Abstract
Fracture healing involves interactions of different cell types, driven by various growth factors and signaling cascades. Periosteal mesenchymal progenitor cells give rise to the majority of osteoblasts and chondrocytes in a fracture callus. Notch signaling has emerged as an important regulator of skeletal cell proliferation and differentiation. We investigated the effects of Notch signaling during the fracture healing process. Increased Notch signaling in osteochondroprogenitor cells driven by overexpression of Notch1 intracellular domain (NICD1) (αSMACreERT2 mice crossed with Rosa-NICD) during fracture resulted in less cartilage, more mineralized callus tissue and stronger and stiffer bones after three weeks. Periosteal cells overexpressing NICD1 showed increased proliferation and migration in vitro. In vivo data confirmed that increased Notch1 signaling caused expansion of αSMA-derived cells including αSMA-derived osteoblasts in the callus without affecting osteoclast numbers. In contrast, anti-NRR1 antibody treatment to inhibit Notch1 signaling resulted in increased callus cartilage area, reduced callus bone mass, and reduced biomechanical strength. Our study shows a positive effect of induced Notch signaling on the fracture healing process, suggesting that stimulating the Notch pathway could be beneficial for fracture repair.
Keywords: Notch signaling, Notch1, bone fracture, periosteal progenitors, osteoblast differentiation, inducible Cre, alpha smooth muscle actin
Introduction
Bone fractures represent a frequent clinical problem with 5–10% of all fractures having delayed healing, which leads to increased medical expenses and lower quality of life.1 Fracture healing is a complex process involving many different cell lineages, growth factors, cytokines, and mechanical stimuli. The periosteum is the main contributor of progenitor cells that participate in fracture healing.2 Mesenchymal progenitor cells (MPCs) in the periosteum surrounding the fracture site rapidly expand followed by differentiation into distinct cell lineages in the callus including chondrocytes and osteoblasts. As healing progresses, soft callus is replaced with a bony callus that remodels until the original structure is restored.3 However, the mechanisms that regulate MPC recruitment, proliferation and differentiation during fracture healing are not well defined.
Recent studies have suggested that the Notch signaling pathway plays a role during bone healing.4–6 Notch signaling is activated when Notch ligands (Jagged 1 and 2, and Delta like ligand1, 3 and 4) bind to one of the Notch (1–4) receptors on a nearby cell. The extracellular Notch receptor domain is important for ligand binding, the transmembrane domain is the site of cleavage by the γ-secretase complex following ligand binding, and the intracellular domain regulates transcriptional activity.7 Upon ligand binding, NICD is cleaved by a γ-secretase complex, then translocates to nucleus where it binds to RBPjκ and mastermind-like (Maml) and activates transcription of downstream target genes including the Hes and Hey family of transcriptional repressors.7
In the osteoblast lineage, the effects of Notch signaling are dependent on the differentiation stage.8 During osteolineage cell differentiation, Notch1 signaling increases proliferation and maintains the progenitor pool, while inhibiting differentiation.9 Furthermore, Notch signaling activation promotes differentiation of osteoblasts to osteocytes and induces bone formation.10–13 Disregulated Notch signaling, specifically due to mutations in Notch2, is responsible for Hajdu Cheney syndrome which causes osteoporosis in humans.14
Despite the accumulated knowledge on Notch signaling effects on bone homeostasis, only a few studies have addressed the role of Notch signaling in injury models.4–6; 15–17 Expression of Notch receptors and ligands have been detected during all phases of fracture healing indicating a potential role of Notch signaling during the bone repair process.4 Genetic inhibition of Notch signaling in most cell types affects the callus size and healing progression due to global inflammatory processes.15 However, inhibition of Notch signaling by PrxCre mediated deletion of RBPJk resulted in non-union due to depletion of progenitor populations.6 Treatment of a bone defect with the Notch ligand Jagged1 promotes osteoblast differentiation and healing without excess bone formation.17 Our previous work showed that periosteal cells derived from alpha smooth muscle actin (αSMA) expressing progenitors have decreased expression of Notch receptors, ligands and downstream genes (Hes1 and Hey1) in the early stages of fracture healing indicating regulation of Notch signaling during healing in mice.5
The majority of the previous studies utilized global or constitutive deletion or activation of Notch signaling, but understanding Notch signaling during the fracture healing requires tissue targeting and evaluation of developmentally unperturbed bones. In contrast to previous studies we used an inducible mouse model to overexpress the Notch1 intracellular domain (NICD1) in MPCs, and evaluated the effects on fracture healing. We have also examined the effect of pharmacological inhibition of Notch1 during fracture.
Materials and Methods
Animal models
All animal procedures were approved by an UConn Helth Institutional Animal Care and Use Committee prior to performing the study. Mice were housed in ventilated cages maintained at 22 ± 2°C, 55 ± 5% humidity, and 12-hour light/dark cycle. To generate mice overexpressing NICD1 we used αSMACreERT2 mice 18 and bred them with previously described Gt(ROSA)26Sortm1(Notch1)Dam/J (termed RosaNICD1) mice obtained from Jackson labs # 008159 19 to generate αSMACreNICD1. Cre− littermates were used as controls. For the lineage tracing experiments we crossed αSMACreNICD1 mice with the Ai9 reporter mice (Jackson labs, stock # 007909) to generate αSMA9/NICD1. We determined genotype for Cre, NICD and DNA recombination by PCR (Supplemental table 1)8. To study the effect of Notch1 signaling inhibition αSMA9 mice were used.5; 20 We utilized males and female mice on a C57Bl/6J background.
Femoral fractures
Fractures were performed in 8–9-week-old animals. Mice were anesthetized with isoflurane and a closed transverse diaphyseal fracture on right femur was created using a drop‐weight blunt guillotine device, after inserting a 24G needle into the intramedullary canal to stabilize the fracture.21 To trigger overexpression in αSMACreNICD1 mice, tamoxifen (Sigma Aldrich, St. Louis, MO, USA) dissolved in corn oil (Acros Organics, Belgium) was injected intraperitoneally (ip) at a dose of 75 μg/g of body weight on 0, 2 and 4 days post fracture (DPF) to experimental and control groups. Buprenorphine (0.1 mg/kg) was given subcutaneously during the first 2 days of healing. Fractures were confirmed immediately after the procedure and the healing process was monitored by X-ray (Faxitron LX 60) on 7, 14, 21- and 42 DPF.
Gene expression analysis
Total RNA from cultured periosteal cells was extracted using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) and RNA isolated by the manufacturers protocol. RNA concentration and purity were assessed on a NanoDrop one (Thermo Fisher Scientific, Waltham, MA, USA). Following DNase treatment, 1 μg of RNA was used for cDNA synthesis by ImProm II (Promega, USA). qRT-PCR was performed with TaqMan probes and Universal Mastermix (Thermo Fisher Scientific, UK) to assess Hey1 (Mm00468845_m1), BSP (Mm00492555_m1), OCN (Mm03413826_mH) and GAPDH (Mm99999915_g1) expression or SYBR Select Master Mix (Thermo Fisher Scientific, Austin, Tx, USA) and primers for Hes1 from Canalis et al.8 and GAPDH from Lin et al (Supplemental table 1).22 Data are presented as relative gene expression to GAPDH housekeeping gene, calculated using 2-ΔΔCt and presented as fold change compared to control group.
Histology
Bones were fixed in 4% paraformaldehyde for 3 days at 4°C, intramedullary pins removed, then incubated in 30% sucrose/PBS overnight and embedded in Cryomatrix (Thermo Scientific, Waltham, MA, USA). Tissue was sectioned using a tape transfer system (Section-lab, Japan). Callus area, mineralization and cartilage area within the callus was determined by von Kossa and Safranin O staining. In brief, for mineral tissue staining, 7 μm sections were stained with 4% silver nitrate, exposed to UV light, washed and coversliped. To determine cartilage area within the periosteal callus, samples were stained with Weigert’s hematoxylin for 5 min, washed, stained with 0.2% Fast green, washed with 1% acetic acid, then stained with 0.1% Safranin O for 30 sec, washed and coversliped. To determine osteoclast numbers, TRAP staining was performed on 14-day fractures with Leukocyte acid phosphatase kit (Sigma Aldrich, St. Louis, MO, USA) according to the manufacturer’s protocol. Sections were evaluated using Osteomeasure software (Osteometrics Inc., Atlanta, GA, USA). For osteocalcin staining, sections were incubated in Epitope Retrieval Solution (IHC World) at 60°C for 6 hr, washed in 0.1% Tween 20/PBS, and blocked with 10% goat serum in 0.1% Tween 20/PBS for 1h at room temperature. Osteocalcin antibody (1:100, Cat. No. PA5–78870, Thermo Fisher Scientific, Waltham, MA, USA) incubated overnight at 4°C, followed by washing in 50 mM Tris-Cl, 150 mM NaCl and incubating with secondary antibody conjugated with Alexa 647 (1:300, Cat. No. A21244, Thermo Fisher, Waltham, MA, USA). A minimum of two sections from the middle of the fractured bone were stained, scanned using Axioscan microscope (Zeiss, Germany) and analyzed using automated cell counting within the callus area using ImageJ software (NIH, USA). Before analyzing scanned images, standardized thresholds were set for each colour, and watershed step applied for segmenting clustered nuclei.23 This allowed calculation of the proportion of αSMA-derived cells (red nuclei/total nuclei), the proportion of OCN+ osteoblasts in the callus (green nuclei/total nuclei), and the proportion of OCN+ osteoblasts derived from αSMA progenitors (dual red-green/green nuclei).
Micro computed tomography (μCT) and torsion testing
Two, three and six weeks after fracture, femurs were dissected and scanned using μCT (μCT40, Scanco Medical AG, Bassersdorf, Switzerland) with a voxel size of 12 μm, 55 kV and intensity of 145 μA. Since the callus tissue is not trabecular, nor cortical, algorithms calculating bone parameters might not reflect reality (analysis would not accurately segment larger and/or more mineralized regions), so density, and callus volume was determined and bone mass within the callus calculated. One hundred slices above and below the fracture site were evaluated. Following μCT imaging, the samples were soaked in PBS and stored at −20°C until torsion testing. Samples were potted in methyl methacrylate bone cement (Orthodontic Resin, Dentisply Caulk Inc. Milford, DE, USA) in custom aluminum jigs. Biomechanical properties were measured using the TestBench™ Torsion Testing system (Bose Corporation ElectroForce Systems Group, Eden Prairie, MN, USA), with data acquisition rate of 10 Hz, and 1 degree/s torsion. Data were plotted as a torque to rotation and bone strength, stiffness, twist to failure and toughness were determined.
Flow cytometry
Callus tissue was pooled from 2 fractured femurs 5 DPF and digested.24 In brief, muscle tissue was dissected from the bone, bone marrow flushed and the thickened periosteum around the fracture site (“periosteal callus”) was scraped and digested in PBS containing 0.05% Collagenase P (Roche, IN, USA) and 0.2% hyaluronidase (Sigma Aldrich, St Louis, MO, USA) for 1 hour at 37°C. Cells were washed and stained with Zombie aqua (1:500, BioLegend, San Diego, CA, USA) to exclude dead cells. To exclude hematopoietic and endothelial cells staining for CD45 violet Fluor 450 (Clone: 30-F11, Tonbo biosciences), Ter119 eFluor 450 (Clone: TER-119, Invitrogen, Waltham, MA, USA) and CD31 eFluor 450 (Clone: 390, eBioscience) was performed. LSR II flow cytometry system was used (BD Bioscience, San Jose, CA, USA). Voltages and gates were set based on unstained samples and single stained controls. Diva8 software (BD Bioscience, San Jose, CA, USA) was used for analyzing the data.
Periosteal progenitor cell culture
Periosteal cells were isolated from both the tibia and femur. Muscle tissue was dissected away and thickened periosteum around the fracture site or periosteum from intact bone was scraped, and digested as described above. 2.5×105 cells were seeded in αMEM/10 % FBS in 6-well plates. Following 48 hr at 37°C with 5% O2, half media was changed and cultures were transferred to ambient oxygen. Cells were treated with 1 μM 4-hydroxytamoxifen (Sigma Aldrich, MO, USA), on day 2 and 4. At 70% confluent, osteogenic medium (50 μg/ml ascorbic acid, 4 mM β-glycerophosphate) was added. After 7, 10 and 14 days of differentiation, cells were harvested for RNA isolation or stained for alkaline phosphatase, von Kossa and crystal violet. For assessing proliferation, after 24 h of serum starvation the cells were labeled with EdU for 4 h, and stained according to the manufacturer’s protocol (Click-iT® EdU Alexa Fluor® 647 Imaging Kit, Thermo Fisher) and EdU incorporation determined by flow cytometry in CD45 negative cells.
To measure migration, confluent cells were cultured for 24h in serum free medium. Next, scratch-wound was made on the monolayer and closure determined after 4, 8, 12, 24 and 30 hours.
Treatment with NRR1 antibody
Fractures were performed in 8–9 week old male C57Bl/6J animals, and on 0, 2 and 4 DPF, 2.5 mg/kg of anti-NRR1 antibody (aNRR1, Genentech, Inc. San Francisco, CA, USA) or isotype control (anti-gD, Genentech, Inc. San Francisco, CA, USA) was injected ip. To determine efficiency of Notch1 signaling inhibition, we evaluated thymocyte differentiation ability 25 by flow cytometry assessing frequency of CD4+CD8+ double positive cells using CD4-PE (Clone: GK1.5, eBioscience) and CD8-biotin (Clone: 53–6.7, Invitrogen, Waltham, MA, USA) and stained with secondary antibody conjugated with APC eFluor780 (eBioscience).
Statistical analysis
All data are expressed as mean ± standard deviation (SD) and assessed by two-tailed Student t-tests. P<0.05 was set as statistically significant difference. Real time PCR data is presented as mean ± standard error of the mean (SEM). Statistical analyses were performed using Graph Prism 6 software (GraphPad software, CA, USA).
Results
Effect of Notch1 Signaling overexpression on fracture healing
We utilized an inducible model overexpressing NICD1 in αSMA osteoprogenitor cells during fracture healing using αSMACreERT2 mice 18 and bred them with previously described RosaNICD1 mice. We have evaluated effects 1, 2, 3- and 6-weeks following fracture using x-ray, histological analysis, μCT and mechanical testing (Figure 1A). We confirmed that DNA recombination had occurred in fracture callus tissue at 7 days post fracture (DPF) (Figure 1A). Significantly increased expression of NICD1, as well as downstream Notch target gene Hes1 in αSMACreNICD1 male animals compared to littermate controls was detected in primary cells derived from periosteal callus tissue 5 DPF. These cells were expanded 3 days to enrich the population of periosteal progenitor cells26 (Figure 1B).
Figure 1. Validation of NICD1 overexpression.
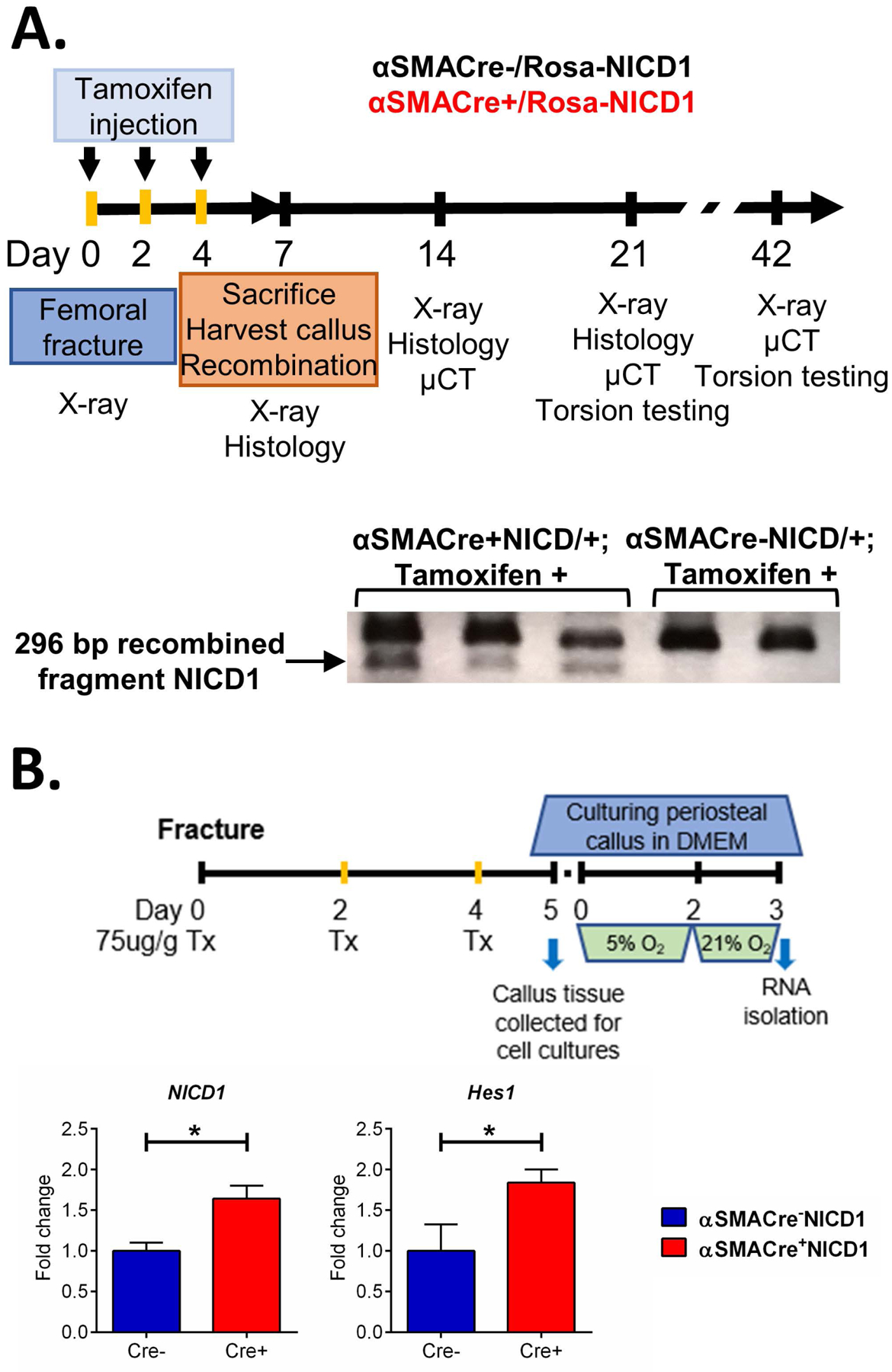
A. Experimental design for Rosa-NICD1 fracture studies. Cre recombination was confirmed by DNA recombination in callus tissue 7 DPF, each lane represents a band from single animal callus tissue sample. B. Relative expression of Notch pathway target genes of male mice from primary periosteal cells derived from callus tissue digested 5 DPF and additionaly expanded for 3 days in cell culture, determined by qRT-PCR. Results are presented as fold change normalized to Cre− expression, n=4–6, *p<0.05.
To visualize the cell population with targeted overexpression of NICD1, αSMACreNICD1 mice were crossed with Ai9 reporter mice and αSMA9/NICD1 expression traced at 5, 10 and 14 DPF (Figure 2B). Consistent with our previous studies 5; 18, αSMACre targeted various mesenchymal populations in the fracture callus including osteoblasts and chondrocytes. Compared to controls, mice overexpressing NICD1 showed an increased proportion of αSMA9+ cells in the callus indicated by histology and flow cytometry at 5 DPF (Figure 2C).
Figure 2. Tracing the αSMA+ cells in fracture healing using αSMACreERT2/Rosa-NICD1 mice crossed with Ai9 reporter mice.
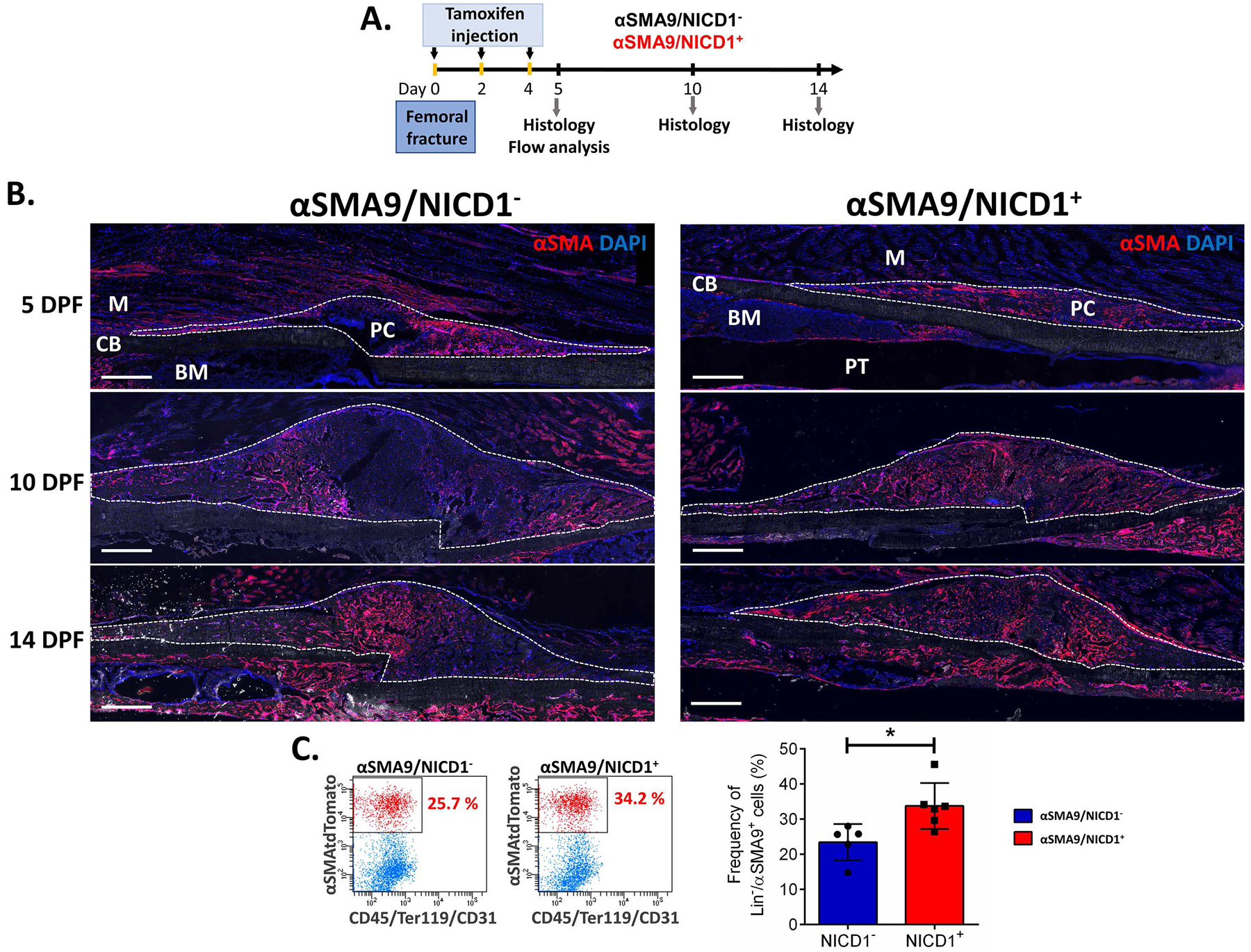
A. Experimental design for αSMA9/NICD1 lineage tracing during the fracture healing. Tamoxifen was injected on the day of the fracture, 2 and 4 DPF, and fractured femurs collected for histological analysis 5, 10 and 14 FPF and flow cytometry 5 DPF. B. Representative images show presence of TdTomato reporter in αSMA cells in controls (αSMA9/NICD1−) and with NICD1 overexpression (αSMA9/NICD1+) 5, 10 and 14 DPF. Dotted lines are surrounding the periosteal callus. Scale bar = 500 μm. PC-periosteal callus, CB-cortical bone, BM-bone marrow, PT-pin track, M-muscle. C. Representative dot plots and graph present αSMA9 cell frequency in αSMA9/NICD1− and αSMA9/NICD1+ from digested periosteal callus tissue 5 DPF, *p<0.05. The results are presented as mean ± SD, (n=5 for Cre- and n=6 for Cre+). **p<0.01.
The in vivo effect of NICD1 overexpression on fracture healing was evaluated by histological analysis. Total callus area was unchanged between the groups at all time-points examined and no difference was observed in cartilage area and bone area on day 7. However, male mice with targeted NICD1 overexpression showed a significantly lower percentage of cartilaginous area 10 DPF, and at 14 DPF had increased mineralized area (Figure 3A). μCT evaluation of fractured femurs 14 DPF did not reveal a significant difference in callus volume, callus bone mass, or bone density between mice overexpressing NICD1 and controls (data not shown). The 21 DPF αSMACre+NICD1 mice had a significant increase in callus bone mass (p<0.05). Furthermore, torsion testing showed increased strength and stiffness (p<0.001) of the fractured femurs 21 DPF in mice overexpressing NICD1 (Figure 3B, C). Six weeks after fracture, we did not detect a difference in the bone volume or bone mass of the remodeling callus (Figure S-2A, B). Furthermore, bone strength and stiffness were not statistically different between αSMACre+NICD1 and control groups (Figure S-2B). This data confirms that NICD1 overexpression in osteoprogenitor cells does not lead to bone tissue overgrowth or abnormal cortical structure and that normal function was established as confirmed by mechanical testing. In contrast to males, NICD1 and Hes1 expression in females did not differ from controls, and μCT analysis 21 DPF did not show difference in healing between αSMACre+NICD1 and their Cre− littermate controls (Figure S-1). Altogether, histological analysis, μCT data, and torsion testing suggest accelerated fracture healing in male mice overexpressing NICD1 in αSMA osteoprogenitor cells. We also determined increase strength and stiffness 21 DPF in contralateral femurs in animals overexpressing NICD1 compared to their littermate controls (Figure S-3A).
Figure 3. NICD1 overexpression enhances fracture healing.
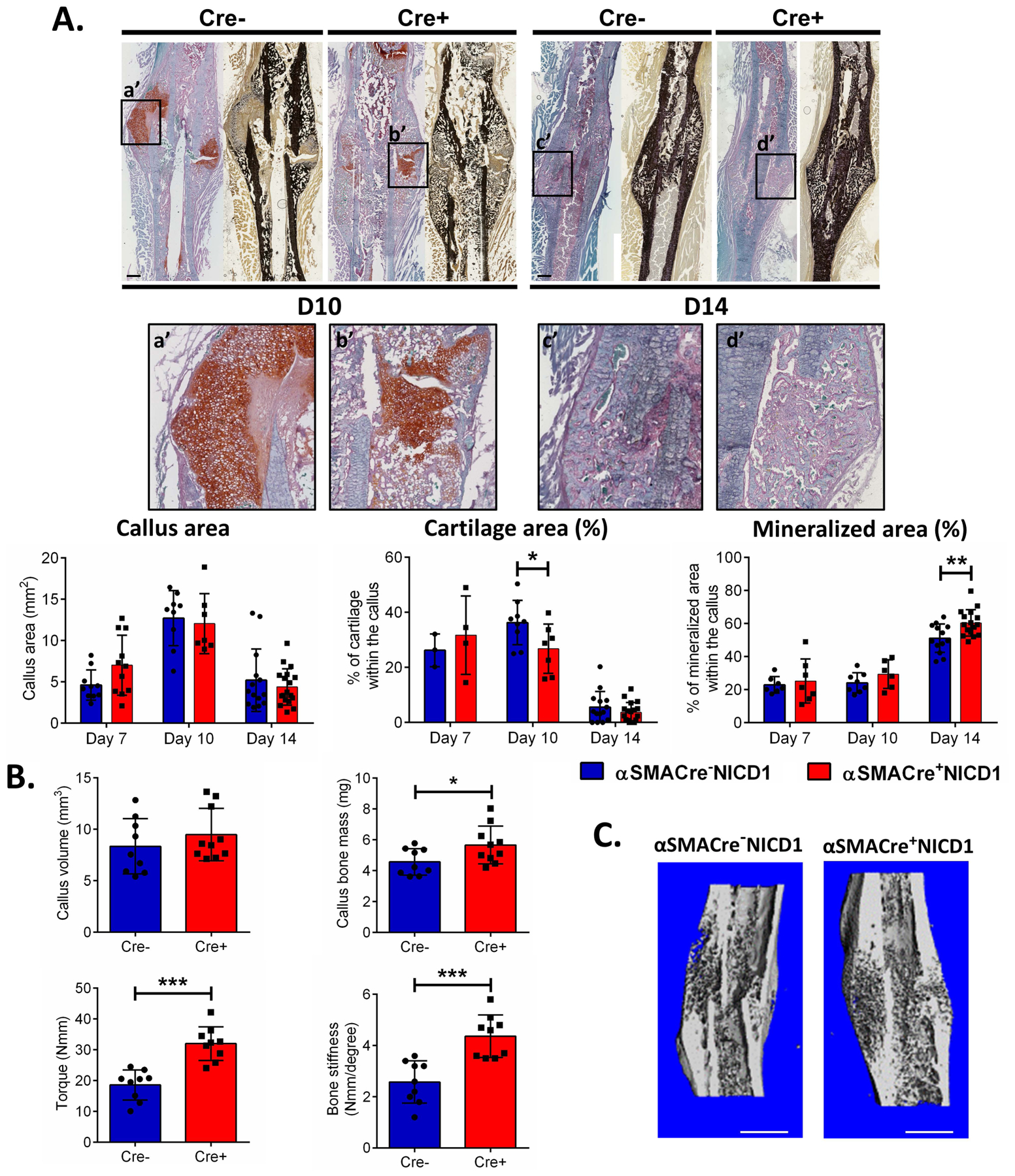
We produced a stabilized fracture on the right femur of male αSMACreNICD1 mice. A. Representative images of Safranin O (left) and von Kossa staining (right) are shown. a’ - d’ shows magnified cartilage area within the callus. Scale bar = 500 μm. Graphs indicate quantification of cartilage and mineralized area from the histological sections. (D7, n=4–7; D10 n=6–7: D14, n=16–18 (Cre+) and D7, n=3–7: D10, n=8–9; D14, n=13–14 for (Cre-)). B. μCT analysis at 21 DPF was used to calculate callus volume and bone mass. Torsion testing was then used to determine strength and stiffness (n=9). C. Reconstruction of representative calluses 21 DPF. Scale bar = 1 mm Results are presented as mean ± SD. * p<0.05, **p<0.01, ***p<0.001.
We used the lineage tracing model to track the frequency of commitment of SMA9/NICD1+ cells into mature osteoblasts by assessing osteocalcin expression. Callus tissue overexpressing NICD1 in αSMA cells had increased frequency of OCN+ cells, and also increased frequency of OCN+ cells derived from αSMA osteoprogenitors (Figure 4). A small proportion of the cells at 10DPF were OCN+. In NICD overexpressing animals 56.5 ± 13.7% osteocalcin positive osteoblasts arise from the αSMA+ osteoprogenitor cells compared to 36.5 ± 11.7% in control animals (Figure 4C). Although osteocalcin was detected in hypertrophic chondrocytes in some studies 27, we stained same slides for Safranin O and did not detect osteocalcin positive cells within the cartilage (Figure S-4), consistent with other published findings.28
Figure 4. NICD1 overexpression in αSMA osteoprogenitor cells increases proportion of αSMA and osteocalcin expressing cells.
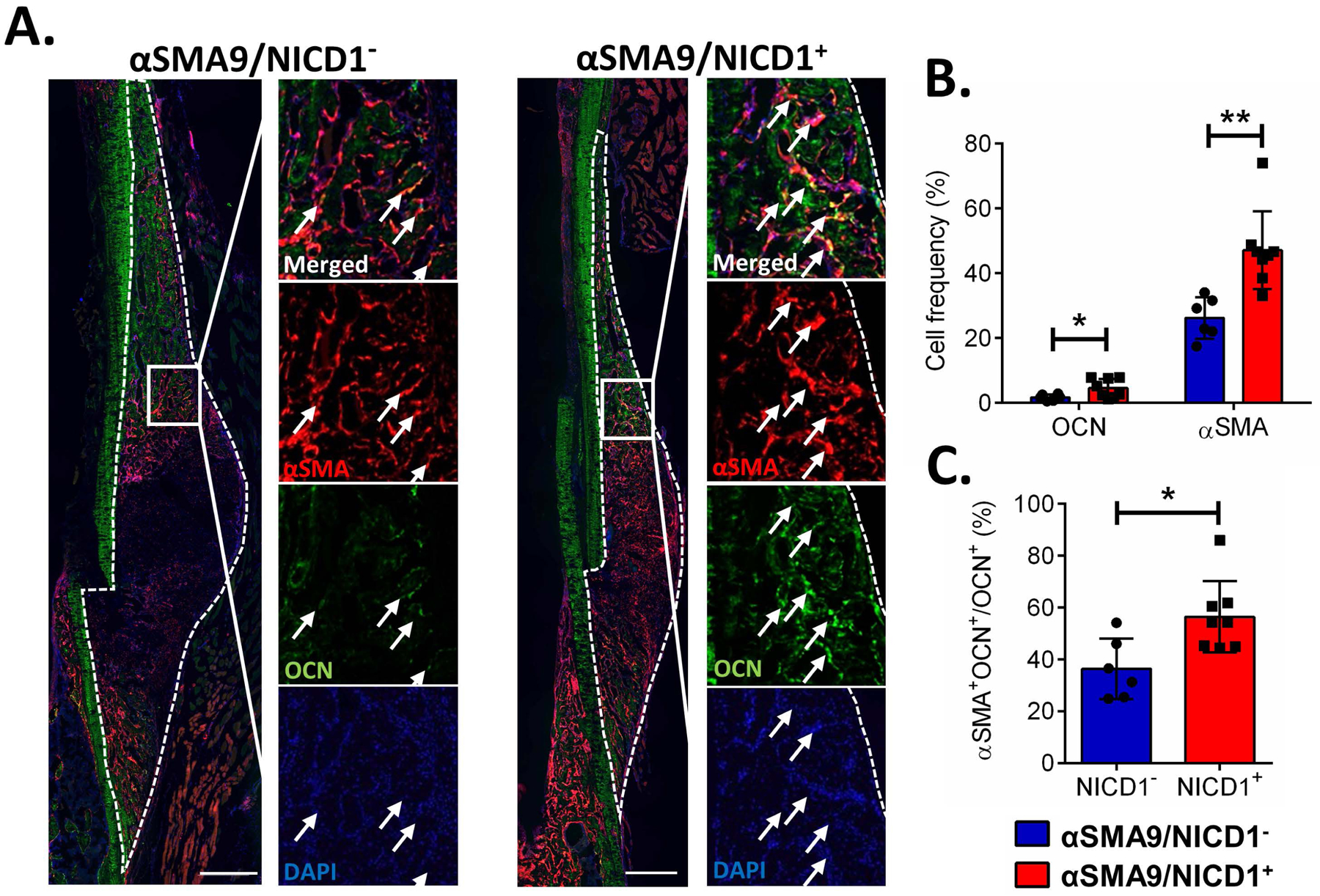
Stabilized femur fracture was produced in male αSMACreERT2/Rosa-NICD1 mice crossed with Ai9 reporter mice and collected 10 DPF. A. Representative images of osteocalcin staining. Scale bar = 500 μm B. Stained cells were quantified as a proportion of DAPI-stained nuclei using image analysis. C. Osteoblasts positive for osteocalcin derived from αSMA cells were determined by counting double positive cells (positive for αSMA+ and OCN+) out of OCN+ cells. Arrows indicate tdTomato+ osteoblasts. n=6 for NICD− and n=8 for NICD+. *p<0.05, **p<0.01.
Previous studies show that activating the Notch signaling pathway in osteocytes increases osteoprotegerin expression and suppresses bone resorption.12 Calluses from αSMACre+NICD1 mice did not show differences in osteoclast perimeter (Oc.Pm), osteoclast number per bone area, or osteoclast number per bone perimeter compared to control animals 14 DPF (Figure S-5).
In order to further elucidate the effects of NICD1 overexpression in periosteal osteoprogenitors, we performed in vitro assays (Figure 5A). Higher expression of Notch downstream target gene Hey1 confirmed that Notch signaling was increased (Figure 5B). Osteogenic differentiation of cells overexpressing NICD1 was reduced as indicated by reduced alkaline phosphatase staining, and expression of bone sialoprotein and osteocalcin (Figure 5A, B). Periosteal cells with NICD1 overexpression in αSMA cells had significantly increased proliferation (Figure 5C), and significantly increased ability for wound closure indicative of enhanced cell migration 24 and 30 hours after the wound was made (p<0.01, and p<0.001) (Figure 5D). Collectively our data indicates that both in vitro and in vivo, NICD1 overexpression is responsible for expansion of progenitor cells.
Figure 5. NICD1 overexpression in αSMA cells inhibits differentiation but enhances migration of periosteal cells in vitro.
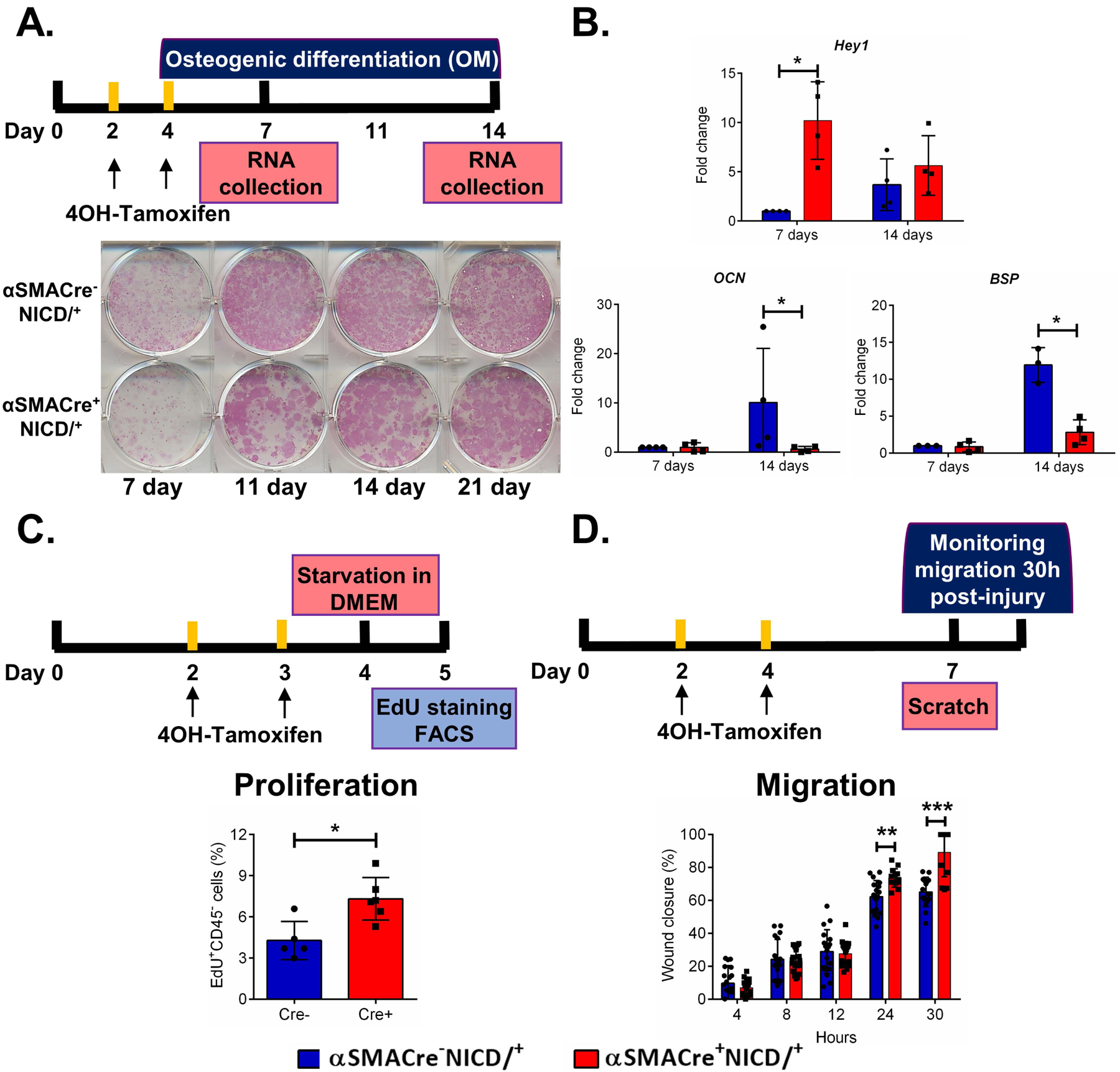
Primary periosteal cells were isolated from 8-week-old male mice, and cultured as indicated in the diagrams. A. Alkaline phosphatase staining. B. Expression of indicated genes during differentiation, mean ± SD (n=3 for Cre-, and n=5 for cre+). C. EdU incorporation over 4h, (n=5 for Cre-, and n=6 for Cre+). D. Wound closure following a scratch assay, n=5.. * p<0.05. Hey1, Hairy/Enhancer-Of-Split Related with YRPW Motif Protein 1; BSP, bone sialoprotein; DOM, days in osteogenic medium; OCN, osteocalcin. Blue bars = Cre- and Red bars = Cre+.
Effect of Notch1 Signaling Inhibition on fracture healing
Using an aNRR1 antibody, we systemically inhibited the Notch1 signaling pathway in the early stages of fracture healing (aNRR1 antibody injected on 0, 2, 4 DPF). Since Notch1 signaling is needed for T cell differentiation, we evaluated thymocyte differentiation 5 DPF. The proportion of CD4+CD8+ thymocytes was 65% lower in the aNRR1 treated group (p<0.01), a confirmation that the dosing regimen caused systemic inhibition of the Notch1 signaling pathway (Figure S-6). In contrast to overexpression of NICD1, we observed that inhibition of Notch1 signaling during the early phases of fracture healing leads to decreased callus area and increased cartilaginous area fraction compared to anti-gD control 10 DPF (Figure 6A). Mineralized area was unchanged. Furthermore, μCT data three weeks after the fracture showed significantly decreased callus volume and bone mass (24.7% smaller callus volume and 24.2% less bone mass, p<0.05, and p<0.005, respectively) in aNRR1 treated animals compared to controls (Figure 6B, C). Bone strength was also significantly decreased 21 DPF in fractures with aNRR1 treatment (Figure 6B), but no change in bone strength and stiffness was observed in unfractured contralateral femora (Figure S-3B).
Figure 6. Notch1 inhibition impairs fracture healing.
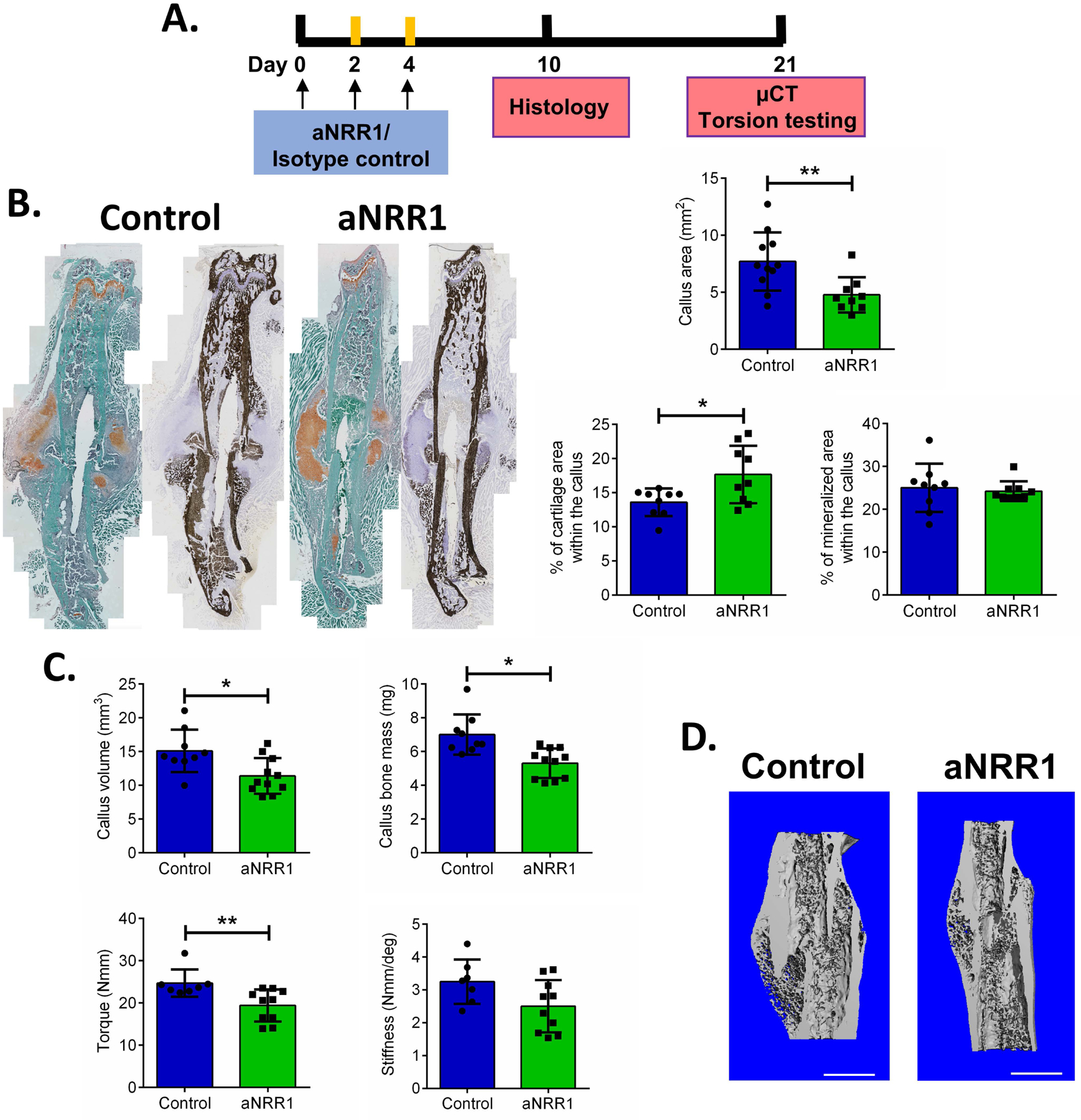
A. Treatment scheme for aNRR1. B. Histology of femur fractures treated with aNRR1 or control antibody at 10 DPF. Cartilaginous and mineralized area within the callus were determined by Safranin O or von Kossa staining and images analyzed in ImageJ software. n=11 for control, and n=9 for aNRR1 treated group. C. Fractured femurs were analysed by μCT to determine callus volume and bone mass, and tested for biomechanical properties by torsion testing at 21 DPF. D. Representative images show reconstructed calluses 21 DPF in control and aNRR1 treated mice. Scale bar = 1 mm, n=7–9 for control, and n=10–11 for aNRR1 treated group. The results are presented as mean ± SD. * p<0.05, ***p<0.001.
To investigate the effect of Notch1 signaling inhibition on cell populations in the fracture callus, we used αSMA9 mice and evaluated proportion of αSMA cells 5 DPF (Figure 7A). Animals treated with aNRR1 had fewer αSMA9 labeled cells (Figure 7B).
Figure 7. Proportion of αSMA cells in fracture healing during early Notch1 signaling inhibition.
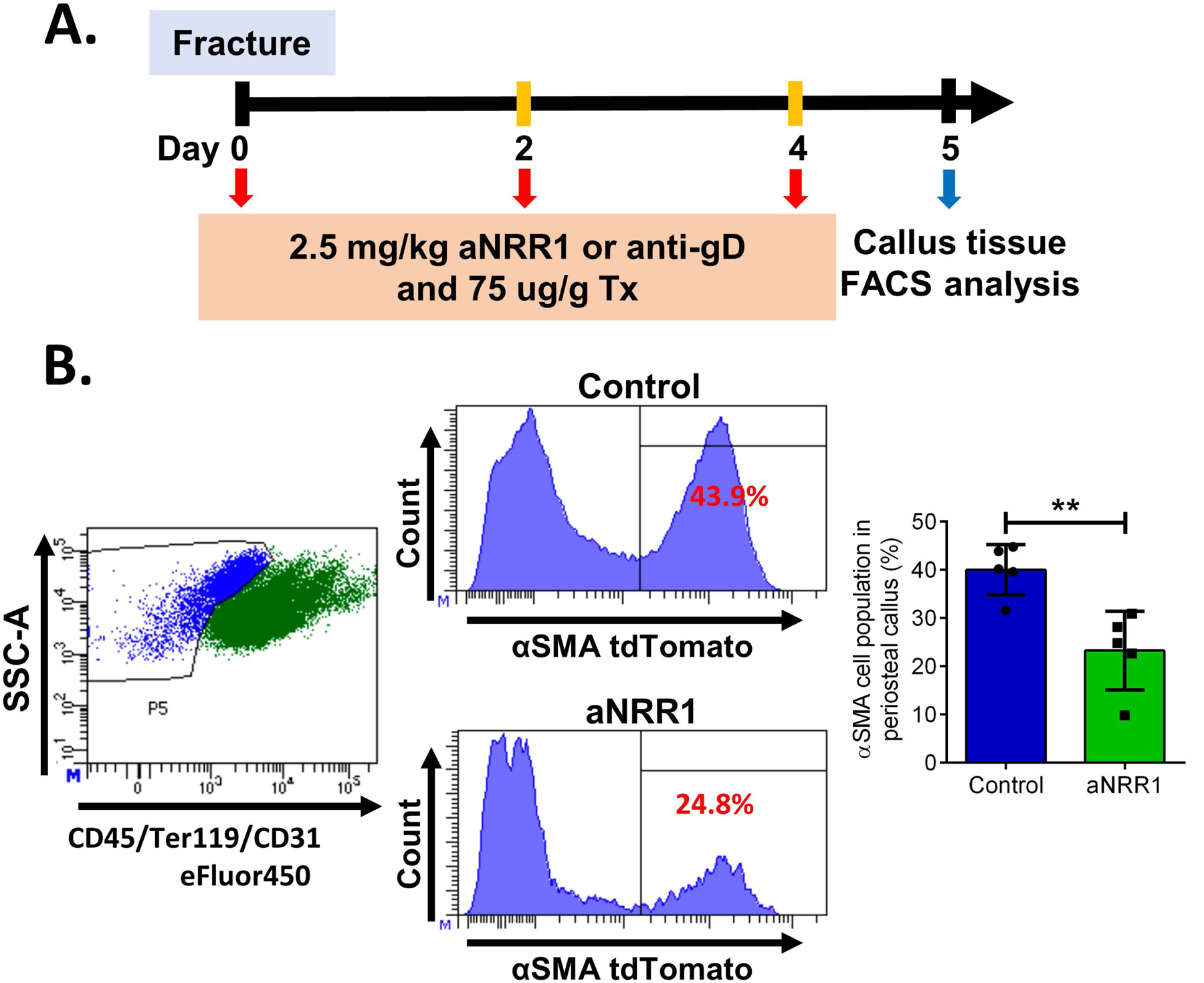
A. Fractures were performed in αSMA9 mice, and tamoxifen and aNRR1 antibody were injected on 0, 2, and 4 DPF, n=5. B. The results are presented as mean ± SD. Representative dot plots are showing gating of (CD45/Ter119/CD31)− cells and histograms of αSMA9 that are (CD45/Ter119/CD31)− in two experimental groups. **p<0.01.
Discussion
We have previously demonstrated that αSMA+ cells are osteoprogenitors that actively participate in bone healing.5; 18 Periosteal αSMA+ osteoprogenitor cells show down-regulation of Notch receptors, ligands and downstream targeting genes (Hes1 and Hey1) after fracture (2 and 6 DPF), compared to cells from uninjured tissue.5 These changes were specific to the αSMA9-labeled population, and were not seen in callus tissue overall. However, Dishowitz et al. showed that expression of Notch signaling genes is upregulated during healing of both tibia fractures and calvarial defects, peaking around day 10 post injury.4 Immunostaining indicated expression of various pathway components in osteoblasts at all stages of differentiation. It is therefore evident that Notch signaling is active during fracture healing, but the level of signaling is likely dependent on the cell type in question as well as its stage of differentiation.
Despite an accumulation of knowledge on role of Notch signaling in development and bone homeostasis and pathology, the mechanisms by which Notch signaling regulates bone healing have been understudied. In part, this is due to the lack of the suitable models for targeting specific lineage of cells during fracture healing. For studying effects on fracture healing it is critical to have animal models that do not have preexisting musculoskeletal phenotypes. Previous studies targeting Notch signaling utilized inhibition using Mx1Cre;dnMAML mice, inhibiting Notch in most cell types during callus formation, resulted in impaired fracture healing, likely due to a prolonged inflammatory phase.15 Mice with constitutively targeted RBPjκ deletion in the skeletal lineage (Prx1-Cre;RBPjκfl/fl mice) also have impaired fracture healing; callus formation is delayed, with more fibrous tissue within the fractured gap, and reduced BMSC numbers, suggesting that the exhausted osteoprogenitor pool is unable to properly heal fractured bone which leads to non-union.6 The disadvantage of the Prx1-Cre based model is that RBPjκ is knocked out in the limb mesenchyme from early in development, which could confound these results. These animals at the age of 8-weeks have a high-bone-mass phenotype with 730% increase in BV/TV, and significantly more active osteoblasts, but with aging their MPC pool diminishes, and they have age-dependent bone loss.29
We used αSMACreERT2 to specifically target MPCs by induction at the time of fracture resulting in an increased proportion of αSMA9 cells when NICD1 is overexpressed. Our data indicate that forced Notch1 activity in osteoprogenitors resulted in improvement of fracture healing parameters, induction of osteogenesis and increased bone formation in the callus as assessed by μCT and mechanical testing. Notch 1 overexpression in αSMA cells did not result in negative effects on healing 6 weeks after fracture such as delayed callus remodeling. Our model uses induction of Notch 1 overexpression and fractures are done in biologically unperturbed adult bone confirming the benefits of using inducible models. In contrast to this, a mouse model reproducing the Hajdu-Cheney syndrome (HCS, mice with 6955C→T gain of function mutation in the Notch2 locus) have shorter femurs than WT and exhibited cancellous and cortical bone osteopenia.30 This phenotypic difference HCS mice from NICD1 overexpression phenotype can be a result of constitutively active mutation in Notch2 receptor but also due to differences in expression and function of different Notch receptors in the skeletal biology. Inducible COIN by inversion model for HCS are available, and these will provide a better comparison to our inducible Notch1 findings.31; 32 In line with our data is recent work by Youngstrom et al. showing that local delivery of Notch ligand Jagged-1 at the time of injury promotes regeneration of femoral and calvarial defects in mice and rats, with more physiological tissue formation than what was achieved with BMP2.17 In contrast, fractures in mice treated systemically during the early phases of healing with the γ-secretase inhibitor DAPT also appeared to have enhanced healing.16 γ-secretase activity is necessary for cleavage of Notch receptors to release the NICD. While Wang and colleagues demonstrate that Notch targets are transiently downregulated following DAPT treatment, confirming the expected inhibition of Notch signaling by this compound, this inhibitor is not specific for Notch signaling.33; 34
In our study, a specific antibody was used to inhibit Notch1 signaling by targeting the negatively regulated region (NRR) of Notch1 receptor using anti-NRR1 antibody.25 When anti-NRR1 antibody binds the NRR1 region of the extracellular domain, it blocks conformational change of the NRR1 region, and protease cleavage is prevented, leading to specific inhibition of Notch1 signaling pathway.25 We have demonstrated that systemic treatment of mice with aNRR1 antibody during days 0–4 of fracture healing leads to impaired fracture healing evidenced by smaller, less mineralized callus formation, and reduced bone strength. The treatment window corresponds to the inflammation and soft callus formation phases, and is very similar to the treatment regimen used for DAPT.16 As well as Notch-independent effects, DAPT would also target signaling by all the Notch receptors, which are also expressed in fracture, and could contribute to the differences between the two studies.
Periosteal cells from these mice make less cartilage, and differentiate to osteoblasts and incorporate more mineral earlier, so the fractured bones are initially stronger than controls. Although Notch signaling in osteoblasts can modulate osteoclastogenesis, this does not appear to be the mechanism in this case. The enhanced healing was unexpected based on our in vitro data in this and our previous study5, as following Cre activity, NICD1 would be permanently overexpressed in the cells which would be expected to impair terminal osteoblast differentiation. The reason for this discrepancy requires further investigation, as it likely relates to interaction of Notch and other signaling pathways present in the microenvironment specific to fracture healing.
While αSMACreERT2 has advantages over constitutive Cre models for fracture studies, it does not specifically target the periosteum, so it is possible that non-periosteal NICD1 overexpression is influencing fracture healing. We have previously shown that in the muscle, αSMA9 labels perivascular and satellite cells.20 The perivascular subset of αSMA-labeled cells are capable of BMP2-stimulated osteogenesis. Muscle may exert supportive effect on fracture healing, with callus tissue mineralizing fastest at the edges of the callus. Muscle can also be a source of osteoprogenitors in cases where injury extends into the adjacent muscle tissue.35; 36 We did not observe any significant changes in other organ systems in which αSMA9 expression was detected up to 42 DPF.
The apparent discrepancy in the effects of NICD1 overexpression in vitro and in vivo, could have a few potential explanations. Our in vivo study suggest that the targeted cells make a greater contribution to osteoblasts following Notch overexpression. Mice overexpressing NICD1 using Osx-Cre;Rosa(Notch) at one month of age had osteopenia, but at 3 months of age had increased bone volume, and increased osteoblasts number.8 Next, Canalis’ group showed that overexpressing NICD1 in DMP1 positive osteocytes lead to increased bone formation and decreased bone resorption increasing expression of OPG and activating Wnt signaling.12 This data suggests that NICD1 inhibition of osteoblast differentiation is lineage stage specific and might be overcomed with sufficient osteogenic signals provided within the cells or by the environment. Overexpression of NICD1 can trigger regulation of other signaling pathways that exert stronger induction of osteogenic differentiation, such as C3H10T1/2 cells that showed significantly improved osteoblastic differentiation after BMP-2 induction with NICD1 overexpression.37 Besides the autocrine effects of Notch signaling overexpression, it is possible that NICD1 overexpressing cells have paracrine effects on other progenitors that promote healing. Complex signaling and cellular diversity present at the fracture site, is different from cultured cells and could be an explanation for persistence of NICD1 inhibitory effect on osteogenesis in vitro.
The majority of data presented are from male mice. As indicated in Figure S-1, females with targeted NICD1 overexpression did not demonstrate a bone healing phenotype. It is well-established that bone mass and microarchitecture are different between male and female C57BL/6 mice (females have lower trabecular number and spacing, BV/TV, and decreased ALP activity and differentiation markers in cultured BMSC)38, although differences in healing are not well defined. A number of bone phenotypes related to dysregulation of Notch signaling are evident in both males and females, including in mice combining Rosa-NICD1 with other Cre drivers.8; 12; 29; 39 However, it has been reported that females have decreased αSMA expression17, and in our studies, we did not see overexpression of Notch1 signaling in the females which would be consistent with lower recombination efficiency resulting in no significant changes in healing (Figure S-1). As most Cre inducible models are not 100% efficient, using in vitro experiments we were determinde recombination efficiency of αSMACreERT2 to about 40–50%.40 In conjunction with no increase in NICD1 and nonsignificant increase of Hes1 this might be a likely reason why we do not see effects on fracture healing in females. None of the other studies looking at modulating Notch signaling during fracture healing systematically compared males and females, in fact most either perform studies only in male mice, or do not report which sex was studied.6; 15–17
In summary, enhancing Notch signaling in early fracture healing via forced NICD1 expression in αSMA+ progenitors accelerated fracture healing in male mice, with earlier loss of cartilage, increased OCN+ osteoblasts, increased mineralization, and stronger and stiffer healing bones. This is likely due to increased expansion of progenitor cells during the early stages of healing. Notch1 inhibition via aNRR1 during the initial stages of fracture has the opposite effect, with smaller fracture calluses, larger cartilaginous area within the callus tissue, and weaker bones. We conclude that regulation of Notch1 signaling is crucial in the fracture healing process. All this data suggests that treatment increasing Notch1 signaling in fractured bone if applied at the appropriate time early in the healing process might have a therapeutic effect.
Supplementary Material
Supplemental figure 1. Fracture healing in female αSMACre/Rosa-NICD1 mice. A. Experimental design for evaluation of Notch1 target genes following fracture. Gene expression was evaluated by qRT-PCR. Results are presented as fold change normalized to Cre- expression, n=3, *p<0.05. B. μCT analysis of fracture callus 21 DPF in female mice, n=5 for Cre-, and n=6 for Cre+.
Supplemental figure 2. Effect of NICD1 overexpression in αSMA cells on healing bone 6 weeks after the fracture. A. Representative X-ray images of a fractured femurs 6 weeks after the fracture. B. μCT analysis at 42 DPF in male mice was used to calculate callus volume and bone mass. Torsion testing was then used to determine strength and stiffness (n=5).
Supplemental figure 3. Biomechanical properties of contralateral bones 21 DPF. A. Torsion testing was used to determine strength and stiffness of contralateral femurs 21 DPF of male αSMACre+NICD1 and their littermate control Cre− mice (n=10) and in B. animals treated with aNRR1 and their controls (aNRR1 n=8; control n=11).
Supplemental figure 4. Chondrocytes lack osteocalcin expression in callus tissue. After OCN staining, slide was stained for Safranin O and when images of OCN staining and Safranin O were merged using Adobe Photoshop CS6 (version 6.0, Adobe Systems Inc., San Jose, CA), osteocalcin positive cells are not present in red cartilage area.
Supplemental figure 5. TRAP staining (purple) is shown in male mice 14 DPF. Histomorphometric parameters of osteoclasts are shown in the right panel. The results are presented as mean value ± SD, n=10.
Supplemental figure 6. Thymocyte differentiation is inhibited by aNRR1 treatment. Experimental design showing treatment protocol. Thymocytes were analyzed 5 DPF for CD4 and CD8 expression. Representative dot plots are showing thymocytes CD4 and CD8 expression in experimental groups. The results are presented as mean value ± SD, n=4 for control and n=6 for aNRR1. **p<0.01.
Acknowledgments
This work has been supported by NIH/NIAMS grants AR055607 and AR070813, Regenerative Medicine Research Fund (RMRF) grant 16-RMB-UCHC-10 to I.K, and Connecticut Stem Cell grant 14-SCA-UCHC-02 to BGM. K.D.H was supported by NIH/NIAMS AR066028 and OR140396 from PRORP-CDMRP. Great thanks to Renata Rydzik for the help with uCT scanning.
Footnotes
Data availability and sharing:
The data that support the findings of this study are available from the corresponding author on request.
Reference
- 1.Mills LA, Simpson AH. 2013. The relative incidence of fracture non-union in the Scottish population (5.17 million): a 5-year epidemiological study. BMJ open 3:e002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colnot C. 2009. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 24:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Einhorn TA, Gerstenfeld LC. 2015. Fracture healing: mechanisms and interventions. Nature reviews Rheumatology 11:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dishowitz MI, Terkhorn SP, Bostic SA, et al. 2012. Notch signaling components are upregulated during both endochondral and intramembranous bone regeneration. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 30:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthews BG, Grcevic D, Wang L, et al. 2014. Analysis of alphaSMA-labeled progenitor cell commitment identifies notch signaling as an important pathway in fracture healing. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 29:1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, Inzana JA, Mirando AJ, et al. 2016. NOTCH signaling in skeletal progenitors is critical for fracture repair. The Journal of clinical investigation 126:1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanotti S, Canalis E. 2016. Notch Signaling and the Skeleton. Endocrine reviews 37:223–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canalis E, Parker K, Feng JQ, et al. 2013. Osteoblast lineage-specific effects of notch activation in the skeleton. Endocrinology 154:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilton MJ, Tu X, Wu X, et al. 2008. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nature medicine 14:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji Y, Ke Y, Gao S. 2017. Intermittent activation of notch signaling promotes bone formation. American journal of translational research 9:2933–2944. [PMC free article] [PubMed] [Google Scholar]
- 11.Shao J, Zhou Y, Xiao Y. 2018. The regulatory roles of Notch in osteocyte differentiation via the crosstalk with canonical Wnt pathways during the transition of osteoblasts to osteocytes. Bone 108:165–178. [DOI] [PubMed] [Google Scholar]
- 12.Canalis E, Adams DJ, Boskey A, et al. 2013. Notch signaling in osteocytes differentially regulates cancellous and cortical bone remodeling. The Journal of biological chemistry 288:25614–25625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu P, Ping Y, Ma M, et al. 2016. Anabolic actions of Notch on mature bone. Proc Natl Acad Sci U S A 113:E2152–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isidor B, Lindenbaum P, Pichon O, et al. 2011. Truncating mutations in the last exon of NOTCH2 cause a rare skeletal disorder with osteoporosis. Nature Genetics 43:306–308. [DOI] [PubMed] [Google Scholar]
- 15.Dishowitz MI, Mutyaba PL, Takacs JD, et al. 2013. Systemic inhibition of canonical Notch signaling results in sustained callus inflammation and alters multiple phases of fracture healing. PloS one 8:e68726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C, Shen J, Yukata K, et al. 2015. Transient gamma-secretase inhibition accelerates and enhances fracture repair likely via Notch signaling modulation. Bone 73:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youngstrom DW, Senos R, Zondervan RL, et al. 2017. Intraoperative delivery of the Notch ligand Jagged-1 regenerates appendicular and craniofacial bone defects. NPJ Regenerative medicine 2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grcevic D, Pejda S, Matthews BG, et al. 2012. In vivo fate mapping identifies mesenchymal progenitor cells. Stem cells 30:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murtaugh LC, Stanger BZ, Kwan KM, et al. 2003. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A 100:14920–14925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews BG, Torreggiani E, Roeder E, et al. 2016. Osteogenic potential of alpha smooth muscle actin expressing muscle resident progenitor cells. Bone 84:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnarens F, Einhorn TA. 1984. Production of a standard closed fracture in laboratory animal bone. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 2:97–101. [DOI] [PubMed] [Google Scholar]
- 22.Lin X, Jia J, Du T, et al. 2015. Overexpression of miR-155 in the liver of transgenic mice alters the expression profiling of hepatic genes associated with lipid metabolism. PloS one 10:e0118417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews BG, Wee NKY, Widjaja VN, et al. 2019. alphaSMA Osteoprogenitor Cells Contribute to the Increase in Osteoblast Numbers in Response to Mechanical Loading. Calcif Tissue Int. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Matthews BG, Yu J, et al. 2019. PDGF Modulates BMP2-Induced Osteogenesis in Periosteal Progenitor Cells. JBMR Plus 3:e10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y, Cain-Hom C, Choy L, et al. 2010. Therapeutic antibody targeting of individual Notch receptors. Nature 464:1052–1057. [DOI] [PubMed] [Google Scholar]
- 26.Short BJ, Brouard N, Simmons PJ. 2009. Prospective isolation of mesenchymal stem cells from mouse compact bone. Methods Mol Biol 482:259–268. [DOI] [PubMed] [Google Scholar]
- 27.Hughes SS, Hicks DG, O’Keefe RJ, et al. 1995. Shared phenotypic expression of osteoblasts and chondrocytes in fracture callus. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 10:533–544. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, von der Mark K, Henry S, et al. 2014. Chondrocytes Transdifferentiate into Osteoblasts in Endochondral Bone during Development, Postnatal Growth and Fracture Healing in Mice. PLOS Genetics 10:e1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tu X, Chen J, Lim J, et al. 2012. Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1. PLOS Genetics 8:e1002577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canalis E, Schilling L, Yee SP, et al. 2016. Hajdu Cheney Mouse Mutants Exhibit Osteopenia, Increased Osteoclastogenesis, and Bone Resorption. The Journal of biological chemistry 291:1538–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J, Zanotti S, Schilling L, et al. 2018. Induction of the Hajdu-Cheney Syndrome Mutation in CD19 B Cells in Mice Alters B-Cell Allocation but Not Skeletal Homeostasis. Am J Pathol 188:1430–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanotti S, Yu J, Sanjay A, et al. 2017. Sustained Notch2 signaling in osteoblasts, but not in osteoclasts, is linked to osteopenia in a mouse model of Hajdu-Cheney syndrome. The Journal of biological chemistry 292:12232–12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beel AJ, Sanders CR. 2008. Substrate specificity of gamma-secretase and other intramembrane proteases. Cellular and molecular life sciences : CMLS 65:1311–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang T, Arslanova D, Gu Y, et al. 2008. Quantification of gamma-secretase modulation differentiates inhibitor compound selectivity between two substrates Notch and amyloid precursor protein. Molecular brain 1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu R, Birke O, Morse A, et al. 2011. Myogenic progenitors contribute to open but not closed fracture repair. BMC Musculoskeletal Disorders 12:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abou-Khalil R, Yang F, Lieu S, et al. 2015. Role of Muscle Stem Cells During Skeletal Regeneration. Stem cells 33:1501–1511. [DOI] [PubMed] [Google Scholar]
- 37.Nobta M, Tsukazaki T, Shibata Yi, et al. 2005. Critical Regulation of Bone Morphogenetic Protein-induced Osteoblastic Differentiation by Delta1/Jagged1-activated Notch1 Signaling. Journal of Biological Chemistry 280:15842–15848. [DOI] [PubMed] [Google Scholar]
- 38.Zanotti S, Kalajzic I, Aguila HL, et al. 2014. Sex and genetic factors determine osteoblastic differentiation potential of murine bone marrow stromal cells. PloS one 9:e86757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canalis E, Schilling L, Zanotti S. 2017. Effects of Sex and Notch Signaling on the Osteocyte Cell Pool. Journal of Cellular Physiology 232:363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinder BP, Novak S, Wee NKY, et al. 2019. Engraftment of skeletal progenitor cells by bone directed transplantation improves osteogenesis imperfecta murine bone phenotype. Stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1. Fracture healing in female αSMACre/Rosa-NICD1 mice. A. Experimental design for evaluation of Notch1 target genes following fracture. Gene expression was evaluated by qRT-PCR. Results are presented as fold change normalized to Cre- expression, n=3, *p<0.05. B. μCT analysis of fracture callus 21 DPF in female mice, n=5 for Cre-, and n=6 for Cre+.
Supplemental figure 2. Effect of NICD1 overexpression in αSMA cells on healing bone 6 weeks after the fracture. A. Representative X-ray images of a fractured femurs 6 weeks after the fracture. B. μCT analysis at 42 DPF in male mice was used to calculate callus volume and bone mass. Torsion testing was then used to determine strength and stiffness (n=5).
Supplemental figure 3. Biomechanical properties of contralateral bones 21 DPF. A. Torsion testing was used to determine strength and stiffness of contralateral femurs 21 DPF of male αSMACre+NICD1 and their littermate control Cre− mice (n=10) and in B. animals treated with aNRR1 and their controls (aNRR1 n=8; control n=11).
Supplemental figure 4. Chondrocytes lack osteocalcin expression in callus tissue. After OCN staining, slide was stained for Safranin O and when images of OCN staining and Safranin O were merged using Adobe Photoshop CS6 (version 6.0, Adobe Systems Inc., San Jose, CA), osteocalcin positive cells are not present in red cartilage area.
Supplemental figure 5. TRAP staining (purple) is shown in male mice 14 DPF. Histomorphometric parameters of osteoclasts are shown in the right panel. The results are presented as mean value ± SD, n=10.
Supplemental figure 6. Thymocyte differentiation is inhibited by aNRR1 treatment. Experimental design showing treatment protocol. Thymocytes were analyzed 5 DPF for CD4 and CD8 expression. Representative dot plots are showing thymocytes CD4 and CD8 expression in experimental groups. The results are presented as mean value ± SD, n=4 for control and n=6 for aNRR1. **p<0.01.


