19804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habib M, Waheed I. Evaluation of anti-nociceptive, anti-inflammatory and antipyretic activities of artemisia scoparia hydromethanolic extract. J Ethnopharmacol 2013; 145:18–24. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Huang H, Ma X, et al. Anti-inflammatory effects and mechanism of the total flavonoids from Artemisia scoparia Waldst. et kit. in vitro and in vivo. Biomed Pharmacother 2018,104390–403. [DOI] [PubMed] [Google Scholar]
- 20.Nam S-Y, Han N-R, Rah S-Y, Seo Y, Kim H-M, Jeong H-J. Anti-inflammatory effects of Artemisia scoparia and its active constituent, 3,5-dicaffeoyl-epi-quinic acid against activated mast cells. Immunopharmacol Immunotoxicol 2018;40:52–58. [DOI] [PubMed] [Google Scholar]
- 21.Cawthorn WP, Sethi JK. TNF-α and adipocyte biology. FEBS Lett 2008;582:117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112:1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escande C, Nin V, Pirtskhalava T, et al. Deleted in breast cancer 1 limits adipose tissue fat accumulation and plays a key role in the development of metabolic syndrome phenotype. Diabetes 2015;64:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreno-Navarrete JM, Moreno M, Vidal M, et al. Deleted in breast cancer 1 plays a functional role in adipocyte differentiation. Am J Physiol Metab 2015;308:E554–E561. [DOI] [PubMed] [Google Scholar]
- 25.Moreno-Navarrete JM, Moreno M, Vidal M, Ortega F, Ricart W, Fernandez-Real JM. DBC1 is involved in adipocyte inflammation and is a possible marker of human adipose tissue senescence. Obesity 2015;23:519–522. [DOI] [PubMed] [Google Scholar]
- 26.Able AA, Richard AJ, Stephens J. Loss of DBC1 (CCAR2) affects TNFα-induced lipolysis and Glut4 gene expression in murine adipocytes. J Mol Endocrinol 2018;61:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mita T, Furuhashi M, Hiramitsu S, et al. FABP4 is secreted from adipocytes by adenyl cyclase-PKA- and guanylyl cyclase-PKG-dependent lipolytic mechanisms. Obesity 2015;23:359–367. [DOI] [PubMed] [Google Scholar]
- 28.Ertunc ME, Sikkeland J, Fenaroli F, et al. Secretion of fatty acid binding protein aP2 from adipocytes through a nonclassical pathway in response to adipocyte lipase activity. J Lipid Res 2015;56:423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 2000;49:424–430. [DOI] [PubMed] [Google Scholar]
- 30.Kilroy G, Kirk-Ballard H, Carter LE, Floyd ZE. The Ubiquitin Ligase Siah2 Regulates PPARγ Activity in Adipocytes. Endocrinology 2012;153:1206–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penfornis P, Marette A. Inducible nitric oxide synthase modulates lipolysis in adipocytes. J Lipid Res 2005;46:135–42. [DOI] [PubMed] [Google Scholar]
- 32.Li D, Yan Sun W, Fu B, Xu A, Wang Y. Lipocalin-2—The myth of its expression and function. Basic Clin Pharmacol Toxicol 2019:bcpt. 13332. [DOI] [PubMed] [Google Scholar]
- 33.Zhao P, Elks CM, Stephens JM. The induction of lipocalin-2 protein expression in vivo and in vitro. J Biol Chem 2014;289:5960–5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burke SJ, Stadler K, Lu D, et al. IL-1 β reciprocally regulates chemokine and insulin secretion in pancreatic β-cells via NF-κB. Am J Physiol Metab 2015;309:E715–E726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burke SJ, Lu D, Sparer TE, et al. NF-κB and STAT1 control CXCL1 and CXCL2 gene transcription. Am J Physiol Metab 2014;306:E131–E149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burke S, Collier J. Transcriptional Regulation of Chemokine Genes: A Link to Pancreatic Islet Inflammation? Biomolecules 2015;5:1020–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burke SJ, Updegraff BL, Bellich RM, et al. Regulation of iNOS gene transcription by IL-1β and IFN-γ requires a coactivator exchange mechanism. Mol Endocrinol 2013;27:1724–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burke SJ, Karlstad MD, Eder AE, et al. Pancreatic β-Cell production of CXCR3 ligands precedes diabetes onset. BioFactors 2016;42:703–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao P, Stephens JM. STAT1, NF-κB and ERKs play a role in the induction of lipocalin-2 expression in adipocytes. Mol Metab 2013;2:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab 2011;13:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chini EN, Chini CCS, Nin V, Escande C. Deleted in breast cancer-1 (DBC-1) in the interface between metabolism, aging and cancer. Biosci Rep 2013;33:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong S, Dong H, Song J, et al. Deleted in Breast Cancer 1 Suppresses B Cell Activation through RelB and Is Regulated by IKKa Phosphorylation. J Immunol 2015;195:3685–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu A, Wang Y, Xu JY, et al. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem 2006;52:405–413. [DOI] [PubMed] [Google Scholar]
- 44.Cao H, Sekiya M, Ertunc ME, et al. Adipocyte lipid chaperone aP2 Is a secreted adipokine regulating hepatic glucose production. Cell Metab 2013;17:768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doyle SL, O’Neill LAJ. Toll-like receptors: From the discovery of NFκB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol 2006;72:1102–1113. [DOI] [PubMed] [Google Scholar]
- 46.Vaure C, Liu Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol 2014;5:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Falvo JV, Tsytsykova AV, Goldfeld AE. Transcriptional control of the TNF Gene. Curr Dir Autoimmun 2010; 11:27–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-κB activation. Science (80-) 2005;309:1854–1857. [DOI] [PubMed] [Google Scholar]
- 49.Ahmed A, Williams B, Hannigan G. Transcriptional Activation of Inflammatory Genes: Mechanistic Insight into Selectivity and Diversity. Biomolecules 2015;5:3087–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boudreau A, Poulev A, Ribnicky DM, et al. Distinct fractions of an artemisia scoparia extract contain compounds with novel adipogenic bioactivity. Front Nutr 2019;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]


